Abstract
Background
Pancreatic cancer (PC) often produces pain that is difficult to control. Celiac neurolysis (CN) is performed with the goal of improving pain control and quality of life while reducing opioid-related side effects.
Objective
We aimed to evaluate whether CN provides a survival advantage for PC patients.
Design
Retrospective case-control study.
Setting
Single tertiary-care referral center.
Patients
Review of a prospectively maintained database identified patients with unresectable PC who underwent CN over a 12-year period. Each patient was matched to 2 control patients with unresectable PC.
Intervention
CN, which included both celiac plexus neurolysis (CPN) and celiac ganglia neurolysis (CGN).
Main Outcome Measurements
Median survival in Kaplan-Meier curves and hazard ratios.
Results
A total of 417 patients underwent CN and were compared with 840 controls with PC. Baseline characteristics were similar except the CN group had greater weight loss and pain requiring opioids. A mean of 16.6 ± 5.8 mL of alcohol was administered. For patients who underwent CN, the median survival from the time of presentation was shorter compared with controls (193 vs 246 days; hazard ratio 1.32; 95% confidence interval, 1.13–1.54). There was no difference in survival with unilateral or bilateral injection. However, EUS-guided CN was associated with longer survival compared with non-EUS approaches, and those who received CPN had longer survival compared with CGN.
Limitations
Single center, retrospective.
Conclusion
Our study suggests that CN is an independent predictor of shortened survival in PC patients. A prospective study is needed to verify the findings and determine whether shortened survival results from CN or from other features such as performance status and tumor-related characteristics. It is also imperative to verify our finding that EUS-guided CN provides a survival advantage over other approaches and whether CPN prolongs survival compared with CGN.
In the United States, pancreatic cancer (PC) is the fourth most common cause of cancer-related mortality.1 The overall 1- and 5-year survival rates of 26% and 6%, respectively, reflect the poor prognosis associated with PC.2 Despite advances in detection and therapies over the past 40 years, mortality rates have not improved.3 Because the majority of PC patients have advanced disease at diagnosis, treatment is limited and focuses primarily on pain management. The World Health Organization recommends a stepwise approach with initial administration of nonsteroidal agents and acetaminophen, then subsequent opioid use for refractory pain.4 Unfortunately, this approach is still associated with inadequate pain relief in 55% of patients.5 Furthermore, although opioids provide some analgesic benefit, they are associated with numerous side effects. Therefore, other modalities have been advocated to help manage cancer-related pain.
Celiac neurolysis (CN) has been used to treat pain related to PC since 1914.6 Although studies demonstrate improved pain relief, reduced need for opioid use, and fewer opioid-related side effects after CN,7–13 the impact on quality of life and patient survival is debated.14 Lillemoe et al15 discovered that treatment with CN resulted in significantly prolonged survival compared with those who received placebo (21 vs 6 months, respectively). In contrast, in a randomized, controlled trial by Wong et al,11 there was no survival advantage for patients who received CN versus a sham procedure. All such studies are limited by the number of patients included and lack of consideration of other confounding variables, potentially masking any difference in survival. Our aim was to overcome the methodological shortcomings by evaluating all patients who have undergone CN at our center and to compare outcomes with those of a large matched cohort to determine the effect of CN on survival of patients with unresectable PC.
METHODS
Patient selection
After approval from the Institutional Review Board, a prospectively maintained Life Sciences System database was reviewed to identify patients with primary PC who underwent CN from January 1, 2001 to March 31, 2013. This list was cross-referenced to prospectively maintained EUS, radiology, and surgical databases to ensure complete representation of the study cohort. All CN procedures were selected regardless of route, technique, or performing physician subspecialty. CN procedures included both celiac plexus neurolysis (CPN) and celiac ganglia neurolysis (CGN).
Patients were included in the study if they were 18 years of age and older with biopsy-proven PC or a classic radiographic and clinical course for PC who underwent CN in which alcohol-based formulations were used. Patients were excluded if they underwent pancreatic resection, participated in the previous randomized, controlled trial by Wong et al,11 were receiving hospice care, or if data were insufficient for analysis. For patients who underwent more than 1 CN procedure, only the index procedure was the included in the analysis. Each patient who underwent CN was matched to 2 patients with PC who did not undergo CN by using the pancreatic cancer Specialized Program of Research Excellence registry. Patients were frequency matched by age, sex, and year and stage grouping at time of presentation to our institution. Stage grouping was determined by the American Joint Committee on Cancer, 7th Edition TNM classification of each patient, as shown in Appendix 1.16
Clinical and survival data
Each patient’s medical chart was reviewed to abstract data concerning their clinical presentation, presence of pain, use of analgesics, radiologic and endoscopic findings, stage grouping at diagnosis and at presentation to our institution, details regarding the CN technique, and all PC-targeted therapies. Appendix 2 details the techniques for EUS and percutaneous CN. The time of symptom onset was approximated. Precise dates for PC diagnosis, presentation to the Mayo Clinic, and mortality were recorded.
Statistical analysis
Continuous variables are reported either as a mean ± standard deviation (SD) or median (interquartile range). Means were reported unless the data were nonparametric. Categorical variables were summarized by using frequency (%). The Student t test or Wilcoxon rank sum test was used to analyze continuous variables, and a Pearson χ2 analysis was used for categorical variables. Survival data are based on the median number of days from the date of initial presentation to the Mayo Clinic for PC until the time of death. The date of presentation to our institution was chosen as the start date for the survival analysis because this is not only a reliable time point, but also best approximated the timing of CN and allowed standardization for both the CN and control groups. Furthermore, because many patients received their diagnosis before their presentation to the Mayo Clinic, by using the date of their diagnosis as the start point would increase the risk of an immortal time bias. Survival from the date of diagnosis was used as the start date in a secondary analysis. Differences in median survival were analyzed by using Kaplan-Meier curves and compared by a log-rank test for statistical significance. Univariate and multivariate analyses were performed by using a Cox proportional hazards regression model and presented as a hazard ratio (95% confidence interval [CI]).
