Abstract
Introduction
Chronic graft-versus-host disease (cGVHD) continues to be the leading cause of late morbidity and mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT), which is an increasingly applied curative method for both benign and malignant hematologic disorders. Biomarker identification is crucial for the development of noninvasive and cost-effective cGVHD diagnostic, prognostic, and predictive test for use in clinic. Furthermore, biomarkers may help to gain a better insight on ongoing pathophysiological processes. The recent widespread application of omics technologies including genomics, transcriptomics, proteomics and cytomics provided opportunities to discover novel biomarkers.
Areas covered
This review focuses on biomarkers identified through omics that play a critical role in target identification for drug development, and that were verified in at least two independent cohorts. It also summarizes the current status on omics tools used to identify these useful cGVHD targets. We briefly list the biomarkers identified and verified so far. We further address challenges associated to their exploitation and application in the management of cGVHD patients. Finally, insights on biomarkers that are drug targetable and represent potential therapeutic targets are discussed.
Expert commentary
We focus on biomarkers that play an essential role in target identification.
Keywords: Chronic graft-versus-host disease (cGVHD), allogeneic hematopoietic stem cell transplantation (allo-HSCT), biomarkers, omics, therapy
1. Introduction
Today, allogeneic hematopoietic stem cell transplantation (allo-HSCT) has become a widely used therapeutic approach for hematologic malignancies and other benign conditions. Since 2013, more than 8000 allogenic transplantations per year are performed in the United States [1]. Unfortunately, 30–70% of patients develop a major complication known as chronic graft-versus-host disease (cGVHD). Clinical manifestations of cGVHD resemble those of autoimmune diseases such as scleroderma. Clinical features involve fibrosis and inflammatory components that affect all target organs (skin, eyes, mouth, lungs, liver, genital, gastrointestinal), thus making the diagnosis complex [2].
cGVHD remains a leading cause of non-relapse mortality (NRM) with 25% of subsequent deaths in 2-year survivors and 11% of subsequent deaths in 5-year survivors. The clinical symptoms of cGVHD can sometimes be challenging to distinguish from other causes such as a drug reaction. Frequently, current diagnostic and staging methods fail to identify patients at high risk of treatment unresponsiveness, GVHD morbidity, or even death. Furthermore, there is a lack of early prognostic tools that could identify patients before the development of cGVHD. Therefore, the identification of biomarkers in patients, particularly sensitive and specific blood-based biomarkers, would be beneficial for the diagnosis, risk prediction, and response to therapy in patients post-HSCT [3–5].
Over the years, multiple biomarkers have thereby been utilized to aid in the diagnosis, risk prediction, prognosis, and response to therapy in cGVHD. The implementation of omics approaches capable of screening for circulating proteins and molecules has sparked a subsequent boost of interest in blood-based biomarkers for cGVHD subjects. Recently, enormous efforts have been made for the detection and validation of novel blood-derived biomarkers that are suitable for the initiation of noninvasive tests to detect cGVHD at an early stage or to monitor cGVHD biodynamics. The identification of specific and sensitive cGVHD biomarkers would facilitate with the diagnosis and the assessment of clinical cGVHD severity in patients, thus allowing for an optimal clinical management during the disease prognosis, especially in patients with an advanced condition.
In this current review, we will provide an update on cGVHD drug-targetable biomarkers identified through omics tools, including the discovery and validation of important biomarkers that could aid in the diagnosis, risk prediction, and therapeutic responses in clinic.
2. Definition of biomarkers
As highlighted in the National Institutes of Health (NIH) Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-host Disease Report, as well as mentioned by the North American and European Consortium, a biomarker is ‘a characteristic that is objectively measured as an indicator of normal biological or pathogenic processes, or biological and clinical responses to a therapeutic intervention.’ ‘Objectively’ is defined as ‘reliably and accurately.’ Commonly, ‘biomarker’ refers to a biochemical variable, such as a circulating protein or a biomolecule.
Biomarkers can be categorized into four distinct groups: diagnostic, prognostic, predictive, and response to treatment [6,7].
Diagnostic biomarkers are used to identify cGVHD patients, and more importantly aid to differentiate their symptoms from other conditions such as an infection or a drug reaction.
Prognostic biomarkers are used for the identification of patients with risk of cGVHD development before the onset, or at the initial stage of the disease.
Predictive biomarkers aid in determining patients that are most likely to benefit from a treatment based on their likelihood to respond to therapy.
Response to treatment biomarkers assay for the response to treatment after the initiation of therapy and could be used to monitor therapeutic response. This could aid with treatment management by detecting when a treatment has not resulted in a response [6–9].
Biomarkers may improve diagnosis and prognosis, monitoring of response to treatment, allowing for treatment that are customized to the patients, and overall leading to reduce healthcare costs [8]. The identification of a biomarker panel as well as cut points for cGVHD would render personalized medicine for adult and pediatric patients achievable. Furthermore, some of the biomarker data indicate that they can be used as targeted therapeutics [8].
3. Blood omics
‘Omics,’ the study of complete sets of molecules, including genomics, transcriptomics, proteomics, and cytomics, has been facilitated by advances in engineering which have allowed for increased data throughput. These approaches are now broadly used in biomedical research to facilitate the understanding of cGVHD mechanisms and identification of biomarkers and molecular targets for diagnostic and therapeutic development [3,4,10]. In cGVHD, genomics would illustrate the diverse landscape of genetic variations in cGVHD and provide a systematic, comprehensive description of the correlations between genome sequence alteration and major clinical phenotypes through large-scale genome-wide resequencing efforts. Proteomics refers to the PROTeins expressed by a genOME and is the large-scale study of proteins applied according to space (serum, plasma, blood) and time (diagnosis, treatment, prognosis), and can be used to decipher the mechanisms involved in cGVHD progression. Cytomics attempt to understand the molecular architecture and functionality of the cell system, which is achieved by using flow cytometry techniques that allow the various molecular phenotypes of a cell to be analyzed. Transcriptomics is the study of gene activity under cGVHD specific circumstances or in a specific cell, applying high-throughput methods, such as microarray analysis.
3.1. Selection of samples
As always, if an unbiased approach is used to identify a signature, a candidate transcript or a candidate protein, one potential limitation is the size and distribution of the discovery cohort. Indeed, cGVHD is a heterogeneous disease with different subtypes which differ in organ involvement, prognosis, and response to treatment, and the discovery cohort may be biased if not well distributed and sufficiently powered. As summarized by the 2014 NIH Consensus on Biomarkers as well as the North American and European consortium, cGVHD biomarker discovery can be affected by different confounding factors, thus creating a limitation in the interpretation of data [6,7]. Therefore, the confounding factors should be controlled as much as possible in any study. In the recipient, several factors including (1) age, (2) the diagnosis of a nonmalignant disorder with bone marrow failure or chromosomal instability, (3) alloimmunization of the recipient, (4) the presence of non-human leukocyte antigen (HLA) polymorphism, and (5) the preparative conditioning of the recipient prior to the transplant with a myeloablative regimen such as antihuman T lymphocyte immune globulin are all factors to be considered. The donor also has characteristics that are worthwhile to consider in cGVHD studies: (1) the donor type, whether related or unrelated; (2) HLA mismatched versus HLA matched; (3) the gender since female are associated with a higher incidence of cGVHD; (4) the type of graft (peripheral blood, bone marrow, umbilical cord blood); (5) the presence of non-HLA polymorphisms; (6) the treatment of the donor product with G-CSF, or other agent such as CXCR4 inhibitors (Plerixafor); and (7) graft manipulation such as T-and B-cell depletion or HLA-haploidentical graft with the administration of cyclophosphamide post-HSCT. Since cGVHD is a heterogenic disease that can affect all organs and tissues in the body, other factors can affect biomarkers. These factors include (1) the type of tissue involved; (2) the NIH grade score; (3) immune reconstitution post-HSCT, thus the importance of time- and age-matched controls; (4) concomitant acute GVHD (aGVHD), which may overlap with classic cGVHD; (5) history of aGVHD and prior treatment of it; (6) any current immunosuppressive treatment, particularly steroids which can affect biomarkers such as sBAFF; (7) the presence of any active infections that can change the cytokine milieu; and (8) the sample processing and storage, where cell populations such as the B cells can be lost post process with Ficoll.
3.2. Genomics
A gene or other fragment of DNA, whose location in the genome is known, is defined as a DNA marker. Gene signatures were previously reported in cGVHD prevention and management. Although HLA-matching is critical to detect cGVHD, recently functional non-HLA immune-associated gene biomarkers have also been exploited in attempts to reveal novel signatures and evaluate their potential as prognostic markers. These biomarkers would facilitate the selection of optimal donors, including the type of graft, the conditioning treatment, and the cGVHD prophylaxis. One study reported that mutations and polymorphisms within these non-HLA-encoded genes affect, for example, the amount of cytokines/chemokines produced in response to alloantigen in cGVHD [11].
