Abstract
This work aims to synthesize new trehalase inhibitors selective towards the insect trehalase versus the porcine trehalase, in view of their application as potentially non-toxic insecticides and fungicides. The synthesis of a new pseudodisaccharide mimetic 8, by means of a stereoselective α-glucosylation of the key pyrrolizidine intermediate 13, was accomplished. The activity of compound 8 as trehalase inhibitor towards C. riparius trehalase was evaluated and the results showed that 8 was active in the μM range and showed a good selectivity towards the insect trehalase. To reduce the overall number of synthetic steps, simpler and more flexible disaccharide mimetics 9–11 bearing a pyrrolidine nucleus instead of the pyrrolizidine core were synthesized. The biological data showed the key role of the linker chain’s length in inducing inhibitory properties, since only compounds 9 (α,β-mixture), bearing a two-carbon atom linker chain, maintained activity as trehalase inhibitors. A proper change in the glucosyl donor-protecting groups allowed the stereoselective synthesis of the β-glucoside 9β, which was active in the low micromolar range (IC50 = 0.78 μM) and 12-fold more potent (and more selective) than 9α towards the insect trehalase.
Keywords: iminosugars, trehalase inhibitors, pseudodisaccharides, pyrrolizidines, pyrrolidines, glycosylation reaction
1. Introduction
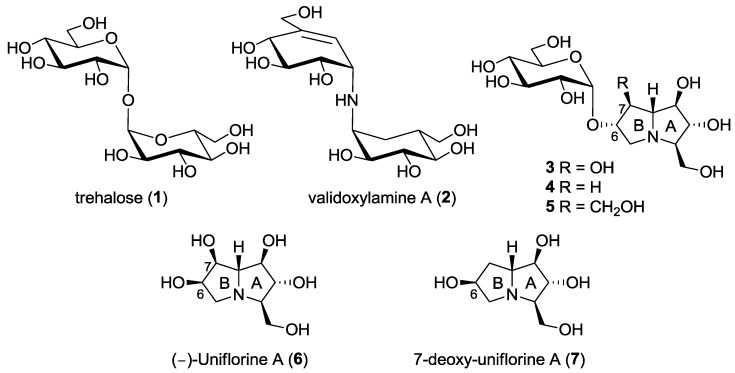
Trehalose (α-d-glucopyranosyl α-d-glucopyranoside, 1, Figure 1) is a disaccharide with a multifunctional physiological role in various organisms (e.g., bacteria, fungi, invertebrates and higher plants) [1]. The role of trehalose in insects is of particular importance connected to its hydrolysis operated by the enzyme α-trehalase (EC 3.2.1.28). Indeed, α-trehalase is an inverting glycosidase [2] that promotes the conversion of trehalose into two molecules of glucose, which is vital for insect flight and essential for larvae resistance to stress factors. While trehalose is not present in mammalian cells, humans do possess the enzyme trehalase, only at intestinal level, probably to hydrolyze occasionally ingested trehalose. Indeed, intolerance to fungi has been correlated with the absence or deficit of trehalases in mammals [1]. For the important role of trehalose-derived glucose in larvae survival and development, trehalase inhibitors have been considered in recent years an interesting target for the identification of novel insecticides and fungicides. However, due to the presence of trehalase also in mammals, specificity towards insect trehalase is crucial for the development of drugs that are safe for plants and mammals, and possibly also for insects that are of benefit in nature [3].
Figure 1.
Trehalose (1), the natural substrate of the enzyme α-trehalase (EC 3.2.1.28), and some natural and non-natural trehalase inhibitors reported to date.
Among the most powerful inhibitors of trehalases there are some natural pseudodisaccharides and analogs shown in Figure 1, such as validoxylamine (2), casuarine-6-O-d-glucoside 3 and its non-natural analogues 4 and 5. The first investigated trehalase three-dimensional structure was the family 37 periplasmic enzyme from E. coli (Tre37A), which was solved in complex with 2 [4], with casuarine-6-O-d-glucoside 3 [5] and with non-natural analogue 5 [6]. Our findings showed that, with E. coli trehalase, casuarine-based inhibitors are placed within the primary catalytic site with the A ring of the pyrrolizidine nucleus that mimics the natural glucose configuration [5,6]. However, subtle changes at ring B (e.g., modification at C-7 as in compound 5) were able to confer both potency and specificity in trehalase inhibition [6]. More interestingly, we later found that simpler pseudomonosaccharide inhibitors such as natural (-)-uniflorine A (6) and non-natural analogue 7-deoxy-uniflorine A (7) showed an excellent inhibitory profile, being completely selective towards the insect trehalase, although less potent in absolute value with respect to casuarine-6-O-d-glucoside derivatives 3–5 [7]. It is worth noting that compounds 6 and 7 bear the opposite configuration at C-6 in ring B with respect to the previously investigated casuarine-6-O-d-glucoside and analogues (compounds 3–5).
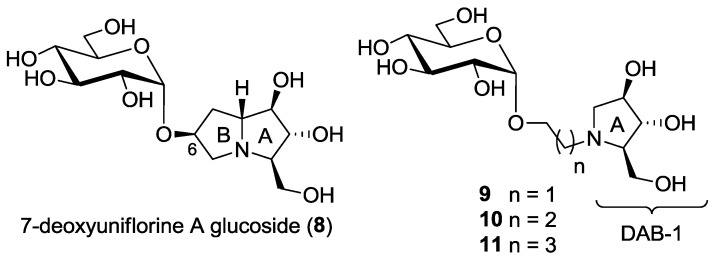
Following our interest in the identification of new more potent and selective trehalase inhibitors, we envisaged that the synthesis of a pseudodisaccharide 8 (7-deoxyuniflorine A glucoside), analogue of disaccharide 4 but with an opposite configuration at C-6, would furnish a promising inhibitor (Figure 2). A straightforward approach to compound 8 is reported in this work.
Figure 2.
This work: synthesis of a new pseudodisaccharide mimetic 8 and of a series of more flexible disaccharide mimics 9–11 bearing the DAB-1 nucleus instead of the pyrrolizidine moiety.
In consideration of the high number of steps required for the synthesis of pseudodisaccharides bearing the pyrrolizidine nucleus such as compounds 3–5 and 8 itself, we also wanted to explore the insect trehalase activity of simpler pseudodisaccharide inhibitors 9–11, that bear the glucose unit linked to the pyrrolidine DAB-1 instead of the pyrrolizidine nucleus. DAB-1 actually has the same structure of ring A of the pyrrolizidine nucleus (Figure 2), and we had previously verified that it is able to mimic glucose by imparting trehalase inhibition in pseudodisaccharide mimetics [8,9]. The aim of the present work was to link the DAB-1 moiety to the glucose moiety through a linker of variable length (2, 3 and 4 carbon atoms), in order to verify which was the more appropriate tether length (Figure 2) for trehalase inhibition.
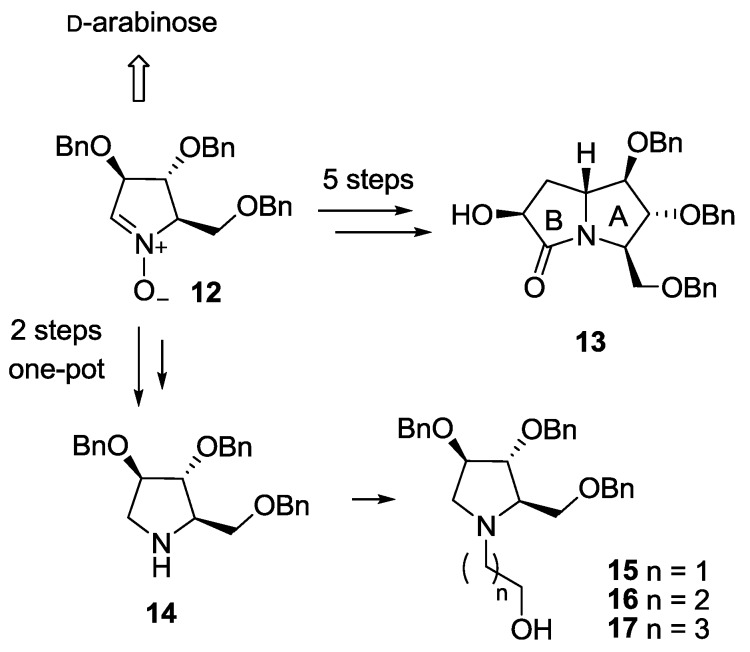
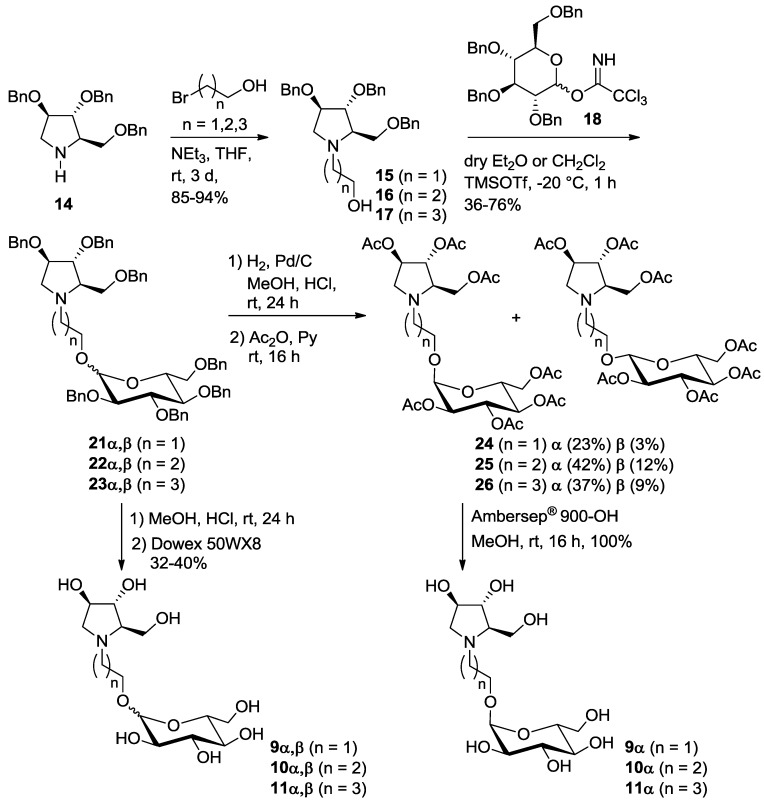
The synthesis of both pyrrolizidine-based (8) and pyrrolidine-based (9–11) pseudodisaccharides involved a common key intermediate: the enantiopure nitrone 12, readily available from d-arabinose in 5 steps (Scheme 1) [10]. However, while on one hand 5 further steps are necessary to access the pyrrolizidine glucosyl acceptor (lactam 13) [7], the hydroxypyrrolidine glucosyl acceptors 15–17 are in principle readily available from amine 14 [11,12]. Pyrrolidine 14, recently synthesized by some of us in a one-pot 2-steps sequence from nitrone 12 (Scheme 1) [13], has the same stereochemical pattern of the hydroxyl groups of ring A in lactam 13, which is responsible for the key interactions within the enzyme active site of the final compounds [5,6]. Therefore, compound 14 is in principle able to mimic the more complex pyrrolizidine skeleton of lactam 13. We also reasoned that pyrrolidine 14 could be easily functionalized at the endocyclic nitrogen atom to afford the desired glucosyl acceptors 15–17, allowing to probe different spatial lengths between the sugar and the iminosugar unit of the target pseudodisaccharides.
Scheme 1.
Synthetic steps to access pyrrolizidine lactam 13 and hydroxypyrrolidines 15–17 (through the key intermediate 14) from nitrone 12.
This work has therefore a dual aim: from one side to investigate the synthesis and biological activity of a new and differently configured pseudodisaccharide mimic (compound 8), and from the other side to explore the possibility to obtain simpler and more flexible inhibitors (compounds 9–11). The results in this direction are shown herein, and highlight the crucial role of the linker length in the design of more flexible inhibitors.
