Abstract
A series of linear dipeptide derivatives (4–10) were prepared and evaluated as antimicrobial agents via the synthesis of N-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl) nicotinamide (4). Compound 4 was reacted with 4-chlorobenzaldehyde or 4-hydroxybenzaldehyde, to give the hydrazones 5 and 6, respectively. On the other hand, Compound 4 was coupled with phenylisocyanate or methylisothiocyanate to give Compounds 7 and 8, respectively. The latter compounds (7 and 8) were coupled with chloroacetic acid to give oxazolidine (9) and thiazolidine (10), respectively. The newly synthesized dipeptide compounds were confirmed by means of their spectral data. The antimicrobial activity of the newly synthesized compounds 4–10 was evaluated by agar well diffusion, and they showed good activity. Compounds 4, 5, and 9 gave the most promising activity in this study. Most of the tested compounds possessed MIC values ranging from 50 to 500 µg/mL. Furthermore, docking studies were carried out on enoyl reductase from E. coli and cytochrome P450 14 α-sterol demethylase (Cyp51) from Candida albicans active sites. The MolDock scores of the seven tested compounds ranged between −117 and −171 and between −107 and −179, respectively.
Keywords: nicotinoyl chloride, amino acids, linear dipeptides, antimicrobial activity, molecular docking studies
1. Introduction
Nicotinic acid (also known as pyridine-3-carboxylic acid, vitamin B3, and niacin) is a widely used in the food, biochemical, and pharmaceutical industries. It is found in various plants and animals [1] and plays a vital role in biological processes such as the production of energy [2]. Nicotinic acid supplements (astatin drug) have not been found useful for decreasing the risk of cardiovascular disease [3] but appear to be highly effective in those not taking a statin, such as ezetimibe, simvastatin, gemfibrozil, cholestyramine, colestipol, or clofibrate [4]. Although nicotinic acid and nicotinamide are similar in their vitamin activity, nicotinamide does not have the same pharmacological properties and lipid-modifying effects as nicotinic acid. However, some of the new heterocyclic and peptide derivatives have been studied with respect to antiviral [5], anti-inflammatory [6], enzymatic peptide [7], and antimicrobial activities [8,9]. Additionally, there have been several successful publications of peptide synthesis functioning as an antimicrobial for therapeutic applications [10]. A large number of antimicrobial peptides show highly broad-spectrum activities against different microorganisms, Gram-positive and Gram-negative bacteria, fungi, and viruses [11]. Many peptide candidates have antimicrobial properties against multiple drug resistances and possess a low tendency for the improvement of said resistances [12,13,14]. Peptides are being used as therapeutic agents such as Lupron™, Sandostatin™, and Zoladex™ [15,16]. Peptides are intrinsically able to interact with biological systems and are therefore potent therapeutics [17,18,19]. Due to the importance of nicotinic acid derivatives, amino acids and peptides act as bioactive compound. The objective of this study was to prepare novel antimicrobial dipeptide compounds that are based on N-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide.
2. Results and Discussion
2.1. Chemistry
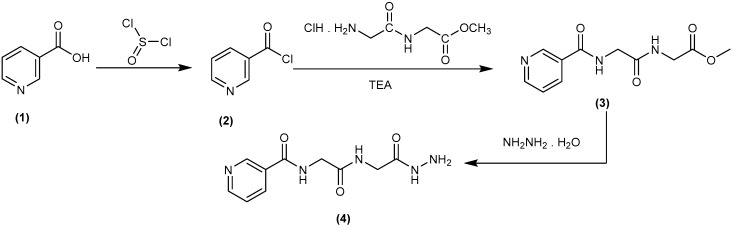
In our previous studies of peptide candidates, good antimicrobial properties [20,21,22,23,24] and anticancer activities [25,26,27,28], as well as, pyrazolo derivatives as anticancer agents [29]. Thus, this study aims to conjugate dipeptides and nicotinic acid. Some of the novel derivatives (5–10) were synthesized based on nicotinyl-glycyl-glycine-hydrazide (4), which may be expected to possess various antibacterial and antifungal properties. Accordingly, a rational design, synthesis, purification, and structural characterization of nicotinyl-glycyl-glycine-hydrazide, were optimally synthesized via conventional synthetic peptide coupling methods (in solution). The compound nicotinyl-glycyl-glycine-hydrazide (4) was synthesized by the conversion of nicotinic acid (1) to nicotinoyl chloride (2) through thionyl chloride, while Compound 2 was then coupled with a free glycyl-glycine methyl ester to give glycyl-glycine-methylester (3). IR of Compound 3 showed absorption bands at 3400 cm−1 due to NH in addition to absorptions of carbonyl groups at 1734, 1660, and 1580 cm−1, respectively. 1H-NMR of the ester (3) revealed two signals in the region δ 3.7–3.3 of 2CH2 in addition to the D2O exchangeable signals of amidic. Hydrazinolysis of Compound 3 with hydrazine hydrate (99%) led to the corresponding dipeptide hydrazide (4) in 65% yield (Scheme 1). 1H-NMR of hydrazide (4) exhibited D2O exchangeable signals at δ 10.67, 9.04, 8.99, and 8.50 in addition to the signals of 2CH2 at δ 3.8–3.5. The mass spectrum of Compound 4 showed a peak equal to its molecular weight at m/z = 252 (M+ + 1).
Scheme 1.
Synthetic routes for N-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide (4).
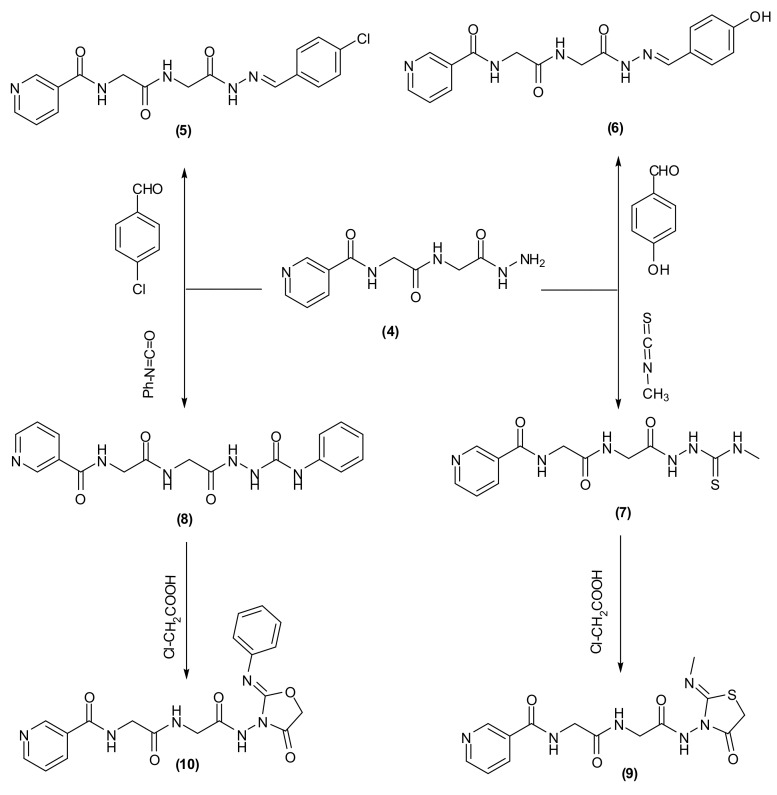
The condensation of the N-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide, (Compound 4) with 4-chlorobenzaldehyde or 4-hydroxybenzaldehyde took place through refluxing in ethanol using piperidine as an organic base. This produced substituted hydrazides (5,6) (Scheme 2). 1H-NMR of the hydrazones 5 and 6 showed the characteristic signal of –N=CH– in the range δ 8.36–8.16.
