Abstract
Background
The lethal thrombocytopenia that accompanies liver xenotransplantation is a barrier to clinical application. Human platelets are bound by the asialoglycoprotein receptor (ASGR) on pig sinusoidal endothelial cells and phagocytosed. Inactivation of the ASGR1 gene in donor pigs may prevent xenotransplantation-induced thrombocytopenia.
Methods
Transcription activator-like effector nucleases (TALENs) were targeted to the ASGR1 gene in pig liver derived cells. ASGR1 deficient pig cells were used for somatic cell nuclear transfer (SCNT). ASGR1 knock out (ASGR1−/−) fetal fibroblasts were used to produce healthy ASGR1 knock out piglets. Human platelet uptake was measured in ASGR1 and ASGR1−/− livers.
Results
Targeted disruption of the ASGR1 gene with TALENs eliminated expression of the receptor. ASGR1−/− livers phagocytosed fewer human platelets than domestic porcine livers during perfusion.
Conclusions
The use of TALENs in liver-derived cells followed by SCNT enabled the production of healthy homozygous ASGR1 knock out pigs. Livers from ASGR1−/− pigs exhibit decreased human platelet uptake. Deletion of the ASGR1 gene is a viable strategy to diminish platelet destruction in pig-to-human xenotransplantation.
Keywords: TALENS, genetically modified, pigs, asialoglycoprotein, liver xenotransplantation, thrombocytopenia, platelet, ASGR1
INTRODUCTION
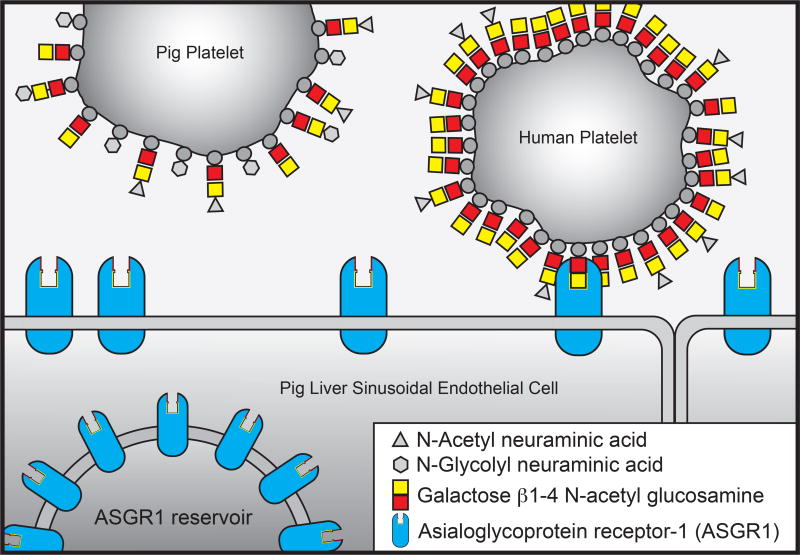
Xenotransplantation using pig organs could allow end-stage liver disease patients to receive a transplant, but a lethal thrombocytopenia that occurs shortly following graft reperfusion prevents clinical application [1, 2]. Pig liver sinusoidal endothelial cells (LSEC) and Kupffer cells (KC) remove human platelets from the circulation by endocytosis [3–5]. In vitro data shows that the mechanism of human platelet removal is partially dependent upon the asialoglycoprotein receptor (ASGR) (Figure 1), and the glycosylation pattern of pig and human platelets [4–6]. ASGR binds glycoproteins lacking terminal sialic acids and clears them from the circulation via receptor-mediated endocytosis [7, 8]. ASGR has roles in clearing prothrombotic blood components, platelets, von Willebrand factor (VWF), and factor VIII from the circulation [8, 9]. It also plays a critical role in the removal of cold stored platelets, limiting the shelf life of banked platelets for transfusion to a few days [10]. In murine models of streptococcal sepsis ASGR plays an important role in desialylated platelet clearance, preventing fatal thrombosis [11], and ASGR may be responsible for the clearance of lipoproteins and chylomicrons in a mechanism distinct from the LDL receptor pathway.
Figure 1.
Model of ASGR-mediated clearance of human versus pig platelets.
The functional heterotrimeric ASGR receptor, consisting of one ASGR2 and two ASGR1 subunits, is expressed in liver and can be disrupted by elimination of the ASGR1 protein [12]. We used TALENs targeted to the ASGR1 gene to create pigs lacking expression of the ASGR1 protein. Liver from these animals were evaluated for platelet uptake in an ex vivo perfusion model.