2. Results and Discussion
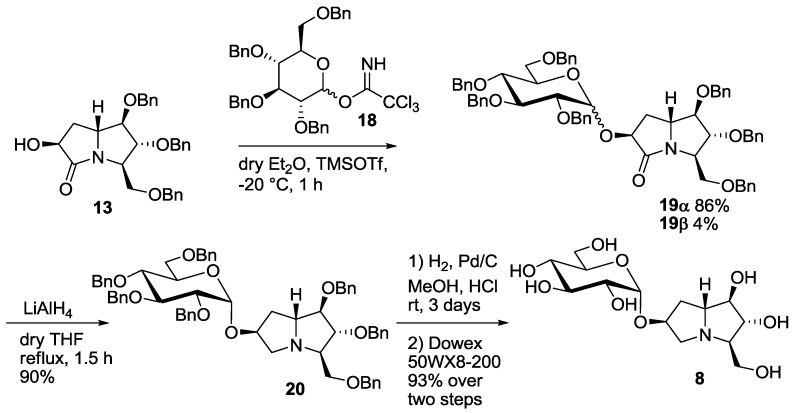
The synthesis of the new disaccharide mimetic 8 started from lactam 13, whose preparation was recently reported by some of us [7]. Compound 13 bears a free OH group at C-6, and was therefore employed as the acceptor in the glucosylation reaction with the benzyl protected glucopyranosyl trichloroacetimidate 18 (Scheme 2) [14]. The reaction, performed in diethyl ether at −20 °C, was highly selective in favor of the α-anomer, as reported before for the casuarine derivatives [5,6], and afforded the α glucoside 19α as the major product (86% yield). Small amounts (4% yield) of the β-anomer 19β were isolated and characterized. Reduction of the C=O double bond in 19α with LiAlH4 in THF at reflux temperature afforded compound 20 in excellent yield (90%). Catalytic hydrogenation, followed by treatment with the ion exchange resin 50WX8-200 gave 7-deoxy-uniflorine A glucoside 8 in 93% yield over two steps (Scheme 2). The 1H-NMR spectrum of compound 8 showed a dd at 3.30 ppm for H-2 signal, with coupling constants of 9.8 and 3.5 Hz, respectively. This indicates an axial-axial relationship with H-3 and an axial-equatorial relationship with β H-1, and therefore confirms the α-configuration of the glucose moiety.
Scheme 2.
Synthesis of the pseudodisaccharide 7-deoxyuniflorine A glucoside (8).
In order to reduce the overall number of synthetic steps necessary to access the glucosyl acceptor in the final glucosylation with trichloroacetimidate 18, we also designed and prepared a series of pseudodisaccharide derivatives 9–11 (Scheme 1) containing a DAB-1 nucleus and a remaining d-glucose unit linked through a 2, 3 or 4-carbon atoms spacer.
Pyrrolidine 14 was N-alkylated with an excess of 2-bromo-1-ethanol, 3-bromo-1-propanol and 4-bromo-1-butanol in THF, using Et3N as the base at room temperature, affording alcohols 15 (85% yield) [12], 16 (94% yield) and 17 (88% yield), with a free hydroxyl group at the end of the aliphatic chain, thus allowing selective glucosylation at this position (Scheme 3).
Scheme 3.
Synthesis of pseudodisaccharides 9–11α, composed by glucose 1-α-linked to the pyrrolidine iminosugar DAB-1.
The reaction of alcohols 15–17 with trichloroacetimidate donor 18 [14] in dry diethyl ether in the presence of trimethylsilyl trifluoromethanesulfonate (TMSOTf) gave 21 (50% yield), 22 (36%) and 23 (66%) as α,β glucoside mixtures, impossible to be separated by flash column chromatography (Scheme 3). In all cases it was not possible to determine the α:β ratios by 1H-NMR analysis because the signals of the anomeric protons of the two anomers were covered by the CH2 benzyl groups signals. The glucosylation reactions were also performed in dry CH2Cl2 affording similar yields (21, 76%, 22, 39% and 23, 50%) of the α,β glucoside mixtures.
Catalytic hydrogenation of 21–23α,β in acidic MeOH with Pd/C gave the corresponding mixture of deprotected α,β isomers 9–11, as hydrochloride salts, that were passed onto an ion exchange resin (Dowex 50WX8-200) eluting successively with MeOH, H2O and 6% aqueous ammonia. The final elution with ammonia afforded pure glucosyl derivatives 9–11 as a mixture of α and β-anomers, in 32–40% yields (9α,β, α:β 2:1; 10α,β, α:β 1:1; 11α,β, α:β 1.2:1 as determined by 1H NMR analysis, see Supplementary Materials).
Due to the impossibility to separate the α and β isomers both with benzylated and free hydroxyl groups, we decided to directly react the crude obtained by catalytic hydrogenation of 9–11α,β with an excess of acetic anhydride in pyridine affording the corresponding peracetylated compounds 24–26 as α,β mixtures. As expected, in all cases, the two isomers were separated by flash column chromatography, affording pure compounds 24α, 25α and 26α in 23%, 42% and 37% yields over two steps and β-isomers (24β, 25β and 26β in 3%, 12% and 9% yield) impure of some traces of the corresponding α-isomers (Scheme 3). The α-isomers 24–26α were subsequently treated with strongly basic Ambersep 900 OH resin in MeOH, leading to pure polyhydroxylated α-pseudodisaccharides 9–11α in quantitative yield.
Synthesized compounds 8, 9α, 10α, 11α and the α/β mixture of compounds 9,10 and 11 were tested for their inhibitory activity against insect (C. riparius) and porcine kidney trehalases and the results are shown in Table 1, together with the previously published data on compounds 4, 6 and 7 [7].
Table 1.
Biological activity (IC50) towards C. riparius and porcine trehalases.
| Entry | Compound | C. riparius Trehalase | Porcine Trehalase | Selectivity 2 |
|---|---|---|---|---|
| entry 1 | 4 | 44 ± 1 nM 1 | 479 ± 45 nM 1 | 10 |
| entry 2 | 6 | 177 ± 18 nM 1 | >1 mM 1 | >5649 |
| entry 3 | 7 | 175 ± 12 nM 1 | >1 mM 1 | >5714 |
| entry 4 | 8 | 29.49 ± 7.26 μM | 190.60± 34.15 μM | 6 |
| entry 5 | 9α,β | 2.30 ± 0.13 μM | 7.67 ± 3.91 μM | 3 |
| entry 6 | 9α | 9.36 ± 1.49 μM | 27.64 ± 5.35 μM | 3 |
| entry 7 | 9β | 0.784 ± 0.059 μM | 5.84 ± 0.26 μM | 7 |
| entry 8 | 10α,β | >1000 μM | n.d. 3 | - |
| entry 9 | 10α | >1000 μM | n.d. 3 | - |
| entry 10 | 11α,β | >1000 μM | n.d. 3 | - |
| entry 11 | 11α | >1000 μM | n.d. 3 | - |
1 Value taken from Ref. [7]. 2 Selectivity is the ratio between IC50 value against porcine trehalase and the IC50 value against C. riparius trehalase. 3 n.d. = not determined.
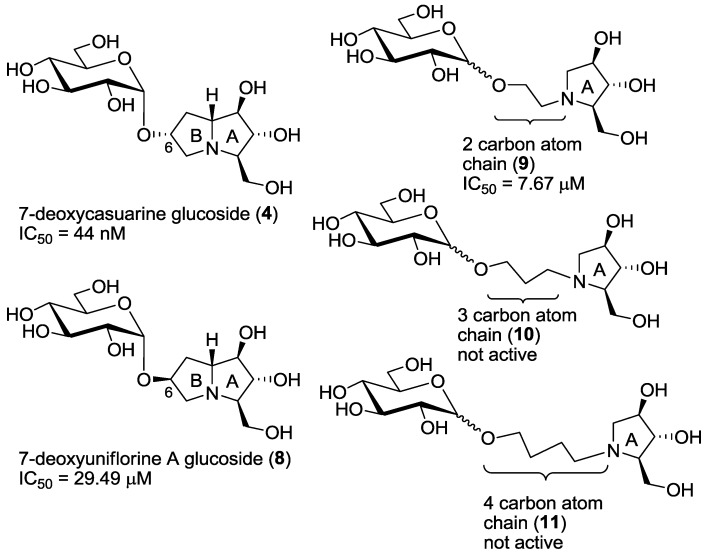
As already mentioned in the introduction, compounds 6 and 7, bearing the opposite configuration at C-6 with respect to the pyrrolizidine portion of compound 4, showed a remarkable selectivity (higher than 5000) towards the insect trehalase with respect to the porcine enzyme. However, they were less active (one order of magnitude) than the pseudodisaccharide mimic 4 [7]. For this reason, we planned the synthesis of compound 8, possessing both a pseudodisaccharide structure and the same configuration at the C-6 carbon atom of compounds 6 and 7. The IC50 value, measured towards insect trehalase, appeared quite disappointing, since compound 8 was active only in the μM range. However, quite a good selectivity was still observed with respect to porcine trehalase (entry 4, Table 1). These results can be rationalized assuming that the active catalytic site of the C. riparius trehalase accommodates the pyrrolizidine portion of the compound, as it happens with recombinant Tre37A trehalase, [5,6]: in this case it appears evident that a pyrrolidizine with such configuration at C-6 (such as 8) is not able to place the glucosyl moiety in a part of the enzyme cavity with favorable interactions.
Derivatives 9–11 were designed in order to simplify the overall synthesis of the inhibitors and the data, shown in Table 1, clearly demonstrate that only compounds 9 are able to maintain inhibitory properties towards C. riparius trehalase, while compounds with a longer linker chain (e.g., 10 and 11) loose completely their inhibitory properties (entries 8–11). Collected data suggest that only the two-carbon chain linker of compounds 9 is able to mimic the pyrrolizidine moiety of compound 8 (see also Figure 3), while its higher flexibility probably allows a better placement of the inhibitor within the active cavity. This is a very good result, which demonstrates the crucial role played by the linker chain’s length joining the iminosugar and the glucosyl moiety. Considering that compounds 9 are more active than the pyrrolizidine-based pseudodisaccharide 8, the advantage of using flexible pyrrolidine-based inhibitors was therefore demonstrated.
Figure 3.
Compounds 4, 8, 9, 10, 11 and their IC50 values towards C. riparius trehalase.
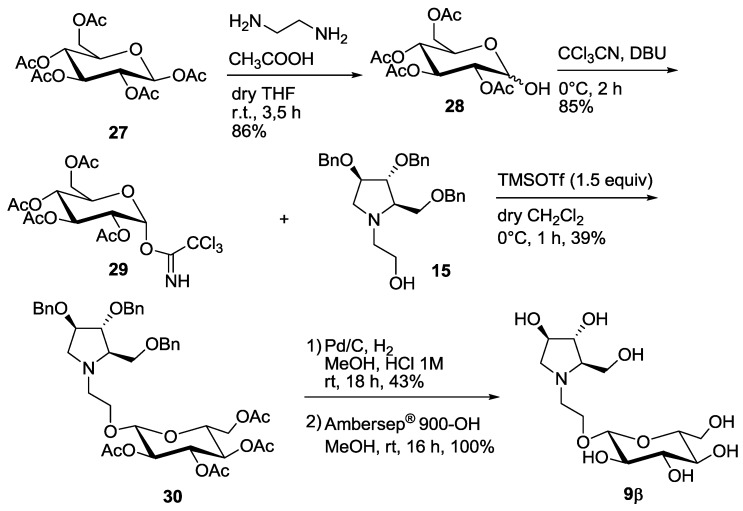
Interestingly, the 9α,β mixture was more active than compound 9α alone (entry 5 vs. entry 6, Table 1). Thus, we reasoned that the pure β-anomer might be even more active. In order to obtain a substantial amount of the β-isomer 9β, we decided to change the protecting groups on the glycosyl donor by employing the O-acetyl protected 1-α-trichloroacetimidate 29 (Scheme 4), which would in principle favor the formation of β-pseudodisaccharide 30 in the following glycosylation reaction through the orthoester procedure (1,2-trans glycosylation) [15,16].
Scheme 4.
Synthesis of the pseudodisaccharide 9β.
