Abstract
The Red Sea specimen of the marine sponge Hyrtios erectus (order Dictyoceratida) was found to contain scalarane-type sesterterpenes. 12-O-deacetyl-12,19-di-epi-scalarin (14), a new scalarane sesterterpenoid, along with fourteen previously-reported scalarane-type sesterterpenes (1–13 and 15) have been isolated. The chemical structures of the isolated compounds were elucidated on the basis of detailed 1D and 2D NMR spectral data and mass spectroscopy, as well as by comparison with reported data. The anti-Helicobacter pylori, antitubercular and cytotoxic activities of all fifteen compounds were evaluated to reveal the potency of Compounds 1, 2, 3, 4, 6, 7 and 10. Amongst these, Compounds 1, 3, 4, 6 and 10 displayed a promising bioactivity profile, possessing potent activities in the antitubercular and anti-H. pylori bioassay. Compounds 2 and 7 showed the most promising cytotoxic profile, while Compounds 1 and 10 showed a moderate cytotoxic profile against MCF-7, HCT-116 and HepG2 cell lines.
Keywords: sponges (Porifera); Hyrtios erectus; scalarane sesterterpenoids; 12-O-deacetyl-12,19-di-epi-scalarin; Helicobacter pylori; antitubercular; cytotoxic
1. Introduction
Marine sponges of the genus Hyrtios, order Dictyoceratida, family Thorectidae, occur often in tropical and subtropical sea waters and frequently form the dominant habitat on coral reefs. Previous biological and chemical investigations of the genus Hyrtios and their derived microbial symbionts have showed the presence of numerous natural products possessing a variety of important biological activities and unique structure including scalarane-type sesterterpenes [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21], sesquiterpenes [22,23,24], acyclic triterpenoids [25], macrolides [26,27,28,29], steroids [30], indole and β-carboline alkaloids [24,31,32,33,34,35,36,37,38]. Many sesterterpenoids of the scalarane class showed a variety of pharmacological activities. Among these is the sesterterpene heteronemin [14,39], which exhibited antimycobacterial activity against M. tuberculosis H37Rv with a MIC value of 6.25 µg/mL [40]. Other biological activities of scalaranes include cytotoxic [3,4,5,13,18,19,20,41,42], antifeedant [43,44], antitubercular [6], antimicrobial [45,46], ichthyotoxic [47], anti-inflammatory [14,48], platelet aggregation inhibition [49,50] and nerve growth factor synthesis-stimulation [21,51].
In the course of our ongoing efforts to identify drugs from the sea [2,3,52], we have studied and investigated the extract of the Red Sea marine sponge Hyrtios erectus (Figure 1). We report herein the characterization, anti-H. Pylori, antitubercular and cytotoxic activities of fifteen scalarane-type sesterterpenes including the new compound 12-O-deacetyl-12,19-di-epi-scalarin (14), together with the previously-reported compounds, sesterstatin 7 (1) [6], heteronemin (2) [39], scalarolide (3) [43], 12-epi-24-deoxyscalarin (4) [53], scalarolide acetate (5) [46], 19 acetylsesterstatin 3 (6) [4], 12-deacetyl-12,18-di-epi-scalaradial (7) [43], 12-deacetyl-12-epi-scalaradial (8) [14], sesterstatin 3 (9) [20], 12β,20α-dihydroxy-16β-acetoxy-17-scalaren-19,20-olide (10) [54], 12-O-acetyl-16-O-methylhyrtiolide (11) [55], 12β-acetoxy,16β-methoxy,20α-hydroxy-17-scalaren-19,20-olide (12) [3], 24-methoxypetrosaspongia C (13) [2] and 12-acetoxy,16-epi-hyrtiolide (15) [3]. The previously-known compounds exhibited diverse cytotoxic activity against several cancer cell lines.
Figure 1.
Red Sea sponge Hyrtios erectus (morphology before methanol extraction).
2. Results and Discussion
2.1. Purification of Compounds 1–15
Successive fractionation of the lipophilic fraction obtained from the methanolic extract of the sponge using silica gel column chromatography followed by final purification on semipreparative reversed phase HPLC column afforded fifteen pure isolated compounds (1–15) containing a scalarane-type framework, of which Compound 14 was assigned as a new scalarane sesterterpenoid.
2.2. Structural Elucidation of Compounds 1–15
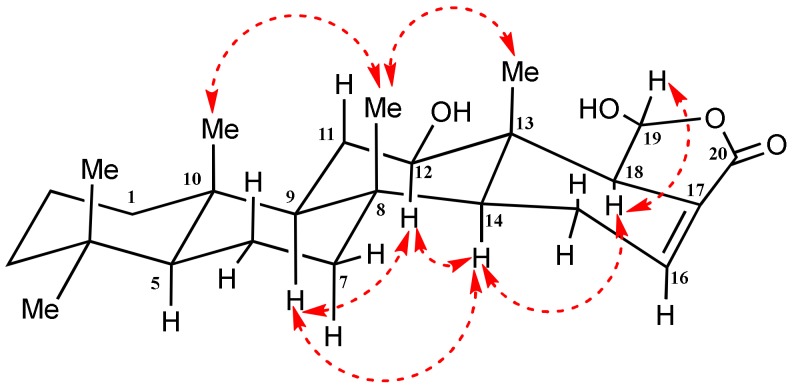
Compound 14 (Figure 2) was isolated and purified as an amorphous powder. The molecular formula, C25H38O4, was established from the positive ESIMS, as well as from 13C-NMR data. In the mass spectrum of Compound 14, the molecular ion peak was of low abundance or absent, but strong peaks corresponding to losses of water were observed at m/z 385.3 [C25H36O3 (M − H2O)⁺] and m/z 367.3 [C25H35O2 (M + H − 2H2O)⁺] (Supplementary Materials, Figure S1). The 1H and 13C-NMR spectra measured in CDCl3 (Table 1), as well as correlations in the HMBC (Table 1, Figure 3) suggested that Compound 14 was also a scalarane-type sesterterpenoid. Thus, five singlets at δH 0.81, 0.83, 0.85, 0.86 and 0.93 were assigned to the five methyl groups of a scalarane skeleton. In the 13C-NMR spectrum of 14 (Table 1), the carbon signals in the low-field region at δC 98.5 (C-19), 127.3 (C-17), 136.3 (C-16) and 166.9 (C-20) were reminiscent of those of scalarin [56] (Figure 2).