RESULTS
Baseline characteristics
Among the 510 patients who underwent CN, 417 patients met study inclusion criteria. Reasons for exclusion are listed in Appendix 3. Patients were matched by using the Specialized Program of Research Excellence database to 840 control patients based on their age, sex, and year and stage grouping at the time of presentation to the Mayo Clinic. The mean age for the entire cohort was 65 ± 10 years, and 57% were male. For the collective 1257 patients, the stage grouping at presentation was IV in 51%, III in 39%, and I or II in 10%. Appendix 4 highlights how the diagnosis of PC was made in the patient cohort.
There were no differences in the length of time between the onset of clinical symptoms and presentation to the Mayo Clinic between the CN and control groups (115 ± 153 days vs 121 ± 158 days; P = .51). However, patients in the CN group had a shorter time interval between diagnosis and presentation to our facility (4 ± 44 days vs 28 ± 90 days, P< .001). Table 1 displays the baseline characteristics of the CN and control groups. As expected, the CN group had a greater proportion of patients experiencing pain (99% vs 80%, P<.001) and taking opioid medications (89% vs 36%, P< .001) at baseline. In addition, patients in the CN group had greater weight loss before presentation (median 20 lb vs 15 lb, P < .001) and were more commonly associated with TNM T4 stage. They also appeared to have more extensive pancreatic tumor involvement based on the percentage of patients whose tumors bridged 2 anatomic segments of the gland, but were not more likely to have lymph node involvement. Compared with those in the control group, patients in the CN group were less likely to undergo chemotherapy or radiotherapy.
TABLE 1.
Baseline characteristics of the CN and control groups
| Baseline characteristics | CN (n = 417) | Controls (n = 840) | P value |
|---|---|---|---|
| Patient characteristics at presentation to the Mayo Clinic | |||
|
| |||
| Age, y, mean (SD) | 65 (11) | 65 (10) | .91 |
|
| |||
| Male, % | 56.6 | 56.8 | .95 |
|
| |||
| White, % | 81.1 | 79.9 | .33 |
|
| |||
| BMI, kg/m2, mean (SD) | 26.3 (5.4) | 26.8 (6.3) | .53 |
|
| |||
| Weight loss, lb, median (IQR) | 20 (10–30) | 15 (5–26) | <.001 |
|
| |||
| Presence of pain, % | 99 | 80 | <.001 |
|
| |||
| Opioid use, % | 89 | 36 | <.001 |
|
| |||
| Cancer characteristics at presentation to the Mayo Clinic | |||
|
| |||
| Stage grouping, % | .80 | ||
|
| |||
| I | 0 | 0 | |
|
| |||
| II | 9 | 10 | |
|
| |||
| III | 38 | 39 | |
|
| |||
| IV | 53 | 51 | |
|
| |||
| TNM T stage, % | .02 | ||
|
| |||
| 0* | 3 | 1 | |
|
| |||
| 1 | 2 | 3 | |
|
| |||
| 2 | 6 | 8 | |
|
| |||
| 3 | 30 | 35 | |
|
| |||
| 4 | 59 | 53 | |
|
| |||
| Site of involvement, % | .005 | ||
|
| |||
| Head/uncinate | 43 | 49 | |
|
| |||
| Neck | 4 | 5 | |
|
| |||
| Body | 20 | 16 | |
|
| |||
| Tail | 8 | 11 | |
|
| |||
| Bridge 2 sites† | 25 | 19 | |
|
| |||
| Lymph node status, % | .18 | ||
|
| |||
| Negative | 62 | 66 | |
|
| |||
| Positive | 38 | 34 | |
|
| |||
| Treatment characteristics | |||
|
| |||
| Chemotherapy, % | <.001 | ||
|
| |||
| Yes | 49 | 49 | |
|
| |||
| No | 20 | 7 | |
|
| |||
| Unsure | 31 | 44 | |
|
| |||
| Radiation therapy, % | <.001 | ||
|
| |||
| Yes | 22 | 20 | |
|
| |||
| No | 70 | 63 | |
|
| |||
| Unsure | 8 | 17 | |
|
| |||
| Surgery, %‡ | .08 | ||
|
| |||
| Yes | 10 | 15 | |
|
| |||
| No | 90 | 85 | |
CN, Celiac neurolysis; SD, standard deviation; BMI, body mass index; IQR, interquartile range.
Primary tumor was not detected by CT, but diagnosed by other imaging modality.
Tumors that were reported to bridge 2 adjacent regions of the pancreas (eg, body and tail).
Surgical procedure other than resection.
CN group
CN was performed by endosonographers in the majority of patients (56%), followed by anesthesiologists (42%), surgeons, and radiologists (1% each), predominantly (96%) in an outpatient setting. CPN was performed in 82% of cases, most often (79%) by using a bilateral approach. CGN was performed under EUS guidance in the remaining 18%. The most common anesthetic used was bupivacaine at concentrations of 0.25% or 0.50% (64% or 28% of patients, respectively). A mean of 12.6 ± 7.4 mL of anesthetic was administered. The most common neurolytic agent was 98% ethyl alcohol (67%) followed by 100% ethyl alcohol (30%). A mean of 16.6 ± 5.8 mL of neurolytic was administered.