MATERIALS AND METHODS
Design of TALENS
Custom TALEN plasmids were designed to bind and cleave the region containing the start codon ATG in exon 2 of ASGR1. The design, cloning, and validation of the TALENs were performed by Cellectis as previously described [13]. The full binding site of the TALEN pair used is: 5’-TTCGAGGTCTAGCCAGCcttagcatgacaaagGAATATCAGGATCTGCA-3’, where the underlined letters are the TALEN binding sites and the lowercase letters are where the double-strand break was created.
Cell Culture and Transfection of Liver-Derived Cells (LDC)
Porcine adult LDC were isolated as previously described [14] and cultured in a combination media SCM (a-MEM:EGM-MV 3:1) (Invitrogen/Lonza, Switzerland) supplemented with 10% FBS (Hyclone, Logan, UT), 10% horse serum (Invitrogen, Carlsbad, CA), 12mM HEPES, and 1% Pen/Strep (Invitrogen). Neon transfection system was used according to the manufacturer’s instruction (Invitrogen). Briefly, LDC were harvested by trypsin digestion, washed with calcium and magnesium free DPBS (Invitrogen) and centrifuged. One million cells were suspended in 120 µl of electroporation buffer (Invitrogen) containing 2 µg DNA of each TALEN and were electroporated at 1300 V, 30 msec, 1 pulse. Cells were transferred in SCM without antibiotics and plated onto collagen I coated plates. They were cultured with 5% CO2 and 10% O2 at 30°C for 3 days and 37°C for 2 days.
SURVEYOR Mutation Detection Assay (CEL I assay)
TALEN-induced mutation was detected using the Surveyor Mutation Detection kit (Transgenomic, Omaha, NE). At day 5 post-transfection, genomic DNA from TALEN-treated and un-transfected cells was extracted and PCR was performed using primers AT-F1 (5’ CCACCTTGAGACCTTCAGCAAG 3’), AT-R1 (5’ GGTCATTCTCCTCATTGTCCAGATG 3’). Pwo SuperYield DNA Polymerase, dNTPack (Roche, Indianapolis, IN) was used and PCR conditions were as follows: 94°C, 2 minutes; 94°C, 15 seconds, 60°C, 30 seconds and 72°C, 50 seconds for 15 cycles; 94°C, 15 seconds, 60°C, 30 seconds and 72°C, 50 seconds with 5 seconds added to each elongation for 25 cycles; and a final extension step of 72°C for 5 minutes. A 334 bp of PCR product was produced. 200–400 ng of PCR product was denatured and annealed using the following program on a MyCycler (Bio-Rad): 95°C, 10 minutes; 95°C to 85°C, −2°C /second; 85°C to 25°C, −0.1°C /second. 1 µl of enhancer and 1 µl of Nuclease S (Transgenomic, Omaha, NE) was added to each reaction and incubated at 42°C for 40 minutes. The product was separated on a 10% polyacrylamide gel and stained with SYBR Safe to assess TALEN-induced mutations.
Screening ASGR1 Mutant cells
TALEN-treated cells were plated at 1 cell/well in ten 96-well plates coated with collagen I (BD, Franklin Lakes, NJ, USA). After 14 days, single cell clones became evident. Some cells were harvested for mutation screening. PCR was performed with a primer pair AT-F2 (5’ CCTCCCACACCCAAGTCTGTTC 3’) and AT-R2 (5’ TCTTCCGCTTACTCCCACGC 3’). A 379 bp of PCR products were resolved on a 1% agarose gel and purified by QIAquick Gel Extraction Lit (Qiagen, Valencia, CA, USA). Primer AT-F2 was used to sequence TALEN-targeted ASGR1 region.
DNA Sequencing analysis of TALEN-targeted ASGR1 region in cloned fetuses and piglets
Genomic DNA from cloned fetuses and piglets was extracted using DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA). PCR, DNA purification and sequencing were performed as described above.