Single nucleotide polymorphisms (SNPs) are the most common type of natural genetic mutation in populations. Donor selection according to SNP genotyping is still not performed clinically. However, recipient–donor non-HLA genetic polymorphisms have been focused as one area of investigation. Multiple studies investigated the role of non-HLA SNPs on outcomes post bone marrow transplantation. The goal of these studies was to provide guidance on the clinical management of patients who were at high risk, and ultimately serve as potential biological targets for novel therapeutics. Although, SNPs were reported to be associated with the risk of GVHD after allo-HSCT in large cohorts of donors and recipients, one group recently conducted the first adequately powered evaluation of previously identified SNP candidates and gene hypotheses using typed and imputed data from an existing genome-wide association study named DISCOVeRY-BMT (Determining the Influence of Susceptibility COnveying Variants Related to one-Year mortality after BMT) to replicate or validate the published associations with survival outcomes after transplant [12]. Finally, using publically available data, they found that candidate SNP associations with survival outcomes after unrelated donor transplant were most likely false-positive findings and over 85% of candidate SNPs were not linked to a biochemical function; of those that are linked, about half are not linked to the candidate gene [12].
3.3. Transcriptomics profiling
Transcriptomics technologies study an organism’s transcriptomes, the sum of all its RNA transcripts (mRNAs, lncRNAs, and small RNAs), and their quantity produced by the genome for a specific developmental stage or physiological condition [13].
MicroRNAs (miRNAs) are small noncoding RNA molecules containing 21–25 nucleotides that function through the interaction with target mRNAs and control gene activity at multiple levels, especially transcription, translation, and protein degradation [14,15]. Circulating miRNAs have recently been reported as potential biomarkers in various disorders including GVHD [16–19]. miRNAs have a high level of sensitivity and specificity allowing for early and reliable screening of pathophysiological state reflecting physiological and pathological changes.
A significant portion of the mammalian genome encodes numerous transcripts with little or no protein-coding capacity, termed long noncoding RNAs (lncRNAs), which initially emerged as regulators of differentiation, development, and disease. Now, it has become apparent that lncRNAs play a critical role in a wide variety of biological processes, such as expression profiles in specific cell types and localizations in specific subcellular compartments. The association between changes in expression of lncRNAs and cGVHD has not been studied and deserves exploiting.
Whole transcriptome analysis of T cells using a nonhuman primate GVHD model revealed the presence of active molecular pathways during GVHD. Among other pathways, the aurora kinase A (AURKA) pathway, which encodes for cell cycle progression regulatory proteins, cell growth, and differential and survival proteins, was identified as a novel druggable therapeutic target implicated in GVHD. This study provided a comprehensive elucidation of the T-cell transcriptome in nonhuman primate GVHD and revealed that AURKA should be considered as a target for preventing GVHD [20].
3.4. Proteomics
3.4.1. Antibody microarrays for discovery
Immunoassays, which focus on the antibody–antigen interaction, can be used for the identification of proteins. The antibody-based approaches have several advantages. Depending on the individual characteristic of the immobilized antibodies, antigens can be tested in picomolar concentrations. Furthermore, this approach (1) is quantitative; (2) allows the detection of low-abundance proteins such as cytokines; (3) is suited for the characterization of complex protein mixtures, such as human blood; and (4) is high throughput. Although this approach has demonstrated a major application in proteomics, the number of antibodies included in the array and the high cross-reactivity between antibodies and nontarget proteins are the major limitations [21].
Antibody microarrays have been used to screen for potential biomarkers in human blood serum because of their advantages with relatively high specificity and extreme sensitivity for low-abundance proteins such as cytokines [22]. For instance, high CXCL9 expression was previously shown to be elevated in newly cGVHD-diagnosed patients using protein microarray. CXCL9, chemokine (C-X-C motif) ligand 9, is an interferon-γ-inducible ligand for chemokine (C-X-C motif) receptor3 (CXC3) which is expressed on effector CD4+ Th1 cells and CD8+ cytotoxic T lymphocytes. This finding suggested the involvement of CXCL9 in the initiation steps of cGVHD [23].
3.4.2. Mass spectrometry for discovery
Mass spectrometry (MS) is a powerful approach used for the characterization and assessment of qualitative and quantitative changes in complex protein mixtures. Significant progress has been made in biomarker identification and acquisition using MS-based technologies within the last decade. MS can be used for both the discovery and validations of biomarkers. In clinical proteomics, two types of MS techniques have been used: 1) the pattern profiling and 2) detailed protein characterization [4,21].
In pattern profiling, polypeptide spectra obtained by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) or surface-enhanced laser desorption/ionization (SELDI-TOF) MS are compared to identify patients suffering from a particular disease without determining the individual components. The advantage with these methods is that they 1) are high throughput and 2) do not require in-depth analysis.
In MS approaches, proteins are generally digested, commonly using trypsin, followed by separation of the generated peptides by reversed-phase liquid chromatography, and MS analysis of the eluted proteins [24]. Several methods are used for the identification and characterization of proteins. In a typical proteomics discovery experiment, a non-targeted approach, shotgun proteomics, is used for the relative quantification of thousands of proteins within a small sample. As a result, hundreds of proteins that are differentially expressed between the healthy and diseases samples are compared and analyzed. Post discovery, the potential biomarkers are further filtered by performing studies on additional patients [25].
MS approaches can be gel based such as two-dimensional polyacrylamide gel electrophoresis and two-dimensional differential gel electrophoresis. The limitations with the gel-based approaches include (1) the lengthy analysis time, (2) a poor separation of the proteins with low molecular weight, or (3) isoelectric point. Gel-free methods such as the liquid chromatography and capillary electrophoresis provide a better separation compared to the gel-based methods. Additionally, they overcome the limitations associated with the gel-based separation methods [21]. MS is the last step in the analytical procedure and reliably allows for the identification of proteins and the determination of their isoforms, including their posttranslational modifications. With MS, ambiguous quantifications are eliminated, especially when tandem MS (MS/MS) is used. Recently, high-resolution instruments such as the linear ion trap Orbitrap, the quadrupole-TOF (Q-TOF), and the Triple-TOF analyzers are commonly used in biomarker discovery studies and ideal for top-down proteomics [26,27].
3.4.3. High-throughput immunoassays
The production of large amounts of results in a short amount of time is of importance in all immunoassays. Therefore, the ideal immunoassay should have two qualities: (1) high throughput and (2) multiplexed. High throughput refers to immunoassays that can assess multiple quantities of a sample at the same time, while multiplexing allows for the analysis of a large quantity of different proteins at the same time.
The enzyme-linked immunosorbent assays (ELISAs), an immunoassay that utilizes two antibodies specific for a candidate protein, remain the most applicable immunoassay for the quantitation of individual proteins for validation. Although ELISAs remain simple and highly reproducible with little inter-and intra-assay variability, it still has several limitations. The main disadvantage of ELISAs is their requirement for a large volume of plasma. Furthermore, ELISAs require antibodies against each individual protein of interest, which might be unavailable. ELISAs can also exhibit cross-reactivity, be expensive, time consuming, with limited multiplexing abilities [25]. Although ELISAs are just one of the techniques used in validation, ELISAs are preferred because they are high throughput and the most validated techniques. Recently, MS methods, the multiple reaction monitoring, or the selected ion monitoring have emerged as potential methods of choice for clinical diagnosis [4,25].
3.5. Cytomics
Cytomics is an astounding analysis tool that has received great attention over the years as it allows for the quantitative and qualitative analysis of pathophysiological responses and status in single cells real time. This approach incorporates biological imaging, bioengineering resources for cellular implements, and cell analysis tools including computational resources for flow cytometry.
Flow cytometry is a common method where a panel of multiple antibodies is used to stain cells. Each antibody is labeled with a different fluorescent dye. The stained cells are then introduced into the instrument and the individual cells are recorded according to their laser excitation region and their emission wavelength. Flow cytometry can detect 8–14 parameters. Recently, mass cytometry by time-of-flight (CYTOF), a novel flow-cytometry next-generation technique, was suggested. This approach is used to assess the two-dimensional phenotype and the functional analysis of single cells. In CYTOF, metal-conjugated antibodies are used to probe multiple internal and external cellular marker cells such as immune cells, cytokines, transcription factors, death, and proliferation. These are then simultaneously quantified at the single-cell level using CYTOF. Commonly used analysis tools for CYTOF are spanning-tree progression analysis of density normalized events, t-distributed stochastic neighbor embedding-based visualization, and cluster identification, characterization and regression [28]. Contrary to flow cytometry, CYTOF allows for the detection of 42 or more parameters. Other advantages of CYTOF are (1) minimal background noise from signal overlap or endogenous cellular components; (2) low-cost per probe, per test, and per cell analysis; and 3) the simultaneous measurement of multiple cellular parameters within a cell. The limitations with CYTOF are that cells are destroyed through the process and cannot be cultured after data acquisition. Additionally, some cellular parameters such as pH and ion concentration cannot be measured, only 30–60% of cells can be measured (low efficiency), slow sample throughput, and the requirement of new analysis tools [28]. Several studies have examined the correlation of the cellular content of the donor allograft with the risk of chronic GVHD using both flow cytometry and CYTOF [29,30].