By using the definitions of adverse events adopted by the American Society for Gastrointestinal Endoscopy, mild adverse events requiring overnight observation for pain exacerbation, nausea or vomiting, or orthostasis occurred in 7 patients.17 Moderate to severe adverse events occurred in 5 patients, requiring hospitalizations for 1 to 13 days for pain exacerbation (n = 2), duodenal perforation (n = 1), alcohol tracking to the lumbar roots causing persistent T12/L1 numbness (n = 1), and anterior spinal cord infarction with permanent paralysis (n = 1). After CN performed by an anesthesiologist, 1 patient had persistent numbness but no motor deficits in her thigh in a T12/L1 distribution, which improved slightly after 1 month. The patient with an endoscopic duodenal perforation required surgical repair with omental patch duodenotomy closure. The patient with permanent paralysis underwent EUS-guided CGN and was previously presented as a case report.18
Survival analysis
Survival from the date of presentation
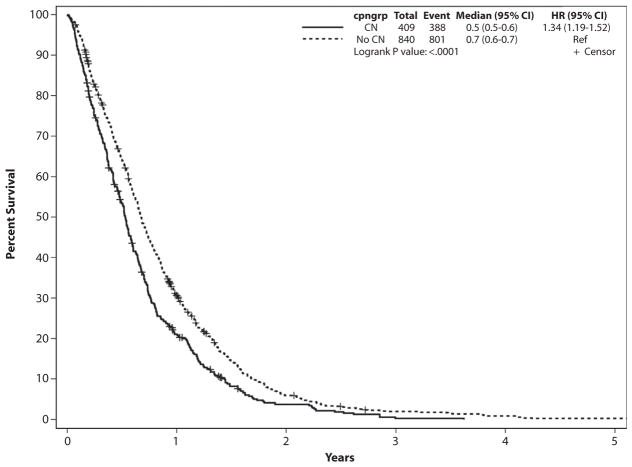
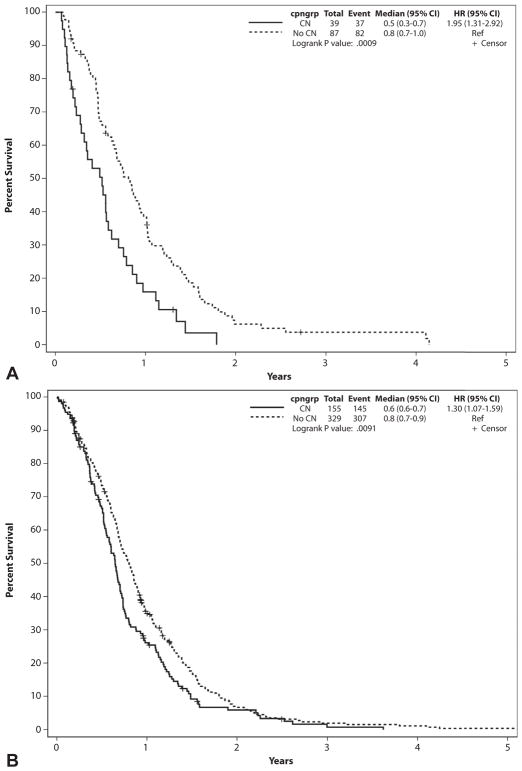
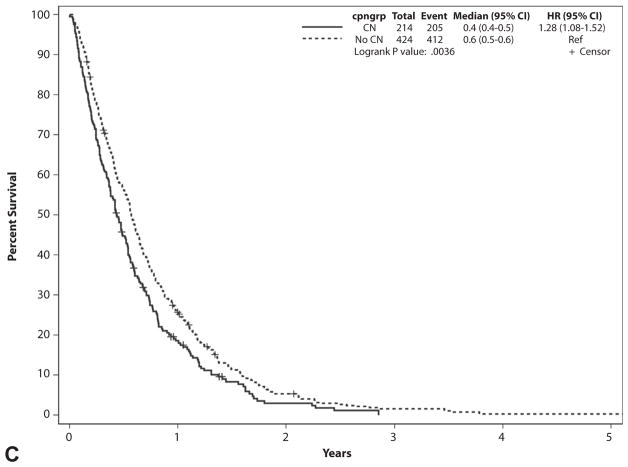
The median survival from the date of presentation to the Mayo Clinic was significantly lower for patients who underwent CN compared with controls (193 vs 246 days, P < .001) (Fig. 1). The survival difference was consistent in all stage grouping categories (Table 2, Figs. 2A–C).
Figure 1.
Survival of celiac neurolysis group and controls from date of presentation to the Mayo Clinic. CI, confidence interval; CN, celiac neurolysis; cpngroup, whether CN was performed; HR, hazard ratio.
TABLE 2.
Survival (days) from presentation based on stage grouping
| N | No. of events | Censors | Median survival | Survival difference | P value | |
|---|---|---|---|---|---|---|
| Stages I and II | ||||||
|
| ||||||
| CN group | 39 | 37 | 2 | 188 | 3.6 mo | <.001 |
|
| ||||||
| Control group | 87 | 82 | 5 | 296 | ||
|
| ||||||
| Stage III | ||||||
|
| ||||||
| CN group | 155 | 144 | 11 | 236 | 1.9 mo | .012 |
|
| ||||||
| Control group | 329 | 307 | 25 | 292 | ||
|
| ||||||
| Stage IV | ||||||
|
| ||||||
| CN group | 214 | 205 | 9 | 158 | 1.6 mo | .003 |
|
| ||||||
| Control group | 424 | 411 | 13 | 207 | ||
CN, Celiac neurolysis.
Figure 2.
Survival from date of presentation to the Mayo Clinic based on stage grouping. A, Stage grouping I and II patients. B, Stage grouping III patients. C, Stage grouping IV patients. CI, confidence interval; CN, celiac neurolysis; cpngroup, whether CN was performed; HR, hazard ratio.