Somatic cell nuclear transfer (SCNT)
The Institutional Biosafety and Institutional Animal Care and Use Committee of the Indiana University School of Medicine approved animals used in this study, and housing facilities are AAALAC accredited. SCNT was performed using in vitro matured oocytes (DeSoto Biosciences Inc, St. Seymour, TN.). Cumulus cells were removed from the oocytes by pipetting in 0.1% hyaluronidase. Only oocytes with normal morphology and a visible polar body were selected for SCNT. Oocytes were incubated in manipulation media (Ca-free NCSU-23 with 5% FBS) containing 5 mg/mL bisbenzimide and 7.5 mg/mL cytochalasin B for 15 min. Oocytes were enucleated by removing the first polar body plus metaphase II plate, and one cell was injected into each enucleated oocyte. Couples were fused by two DC pulses of 140V for 50 µsec (BTX cell electroporator, Harvard Apparatus, Holliston, MA, USA) in 280mM mannitol, 0.001mM CaCl2, and 0.05mM MgCl2. One hour later, reconstructed oocytes were activated by two DC pulses of 120V for 60 µsec in 280mM mannitol, 0.1mM CaCl2, and 0.05mM MgCl2 [18]. After activation, oocytes were placed back in NCSU-23 medium with 0.4% bovine serum albumin (BSA) and cultured at 38.5 °C, 5% CO2 in a humidified atmosphere for less than 1 h, before being transferred into the recipient. Recipients were synchronized occidental gilts on their first day of estrus. Adult liver-derived cells (LDC) genetically modified were used to produce the ASGR1 knock out fetuses by SCNT. Pregnancy was interrupted at day 32 of gestation for fetus collection. Samples from fetuses were taken for genotype analysis. Fetal fibroblasts from collected fetuses were cultured and used to produce ASGR1−/− pigs by SCNT.
Liver sources
Livers were obtained as previously described from an abattoir (domestic) or from genetically modified pigs [3–5, 15]. Porcine livers were flushed with saline and cold Histidine-Tryptophan-Ketoglutarate (HTK) (Essential Pharmaceuticals, Newtown, PA) preservation solution or cold HTK only.
Confocal analysis of ASGR1 knockout pig tissue
Frozen tissue sections (8µm) from pig liver biopsies were fixed in 4% freshly prepared paraformaldehyde and blocked in 2 % IgG-free BSA in HBSS for 2 hours at room temperature. Tissue sections were labeled with anti-ASGR1 antibody (Genway Biotech Inc., San Diego, CA) followed by detection with anti-rabbit secondary antibody conjugated to DyLight 649 (Jackson Immunoresearch, West Grove, PA). Nuclei were stained with DAPI (Invitrogen, Grand Island, NY) and tissue sections were washed three times with HBSS and mounted with Prolong Gold (n=2)(Invitrogen, Grand Island, NY). Confocal settings were determined by comparison to control tissue sections that received isotype control antibody.
Western blot analysis of ASGR1−/− pig liver tissue
Liver tissue was collected from domestic or ASGR1 knockout pigs and frozen at −80°C. Liver tissue (5.0mg) was homogenized in ice-cold RIPA lysis buffer. Proteins from lysates were separated on a 4–20% TGX Criterion (Bio-Rad) by SDS PAGE and transferred to PVDF membrane. Membranes were probed with anti-ASGR-1 (GWB-1C1B16 Genway Biotech INC) and anti-GAPDH (Millipore). Secondary antibodies used were donkey anti-rabbit 800 and donkey anti-mouse 680 (LI-COR) and visualized using the LI-COR Odyssey scanner.
DNA sequence analysis
Total RNA was purified using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). RNA samples were reverse transcribed into cDNA using OneStep RT-PCR Kit (Qiagen, Valencia, CA) following manufacturer’s protocol. All PCR products were electrophoresed in 1% agarose gel and purified by QIaquick Gel Extraction Kit (Qiagen, Valencia, CA). Purified PCR products were ligated into the pCR4-TOPO TA plasmid vector and transformed into the TOP10 One Shot chemically competent bacterial cells using the TOPO TA PCR Cloning Kit for Sequencing (Invitrogen, Carlsbad, CA). Transformed bacteria were plated onto Luria–Bertani agar containing 50 µg/ml Kanamycin for clone selection. Plasmids were isolated using the QIAprep Spin Miniprep Kit (Qiagen, Valencia, CA, USA). Nucleotide sequences were performed by the Sanger method using custom sequencing service (Core Facility at IUPUI).