Selective deprotection of 27 with ethylendiamine in acetic acid following the procedure reported by Sun et al. [17] afforded in 86% yield compound 28, that was treated with trichloroacetonitrile to obtain 29 in 85% yield. Glycosylation reaction with 15 at 0 °C in dry CH2Cl2 in the presence of TMSOTF (1.5 equiv) afforded 39% yield of the β-glucoside 30 as the major compound, although impure of a partially deacetylated isomer (See Supplementary Materials). The 1H-NMR spectrum of compound 30 showed a dd at 5.05 ppm for H-2′ signal (appearing as a pseudo triplet), with coupling constants of 9.6 and 9.8 Hz. This indicates an axial-axial relationship with both H-1′ and H-3′, and therefore confirms the β-configuration of the glucose moiety.
Deprotection of the benzyl groups by catalytic hydrogenation and of the acetyl groups by treatment with Ambersep 900-OH, allowed to isolate pure disaccharide 9β in 43% yield over 2 steps (Scheme 4). To our delight and accordingly to our expectation, compound 9β was 12-fold more active than its α-anomer towards C. riparius trehalase and was the most potent insect trehalase inhibitor of the pseudodisaccharide pyrrolidine series, with an IC50 in the low micromolar range (IC50 = 0.784 μM, entry 7, Table 1). Moreover, 9β showed also a good 7-fold selectivity towards the insect trehalase vs. the porcine enzyme.
To evaluate the inhibitory pattern we performed kinetics measurements by varying the trehalose concentration, at two different concentrations of compounds 9α and 9β. Results showed a pure competitive inhibitory pattern. To determine the inhibition type, data were plotted according to the Lineweaver-Burk plot, replots were built and an inhibition constant (Ki) was calculated equal to 2.56 ± 0.42 µM for 9α and 0.40 ± 0.022 µM for 9β (see supplementary materials).
3. Materials and Methods
3.1. General Experimental Procedures
All the starting reactants, solvents, and catalysts were commercially available unless otherwise stated. All reactions were carried out under magnetic stirring and monitored by TLC on 0.25 mm silica gel plates (Merck F254). Column chromatographies were carried out on Silica Gel 60 (32–63 μm) or on silica gel (230–400 mesh, Merck, Kenilworth, NJ, USA). Yields refer to spectroscopically and analytically pure compounds unless otherwise stated. 1H-NMR spectra were recorded on a Varian Mercury-400 or on a Varian INOVA 400 instruments (Agilent Technologies, Santa Clara, CA, USA) at 25 °C. 13C-NMR spectra were recorded on a Varian Gemini-200 spectrometer (Agilent Technologies, Santa Clara, CA, USA). Chemical shifts are reported relative to TMS (1H: δ = 0.00 ppm) and CDCl3 (13C: δ = 77.0 ppm). Integrals are in accordance with assignments, coupling constants are given in Hz. For detailed peak assignments 2D spectra were measured (COSY, HSQC, NOESY, and NOE as necessary). IR spectra were recorded with a BX FTIR Perkin-Elmer system spectrophotometer (Perkin-Elmer, Waltham, MA, USA). ESIMS spectra were recorded with a Thermo Scientific™ LCQ fleet ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Elemental analyses were performed with a Perkin-Elmer 2400 analyzer (Perkin-Elmer, Waltham, MA, USA). Optical rotation measurements were performed on a JASCO DIP-370 polarimeter (JASCO, Easton, MD, USA).
3.2. Synthesis and Purification of 7-Deoxyuniflorine A Glucoside (8)
3.2.1. Synthesis of Compounds 18
To a solution of 2,3,4,6-tetra-O-benzyl-d–glucopyranose (300 mg, 0.55 mmol) in 7 mL of dry CH3CN, trichloroacetonitrile (568 μL, 7.15 mmol) and K2CO3 (380 mg, 2.75 mmol) were added under nitrogen atmosphere and the mixture was stirred at room temperature for 4 h. When a TLC analysis (CH2Cl2/MeOH 30:1) showed the disappearance of the starting material (Rf = 0.06) and formation of a new product (Rf = 0.33), the mixture was filtered through Celite® and the solvent was removed under reduced pressure affording a crude oil. The crude, obtained in a quantitative yield, was used directly for the next step without further purification.
3.2.2. Synthesis of Compounds 19
A solution of alcohol 13 [7] (100 mg, 0.21 mmol) and glucopyranosyl tricholoroacetimidate 18 (230 mg, 0.35 mmol) in 4.2 mL of dry Et2O was stirred for 10 min at room temperature under nitrogen atmosphere in the presence of 3 Å molecular sieves. After cooling to −20 °C and addition of trimethylsilyl trifluoromethanesulfonate (20 µL, 0.11 mmol), stirring was continued for 1 h; during this period the temperature was raised to room temperature. A TLC analysis (PE/EtOAc 1:1) showed the disappearance of the starting material (Rf = 0.22) and formation of two new products (Rf = 0.72 and 0.66). Then 2 mL of a saturated Na2CO3 solution were added, the mixture was extracted with Et2O (2 × 15 mL) and the organic layers were dried on Na2SO4. Filtration on cotton and evaporation under reduced pressure afforded the crude 19 that was purified by flash column chromatography on silica gel (PE/EtOAc from 3:1 to 1:1) to afford pure 19β (Rf = 0.25 PE/EtOAc 3:1, 9 mg, 0.009 mmol, 4% yield) and 19α (Rf = 0.18 PE/EtOAc 3:1, 179 mg, 0.18 mmol, 86% yield) as yellow oils.
(1R,2R,3R,6S,7aR)-1,2-bis(benzyloxy)-3-[(benzyloxy)methyl]-5-oxohexahydro-1H-pyrrolizin-6-yl 2,3,4,6-tetra-O-benzyl-α-hexopyranoside. 19α: [α = +18.3 (c = 0.06, CHCl3). 1H-NMR (400 MHz, CDCl3): δ = 7.38–7.11 (m, 35 H, H-Ar), 4.92–4.40 (m, 14 H, H-Bn), 4.83–4.80 (m, 1H, H-1), 4.33 (dd, J = 4.7, 4.1, 1H, H-1′), 4.25–4.23 (m, 1 H, H-6′), 4.08–4.02 (m, 3 H, H-7a′, H-5, H-3′), 3.95 (t, J = 9.4 Hz, 1H, H-3), 3.77 (dd, J = 10.8, 2.6 Hz, 1 H, Ha-8′), 3.72 (t, J = 9.7 Hz, 1 H, H-4), 3.68–3.65 (m, 1 H, Hb-8′), 3.63–3.56 (m, 4 H, H-2, H-2′, H-6), 2.23–2.18 (m, 1 H, Ha-7′), 1.95 (dt, J = 13.8, 6.7 Hz, 1 H, Hb-7′) ppm. 13C-NMR (50 MHz, CDCl3): 171.3 (s, C=O), 139.1–137.6 (s, 7 C, C-Ar), 128.6–127.6 (d, 35 C, C-Ar), 96.7 (d, C-1), 88.8 (d, C-2′), 87.2 (d, C-1′), 82.0 (d, C-3), 80.2 (d, C-2), 77.8 (d, C-6′), 77.2 (d, C-4), 76.5–72.4 (t, 7 C, C-Bn), 70.5 (d, C-3′), 69.2 (t, C-6), 68.3 (t, C-8), 62.0 (d, C-7a′), 58.9 (d, C-5), 33.3 (t, C-7′) ppm. MS (ESI): m/z (%) = 1018.75 (100) [M + Na]+. IR (CDCl3): 3088, 3066, 3032, 2915, 2867, 1700, 1602, 1496, 1453, 1363, 1098, 1071, 1028 cm−1. Elemental analysis (%) for C63H65NO10 (996.19): calcd. C 75.96, H 6.58, N 1.41; found C 75.92, H 6.55, N 1.43.
(1R,2R,3R,6S,7aR)-1,2-bis(benzyloxy)-3-[(benzyloxy)methyl]-5-oxohexahydro-1H-pyrrolizin-6-yl 2,3,4,6-tetra-O-benzyl-β-hexopyranoside. 19β: [α = −40.0 (c = 0.04, CHCl3). 1H-NMR (400 MHz, CDCl3): δ = 7.36–7.12 (m, 35 H, H-Ar), 4.91–4.49 (m, 14 H, H-Bn), 4.82–4.79 (m, 1 HH-1), 4.46–4.44 (m, 1 H, H-6′), 4.32 (dd, J = 4.7, 3.8, 1H, H-1′), 4.10–4.04 (m, 2 H, H-7a′ and H-5), 3.70–3.59 (m, 5 H, H-2′, H-3, H-4 and H-8), 3.50–3.39 (m, 4 H, H-3′, H-2 and H-6), 2.46–2.41 (m, 1 H, Ha-7′), 2,04 (dt, J = 14.7, 7.0 Hz, 1 H, Hb-7′) ppm. 13C-NMR (100 MHz, CDCl3): 171.5 (s, C=O), 138.6–137.5 (s, 7 C, C-Ar), 128.5–127.5 (d, 35 C, C-Ar), 102.2 (d, C-1), 88.8 (d, C-2′), 87.3 (d, C-1′), 84.5 (d, C-4), 81.8 (d, C-2), 78.6 (d, C-6′), 77.3 (d, C-3), 75.7–72.2 (t, 7 C, C-Bn), 74.3 (d, C-3′), 69.0 (t, C-6), 68.7 (t, C-8), 62.4 (d, C-7a′), 58.5 (d, C-5), 34.8 (t, C-7′) ppm. MS (ESI): m/z (%) = 1018.58 (100) [M + Na]+. IR (CDCl3): 3089, 3066, 3032, 2905, 2868, 1697, 1602, 1496, 1454, 1362, 1095, 1069, 1028 cm−1. Elemental analysis (%) for C63H65NO10 (996.19): calcd. C 75.96, H 6.58, N 1.41; found C 75.94, H 6.57, N 1.41.
3.2.3. Synthesis of Compound 20
A solution of 19α (118 mg, 0.12 mmol) in 1.2 mL of dry THF was stirred under nitrogen atmosphere at 0 °C and LiAlH4 (1 M solution in THF, 0.56 mL, 0.56 mmol) was added drop wise. The mixture was then heated at reflux temperature for 1.5 h, until a TLC analysis (PE/EtOAc 2:1) showed the disappearance of the starting material (Rf = 0.57) and the formation of a new product (Rf = 0.75). The reaction was then quenched at 0 °C with a saturated aqueous solution of Na2SO4 (5 mL). After extraction with EtOAc (3 × 15 mL), the organic layers were dried on Na2SO4 and concentrated at reduced pressure affording the crude that was purified by flash column chromatography on silica gel (PE/EtOAc 4:1) to afford 20 pure (Rf = 0.25, 108 mg, 0.11 mmol, 90% yield) as a waxy white solid.
(1R,2R,3R,6S,7aR)-1,2-bis(benzyloxy)-3-[(benzyloxy)methyl]hexahydro-1H-pyrrolizin-6-yl 2,3,4,6-tetra-O-benzyl-α-hexopyranoside 20: [α = +50.6 (c = 0.17, CHCl3). 1H-NMR (400 MHz, CDCl3): δ = 7.36–7.12 (m, 35 H, H-Ar), 4.98–4.39 (m, 14 H, H-Bn), 4.84–4.80 (m, 1H, H-1), 4.36–4.30 (m, 1H, H-6), 4.03 (t, J = 5.3 Hz, 1 H, H-2′), 3.96–3.90 (m, 1 H, H-1′), 3.84–3.63 (m, 5 H, H-4, H-5, H-3, H-7a′, Ha-6), 3.59–3.54 (m, 3 H, 2-H, Hb-6, Ha-8), 3.49 (dd, J = 9.4, 5.9 Hz, 1 H, Hb-8), 3.16 (dd, J = 11.7, 3.5 Hz, 1 H, Ha- 5′), 3.01 (dd, J = 12.3, 5.3 Hz, 1 H, Hb-5′), 2.94 (q, J = 5.9 Hz, 1 H, H-3′), 2.09–2.05 (m, 1 H, Ha-7′), 1.82 (ddd, J = 13.5, 7.6, 5.9 Hz, 1 H, Hb-7′) ppm. 13C-NMR (50 MHz, CDCl3): 139.1–138.1 (s, 7 C, C-Ar), 128.4–127.0 (d, 35 C, C-Ar), 96.5 (d, C-1), 88.5 (d, C-3), 86.1 (d, C-2′), 82.0 (d, C-1′), 80.3 (d, C-2), 78.4 (t, C-6′), 77.9 (d, C-4), 75.7–71.8 (t, 8 C, C-Bn and C-8), 70.6 (d, C-5), 69.4 (d, C-3′), 68.6 (t, C-6), 66.4 (d, C-7a′), 60.6 (t, C-5′), 37.0 (t, C-7′) ppm. MS (ESI): m/z (%) = 982.50 (100) [M + H]+. IR (CDCl3): 3089, 3067, 3032, 2915, 2867, 1605, 1496, 1454, 1363, 1144, 1070, 1028 cm−1. Elemental analysis (%) for C63H67NO9 (982.21): calcd. C 77.04, H 6.88, N 1.43; found C 77.01, H 6.87, N 1.41.
