Abstract
Cell viability studies for benzo[1,2,4]triazin-7-ones and 1,2,4-benzotriazinyl (Blatter-type) radical precursors are described with comparisons made with 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO). All of the stable free radicals were several orders of magnitude less cytotoxic than the benzo[1,2,4]triazin-7-ones. The synthesis and evaluation of two new pyrid-2-yl benzo[1,2,4]triazin-7-ones are described, where altering the 1,3-substitution from phenyl to pyrid-2-yl increased cytotoxicity against most cancer cell lines, as indicated using National Cancer Institute (NCI) one-dose testing. COMPARE analysis of five-dose testing data from the NCI showed very strong correlations to the naturally occurring anti-cancer compound pleurotin. COMPARE is program, which analyzes similarities in cytotoxicity data of compounds, and enables quantitative expression as Pearson correlation coefficients. Compounds were also evaluated using the independent MTT assay, which was compared with SRB assay data generated at the NCI.
Keywords: anti-tumour, blatter-type radical, heterocyclic compound, NCI, pleurotin, TEMPO
1. Introduction
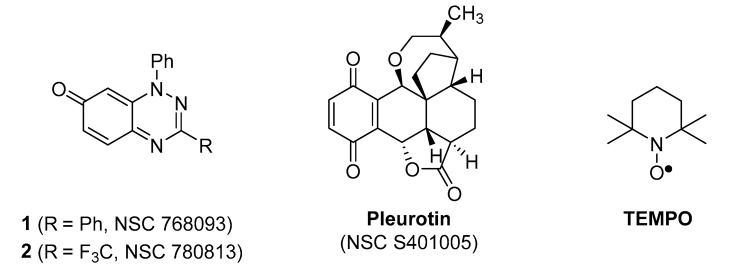
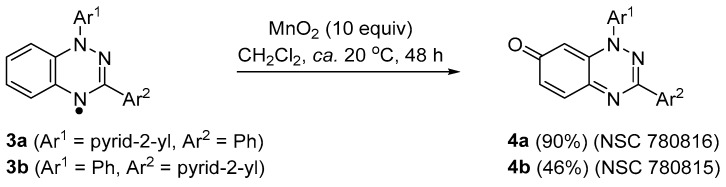
The cytotoxicity of the 3-substituted 1-phenylbenzo[1,2,4]triazin-7-ones 1 and 2 (Figure 1) showed very strong correlation (Pearson correlation coefficients ~0.8) to the naturally occurring antibiotic and anti-cancer agent pleurotin using the National Cancer Institute (NCI) COMPARE analysis. The origin for the anti-cancer activity is likely to be thioredoxin reductase (TrxR) inhibition, with the 3-Ph 1 and 3-F3C 2 substituted benzotriazinones exhibiting strong reversible mixed and uncompetitive inhibition [1]. Our anti-cancer studies followed evidence that analogues of scaffold 1 were multi-target inhibitors of Alzheimer’s disease [2]. Benzotriazinones are easily prepared by treating Blatter-type (benzotriazin-4-yl) radicals with manganese dioxide in dichloromethane at room temperature (Scheme 1) [3]. Given that stable free radicals such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) have shown cytotoxicity, including against breast (MDA-MB 231) [4] and prostate cancer (DU-145) cell lines [5], in this article we investigate the cytotoxicity of benzotriazin-4-yl radicals 3a and 3b and contrast them with the oxidation products 4a and 4b. Herein, we report the synthesis and cytotoxicity evaluation of new benzotriazinones, 3-phenyl-1-(pyrid-2-yl)benzo[1,2,4]triazin-7-one (4a) and 1-phenyl-3-(pyrid-2-yl)benzo[1,2,4]triazin-7-one (4b). Incorporating pyridine is reported to significantly alter cytotoxicity against certain cancer cell lines, hence we made the choice of replacing phenyl in the parent structure 1 with the pyrid-2-yl substituent in 4a and 4b [6,7,8].