Platelet sources, and isolation
Platelets were purchased from the Indiana Blood Bank. Platelets were counted and concentration adjusted to 1×1010/L in modified Krebs solution 2.0 g/L D-glucose, 0.141g/L MgSO4, 0.16 g/L KH2PO4, 0.35g/L KCl, 6.9 g/L NaCl, 2.10 g NaHCO3, 2.38g/L HEPES, 1 U/mL Heparin (Sagent Pharmaceuticals, Schaumburg, IL) pH 7.2–7.4.
ex vivo perfusion
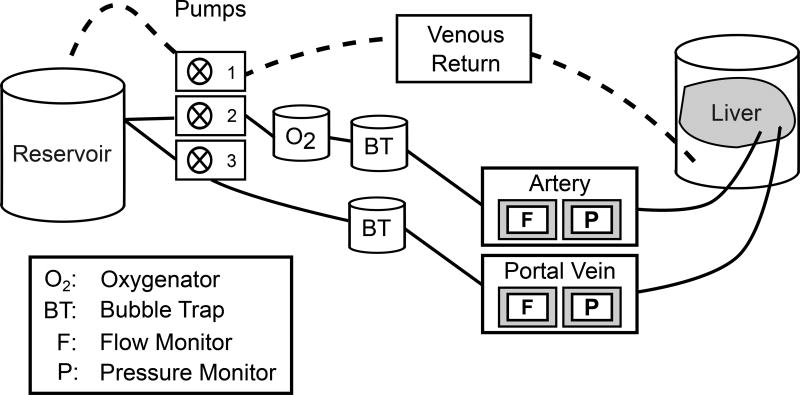
Porcine livers were removed from cold preservation and fitted with cannulas at the portal vein, hepatic artery, and suprahepatic inferior vena cava. The liver was placed in the perfusion apparatus, and the portal vein was connected to a centrifugal blood pump (Ismatec SA, Glattbrugg, Switzerland) and the hepatic artery to a pulsatile pump (Harvard Apparatus, Holliston, MA, USA). The average hepatic artery pressure was maintained manually at 60–80 mmHg by adjusting the flow rate. The portal circuit was set to constant pressure (9 mm Hg) with variable flow based on feedback from a pressure meter (Hugo Sachs Elektronik-Harvard Apparatus GmbH, March-Hugstetten, Germany). A water-jacketed hollow-fiber, oxygenator (Capiox® RX25, Terumo) was used. Livers were perfused for 15–20 minutes with 5 L warmed modified Krebs solution (described above) supplemented with 50 U/mL Penicillin/50µg Streptomycin (Gibco, Grand Island, NY), 0.1U/L Humulin® R (Lilly, Indianapolis, IN). A 2 liter water-jacketed reservoir was maintained in the circuit to facilitate addition of material to the system. This reservoir had a stir bar to facilitate mixing. Fifty mL packed washed autologous swine RBC were added to the reservior. After perfusion for another 15–20 minutes platelets were added. Typical flow rates were approximately 600mls per minute. Samples were taken from the reservoir at indicated times. Platelets were counted and total platelet number calculated at each time point. Platelet uptake in wild type versus ASGR1 deficient livers was compared using 2-factor ANOVA with repeated measures and platelets remaining at each time point were compared using the Holm-Sidak multiple comparison test using Graph Pad Prism 6.0 software.
Results
Evaluation of TALEN activity in porcine LDCs and generation of ASGR1−/− cells
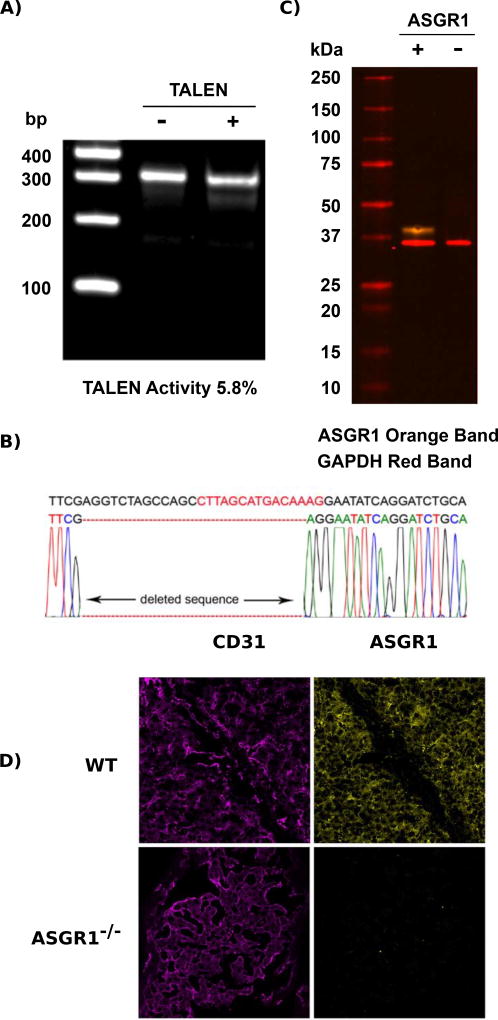
TALEN transfected and non-transfected porcine LDCs were cultured at 30°C for 3 days followed by 37°C for 2 days [16, 17]. To evaluate the success of initial transfection and determine what percent of cells had a mutated ASGR1 gene, genomic DNA from transfected (TALEN) and non-transfected (control) cells was extracted and used in the Surveyor assay. The Surveyor assay examines the TALEN-induced mutagenesis efficiency. PCR products were hybridized to generate mismatches in heteroduplexed DNA that was then treated and cleaved by the SURVEYOR nuclease. Our results demonstrated that TALEN pair cleavage efficiency was 5.8% for a mutation in the ASGR1 coding region (Figure 2A).