3.2.4. Synthesis of Compound 8
To a solution of compound 20 (153 mg, 0.16 mmol) in 15 mL of methanol, 10% Pd/C (77 mg, 50 % weight) and two drops of 37% HCl were added under nitrogen atmosphere, then the mixture was stirred under hydrogen atmosphere at room temp for three days. At completion of the reaction, the mixture was filtered through Celite® and the solvent was removed under reduced pressure affording a crude oil. Free amine 8 (53 mg, 0.15 mmol, 93% yield) was obtained by purification with ion exchange resin Dowex 50WX8-200, eluting successively with MeOH, H2O and 6% NH4OH.
(1R,2R,3R,6S,7aR)-1,2-dihydroxy-3-(hydroxymethyl)hexahydro-1H-pyrrolizin-6-yl-α-hexopyranoside 8: [α = +102.3 (c = 0.39, H2O). 1H-NMR (400 MHz, H2O): δ = 4.78 (d, J = 3.9 Hz, 1H, H-1), 4.36–4.30 (m, 1H, H-6′), 3.68–3.63 (m, 2 H, H-1′, H-2′), 3.63–3.44 (m, 4 H, H-4, H-5, H-6), 3.57 (dd, J = 11.7, 3.9 Hz, 1H, Ha-8), 3.41 (dd, J = 11.7, 6.9 Hz, 1 H, Hb-8), 3.30 (dd, J = 9.8, 3.5 Hz, 1 H, H-2), 3.18–3.12 (m, 1 H, H-7a′), 3.16 (t, J = 9.7 Hz, 1 H, H-3), 3.01–2.98 (m, 1 H, Ha-5′), 2.76 (dd, J = 11.7, 3.9 Hz, 1 H, Hb-5′), 2.63–2.56 (m, 1 H, H-3′), 2.13–2.07 (m, 1 H, Ha-7′), 1.71 (ddd, J = 13.2, 8.3, 4.9, 1 H, Hb-7′) ppm. 13C-NMR (100 MHz, H2O): 96.0 (d, C-1), 80.3 (d, C-1′), 78.0 (d, C-6′), 77.6 (d, C-2′), 72.9 (d, C-4), 71.9 (d, C-5), 71.1 (d, C-2), 70.1 (d, C-3′), 69.5 (d, C-3), 64.9 (d, C-7a′), 62.5 (t, C-8), 60.6 (t, C-6), 60.5 (t, C-5′), 34.2 (t, C-7′) ppm. MS (ESI): m/z (%) = 374.25 (100) [M + Na]+. Elemental analysis (%) for C14H25NO9 (351.15): calcd. C 47.86, H 7.17, N 3.99; found C 47.83, H 7.16, N 3.98.
3.3. Synthesis and Purification of Pyrrolidine-based Pseudodisacharides 9α, 10α and 11α
3.3.1. Synthesis of Compound 15
To a solution of 14 [13] (82 mg, 0.20 mmol) in 3 mL of THF, NEt3 (141 μL, 1.00 mmol) and 2-bromo-1-etanol (85 μL, 1.22 mmol) were added. The reaction mixture was stirred at room temperature for 3 days until a TLC analysis (CH2Cl2:MeOH 30:1) showed the disappearance of the starting material (Rf = 0.43) and the formation of a new product (Rf = 0.81). After evaporation under reduced pressure, the crude was purified by FCC (AcOEt:PE 1:1) affording pure 15 (Rf = 0.30, 75 mg, 0.17 mmol, 85% yield) as a yellow oil.
2-{(2R,3R,4R)-3,4-bis(benzyloxy)-2-[(benzyloxy)methyl]-1-[(2-hydroxy)etyl]-1H-pyrrolidine 15 [12]: [α = −20.3 (c = 0.92 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 7.35–7.26 (m, 15H, H-Ar), 4.55–4.42 (m, 6H, H-Bn), 3.99–3.97 (m, 1H, H-4), 3.89 (dd, J = 3.6, 1.2 Hz, 1H, H-3), 3.64–3.48 (m, 4H, H-7, H-8), 3.25 (d, J = 10.4 Hz, 1H, Ha-5), 3.06 (ddd, J = 12.9, 9.1, 4.7 Hz, 1H, Ha-6), 2.88 (dd, J = 9.6, 5.6 Hz, 1H, H-2), 2.67 (dd, J = 10.4, 5.2 Hz, 1H, Hb-5), 2.58 (dt, J = 12.6, 3.8 Hz, 1H, Hb-6).
3.3.2. Synthesis of Compound 16
To a solution of 14 [13] (132 mg, 0.33 mmol) in 6 mL of THF, NEt3 (230 μL, 1.65 mmol) and 3-bromo-1-propanol (179 μL, 1.98 mmol) were added. The reaction mixture was stirred at room temperature for 3 days until a TLC analysis (CH2Cl2:MeOH 10:1) showed the disappearance of the starting material (Rf = 0.51) and the formation of a new product (Rf = 0.85). After evaporation under reduced pressure, the crude was purified by FCC (AcOEt:PE 1:1) affording pure 16 (Rf = 0.30, 143 mg, 0.31 mmol, 94% yield) as a yellow oil.
(2R,3R,4R)-3,4-bis(benzyloxy)-2-[(benzyloxy)methyl]-1-[(3-hydroxy)propyl]-1H-pyrrolidine 16: [α = −39.1 (c = 0.47 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 7.34–7.24 (m, 15H, H-Ar), 4.56–4.48 (m, 6H, H-Bn), 3.93 (d, J = 4.8 Hz, 1H, H-4), 3.82–3.77 (m, 3H, H-9 and H-3), 3.63 (dd, J = 9.7, 5.8 Hz, 1H, Ha-6), 3.55 (dd, J = 9.8, 6.4 Hz, 1H, Hb-6), 3.45 (d, J = 10.8 Hz, 1H, Ha-5), 3.14 (td, J = 11.7, 3.9 Hz, 1H, Ha-7), 2.73 (q, J = 5.2 Hz, 1H, H-2), 2.64 (dt, J = 12.2, 3.9 Hz, 1H, Hb-7), 2.50 (dd, J = 10.8, 4.7 Hz, 1H, Hb-5), 1.94–1.82 (m, 1H, Ha-8), 1.56–1.49 (m, 1H, Hb-8); 13C-NMR (50 MHz, CDCl3): δ = 138.3, 138.2, 138.1 (s, 3C, C-Ar), 128.3–127.5 (d, 15C, C-Ar), 85.3 (d, C-3), 81.4 (d, C-4), 73.3, 71.4, 71.2, 70.9 (t, 4C, C-Bn and C-6), 69.7 (d, C-2), 64.0 (t, C-9), 56.7 (t, C-5), 55.2 (t, C-7), 29.3 (t, C-8); IR (CDCl3): ν = 3312, 3066, 3032, 2923, 2861, 1496, 1453, 1366, 1333, 1282, 1208, 1100, 1071, 1028 cm−1; MS (ESI): m/z calcd (%) for C29H35NO4 + Na+ 484.26 [M + Na]+; found: 484.33; elemental analysis calcd (%) for C29H35NO4 (461.59): C 75.46, H 7.64, N 3.03; found: 75.43, H 7.62, N 3.02.
3.3.3. Synthesis of Compound 17
To a solution of 14 [13] (100 mg, 0.25 mmol) in 1 mL of THF, NEt3 (174 μL, 1.25 mmol) and 4-bromo-1-butanol (158 μL, 1.5 mmol) were added. The reaction mixture was stirred at room temperature for 3 days until a TLC analysis (CH2Cl2:MeOH 10:1) showed the disappearance of the starting material (Rf = 0.51) and the formation of a new product (Rf = 0.65). After evaporation under reduced pressure, the crude was purified by FCC (AcOEt:PE 2:1) affording pure 17 (Rf = 0.35, 105 mg, 0.22 mmol, 88% yield) as a yellow oil.
(2R,3R,4R)-3,4-bis(benzyloxy)-2-[(benzyloxy)methyl]-1-[(4-hydroxy)butyl]-1H-pyrrolidine 17: [α = −26.2 (c = 0.60 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 7.34–7.24 (m, 15H, H-Ar), 4.56–4.39 (m, 6H, H-Bn), 3.94 (d, J = 4.8 Hz, 1H, H-4), 3.86 (d, J = 3.9 Hz, 1H, H-3), 3.67–3.63 (m, 2H, Ha-10 and Ha-6), 3.57–3.51 (m, 2H, Hb-10 and Hb-6), 3.30 (d, J = 10.8 Hz, 1H, Ha-5), 2.94–2.87 (m, 1H, Ha-7), 2.81–2.77 (m, 1H, H-2), 2.54 (dd, J = 10.7, 4.8 Hz, 1H, Hb-5), 2.46 (dt, J = 11.7, 4.4 Hz, 1H, Hb-7), 1.76–1.66 (m, 2H, Ha-8 and Ha-9), 1.61–1.51 (m, 2H, Hb-8 and Hb-9); 13C-NMR (50 MHz, CDCl3): δ = 138.4, 138.3, 138.2 (s, 3C, C-Ar), 128.2–127.5 (d, 15C, C-Ar), 85.4 (d, C-3), 81.6 (d, C-4), 73.2 (t, C-Bn), 71.4, 71.1, 71.0 (t, 3C, C-Bn and C-6), 69.7 (d, C-2), 62.6 (t, C-10), 56.9 (t, C-5), 56.0 (t, C-7), 31.9, 26.0 (t, 2C, C-8 and C-9); IR (CDCl3): ν = 3392, 3066, 3032, 2925, 2864, 1496, 1453, 1365, 1206, 1100 cm−1; MS (ESI): m/z calcd (%) for C30H37NO4 + H+ 476.27 [M + H]+; found: 476.60; elemental analysis calcd (%) for C30H37NO4 (475.62): C 75.76, H 7.84, N 2.94; found: C 75.74, H 7.82, N 2.93.
3.3.4. Synthesis of Compounds 2123α/β
General procedure for the glycosylation reaction: A solution of glucopyranosyl tricholoroacetimidate 18 (1.65 equiv.) and alcohol (1 equiv.) in dry diethyl ether or dry CH2Cl2, was stirred for 10 min at room temperature under nitrogen atmosphere in the presence of 3 Å molecular sieves. After cooling to −20 °C trimethylsilyl trifluoromethanesulfonate (1.5 equiv.) was added and the mixture was stirred for 1 h; letting the temperature to rise. The mixture was washed with a saturated Na2CO3 solution, dried over Na2SO4, filtered and concentrated to dryness. The crude was purified by flash column chromatography on silica gel to afford the pure glycosylated compound as α,β mixture.
21α,β. Obtained as a brown oil in 50% (Et2O), 76% (CH2Cl2), 23% (CH3CN) yields on 0.22–0.34 mmol of alcohol 15. Rf = 0.40 (EP:AcOEt 4:1); 1H-NMR (400 MHz, CDCl3): δ = 7.34–7.05 (m, 70H, H-Ar), 4.98–4.26 (m, 30H, H-Bn and OCHO), 3.97 (t, J = 9.3 Hz, 2H), 3.89–3.84 (m, 4H), 3.78–3.42 (m, 18H), 3.23–3.12 (m, 4H), 2.82–2.78 (m, 2H), 2.71–2.63 (m, 4H).