Figure 2.
Scalarane Sesterterpenes 1–15.
Table 1.
NMR data and HMBC correlations of Compound 14 (CDCl3).
| Position | δC | δH (m, J in Hz) | HMBC (H→C) a |
|---|---|---|---|
| 1 | 39.8, CH2 | 1.73, 0.83 (m) | C-10 |
| 2 | 18.5, CH2 | 1.63, 1.46 (m) | C-4, C-10 |
| 3 | 42.0, CH2 | 1.39, 1.15 (m) | C-4 |
| 4 | 33.2, C | - | - |
| 5 | 56.4, CH | 0.83 (m) | C-4 |
| 6 | 18.0, CH2 | 1.58, 1.42 (m) | |
| 7 | 41.4, CH2 | 1.74, 0.95 (m) | C-8 |
| 8 | 37.4, C | - | - |
| 9 | 58.8, CH | 0.93 (m) | C-10, C-12 |
| 10 | 37.4, C | - | - |
| 11 | 26.1, CH2 | 1.79, 1.50 (m) | C-10, C-12 |
| 12 | 80.4, CH | 3.57 (dd, 11.05, 4.25) | C-9, C-11, C-18, C-25 |
| 12-OH | 4.19 (s) | ||
| 13 | 39.9, C | - | - |
| 14 | 52.7, CH | 1.25 (m) | C-8, C-9, C-13, C-16, C-18 |
| 15 | 23.5, CH2 | 2.17, 2.37 (m) | C-16 |
| 16 | 136.3, CH | 6.87 (dd, 6.80, 3.40) | C-14, C-15, C-18, C-20 |
| 17 | 127.3, C | - | - |
| 18 | 58.7, CH | 2.54 (m) | C-12, C-13, C-25 |
| 19 | 98.5, CH | 5.74 (d, 5.10) | C-18 |
| 20 | 166.9, C | - | - |
| 21 | 21.3, CH3 | 0.81 (s) | C-4 |
| 22 | 33.2, CH3 | 0.83 (s) | C-4 |
| 23 | 16.5, CH3 | 0.85 (s) | C-1, C-5, C-9, C-10 |
| 24 | 16.7, CH3 | 0.93 (s) | C-7, C-8, C-9, C-14 |
| 25 | 9.1, CH3 | 0.86 (s) | C-12, C-13, C-14, C-18 |
a HMBC correlations are from proton(s) stated for the indicated carbons.
Figure 3.
Selected HMBC correlations observed for Compound 14.
The NMR spectra of Compound 14 (Supplementary Materials, Figures S2–S5) were quite similar to those of scalarin [56] (Figure 2). However, several diagnostic differences were observed. The most evident were the absence of the signals due to the acetyl group, the upfield shift of H-12 at δH 3.57, the chemical shift of CH-18 at δC 58.7, δH 2.54/(δC 50.8, δH 3.14, in scalarin), and the 13C chemical shift of CH-19 at δC 98.5/(δC 98.9 in scalarin).
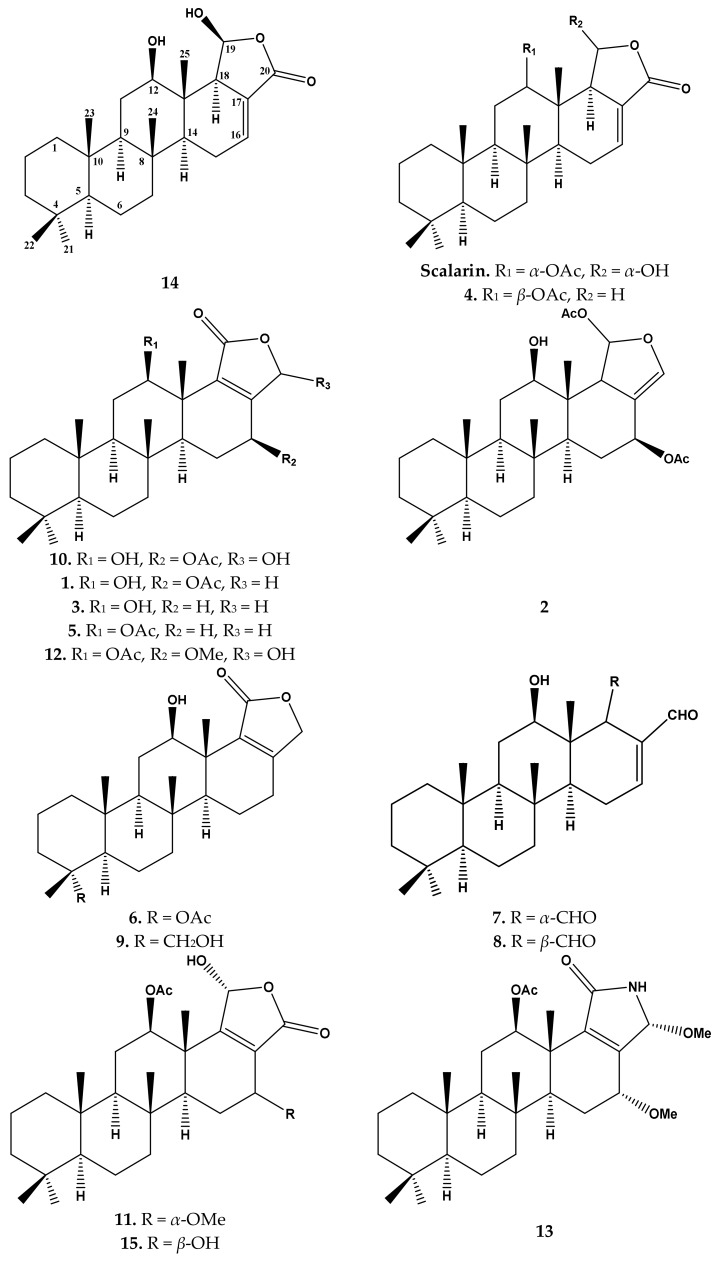
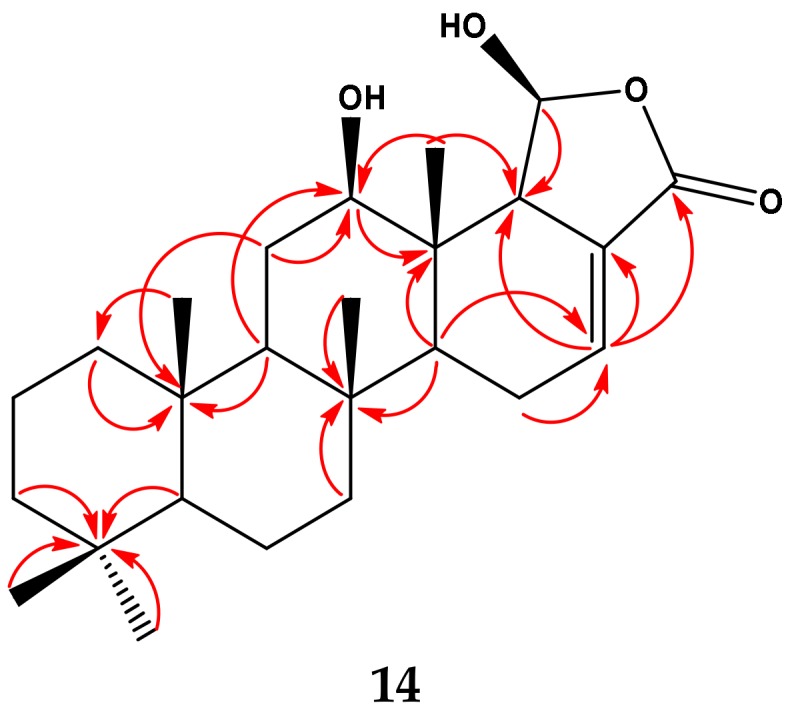
The relative configuration of H-12 and H-19 was detected by coupling constants (for H-12) and confirmed by interpreting the NOESY spectrum (Supplementary Materials, Figure S6). The α-configuration of H-12 was deduced on the basis of the diaxial coupling of H-12 (δH 3.57; dd, J = 11.05 and 4.25 Hz) with H-11 and cross-peaks with α-oriented H-9 and H-14 in NOESY (Figure 4). Finally, the signal assigned to H-19 was correlated in the NOESY spectrum with H-18, which in turn was correlated with H-14, and H-12 indicated the α orientation of H-19 (Figure 4); therefore, the configuration at the C-12/C-19 carbons was opposite to that of scalarin (Figure 2). To the best of our knowledge, Compound 14 has not been reported before in the literature; therefore, it is considered as a new scalarane sesterterpene analogue. The name 12-O-deacetyl-12, 19-di-epi-scalarin was assigned for this compound.