The unadjusted hazard ratio of survival from the date of presentation to the Mayo Clinic in the CN group was 1.32 (95% CI, 1.15–1.52) compared with controls. On multivariate analysis with adjustment for age, sex, white race, body mass index at presentation, weight loss before presentation, stage grouping, TNM T stage, site of pancreas involvement, lymph node status, and whether the patient was treated with chemotherapy, radiation, and/or a surgical procedure other than resection, the HR remained approximately the same at 1.35 (95% CI, 1.14–1.60).
Univariate analyses of risk factors for shortened survival from date of presentation
For the composite group of 1257 patients, factors that correlated with a shortened survival included older age at presentation, presence of pain, opioid use, stage grouping IV, tumors located within the pancreatic tail, positive lymph node status, and not undergoing chemotherapy or radiotherapy (Table 3).
TABLE 3.
Survival from date of presentation to the Mayo Clinic (all patients)
| N | No. of events | No. of censors | Median survival, days | P value | |
|---|---|---|---|---|---|
| Patient characteristics at presentation to the Mayo Clinic | |||||
|
| |||||
| Age, y (quartiles) | .004 | ||||
|
| |||||
| ≤58 | 364 | 343 | 21 | 252 | |
|
| |||||
| 59–65 | 282 | 272 | 10 | 242 | |
|
| |||||
| 66–72 | 307 | 290 | 17 | 221 | |
|
| |||||
| ≥73 | 296 | 282 | 14 | 175 | |
|
| |||||
| Sex | .57 | ||||
|
| |||||
| Male | 707 | 664 | 43 | 234 | |
|
| |||||
| Female | 542 | 523 | 19 | 219 | |
|
| |||||
| Race | .26 | ||||
|
| |||||
| White | 1004 | 950 | 54 | 234 | |
|
| |||||
| Other | 45 | 43 | 2 | 194 | |
|
| |||||
| BMI, kg/m2 (quartiles) | .26 | ||||
|
| |||||
| ≤22.7 | 303 | 287 | 16 | 236 | |
|
| |||||
| 22.8–25.6 | 302 | 287 | 15 | 218 | |
|
| |||||
| 25.7–28.9 | 303 | 284 | 19 | 238 | |
|
| |||||
| ≥29 | 302 | 290 | 12 | 229 | |
|
| |||||
| Weight loss, lb (quartiles) | .46 | ||||
|
| |||||
| ≤7 | 280 | 267 | 13 | 229 | |
|
| |||||
| 7–15 | 279 | 262 | 17 | 243 | |
|
| |||||
| 16–30 | 329 | 319 | 10 | 211 | |
|
| |||||
| >30 | 208 | 194 | 14 | 203 | |
|
| |||||
| Presence of pain | .04 | ||||
|
| |||||
| Yes | 1075 | 1028 | 47 | 223 | |
|
| |||||
| No | 172 | 157 | 15 | 255 | |
|
| |||||
| Opioid use | <.001 | ||||
|
| |||||
| Yes | 637 | 617 | 20 | 202 | |
|
| |||||
| No | 570 | 529 | 41 | 261 | |
|
| |||||
| Cancer characteristics at presentation to the Mayo Clinic | |||||
|
| |||||
| Stage grouping | <.001 | ||||
|
| |||||
| I | 5 | 5 | 0 | 172 | |
|
| |||||
| II | 121 | 114 | 7 | 247 | |
|
| |||||
| III | 484 | 451 | 33 | 263 | |
|
| |||||
| IV | 638 | 616 | 22 | 195 | |
|
| |||||
| TNM T stage | .003 | ||||
|
| |||||
| 0* | 17 | 17 | 0 | 289 | |
|
| |||||
| 1 | 30 | 29 | 1 | 219 | |
|
| |||||
| 2 | 88 | 88 | 0 | 190 | |
|
| |||||
| 3 | 373 | 361 | 12 | 204 | |
|
| |||||
| 4 | 625 | 580 | 45 | 253 | |
|
| |||||
| Site of cancer | .006 | ||||
|
| |||||
| Head/uncinate | 579 | 553 | 26 | 234 | |
|
| |||||
| Neck | 52 | 49 | 3 | 237 | |
|
| |||||
| Body | 213 | 198 | 15 | 253 | |
|
| |||||
| Tail | 124 | 122 | 2 | 178 | |
|
| |||||
| Bridge 2 sites† | 256 | 241 | 15 | 226 | |
|
| |||||
| Lymph node status | <.001 | ||||
|
| |||||
| Negative | 739 | 697 | 42 | 252 | |
|
| |||||
| Positive | 402 | 386 | 16 | 204 | |
|
| |||||
| Treatment characteristics | |||||
|
| |||||
| Chemotherapy | <.001 | ||||
|
| |||||
| Yes | 582 | 553 | 29 | 281 | |
|
| |||||
| No | 133 | 131 | 2 | 71 | |
|
| |||||
| Unsure | 471 | 443 | 28 | 211 | |
|
| |||||
| Radiation therapy | <.001 | ||||
|
| |||||
| Yes | 243 | 232 | 11 | 339 | |
|
| |||||
| No | 775 | 738 | 37 | 197 | |
|
| |||||
| Unsure | 170 | 159 | 11 | 242 | |
BMI, Body mass index.
Primary tumor was not detected by CT, but diagnosed by other imaging modality.
Tumors that were reported to bridge 2 adjacent regions of the pancreas (eg, body and tail).
Among the CN group alone, there was no survival difference based on the volume of neurolytic administered or technique of bilateral versus unilateral injection (Table 4). However, patients who underwent EUS CN had longer survival times compared with those who underwent neurolysis by using non-EUS approaches. In addition, patients who underwent CPN had longer survival duration than those who underwent CGN. Notably, patients who experienced more weight loss after CN survived longer than those who experienced little or no subsequent weight loss.