Scheme 2.
Synthetic routes for N-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide derivatives (5–10).
On the other hand, Compound 4 was reacted with methyl isothiocyanate or phenylisocyanate in dry dioxane, which produced hydrazinecarbothioamide or hydrazinecarboxamide derivatives (7,8), respectively (Scheme 2). The structure of Compounds 7 and 8 was established under the basis of their spectral data. Compounds 7 and 8 were reacted with chloroacetic acid in ethanol to give thiazolidine or oxazolidin derivatives (9,10), respectively (Scheme 2). 1H-NMR spectra showed characteristic singlets at δ 4.45 (CH2 of thiazolidine ring), 3.17, 2.95 (2CH2 of α-gly) for Compound 9 and at δ 4.29 (CH2 of oxazolidine ring), 3.67, 3.05 (2CH2 of α-gly) for Compound 10.
2.2. Biological Evaluations
The antibacterial and antifungal activities of the synthesized Compounds 5–10 and the starting Compound 4 against a panel of pathogenic tested organisms are represented in Table 1. The results reveal that some of these synthesized candidates are highly biologically active with different spectrum activities.
Table 1.
The antimicrobial activities of the synthesized candidates expressed as inhibition zones of growth in millimeters against the used test organisms.
| Compounds | Test Organisms | |||
|---|---|---|---|---|
| Bacteria | Fungi | |||
| Gram-Positive | Gram-Negative | Unicellular | Filamentous | |
| B. subtilis | E. Coli | C. albicans | A. niger | |
| Inhibition zone (mm) | ||||
| 4 | 29 | 30 | 28 | 16 |
| 5 | 20 | 19 | 25 | 20 |
| 6 | 16 | 15 | 17 | 00 |
| 7 | 20 | 20 | 18 | 00 |
| 8 | 18 | 17 | 18 | 00 |
| 9 | 30 | 15 | 30 | 00 |
| 10 | 20 | 20 | 18 | 00 |
| Standard Antimicrobial Antibiotics | ||||
| NA = 30 µg | 20 | 16 | 00 | 00 |
| S = 10 µg | 14 | 00 | 12 | 00 |
| NV = 30 µg | 29 | 30 | 00 | 00 |
| T = 30 µg | 30 | 27 | 00 | 00 |
| CDZ = 30 µg | 00 | 20 | 00 | 00 |
| VA = 30 µg | 21 | 23 | 00 | 00 |
| Ny = 100 µg | 00 | 00 | 14 | 15 |
| CLT = 50 µg | 00 | 00 | 12 | 10 |
| FLC = 25 µg | 00 | 00 | 13 | 11 |
Standard bacterial and fungal antibiotics. NA = Negram (nalidixic acid); S = Streptomycin; NV = Novobiocin; T = Oxytetracycline; CDZ = Cefodizime; VA = Vancomycin; Ny = Nystatin; CLT = Clotrimazole; FLC = Fluconazole.
The results in Table 1 showed variations in antimicrobial effect against each test pathogenic microorganisms. In the case of Bacillusubtilis, Compounds 4 and 9 showed a strong inhibitory effect, and an inhibition zone (IZ) diameters of 29 mm and 30 mm were recorded. The other compounds recorded a moderate inhibitory effect and ranged from 16–20 mm in comparison to the antibiotic drug used as the standard antibacterial in this study. On the other hand, in the case of E. coli, Compound 4 showed a strong inhibitory effect, and Compounds 6 and 9 showed a weak inhibitory effect as well. The other tested compounds recorded a moderate inhibition effect and ranged from 17–20 mm in comparison to the reference drug.
By testing the compounds against Candida albicans, Compounds 4, 5, and 9 showed strong inhibitory effects and had IZ diameters of 25–30 mm. Moreover, Compounds 6, 7, 8, and 10 showed a moderate inhibition effect, and the IZ diameters ranged between 16 and 18 mm in comparison to the antifungal drug that was used in this study. In the case of the pathogenic fungi Aspergillus niger, most of the compounds had a negative effect against the test pathogen except Compounds 4 and 5, which showed a moderate inhibition effect and ranged from 16–20 mm.
Finally, all compounds of this series, the newly synthesized Compounds 4–10, displayed activity against Gram-positive, Gram-negative, and fungi. The results for the inhibitory activity represented a promising indication in Gram-positive, Gram-negative, and fungi pathogens.
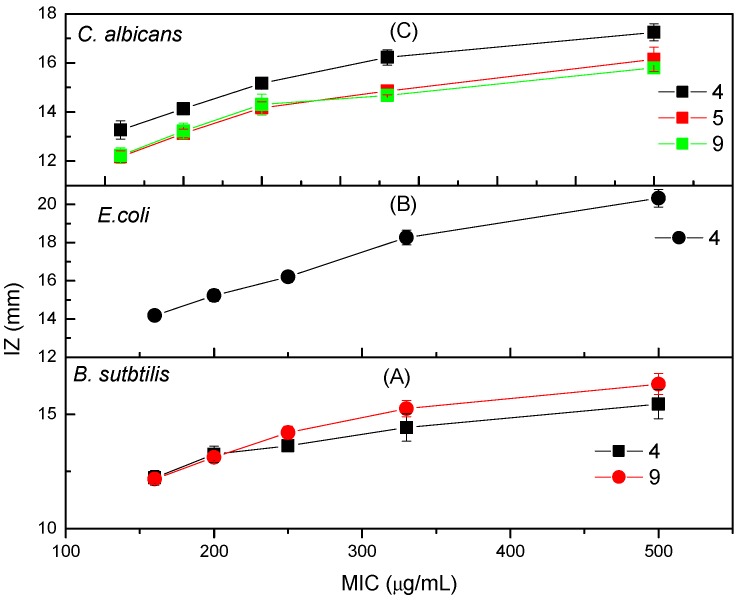
The minimum inhibitory concentration (MIC) of the newly synthesized peptide derivatives are presented in (Table 1 and Figure 1a–c). The MIC was 160 µg/mL based on the compounds tested, but the inhibition zones obtained were different and ranged from 12–14 mm. The MIC of Compound 4 was 160 µg/mL against B. subtilis, E. coli, and C. albicans. The MIC of N-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide (5) was 160 µg/mL against B. subtilis, and the inhibition zone was 12 mm. Finally, the MIC of N-(2-oxo-2-(2-oxo-2-(2-(phenylcarbamoyl)-hydrazinyl)-ethylamino)-ethyl)-nicotinamide (9) was 160 µg/mL against B. subtilis and C. albicans, and the inhibition zone obtained was 12 mm.
Figure 1.
The minimum inhibitory concentrations (MICs) of Compounds 4, 5, and 9 against differently tested microorganisms expressed as inhibition zones of growth in millimeters.