Figure 4.
Important NOESY NMR correlations observed for Compound 14.
The other known Compounds 1–13 and 15 (Figure 2) were identified by extensive study of their spectral data, including ESIMS, 1D and 2D NMR data, as well as by comparison with the published data.
2.3. Biological Activities of the Isolated Compounds 1–15
The isolated Compounds 1–15 were evaluated for their antitubercular, anti-H. pylori and cytotoxic activities (Table 2). Anti-H. pylori compounds are reported herein for the first time for scalarane sesterterpene analogues. Among these, Compounds 1, 3, 4, 6 and 10 displayed a promising bioactivity profile, possessing potent activities in the antitubercular and anti-H. pylori bioassays. Compound 7 displayed an interesting bioactivity profile, possessing potent antitubercular and cytotoxic activities and being practically slightly active in the anti-H. pylori bioassay.
Table 2.
Anti-H. pylori, antitubercular and cytotoxic activities of Compounds 1–15 in µM.
| Compound | Anti-H. pylori (MIC) | Anti-TB (MIC) | Cytotoxic (IC50 ± SEM) | ||
|---|---|---|---|---|---|
| MCF-7 | HCT-116 | HepG2 | |||
| 1 | 4.39 | 0.54 | 24.6 ± 2.3 | 25.5 ± 3.3 | 19.8 ± 1.4 |
| 2 | NA | 16.00 | 1.2 ± 0.1 | 0.4 ± 0.1 | 1.1 ± 0.1 |
| 3 | 10.10 | 5.05 | NA | NA | NA |
| 4 | 9.11 | 1.12 | 25.9 ± 1.9 | 17.5 ± 1.3 | 24.7 ± 4.8 |
| 5 | 146.02 | 9.13 | 22.0 ± 0.4 | 15.2 ± 2.0 | 15.3 ± 1.1 |
| 6 | 8.78 | 4.39 | - | - | - |
| 7 | 20.23 | 5.05 | 1.6 ± 0.1 | 1.4 ± 0.05 | 1.6 ± 0.5 |
| 8 | 80.95 | 10.12 | 32.7 ± 3.2 | 34.5 ± 7.5 | 23.5 ± 1.8 |
| 9 | 77.73 | 19.42 | NA | NA | NA |
| 10 | 8.47 | 4.23 | 12.4 ± 0.9 | 2.9 ± 0.7 | 5.5 ± 0.9 |
| 11 | 263.71 | 16.47 | 55.6 ± 2.1 | 17.8 ± 1.9 | 20.1 ± 1.5 |
| 12 | 32.97 | 8.24 | 37.3 ± 5.5 | 22.8 ± 2.2 | 34.9 ± 6.1 |
| 13 | 16.03 | 8.02 | 54.2 ± 3.3 | 26.5 ± 1.1 | 26.6 ± 3.8 |
| 14 | 81.38 | 20.33 | NA | NA | NA |
| 15 | 33.97 | 16.97 | 34.9 ± 4.9 | 48.6 ± 7.2 | 27.3 ± 3.3 |
| isoniazid | ---- | 0.87 | ---- | ---- | ---- |
| clarithromycin | 1.31 | ---- | ---- | ---- | ---- |
| doxorubicin | ---- | ---- | 0.41 ± 0.1 | 0.11 ± 0.04 | 0.85 ± 0.1 |
NA: No activity within the range of concentration used; data are presented as the mean ± SD; n = 3.