Figure 1.
Background: Anti-cancer agents.
Scheme 1.
Synthesis of pyrid-2-yl-substituted benzo[1,2,4]triazin-7-ones.
2. Results and Discussion
2.1. Synthesis of Pyrid-2-yl-Substituted Benzo[1,2,4]triazin-7-ones
The preparation of benzotriazinyl radicals 3a and 3b has previously been reported [9]. New pyridyl-substituted benzotriazinones 4a and 4b were prepared from the MnO2-mediated oxidation [3] of benzotriazin-4-yls 3a and 3b in 90 and 46% yields, respectively (Scheme 1). It should be noted that 1,3-bis(pyrid-2-yl)benzo[1,2,4]triazin-7-one is derived from the MnO2-mediated oxidation of 1,3-bis(pyrid-2-yl)-1,4-dihydro-1,2,4-benzotriazine (i.e., the 4-NH, and not the free radical). However, the latter di-substituted pyrid-2-yl derivative was not selected for evaluation at the National Cancer Institute (NCI, see below), and thus is not a subject of the present article.
2.2. Development Therapeutic Program (DTP) National Cancer Institute (NCI) 60 Human Tumor Cell Line Screen and COMPARE Analysis
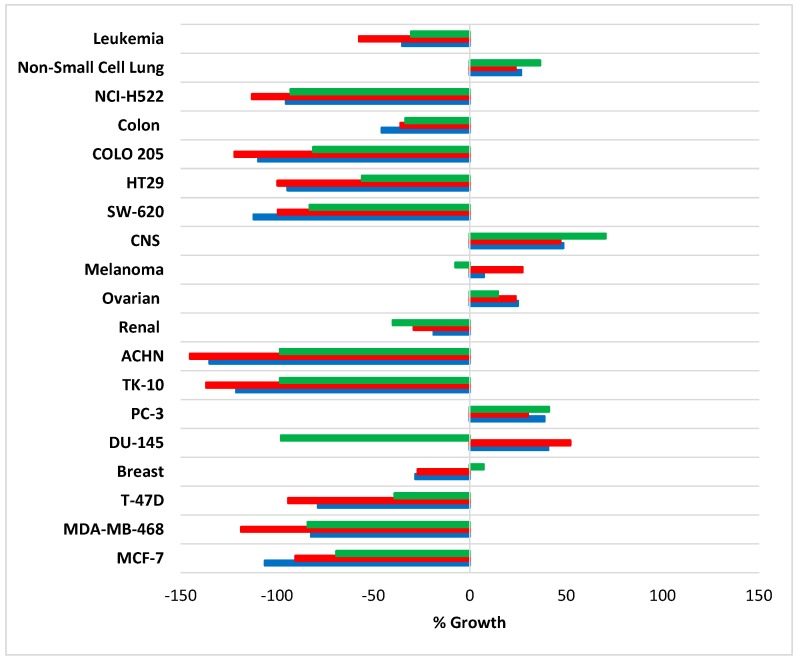
Radicals 3a and 3b were not selected by the NCI for cytotoxicity evaluation, although benzotriazinones 4a and 4b were used for one-dose (10 µM) screening against the DTP 60 cell line panel. Pyrid-2-yl-substituted compounds 4a and 4b exhibited similar variable cytotoxicity profiles, with overall cytotoxicity greater than the 1,3-diphenyl-substituted compound 1 [1] against most of the nine major histological tissue types at the NCI DTP (Figure 2). There was particularly strong growth inhibition exhibited by 4a and 4b in certain cancer cell lines, NCI-H522 (non-small cell lung cancer), COLO 205 (colon cancer), HT29 (colon cancer), SW-620 (colon cancer), ACHN (renal cancer), TK-10 (renal cancer), T-47D (breast cancer), MDA-MB-468 (breast cancer), and MCF-7 (breast cancer). Pyridyl-substituted compounds 4a and 4b, however, showed little growth inhibition of the DU-145 (prostate cancer) cell line after one-dose testing in comparison to 1. However, IC50 values after NCI five-dose testing (see below) showed that 4b had comparable cytotoxicity to 1, with 4a being over eight times less cytotoxic than 1 against the DU-145 cell line (Table 1). The DU-145 and MCF-7 cell lines were available to us (see below), and the MTT assay was used to obtain independent IC50 values.