TABLE 4.
Survival from date of presentation (CN patients only)
| N | No. of events | No. of censors | Median survival, days | P value | |
|---|---|---|---|---|---|
| Approach | <.001 | ||||
|
| |||||
| EUS | 230 | 208 | 22 | 206 | |
|
| |||||
| Anesthesia | 170 | 170 | 0 | 177 | |
|
| |||||
| Radiology | 4 | 4 | 0 | 60 | |
|
| |||||
| Surgery | 5 | 5 | 0 | 153 | |
|
| |||||
| Type | .03 | ||||
|
| |||||
| CPN | 330 | 313 | 17 | 200 | |
|
| |||||
| CGN | 73 | 68 | 5 | 154 | |
|
| |||||
| Location | .72 | ||||
|
| |||||
| Unilateral | 56 | 55 | 1 | 187 | |
|
| |||||
| Bilateral | 208 | 208 | 0 | 190 | |
|
| |||||
| Neurolytic volume, mL | .32 | ||||
|
| |||||
| <10 | 47 | 46 | 1 | 193 | |
|
| |||||
| 10–19 | 84 | 83 | 1 | 171 | |
|
| |||||
| 20 | 233 | 214 | 19 | 197 | |
|
| |||||
| >20 | 7 | 7 | 0 | 159 | |
| Weight loss after CN, lb (quartiles) | .002 | ||||
|
| |||||
| ≥12 | 39 | 38 | 1 | 341 | |
|
| |||||
| 5.7–12 | 38 | 36 | 2 | 252 | |
|
| |||||
| 1.5–5.6 | 39 | 35 | 4 | 255 | |
|
| |||||
| ≤1.5 | 35 | 32 | 3 | 194 | |
CN, Celiac neurolysis; CPN, celiac plexus neurolysis; CGN, celiac ganglia neurolysis.
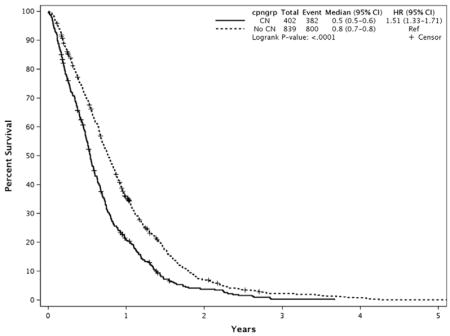
Survival from time of diagnosis
Secondary analysis looking at survival from the time of diagnosis revealed a similar decrease in survival in patients who underwent CN compared with those who did not receive CN (196 vs 281 days, P < .001) (Appendix 5). The same patient and CN characteristics that were statistically significant on univariate analyses performed by using the date of presentation to the Mayo Clinic as the start date for survival remained significant when the date of diagnosis was used, except that the presence of pain at presentation only trended toward significance (245 vs 282 days in patients with and without pain, respectively, P = .08).
DISCUSSION
The sensation of pain arising from the pancreas and most intra-abdominal organs (excluding the left side of the colon, rectum, and pelvic organs) is transmitted via the celiac plexus.19 Although the pain associated with PC is multifactorial, neural tumor involvement is considered the key contributor. Perineural invasion of intra- and ex-trapancreatic nerves is seen in 90% to 100% and 52% to 80%, respectively, with remote microscopic perineural migration to the celiac ganglia also reported.20–22 Surgical and autopsy data reveal that patients with intra- and/or extrapancreatic neural involvement demonstrate a worse prognosis, shortened survival, and increased risk of tumor recurrence compared with those without neural invasion.20,21
Contrary to the theory that CN prolongs survival, in our study, patients with unresectable PC who underwent CN had a shorter survival compared with control patients who did not undergo CN. The shortened survival persisted on multivariate analysis. This shorter survival could not be attributed to the length of time between the onset of clinical symptoms and presentation to our facility.
It is unclear whether the shortened survival was a consequence of the CN itself, or whether the use of CN reflects a patient cohort that possessed other clinical and/or tumor related characteristics that conferred worse prognosis. For instance, although patients were stage matched, the shorter survival among the CN group may be related to a lower performance status as indicated by the greater initial weight loss, presence of pain, opioid use, and less-common use of chemotherapy and/or radiotherapy. Patients who underwent CN also probably had larger and more locally advanced tumors because they were more likely to have T4 tumors and tumors involving more than 1 anatomic segment of the pancreas. However, patients with CN were not more likely to have a positive lymph node status. In addition, physicians seeing patients with PC may have been biased toward offering CN to those who appeared sicker and were experiencing more discomfort. This may have been particularly true in patients with stage grouping I and II cancers who might have been unfit for surgery due to their poor performance status, comorbidities, and debilitation, leading to a lower than expected median survival of patients in this stage grouping.
Given that CN is typically reserved for patients with moderate to severe pain, our findings may also reflect a disparate disease course among patients with no to mild pain compared with patients with moderate to severe pain. Significant pain and opioid use have been previously shown to be independent risk factors for poorer outcomes. In patients with pancreatic cancer, the presence of abdominal or back pain may reflect intra- and/or extrapancreatic neural involvement and has been shown to be a predictor of unresectability and lower survival.23,24 Furthermore, pain is closely related to mood, functional ability, and stress, all factors that can affect quality of life and survival. Although controversial, opioid medications may indirectly and directly affect tumor growth and recurrence.25,26
In our study, patient and cancer characteristics that were associated with shortened survival included advanced age, presence of pain and opioid use at presentation, T4 status, tumors within the pancreatic tail, positive lymph node involvement, and chemotherapy or radiotherapy. Although other studies have also identified each of these factors as predictors of shortened survival,27–29 this is the first study to demonstrate that CN is an independent predictor of shortened survival.