In the cytotoxic assay, the compounds tested against various cell lines (MCF-7, HCT-116 and HepG2) showed variable cytotoxic activity. Amongst these, Compounds 2 and 7 showed the most promising cytotoxic profile with IC50 values ranging from 0.4 ± 0.1–1.2 ± 0.1 µM and from 1.4 ± 0.05–1.6 ± 0.1 µM against the cell lines under investigation, respectively. Compounds 1, 5 and 10 showed a moderate cytotoxic profile with IC50 values less than, or approximately, 20 µM against all cell lines under investigation. Other compounds showed weak to no activity against the cell lines under investigation (Table 2).
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotation was measured on the automatic high-speed laboratory polarimeter P3000 (A.KRUSS Optronic Gmbh, Hamburg, Germany). UV spectra were measured on a Hitachi 300 Spectrophotometer (Hitachi High-Technologies Corporation, Kyoto, Japan). High-resolution ESIMS data were recorded with an ultra-high resolution (UHR) TOF spectrometer (Impact, Bruker, Bremen, Germany). NMR spectra were obtained in CDCl3 on a Bruker Avance DRX 600-MHz spectrometer (Bruker, Bremen, Germany) at 600 MHz for 1H-NMR and 150 MHz for 13C-NMR. NMR chemical shifts were expressed in parts per million (ppm) referenced to residual CDCl3 solvent signals (δH 7.26 for 1H and δC 77.0 for 13C). Precoated SiO2 60 F254 plates (Merck, Darmstadt, Germany) were used for TLC. For column chromatography, SiO2 (70–230 mesh, Merck, Darmstadt, Germany) was used. HPLC purifications were performed on an HPLC column (5-µm ZORBAX Eclipse XDB-C18, 250 × 4.6 mm, Agilent, Santa Clara, CA, USA).
3.2. Biological Materials
The marine sponge specimens used in this study (Figure 1) were collected from the Red Sea, Egypt, by scuba diving. The sponge material was immediately frozen after collection and kept at −20 °C until investigation. The sponge specimen was later identified to be Hyrtios erectus (class: Demospongiae, order: Dictyoceratida, family: Thorectidae) by Dr. Rob van Soest (Institute of Systematic Population Biology, Amsterdam University, The Netherlands). A voucher specimen was preserved at the Zoological Museum of the University of Amsterdam, under Registration Number ZMAPOR19761.
3.3. Purification of Compounds 1–15
The collected sponge specimen (0.90 kg, wet wt.) was cut into small pieces and was macerated exhaustively at room temperature in MeOH. The combined extracts were concentrated under reduced pressure to yield the organic crude extract (85 g). The total crude extract was subjected to silica gel column using VLC (vacuum liquid chromatography) gradient elution (n-hexane–CHCl3–MeOH) to afford Fractions 1–9.
Fraction 4 (n-hexane–CHCl3, 1:3) was chromatographed on silica gel column with n-hexane–CHCl3–MeOH gradient elution to give 8 further fractions. Of these, Fraction 3 (700 mg) was fractionated over a silica gel column chromatography (n-hexane–CHCl3), then finally purified by HPLC (ODS XDP-Zorbax column, 5 µm, 250 × 4.6 mm, 80% CH3CN/H2O, 1.5-mL/min flow rate and 220-nm UV detection), and Compounds 1–8 were obtained (4.6, 14, 3.4, 3, 4, 2.9, 2.3 and 3.5 mg, respectively). In turn, Fraction 5 (137 mg) was further chromatographed on silica gel column chromatography followed by final purification on HPLC the same as Fraction 3, and compounds 9–14 were obtained (2.2, 4, 3, 2.3, 2.7 and 1.3 mg, respectively). Finally, Fraction 6 (55 mg) was also chromatographed and purified as Fraction 3 to obtain Compound 15 (3.5 mg).
Compound 14: Amorphous solid (1.3 mg); +112.0 (c 0.1, CHCl3); UV (λmax, MeOH) (log ε): 226 (4.31), 285 (2.54) nm; NMR data: see Table 1; ESI-MS: m/z 385.3 [M − H2O]⁺. HRESIMS: m/z 403.2851 (calculated for C25H39O4 [M + H]⁺, 403.2848).
3.4. Biological Activity of Compounds 1–15
3.4.1. Anti-Helicobacter pylori Activity Assessment
The anti-H. pylori activity was assessed against a strain of Helicobacter pylori (American Type Culture Collection, H.b., ATCC 700392) using a micro-well dilution method as previously described [57]. Clarithromycin was used as a positive control and exhibited an MIC of 1.31 µM.
3.4.2. Antitubercular Activity Assessment
A nonvirulent strain Mycobacterium tuberculosis (ATCC 25177, H37Ra) was obtained from the American Type Culture Collection. The antitubercular activity was assessed according to the protocol described by Franzblau [58,59] using the microplate Alamar blue assay (MABA). Isoniazid was used as positive control and exhibited an MIC of 0.87 µM.