Figure 2.
Summary of Development Therapeutic Program (DTP) National Cancer Institute (NCI)-single dose (10 µM) screening results for benzo[1,2,4]triazin-7-ones 1 (green), 4a (red), and 4b (blue) expressed as an average percent growth of each cancer cell type or certain cancer cell line relative to untreated cancer cells.
Table 1.
NCI IC50 data after five-dose testing: DMSO solution of compound (100 µL) was added to plates, which were incubated for 48 h at 37 °C using 5% CO2 (humidified atmosphere). Sulforhodamine B (SRB) assay was used to evaluate cytotoxicity.
| Compound | IC50 DU-145 (µM) | IC50 MCF-7 (µM) |
|---|---|---|
| 1 | 3.388 | 0.295 |
| 4a | 27.542 | 3.388 |
| 4b | 5.248 | 1.348 |
The NCI selection of 4a and 4b for subsequent five-dose testing established key parameters used in the NCI COMPARE algorithm to determine closely matching cytotoxicity profiles [1,10] (complete one- and five-dose data for 4a and 4b can be found in the Supplementary Information accompanying this article). The COMPARE analysis facilitated comparisons of cytotoxicity with the NCI’s vast database of over 250,000 synthetic compounds. The degree of similarity between two cytotoxicity profiles is described by the Pearson product-moment correlation coefficient (0 to ±1), with values above ±0.5 considered to be strong. On par with the Pearson correlation coefficient of 1 [1], very strong correlations to pleurotin of 0.84 and 0.73 were obtained for 4a and 4b, respectively (Table 2). Altering the 1,3-substitution from Ph to pyrid-2-yl in the benzo[1,2,4]triazin-7-one scaffold did not appear to alter the compound’s mechanism of action, with cytotoxicity profiles closely matching that of the irreversible TrxR inhibitor pleurotin.
Table 2.
NCI Pearson correlation coefficients obtained by COMPARE analysis to pleurotin.
| Compound | Pleurotin |
|---|---|
| 1 | 0.841 |
| 4a | 0.842 |
| 4b | 0.730 |
2.3. Cytotoxicity against DU-145 and MCF-7 Cell Lines Using the MTT Assay
The DU-145 (prostate cancer) and MCF-7 (breast cancer) cell lines, available at the National University of Ireland Galway (NUI Galway), were used to independently determine IC50 values. The cytotoxicity of benzotriazin-4-yl radicals 3a and 3b, TEMPO, and iminoquinones 4a and 4b was determined using the MTT assay. Radicals 3a and 3b exhibited very similar cytotoxicity profiles (see Supplementary Information), and were on average 13 and 105 times less cytotoxic towards the DU-145 and MCF-7 cell lines than oxidation products 4a and 4b (Table 3). Radicals 3a and 3b exhibited an approximate 10-fold greater cytotoxicity towards the prostate cancer compared to the breast cancer cell line. Benzotriazinones 4a and 4b gave similar submicromolar IC50 values against both cell lines, which were similar in magnitude to that of the previously evaluated compound 1 using the MTT assay [1]. In comparison, TEMPO was found to be relatively non-toxic. This is perhaps not surprising given that high concentrations of TEMPO (of 2.5 mM for 24 h) were required in order to induce a 3.4-fold increase in both early apoptotic cells and late apoptotic/necrotic cells compared with untreated DU-145 cell controls [5]. Literature reports support the requirement for high concentrations (2.5–10 mM) of nitroxide radicals (such as TEMPO) to achieve a therapeutic dose against various breast and prostate cancer cell lines [4,5]. Nevertheless, all radicals were significantly more cytotoxic towards the DU-145 cell line than the MCF-7 cell line.