2.3. Molecular Docking Studies
Molecular docking studies are currently gaining much attention in the field of medicinal chemistry. The significant experimental antibacterial and antifungal activities of Compounds 4–10 provide a hint to performing molecular docking studies to understand the protein–ligand interactions. The docking was performed via the Molegro Virtual Docker for the active sites of enoyl reductase from E. coli (PDB 1C14) and cytochrome P450 14-α-sterol demethylase (Cyp51) from Candida albicans (PDB 5TZ1) in order to predict the binding mode and support the biological results. Bacterial enoyl reductase catalyzes an important step in bacterial fatty acid biosynthesis. Enoyl reductase is an attractive target for antibacterial drug discovery because of its essential role in metabolism and its sequence conservation across many bacterial species. On the other hand, cytochrome P450 14α-sterol demethylase (Cyp51) catalyzes the oxidative removal of the 14-α-methyl group of lanosterol and/or eburicol in fungi by a typical P450 mono-oxygenase activity. This protein contains an iron protoporphyrin moiety located at the active site, and our compounds may bind to the iron atom via a nitrogen atom in the nicotinamide ring. After reviewing the different chemical classes that docked into the enoyl reductase active site, such as amides, triazoles, azoles, and pyridone derivatives [30,31,32,33], we examined our compounds on the active site because some structural similarities exist. The same was done with respect to the cytochrome P450 14-α-sterol demethylase active site, which was examined with different groups of compounds from different chemical classes, such as imidazoles, pyrimidines, triazoles, thiosemicarbazides, and acetamide derivatives [34,35,36,37].
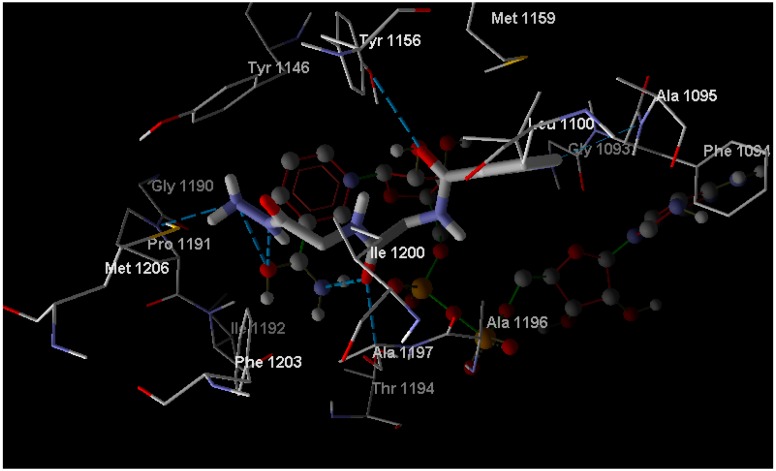
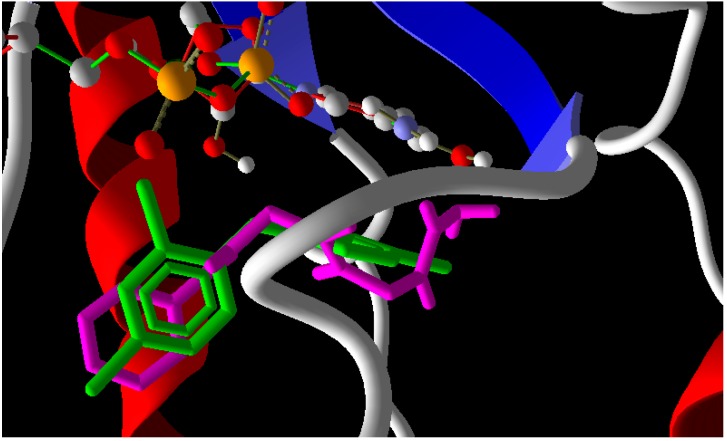
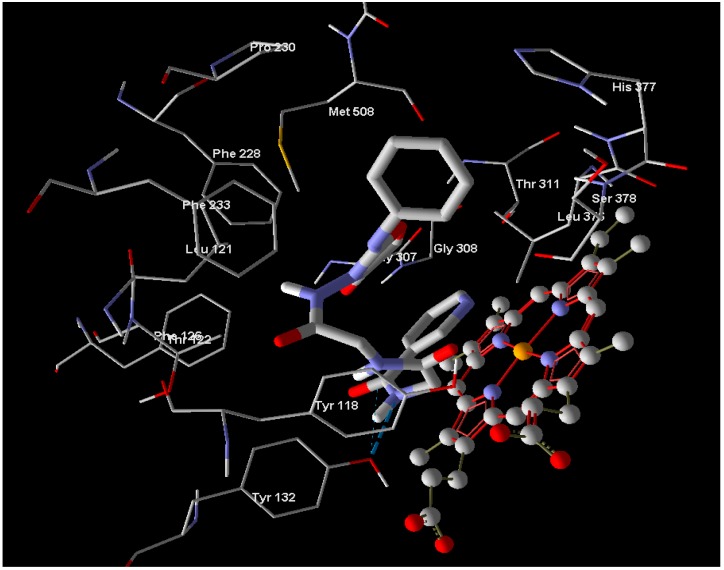
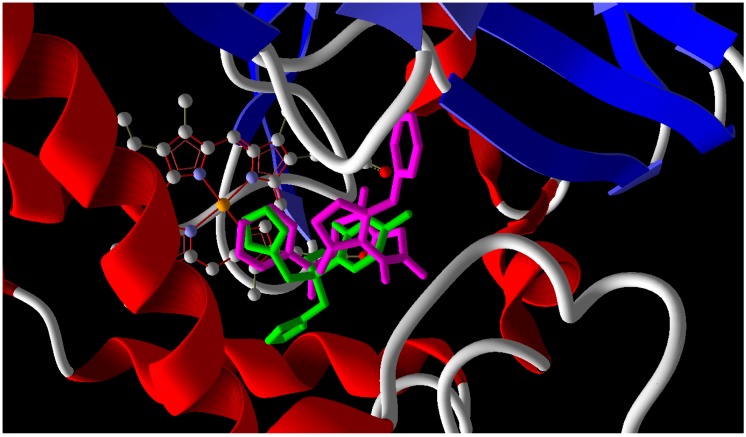
In the case of enoyl reductase from E. coli, the seven compounds interact with the amino acids of its active site and give MolDock scores ranging from −171 to −117 (Table 2). The most stable complex was formed with Compound 10, but Compound 4, which has the best biological activity, makes four hydrogen bonds with the Ala1095, Tyr1156, Pro1191, and Thr1194 amino acids and four hydrogen bonds with the NAD+ coenzyme (Figure 2). Compound 4 and the reference drug (triclosan) were superimposed in the active site as shown in Figure 3. On the other hand, the docked compounds on the active site of cytochrome P450 14-α-sterol demethylase also gave MolDock scores ranging from −179 to −107 (Table 2), which is slightly better than those of the enoyl reductase active site. Compound 10 also has the best Moldock score (−179) and forms two hydrogen bonds with Tyr132 amino acid and hydrophobic interactions with Thr118, Phe126, Phe228, and Phe233 (Figure 4). Compound 10 and fluconazole (the reference drug) were superimposed in the active site as shown in Figure 5. This superior interaction between Compound 10 and the enzymes active sites can be explained by the increase in the chemical building of the molecule, which makes extra bonding with amino acid residues of the active site.
Table 2.