3.4.3. Cytotoxic Activity Assessment
3.4.3.1. Cell Culture
Human hepatocellular carcinoma cells (HepG2), colorectal adenocarcinoma cells (HCT-116) and human breast adenocarcinoma cells (MCF-7) were obtained from the VACSERA (Giza, Egypt). HCT-116 cells were maintained in Roswell Park Memorial Institute Media (RPMI-1640), while HepG2 and MCF-7 cells were maintained in Dulbecco Modified Eagle’s Media (DMEM). All culture media were supplemented with heat-inactivated fetal bovine serum (10% v/v), penicillin-G (100 units/mL) and streptomycin sulfate (100 µg/mL). Cells were passaged in a humidified chamber (37 °C and 5% (v/v) CO2).
3.4.3.2. Trypan-Blue Exclusion Assay
Cell viability was measured prior to seeding using the trypan-blue exclusion method. Briefly, exponentially-growing cells were trypsinized by 0.25% trypsin-EDTA solution. Aliquots of cell suspensions were mixed with trypan blue solution (0.4% w/v), and blue color (positive cells) was determined. Cells were not seeded unless viability was greater than 95%.
3.4.3.3. Cytotoxic Assessment
The cytotoxic effects of Compounds 1–15 on breast (MCF-7), colorectal (HCT-116) and liver cancer cells (HepG2) were evaluated using the sulforhodamine B (SRB) method as previously described [60]. Briefly, mid-exponentially-proliferating cells were trypsinized by trypsin-EDTA (0.25% w/v) and seeded in 96-well plates (1000–2000 cells/well). Cells were treated with serial concentrations of the compounds under investigation for 72 h and subsequently fixed with TCA (10% w/v) for 1 h at 4 °C. After washing three times, cells were stained with 0.4% SRB solution for 10 min in a dark place and then washed with glacial acetic acid (1% v/v). After drying, Tris-HCl (50 mM, pH 7.4) was used to dissolve the SRB-stained cells, and color intensity was measured at 540 nm. The dose response relationship of test compounds was analyzed using the Emax model (Equation (1)).
| (1) |
where (R) is the residual unkilled fraction (the resistance fraction), (D) is the drug concentration used, (Kd) or IC50 is the drug concentration needed to produce 50% viability reduction and (m) is the Hill-type coefficient. IC50 is defined as the drug concentration required to reduce maximum absorbance by 50% compared to untreated control cells [61].
3.4.3.4. Statistical Analysis
Data are presented as the mean ± SEM using GraphPad prism™ software Version 5.00 (GraphPad software Inc., La Jolla, CA, USA) for Windows Version 10.00. Analysis of variance (ANOVA) with the Tukey–Kremer post hoc test was used to calculate the significance using SPSS® for Windows, Version 17.0.0. p < 0.05 was taken as a cut-off value for significance.
4. Conclusions
Chemical investigation of the bioactive extract of the sponge Hyrtios erectus, collected in the Red Sea, Egypt, yielded fifteen compounds of scalarane-type sesterterpenes. Compounds 1–15 including a new one (14) were purified, and their chemical structures were characterized using spectroscopic studies. The isolated compounds belong to the class of scalarane sesterterpenes and displayed diverse biological activities including anti-H. pylori, antitubercular and cytotoxic. Anti-H. pylori compounds are reported herein for the first time for scalarane sesterterpene analogues. Among these, Compounds 1, 3, 4, 6 and 10 displayed a promising bioactivity profile, possessing potent activities in the antitubercular and anti-H. pylori bioassays. Compound 7 displayed an interesting bioactivity profile, possessing potent antitubercular and cytotoxic activities and being practically slightly active in the anti-H. pylori bioassay. Moreover, Compounds 2 and 7 showed the most promising cytotoxic profile, while Compounds 1, 5 and 10 showed a moderate cytotoxic profile against MCF-7, HCT-116 and HepG2 cell lines. Other compounds showed weak to no activity against the cell lines under investigation.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under Grant No. G-321-166-38. The authors, therefore, acknowledge with thanks DSR for technical and financial support. We thank Rob van Soest for taxonomic identification of the sponge specimen. We also thank Alaa Khedr for mass spectrometric analysis of the compounds.
Supplementary Materials
The supplementary materials are available online.
Author Contributions
S.S.E. and S.A.A. designed the experiments. S.A.A. collected the sponge specimen. S.S.E. performed the experiments. A.O.N., A.M.A.-A. and M.A.E. performed the biological activity study. S.S.E., H.Z.A. and S.A.A. analyzed the data. A.M.A. and S.S.E. wrote and edited the manuscript.
Conflicts of Interest
All contributing authors declare no conflicts of interest to disclose, whether financial or of any other nature.
Footnotes
Sample Availability: Not available.