Table 3.
Cytotoxicity evaluation (NUI Galway) using the MTT colorimetric assay. IC50 values were obtained after the incubation of cells with the test compounds in DMSO for 72 h. IC50 for 1 were previously obtained under identical conditions [1].
| Compound | IC50 DU-145 (µM) | IC50 MCF-7 (µM) |
|---|---|---|
| 1 | 0.230 ± 0.03 | 0.810 ± 0.08 |
| TEMPO | 151.400 ± 7.480 | >999 |
| 3a | 3.140 ± 0.179 | 34.740 ± 6.236 |
| 3b | 3.177 ± 0.154 | 26.415 ± 2.538 |
| 4a | 0.245 ± 0.003 | 0.303 ± 0.009 |
| 4b | 0.241 ± 0.011 | 0.277 ± 0.002 |
Using the MTT assay (Table 3), 1, 4a, and 4b exhibited greater cytotoxicity towards DU-145 and MCF-7 cell lines than that shown by the NCI five-dose data (except for 1 against MCF-7 at the NCI Table 1). The discrepancies in IC50 values were expected given the longer exposure time of cancer cells to the cytotoxic agent in the MTT assay (72 h versus 48 h at the NCI), as well as fundamental differences in the assays [11,12,13]. The SRB assay used by the NCI measures the amount of dye (sulforhodamine B) bound onto cellular protein, and is reported to be more sensitive than MTT [12,13]. In contrast, MTT relies on the ability of viable cells to reduce the tetrazolium dye MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) [11].
3. Experimental Section
3.1. General Procedures
Reactions were protected from atmospheric moisture using CaCl2 drying tubes. All volatiles were removed under reduced pressure. All reaction mixtures and column eluents were monitored by TLC (thin layer chromatography) using commercial glass-backed thin layer chromatography (TLC) plates (Merck Kieselgel 60 F254, Darmstadt, Germany). The plates were observed under UV light at 254 and 365 nm. The technique of dry flash chromatography was used throughout for all non-TLC scale chromatographic separations using Merck Silica Gel 60 (less than 0.063 mm) (Merck, Darmstadt, Germany) [14]. Melting and decomposition points were determined using either a PolyTherm-A, Wagner & Munz, Kofler-Hotstage Microscope apparatus (Wagner & Munz, Munich, Germany) or a TA Instruments DSC Q1000 (TA Instrument, New Castle, DE, USA) with samples hermetically sealed in aluminum pans under an argon atmosphere, using heating rates of 5 °C/min. Solvents used for recrystallization are indicated after the melting point. UV spectra were obtained using a Shimadzu UV-1601 UV/Vis spectrophotometer (Shimadzu, Kyoto, Japan) and inflections are identified by the abbreviation “inf”. IR spectra were recorded on a Shimadzu FTIR-NIR Prestige-21 spectrometer (Shimadzu, Kyoto, Japan) with a Pike Miracle Ge ATR accessory and strong, medium, and weak peaks are represented by s, m, and w, respectively. 1H and 13C-NMR spectra were recorded on a Bruker Avance 500 machine (at 500 and 125 MHz, respectively) (Bruker, Billerica, MA, USA). Deuterated solvents were used for homonuclear lock and the signals are referenced to the deuterated solvent peaks. MALDI-TOF MS was conducted on a Bruker BIFLEX III time-of-flight (TOF) mass spectrometer (Bruker, Billerica, MA, USA). 3-Phenyl-1-(pyrid-2-yl)-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl (3a) and 1-phenyl-3-(pyrid-2-yl)-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl (3b) were prepared according to literature procedures [9].