22α,β. Obtained as a brown oil in 36% (Et2O), 39% (CH2Cl2) yields on 0.27-0.31 mmol of alcohol 16. Rf = 0.42 (EP:AcOEt 4:1); 1H-NMR (400 MHz, CDCl3): δ = 7.39–7.15 (m, 70H, H-Ar), 5.02–4.41 (m, 30H, H-Bn and OCHO), 4.03–3.99 (m, 2H), 3.95–3.91 (m, 4H), 3.81–3.44 (m, 18H), 3.24–3.21 (m, 2H), 3.05–2.93 (m, 2H), 2.79–2.73 (m, 2H), 2.63–2.57 (m, 2H), 2.51–2.45 (m, 2H), 1.98–1.84 (m, 4H).
23α,β. Obtained as a brown oil in 66% (Et2O), 50% (CH2Cl2) yields on 0.21–0.33 mmol of alcohol 17. Rf = 0.45 (EP:AcOEt 4:1); 1H-NMR (400 MHz, CDCl3): δ = 7.30–7.05 (m, 70H, H-Ar), 4.92–4.30 (m, 30H, H-Bn and OCHO), 3.91 (t, J = 9.3 Hz, 2H), 3.83–3.79 (m, 4H), 3.70–3.31 (m, 18H), 3.13–3.11 (m, 2H), 2.81–2.74 (m, 2H), 2.65–2.62 (m, 2H), 2.45 (dd, J = 10.3, 4.9 Hz, 2H), 2.30–2.24 (m, 2H), 1.56–1.43 (m, 8H).
3.3.5. Synthesis of Peracetylated Compounds 24–26
General procedure for the synthesis of peracetylated derivatives: To a solution of the α,β mixture of benzylated derivatives 21–23 (1 equiv.) in 10–20 mL of methanol, 10% Pd/C (50% w/w) and 37% HCl were added under nitrogen atmosphere, then the mixture was stirred under hydrogen atmosphere at room temperature for 24 h. After that an 1H-NMR analysis showed the disappearance of the starting material, the mixture was filtered through Celite® and the solvent was removed under reduced pressure. The crude yellow oil was dissolved in pyridine (2–6 mL) and acetic anhydride (1–2 mL) was added. The solution was stirred at room temperature overnight. Then, after concentration under reduced pressure, the α- and β- isomers were separated by flash column chromatography on silica gel affording pure peracetylated 24–26α (23–42% yields over two steps) and 24–26β (3–12% yields over two steps).
Compound 24α. Obtained as a yellow oil in 23% yield starting from 0.26 mmol of 21α,β. Rf = 0.32 (Et2O:CH2Cl2 2:1); [α = +61.3 (c = 0.39 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 5.42 (t, J = 9.8 Hz, 1H, H-3′), 5.09 (d, J = 3.9 Hz, 1H, H-1′), 5.06–5.01 (m, 3H, H-3, H-4 and H-4′), 4.85 (dd, J = 10.3, 3.9 Hz, 1H, H-2′), 4.24 (dd, J = 12.2, 4.4 Hz, 1H, Ha-6′), 4.20 (dd, J = 11.2, 5.9 Hz, 1H, Ha-6), 4.10 (dd, J = 11.2, 4.9 Hz, 1H, Hb-6), 4.07 (dd, J = 12.2, 1.9 Hz, 1H, Hb-6′), 3.97 (ddd, J = 10.3, 4.4, 2.0 Hz, 1H, H-5′), 3.74 (dt, J = 11.3, 5.6 Hz, 1H, Ha-8), 3.57 (dt, J = 11.3, 5.9 Hz, 1H, Hb-8), 3.18 (d, J = 11.2 Hz, 1H, Ha-5), 3.03 (dt, J = 12.7, 6.4 Hz, 1H, Ha-7), 2.89 (dd, J = 11.2, 5.3 Hz, 1H, Hb-5), 2.82 (q, J = 4.9 Hz, 1H, H-2), 2.68 (dt, J = 12.7, 5.9 Hz, 1H, Hb-7), 2.07 (s, 3H, OAc), 2.06–2.05 (m, 12H, OAc), 2.00 (s, 3H, OAc), 1.99 (s, 3H, OAc); 13C-NMR (50 MHz, CDCl3): δ = 170.6, 170.4, 170.1, 170.0, 169.9, 169.5, 169.4 (s, 7C, C=O), 95.7 (d, C-1′), 78.7 (d, C-4), 76.3 (d, C-3), 70.8 (d, C-2′), 70.1 (d, C-3′), 68.7 (d, C-4′), 67.8 (d, C-2), 67.5 (d, C-5′), 66.6 (t, C-8), 63.2 (t, C-6), 62.0 (t, C-6′), 58.0 (t, C-5), 53.2 (t, C-7), 20.8, 20.7, 20.6, 20.5 (q, 7C, CH3); IR (CDCl3): ν = 2958, 2827, 2259, 1743, 1454, 1369, 1230, 1036 cm−1; MS (ESI): m/z calcd (%) for C27H39NO16 + Na+ 656.23 [M + Na]+; found: 656.33; elemental analysis calcd (%) for C27H39NO16 (633.60): C 51.18, H 6.20, N 2.21; found: C 51.15, H 6.18, N 2.20. Compound 24β. Obtained as a yellow oil in 3% yield starting from 0.26 mmol of 21α,β. Rf = 0.23 (Et2O:CH2Cl2 2:1); [α = −4.3 (c = 0.47 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 5.19 (t, J = 9.3 Hz, 1H, H-3′), 5.10-5.02 (m, 3H, H-3, H-4, H-4′), 4.98 (dd, J = 9.2, 8.3 Hz, 1H, H-2′), 4.52 (d, J = 8.3 Hz, 1H, H-1′), 4.25 (dd, J = 12.2, 4.4 Hz, 1H, Ha-6′), 4.22 (dd, J = 11.2, 5.8 Hz, 1H, Ha-6), 4.14 (dd, J = 12.2, 2.4 Hz, 1H, Hb-6′), 4.09 (dd, J = 11.2, 4.4 Hz, 1H, Hb-6), 3.97 (dt, J = 9.8, 4.9 Hz, 1H, Ha-8), 3.68 (ddd, J = 9.7, 4.9, 2.4 Hz, 1H, H-5′), 3.61 (ddd, J = 10.2, 7.3, 4.4 Hz, 1H, Hb-8), 3.12 (d, J = 11.7 Hz, 1H, Ha-5), 3.00 (dt, J = 13.2, 4.9 Hz, 1H, Ha-7), 2.84 (dd, J = 11.7, 5.4 Hz, 1H, Hb-5), 2.80–2.76 (m, 1H, H-2), 2.66 (ddd, J = 13.2, 7.8, 4.4 Hz, 1H, Hb-7), 2.08 (s, 3H, OAc), 2.07 (m, 6H, OAc), 2.06 (s, 3H, OAc), 2.03 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.99 (s, 3H, OAc); 13C-NMR (100 MHz, CDCl3): δ = 170.8, 170.6, 170.2, 170.1, 169.5, 169.4, 169.2 (s, 7C, C=O), 100.5 (d, C-1′), 78.5 (d, C-4), 76.5 (d, C-3), 72.8 (d, C-3′), 71.9 (d, C-5′), 71.2 (d, C-2′), 68.8 (t, C-8), 68.4 (d, C-4′), 68.0 (d, C-2), 62.9 (t, C-6), 61.9 (t, C-6′), 58.3 (t, C-5), 53.3 (t, C-7), 21.0, 20.9, 20.8, 20.7, 20.6 (q, 7C, CH3); IR (CDCl3): ν = 2958, 2827, 2259, 1747, 1454, 1369, 1225, 1036 cm−1; MS (ESI): m/z calcd (%) for C27H39NO16 + Na+ 656.23 [M + Na]+; found: 656.42; elemental analysis calcd (%) for C27H39NO16 (633.60): C 51.18, H 6.20, N 2.21; found: C 51.15, H 6.18, N 2.19.
Compound 25α. Obtained as a yellow oil in 42% yield starting from 0.12 mmol of 22α,β. Rf = 0.34 (Et2O:CH2Cl2 3:1); [α = +48.6 (c = 0.42 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 5.44 (t, J = 9.8 Hz, 1H, H-3′), 5.06–5.01 (m, 4H, H-3, H-4, H-1′ and H-4′), 4.86 (dd, J = 10.2, 3.4 Hz, 1H, H-2′), 4.23 (dd, J = 12.2, 4.4 Hz, 1H, Ha-6′), 4.15 (dd, J = 11.2, 6.3 Hz, 1H, Ha-6), 4.10 (dd, J = 11.2, 4.9 Hz, 1H, Hb-6), 4.07 (dd, J = 12.2, 2.4 Hz, 1H, Hb-6′), 3.97 (ddd, J = 10.3, 4.4, 2.4 Hz, 1H, H-5′), 3.71 (dt, J = 10.3, 6.4 Hz, 1H, Ha-9), 3.44 (dt, J = 10.3, 6.6 Hz, 1H, Hb-9), 3.12 (d, J = 11.2 Hz, 1H, Ha-5), 2.87 (dt, J = 12.2, 7.9 Hz, 1H, Ha-7), 2.76 (dd, J = 11.2, 4.9 Hz, 1H, Hb-5), 2.75–2.71 (m, 1H, H-2), 2.45 (dt, J = 12.2, 6.4 Hz, 1H, Hb-7), 2.08 (m, 6H, OAc), 2.06 (m, 6H, OAc), 2.04 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.77 (quint, J = 6.9 Hz, 2H, H-8); 13C-NMR (100 MHz, CDCl3): δ = 170.6, 170.4, 170.3, 170.2, 170.1, 169.9, 169.4 (s, 7C, C=O), 95.8 (d, C-1′), 78.9 (d, C-4), 76.3 (d, C-3), 70.9 (d, C-2′), 70.3 (d, C-3′), 68.8 (d, C-4′), 67.8 (d, C-2), 67.3 (d, C-5′), 66.3 (t, C-9), 63.2 (t, C-6), 62.0 (t, C-6′), 57.4 (t, C-5), 51.2 (t, C-7), 28.0 (t, C-8), 20.8, 20.7, 20.6, 20.5 (q, 7C, CH3); IR (CDCl3): ν = 2952, 2872, 2261, 1743, 1455, 1374, 1234, 1039 cm−1; MS (ESI): m/z calcd (%) for C28H41NO16 + Na+ 670.24 [M + Na]+; found: 669.72; elemental analysis calcd (%) for C28H41NO16 (647.62): C 51.93, H 6.38, N 2.16; found: C 51.92, H 6.29, N 2.15. Compound 25β. Obtained as a yellow oil in 12% yield starting from 0.12 mmol of 22α,β. Rf = 0.26 (Et2O:CH2Cl2 3:1); [α = −24.2 (c = 0.12 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 5.19 (t, J = 9.7 Hz, 1H, H-3′), 5.07 (t, J = 9.7 Hz, 1H, H-4′), 5.07–5.03 (m, 2H, H-3 and H-4), 4.96 (dd, J = 9.7, 7.8 Hz, 1H, H-2′), 4.48 (d, J = 7.8 Hz, 1H, H-1′), 4.25 (dd, J = 12.2, 4.9 Hz, 1H, Ha-6′), 4.15–4.12 (m, 2H, H-6), 4.13 (dd, J = 12.2, 2.4 Hz, 1H, Hb-6′), 3.87 (dt, J = 9.4, 6.4 Hz, 1H, Ha-9), 3.68 (ddd, J = 10.2, 4.4, 2.4 Hz, 1H, H-5′), 3.53 (dt, J = 9.7, 6.4 Hz, 1H, Hb-9), 3.11 (d, J = 10.7 Hz, 1H, Ha-5), 2.83 (dt, J = 12.2, 7.8 Hz, 1H, Ha-7), 2.76–2.72 (m, 1H, H-2), 2.75 (dd, J = 11.2, 4.8 Hz, 1H, Hb-5), 2.43 (dt, J = 12.2, 6.4 Hz, 1H, Hb-7), 2.08 (m, 6H, OAc), 2.07 (s, 3H, OAc), 2.06 (s, 3H, OAc), 2.04 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.74 (quint, J = 6.8 Hz, 2H, H-8); 13C-NMR (100 MHz, CDCl3): δ = 170.8, 170.6, 170.2, 170.0, 169.5, 169.3, 169.1 (s, 7C, C=O), 100.7 (d, C-1′), 78.8 (d, C-4), 76.3 (d, C-3), 72.8 (d, C-3′), 71.8 (d, C-5′), 71.3 (d, C-2′), 68.5 (d, C-4′), 67.8 (t, C-9), 67.7 (d, C-2), 63.3 (t, C-6), 62.0 (t, C-6′), 57.4 (t, C-5), 51.3 (t, C-7), 28.1 (t, C-8), 21.0, 20.9, 20.8, 20.6, 20.5 (q, 7C, CH3); IR (CDCl3): ν = 2954, 2882, 2261, 1743, 1376, 1234, 1039 cm−1; MS (ESI): m/z calcd (%) for C28H41NO16 + Na+ 670.24 [M + Na]+; found: 670.36; elemental analysis calcd (%) for C28H41NO16 (647.62): C 51.93, H 6.38, N 2.16; found: C 51.90, H 6.32, N 2.14.