References
- 1.Mehbub M.F., Perkins M.V., Zhang W., Franco C.M.M. New marine natural products from sponges (Porifera) of the order Dictyoceratida (2001 to 2012); a promising source for drug discovery, exploration and future prospects. Biotechnol. Adv. 2016;34:473–491. doi: 10.1016/j.biotechadv.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Elhady S.S., El-Halawany A.M., Alahdal A.M., Hassanean H.A., Ahmed S.A. A new bioactive metabolite isolated from the Red Sea marine sponge Hyrtios erectus. Molecules. 2016;21:82. doi: 10.3390/molecules21010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elhady S.S., Al-Abd A.M., El-Halawany A.M., Alahdal A.M., Hassanean H.A., Ahmed S.A. Antiproliferative scalarane-based metabolites from the Red Sea sponge Hyrtios erectus. Mar. Drugs. 2016;14:130. doi: 10.3390/md14070130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youssef D.T.A., Yamaki R.K., Kelly M., Scheuer P.J. Salmahyrtisol A, a novel cytotoxic sesterterpene from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2002;65:2–6. doi: 10.1021/np0101853. [DOI] [PubMed] [Google Scholar]
- 5.Pettit G.R., Tan R., Cichacz Z.A. Antineoplastic agents. 542. isolation and structure of sesterstatin 6 from the Indian Ocean sponge Hyrtios erecta. J. Nat. Prod. 2005;68:1253–1255. doi: 10.1021/np0402221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youssef D.T.A., Shaala L.A., Emara S. Antimycobacterial scalarane-based sesterterpenes from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2005;68:1782–1784. doi: 10.1021/np0502645. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Y., Deng Z., Pei Y., Fu H., Li J., Proksch P., Lin W. Sesterterpenoids from the marine sponge Hyrtios erectus. J. Nat. Prod. 2004;67:921–924. doi: 10.1021/np030457x. [DOI] [PubMed] [Google Scholar]
- 8.Miyaoka H., Nishijima S., Mitome H., Yamada Y. Three new scalarane sesterterpenoids from the Okinawan sponge Hyrtios erectus. J. Nat. Prod. 2000;63:1369–1372. doi: 10.1021/np000115g. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi M., Okamoto T., Hayashi K., Yokoyama N., Sasaki T., Kitagawa I. Marine natural products. XXXII. Absolute configurations of C-4 of the manoalide family, biologically active sesterterpenes from the marine sponge Hyrtios erecta. Chem. Pharm. Bull. 1994;42:265–270. doi: 10.1248/cpb.42.265. [DOI] [PubMed] [Google Scholar]
- 10.Bourguet-Kondracki M.L., Martin M.T., Debitus C., Guyot M. 12-epi-heteronemin: New sesterterpene from the marine sponge Hyrtios erecta. Tetrahedron Lett. 1994;35:109–110. doi: 10.1016/0040-4039(94)88175-8. [DOI] [Google Scholar]
- 11.Iguchi K., Shimada Y., Yamada Y. Hyrtiosal, a new sesterterpenoid with a novel carbon skeleton from the Okinawan marine sponge Hyrtios erectus. J. Org. Chem. 1992;57:522–524. doi: 10.1021/jo00028a023. [DOI] [Google Scholar]
- 12.Crews P., Bescansa P., Bakus G.J. A non-peroxide norsesterterpene from a marine sponge Hyrtios erecta. Experientia. 1985;41:690–691. doi: 10.1007/BF02007724. [DOI] [PubMed] [Google Scholar]
- 13.Ryu G., Matsunaga S., Fusetani N. Three new cytotoxic sesterterpenes from the marine sponge Hyrtios cf. erectus. J. Nat. Prod. 1996;59:515–517. doi: 10.1021/np960130e. [DOI] [PubMed] [Google Scholar]
- 14.Crews P., Bescansa P. Sesterterpenes from a common marine sponge, Hyrtios erecta. J. Nat. Prod. 1986;49:1041–1052. doi: 10.1021/np50048a012. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiya N., Sato A., Hata T., Sato N., Sasagawa K., Kobayashi T. Cytotoxic scalarane sesterterpenes from a sponge, Hyrtios erecta. J. Nat. Prod. 1998;61:468–473. doi: 10.1021/np970462z. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch G., Koeng G.M., Wright A.D., Kaminsky R. A new bioactive sesterterpene and antiplasmodial alkaloids from the marine sponge Hyrtios cf. erecta. J. Nat. Prod. 2000;63:825–829. doi: 10.1021/np990555b. [DOI] [PubMed] [Google Scholar]
- 17.Fontana A., Cavaliere P., Ungur N., D’Souza L., Parameswaram P.S., Cimino G. New scalaranes from the nudibranch Glossodoris atromarginata and its sponge prey. J. Nat. Prod. 1999;62:1367–1370. doi: 10.1021/np9900932. [DOI] [PubMed] [Google Scholar]
- 18.Pettit G.R., Cichaz Z.A., Tan R., Herald D.L., Melody N., Hoard M.S., Doubek D.L., Hooper J.N.A. Antineoplastic agents. 385. The isolation and structure of a scalarane-type sesterterpene from the Indian Ocean porifera Hyrtios erecta. Collect. Czech. Chem. Commun. 1998;63:1671–1677. doi: 10.1135/cccc19981671. [DOI] [Google Scholar]
- 19.Pettit G.R., Tan R., Melody N., Cichacz Z.A., Herald D.L., Hoard M.S., Pettit R.K., Chapuis J.-C. Antineoplastic agents. 397: Isolation and structure of sesterstatins 4 and 5 from Hyrtios erecta (the Republic of Maldives) Bioorg. Med. Chem. Lett. 1998;8:2093–2098. doi: 10.1016/S0960-894X(98)00373-4. [DOI] [PubMed] [Google Scholar]
- 20.Pettit G.R., Cichacz Z.A., Tan R., Hoard M.S., Melody N., Pettit R.K. Antineoplastic agents. 386. isolation of sesterstatins 1-3 from the marine sponge Hyrtios erecta. J. Nat. Prod. 1998;61:13–16. doi: 10.1021/np970203+. [DOI] [PubMed] [Google Scholar]