3.2. Synthesis of Phenyl- and Pyrid-2-yl-Substituted Benzo[1,2,4]triazin-7-ones 4a and 4b
3-Phenyl-1-(pyrid-2-yl)benzo[e][1,2,4]triazin-7(1H)-one (4a). To a stirred solution of 3-phenyl-1-(pyrid-2-yl)-1,4-dihydro-1,2,4-benzotriazin-4-yl (3a) (285 mg, 1.0 mmol) in dichloromethane (10 mL), MnO2 (869 mg, 10.0 mmol) was added and the reaction mixture was stirred at ca. 20 °C for two days. The reaction mixture was filtered through Celite® (Honeywell Specialty Chemicals Seelze GmbH, Seelze, Germany), rinsed with additional dichloromethane (50 mL), and volatiles were removed in vacuo. The residue was chromatographed on a short pad of silica with tert-butyl methyl ether (TBME) to give the title compound 4a (270 mg, 90%) as purple needles, m.p. (hot-stage): 210.1–213.8 °C (from PhH); m.p. (DSC) onset: 209.9 °C, peak max: 210.5 °C, decomp. onset: 225.8 °C, decomp. peak max: 227.7 °C (from PhH); Rf 0.58 (TBME, 100%); found: C, 71.87; H, 4.12; N, 18.70. C18H12N4O requires C, 71.99; H, 4.03; N, 18.66%; λmax(DCM)/nm 295 (logε 4.58), 310 inf (4.46), 339 inf (3.91), 356 inf (3.91), 544 (3.75), 582 (3.70), 635 inf (3.33); νmax/cm−1 3073w (aryl C-H), 1624s, 1599s, 1587s, 1570m, 1547s, 1526m, 1497w, 1468m, 1437s, 1398w, 1385w, 1333w, 1310w, 1281w, 1238m, 1192m, 1150w, 1115w, 1103w, 1094w, 1072w, 1028w, 995w, 974w, 905w, 856s, 787s,781m, 760s, 737s; δH (500 MHz, CDCl3) 8.63 (1H, d, J 3.5 Hz, Ar H), 8.26–8.25 (2H, m, Ar H), 8.02 (1H, ddd, J 8.0, 8.0, 1.5 Hz, Ar H), 7.80 (1H, d, J 8.0 Hz, Ar H), 7.66 (1H, d, J 9.5 Hz, Ar H), 7.48–7.46 (4H, m, Ar H), 7.25 (1H, CH, overlap with CDCl3), 6.78 (1H, d, J 2.0 Hz, CH); δC (125 MHz, CDCl3) 183.2 (s, C=O), 156.5 (s), 154.6 (s), 150.0 (s), 148.5 (d), 141.5 (d), 139.5 (d), 134.3 (s), 133.7 (s), 132.7 (d), 130.6 (d), 128.8 (d), 126.7 (d), 124.3 (d), 120.0 (d), 99.9 (d); m/z (MALDI-TOF) 302 (MH+ + 1, 16%), 301 (MH+, 100), 300 (M+, 21), 299 (M+ − 1, 39), 272 (16).