Figure 2.
Analysis of ASGR1 disruption. Surveyor mutation detection assay examining TALEN activity in LDCs. (A) A 334 bp PCR product was cleaved into two fragments: 283 bp and 51 bp. (B) DNA sequence of the ASGR1 region in a mutated single clone. The letters in red are where the double-strand break was created and the black letters that flank this site represent the TALEN binding sequences. Base deletion is shown as red dashes. (C) Immuno-blot examination of whole liver tissue lysates from wild type and ASGR1−/− pigs. ASGR1 is the green band and the loading control protein GAPDH is in red. (D) Porcine frozen tissue sections were fixed and labeled with anti-CD31 and anti-ASGR1 antibodies, detected with fluorescently conjugated secondary antibodies and analyzed by confocal microscopy. Domestic pig liver samples were positive for CD31 (magenta) and ASGR1 (yellow). ASGR1−/− pig liver tissue expressed CD31 but was negative for ASGR1. Image intensity was determined by comparison to the isotype control.
Clones were isolated using single cell populations in 96 well plates. A total of 112 clones were screened of which eight clones were ASGR1 mutants. A single clone having a 26 bp deletion that included the ASGR1 start codon was selected as a nuclear donor for SCNT (Figure 2B). Both alleles of the ASGR1 are likely identically mutated as this analysis revealed only the single mutated sequence.
Production of ASGR1 knockout fetuses and piglets by SCNT
Embryos made with cloned ASGR1−/− LDC were transferred into two gilts resulting in one pregnancy (Table 1). Six fetuses from this gilt were collected at day 32 of gestation. Five of six fetuses were well developed. Fibroblasts derived from these fetuses were used to produce ASGR1−/− pigs by SCNT. Four of six gilts transferred with the isolated fetal cells became pregnant; three of them went to term and delivered 22 piglets. One aborted at day 30 post embryo transfer (Table 1). Two of the piglets developed foreleg issues and were euthanized. Sequence analysis of genomic DNA indicated that the mutation in ASGR1 eliminated 26 bases containing the start codon (ATG) and surrounding sequences (Figure 2B). Western blotting and confocal microscopy verified that animals containing this mutation did not express ASGR1 protein in their livers (Figures 2C and 2D).
Table 1.
Somatic cell nuclear transfer with cells genetically modified with TALEN technology. Out of 22 piglets born, 19 were alive (86%). Eighteen healthy piglets were weaned at about 25 days; one piglet from the first litter was euthanized when one day old.