- 21.Doi Y., Shigemori H., Ishibashi M., Mizobe F., Kawashima A., Nakaike S., Kobayashi J.I. New sesterterpenes with nerve growth factor synthesis-stimulating activity from the Okinawan marine sponge Hyrtios sp. Chem. Pharm. Bull. 1993;41:2190–2191. doi: 10.1248/cpb.41.2190. [DOI] [PubMed] [Google Scholar]
- 22.Youssef D.T.A., Singab A.N.B., van Soest R.W.M., Fusetani N. Hyrtiosenolides A and B, two new sesquiterpene γ-methoxybutenolides and a new sterol from a Red Sea sponge Hyrtios species. J. Nat. Prod. 2004;67:1736–1739. doi: 10.1021/np049853l. [DOI] [PubMed] [Google Scholar]
- 23.Piña I.C., Sanders M.L., Crews P. Puupehenone congeners from an Indo-Pacific Hyrtios sponge. J. Nat. Prod. 2003;66:2–6. doi: 10.1021/np020279s. [DOI] [PubMed] [Google Scholar]
- 24.Salmoun M., Devijver C., Daloze D., Braekman J.C., Gomez R., de Kluijver M., Van Soest R.W.M. New sesquiterpene/quinones from two sponges of the genus Hyrtios. J. Nat. Prod. 2000;63:452–456. doi: 10.1021/np9903346. [DOI] [PubMed] [Google Scholar]
- 25.Williams D.E., Tahir A., Andersen R.J. A new acyclic diketotriterpenoid isolated from the Indonesian marine sponge Hyrtios erectus. J. Nat. Prod. 1999;62:653–654. doi: 10.1021/np980526l. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi M., Aoki S., Sakai H., Kawazoe K., Kihara N., Sasaki T., Kitagawa I. Altohyrtin A, a potent anti-tumor macrolide from the Okinawan marine sponge Hyrtios altum. Tetrahedron Lett. 1993;34:2795–2798. doi: 10.1016/S0040-4039(00)73564-7. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M., Aoki S., Sakai H., Kihara N., Sasaki T., Kitagawa I. Altohyrtins B and C and 5-desacetylaltohyrtin A, potent cytotoxic macrolide congeners of altohyrtin A, from the Okinawan marine sponge Hyrtios altum. Chem. Pharm. Bull. 1993;41:989–991. doi: 10.1248/cpb.41.989. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi M., Aoki S., Kitagawa I. Absolute stereostructures of altohyrtin A and its congeners, potent cytotoxic macrolides from the Okinawan marine sponge Hyrtios altum. Tetrahedron Lett. 1994;35:1243–1246. doi: 10.1016/0040-4039(94)88034-4. [DOI] [Google Scholar]
- 29.Kobayashi M., Aoki S., Gato K., Kitagawa I. Marine natural products. XXXVIII. Absolute stereostructures of altohyrtins A, B, and C and 5-desacetylaltohyrtin A, potent cytotoxic macrolides, from the Okinawan marine sponge Hyrtios altum. Chem. Pharm. Bull. 1996;44:2142–2149. doi: 10.1248/cpb.44.2142. [DOI] [Google Scholar]
- 30.Koch P., Djerassi C., Lakshmi V., Schmitz F.J. Identification of sterols with oxygenated side chain in a sponge of the species Hyrtios. Helv. Chim. Acta. 1983;66:2431–2436. doi: 10.1002/hlca.19830660806. [DOI] [Google Scholar]
- 31.Kobayashi J., Murayama T., Ishibashi M., Kosuge S., Takamatsu M., Ohizumi Y., Kobayashi H., Ohta T., Nozoe S., Sasaki T. Hyrtiosins A and B, new indole alkaloids from the Okinawan marine sponge Hyrtios erecta. Tetrahedron. 1990;46:7699–7702. doi: 10.1016/S0040-4020(01)90065-1. [DOI] [Google Scholar]
- 32.Kobayashi M., Aoki S., Gato K., Matsunami K., Kurosu M., Kitagawa I. Marine natural products. XXXIV. Trisindoline, a new antibiotic indole trimer, produced by a bacterium of Vibrio sp. separated from the marine sponge Hyrtios altum. Chem. Pharm. Bull. 1994;42:2449–2451. doi: 10.1248/cpb.42.2449. [DOI] [PubMed] [Google Scholar]
- 33.Bourguet-Kondracki M.L., Martin M.T., Guyot M. A new β-carboline alkaloid isolated from the marine sponge Hyrtios erecta. Tetrahedron Lett. 1996;37:3457–3460. doi: 10.1016/0040-4039(96)00573-4. [DOI] [Google Scholar]
- 34.Varoglu M., Corbett T.H., Valeriote F.A., Crews P. Asperazine, a selective cytotoxic alkaloid from a sponge-derived culture of Aspergillus niger. J. Org. Chem. 1997;62:7078–7079. doi: 10.1021/jo970568z. [DOI] [PubMed] [Google Scholar]
- 35.Youssef D.T.A. Hyrtioerectines A-C, cytotoxic alkaloids from the Red Sea sponge Hyrtios erectus. J. Nat. Prod. 2005;68:1416–1419. doi: 10.1021/np050142c. [DOI] [PubMed] [Google Scholar]
- 36.Sauleau P., Martin M.-T., Dau M.-E.T.H., Youssef D.T.A., Bourguet-Kondracki M.-L. Hyrtiazepine, an azepino-indole-type alkaloid from the Red Sea marine sponge Hyrtios erectus. J. Nat. Prod. 2006;69:1676–1679. doi: 10.1021/np060132r. [DOI] [PubMed] [Google Scholar]
- 37.Salmoun M., Devijver C., Daloze D., Braekman J.-C., Van Soest R.W.M. 5-Hydroxytryptamine-derived alkaloids from two marine sponges of the genus Hyrtios. J. Nat. Prod. 2002;65:1173–1176. doi: 10.1021/np020009+. [DOI] [PubMed] [Google Scholar]
- 38.Aoki S., Ye Y., Higuchi K., Takashima A., Tanaka Y., Kitagawa I., Kobayashi M. Novel neuronal nitric oxide synthase (nNOS) selective inhibitors, aplysinopsin-type indole alkaloids, from marine sponge Hyrtios erecta. Chem. Pharm. Bull. 2001;49:1372–1374. doi: 10.1248/cpb.49.1372. [DOI] [PubMed] [Google Scholar]