1-Phenyl-3-(pyrid-2-yl)benzo[e][1,2,4]triazin-7(1H)-one (4b). To a stirred solution of 1-phenyl-3-(pyrid-2-yl)-1,4-dihydrobenzo[e][1,2,4]triazin-4-yl (3b) (285 mg, 1.0 mmol) in dichloromethane (10 mL), MnO2 (896 mg, 10.0 mmol) was added and the reaction mixture was stirred at ca. 20 °C for two days. Upon completion, the reaction mixture was filtered through a short pad of Celite®, rinsed with additional dichloromethane (50 mL), and volatiles were removed in vacuo. The residue was chromatographed on a short pad of silica (acetone) to give the title compound 4b (138 mg, 46%) as purple needles; m.p. (hot-stage): decomp: 199.8–204.0 °C (from PhH); m.p. (DSC) decomp. onset: 205.8 °C, peak max: 210.8 °C (from PhH); Rf 0.69 (CHCl3/MeOH, 80:20); found: C, 71.85; H, 4.13; N, 18.48. C18H12N4O requires C, 71.99; H, 4.03; N, 18.66%; λmax(DCM)/nm 240 (logε 4.15), 300 (4.46), 310 inf (4.41), 338 inf (4.02), 351 inf (4.05), 374 inf (3.75), 534 (3.64), 572 (3.60), 628 inf (3.24); νmax/cm−1 3040w (aryl C-H), 1624m, 1611m, 1589m, 1584m, 1541s, 1537s, 1522m, 1493m, 1477m, 1456m, 1435m, 1395m, 1331m, 1304m, 1233m, 1200m, 1150w, 1117m, 1096w, 1072w, 1045w, 1026w, 995w, 978w, 928w, 907w, 856s, 822m, 814m, 800m, 779s, 764m, 745m; δH(500 MHz, CDCl3) 8.84 (1H, d, J 4.4 Hz, Ar H), 8.31 (1H, d, J 7.9 Hz, Ar H), 7.88–7.83 (2H, m, Ar H), 7.65–7.55 (5H, m, Ar H), 7.41 (1H, dd, J 6.9, 5.2 Hz, Ar H), 7.32 (1H, dd, J 9.8, 1.9 Hz, CH), 6.09 (1H, d, J 1.7 Hz, CH); δC(125 MHz, CDCl3) 182.2 (s), 155.3 (s), 151.6 (s), 150.2 (d), 149.8 (s), 142.5 (d), 140.7 (s), 137.0 (d), 136.6 (s), 132.4 (d), 130.3 (d), 130.1 (d), 125.7 (d), 124.7 (d), 122.3 (d), 98.4 (d); m/z (MALDI-TOF) 302 (MH+ + 1, 13%), 301 (MH+, 100), 300 (M+, 19), 285 (19), 273 (28), 242 (35).
3.3. Cell Culture and Cytotoxicity Evaluation
3.3.1. Materials and Cell Lines
2,2,6,6-Tetramethyl-1-piperidinyloxy (TEMPO, CAS number 2564-83-2) was obtained from Sigma-Aldrich (Darmstadt, Germany). The cytotoxicity evaluation of 1,3-bisphenylbenzo[1,2,4]triazin-7-one 1 was previously reported [1]. The MCF-7 breast cancer cell line and DU-145 prostate cancer cell line were obtained from Dr. Stephen Rea, National University of Ireland Galway (Galway, Ireland).
DU-145 was grown in RPMI-1640 medium and supplemented with 1% 2 Mm L-glutamine, 1% penicillin-streptomycin, and 10% non-heat inactivated fetal bovine serum (FBS). MCF-7 was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing high glucose (4.5 g/mL) and supplemented with 1% penicillin-streptomycin and 10% heat-inactivated fetal bovine serum (FBS). All cells grew as adherent cultures. Cell culture reagents were obtained from Sigma-Aldrich. Disposable sterile plastic ware was obtained from Sarstedt (Numbrecht, Germany).
3.3.2. Cytotoxicity Measurements Using the MTT Assay
The MTT colorimetric assay was used to determine cell viability [11]. Cells were added to 96-well plates at a cell density of 1000 cells per well for MCF-7 (200 µL per well) and 2000 cells per well for DU-145 (200 µL per well), and allowed to adhere over 24 h. Compound solutions were added in DMSO (1% v/v final concentration in well). The control cells were exposed to the same concentration of the vehicle control alone (DMSO). All cells were incubated at 37 °C and 5% CO2 (humidified atmosphere) for 72 h. MTT (20 µL, 5 mg/mL solution) was added and the cells were incubated for a further 3 h. The supernatant was then removed using a multi-transfer pipette and DMSO (100 µL) added to dissolve the MTT formazan crystals. The absorbance was determined using a plate reader at 550 nm with a reference at 690 nm. Cell viability is expressed as a percentage of the vehicle-only treated control (DMSO). Dose-response curves were analyzed by non-linear regression analysis and IC50 values were determined using GraphPad Prism software, v 8.0 (GraphPad Inc., San Diego, CA, USA). The in vitro activity of the drugs towards all cell lines is expressed as IC50 (i.e., concentration required for the reduction of the mean cell viability to 50%).