MolDock scores of the tested compounds.
| Compounds | Moldock Score with Enoyl Reductase from E. coli (PDB 1C14) | Moldock Score with Cytochrome P450 14-α-Sterol Demethylase (Cyp51) from Candida (PDB 5TZ1) |
|---|---|---|
| 4 | −117 | 107 |
| 5 | −147 | 147 |
| 6 | −156 | 151 |
| 7 | −139 | 140 |
| 8 | −153 | 161 |
| 9 | −142 | 151 |
| 10 | −171 | 179 |
| Reference | −127 (triclosan) | 133 (fluconazole) |
Figure 2.
A binding mode of 4 into the binding site of enoyl reductase (Red: Oxygen, Blue: Nitrogen, Gray: Carbon, Dash line: Hydroegen bonds).
Figure 3.
Overlay of Compound 4 (purple) and triclosan (green) in the groove of enoyl reductase (Red: alpha helix Blue: Beta sheets).
Figure 4.
Binding mode of Compound 10 into the binding site cytochrome P450 14-α-sterol demethylase (Red: Oxygen, Blue: Nitrogen, Gray: Carbon, Dash line: Hydroegen bonds).
Figure 5.
Overlay of Compound 10 (purple) and fluconazole (green) in the groove of cytochrome P450 14-α-sterol demethylase (Red: alpha helix Blue: Beta sheet).
3. Materials and Methods
3.1. Chemistry
The solvents, chemicals, and thin layer chromatography used in this work were obtained from international chemical companies: Sigma (Ronkonkoma, NY, USA), Fluka (Buchs, Switzerland), and E. Merck (Hohenbrunn, Germany). The melting points were determined using the Digital Electro thermal melting point apparatus in opened glass capillary tubes and are uncorrected. Elemental micro-analyses for carbon, nitrogen, and hydrogen (Micro-Analytical Unit, Cairo University, Cairo, Egypt) were obtained within good limits of the theoretical values (±0.4%). Infrared (IR) spectra were listed as KBr disks using the Fourier transform infrared spectrophotometer (Shimadzu; Model: IRAffinity-1S) at the Micro-Analytical Unit at Cairo University in Egypt. The measurements of mass spectra occurred on a gas chromatograph–mass spectrometer (Shimadzu, Kyoto, Japan; Model: QP2010 ultra) at the Micro-analytical Unit at Cairo University in Egypt. The 1H-NMR and 13C-NMR spectra were run on JEOL, JöEL500 MHz instruments (Tokyo, Japan) in DMSO-d6.
Synthesis of nicotinoyl chloride (2) and glycyl-glycine-methyl-ester-hydrochloride: These compounds were prepared according to previously reported methods [38,39].
Synthesis of nicotinyl-glycyl-glycine-methyl ester (3): A cold dichloromethane (DCM) solution of free glycyl-glycine methyl ester (4.13 gm, ~30 mmol, −20 °C) was added carefully under control conditions to a cold DCM solution of the nicotinoyl chloride (2) (−20 °C, 3 gm, 14.78 mmol). The reacted mixture was stirred for 3 h at −20 °C and subsequently for 24 h at room temperature. It was then washed with water, 1 N sodium bicarbonate, and 1 N potassium hydrogen sulfate followed by water again and then dried over sodium sulfate. Next, the solvent was evaporated and the obtained compound was solidified by petroleum ether (boiling point: 40–60 °C). The solid was filtered off, dissolved in methanol (MeOH), and precipitated by petroleum ether to give Compound 3.
Methyl-2-(2-(nicotinamido)-acetamido)-acetate (3): Yield: 80%; melting point: 122–124 °C, IR (cm−1): (KBr): ν = 3400 (NH stretching), 3030 (CH, aromatic), 2900 (CH, aliphatic), 1734 (C=O, ester), 1660, and 1580 (C=O amide I and II, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.22−9.00 (s, 1H, 1NH, D2O exchangeable, amide), 8.90 (1H, aromatic H, C1, Pyr.), 8.81 (1H, aromatic H, C5, Pyr.), 8.65 (1H, aromatic H, C3, Pyr.), 8.30 (s, 1H, 1NH, D2O exchangeable, Py–CONH amide), 7.30 (1H, aromatic H, C4, Pyr.), 3.7–3.3 (m, 7H, 2CH2 α-gly + MeO). MS (EI, 70 eV): m/z (%) = 251 (M+, 3.55), 220 (1.66), 160 (11.44), 78 (12.90), 63 (100), 50 (2.00).Molecular formula (molecular weight): C11H13N3O4 (251.2). Calculated analysis: C, 52.59; H, 5.22; N, 16.73; Found: C, 52.62; H, 5.25; N, 16.70.
Synthesis of nicotinyl-glycyl-glycine-hydrazide (4): To a stirred methanolic solution (50 mL) of the corresponding dipeptide methyl ester (3) (1 mmol), hydrazine hydrate 99% (0.35 mL, 10 mmol) was added. The mixture was then refluxed for 3 h, the solvent was evaporated and the obtained residue was triturated with diethylether, filtered off, and precipitated from MeOH/diethylether to give nicotinyl-glycyl-glycine-hydrazide (4).
N-(2-(2-Hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide (4): Yield: 65%; melting point: 261–263 °C, IR (cm−1): (KBr): ν = 3425 (NH stretching), 2997 (CH, aromatic), 2913 (CH, aliphatic), 1655, 1542 and 1534 (C=O amide I, II, and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 10.67 (s, 1H, 1NH, D2O exchangeable, Hydrazide), 9.04 (s, 1H, 1NH, D2O exchangeable, NHCH2CONHNH2, amide), 8.99 (1H, aromatic H, C1, Pyr.), 8.77 (1H, aromatic H, C5, Pyr.), 8.70 (1H, aromatic H, C3, Pyr.), 8.50 (s, 1H, 1NH, D2O exchangeable, Py–CONH amide), 7.50 (1H, aromatic H, C4, Pyr.), 4.24–4.23 (s, 2H, NH2), 3.8–3.5 (t, 4H, 2CH2, α-gly). MS (EI, 70 eV): m/z (%) = 252 (M+ + 1, 0.59), 251 (M+, 7.22), 179 (0.48), 78 (42.94), 63 (100), 49 (2.36). Molecular formula (molecular weight): C10H13N5O3 (251.2). Calculated analysis: C, 47.81; H, 5.22; N, 27.87; found: C, 47.80; H, 5.24; N, 27.90.
The general procedures for the synthesis of Compounds 5 and 6: A mixture of N-(2-(2-hydrazinyl-2-oxoethylamino)-2-oxoethyl)-nicotinamide (4) (0.01 mol) and aromatic aldehydes of equimolar amount (namely, P-chlorobenzaldehyde and/or P-hydroxybenzaldehyde) in ethanol (20 mL) was refluxed for 8 h. The mixture was cooled down and the formed precipitate was filtered off. Finally, the precipitate was recrystallized from dimethylformamide (DMF) to give Compounds 5 and 6.