Factors related to the CN procedures that were associated with prolonged survival included EUS-guided CN versus non-EUS techniques, CPN versus CGN, and greater postprocedure weight loss. No prospective studies have been conducted directly comparing EUS-guided and percutaneous neurolysis. The improved survival with EUS-guided CN may be partly attributed to the more precise targeting of the injectate. The practice of often performing CN at the time of EUS-guided FNA diagnosis may have resulted in a lead time bias. However, because the time between the onset of clinical symptoms and presentation to our institution did not differ between the 2 groups, it is not believed that a significant lead time bias would account for this difference in survival. In addition, patients with larger tumors, those with metastases amenable to percutaneous biopsy, or those with comorbidities prohibiting monitored anesthesia care may have been less likely to undergo EUS versus percutaneous biopsy and neurolysis. Our data indicate a need to prospectively compare EUS with percutaneous approaches. One study that compared CGN with CPN found that patients who underwent CGN had greater pain relief.30 However, our finding that patients undergoing CGN had shortened survival raises concern regarding this approach and indicates a need for additional study. Because greater weight loss is typically associated with worse nutritional status and survival in patients with cancer, the finding that patients with increased weight loss after CN had longer survival was unexpected and not readily explicable.31–35 Data are conflicting as to whether unilateral or bilateral injection more effectively relieves pain, but in our study, neither technique resulted in a survival advantage.36–39 The ideal volume of neurolytic that should be injected during CN has not been established, with most studies using a total of 10 to 20 mL. Similarly, our data did not identify a dose of injectate that affected survival.
This study has several limitations. Despite the fact that the shortened survival among CN patients persisted on multivariate analysis after controlling most known key variables, the retrospective nature did not allow us to evaluate other potential factors such as the performance status. Potential surrogate markers of performance status including greater initial weight loss, presence of pain, opioid use, and less common use of chemotherapy and/or radiotherapy may suggest a difference in clinical status between those managed with and without CN. Furthermore, due to the nature of a tertiary referral hospital and retrospective study, the medical records could not adequately address this particular variable for many patients. The impact of this variable was addressed by performing the multivariate analysis. Also, our study cannot address whether pain severity and duration, analgesic dose, or the success of CN in terms of pain relief, analgesic use, and other endpoints may have affected survival.
In summary, this is the first study to demonstrate that CN is an independent predictor of shortened survival in patients with PC. It is unclear whether the shortened survival was a direct or indirect consequence of the CN itself or whether the patients who underwent CN possessed other unidentified clinical and/or tumor-related characteristics that conferred the worse prognosis. Although the shortened survival among CN patients persisted on multivariate analysis after controlling most key patient and tumor characteristics, the impact of variables such as performance status and the success of CN in managing pain and analgesic dosing remains uncertain. Overall, the use of CN in patients with pancreatic cancer–related pain should be carefully considered until prospective, randomized data are available to clarify whether (1) the shortened survival results from CN or from other features such as performance status and tumor-related characteristics, (2) EUS-guided CN provides a survival advantage over other approaches, and (3) CPN prolongs survival compared with CGN. These data are needed to develop more effective approaches to pain management and improve outcomes in patients with PC.
Abbreviations
- CI
confidence interval
- CGN
celiac ganglia neurolysis
- CN
celiac neurolysis
- CPN
celiac plexus neurolysis
- PC
pancreatic cancer
- SD
standard deviation
APPENDIX 1. AMERICAN JOINT COMMITTEE ON CANCER, 7TH EDITION PANCREATIC CANCER STAGING
| Primary tumor (T)* | |||
| TX: Primary tumor cannot be assessed | |||
| T0: No evidence of primary tumor | |||
| Tis: Carcinoma in situ or PanInIII | |||
| T1: Tumor limited to the pancreas and ≤2 cm in greatest diameter | |||
| T2: Tumor limited to the pancreas and >2 cm in greatest diameter | |||
| T3: Tumor extends beyond the pancreas but does not involve the celiac axis or SMA | |||
| T4: Tumor involves the celiac axis or SMA | |||
| Regional lymph nodes (N) | |||
| NX: Regional lymph nodes cannot be assessed | |||
| N0: No regional lymph node involvement | |||
| N1: Regional lymph node metastases | |||
| Distant metastases (M) | |||
| M0: No distant metastases | |||
| M1: Distant metastases | |||
|
| |||
| Stage grouping based on TNM classification | |||
| 0 | T0, Tis | N0 | N0 |
|
| |||
| I | T1 or T2 | N0 | M0 |
|
| |||
| II | T3 | N0 | M0 |
|
| |||
| T1–T3 | N1 | M0 | |
|
| |||
| III | T4 | Any N | M0 |
|
| |||
| IV | Any T | Any N | M1 |
Primary tumor was assessed by contrast-enhanced CT scan.
APPENDIX 2. CELIAC NEUROLYSIS TECHNIQUE
Some aspects of the procedures were uniform regardless of the approach, including need for informed consent, routine administration of intravenous fluids to decrease the risk of post–celiac neurolysis (CN) hypotension, and 1- to 2-hour postprocedure observation to assess for adverse events. The following is a brief discussion regarding the general techniques adopted at our institution, realizing that minor technical variance exists among physicians and for individual patients.