3.6. Imaging as cGVHD biomarkers
Biomarkers are mostly considered circulating blood biomarkers. However, according to the NIH Consensus of cGVHD biomarker discovery group, ‘routine evaluations that aid in the assessment of chronic GVHD such pulmonary function testing and radiographic test, including computer tomography scans’ are also defined as biomarkers [31]. Recently, imaging biomarkers (IBs) have been shown to facilitate the testing of cGVHD research hypotheses in clinical trials and research studies. An IB is defined as a measurement that is acquired from one or more medical imaging. Additionally, IBs assist with decision-making in clinical practice, by providing cost-effective and noninvasive screening, detection of disease, and monitoring of patients. IBs are also beneficial in the predictions of a patient’s response to therapy and complications [32].
4. Statistical considerations
Over the years, many engineering and molecular technologies continue to arise and contribute to the identification of biomarkers with clinical application. However, fewer biomarkers with clinical application have been discovered, mostly due to a poor lack of study design. Several statistical factors should be noted during the discovery of biomarker with translational potential into clinical practice.
4.1. Sample size
In a biomarker discovery study, the selection of the specimen and the controls can be affected by the objective of the study and the extent of biomarker variability. Different factors affect variability in the selection of biomarkers from a list of candidates: (1) the disease’s subtypes (skin, liver, or gastrointestinal GVHD), (2) the ability of the biomarkers to allow the distinction between the different disease subtypes, (3) the number of biomarkers being studied, (4) the number of cases and controls, and (5) the statistical algorithm used for the selection of the potential biomarkers [4]. In order to minimize variability, Pepe et al. 2015, recommended the use of PRoBE, a computational tool that can minimize bias during the selection of sample size. The principles of PRoBe are the following: (1) the group proposed that biological samples should be drawn and stored using standard operating procedures that require no knowledge of case-controls information, (2) a random selection of cases and controls from all the cases and controls, (3) measurement of the biomarkers by ensuring blinding of cases–controls in the cohort, and (4) the measure of biomarkers performance should be conducted in a similar setting to the clinic. More importantly, sample size should be large to allow for a reliable recommendation about promising biomarkers [33].
4.2. Receiver operating characteristics curve
The receiver operating characteristics (ROC) curve is one of the most objective and statistically valid methods for biomarker performance evaluation. The ROC curve is a plot that denotes the true positive rate versus the false-positive rate, and often used in the classification of individuals as ‘positive’ or ‘negative’ by providing an optimal cutoff point [4,34]. ROC curves are often denoted by a single metric unit, the area under the curve (AUC). In a perfect scenario, where all positive biomarkers are ranked before the negative ones, the AUC is 1. Therefore, the utility of a biomarker can be assessed using the following guide: 0.9–1.0 = excellent; 0.8–0.9 = good; 0.7–0.8 = fair; 0.6–0.7 = poor; and 0.5–0.6 = fail [35].
4.3. Single versus multiple biomarkers of GVHD
In a diagnostic or predictive test, the use of several biomarkers simultaneously may increase predictability, diagnostic performance, or specificity. Therefore, in most cases, single biomarkers are not sufficiently sensitive or specific on their own. In order to develop a comprehensive cGVHD biomarker panel, proportional odds logistic regression models are utilized to create a composite panel. When evaluating multiple biomarkers, ROC curves can be calculated for each, and then potential biomarkers can be selected by using the ones with the highest AUC. However, the probability of identifying false positives increases due to the extensive list of potential biomarkers. The primary goal when simultaneously comparing multiple biomarkers is to minimize false positives while maximizing the power of meaningful correlations. Statistical methods such as the Bonferroni correction and the Benjamini–Hochberg false discovery rate assist to control false-positive rate in multiple biomarker comparison. However, it is noteworthy to add that if a biomarker is not highly correlated with other biomarkers or clinical predictors, one or two biomarkers can be sufficient for either diagnostic or predictive tests [21,35,36].
4.4. Training, validation, and independent sets
The validation of biomarker can be best performed by randomly dividing patients into training and validation sets. The training set is used to develop the statistical model, and subsequently tested in the validation, creating a blinded measure of biomarker performance. Additionally, the biomarkers must be tested into independent sets. Last, in order to eliminate center-specific effects, biomarkers must be validated in prospective multiple centers [4,21].
4.5. Risk stratification
Risk-predictive models can provide information on prognostic and diagnostic outcomes and aid in clinical decision-making, diagnosis, and therapy choice. For instance, patients with high risk and low risk of developing a future outcome such as death post-HSCT can be identified. The most widely used risk-predictive method is the AUC. Although the AUC estimates the probability that a patient with a particular outcome has a higher risk than a patient without the outcome, its main limitation remains the lack of clinical interpretability. Recently, new methods such as the Net Reclassification Improvement (NRI), weighted NRI, Net Benefit, and Relative Utility have been proposed [4,37].
5. Useful cGVHD biomarkers
5.1. Plasma cGVHD biomarkers
Only a few cGVHD biomarker studies have attempted to identify candidate biomarkers using proteomics discovery. Some studies have also validated these biomarkers in one or more independent sets. These studies are summarized in Table 1.
Table 1.
Plasma candidate biomarkers in cGVHD validated in at least two cohorts.
| Protein | Study | No. of patients in the study | Association direction | Setting of identification | Diagnosis/predictive time point (median day post-HSCT) | Prognosis time point (median day post-HSCT) | References |
|---|---|---|---|---|---|---|---|
| Protein pattern | Weissinger 2017 | 57 + 250a,d + 162a,d | 10 peptides increased, 4 peptides decreased, not validated | Diagnostic and Prognostic | 193 (extensive), 228 (limited) | 55 (extensive), 18 (limited) | [38] |
| sBAFF | Sarantopoulos 2007 | 104 + 24a,d + 24a,d | Increased | Diagnostic and Prognostic | 210–360 | 90,180 | [39] |
| Fujii 2008 | 80 | Increased | Diagnostic | 171 (earlyc), 429 (latec) | NA | [40] | |
| Kitko 2014 | 35 + 109a + 211a | Increased, and not validated in independent cohort | Diagnostic | 154b, 256 (earlyc), 619 (latec) | NA | [23] | |
| Kariminia 2016 | 23 + 198a + 83a | Increased | Diagnostic | 203, 174 | NA | [41] | |
| Ahmed 2016 | 78 + 37 | Increased | Diagnostic | 90, 180, 365 | NA | [42] | |
| Saliba 2017 | 341 | Increased | Diagnostic and Predictive | 189 | NA | [43] | |
| sCD13 | Fujii 2008 | 80 (enzymatic assay) | Increased | Diagnostic | 171 (earlyc), 429 (latec) | NA | [40] |
| Kitko 2014 | 35 + 109a + 211a (ELISA) | Increased, and not validated in independent cohort | Diagnostic | 154b, 256 (earlyc), 619 (latec) | NA | [23] | |
| Kariminia 2016 | 38 + 23 (enzymatic assay) | Increased, and significance not validated in both replication tests | Diagnostic | 362, 281 | NA | [41] | |
| sIL-2Ra | Liem 1998 | 46 | Increased | Diagnostic | 575 | NA | [44] |
| Fujii 2008 | 80 | Increased (early), NS(late) | Diagnostic | 171 (early), 429 (late) | NA | [40] | |
| Kitko 2014 | 35 + 109a + 211a | Increased, and not validated in independent cohort | Diagnostic | 154b, 256 (earlyc), 619 (latec) | NA | [23] | |
| Elafin | Kitko 2014 | 35 + 109a + 211a | Increased, and not validated in independent cohort | Diagnostic | 154b, 256 (earlyc), 619 (latec) | NA | [23] |
| CXCL9 | Kitko 2014 | 35 + 109a + 211a | Increased | Diagnostic | 154b, 256 (earlyc), 619 (latec) | NA | [23] |
| Yu 2016 | 53 + 211a + 180a,d | Increased | Diagnostic and prognostic | 210b,203c | 100 | [45] | |
| Kariminia 2016 | 23 + 198a + 83a | Increased, and not validated in independent cohort | Diagnostic | 203b,174c | NA | [41] | |
| Hakim 2016 | 26 + 83a | Increased | Diagnostic | 132 | NA | [46] | |
| Abu 2017 | 211d | Increased | Prognostic | NA | 100 | [47] | |
| CXCL10 | Kariminia 2016 | 23 + 198a + 83a | Increased | Diagnostic | 203b,174c | NA | [41] |
| Hakim 2016 | 26 + 83a | Increased | Diagnostic | 132 | NA | [46] | |
| Ahmed 2016 | 78 + 37 | Increased | Diagnostic | 90,180,365 | NA | [42] | |
| CXCL11 | Ahmed 2016 | 78 + 37 | Increased | Diagnostic | 90,180,365 | NA | [42] |
| ST2 | Yu 2016 | 53 + 211a + 180a,d | Increased | Diagnostic and prognostic | 210b,203c | 100 | [45] |
| MMP3 | Liu 2016 | 76 (BOS) | Increased | Diagnostic | 531 | NA | [51] |
| Yu 2016 | 53 + 211a + 180a,d | Increased, and not validated in independent cohort | Diagnostic and prognostic | 210b,203c | 100 | [45] | |
| OPNe | Yu 2016 | 53 + 211a + 180a,d | Increased, and not validated in independent cohort | Diagnostic and prognostic | 210b,203c | 100 | [45] |
| CD163 | Inamoto 2017 | 40 + 127a,d | Increased | Prognostic | NA | 80 | [52] |
| CCL15e | Du 2018 | 211 + 792d | Increased at onset but not prognostic | Diagnostic | 203 | 100 | [54] |
| AREG/EGF ratio (late aGVHD) | Holtan 2016 | 105 + 50a | Increased | Diagnostic | 160 | N/A | [56] |
SPON1: spondin-1; AREG: amphiregulin; EGF: epidermal growth factor; NA: not applicable; NS: not significant; SSc: systemic sclerosis.