| Recipient | Donor Cells* |
Transferred Embryos |
Pregnancy | Fetuses | Piglets | ASGR1−/− | Cloning Efficiency** |
|---|---|---|---|---|---|---|---|
| 1 | LDC | 82 | No | -- | -- | 0 | |
| 2 | LDC | 108 | Yes | 6 | 6 | 5.6 | |
| 3 | FF | 111 | No | ||||
| 4 | FF | 119 | Yes | Aborted | |||
| 5 | FF | 130 | No | ||||
| 6 | FF | 117 | Yes | -- | 6 | 6 | 5.1 |
| 7 | FF | 115 | Yes | -- | 9 | 9 | 7.8 |
| 8 | FF | 103 | Yes | -- | 7 | 7 | 7.0 |
| Total | 885 | 5 | 22 | 28 | 3.2 |
LDC (Liver Derived Cells); FF (Fetal Fibroblasts)
Fetuses or piglets / total embryos transferred
Analysis of xenogeneic human platelet uptake by ASGR1−/− livers and liver cells
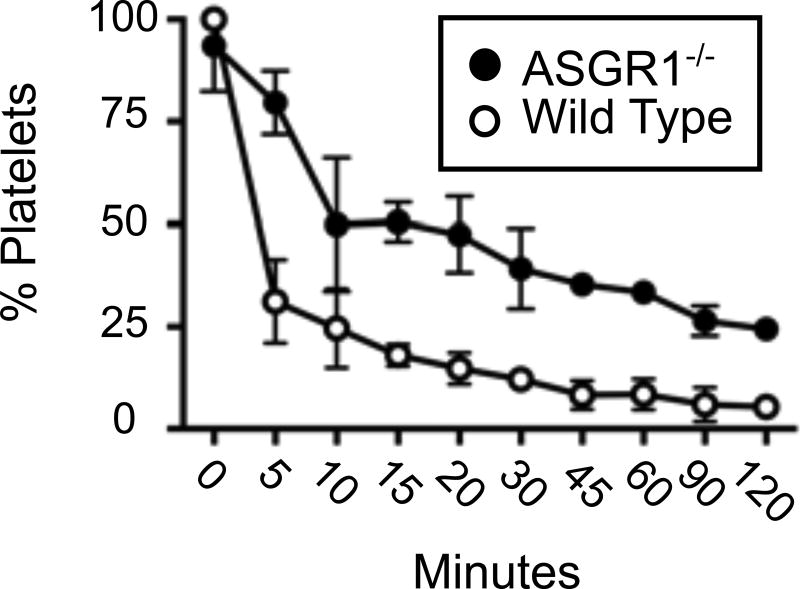
The perfusion circuit used to pass fluorescent human platelets through pig livers is shown in figure 3. The circuit was designed to measure platelet disappearance in the reservoir as an indicator of platelet consumption by the organ (Figure 3). The means and standard deviations for N=3 (control and ASGR1−/− livers) are shown in Figure 4. Platelet disappearance from the circuit was analyzed using a two-factor ANOVA with repeated measures (Table 2). Platelet loss from the perfusion circuit was significantly reduced in the presence of ASGR1 deficient livers vs. wild type livers (Table 2 row labeled Liver, p=0.02110). In addition, the Holm-Sidak’s multiple-comparison test revealed that, except for time 0, remaining platelets at each time point were different in wild type and ASGR1−/− circuits (p-values less than 0.05 at each timepoint). Platelets rapidly disappeared from both circuits within the first ten minutes of addition, though wild type livers eliminated more platelets during this initial phase than the ASGR1−/− organs. Dilution also contributes to this rapid depletion because the platelets are added to a 2-liter reservoir and are diluted by 3 additional liters of platelet-deficient buffer residing in the remainder of the perfusion apparatus at time zero. Given the flow rate of approximately 600mls/min, 9 minutes were required to ensure platelets were present throughout the circuit, closely matching the time when dilution of platelets in the reservoir slowed markedly. Beyond one hour, consumption of platelets by the WT liver appeared to be nearly complete. Notably, even with prolonged time, 2 hours, the ASGR1-deficient livers did not achieve the same the amount of platelet consumption seen in the WT livers.
Figure 3.
Diagram of the perfusion circuit setup.
Figure 4.
Examination of human platelet loss when perfused through pig livers. Platelet loss from perfusion circuits containing either ASGR1−/− or WT livers is compared. Means and standard deviations are shown (N=3 animals for each group).
Table 2.
Two-Factor ANOVA with Repeated Measures: Genetically Modified Liver vs. Time
| ANOVA table | SS | DF | MS | F (DFn, DFd) | P value |
|---|---|---|---|---|---|
| minutes | 3.279 | 9 | 0.3643 | F (9, 18) = 145.9 | P < 0.0001 |
| liver | 0.9463 | 1 | 0.9463 | F (1, 2) = 45.84 | P = 0.0211 |
| Interaction: minutes × liver | 0.2576 | 9 | 0.02862 | F (9, 18) = 5.115 | P = 0.0016 |
| Interaction: minutes × Subjects | 0.04495 | 18 | 0.002497 | ||
| Interaction: liver×Subjects | 0.04129 | 2 | 0.02064 | ||
| Subjects | 0.003532 | 2 | 0.001766 | ||
| Residual | 0.1007 | 18 | 0.005596 |
Discussion
The thrombocytopenia following graft reperfusion is a barrier to the application of liver xenotransplantation [1, 4, 6]. Because pig ASGR, MAC-1, and VWF have been implicated as mediators of the binding of human or baboon platelets to porcine LSEC and Kupffer cells, inactivation or modification of these molecules could reduce this barrier [4, 6]. The ASGR1 gene was disrupted in LDC using a single transfection with TALENS, and ASGR1 knock out pigs were created using SCNT of ASGR1 deficient LDCs. Off-target effects have not been examined in ASGR1 knock out pigs, but 17 of 19 pigs were healthy with no signs of illness. Knocking out the ASGR1 gene does not affect animal viability as demonstrated by the percentage of piglets born alive (86%) and the percentage of piglets weaned (94.7%). Two pigs developed foreleg problems that lead to early euthanasia; one was one day old and the second was 5 weeks old.