N-(2-(2-(2-(4-Chlorobenzylidene)-hydrazinyl)-2-oxoethylamino)-2-oxoethyl)-nicotinamide (5): Yield: 60%; melting point: 280–281 °C, IR (cm−1): (KBr): ν = 3425 (NH stretching), 2999 (CH, aromatic), 2913 (CH, aliphatic), 1655, 1533 and 1511 (C=O amide I, II, and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.1, 9.09 (s, 2H, 2NH, D2O exchangeable, NHCH2CONHN=CH–Phe, amide), 8.84 (1H, aromatic H, C1, Pyr.), 8.82 (1H, aromatic H, C5, Pyr.), 8.72 (1H, aromatic H, C3, Pyr.), 8.38 (s, 1H, 1NH, D2O exchangeable, Py–CONH amide), 8.36 (d, 1H, N=CH–Phe), 8.29 (1H, aromatic H, C4, Pyr.), 7.95–7.15 (4H, the remaining aromatic H), 2.26–2.00 (t, 4H, 2CH2, α-gly). MS (EI, 70 eV): m/z (%) = 373 (M+, 10.71), 232 (18.37), 134 (18.03), 77 (33.33), 63 (100), 50 (54.59). Molecular formula (molecular weight): C17H16ClN5 (373.8). Calculated analysis: C, 54.62; H, 4.31; Cl, 9.48; N, 18.74; found: C, 54.64; H, 4.34; Cl, 9.52; N, 18.72.
N-(2-(2-(2-(4-Hydroxybenzylidene)-hydrazinyl)-2-oxoethylamino)-2-oxoethyl)-nicotinamide (6): Yield: 62%; melting point: 280–281°C, IR (cm−1): (KBr): ν = 3420 (OH), 3200 (NH stretching), 2951 (CH, aromatic), 2839 (CH, aliphatic), 1645, 1593, and 1558 (C=O amide I, II, and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.15 (s, 1H, OH, D2O exchangeable, aromatic C–OH), 9.12, 9.00 (s, 2H, 2NH, D2O exchangeable, NHCH2CONHN=CH–Phe, amide), 8.98 (1H, aromatic H, C1, Pyr.), 8.62 (1H, aromatic H, C5, Pyr.), 8.55 (1H, aromatic H, C3, Pyr.), 8.39 (s, 1H, 1NH, D2O exchangeable, Py–CONH amide), 8.18–8.16 (d, 1H, N=CH–Phe), 8.08 (1H, aromatic H, C4, Pyr.), 7.55–7.40 (4H, the remaining aromatic H), 2.96–2.93 (t, 4H, 2CH2, α-gly). 13C-NMR (125 MHz, δ, ppm, DMSO-d6): δ = 173–170 (3CO), 160–122 (11aromatic C), 140.5 (N=CH, Hydrazide), 41.5–40.0 (aliphatic C, 2CH2, α-gly). MS (EI, 70 eV): m/z (%) = 356 (M+ + 1, 7.55), 355 (M+, 11.05), 224 (6.70), 155 (6.15), 68 (11.14), 56 (100), 50 (14.49). Molecular formula (molecular weight): C17H17N5O4 (355.3). Calculated analysis: C, 57.46; H, 4.82; N, 19.71; found: C, 57.45; H, 4.84; N, 19.75.
The general procedure for the synthesis of Compounds 7 and 8: A mixture of Compound 4 (0.01 mol), phenyl isocyanate, and/or methyl isothiocyanate (0.01 mol) and triethyl amine as a catalytic amount in dry benzene (20 mL) was refluxed for 7 h. The mixture was thereafter concentrated. The precipitated was filtered off and recrystallized from ethanol to give the new Compounds 7 and 8.
N-(2-(2-(2-(Methylcarbamothioyl)-hydrazinyl)-2-oxoethylamino)-2-oxoethyl)-nicotinamide (7): Yield: 77%; melting point: 202–204 °C, IR (cm−1): (KBr): ν = 3425 (NH stretching), 3251 (CH, aromatic), 3143 (CH, aliphatic), 1590, 1542, and 1515 (C=O amide I, II, and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.50–8.90 (5s, 5H, 5NH, D2O exchangeable, amide), 8.75 (1H, aromatic H, C1, Pyr.), 8.60 (1H, aromatic H, C5, Pyr.), 8.45, 7.80 (2H, aromatic H, C3 and C4, Pyr.), 3.50–3.42 (t, 4H, 2CH2, α-gly), 2.00 (s, 3H, CH3,). 13C-NMR (125MHz, δ, ppm, DMSO-d6): δ = 183 (CS), 165 (3CO), 145–140 (5 aromatic C), 45.7 (aliphatic C, 2CH2, α-gly), 31.1 (aliphatic C, CH3). MS (EI, 70 eV): m/z (%) = 325 (M+ + 1, 57.45), 324 (M+, 63.30), 299 (60.64), 199 (100), 144 (47.87), 75 (57.45), 65 (81.91), 51 (46.28). Molecular formula (molecular weight): C12H16N6O3S (324.4). Calculated analysis: C, 44.43; H, 4.97; N, 25.91; S, 9.89; found: C, 44.44; H, 5.00; N, 25.95; S, 9.86.
N-(2-oxo-2-(2-oxo-2-(2-(Phenylcarbamoyl)-hydrazinyl)-ethylamino)-ethyl)-nicotinamide (8): Yield: 55%; melting point: 230–231 °C, IR (cm−1): (KBr): ν = 3290 (NH stretching), 3056 (CH, aromatic), 2677 (CH, aliphatic), 1688, 1660, 1630 and 1599 (C=O amide I, II, III, and IV, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.22–8.85 (5s, 5H, 5NH, D2O exchangeable, amide), 8.80 (1H, aromatic H, C1, Pyr.), 8.51 (1H, aromatic H, C5, Pyr.), 8.39 (1H, aromatic H, C3, Pyr.), 7.62–6.93 (6H, the remaining aromatic H), 3.83–3.66 (t, 4H, 2CH2, α-gly). MS (EI, 70 eV): m/z (%) = 371 (M+ + 1, 1.77), 370 (M+, 2.79), 322 (100), 236 (4.24), 91 (12.08), 77 (17.96), 64 (43.17), 50 (10.19). Molecular formula (molecular weight): C17H18N6O4 (370.4). Calculated analysis: C, 55.13; H, 4.90; N, 22.69; found: C, 55.15; H, 4.94; N, 22.73.
The general procedure for the synthesis of Compounds 9 and 10: A mixture of Compound 7 or 8 (0.01 mol) in ethanol (30 mL) and chloroacetic acid (0.01 mol) was refluxed for 10 h. The reaction mixture was concentrated. The precipitate was filtered off and recrystallized from the ethanol to give the corresponding candidates (9,10), respectively.
N-(2-(2-(2-(Methylimino)-4-oxothiazolidin-3-ylamino)-2-oxoethylamino)-2-oxoethylamino)-2-oxoethyl)nicotinamide (9): Yield: 70%; melting point: 151–153 °C, IR (cm−1): (KBr): ν = 3429 (NH stretching), 2981 (CH, aromatic), 2943 (CH, aliphatic), 1616, 1603 and 1524 (C=O amide I, II and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.40–9.21 (3s, 3H, 3NH, D2O exchangeable, amide), 8.72–8.24 (m, 4H, aromatic H, Pyr.), 4.45 (t, 2H, CH2, oxazolidine ring), 3.17, 2.95 (t, 4H, 2CH2, α-gly), 1.34–1.10 (s, 3H, CH3). MS (EI, 70 eV): m/z (%) = 365 (M+ + 1, 2.37), 364 (M+, 6.11), 258 (24.53), 73 (100), 57 (25.80), 50 (2.08). Molecular formula (molecular weight): C14H16N6O4S (364.4). Calculated analysis: C, 46.15; H, 4.43; N, 23.06; S, 8.80; found: C, 46.17; H, 4.45; N, 23.03; S, 8.85.