Patient number in validation cohort.
Cohort 1.
Cohort 2.
Prognostic.
Candidate biomarkers that have not met the criteria of two independent cohort verification.
Peptides pattern. Urine proteins has been used to correlate a 14 peptides fragment pattern with cGVHD in 2 independent multicenter European cohorts, but the peptides pattern failed to correlate with cGVHD in a US multicenter cohort (Lee, SJ, verbal communication) [38]. One possible explanation is that classifiers using a machine-based algorithm can be overfitted.
sBAFF. Six studies demonstrated the role of B-cell activating factor (BAFF) in chronic GVHD patients. In the first study to ever investigate the role of soluble B-cell activating factor (sBAFF) in cGVHD, high levels of sBAFF were associated with active cGVHD [39]. Another study of 80 patients confirmed that higher levels of sBAFF were associated with both the early onset of cGVHD (3–8 months) and late cGVHD (≥9 months). Moreover, sBAFF was sensitive to steroids, as patients on corticosteroids at the onset of cGVHD showed decreased levels of sBAFF [40]. Using a protein microarray and sequential ELISA, higher levels of sBAFF were recorded into 3 different cohorts of 35, 109, and 211 patients, in newly diagnosed de novo-onset cGVHD patients compared those without cGVHD [23]. sBAFF was markedly elevated in cGVHD patients in two other studies [41,42]. More recently, another study demonstrated that increased levels of cGVHD can be essential for risk stratification as higher sBAFF at the time of diagnosis was associated with NRM [43].
sCD13. In three of those studies, increased sCD13 levels were also found in cGVHD patients. Two of the studies detected high CD13 at the onset of cGVHD, one by enzymation activity and the other using ELISA [23,40]. The third study also confirmed the association of CD13 and the development of cGVHD into two replication test cohorts by enzymatic activity. However, the significance of the data in the latter was contradictory and CD13 was not moved forward into the biomarker testing; in replication test one, CD13 was not significant (p = 0.99), while it was found to be significant into the second replication test two (p = 0.02) [41]. Overall, CD13 was not validated by high throughput. The only validated study was by enzymatic activity. However, one of the groups showed high correlation using both enzymatic and ELISA test that they developed, and CD13 did not validate in an independent cohort different from the other group [23].
sIL-2Rα. Similarly, elevated soluble IL-2 receptor α chain (sIL-2Rα) was identified in three different studies to be associated with cGVHD. Using 329 sera of 46 patients post HLA-identical bone marrow transplant, sIL-2Ra was elevated in cGVHD. In the same cohort, other cytokines, IL-10 and the cytokine-related molecule IL-1ra, were also elevated during cGVHD, demonstrating the complexity of the cytokine cascade activated during cGVHD [44]. Fujii et al. confirmed that early onset of cGVHD patients showed high sIL-2Rα along with three other proteins, including sBAFF. While sBAFF was elevated in both early and late cGVHD patients, sIL-2Rα was only elevated in early onset cGVHD patients [40]. Four additional biomarkers were elevated in patients with newly diagnosed de novo-onset cGVHD compared to those without cGVHD: sIL-2Rα, Elafin, CD13, and CXCL9. Analysis of the area under the curve revealed that CXCL9 had the best correlation with de novo-onset cGVHD in this analysis with an AUC of 0.83.
CXCR3+ expressing cells and recruiting chemokines. CXCL9 is an interferon-γ-inducible ligand for chemokine (C-X-C motif) receptor 3 (CXCR3), which is expressed on effector CD4 Th1 cells and CD8 cytotoxic T lymphocytes. Further analysis using a larger independent cohort consisting of patients with different cGVHD presentation (de novo, quiescent, or progressive) indeed confirmed that elevated CXCL9 levels were associated with de novo cGVHD. More specifically, increased CXCL9 levels correlated with increased cGVHD severity [23]. Elevated CXCL9 was associated with cGVHD in four other studies [41,45–47]. Using a quantitative proteomics approach, iTRAQ, in a cohort of 53 patients, CXCL9 along with 3 other biomarkers were found to be increased in cGVHD patient’s plasma compared to the non-GVHD. The other biomarkers also found to be upregulated were: ST2, MMP3, and Osteopontin. However, a second confirmatory cohort consisting of samples from eight different sites revealed that only ST2, CXCL9, and MMP3 were associated with cGVHD, whereas OPN was not [45]. CXCL10 is an inflammatory chemokine that also binds to CXCR3 and is involved with the activation and recruitment of T cells, NK cells, eosinophils, and monocytes. Three different groups showed that increased CXCL10 levels were present in cGVHD. In one of the studies, CXCL10 and another CXCR3-binding protein, CXCL9, were both increased. However, only CXCL10 had an ROC curve AUC of ≥0.75, and was validated in different groups [41,42,46]. Another CXCR3 ligand involved in the pathogenesis of cGVHD is CXCL11. Two studies showed increased CXCL11 levels in patients with active skin cGVHD. In one of the studies, all three CXCR3 ligands (CXCL11, CXCL9, and CXCL10) were increased in those patients [48]. The second study showed that CXCL10 and CXL11 along with sBAFF were increased in cGVHD patients [42].
ST2. sST2, also known as IL-33R, is a member of the interleukin-type I family and previously shown to be increased in patients with gastrointestinal diseases, small bowel transplant allograft rejection, and GVHD and can be secreted by proinflammatory T cells during gut inflammation [49]. As early at 100 post-HSCT, measurement of the biomarkers, particularly ST2 and CXCL9 could predict the development of cGVHD within 3 months with an AUC of 0.67 and 0.79 without and with known clinical risk factors, respectively [45].
MMP3. MMP3 is a metalloproteinase, a group of proteins known to degrade the extracellular membrane and the basement membrane in order to facilitate cell migration, infiltration, and tissue remodeling [50]. MMP3 levels were increased in cGVHD and bronchiolitis obliterans syndrome (BOS) patients [45,51].
CD163. CD163 is a macrophage scavenger receptor elevated in oxidative conditions [52]. The cumulative incidence of de novo-onset cGVHD was higher in patients with higher plasma soluble CD163 concentrations at day 80 than those with lower concentrations. The group suggested that monocyte or macrophage activation or increased oxidative stress may contribute to the pathogenesis of cGVHD [52].
SPON1. A prominent clinical feature of chronic GVHD is a debilitating fibrosing skin disease whose histologic features are similar to scleroderma. Spondin-1 was increased in diffuse cutaneous systemic sclerosis skin disease over time [53].
CCL15. Another candidate biomarker recently identified is CCL15. Increased CCL15 was detected in cGVHD patients compared to allo-HSCT patients without cGVHD. Additionally, patients with higher than median levels of CCL15 had a higher risk of NRM than patients with low CCL15 levels. Measurement of CCL15 levels day+100 in a second independent cohort showed no association of CCL15 and future cGVHD occurrence. CCL15 is cytokine produced by several tissue and cells in normal condition. However, high expression of CCL15 is detected in autoimmune diseases including asthma and rheumatoid arthritis [54].
Others. An independent verification study used previously reported RNA and protein cGVHD diagnostic biomarkers and identified with high accuracy three sets of RNA panel (PLEKHF1, IL1R2, and IRS2) that can be used to identify cGVHD patients. PLEKHF1 is a proapoptotic protein that functions through the lysosomal–mitochondrial pathway, and IRS2 is an important component of the IL-4 receptor signaling required for IL-4-mediated proliferation. Last, IL1R2 functions as a decoy receptor for IL-1 and has been shown to be overexpressed in multiple conditions such as autoimmune diseases and organ rejections [55].
AREG/EGF ratio in late aGVHD. In a prospective cohort study of 909 patients, 83 patients (11%) developed late onset aGVHD. The study group measured angiogenic factors’ (AREG, EGF, and AREG/EGF) ratio in 2 different cohorts of 105 and 50 patients, respectively. High ratio of AREG/EGF in late aGVHD patients was elevated compared to controls. In addition, they compared AREG/EGF ratio in classic aGVHD and cGVHD, and found that AREG/EGF ratio was also elevated in classic aGVHD, but not in cGVHD [56].
5.2. Cellular biomarkers
Cellular biomarkers associated with cGVHD are summarized in Table 2. Several immune cells have been identified as potential biomarkers in cGVHD using cytomics.
Table 2.