Endoscopic ultrasound
EUS-guided CN was performed by using a curvilinear echoendoscope (GF-UC30P, GF-UC140P-AL5, GF-UCT180, or GF-UC160P-AT8; Olympus America, Center Valley, Pa) and a 22-gauge FNA needle (EUSN-3; Cook Medical Inc, Winston-Salem, NC). Doppler imaging was used to avoid intervening vessels along the needle path. Either celiac ganglia neurolysis (CGN) or celiac plexus neurolysis (CPN) was performed. If CGN was performed, then treatment was delivered to as many ganglia as could be accessed, injecting approximately 1 to 3 mL of injectate into each ganglion, while the remaining was injected unilaterally or bilaterally in the region of the celiac artery takeoff. If only CPN was performed, the same technique was used to inject the entire solution into the celiac plexus area by using either a bilateral or unilateral approach.
Percutaneous neurolysis
Percutaneous neurolysis was performed by radiologists or anesthesiologists under the guidance of fluoroscopy. Patients were placed in the supine position and by using fluoroscopy, the L1 vertebral body was identified. An entry point approximately 7 cm lateral to the L1 caudal border and below the 12th rib was marked, and the area was sterilely prepped and draped. After lidocaine was injected into each entry point, a 22-gauge spinal needle was advanced under fluoroscopic view to the midbody of L1. Typically after aspirating to ensure no return of blood, Omnipaque 180 contrast was injected to confirm linear spread along the anterior vertebral body with no intravascular, intrathecal, intracrural, intramuscular, or intradiscal spread. Furthermore, the percutaneous technique uses digital subtraction angiography (DSA) to detect any flow into the anterior spinal artery. After confirmation of no spread outside of the targeted area, bupivacaine was injected. After waiting 15 minutes and after a sensorimotor examination to ensure no neurological complications, sterile dehydrated alcohol was injected. This was usually performed bilaterally unless prohibited by patient anatomy or discomfort.
APPENDIX 3. REASONS TO EXCLUDE CELIAC NEUROLYSIS PATIENTS FROM ANALYSIS
| Reason for exclusion | No. |
|---|---|
| Repeat celiac neurolysis | 33 |
|
| |
| Nonadenocarcinoma tumor type | 27 |
|
| |
| Surgically resected tumor | 26 |
|
| |
| Receiving hospice care | 7 |
APPENDIX 4. DIAGNOSTIC TECHNIQUES TO DIAGNOSE PC
| Diagnostic modality | % |
|---|---|
| EUS-guided FNA | 52 |
|
| |
| Pancreatic mass | 47 |
|
| |
| Metastatic site (eg, liver, lymph node) | 5 |
|
| |
| Percutaneous biopsy | 34 |
|
| |
| Pancreatic mass | 14 |
|
| |
| Metastatic site (eg, liver) | 20 |
|
| |
| Surgical biopsy | 7 |
|
| |
| Brushing/biopsy during ERCP | 4 |
|
| |
| Radiographic and/or EUS imaging and clinical course consistent with pancreatic cancer | 3 |
APPENDIX 5. SURVIVAL OF CELIAC NEUROLYSIS GROUP AND CONTROLS FROM DATE OF DIAGNOSIS
Footnotes
DISCLOSURE: All authors disclosed no financial relationships relevant to this article. Supported in part by the Mayo Clinic Specialized Program of Research Excellence (SPORE) in Pancreatic Cancer (NCI grant P50 CA102701).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts and figures 2012. American Cancer Society; 2012. p. 19. [Google Scholar]
- 3.Ma J, Siegel R, Jemal A. Pancreatic cancer death rates by race among US men and women, 1970–2009. J Natl Cancer Inst. 2013;105:1694–700. doi: 10.1093/jnci/djt292. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Cancer pain relief. Geneva, Switzerland: WHO Office of Publications; 1986. [Google Scholar]
- 5.Azevedo São Leão Ferreira K, Kimura M, Jacobsen Teixeira M. The WHO analgesic ladder for cancer pain control, twenty years of use. How much pain relief does one get from using it? Support Care Cancer. 2006;14:1086–93. doi: 10.1007/s00520-006-0086-x. [DOI] [PubMed] [Google Scholar]
- 6.Kappis M. Erfahrungen mit local anasthesie bie bauchoperationen. Vehr Dtsch Gesellsch Chir. 1914;43:87–9. [Google Scholar]
- 7.Mercadante S. Celiac plexus block versus analgesics in pancreatic cancer pain. Pain. 1993;52:187–92. doi: 10.1016/0304-3959(93)90130-H. [DOI] [PubMed] [Google Scholar]
- 8.Kawamata M, Ishitani K, Ishikawa K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer pain. Pain. 1996;64:597–602. doi: 10.1016/0304-3959(95)00189-1. [DOI] [PubMed] [Google Scholar]
- 9.Gunaratnam NT, Sarma AV, Norton ID, et al. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–24. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 10.Staats PS, Hekmat H, Sauter P, et al. The effects of alcohol celiac plexus block, pain, and mood on longevity in patients with unresectable pancreatic cancer: a double-blind, randomized, placebo-controlled study. Pain Med. 2001;2:28–34. doi: 10.1046/j.1526-4637.2001.002001028.x. [DOI] [PubMed] [Google Scholar]
- 11.Wong GY, Schroeder DR, Carns PE, et al. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092–9. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 12.Zhang CL, Zhang TJ, Guo YN, et al. Effect of neurolytic celiac plexus block guided by computerized tomography on pancreatic cancer pain. Dig Dis Sci. 2008;53:856–60. doi: 10.1007/s10620-007-9905-2. [DOI] [PubMed] [Google Scholar]