N-(2-oxo-2-(2-oxo-2-(4-oxo-2-(Phenylimino)-oxazolidin-3-ylamino)-ethylamino)-ethyl)-nicotinamide (10): Yield: 80%; melting point: 98–100 °C, IR (cm−1): (KBr): ν = 3431 (NH stretching), 3295 (CH, aromatic), 3219 (CH, aliphatic), 1687, 1669, and 1600 (C=O amide I, II and III, respectively). 1H-NMR (500 MHz, δ, ppm, DMSO-d6): δ = 9.12–8.92 (3s, 3H, 3NH, D2O exchangeable, amide), 8.28–8.04 (m, 4H, aromatic H, Pyr.), 7.62–6.65 (m, 5H, the remaining aromatic H), 4.29 (t, 2H, CH2, oxazolidine ring), 3.67, 3.05 (t, 4H, 2CH2, α-gly). 13C-NMR (125 MHz, δ, ppm, DMSO-d6): δ = 169.2–165.3 (4CO), 156.5 (CH, Oxazolidine ring), 140–118 (11aromatic C), 55.5 (CH2, Oxazolidine ring), 40–39 (aliphatic C, 2CH2, α-gly). MS (EI, 70 eV): m/z (%) = 410 (M+, 0.26), 322 (100), 235 (0.44), 91 (1.76), 64 (7.69), 50 (1.46). Molecular formula (molecular weight): C19H18N6O5 (410.4). Calculated analysis: C, 55.61; H, 4.42; N, 20.48; found: C, 55.64; H, 4.45; N, 20.47.
3.2. Biological Evaluations
3.2.1. Antibacterial and Antifungal Activity (Agar Well Diffusion Assay)
All samples were dissolved in dimethyl sulfoxide (DMSO) at a 1 mg/1 mL concentration in comparison to different antibiotic drugs as standard samples. The strain used and test organisms: A-Bacteria such as E. coli (ATCC 25922) and B. subtilis (NRRL-B-4219); B-Test fungi such as A. niger (ATCC 16888) and C. albicans (ATCC 10231). The antimicrobial activity of the candidates (4–10) was evaluated by use of an agar well diffusion method [40,41]. The samples were dissolved in DMSO. Briefly, a one-day-old culture of bacteria and a two-day-old culture of fungi were then mixed with sterile physiological saline (NaCl 0.9%), and the turbidity was adjusted to the standard inoculums of a MacFarland scale of 0.5 (106 colony forming units/mL). Briefly, agar plates containing 20 mL of Mueller Hinton Agar (Lab M., Bury, Lancashire, UK) and Sabouraud-dextrose agar (Lab M., Bury, Lancashire, UK) were inoculated with bacterial and fungal strains under aseptic conditions and wells (diameter = 9 mm) were filled with 100 µL of the test samples. Standard antibacterial antibiotics, NA = Negram (nalidixic acid), S = Streptomycin; T = Oxytetracycline, VA = Vancomycin, CDZ = Cefodizime, and NV = Novobiocin, and fungal antibiotics, Ny = Nystatin; CLT = Clotrimazole, and FLC = Fluconazole were used as positive controls over bacteria and fungi. A zone of inhibition was recorded after incubating bacterial strains at 37 °C (24 h) and fungal strains at 25 °C, 72 h [42,43].
3.2.2. Determination of the Minimum Inhibitory Concentration (MIC)
The synthesized compounds showed antibacterial activities and were subjected to MIC (minimum inhibitory concentration) assays [44]. Serial dilutions were prepared with concentrations ranging from 100 to 500 μg/mL. DMSO was used as a negative control (Blank DMSO). Each prepared concentration in tubes was applied in plates that were inoculated with 100 μL each of the 106 cfu/mL bacterial and spore suspension from fungal strains, and the assay was applied by an agar well diffusion method. The plates were incubated aerobically at 37 °C (18–24 h) for bacterial strains and 25 °C (48 h) for fungal strains. The MIC values which represent the lowest compound concentration that completely inhibits the growth of microorganisms. All tests were performed in triplicates.
3.3. Molecular Docking Studies
The crystal structures of E. coli enoyl reducates including triclosan (PDB 1C14) and cytochrome P450 14-𝛼-sterol demethylase (Cyp51) from Candida albicans (PDB 5TZ1) were provided from the Brookhaven protein data bank (PDB; http://www.rcsb.org/pdb) and loaded to the Molegro Virtual Docker (MVD 2013.6.0.0 (win32), (Molegro ApS, Aarhus C, Denmark) program. We used a fully functional free trial version with a limited-term license [45]. The non-bonded oxygen atoms of water present in the crystal structure were removed. ChemBio3D Ultra 10 (Billerica, MA, USA) [46] was used to draw the 3D structures of different ligands. The ligands were further pre-optimized using the free version of Marvinsketch 4.1.13 from Chemaxon Ltd. (Budapest, Hungary) [47] with an MM force field and saved in the Tripos mol2 file format. The MolDock score functions were used with a 0.3° grid resolution. The binding sites were defined to any residues that were a distance of 12 Å away from the co-crystallized tetrazole-based antifungal drug and fluconazole in the complex crystal structures of the enzymes [48,49,50,51].
4. Conclusions
In the present study, seven novel dipeptide Compounds 4–10 were synthesized using a solution phase method in peptide synthesis. The compounds were evaluated for their antibacterial activity against Gram-positive, Gram-negative, and fungi by the agar well diffusion method, all the synthesized candidates showed a significant antimicrobial inhibitor activity. Docking studies have been carried out on enoyl reeducates from E. coli and P450 14-α-sterol demethylase (Cyp51) from Candida albicans active sites. The MolDock scores of the seven tested compounds range between −117 and −171 and between −179 and −107, respectively.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourahbint Abdulrahman University, through the Research Groups Program Grant No. (RGP-1438-0003), and was funded by the National Research Centre at Dokki, Cairo, Egypt.