Blood cellular candidate biomarkers in cGVHD.
| Phenotype | Subset | Study | No. of patients in the study | Association direction | Setting of Identification | Diagnosis endpoint (median day post-HSCT) | Prognostic time point (median day post-HSCT) | References |
|---|---|---|---|---|---|---|---|---|
| B cells | TLR9+ | She 2007 | 54 | Increased | Diagnostic and response to treatment | 171 (early), 429 (late) | NA | [57] |
| CD21low | Greinix 2008 | 70 | Increased | Diagnostic | 1428 | NA | [58] | |
| Kuzmina 2013 | 136 | Increased | Diagnostic | 143 | NA | [59] | ||
| IgM memory (CD19+CD27+IgM+) | D’Orsogna 2009 | 37 | Decreased | Diagnostic | 180 | NA | [60] | |
| BAFF/B-cell ratio | Sarantopoulos 2009 | 57 | Increased | Diagnostic | 180 | NA | [61] | |
| T effector cells | Th17 | Dander 2009 | 51 | Increased | Diagnostic | 281 | NA | [62] |
| Zhao 2013 | 59 | Increased | Predictive | NA | 30 | [63] | ||
| Malard 2014 | 17 | Increased | Diagnostic | 258 | NA | [64] | ||
| CD4+CD146 +CCR5+ | Forcade 2017 | 40 | Increased | Diagnostic | 942 | NA | [65] | |
| CXCR3+ T cells | Croudace 2012 | 38 + 28 | Decreased | Diagnostic | 362, 281 | NA | [48] | |
| Hakim 2016 | 26 + 83 | Decreased | Diagnostic | 132 | NA | [46] | ||
| CD4+CD45RA+CD31+ | Greinix 2015 | 227 | Increased | Diagnostic and prognostic | 171 | 100 | [66] | |
| T regulatory cells | CD4+CD25+ Foxp3+ | Zorn 2005 | 57 | Decreased | Diagnostic | 720 | NA | [67] |
| Matsuoka 2010 | 78 | Decreased | Diagnostic | 270 | NA | [68] | ||
| Treg/Tcons | Treg/Th17 | Malard 2014 | 17 | Decreased | Diagnostic | 264 | NA | [64] |
| Alho 2016 | 107 | Decreased | Diagnostic | 228 | NA | [70] | ||
| TFH | Forcade 2016 | 66 | Decreased | Diagnostic | 867 | NA | [71] | |
| Other NK and NKT cells | CD56+ | Hirakawa 2016 | 14 | Decreased | Diagnostic and predictive response to treatment | 237 | NA | [72] |
| CD56+ | Huenecke 2017 | 74 | Decreased | Prognostic | 378 | ≤60 | [73] | |
| CXCR3+CD56bright | Kariminia 2016 | 76 | Decreased | Diagnostic | >270, <270 | NA | [41] | |
| CXCR3+CD56bright | Kariminia 2018 | 223 | Decreased | Diagnostic | 730 | NA | [41,74] | |
| CD3+CD28+ T cells | Skert 2009 | 30 | Increased | Diagnostic and prognostic | 180 | 120 | [75] | |
| CD8+CD38+ T cells | Stikvoort 2017 | 68 | Increased | Diagnostic | Mild:201 Moderate: 207 Severe:208 |
NA | [76] | |
| MAIT | Stikvoort 2017 | 68 | Decreased | Diagnostic | Mild:201 Moderate: 207 Severe:208 |
NA | [76] | |
| Myeloid dendritic cells | CD1a+ | Botari 2014 | 26 | Increased | Diagnostic | 100–200 | NA | [77] |
| Plasmacytoid dendritic cells (pDCs) | CD123+ | Waller 2012 | 113 | Decreased | Diagnostic | 182 | N/A | [78] |
| Chan 2003 | 21 | Increased | Diagnostic | 100 | NA | [79] | ||
| Monocytes | Monocytes CD86+CD14+ | Arpinati 2008 | 41 | Increased | Diagnostic | 289 | NA | [80] |
TFH: T follicular helper cells; NA: not applicable; MAIT: mucosal-associated invariant.
B cells. High expression of Toll-like receptor 9 (TLR9) in B cells positively correlated with the development of cGVHD in patients [57]. The presence of immature B cells, characterized as CD19+/CD21− cells, in patients was also associated with high cGVHD development [58,59]. In another study, allo-HSCT patients with a history of cGVHD showed a deficiency in IgM memory cells was found in allo-HSCT also associated with the development of cGVHD [60]. Last, high plasma BAFF/B-cell ratio was detected in cGVHD patients compared to healthy patients [61].
Effector and regulatory T cells. Several studies showed high expression of pathogenic TH17 in cGVHD patients compared non-GVHD patients [62–64]. Recently, a CD4 T-cell population, expressing CD146, an adhesion molecule and CCR5, a chemokine receptor, was identified in cGVHD patients. More importantly, this T-cell population is TH17-prone [65]. Another subset of T cells, CXCR3+ T cells were identified in two studies, where their levels were significantly decreased in cGVHD patients compared to non-GVHD patients [46,48]. One study showed that naive thymic T cells, CD4+CD45RA+CD31+ T cells, were significantly increased in patients on day 100 post-HSCT and this correlated with later development of cGVHD (day 171) [66]. In three other studies, Tregs, characterized as CD4+CD25+forkheadbox protein3+ (Foxp3+) were profoundly reduced in cGVHD patients compared to the healthy patients and said to be critical for the tolerance of cGVHD post-HSCT [67,68]. Of note, one small sample-sized cohort study showed the opposite, possibly because they only used CD25 high as a marker for Tregs. However, activated T cells also express high CD25 [69]. Furthermore, two studies detected a high ratio of TH17/Treg in cGVHD patients, suggesting the critical role of a balance in Tregs ratio in the prevention of cGVHD [64,70].
T follicular helper cells. Circulating T follicular helper cells (TFH) are subset of cells that play a role in B-cell immunity. In cGVHD patients, the ratio of circulation TFH cells was markedly decreased compared to non-GVHD patients [71].
Other NK and NKT cells. Four studies associated a decrease in CD58bright NK cells with the development of cGVHD [41,72–74]. In one of the studies, CXCR3+CD56bright NK cells inversely correlated with CXCL10 as a significant biomarker panel for the diagnosis of cGVHD [41]. Four other studies confirmed the role of natural killer cells in cGVHD where patient biopsies revealed the presence of natural killer cells among the lymphocytes that infiltrated into the skin, liver, and the intestine in those GVHD target tissues [72,73,75,76],.
Myeloid dendritic cells. High expression of both myeloid dendritic cells and natural killer cells was also previously found in oral cGVHD patients [77].
Plasmacytoid dendritic cells (pDCS). pDCs defined as (Lin−HLA−DR+CD11c−CD123+) have a controversial role in cGVHD. High numbers of pDCs in donor bone marrow has been associated with both increased and decreased cGVHD in patients post allogeneic bone marrow transplants [78,79].
Monocytes. Last, using peripheral blood and bone marrow cells, one study showed the presence of higher levels of monocytes in cGVHD compared to the non-cGVHD patients [80].
6. Applications of cGVHD biomarkers in clinical practice
The analysis of thousands of biological molecular biomarkers can be simultaneously yielded due to the emergence of powerful proteomic and genomic strategies along with advanced bioinformatics algorithms. This can help to exploit new cGVHD biomarkers which are specific and sensitive enough for patient-risk stratification, early cGVHD assessment as potential acceptable clinical endpoints, for monitoring cGVHD progression and for cost-effective management decision-making.
The process of cGVHD biomarker identification, validation, and clinic approval is complicated, expensive, and time consuming (Figure 1). The acquisition to high-quality cGVHD bio-samples which are selected, stored, and processed in rigorous ways to minimize confounding variables or potential bias is a key point that requires to be considered before the study is performed [81]. All candidate biomarkers require to be thoroughly validated from preclinical investigation to independent clinical research in large multicenter cohort setting(s) [6,7]. Additionally, collaboration between scientists and clinicians is a necessity to validate a cGVHD biomarker or panel from the bench to clinical use. Undoubtedly, recent approaches have great merit in the improved cGVHD decision-making, early diagnosis, risk prediction, therapy selection, and monitoring of cGVHD patients. A schema for treating newly diagnosed chronic GVHD using biomarkers is shown in Figures 2 and 3.
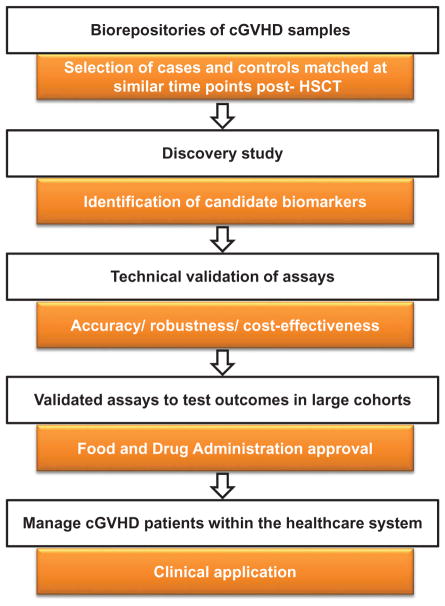
Figure 1.
Overview of cGVHD biomarkers workflow from discovery to validation studies.
Biorepositories of cGVHD patients are used to select cases and controls samples matched at similar time points post-HSCT for the discovery of candidate cGVHD biomarkers. Analytical assays are validated by assessing their accuracy, robustness and cost-effectiveness. Validated assays will move forward for validation in large cGVHD cohorts. Last, after approval from the Food and Drug Administration, tests are used in standard practice in cGVHD clinics.