- 39.Kazlauskas R., Murphy P., Quinn R., Wells R. Heteronemin, a new scalarin type sesterterpene from the sponge Heteronema erecta. Tetrahedron Lett. 1976;17:2631–2634. doi: 10.1016/S0040-4039(00)91753-2. [DOI] [Google Scholar]
- 40.El Sayed K.A., Bartyzel P., Shen X., Perry T.L., Zjawiony J.K., Hamann M.T. Marine natural products as antituberculosis agents. Tetrahedron. 2000;56:949–953. doi: 10.1016/S0040-4020(99)01093-5. [DOI] [Google Scholar]
- 41.Nasu S.S., Yeung B.K.S., Hamann M.T., Scheuer P.J., Kelly-Borges M., Goins K. Puupehenone-related metabolites from two Hawaiian sponges, Hyrtios spp. J. Org. Chem. 1995;60:7290–7292. doi: 10.1021/jo00127a039. [DOI] [Google Scholar]
- 42.Evidente A., Kornienko A., Lefranc F., Cimmino A., Dasari R., Evidente M., Mathieu V., Kiss R. Sesterterpenoids with anticancer activity. Curr. Med. Chem. 2015;22:3502–3522. doi: 10.2174/0929867322666150821101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker R.P., Thompson J.E., Faulkner D.J. Sesterterpenes from Spongia idia. J. Org. Chem. 1980;45:4976–4979. doi: 10.1021/jo01312a032. [DOI] [Google Scholar]
- 44.Terem B., Scheuer P.J. Scalaradial derivatives from the nudibranch Chromodoris youngbleuthi and the sponge Spongia oceania. Tetrahedron. 1986;42:4409–4412. doi: 10.1016/S0040-4020(01)87279-3. [DOI] [Google Scholar]
- 45.Hochlowski J.E., Faulkner D.J., Bass L.S., Clardy J. Metabolites of the dorid nudibranch Chromodoris sedna. J. Org. Chem. 1983;48:1738–1740. doi: 10.1021/jo00158a030. [DOI] [Google Scholar]
- 46.Bergquist P.R., Cambie R.C., Kernan M.R. Scalarane sesterterpenes from Collospongia auris, a new thorectid sponge. Biochem. Syst. Ecol. 1990;18:349–357. doi: 10.1016/0305-1978(90)90008-4. [DOI] [Google Scholar]
- 47.Braekman J.C., Daloze D., Kaisin M., Moussiaux B. Ichthyotoxic sesterterpenoids from the neo guinean sponge Carteriospongia foliascens. Tetrahedron. 1985;41:4603–4614. doi: 10.1016/S0040-4020(01)82355-3. [DOI] [Google Scholar]
- 48.Kikuchi H., Tsukitani Y., Shimizu I., Kobayashi M., Kitagawa I. Marine Natural Products. XI. An antiinflammatory scalarane-type bishomosesterterpene, foliaspongin, from the Okinawan marine sponge Phyllospongia foliascens (PALLAS) Chem. Pharm. Bull. 1983;31:552–556. doi: 10.1248/cpb.31.552. [DOI] [Google Scholar]
- 49.Nakagawa M., Hamamoto Y., Ishihama M., Hamasaki S., Endo M. Pharmacologically active homosesterterpenes from Palauan sponges. Tetrahedron Lett. 1987;28:431–434. doi: 10.1016/S0040-4039(00)95747-2. [DOI] [Google Scholar]
- 50.Kazlauskas R., Murphy P., Wells R. Five new C26 tetracyclic terpenes from a sponge (Lendenfeldia sp.) Aust. J. Chem. 1982;35:51–59. doi: 10.1071/CH9820051. [DOI] [Google Scholar]
- 51.A Gonzalez M. Scalarane sesterterpenoids. Curr. Bioact. Compd. 2010;6:178–206. doi: 10.2174/157340710793237362. [DOI] [Google Scholar]
- 52.Alahdal A.M., Shaala L.A., Noor A.O., Elfaky M.A., Elhady S.S., Almohammadi A., Bagalagel A., Lashkar M.O., Almasri D.M., Youssef D. Evaluation of the antiproliferative and cytotoxic activities of marine invertebrates-derived fungi. Pak. J. Pharm. Sci. 2017;30:1001–1006. [PubMed] [Google Scholar]
- 53.Cimino G., De Stefano S., Minale L., Trivellone E. 12-epi-Scalarin and 12-epi-deoxoscalarin, sesterterpenes from the sponge Spongia nitens. J. Chem. Soc. Perkin Trans. 1977;1:1587–1593. doi: 10.1039/p19770001587. [DOI] [PubMed] [Google Scholar]
- 54.Mahidol C., Prawat H., Sangpetsiripan S., Ruchirawat S. Bioactive scalaranes from the Thai sponge Hyrtios gumminae. J. Nat. Prod. 2009;72:1870–1874. doi: 10.1021/np900267v. [DOI] [PubMed] [Google Scholar]
- 55.Tsukamoto S., Miura S., Van Soest R.W., Ohta T. Three new cytotoxic sesterterpenes from a marine sponge Spongia sp. J. Nat. Prod. 2003;66:438–440. doi: 10.1021/np020497l. [DOI] [PubMed] [Google Scholar]
- 56.Fattorusso E., Magno S., Santacroce C., Sica D. Scalarin, a new pentacyclic C-25 terpenoid from the sponge Cacospongia scalaris. Tetrahedron. 1972;28:5993–5997. doi: 10.1016/0040-4020(72)88132-8. [DOI] [Google Scholar]
- 57.Bonacorsi C., Raddi M.S.G., Carlos I.Z., Sannomiya M., Vilegas W. Anti-helicobacter pylori activity and immunostimulatory effect of extracts from Byrsonima crassa Nied. (Malpighiaceae) BMC Complement. Altern. Med. 2009;9:2. doi: 10.1186/1472-6882-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Franzblau S.G., Witzig R.S., McLaughlin J.C., Torres P., Madico G., Hernandez A., Degnan M.T., Cook M.B., Quenzer V.K., Ferguson R.M., et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins L., Franzblau S.G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997;41:1004–1009. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Vistica D., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 61.Mahmoud A.M., Al-Abd A.M., Lightfoot D.A., El-Shemy H.A. Anti-cancer characteristics of mevinolin against three different solid tumor cell lines was not solely p53-dependent. J. Enzyme Inhib. Med. Chem. 2012;27:673–679. doi: 10.3109/14756366.2011.607446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.