4. Conclusions
All three stable free radicals evaluated were significantly more cytotoxic towards DU-145 than the MCF-7 cell line. Benzotriazin-4-yl radicals 3a and 3b were significantly less cytotoxic than their oxidation products, benzo[1,2,4]triazin-7-ones 4a and 4b, towards the cancer cell lines evaluated. Pyridyl-substituted benzotriazin-7-ones exhibited submicromolar cytotoxicity using the MTT assay on par with 1,3-bisphenylbenzo[1,2,4]triazin-7-one 1. The variable DTP-NCI one-dose testing cytotoxicity profiles for 4a and 4b led to their selection for five-dose testing. COMPARE analysis demonstrated very strong correlations to pleurotin, despite the overall greater cytotoxicity of the pyrid-2-yl-substituted compounds compared to 1 after one-dose testing.
Acknowledgments
L-A.J.K. is funded by the College of Science, National University of Ireland Galway. M.S. and S.I.M. are funded by the Irish Research Council (IRC) Government of Ireland Postgraduate and Postdoctoral Scholarship Schemes, respectively. We thank the National Cancer Institute (USA), Development Therapeutic Program for cytotoxicity evaluation. P.A.K. thanks the Cyprus Research Promotion Foundation (Grants: NEAYPODOMH/NEKYP/0308/02 and YGEIA/BIOS/0308(BIE)/13), the University of Cyprus (Medium Sized Grant), and the following organizations in Cyprus for generous donations of chemicals and glassware: the State General Laboratory, the Agricultural Research Institute, the Ministry of Agriculture, Medochemie Ltd., and Biotronics Ltd. Furthermore, P.A.K. thanks the A. G. Leventis Foundation for helping to establish the NMR facility in the University of Cyprus.
Supplementary Materials
Supplementary materials are available online. Figures S1–S4: 1H and 13C-NMR Spectra for Compounds 4a and 4b, Figure S5–S6: NCI-60 One-Dose Mean Graph Data for Compounds 4a and 4b, Figures S7–S8: NCI-60 Five-Dose Mean Graph Data for Compounds 4a and 4b, Figures S9–S13: Viability of DU145 and MCF-7 cell lines as determined using the MTT assay for Compounds TEMPO, 3a, 3b, 4a and 4b.
Author Contributions
L-A.J.K. carried out cytotoxicity assays assisted by S.I.M.; L-A.J.K. and M.S. analyzed cytotoxicity data (including data from the NCI) in consultation with M.P.C. Synthetic and associated analytical work was carried out by G.A.Z. and A.A.B. under the direction of P.A.K.; F.A. conceived the project and wrote the manuscript. All authors read and approved the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds within are available from the authors (or from MDPI).