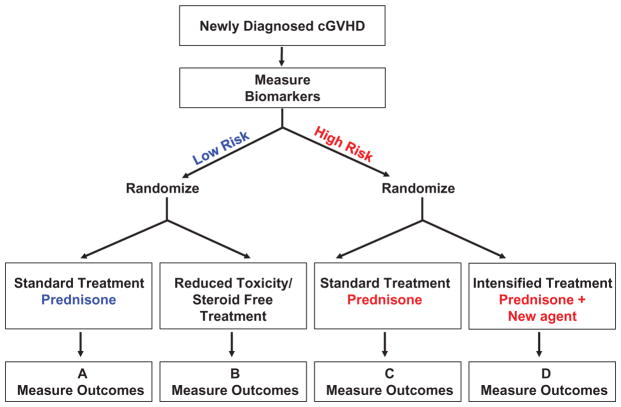
Figure 2.
Predictive biomarker strategy design for newly diagnosed cGVHD.
At the onset of cGVHD, biomarkers are measured to stratify patients in low and high risk. Low risk patients on the left, will be randomized to receive either a standard treatment or a reduced toxicity/steroid free treatment. Comparison between A and B will show if steroid free treatment is as efficient as the steroid treatment and if it reduces the risk of relapse and infections rates. On the other hand, high risk patients will receive either a standard treatment or an intensified treatment. Comparison of C and D will show whether intensification of treatment increases the ratio of responders and effectiveness on cGVHD signs without increasing relapse and infections rates.
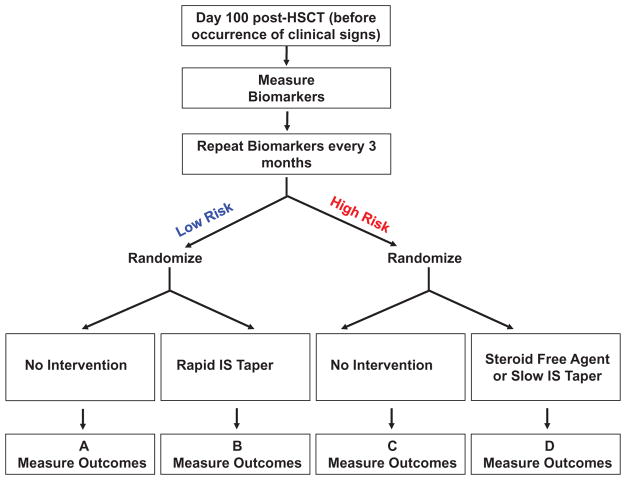
Figure 3.
Prognostic biomarker-based cGVHD preemptive trial design.
Before the occurrence of any clinical signs, biomarkers will be measured starting at D100 post-HSCT and repeated every 3 months. Then, biomarkers cutpoints will be used to identify low and high risk patients. Low risk patients, on the left, will receive no intervention or a rapid immunosuppressive (IS) drugs taper. Comparison of A and B will indicate if a rapid taper immunosuppressive drugs regimen is less toxic and lowers infections rates while achieving tolerance sooner (no development of cGVHD). High risk patients, on the right, will be randomized for either no intervention or a preemptive treatment with a steroid-free agent in option 1. In this case, comparison of A and B will show if preemptive intervention before the onset of cGVHD will lower the incidence of cGVHD as compared to no intervention. In option 2, randomization will compare no intervention versus slow immunosuppressive drugs taper. In this case, comparison of C and D will show if the slower taper will decrease the incidence of cGVHD compared to expected incidence.
7. Biomarkers as potential therapeutic target
Pharmacogenomics exploration based on cGVHD biomarkers has increasingly become an important part of research and the development for novel targeted therapeutics over the last decade. Table 3 summarizes cGVHD biomarkers that can be targeted using recent novel therapeutic approaches.
Table 3.
cGVHD biomarkers that can be targeted.
| Drugs | Targets and related findings | Mechanism of action | References |
|---|---|---|---|
| Currently available targets
| |||
| BTK inhibitors (Ibrutinib) | BAFF, BLNK, BTK, naive B-cell, T cell | Inhibits of BTK Tyrosine Kinase in B cells and interleukin-2-inducible kinase (ITK) in T cell | [82–84] |
| Syk inhibitors (Fostamatinib, Entospletinib) | Luciferase+ donor Tc CXCR4, CD4 T cells, CD11b | Promotes apoptosis and prevents hyperresponsiveness in human chronic GVHD B cells | [87–89] |
| Belimumab | BAFF | Blocks B-cell activation | [90] |
| JAK inhibitors (Ruxolitinib, Pacritinib, Baricitinib, INCB039110) | IFN-γ, IL-2, IL-12, and IL-23, CXCR3, CXCL9 and CXCL10, and Th17 | Blocks Janus Kinase signal transducer and activator of transcription factor (STAT) | [92] |
| Proteasome inhibitors (Bortezomib, Carfilzomib, Ixazomib) | IL-6 and BAFF | Decreases T-cell differentiation mediated by IL-6, and decrease BAFF levels | [93,94] |
| Hedgehog inhibitors (Vismodegib, Sonidegib) | Transcription factors Gli-1 and Gli-2 | Inhibits downstream effector proteins of hedge pathway – the hedgehog coreceptor Smoothened (Smo) | [96–98] |
| IL-2 | Tregs | Promotes the survival, development, and survival of Tregs | [102–104] |
| Neutrophil elastase inhibitor (AZD9668) | IL-2, IRF4, Th17 | Inhibits neutrophil elastase | [106] |
| Future potential new targets | |||
| Anti-RORγt | RORγt | Inhibits the production of IL-17 | [65] |
| Anti-IL-17 | IL-17 | Inhibits IL-17 secretion by T cells | [65] |
| Anti-STAT3 | STAT3 | Inhibits RORγt and activation of IL-17 | [109] |
| Anti-ROCK2 | ROCK2 | Inhibits the production of IL-21 and IL-17 through inhibition of pSTAT3 | [109] |
Ibrutinib, a Bruton’s tyrosine kinase (BTK) inhibitor in B cells and interleukin-2-inducible T-cell kinase inhibitor in T cells, is the first ever US FDA-approved drug for the treatment of cGVHD. In a murine model, ibrutinib ameliorated the development of cGVHD [82]. Another study showed that administration of Ibrutinib in cGVHD murine model significantly reduced B cells and CD4 T cells, resulting in the treatment of sclerodermatous cGVHD [83]. In a multicenter study, patients that failed to respond to prior corticosteroid-containing therapy were given 450 mg of ibrutinib once daily. As high as 71% of those patients had a multiorgan response, while 67% of the responsive patients sustained response for ≥20 weeks. Plasma levels of soluble factors associated with inflammation, fibrosis, and cGVHD were also significantly reduced post-ibrutinib treatment. This study led to the FDA approval of ibrutinib as the first drug for the treatment cGVHD in patients that failed to respond to the corticosteroids [84].
B-cell hyperresponsiveness, caused by the production of immunoglobulin in isotype-switched antibody by hypermutated B cells in the germinal center, is characterized by a prolonged and uncontrolled activation of B cells [2]. Increased germinal center B cells have been associated with the development of cGVHD in a murine model and in patients. More specifically, the expression of spleen tyrosine kinase (Syk) on B cells is essential for the development and pathology of cGVHD. This sparked interest by many to investigate the role of Syk inhibitors, fostamatinib, and entospletinib, as potential therapeutic agents in cGVHD [85,86]. In a murine model, Syk inhibitor, fostamatinib reduced pathology and improved death post allo-HSCT. In patients, fostamatinib induced apoptosis of cGVHD B cells [87–89]. Fostamatinib and entospletinib are both currently in clinical trials for the therapy of cGVHD (ClinicalTrials.gov numbers, NCT02611063 NCT02701634). Belimumab is a recently FDA-approved B-cell activation inhibitor for the treatment of systemic lupus erythematosus (SLE). Belimumab is also currently in clinical trial for the prevention of cGVHD post allo-HSCT [90] (ClinicalTrials.gov number, NCT03207958).
Other studies investigated the use of Janus Kinase (JAK) inhibitors particularly ruxolitinib in the treatment of GVHD. JAK inhibitors block JAK signal transducer and activator of transcription (STAT), thus leading to decreased expression of proinflammatory factors such as IFN-γ, IL-2, and IL-6. JAK inhibitors have also been shown to inhibit T cells, Th1 and Th17 responses, and dendritic cells [91]. A clinical study showed that out of the 41 cGVHD patients who received ruxolitinib, 35 patients or 85.4% responded to the treatment. Additionally, the 6-month overall survival was 97.4% for patients treated with ruxolitinib [92]. Ruxolitinib is currently in a phase III clinical trial in corticosteroid-refractory cGVHD patients (ClinicalTrials.gov number, NCT03112603).