Compound 26α. Obtained as a yellow oil in 37% yield starting from 0.095 mmol of 23α,β. Rf = 0.25 (Et2O:CH2Cl2 4:1); [α = +44.6 (c = 0.74 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 5.46 (t, J = 9.8 Hz, 1H, H-3′), 5.07–5.02 (m, 4H, H-3, H-4, H-1′ and H-4′), 4.84 (dd, J = 10.2, 3.9 Hz, 1H, H-2′), 4.25 (dd, J = 12.7, 4.4 Hz, 1H, Ha-6′), 4.19 (dd, J = 11.7, 6.4 Hz, 1H, Ha-6), 4.12 (dd, J = 11.7, 4.8 Hz, 1H, Hb-6), 4.08 (dd, J = 12.7, 2.4 Hz, 1H, Hb-6′), 3.99 (ddd, J = 10.2, 4.4, 2.4 Hz, 1H, H-5′), 3.68 (dt, J = 9.7, 6.3 Hz, 1H, Ha-10), 3.42 (dt, J = 9.7, 6.8 Hz, 1H, Hb-10), 3.14 (d, J = 11.2 Hz, 1H, Ha-5), 2.80 (dt, J = 11.7, 7.8 Hz, 1H, Ha-7), 2.73 (dd, J = 10.8, 4.9 Hz, 1H, Hb-5), 2.72–2.70 (m, 1H, H-2), 2.40–2.34 (m, 1H, Hb-7), 2.08 (m, 6H, OAc), 2.07 (s, 3H, OAc), 2.06 (s, 3H, OAc), 2.05 (s, 3H, OAc), 2.02 (s, 3H, OAc), 2.00 (s, 3H, OAc), 1.69–1.48 (m, 4H, H-8 and H-9); 13C-NMR (50 MHz, CDCl3): δ = 170.7, 170.6, 170.5, 170.2, 170.1, 170.0, 169.5 (s, 7C, C=O), 95.8 (d, C-1′), 78.9 (d, C-4), 76.3 (d, C-3), 71.0 (d, C-2′), 70.3 (d, C-3′), 68.8 (d, C-4′), 68.4 (t, C-10), 67.8 (d, C-2), 67.3 (d, C-5′), 63.2 (t, C-6), 62.0 (t, C-6′), 57.3 (t, C-5), 54.4 (t, C-7), 27.1 (t, C-9), 24.7 (t, C-8), 20.9, 20.7, 20.5 (q, 7C, CH3); IR (CDCl3): ν = 2953, 2872, 2260, 1742, 1455, 1373, 1233, 1038 cm−1; MS (ESI): m/z calcd (%) for C29H43NO16 + Na+ 684.26 [M + Na]+; found: 683.63; elemental analysis calcd (%) for C29H43NO16 (661.65): C 52.64, H 6.55, N 2.12; found: C 52.61, H 6.59, N 2.17.
Compound 26β. Obtained as a yellow oil in 9% yield starting from 0.095 mmol of 23α,β. Rf = 0.20 (Et2O:CH2Cl2 4:1); [α = −35.2 (c = 0.17 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ = 5.19 (t, J = 9.8 Hz, 1H, H-3′), 5.07 (t, J = 9.8 Hz, 1H, H-4′), 5.07–5.03 (m, 2H, H-3 and H-4), 4.97 (dd, J = 9.8, 7.8 Hz, 1H, H-2′), 4.48 (d, J = 7.8 Hz, 1H, H-1′), 4.25 (dd, J = 12.2, 4.4 Hz, 1H, Ha-6′), 4.19 (dd, J = 11.2, 6.3 Hz, 1H, Ha-6), 4.12 (dd, J = 12.2, 2.4 Hz, 1H, Hb-6′), 4.10 (dd, J = 11.2, 4.9 Hz, 1H, Hb-6), 3.87 (dt, J = 9.8, 5.9 Hz, 1H, Ha-10), 3.68 (ddd, J = 9.8, 4.4, 2.4 Hz, 1H, H-5′), 3.47 (dt, J = 9.8, 6.8 Hz, 1H, Hb-10), 3.11 (d, J = 11.2 Hz, 1H, Ha-5), 2.77 (dt, J = 12.2, 7.8 Hz, 1H, Ha-7), 2.70 (dd, J = 11.2, 5.2 Hz, 1H, Hb-5), 2.70–2.67 (m, 1H, H-2), 2.33 (ddd, J = 12.2, 8.8, 4.4 Hz, 1H, Hb-7), 2.07 (m, 6H, OAc), 2.06 (s, 3H, OAc), 2.03 (s, 3H, OAc), 2.02 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.63–1.42 (m, 4H, H-8 and H-9); 13C-NMR (50 MHz, CDCl3): δ = 170.7, 170.5, 170.1, 170.0, 169.5, 169.3, 169.1 (s, 7C, C=O), 100.7 (d, C-1′), 78.9 (d, C-4), 76.3 (d, C-3), 72.9 (d, C-3′), 71.8 (d, C-5′), 71.4 (d, C-2′), 69.6 (t, C-10), 68.6 (d, C-4′), 67.8 (d, C-2), 63.1 (t, C-6), 62.0 (t, C-6′), 57.2 (t, C-5), 54.2 (t, C-7), 27.1 (t, C-9), 24.3 (t, C-8), 20.9, 20.8, 20.7, 20.6, 20.5, 20.4 (q, 7C, CH3); IR (CDCl3): ν = 2957, 2886, 2816, 2261, 1743, 1454, 1373, 1233, 1039 cm−1; MS (ESI): m/z calcd (%) for C29H43NO16 + Na+ 684.26 [M + Na]+; found: 683.90; elemental analysis calcd (%) for C29H43NO16 (661.65): C 52.64, H 6.55, N 2.12; found: C 52.60, H 6.52, N 2.11.
3.3.6. Synthesis of Compounds 9–11
General procedure for the synthesis of the α,β mixture of polyhydroxylated pseudodisaccharides: To a 0.01 M solution of the α,β mixture of benzylated derivatives 21–23α,β in methanol, Pd/C (50%, w/w) and two drops of 37% HCl were added under nitrogen atmosphere, then the mixture was stirred under hydrogen atmosphere at room temperature for 1–3 days, until a NMR control showed the disappearance of the starting material. The mixture was then filtered through Celite® and the solvent was removed under reduced pressure affording a crude yellow oil. The α,β mixture of free amines was obtained by passing the corresponding hydrochloride salts mixture through a Dowex 50WX8 ion-exchange resin. Elution with 6% NH4OH afforded the free bases 9α,β, 10α,β and 11α,β with 32–40% yields.
Compounds 9α,β: The general procedure applied to 144 mg (0.146 mmol) of 21α,β, stirring the mixture for 3 days, afforded 17 mg (0.05 mmol) of a 2:1 9α,β mixture in 35% yield.
Compounds 10α,β: The general procedure applied to 100 mg (0.102 mmol) of 22α,β, stirring the mixture for one day, afforded 14 mg (0.04 mmol) of a 1:1 10α,β mixture in 40% yield.
Compounds 11α,β: The general procedure applied to 192 mg (0.192 mmol) of 23α,β, stirring the mixture for 3 days, afforded 21 mg (0.06 mmol) of a 1.2:1 11α,β mixture in 32% yield.
General procedure for the synthesis of polyhydroxylated α-pseudodisaccharides: A suspension of α-peracetylated derivatives 24α–26α (1 equiv.) and ion exchange resin Ambersep 900 OH (600 mg) in 5–10 mL of methanol was slowly stirred at room temperature for 16 h. After filtration through Celite®, the solvent was removed under reduced pressure affording pure α-pseudodisaccharides 9α–11α in quantitative yields.
Compound 9α. Obtained as a yellow oil in quantitative yield starting from 0.063 mmol of 24α. [α = +48.4 (c = 1.11 in MeOH); 1H-NMR (400 MHz, D2O): δ = 4.72 (d, J = 3.9 Hz, 1H, H-1′), 3.93 (ddd, J = 5.4, 2.9, 2.4 Hz, 1H, H-4), 3.73 (dd, J = 4.9, 2.9 Hz, 1H, H-3), 3.67 (dd, J = 12.2, 2.4 Hz, 1H, Ha-6′), 3.67–3.64 (m, 1H, Ha-8), 3.56 (dd, J = 12.2, 5.4 Hz, 1H, Hb-6′), 3.55–3.50 (m, 3H, H-3′ and H-6), 3.47 (ddd, J = 9.7, 5.4, 2.4 Hz, 1H, H-5′), 3.39 (ddd, J = 12.2, 8.3, 4.4 Hz, 1H, Hb-8), 3.34 (dd, J = 9.7, 3.9 Hz, 1H, H-2′), 3.21 (t, J = 9.7 Hz, 1H, H-4′), 2.97 (ddd, J = 13.2, 7.8, 4.4 Hz, 1H, Ha-7), 2.86 (dd, J = 11.3, 2.0 Hz, 1H, Ha-5), 2.61 (dd, J = 11.3, 5.9 Hz, 1H, Hb-5), 2.46 (dt, J = 13.2, 4.4 Hz, 1H, Hb-7), 2.38 (q, J = 5.4 Hz, 1H, H-2); 13C-NMR (100 MHz, D2O): δ = 98.4 (d, C-1′), 78.9 (d, C-3), 75.4 (d, C-4), 73.1 (d, C-3′), 72.0 (d, C-5′), 71.9 (d, C-2′), 71.4 (d, C-2), 69.5 (d, C-4′), 65.6 (t, C-8), 61.1 (t, C-6), 60.5 (t, C-6′), 58.6 (t, C-5), 54.1 (t, C-7); MS (ESI): m/z calcd (%) for C13H25NO9 + Na+ 362.15 [M + Na]+; found: 361.52; elemental analysis calcd (%) for C13H25NO9 (339.34): C 46.01, H 7.43, N 4.13; found: C 45.98, H 7.48, N 4.16.