Author Contributions
Gaber Moustafa and Ahmed Naglah designed and observed the proposal and contributed to data analysis and interpretation. Gaber Moustafa and Hemat Khalaf performed the experiments. Hasan Awad conducted the biological study and wrote the biological sections of the paper. Asma Al-Wasidi and Nawal Al-Jafshar surveyed the database and performed the spectral analysis. Gaber Moustafa and Ahmed Naglah provided conceptual advice and wrote the paper. All authors discussed the results and implications, and commented on the manuscript at all stages.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Kumar S., Babu B.V. Extraction of Pyridine-3-carboxylic Acid Using 1-Dioctylphosphoryloctane (TOPO) with Different Diluents: Equilibrium Studies. J. Chem. Eng. Data. 2009;54:2669–2677. doi: 10.1021/je900205h. [DOI] [Google Scholar]
- 2.Yadav R., France M., Younis N., Hama S., Ammori B.J., Kwok S., Soran H. Extended-release niacin with laropiprant: A review on efficacy, clinical effectiveness and safety. Expert Opin. Pharmacother. 2012;13:1345–1362. doi: 10.1517/14656566.2012.690395. [DOI] [PubMed] [Google Scholar]
- 3.Keene D., Price C., Shun-Shin M.J., Francis D.P. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: Meta-analysis of randomised controlled trials including 117 411 patients. BMJ (Clin. Res.) 2014;349:4379–4391. doi: 10.1136/bmj.g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruckert E., Labreuche J., Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210:353–361. doi: 10.1016/j.atherosclerosis.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 5.Lin Q., Fang D., Hou X., Le Y., Fang J., Wen F., Gong W., Chen K., Wang J.M., Su S.B. HCV Peptide (C5A), an Amphipathic α-Helical Peptide of Hepatitis Virus C, Is an Activator of N-Formyl Peptide Receptor in Human Phagocytes. J. Immunol. 2011;186:2087–2094. doi: 10.4049/jimmunol.1002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruchala P., Navab M., Jung C., Hama-Levy S., Micewicz E.D., Luong H., Reyles J.E., Sharma S., Waring A.J., Fogelman A.M., et al. Oxpholipin 11D: An anti-inflammatory peptide that binds cholesterol and oxidized phospholipids. PLoS ONE. 2010;5:e10181. doi: 10.1371/journal.pone.0010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen F., Zhang F., Wang A., Li H., Wang Q., Zeng Z., Wang S., Xie T. Recent progress in the chemo-enzymatic peptide synthesis. Afr. J. Pharm. Pharacol. 2010;4:721–730. [Google Scholar]
- 8.Burrows L.L., Stark M., Chan C., Glukhov E., Sinnadurai S., Deber C.M. Activity of novel non-amphipathic cationic antimicrobial peptides againstCandidaspecies. J. Antimicrob. Chemother. 2006;57:899–907. doi: 10.1093/jac/dkl056. [DOI] [PubMed] [Google Scholar]
- 9.Krishnakumari V., Singh S., Nagaraj R. Antibacterial activities of synthetic peptides corresponding to the carboxy-terminal region of human beta-defensins 1-3. Peptides. 2006;27:2607–2613. doi: 10.1016/j.peptides.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Seo M.D., Won H.S., Kim J.H., Mishig-Ochir T., Lee B.J. Antimicrobial Peptides for Therapeutic Applications: A Review. Molecules. 2012;17:12276–12286. doi: 10.3390/molecules171012276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 12.Marr A.K., Gooderham W.J., Hancock R.E. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006;6:468–472. doi: 10.1016/j.coph.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Mygind P.H., Fischer R.L., Schnorr K.M., Hansen M.T., Sonksen C.P., Ludvigsen S., Raventos D., Buskov S., Christensen B., De Maria L., et al. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437:975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- 14.Van’t Hof W., Veerman E.C., Helmerhorst E.J., Amerongen A.V. Antimicrobial peptides: Properties and applicability. Biol. Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 15.Kimmerlin T., Seebach D. 100 years of peptide syntheses: Ligation methods for peptide and protein synthesis with applications to beta-peptide assemblies. J. Pept. Res. 2005;65:229–260. doi: 10.1111/j.1399-3011.2005.00214.x. [DOI] [PubMed] [Google Scholar]
- 16.Monbaliu J.M., Katritzky A.R. Recent trends in Cys- and Ser/Thr-based synthetic strategies for the elaboration of peptide constructs. Chem. Commun. 2012;48:11601–11622. doi: 10.1039/c2cc34434c. [DOI] [PubMed] [Google Scholar]
- 17.Fjell C.D., Hiss J.A., Hancock R.E.W., Schneider G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Disc. 2012;11:37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- 18.Castanho M., Santos N.C. Peptide Drug Discovery and Development Translational Research in Academia and Industry. 1st ed. Wiley-VCH; Weinheim, Germany: 2011. p. 390. [Google Scholar]
- 19.Gademann K., Kimmerlin T., Hoyer D., Seebach D. Peptide Folding Induces High and Selective Affinity of a Linear and Small β-Peptide to the Human Somatostatin Receptor 4. J. Med. Chem. 2001;44:2460–2468. doi: 10.1021/jm010816q. [DOI] [PubMed] [Google Scholar]
- 20.Naglah A.M., Moustafa G.O., Al-Omar M.A., Al-Salem H.S.A., Hozzein W.N. Synthesis, Characterization andInVitroAntimicrobial Investigation of Novel Amino Acids and Dipeptides Based on Dibenzofuran-2-Sulfonyl-Chloride. J. Comput. Theor. Nanosci. 2017;14:3183–3190. doi: 10.1166/jctn.2017.6613. [DOI] [Google Scholar]
- 21.Al-Salem H.S.A., Naglah A.M., Moustafa G.O., Mahmoud A.Z., Al-Omar M.A. Synthesis of Novel Tripeptides Based on Dibenzofuran-2-Sulfonyl-[Aromatic and Hydroxy Aromatic Residues]: Towards Antimicrobial and Antifungal Agents. J. Comput. Theor. Nanosci. 2017;14:3958–3966. doi: 10.1166/jctn.2017.6702. [DOI] [Google Scholar]
- 22.Naglah A.M., Awad H.M., Bhat M.A., Al-Omar M.A., Amr A.E. Microwave-Assisted Synthesis and Antimicrobial Activity of Some Novel Isatin Schiff Bases Linked to Nicotinic Acid via Certain Amino Acid Bridge. J. Chem. 2015;2015:1–8. doi: 10.1155/2015/364841. [DOI] [Google Scholar]
- 23.Abd El Rahman S.E., Eissa A.M.F., Naglah A.M. Synthesis, Physicochemical Properties and Biological Evaluation of Some Peptide Candidates by Use of Liquid Phase Method as Potential Antimicrobial and Surface Active Agents. J. Pharmacol. 2015;11:726–731. doi: 10.3923/ijp.2015.726.731. [DOI] [Google Scholar]
- 24.Naglah A.M., Khalifa N.M., Al-Omar M.A., Awad H.M., Amr A.E. In vitro Antimicrobial Activity of Some Newly Synthesized Polypeptide Candidates. Dig. J. Nanomater. Biostruct. 2014;9:433–442. [Google Scholar]
- 25.Abo-Ghalia M.H., Moustafa G.O., Alwasidi A.S., Naglah A.M. Cytotoxic Investigation of Isophthaloyl Cyclopentapeptides. Lat. Am. J. Pharm. 2017;36:1957–1962. [Google Scholar]
- 26.Moustafa G.O., El-Sawy A.A., Abo-Ghalia M.H. Synthesis of novel cyclopeptide candidates: I-cyclo-[Nα-isophthaloyl-bis-(Glycine-amino acid)-L-lysine] derivatives with expected anticancer activity. Egypt. J. Chem. 2013;5:473–494. [Google Scholar]