- 13.Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011:CD007519. doi: 10.1002/14651858.CD007519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in unresectable pancreatic cancer. Am J Gastroenterol. 2007;102:430–8. doi: 10.1111/j.1572-0241.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 15.Lillemoe KD, Cameron JL, Kaufman HS, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217:447–55. doi: 10.1097/00000658-199305010-00004. discussion 456–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge S, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 17.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Fujii L, Clain JE, Morris JM, et al. Anterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysis. Endoscopy. 2012;44(Suppl 2):E265–6. doi: 10.1055/s-0032-1309708. [DOI] [PubMed] [Google Scholar]
- 19.Gebhart GF. Visceral pain management. New York: Raven Press; 1993. [Google Scholar]
- 20.Nakao A, Harada A, Nonami T, et al. Clinical significance of carcinoma invasion of the extrapancreatic nerve plexus in pancreatic cancer. Pancreas. 1996;12:357–61. doi: 10.1097/00006676-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Ishikura H, Motohara T, et al. Perineural invasion by ductal adenocarcinoma of the pancreas. J Surg Oncol. 1997;65:164–70. doi: 10.1002/(sici)1096-9098(199707)65:3<164::aid-jso4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Levy MJ, Topazian M, Keeney G, et al. Preoperative diagnosis of extrapancreatic neural invasion in pancreatic cancer. Clin Gastroenterol Hepatol. 2006;4:1479–82. doi: 10.1016/j.cgh.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Kalser MH, Barkin J, MacIntyre JM. Pancreatic cancer. Assessment of prognosis by clinical presentation. Cancer. 1985;56:397–402. doi: 10.1002/1097-0142(19850715)56:2<397::aid-cncr2820560232>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 24.Ridder GJ, Klempnauer J. Back pain in patients with ductal pancreatic cancer. Its impact on resectability and prognosis after resection. Scand J Gastroenterol. 1995;30:1216–20. doi: 10.3109/00365529509101634. [DOI] [PubMed] [Google Scholar]
- 25.Afsharimani B, Cabot P, Parat MO. Morphine and tumor growth and metastasis. Cancer Metastasis Rev. 2011;30:225–38. doi: 10.1007/s10555-011-9285-0. [DOI] [PubMed] [Google Scholar]
- 26.Lennon FE, Moss J, Singleton PA. The mu-opioid receptor in cancer progression: is there a direct effect? Anesthesiology. 2012;116:940–5. doi: 10.1097/ALN.0b013e31824b9512. [DOI] [PubMed] [Google Scholar]
- 27.Tas F, Sen F, Keskin S, et al. Prognostic factors in metastatic pancreatic cancer: Older patients are associated with reduced overall survival. Mol Clin Oncol. 2013;1:788–92. doi: 10.3892/mco.2013.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganti AK, Potti A, Koch M, et al. Predictive value of clinical features at initial presentation in pancreatic adenocarcinoma: a series of 308 cases. Med Oncol. 2002;19:233–7. doi: 10.1385/MO:19:4:233. [DOI] [PubMed] [Google Scholar]
- 29.Rohan VS, Hsu JT, Liu KH, et al. Long-term results and prognostic factors in resected pancreatic body and tail adenocarcinomas. J Gastrointest Cancer. 2013;44:89–93. doi: 10.1007/s12029-012-9448-4. [DOI] [PubMed] [Google Scholar]
- 30.Doi S, Yasuda I, Kawakami H, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multi-center trial. Endoscopy. 2013;45:362–9. doi: 10.1055/s-0032-1326225. [DOI] [PubMed] [Google Scholar]
- 31.Choi Y, Kim TY, Lee KH, et al. The impact of body mass index dynamics on survival of patients with advanced pancreatic cancer receiving chemotherapy. J Pain Sympt Manage. 2014;48:13–25. doi: 10.1016/j.jpainsymman.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Topkan E, Parlak C, Selek U. Impact of weight change during the course of concurrent chemoradiation therapy on outcomes in stage IIIB non-small cell lung cancer patients: retrospective analysis of 425 patients. Int J Radiat Oncol Biol Phys. 2013;87:697–704. doi: 10.1016/j.ijrobp.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Gourin CG, Couch ME, Johnson JT. Effect of weight loss on short-term outcomes and costs of care after head and neck cancer surgery. Ann Otol Rhinol Laryngol. 2014;123:101–10. doi: 10.1177/0003489414523564. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto D, Chikamoto A, Ohmuraya M, et al. Impact of postoperative weight loss on survival after resection for pancreatic cancer. JPEN J Parenter Enteral Nutr. doi: 10.1177/0148607114520992. Epub 2014 Jan 31. [DOI] [PubMed] [Google Scholar]
- 35.Lu Z, Yang L, Yu J, et al. Change of body weight and macrophage inhibitory cytokine-1 during chemotherapy in advanced gastric cancer: what is their clinical significance? PloS One. 2014;9:e88553. doi: 10.1371/journal.pone.0088553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Puli SR, Batapati Krishna Reddy J, Bechtold ML, et al. Endoscopic ultrasound: its accuracy in evaluating mediastinal lymphadenopathy?. A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028–37. doi: 10.3748/wjg.14.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahai AV, Lemelin V, Lam E, et al. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326–9. doi: 10.1038/ajg.2008.64. [DOI] [PubMed] [Google Scholar]
- 38.LeBlanc JK, DeWitt J, Johnson C, et al. A prospective randomized trial of 1 versus 2 injections during EUS-guided celiac plexus block for chronic pancreatitis pain. Gastrointest Endosc. 2009;69:835–42. doi: 10.1016/j.gie.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 39.Bhatnagar S, Joshi S, Rana SP, et al. Bedside ultrasound-guided celiac plexus neurolysis in upper abdominal cancer patients: a randomized, prospective study for comparison of percutaneous bilateral paramedian vs. unilateral paramedian needle-insertion technique. Pain Practice. 2014;14:E63–8. doi: 10.1111/papr.12107. [DOI] [PubMed] [Google Scholar]