Compound 10α. Obtained as a yellow oil in quantitative yield starting from 0.046 mmol of 25α. [α = +34.1 (c = 0.81 in MeOH); 1H-NMR (400 MHz, D2O): δ = 4.71 (d, J = 3.4 Hz, 1H, H-1′), 3.92 (ddd, J = 5.4, 2.9, 2.0 Hz, 1H, H-4), 3.73 (dd, J = 5.4, 2.9 Hz, 1H, H-3), 3.66 (dd, J = 12.2, 2.4 Hz, 1H, Ha-6′), 3.56 (dd, J = 12.2, 5.4 Hz, 1H, Hb-6′), 3.54–3.50 (m, 4H, H-3′, H-6 and Ha-9), 3.46 (ddd, J = 9.7, 5.4, 2.4 Hz, 1H, H-5′), 3.40–3.36 (m, 1H, Hb-9), 3.35 (dd, J = 9.7, 3.5 Hz, 1H, H-2′), 3.21 (t, J = 9.7 Hz, 1H, H-4′), 2.82 (dd, J = 11.2, 1.9 Hz, 1H, Ha-5), 2.82–2.76 (m, 1H, Ha-7), 2.55 (dd, J = 11.2, 5.9 Hz, 1H, Hb-5), 2.35 (q, J = 5.4 Hz, 1H, H-2), 2.26 (ddd, J = 11.7, 9.8, 5.9 Hz, 1H, Hb-7), 1.72–1.60 (m, 2H, H-8); 13C-NMR (100 MHz, D2O): δ = 98.0 (d, C-1′), 79.0 (d, C-3), 75.3 (d, C-4), 73.0 (d, C-3′), 71.8 (d, C-5′), 71.3 (d, 2C, C-2′ and C-2), 69.5 (d, C-4′), 66.2 (t, C-9), 61.2 (t, C-6), 60.5 (t, C-6′), 58.2 (t, C-5), 52.2 (t, C-7), 26.9 (t, C-8); MS (ESI): m/z calcd (%) for C14H27NO9+ 353.17 [M]+; found: 352.96; elemental analysis calcd (%) for C14H27NO9 (353.37): C 47.59, H 7.70, N 3.96; found: C 47.55, H 7.68, N 3.93.
Compound 11α. Obtained as a yellow oil in quantitative yield starting from 0.023 mmol of 26α. [α = +33.1 (c = 0.81 in MeOH); 1H-NMR (400 MHz, D2O): δ = 4.72 (d, J = 3.9 Hz, 1H, H-1′), 3.92 (ddd, J = 5.4, 2.4, 2.0 Hz, 1H, H-4), 3.73 (dd, J = 4.9, 3.0 Hz, 1H, H-3), 3.67 (dd, J = 12.2, 2.5 Hz, 1H, Ha-6′), 3.58–3.46 (m, 6H, H-3′, Hb-6′, H-5′, H-6 and Ha-10), 3.35 (dd, J = 9.7, 3.9 Hz, 1H, H-2′), 3.35–3.32 (m, 1H, Hb-10), 3.21 (t, J = 9.7 Hz, 1H, H-4′), 2.82 (dd, J = 11.2, 1.5 Hz, 1H, Ha-5), 2.66 (ddd, J = 11.7, 10.7, 5.4 Hz, 1H, Ha-7), 2.55 (dd, J = 11.2, 5.3 Hz, 1H, Hb-5), 2.34 (q, J = 5.4 Hz, 1H, H-2), 2.21 (dt, J = 11.7, 4.4 Hz, 1H, Hb-7), 1.49–1.41 (m, 2H, H-8), 1.40–1.30 (m, 2H, H-9); 13C-NMR (100 MHz, D2O): δ = 98.0 (d, C-1′), 79.1 (d, C-3), 75.4 (d, C-4), 73.1 (d, C-3′), 71.8 (d, C-5′), 71.7 (d, C-2), 71.3 (d, C-2′), 69.6 (d, C-4′), 67.8 (t, C-10), 61.3 (t, C-6), 60.5 (t, C-6′), 58.2 (t, C-5), 52.4 (t, C-7), 26.7 (t, C-8), 23.6 (t, C-9); MS (ESI): m/z calcd (%) for C15H29NO9 + Na+ 390.18 [M + Na]+; found: 390.42; elemental analysis calcd (%) for C15H29NO9 (367.39): C 49.04, H 7.96, N 3.81; found: C 49.03, H 7.98, N 3.83.
3.4. Synthesis of Compound 9β
3.4.1. Synthesis of 2,3,4,6-Tetra-O-acetyl-α/β-d-glucopyranose 28 [18]
To a solution of ethylenediamine (93 mg, 1.54 mmol) in 10 mL of dry THF, acetic acid (102 μL, 1.79 mmol) was slowly added dropwise over 10 min and the reaction mixture was stirred under nitrogen atmosphere at room temperature for 1 h. Then 27 (500 mg, 1.28 mmol) was added and the mixture was stirred for 1 h, until TLC analysis (PE/EtOAc 3:2) showed the disappearance of the starting material (Rf = 0.48) and the formation of a new compound (Rf = 0.24). After washing with HCl 0.1 M (2 × 2 mL) and NaHCO3 (2 × 2 mL), the combined organic layers were dried on Na2SO4, concentrated at reduced pressure and the crude mixture was purified by FCC (PE/AcOEt 1:1) affording pure 28 (α/β 1:9 Rf = 0.30, PE/EtOAc 1:1, 383 mg, 1.100 mmol, 86% yield).
1H-NMR (200 MHz, CDCl3): δ = 5.62 (d, J= 9.8 Hz 1H, H-1α), 5.55 (t, J= 3.8 Hz, 1H, H-3α), 5.36–5.27 (m, 1H, H-3β), 5.14 (t, J = 9.6 Hz, 1Hα + 1Hβ, H-4), 4.96 (dd, J = 9.8, 3.4 Hz, 1Hα + 1 Hβ, H-2), 4.80 (t, J= 8.1 Hz, 1H, H-1β), 4.37–4.12 (m, 5H, H-6α, H-6 β, H-5α), 3.85–3.77 (m, 1H, H-5β), 2.16 (s, 3Hα + 3 Hβ, OAc), 2.15 (s, 3Hα + 3 Hβ, OAc), 2.09 (s, 3Hα + 3 Hβ, OAc), 2.08 (s, 3Hα + 3 Hβ, OAc) ppm.
3.4.2. Synthesis of 2,3,4,6-Tetra-O-acetyl-α-d-glucopyranosyltrichloroacetimidate 29 [19]
A solution of 28 (383 mg, 1.10 mmol) in 8 mL of dry CH2Cl2 was cooled to 0 °C and DBU (32 μL, 0.22 mmol) and trichloroacetonitrile (612 μL, 7.70 mmol) were added. The reaction mixture was stirred under nitrogen atmosphere at room temperature for 2.5 h, when a TLC analysis (Hex/EtOAc 1:1) showed the disappearance of the starting material (Rf = 0.26) and the formation of a new compound (Rf = 0.62). A saturated NH4Cl solution was added and the mixture was transferred to a separating funnel, washing with CH2Cl2. The organic layer was washed with water (3 × 5 mL) and dried over Na2SO4. After concentration under reduced pressure, the crude was purified by flash column chromatography on silica gel (Hex/EtOAc from 3:1 to 2:1) to afford pure 29 (Rf = 0.25 Hex/EtOAc 2:1, 461 mg, 0.939 mmol, 85% yield).
1H-NMR (200 MHz, CDCl3): δ = 8.69 (s, 1H, NH), 6.56 (d, J = 3.7 Hz, 1H, H-1), 5.57 (t, J = 9.8 Hz, 1H, H-4), 5.23–5.10 (m, 2H, H-2, H-3), 4.32–4.06 (m, 3H, H-5, H-6 ), 2.08 (s, 3H, OAc), 2.05 (s, 3H, OAc), 2.04 (s, 3H, OAc), 2.02 (s, 3H, OAc) ppm.
3.4.3. Synthesis of Compound 30
A solution of glucopyranosyl tricholoroacetimidate 29 (2 equiv.) and alcohol 15 (1 equiv.) in dry CH2Cl2, was stirred for 10 min at room temperature under nitrogen atmosphere in the presence of 3 Å molecular sieves. After cooling to 0 °C trimethylsilyl trifluoromethanesulfonate (1.5 equiv.) was added and the mixture was stirred for 1.5 h, letting the temperature to rise. To the reaction mixture 1.8 mL of triethylamine was added and the mixture was transferred to a separating funnel, washing with CH2Cl2. The organic layer was washed with HCl 1M (3 × 3 mL), NaOH 1M (1 × 3 mL) and brine (2 × 3 mL). The combined organic layers were dried over Na2SO4 and the solvent was removed under reduced pressure. The resulting crude oil was dissolved in pyridine (3 mL), and acetic anhydride (2 mL) and DMAP (30 μL) were added. The solution was stirred at room temperature overnight, and after concentration under reduced pressure, the crude was purified by flash column chromatography on silica gel (Hex/EtOAc from 1:1 to 1:2) to afford the β compound 30 (Rf = 0.516 Hex/EtOAc 1:1, 34 mg, 0.044 mmol, 39% yield) as colorless oil, contaminated with small amounts of partially deacetylated glucoside (see supplementary information).
Compound 30: [α = −15.2 (c = 0.80 in CHCl3); 1H-NMR (400 MHz, CDCl3): δ =7.32–7.24 (m, 15H, H-Ar), 5.16 (t, J = 9.5 Hz, 1H, H-4′), 5.05 (dd, J = 9.8, 9.6 Hz, 1H, H-2′), 4.97 (t, J = 9.6 Hz, 1H, H-3′), 4.54–4.43 (m, 7H, H-Bn, H-1′), 4.23 (dd, J = 12.2, 4.7 Hz, 1H, Ha-6′), 4.09 (dd, J = 12.2, 2.2 Hz, 1H, Hb-6′), 4.00–3.95 (m, 1H, Ha-8), 3.88 (d, J = 5.0 Hz, 1H, H-4), 3.81 (d, J = 3.8 Hz, 1H, H-3), 3.68–3.58 (m, 2H, Hb-8, H-5′), 3.56–3.43 (m, 2H, H-6), 3.16 (d, J = 10.7 Hz, 1H, Ha-5), 3.10–3.04 (m, 1H, Ha-7), 2.77 (q, J = 5.7 Hz, 1H, H-2), 2.69–2.61 (m, 2H, Hb-5, Hb-7), 2.06 (s, 3H, OAc), 2.01 (s, 3H, OAc), 1.99 (s, 3H, OAc), 1.93 (s, 3H, OAc); 13C-NMR (100 MHz, CDCl3 ): δ = 170.6, 170.2, 169.4, 169.3 (s, 4C, C=O), 138.3, 138.2, 138.1 (t, 3C, C-Ar), 128.3, 127.8, 127.7, 127.6, 127.5 (d, 15C, C-Ar), 100.6 (d, C-1′), 84.7 (d, C-4), 81.6 (d, C-3), 77.4 (C-2′), 77.1 (d, C-3′), 73.2 (d, C-4′), 72.8 (d, C-2), 71.2, 71.1, 71.0 (s, 3C, C-Ar), 69.4 (d, C-5′), 68.9 (t, C-8), 68.4 (t, C-6), 61.9 (t, C-6′), 58.4 (t, C-5), 54.0 (t, C-7), 20.7, 20.6, 20.6, 20.6 (q, 4C, CH3); IR (CDCl3): ν = 3031, 2945, 2866, 2360, 2331, 1755, 1497, 1454, 1375, 1231, 1171,1039 cm−1. MS (ESI): m/z calcd (%) for C42H51NO13 + Na+ 800.33 [M + Na]+; found: 800.63.
3.4.4. Synthesis of Compound 9β
To a solution of 30 (43 mg, 0.055 mmol) in 10 mL of methanol, Pd/C (50%, w/w) and 0.5 mL of HCl 1 M were added under nitrogen atmosphere, then the mixture was stirred under hydrogen atmosphere at room temperature overnight, until an 1H-NMR control showed the disappearance of the starting material. The mixture was then filtered through Celite® and the solvent was removed under reduced pressure. The crude was purified by flash column chromatography on silica gel (CH2Cl2/MeOH 10:1, Rf = 0.585) to afford 12 mg, which were dissolved in 10 mL of methanol, ion exchange resin Ambersep 900-OH was added and the suspension was slowly stirred at room temperature for 16 h. The mixture was filtered through cotton and the solvent was removed under reduced pressure, affording pure 9β (8 mg, 0.024 mmol, 43% yield) as colorless oil.