Proteasome inhibitors also show promise in the treatment of cGVHD. In a 20 patients phase II clinical trial, administration of Bortezomib, an inhibitor of nuclear factor kappa B (NFκB) and main regulator of cytokine signaling and T-cell and B-cell differentiation, along with corticosteroids was significantly effective in the treatment of cGVHD. Sixteen patients (80%) had a positive response to the treatment. As high as 100% of patients with skin cGVHD (n = 3) experienced a complete resolution of the skin cGVHD. Several improvements were recorded in different organs such as GI GVHD. However, at 15 weeks, an immunoregulation was noted post coadministration of bortezomib and the corticosteroid, prednisone. Peripheral dendritic cells count was reduced throughout the study. Moreover, the ratio of Treg:Tconvs was also decreased. However, naive Tregs (nTregs) and B-cell levels remained unchanged [93]. Although nTregs have been shown to prevent GVHD, a decreased Tregs:Tconvs ratio is associated with cGVHD. One study showed that Bortezomib increases the Tregs and T convs ratio in murine cGVHD model, resulting in the alleviation of cGVHD. In the same study, Bortezomib provided a therapeutic benefit to cGVHD patients [94].
Another target pathway in the therapy of cGVHD is the Hedgehog pathway. Sonic Hedgehog pathway is a major regulator of cellular development, differentiation, and polarity. Aberrant regulation of cellular development and differentiation can result in different malignancies, such as the basal cell carcinoma. Activated hedgehog upregulates GLi-1 and GLi-2, two transcription factors that promote pathologic fibrosis, a known hallmark pathophysiology of cGVHD. Currently, two hedgehog inhibitors have been FDA-approved for the treatment of basal cell carcinoma: vismodegib and sonidegib (LDE225) [95]. The role of hedgehog inhibitors in the therapy of cGVHD has also been investigated. Treatment with hedgehog inhibitor, LDE223, almost completely abrogated the clinical manifestation and histological features of human and murine sclerodermatous cGVHD [96,97]. In a phase I clinical trial, 47% of sclerodermatous cGVHD patients treated with a hedgehog pathway inhibitor, sonidegib, exhibited a positive response to the therapy [98]. The results were encouraging and they are currently an ongoing clinical trial investigating the role of vismodegib in the treatment of patients with steroids-refractory cGVHD (ClinicalTrials.gov number NCT02337517).
Interleukin-2 (IL-2) is mainly produced by CD4+ T helper (TH) cells in the steady state and is essential for the homeostasis of regulatory T cells (Tregs), characterized as CD4+, CD25+, and FOXP3+ cells. During an immune response, activated CD4+ T cells produce large amounts of IL-2, which then bind to the IL2-R expressed on CD25+ effectors T cells, including Tregs. IL-2 deficiency in mice has been shown to hinder the development, survival, and function of Tregs [99,100]. In cGVHD, low expression of Tregs was previously identified in patients [101]. In a phase I clinical trial, subcutaneous administration of IL-2 in cGVHD patients resulted to a 52% positive response, including decreased sclerodermatous, softening of the skin and subcutaneous tissue, and improved liver function. In the same study, Tregs and Tconvs count in patients showed an extensive increase (20 times) in the basal levels of Tregs at 2 weeks posttreatment with low dose of IL-2 [102]. Two studies reported an expansion of Tregs and a rapid elevation of Tregs:Tconvs ratio in patients treated with low dose of IL-2 [103,104]. Currently, there is a lot of interest in the adoptive transfer of immunomodulated Tregs through their expansion using low dose of IL-2 for the therapy of cGVHD.
Neutrophil elastase (NE) is a cytotoxic serine protease stored in the granules of neutrophils and released during inflammation. NE has been shown to be the cause of BOS, the main manifestation of cGVHD in the lungs. AZD9668 is a selective inhibitor of NE, which was initially approved for the treatment of inflammatory lung diseases, prevented lung diseases, and inflammation in murine models. Additionally, it was proven effective and well tolerated in the treatment of other lung-related diseases including COPD, cystic fibrosis, and bronchiectasis [105–108]. Although the therapeutic use of AZD9668 in cGVHD patients post-HSCT has not been investigated, there is currently an ongoing phase II clinical trial (ClinicalTrials.gov number, NCT02669251).
In addition, there are potential new therapeutic targets that deserve further investigation in cGVHD. The pathogenesis of Th17-prone CD4+CD145+CCR5+ T cells has been established in cGVHD [65]. Inhibition of IL-17 production using anti-RORγt, anti-IL-17, and anti-STAT3 has all shown effectiveness in the inhibition of cGVHD. For instance, treatment with TMP778, a pharmacological inhibitor that binds to RORγt to block downstream gene expression including IL-17, significantly diminished both IL-17 production and the CD4+CCR5+CD146+ T cells in vitro. Additionally, TMP778 significantly alleviated murine lung cGVHD. The use of anti-IL-17 antibody showed similar results [65]. In cGVHD, activation of STAT3 and production of IL-21 by pathogenic T cells is also important for the pathogenesis. Rho-associated kinase 2 (ROCK2) inhibitor is a small molecule that targets IL-17 and IL-21 production, and that was previously significantly decreased in murine and human cGVHD through the inhibition of STAT3 pathway. There is currently an ongoing phase II clinical trial evaluating the safety and effects of ROCK2 inhibition in cGVHD patients (ClinicalTrials.gov number, NCT02841995) [109].
8. Expert commentary
During the last decade, there has been a tremendous progress in the discovery and validation of more specific and sensitive biomarkers for cGVHD due to major advances in omics technologies. Compared to biomarkers identified in aGVHD, the biology of cGVHD is more complex; multiple organs are affected and a greater number of soluble and cellular factors are produced in patients. Therapeutic approaches have largely been limited to nonspecific immunosuppression. Therefore, corticosteroids remained the main line of therapy until recently. However, in 2017, the FDA approved ibrutinib targeting interleukin-2-inducible T-cell kinase (ITK) and Bruton’s tyrosine (BTK) as the first ever drug for the treatment of corticosteroid refractory cGVHD [84]. In addition, other new cGVHD therapeutic approaches have recently emerged. These agents particularly target pathways and molecules that contribute to the development of cGVHD such as T-cell and B-cell signaling pathways (JAK inhibitors, Syk inhibitors), fibrosis (hedgehog inhibitors), and IL-17 production (anti-ROCK-2, anti-RORyt, and anti-IL-17). The use of low-dose IL-2 to increase Tregs is also a promising therapeutic approach in cGVHD. Furthermore, there have been some encouraging strides in the development of cGVHD biomarkers that would risk stratify patients for the development of cGVHD and possibly response to treatment. But, their sensitivity and specificity is not yet at the comprehensive level that has been seen in aGVHD biomarkers. Although, it is likely that in few years, additional cGVHD biomarkers with good specificity and sensitivity would be discovered and validated to be applied in clinical trials. Focus should particularly be given to biomarkers that could indicate more on the pathophysiology of cGVHD and be targeted using therapeutic approaches.
9. Five-year view
FDA has recently approved ibrutinib; the first ever cGVHD treatment for corticosteroid refractory patients [84]. The use of ibrutinib in clinical trials for first line of therapy is under way. The focus within the next 5 years will be in the discovery and validation of more specific and sensitive cGVHD biomarkers, particularly the drug-targetable cGVHD biomarkers.
Key issues.
Incidence of cGVHD has been decreasing recently due to changes in prophylaxis (i.e. cyclophosphamide post-transplant).
However, cGVHD remains the most common long term complication of allo-HSCT and the leading cause of mortality in patients that survive 2 years without relapse.
Clinical manifestations of cGVHD resemble those of auto-immune diseases such as scleroderma. Clinical components involve inflammation and fibrosis that affect all target organs (skin, eyes, mouth, lungs, genital), thus making the diagnosis complex.
Therapeutic approaches for cGVHD have been limited due to nonspecific targeting of immune cells. For example, corticosteroids remain the main line of therapy until recently.
In 2017, the FDA approved ibrutinib, an ITK and BTK inhibitor for the treatment of corticosteroids refractory cGVHD patients, launching a new era for cGVHD treatments and prevention.
Blood omics tools (genomics, transcriptomics, proteomics, and cytomics) have been utilized the last five years in the identification of relevant cGVHD biomarkers. There still remains a need for more specific and sensitive cGVHD biomarkers.
Several of these cGVHD biomarkers can be targeted by different drugs as shown in Table 3.
Acknowledgments
Funding
This article was funded by National Cancer Institute [R01CA 168814, to S. P.], and National Institute of Diabetes and Digestive and Kidney Diseases [T32 DK007519, to D.A.], Leukemia and Lymphoma Scholar Award [grant1293-15, to S.P.], Lilly Physician Scientist Initiative Award (to S.P.).
Footnotes
Declaration of interest
S Paczesny has a patent on ‘Methods of detection of graft-versus-host disease’ licensed to Viracor-IBT Laboratories. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.D’Souza AZX. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides. 2016 [cited 2017 Oct 10]. Available from: http://www.cibmtr.org.