ASGR has been implicated in the clearance of platelets from circulation to lessen the severity of coagulopathy during sepsis, and also in the removal of platelets that have been cold stored [8, 10, 11, 18]. A stable complex of ASGR1 and ASGR2 in a 2:1 ratio binds Galβ oligosaccharides. Expression of ASGR1 and ASGR2 is necessary for a functional trimer [13]. Targeted deletion of ASGR1 in mice decreased platelet phagocytosis by the liver, exacerbating the lethal thrombosis associated with sepsis [11]. We show that disruption of swine ASGR1 also reduces the loss of human platelets in a normothermic ex vivo perfusion system.
Reduced consumption by ASGR1-deficient swine livers confirms previous experiments linking this receptor to the destruction of human platelets [3–5]. We have previously seen that blocking of ASGR with either antibodies or asialofetuin, or reducing ASGR1 abundance with siRNA, diminishes the uptake of human platelets by swine liver endothelial cells [5]. Inhibition by asialofetuin, a molecule containing carbohydrate ligands of ASGR, suggests that the destruction of human platelets by the swine liver is not the consequence of a species-specific incompatibility resulting in abnormal function of swine ASGR. The half-life of human platelets may be affected by ASGR1 simply because when compared to pigs, human platelets contain increased amounts of the proposed ASGR ligands β1–4, N-acetyl glucosamine and galactose β1–4, N-acetyl glucosamine [15].
Though inactivation of ASGR1 is a promising first step in eliminating xenotransplant-associated thrombocytopenia, ASGR1-deficient pig livers continued to consume human platelets. Additional modifications are likely necessary to eliminate the destruction of human platelets by the porcine livers. Other receptors such as the CD18 receptor that have been implicated in this process are attractive candidates to pursue [5].
Acknowledgments
We thank Hershel Raff, Ph.D. for help with the statistical analyses. This study was supported by IU Health Transplant Institute. The authors thank MRI (Methodist Research Institute) and LARC (Laboratory Animal Research Center) staff for care of the animals. This investigation utilized a facility constructed with support from Research Facilities Improvement Program grant number C06RR10601-01 from the National Center for Research Resources, National Institutes of Health. Funding for this project was provided by Indiana University Health, the Indiana University Health Transplant Institute, and a Liver Scholar Award from the American Association for the Study of Liver Diseases to LLP.
Joseph Tector MD, PhD, is the founder of Xenobridge, LLC which applies for patents in the field of xenotransplantation including patents relevant to this work. Chris Burlak Ph.D., also is a named inventor on a submitted patent application regarding the topic of this manuscript. Richard Sidner, Ph.D. receives consultant fees specifically related to xenotransplantation.
Abbreviations
- ASGR
asialoglycoprotein receptor
- ASGR1
asialoglycoprotein receptor 1 gene
- ASGR1
asialoglycoprotein receptor 1 protein
- RME
receptor-mediated endocytosis
- VWF
von Willebrand factor
- SCNT
somatic cell nuclear transfer
- ZFN
Zinc Finger Nucleases
- NHEJ
non-homologous end joining
- TALENS
Transcription activator-like effector nucleases
- LDC
liver-derived cells
Footnotes
Author contributions
All Authors participated in the drafting and critical review of the manuscript. PL, JBM, SMD and JLE were responsible for creating genetically engineered animals. LLP, RLB, RAS, LMR, CB, JRB, ZYW, and SMD participated in sample collection and experimental analyses. AJT, MT, and LLP designed experiments.