- 27.Naglah A.M., Shinwari Z., Bhat M.A., Al-tahhan M., Al-omar M.A., Al-dhfyan A. Targeting leukemic side population cells by isatin derivatives of nicotinic acid amide. J. Biol. Regul. Homeost. Agents. 2016;30:353–363. [PubMed] [Google Scholar]
- 28.Bhat M.A., Al-dhfyan A., Naglah A.M., Khan A.A., Al-Omar M.A. Targeting leukemic side population cells by isatin derivatives of nicotinic acid amide. Molecules. 2015;20:18246–18263. doi: 10.3390/molecules201018246. [DOI] [PubMed] [Google Scholar]
- 29.Hassan A.S., Moustafa G.O., Awad H.M. Synthesis and in vitro anticancer activity of pyrazolo[1,5-a]pyrimidines and pyrazolo [3,4-d] [1, 2, 3] triazines. Synth. Commun. 2017;47:1963–1972. doi: 10.1080/00397911.2017.1358368. [DOI] [Google Scholar]
- 30.Takhi M., Sreenivas K., Reddy C.K., Munikumar M., Praveena K., Sudheer P., Rao B.N., Ramakanth G., Sivaranjani J., Mulik S., et al. Discovery of azetidine based ene-amides as potent bacterial enoyl ACP reductase (FabI) inhibitors. Eur. J. Med. Chem. 2014;84:382–394. doi: 10.1016/j.ejmech.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 31.Panicker C.Y., Varghese H.T., Manjula P.S., Sarojini B.K., Narayana B., War J.A., Srivastava S.K., Van Alsenoy C., Al-Saadi A.A. FT-IR, HOMO–LUMO, NBO, MEP analysis and molecular docking study of 3-Methyl-4-{(E)-[4-(methylsulfanyl)-benzylidene] amino} 1H-1, 2, 4-triazole-5 (4H)-thione. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;151:198–207. doi: 10.1016/j.saa.2015.06.076. [DOI] [PubMed] [Google Scholar]
- 32.Kini S.G., Bhat A.R., Bryant B., Williamson J.S., Dayan F.E. Synthesis, antitubercular activity and docking study of novel cyclic azole substituted diphenyl ether derivatives. Eur. J. Med. Chem. 2009;44:492–500. doi: 10.1016/j.ejmech.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Hirschbeck M.W., Kuper J., Lu H., Liu N., Neckles C., Shah S., Wagner S., Sotriffer C.A., Tonge P.J., Kisker C. Structure of the Yersinia pestisFabVenoyl-ACP reductase and its interaction with two 2-pyridone inhibitors. Structure. 2012;20:89–100. doi: 10.1016/j.str.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pathan N.B., Rahatgaonkar A.M. Solid supported microwave induced synthesis of imidazole–pyrimidine hybrids: Antimicrobial evaluation and docking study as 14DM-CPY51 inhibitors. Arab. J. Chem. 2016;9:S100–S108. doi: 10.1016/j.arabjc.2011.02.013. [DOI] [Google Scholar]
- 35.Cao X., Chen C., Lu W., Ke S. Chiral β-arylalkyl-1H-1, 2, 4-triazoles as demethylase inhibitors: Biological evaluation and its stereoselective interaction with sterol 14α-demethylase from Penicilliumdigitatum. Pestic. Biochem. Physiol. 2011;99:189–193. doi: 10.1016/j.pestbp.2010.12.003. [DOI] [Google Scholar]
- 36.Pishawikar S.A., More H.N. Synthesis, docking and in-vitro screening of mannich bases of thiosemicarbazide for anti-fungal activity. Arab. J. Chem. 2017;10:S2714–S2722. doi: 10.1016/j.arabjc.2013.10.016. [DOI] [Google Scholar]
- 37.Saha S., Priyadharshini A., Dhanasekaran D., Thajuddin N., Chandraleka S., Chandramohan G., Panneerselvam A. Preclinical evaluation and molecular docking of 4-phenyl-1-Napthyl phenyl acetamide (4P1NPA) from Streptomyces sp. DPTB16 as a potent antifungal compound. Comput. Boil. Med. 2012;42:542–547. doi: 10.1016/j.compbiomed.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z., Yang L., Cui S., Liang Y., Zhang X. Synthesis and Anti-hypertensive Effects of the Twin Drug of Nicotinic Acid and Quercetin Tetramethyl Ether. Molecules. 2014;19:4791–4801. doi: 10.3390/molecules19044791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalton N., Gordon C.P., Boyle T.P., Vandegraaf N., Deadman J., Rhodes D.I., Coates J.A., Pyne S.G., Keller P.A., Bremner J.B. The discovery of allyl tyrosine based tripeptides as selective inhibitors of the HIV-1 integrase strand-transfer reaction. Org. Biomol. Chem. 2016;14:6010–6023. doi: 10.1039/C6OB00950F. [DOI] [PubMed] [Google Scholar]
- 40.Othman M., Lohb H.S., Wiartc C., Khooa T.J., Lima K.H., Ting K.N. Optimal methods for evaluating antimicrobial activities from plant extracts. J. Microbiol. Methods. 2011;84:161–166. doi: 10.1016/j.mimet.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Valgas C., De Souza S.M., Smânia E.F.A., Smânia A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007;38:369–380. doi: 10.1590/S1517-83822007000200034. [DOI] [Google Scholar]
- 42.Rocha L., Marston A., Potterat O., Kaplan M.A.C., Stoeckli-Evans H., Hostettmann K. Antibacterial phloroglucinols and flavonoids from Hypericum brasiliense. Phytochemistry. 1995;40:1447–1452. doi: 10.1016/0031-9422(95)00507-4. [DOI] [PubMed] [Google Scholar]
- 43.Mac Lowry D.J., Jaqua M.J., Selepak S.T. Detailed Methodology and Implementation of a Semiautomated Serial Dilution Microtechnique for Antimicrobial Susceptibility Testing. Appl. Microbiol. 1970;20:46–53. doi: 10.1128/am.20.1.46-53.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones N., Ray B., Ranjit K.T., Manna A.C. Antibacterial activity of ZnOnanoparticle suspensions on a broad spectrum of microorganisms. FEMS Fems Microbiol Lett. 2008;279:71–76. doi: 10.1111/j.1574-6968.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 45.Molegro Virtual Docker (MVD 2013.6.0.0), Molegro Bioinformatics Solutions. [(accessed on 15 October 2013)];2013 Available online: http://www.molegro.com. (In Danish)
- 46.Kerwin S.M. Computer Software Review: Chem Bio Office ultra 2010 suite. J. Am. Chem. Soc. 2010;132:2466–2467. doi: 10.1021/ja1005306. [DOI] [PubMed] [Google Scholar]
- 47.Marvinsketch, “Version 6.1.0, Chemaxon Company Cheminformatics Technology Products Services”. [(accessed on 10 October 2013)];2013 Available online: http://www.chemaxon.com/
- 48.Banfi E., Scialino G., Zampieri D., Mamolo M.G., Vio L., Ferrone M., Fermeglia M., Paneni M.S., Pricl S. Antifungal and antimycobacterial activity of new imidazole and triazole derivatives. A combined experimental and computational approach. J. Antimicrob. Chemother. 2006;58:76–84. doi: 10.1093/jac/dkl182. [DOI] [PubMed] [Google Scholar]
- 49.Stana A., Enache A., Vodnar D.C., Nastasă C., Benedec D., Ionuț I., Login C., Marc G., Oniga O., Tiperciuc B. New thiazolyl-triazoleschiff bases: Synthesis and evaluation of the anti-candida potential. Molecules. 2016;21:1595. doi: 10.3390/molecules21111595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nastasa C., Vodnar D.C., Ionuţ I., Stana A., Benedec D., Tamaian R., Oniga O., Tiperciuc B. Antibacterial Evaluation and Virtual Screening of New Thiazolyl-Triazole Schiff Bases as Potential DNA-Gyrase Inhibitors. Int. J. Mol. Sci. 2018;19:222. doi: 10.3390/ijms19010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hargrove T.Y., Friggeri L., Wawrzak Z., Qi A., Hoekstra W.J., Schotzinger R.J., York J.D., Guengerich F.P., Lepesheva G.I. Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J. Biol. Chem. 2017;292:6728–6743. doi: 10.1074/jbc.M117.778308. [DOI] [PMC free article] [PubMed] [Google Scholar]