References
- 1.Sweeney M., Coyle R., Kavanagh P., Berezin A.A., Lo Re D., Zissimou G.A., Koutentis P.A., Carty M.P., Aldabbagh F. Discovery of anti-cancer activity for benzo[1,2,4]triazin-7-ones: Very strong correlation to pleurotin and thioredoxin reductase inhibition. Bioorg. Med. Chem. 2016;24:3565–3570. doi: 10.1016/j.bmc.2016.05.066. [DOI] [PubMed] [Google Scholar]
- 2.Catto M., Berezin A.A., Lo Re D., Loizou G., Demetriades M., De Stradis A., Campagna F., Koutentis P.A., Carotti A. Design, synthesis and biological evaluation of benzo[e][1,2,4]triazin-7(1H)-one and [1,2,4]-triazino[5,6,1-jk]carbazol-6-one derivatives as dual inhibitors of β-amyloid aggregation and acetyl/butyryl cholinesterase. Eur. J. Med. Chem. 2012;58:84–97. doi: 10.1016/j.ejmech.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Koutentis P.A., Lo Re D. Catalytic oxidation of N-phenylamidrazones to 1,3-diphenyl-1,4-dihydro-1,2,4-benzotriazin-4-yls: An improved synthesis of Blatter’s radical. Synthesis. 2010:2075–2079. doi: 10.1055/s-0029-1218782. [DOI] [Google Scholar]
- 4.Suy S., Mitchell J.B., Ehleiter D., Haimovitz-Friedman A., Kasid U. Nitroxides tempol and tempo induce divergent signal transduction pathways in MDA-MB 231 breast cancer cells. J. Biol. Chem. 1998;273:17871–17878. doi: 10.1074/jbc.273.28.17871. [DOI] [PubMed] [Google Scholar]
- 5.Suy S., Mitchell J.B., Samuni A., Mueller S., Kasid U. Nitroxide Tempo, a small molecule, induces apoptosis in prostate carcinoma cells and suppresses tumor growth in athymic mice. Cancer. 2005;103:1302–1313. doi: 10.1002/cncr.20898. [DOI] [PubMed] [Google Scholar]
- 6.Garuti L., Roberti M., Malagoli M., Rossi T., Castelli M. Synthesis and antiproliferative activity of some benzimidazole-4,7-dione derivatives. Bioorg. Med. Chem. Lett. 2000;10:2193–2195. doi: 10.1016/S0960-894X(00)00429-7. [DOI] [PubMed] [Google Scholar]
- 7.Moriarty E., Carr M., Bonham S., Carty M.P., Aldabbagh F. Synthesis and toxicity towards normal and cancer cell lines of benzimidazolequinones containing aromatic rings and 2-aromatic ring substituents. Eur. J. Med. Chem. 2010;45:3762–3769. doi: 10.1016/j.ejmech.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 8.Dam J., Ismail Z., Kurebwa T., Gangat N., Harmse L., Marques H.M., Lemmerer A., Bode M.L., de Koning C.B. Synthesis of copper and zinc 2-(pyridine-2-yl)imidazo[1,2-a]pyridine complexes and their potential anti-cancer activity. Eur. J. Med. Chem. 2017;126:353–368. doi: 10.1016/j.ejmech.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 9.Berezin A.A., Zissimou G., Constantinides C.P., Beldjoudi Y., Rawson J.M., Koutentis P.A. Route to benzo- and pyrido-fused 1,2,4-triazinyl radicals via N′-(het)aryl-N′′-[2-nitro(het)aryl]hydrazides. J. Org. Chem. 2014;79:314–327. doi: 10.1021/jo402481t. [DOI] [PubMed] [Google Scholar]
- 10.Fagan V., Bonham S., Carty M.P., Saenz-Méndez P., Eriksson L.A., Aldabbagh F. COMPARE analysis of the toxicity of an iminoquinone derivative of imidazo[5,4-f]benzimidazoles with NAD(P)H:quinone oxidoreductase 1 (NQO1) activity and computational docking of quinones as NQO1 substrates. Bioorg. Med. Chem. 2012;20:3223–3232. doi: 10.1016/j.bmc.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 12.Skehan P., Storeng R., Scudiero D., Monks A., McMahon J., Warren J.T., Bokesch H., Kenney S., Boyd M.R. New colorimetric cytotoxicity assay for anti-cancer screening. J. Natl. Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 13.Keepers Y.P., Pizao P.E., Peters G.J., Van Ark-Otte J., Winograd B., Pinedo H.M. Comparison of the sulforhodamine B protein and tetrazolium (MTT) assays for in vitro chemosensitivity testing. Eur. J. Cancer. 1991;27:897–900. doi: 10.1016/0277-5379(91)90142-Z. [DOI] [PubMed] [Google Scholar]
- 14.Harwood L.M. Dry-column flash chromatography. Aldrichimica Acta. 1985;18:25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.