References
- 1.Tector AJ, Fridell JA, Elias N, et al. Aberrations in hemostasis and coagulation in untreated discordant hepatic xenotransplantation: Studies in the dog-to-pig model. Liver transplantation. 2002;8(2):153–159. doi: 10.1053/jlts.2002.30881. [DOI] [PubMed] [Google Scholar]
- 2.Ekser B, Long C, Echeverri GJ, et al. Impact of thrombocytopenia on survival of baboons with genetically modified pig liver transplants: clinical relevance. American journal of transplantation. 2010;10(2):273–285. doi: 10.1111/j.1600-6143.2009.02945.x. [DOI] [PubMed] [Google Scholar]
- 3.Burlak C, Paris LL, Chihara RK, et al. The fate of human platelets perfused through the pig liver: implications for xenotransplantation. Xenotransplantation. 2010;17(5):350–361. doi: 10.1111/j.1399-3089.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- 4.Paris LL, Chihara RK, Reyes LM, et al. ASGR1 expressed by porcine enriched liver sinusoidal endothelial cells mediates human platelet phagocytosis in vitro. Xenotransplantation. 2011;18(4):245–251. doi: 10.1111/j.1399-3089.2011.00639.x. [DOI] [PubMed] [Google Scholar]
- 5.Chihara RK, Paris LL, Reyes LM, et al. Primary porcine Kupffer cell phagocytosis of human platelets involves the CD18 receptor. Transplantation. 2011;92(7):739–744. doi: 10.1097/TP.0b013e31822bc986. [DOI] [PubMed] [Google Scholar]
- 6.Peng Q, Yeh H, Wei L, et al. Mechanisms of xenogeneic baboon platelet aggregation and phagocytosis by porcine liver sinusoidal endothelial cells. PloS one. 2012;7(10):e47273. doi: 10.1371/journal.pone.0047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashwell G, Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–54. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- 8.Sørensen AL, Rumjantseva V, Nayeb-Hashemi S, et al. Role of sialic acid for platelet life span: exposure of β-galactose results in the rapid clearance of platelets from the circulation by asialoglycoprotein receptor–expressing liver macrophages and hepatocytes. Blood. 2009;114(8):1645–1654. doi: 10.1182/blood-2009-01-199414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellies LG, Ditto D, Levy GG, et al. Sialyltransferase ST3Gal-IV operates as a dominant modifier of hemostasis by concealing asialoglycoprotein receptor ligands. Proceedings of the National Academy of Sciences. 2002;99(15):10042–10047. doi: 10.1073/pnas.142005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grozovsky R, Hoffmeister KM, Falet H. Novel clearance mechanisms of platelets. Current opinion in hematology. 2010;17(6):585–589. doi: 10.1097/MOH.0b013e32833e7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nature medicine. 2008;14(6):648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tozawa RI, Ishibashi S, Osuga JI, et al. Asialoglycoprotein receptor deficiency in mice lacking the major receptor subunit its obligate requirement for the stable expression of oligomeric receptor. Journal of Biological Chemistry. 2001;276(16):12624–12628. doi: 10.1074/jbc.M011063200. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Lau CH, Goh SL, et al. Baculoviral transduction facilitates TALEN-mediated targeted transgene integration and Cre/LoxP cassette exchange in human-induced pluripotent stem cells. Nucleic acids research. 2013;41(19):e180–e180. doi: 10.1093/nar/gkt721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P, Estrada J, Zhang F, Waghmare SK, Mir B. Isolation, characterization, and nuclear reprogramming of cell lines derived from porcine adult liver and fat. Cellular Reprogramming (Formerly" Cloning and Stem Cells") 2010;12(5):599–607. doi: 10.1089/cell.2010.0006. [DOI] [PubMed] [Google Scholar]
- 15.Paris LL, Chihara RK, Sidner RA, Joseph Tector A, Burlak C. Differences in human and porcine platelet oligosaccharides may influence phagocytosis by liver sinusoidal cells in vitro. Xenotransplantation. 2012;19(1):31–39. doi: 10.1111/j.1399-3089.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- 16.Doyon Y, Choi VM, Xia DF, et al. Transient cold shock enhances zinc-finger nuclease–mediated gene disruption. Nature methods. 2010;7(6):459–460. doi: 10.1038/nmeth.1456. [DOI] [PubMed] [Google Scholar]
- 17.Carlson DF, Tan W, Lillico SG, et al. Efficient TALEN-mediated gene knockout in livestock. Proceedings of the National Academy of Sciences. 2012;109(43):17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rumjantseva V, Grewal PK, Wandall HH, et al. Dual roles for hepatic lectin receptors in the clearance of chilled platelets. Nature medicine. 2009;15(11):1273–1280. doi: 10.1038/nm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]