Compound 9β: [α = −28.5 (c = 0.67 in H2O); 1H-NMR (400 MHz, D2O): δ = 4.34 (d, J = 8.0 Hz, 1H, H-1′), 4.02–3.98 (m, 1H, H-4), 3.92 (ddd, J = 11.0, 6.4, 4.4 Hz, 1H, Ha-8), 3.82–3.81 (m, 1H, H-3), 3.77 (d, J = 2 Hz, 1H, Ha-6′), 3.68–3.56 (m, 4H, Hb-8, H-6, Hb-6′), 3.38–3.21 (m, 3H, H-3′, H-4′, H-5′), 3.15 (t, J = 8.4 Hz, 1H, H-2′), 3.05–2.97 (m, 2H, Ha-7, Ha-5), 2.74 (dd, J = 11.4, 5.7 Hz, 1H, Hb-5), 2.61–2.53 (m, 2H, Hb-7, H-2); 13C-NMR (100 MHz, D2O): δ = 102.32 (d, C-1′), 79.11 (d, C-3), 76.06 (d, C-3′), 75.79 (d, C-5′), 75.62 (d, C-4), 73.20 (d, C-2′), 72.01 (d, C-2), 69.78 (d, C-4′), 67.88 (t, C-8), 61.19 (t, C-6), 60.88 (t, C-6′), 58.94 (t, C-5), 54.04 (t, C-7); MS (ESI): m/z calcd (%) for C13H25NO9 + Na+ 362.15 [M + Na]+; found: 362.47; elemental analysis calcd (%) for C13H25NO9 (339.34): C 46.01, H 7.43, N 4.13; found: C 46.67, H 7.45, N 3.20.
3.5. Biological Evaluation of Compounds 8, 9α, 9β, 10α, 11α and the α/β Mixture of 9,10 and 11
Compounds were tested for their inhibitory activity against insect trehalase of midge larvae of C. riparius, a good model for biochemical studies [20], and porcine kidney trehalase (purchased from Sigma-Aldrich, St. Louis, MO, USA) as the mammalian counterpart.
Trehalase activity was measured through a coupled assay with glucose-6-phosphate dehydrogenase and hexokinase according to Wegener at al. [21] To examine the potential of each compound as a trehalase inhibitor, dose-response curves were established to determine the IC50 values. Experiments were performed at a fixed substrate concentration close to the Km value (0.5 mM for C. riparius and 2.5 mM for porcine trehalase), in the presence of increasing inhibitor concentrations.Initial rates as a function of inhibitor concentration were fitted to the following equation:
| (1) |
where vi and v are the initial rate in the presence and in the absence of inhibitor, respectively, [I] is the inhibitor concentration, IC50 is the inhibitor concentration producing half-maximal inhibition, and n is the Hill coefficient.
Kinetic experiments were performed using C. riparius trehalase, measuring enzymatic activity at different trehalose concentrations from 0.05 to 20 mM in the presence of fixed inhibitor concentrations (5–10 µM for the compound 9α and 0.5–1 µM for the compound 9β). Kinetic parameters were calculated using a multiparameter, iterative, non-linear regression program based on the Marquardt-Levenberg algorithm (Sigma Plot, Jandel, CA, USA). Data are given ± S.D. of three independent experiments.
All enzyme assays were performed in triplicates at 30 °C by using sample volumes varying from 5 to 20 μL in 1 mL test and using a Cary3 UV/Vis Spectrophotometer. Enzyme activities were analyzed by Cary Win UV application software for Windows XP.
4. Conclusions
In conclusion, the synthesis of new pseudodisaccharide mimics 8, 9, 10 and 11, bearing a glucosyl moiety and a pyrrolizidine or pyrrolidine portion, was undertaken and their biological activity as insect trehalase inhibitors was evaluated. Inversion of configuration at C-6 in compound 8 (with respect to previously synthesized compound 4) resulted in a decrease of potency and selectivity towards the insect trehalase, showing the key role played by the stereochemical configuration at C-6 of the pyrrolizidine nucleus.
Moreover, a simple synthetic strategy was developed to obtain new pseudodisaccharide inhibitors with a pyrrolidine core, demonstrating the pivotal function played by the distance between the glucosyl and the iminosugar pyrrolidine moiety in these flexible inhibitors. Indeed, among a series of new compounds 9–11, only compounds 9 (with a two-carbon atom linker) maintained their inhibitory activity, while compounds 10 and 11 were completely inactive. In particular, the stereoselective synthesis of compound 9β allowed the identification of a new and selective insect trehalase inhibitor with IC50 in the low micromolar range (IC50 = 0.78 μM).
Acknowledgments
Ente Cassa di Risparmio di Firenze is acknowledged for a fellowship to GD (grant No. 2014/0303). CM thanks Accademia dei Lincei/Fondazione Donegani for a fellowship.
Supplementary Materials
The following are available online at www.mdpi.com/link, 1H and 13C-NMR spectra of new compounds and Figures S1–S4. Inhibition kinetics of insect trehalase in the presence of compounds 9α and 9β and dose-response curves of compounds 8, 9α,β, 9α and 9β for insect and porcine trehalase.
Author Contributions
F.C. and C.M. planned the synthetic strategy and wrote the manuscript. G.D., X.F. and C.V. made the syntheses, C.M. contributed to the N.M.R. characterization of the new compounds, M.F., P.F. and P.P. performed the biological data and contributed to the writing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Elbein A.D., Pan Y.T., Pastuszak I., Carroll D. New insights on trehalose: A multifunctional molecule. Glycobiology. 2003;13:17R–27R. doi: 10.1093/glycob/cwg047. [DOI] [PubMed] [Google Scholar]
- 2.Defaye J., Driguez H., Henrissat B., Bar-Guilloux E. Stereochemistry of the hydrolysis of α,α-trehalose by trehalase, determined by using a labelled substrate. Carbohydr. Res. 1983;124:265–273. doi: 10.1016/0008-6215(83)88462-6. [DOI] [Google Scholar]
- 3.Bini D., Cardona F., Gabrielli L., Russo L., Cipolla L. Trehalose mimetics as inhibitors of trehalose processing enzymes. In: Rauter A.P., Lindhorst T., editors. Carbohydrate Chemis Try: Volume 37. Royal Society of Chemistry; Cambridge, UK: 2012. pp. 259–302. [Google Scholar]
- 4.Gibson R.P., Gloster T.M., Roberts S., Warren R.A., de Gracia S., García Á., Chiara J.L., Davies G.J. Molecular basis for trehalase inhibition revealed by the structure of trehalase in complex with potent inhibitors. Angew. Chem. Int. Ed. 2007;46:4115–4119. doi: 10.1002/anie.200604825. [DOI] [PubMed] [Google Scholar]
- 5.Cardona F., Parmeggiani C., Faggi E., Bonaccini C., Gratteri P., Sim L., Gloster T.M., Roberts S., Davies G.J., Rose D.R., et al. Total syntheses of casuarine and its 6-O-α-glucoside: Complementary inhibition towards glycoside hydrolases of families GH31 and GH37. Chem. Eur. J. 2009;15:1627–1636. doi: 10.1002/chem.200801578. [DOI] [PubMed] [Google Scholar]
- 6.Cardona F., Goti A., Parmeggiani C., Parenti P., Forcella M., Fusi P., Cipolla L., Roberts S.M., Davies G.J., Gloster T.M. Casuarine-6-O-α-d-glucoside and its analogues are tight binding inhibitors of insect and bacterial trehalases. Chem. Commun. 2010;46:2629–2631. doi: 10.1039/b926600c. [DOI] [PubMed] [Google Scholar]
- 7.D’Adamio G., Sgambato A., Forcella M., Caccia S., Parmeggiani C., Casartelli M., Parenti P., Bini D., Cipolla L., Fusi P., et al. New synthetic and biological evaluation of uniflorine A derivatives: Towards specific insect trehalase inhibitors. Org. Biomol. Chem. 2015;13:886–892. doi: 10.1039/C4OB02016B. [DOI] [PubMed] [Google Scholar]
- 8.Bini D., Forcella M., Cipolla L., Fusi P., Matassini C., Cardona F. Synthesis of novel iminosugar-based trehalase inhibitors by Cross-Metathesis reactions. Eur. J. Org. Chem. 2011:3995–4000. doi: 10.1002/ejoc.201100484. [DOI] [Google Scholar]
- 9.Bini D., Cardona F., Forcella M., Parmeggiani C., Parenti P., Nicotra F., Cipolla L. Synthesis and biological evaluation of nojirimycin- and pyrrolidine-based trehalase inhibitors. Beilstein J. Org. Chem. 2012;8:514–521. doi: 10.3762/bjoc.8.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardona F., Faggi E., Liguori F., Cacciarini M., Goti A. Total syntheses of hyacinthacine A2 and 7-deoxycasuarine by cycloaddition to a carbohydrate derived nitrone. Tetrahedron Lett. 2003;44:2315–2318. doi: 10.1016/S0040-4039(03)00239-9. [DOI] [Google Scholar]
- 11.Overkleeft H.S., van Wiltenburg J., Pandit U.K. A facile transformation of sugar lactones to azasugars. Tetrahedron. 1994;50:4215–4224. doi: 10.1016/S0040-4020(01)86715-6. [DOI] [Google Scholar]
- 12.Zhou X., Liu W.-J., Ye J.-L., Huang P.-Q. A versatile approach to pyrrolidine azasugars and homoazasugars based on a highly diastereoselective reductive benzyloxymethylation of protected tartarimide. Tetrahedron. 2007;63:6346–6357. doi: 10.1016/j.tet.2007.02.087. [DOI] [Google Scholar]
- 13.D’Adamio G., Matassini C., Parmeggiani C., Catarzi S., Morrone A., Goti A., Paoli P., Cardona F. Evidence for a Multivalent Effect in Inhibition of Sulfatases Involved in Lysosomal Storage Disorders (LSDs) RSC Adv. 2016;6:64847–64851. doi: 10.1039/C6RA15806D. [DOI] [Google Scholar]
- 14.Damager I., Olsen C.E., Møller B.L., Motawia M.S. Chemical synthesis of 6‴-α-maltotriosyl-maltohexaose as substrate for enzymes in starch biosynthesis and degradation. Carbohydr. Res. 1999;320:19–30. doi: 10.1016/S0008-6215(99)00131-7. [DOI] [PubMed] [Google Scholar]
- 15.Stick R.V., Williams S.J. Formation of the glycosidic linkage. In: Stick R.V., Williams S.J., editors. Carbohydrates the Essential Molecules of Life. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2009. pp. 1133–1202. [Google Scholar]
- 16.Wang W., Kong F. New Synthetic Methodology for Regio- and Stereoselective Synthesis of Oligosaccharides via Sugar Ortho Ester Intermediates. J. Org. Chem. 1998;63:5744–5745. doi: 10.1021/jo981135e. [DOI] [PubMed] [Google Scholar]
- 17.Sun Q., Yang Q., Gong S., Fu Q., Xiao Q. Synthesis and enzymatic evaluation of phosphoramidon and its β anomer: Anomerization of α-l-rhamnose triacetate upon phosphitylation. Bioorg. Med. Chem. 2013;21:6778–6787. doi: 10.1016/j.bmc.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-Lorente G., Palomo J.M., Cocca J., Mateo C., Moro P., Terreni M., Fernandez-Lafuenteb R., Guisanb J.M. Regio-selective deprotection of peracetylated sugars via lipase hydrolysis. Tetrahedron. 2003;59:5705–5711. doi: 10.1016/S0040-4020(03)00876-7. [DOI] [Google Scholar]
- 19.Koketsu M., Kuwahara M. First Synthesis of a Trisaccharide of Glycosylkaemferide: A Resistance Factor in Carnations. Synth. Commun. 2004;34:239–245. doi: 10.1081/SCC-120027259. [DOI] [Google Scholar]
- 20.Forcella M., Mozzi A., Bigi A., Parenti P., Fusi P. Molecular cloning of soluble trehalase from Chironomus riparius larvae, its heterologous expression in Escherichia coli and bioinformatic analysis. Arch. Insect Biochem. Physiol. 2012;81:77–89. doi: 10.1002/arch.21041. [DOI] [PubMed] [Google Scholar]
- 21.Wegener G., Tschiedel V., Schlöder P., Ando O.J. The toxic and lethal effects of the trehalase inhibitor trehazolin in locusts are caused by hypoglycaemia. J. Exp. Biol. 2003;206:1233–1240. doi: 10.1242/jeb.00217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.