- 2.Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med. 2017;377(26):2565–2579. doi: 10.1056/NEJMra1703472. [DOI] [PubMed] [Google Scholar]
- 3.Levine JE, Paczesny S, Sarantopoulos S. Clinical applications for biomarkers of acute and chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18(1):S116–S124. doi: 10.1016/j.bbmt.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paczesny S, Raiker N, Brooks S, et al. Graft-versus-host disease biomarkers: omics and personalized medicine. Int J Hematol. 2013;98(3):275–292. doi: 10.1007/s12185-013-1406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald KPA, Hill GR, Blazar BR. Chronic graft-versus-host disease: biological insights from preclinical and clinical studies. Blood. 2017;129(1):13–21. doi: 10.1182/blood-2016-06-686618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paczesny S, Hakim FT, Pidala J, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. The 2014 Biomarker Working Group Report. Biol Blood Marrow Transplant. 2015;21(5):780–792. doi: 10.1016/j.bbmt.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolff D, Greinix H, Lee SJ, et al. Biomarkers in chronic graft-versus-host disease: quo vadis? Bone Marrow Transplant. 2018;24(10):018–0092. doi: 10.1038/s41409-018-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paczesny S. Graft-versus-host disease in children after hematopoietic cell transplantation: potential clinical utility of biomarkers. Int J Hematol Oncol. 2015;4(2):51–54. [Google Scholar]
- 9.Schultz KR, Miklos DB, Fowler D, et al. Toward biomarkers for chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. Biomarker Working Group Report. Biol Blood Marrow Transpl. 2006;12(2):126–137. doi: 10.1016/j.bbmt.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Hu ZZ, Huang H, Wu CH, et al. Omics-based molecular target and biomarker identification. Methods Mol Biol. 2011;719:547–571. doi: 10.1007/978-1-61779-027-0_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogunia-Kubik K, Mizia S, Gronkowska A, et al. CCR5 gene polymorphism affects the risk of GvHD after haematopoietic stem cell transplantation from an unrelated donor. Br J Haematol. 2015 doi: 10.1111/bjh.13387. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Karaesmen E, Rizvi AA, Preus L, et al. Replication and validation of genetic polymorphisms associated with survival after allogeneic blood or marrow transplant. Blood. 2017;130:1585–1596. doi: 10.1182/blood-2017-05-784637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe R, Shirley N, Bleackley M, et al. Transcriptomics technologies. PLoS Comput Biol. 2017;13(5):e1005457. doi: 10.1371/journal.pcbi.1005457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomuleasa C, Fuji S, Cucuianu A, et al. MicroRNAs as biomarkers for graft-versus-host disease following allogeneic stem cell transplantation. Ann Hematol. 2015;94(7):1081–1092. doi: 10.1007/s00277-015-2369-0. [DOI] [PubMed] [Google Scholar]
- 15.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2011;118(21):SCI-50–SCI-50. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin X-F, Wu N, Wang L, et al. Circulating microRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell Mol Neurobiol. 2013;33(5):601–613. doi: 10.1007/s10571-013-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie LN, Zhou F, Liu XM, et al. Serum microRNA155 is increased in patients with acute graft-versus-host disease. Clin Transplant. 2014;28(3):314–323. doi: 10.1111/ctr.12314. [DOI] [PubMed] [Google Scholar]
- 18.Garchow B, Kiriakidou M. MicroRNA-21 deficiency protects from lupus-like autoimmunity in the chronic graft-versus-host disease model of systemic lupus erythematosus. Clin Immunol. 2016;162:100–106. doi: 10.1016/j.clim.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranganathan P, Ngankeu A, Zitzer NC, et al. Serum miR-29a is upregulated in acute graft-versus-host disease and activates dendritic cells through TLR binding. J Immunology. 2017;198(6):2500–2512. doi: 10.4049/jimmunol.1601778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlan SN, Watkins B, Tkachev V, et al. Transcriptome analysis of GVHD reveals aurora kinase A as a targetable pathway for disease prevention. Sci Transl Med. 2015;7(315):315ra191. doi: 10.1126/scitranslmed.aad3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paczesny S. Discovery and validation of graft-versus-host disease biomarkers. Blood. 2013;121(4):585–594. doi: 10.1182/blood-2012-08-355990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Wang J, Li J, et al. Identification of serum biomarkers for gastric cancer diagnosis using a human proteome microarray. Mol Cell Proteomics. 2016;15(2):614–623. doi: 10.1074/mcp.M115.051250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitko CL, Levine JE, Storer BE, et al. Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood. 2014;123(5):786–793. doi: 10.1182/blood-2013-08-520072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anjo SI, Santa C, Manadas B, et al. SWATH-MS as a tool for biomarker discovery: from basic research to clinical applications. Proteomics. 2017;17:3–4. doi: 10.1002/pmic.201600278. [DOI] [PubMed] [Google Scholar]
- 25.Parker CE, Borchers CH. Mass spectrometry based biomarker discovery, verification, and validation–quality assurance and control of protein biomarker assays. Mol Oncol. 2014;8(4):840–858. doi: 10.1016/j.molonc.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crutchfield CA, Thomas SN, Sokoll LJ, et al. Advances in mass spectrometry-based clinical biomarker discovery. Clin Proteomics. 2016;13:1. doi: 10.1186/s12014-015-9102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savaryn JP, Toby TK, Kelleher NL. A researcher’s guide to mass spectrometry-based proteomics. Proteomics. 2016;16(18):2435–2443. doi: 10.1002/pmic.201600113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matos TR, Liu H, Ritz J. Research techniques made simple: mass cytometry analysis tools for decrypting the complexity of biological systems. J Invest Dermatol. 2017;137(5):e43–e51. doi: 10.1016/j.jid.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Kay AW, Strauss-Albee DM, Blish CA. Application of mass cytometry (cytof) for functional and phenotypic analysis of natural killer cells. Methods Mol Biol. 2016;1441:13–26. doi: 10.1007/978-1-4939-3684-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ornatsky O, Bandura D, Baranov V, et al. Highly multiparametric analysis by mass cytometry. J Immunol Methods. 2010;361(1–20):1–2. doi: 10.1016/j.jim.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Paczesny S, Hakim FT, Pidala J, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: III. The 2014 Biomarker Working Group Report. Biol Blood Marrow Transplant. 2015;21:780–792. doi: 10.1016/j.bbmt.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor JP, Aboagye EO, Adams JE, et al. Imaging biomarker roadmap for cancer studies. Nat Rev Clin Oncol. 2017;14(3):169–186. doi: 10.1038/nrclinonc.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pepe MS, Li CI, Feng Z. Improving the quality of biomarker discovery research: the right samples and enough of them. Cancer Epidemiology, Biomarkers Prevention: Publication American Association Cancer Research, Cosponsored by American Society Preventive Oncology. 2015;24(6):944–950. doi: 10.1158/1055-9965.EPI-14-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pepe M, Longton G, Janes H. Estimation and comparison of receiver operating characteristic curves. Stata J. 2009;9(1):1. [PMC free article] [PubMed] [Google Scholar]
- 35.Xia J, Broadhurst DI, Wilson M, et al. Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics: Official Journal of the Metabolomic Society. 2013;9(2):280–299. doi: 10.1007/s11306-012-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ensor JE. Biomarker validation: common data analysis concerns. Oncologist. 2014;19(8):886–891. doi: 10.1634/theoncologist.2014-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Calster B, Vickers AJ, Pencina MJ, et al. Evaluation of markers and risk prediction models: overview of relationships between NRI and decision-analytic measures. Medical Decision Making: an International Journal of the Society for Medical Decision Making. 2013;33(4):490–501. doi: 10.1177/0272989X12470757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weissinger EM, Human C, Metzger J, et al. The proteome pattern cGvHD_MS14 allows early and accurate prediction of chronic GvHD after allogeneic stem cell transplantation. Leukemia. 2017;31(3):654–662. doi: 10.1038/leu.2016.259. [DOI] [PubMed] [Google Scholar]
- 39•.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13(20):6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. The first study to identify a cGVHD biomarker; elevated sBAFFs were detected in 104 patients post-HSCT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujii H, Cuvelier G, She K, et al. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children’s Oncology Group. Blood. 2008;111(6):3276–3285. doi: 10.1182/blood-2007-08-106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Kariminia A, Holtan SG, Ivison S, et al. Heterogeneity of chronic graft-versus-host disease biomarkers: the only consistent association is with CXCL10 and CXCR3+ NK cells. Blood. 2016;127(24):3082–3091. doi: 10.1182/blood-2015-09-668251. This study validated published biomarkers (discovery analysis did not find new biomarkers). These biomarkers were validated in 2 cohorts of 198 and 83 patients; however, cellular biomarkers were tested in only one cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed SS, Wang XN, Norden J, et al. Identification and validation of biomarkers associated with acute and chronic graft versus host disease. Bone Marrow Transplant. 2015;50(12):1563–1571. doi: 10.1038/bmt.2015.191. [DOI] [PubMed] [Google Scholar]
- 43.Saliba RM, Sarantopoulos S, Kitko CL, et al. B-cell activating factor (BAFF) plasma level at the time of chronic GvHD diagnosis is a potential predictor of non-relapse mortality. Bone Marrow Transplant. 2017;52:1010–1015. doi: 10.1038/bmt.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liem L, Van Houwelingen H, Goulmy E. Serum cytokine levels after HLA-identical bone marrow transplantation. Transplantation. 1998;66(7):863–871. doi: 10.1097/00007890-199810150-00009. [DOI] [PubMed] [Google Scholar]
- 45•.Yu J, Storer BE, Kushekhar K, et al. Biomarker panel for chronic graft-versus-host disease. J Clinical Oncology: Official Journal Am Soc Clin Oncol. 2016;34(22):2583–2590. doi: 10.1200/JCO.2015.65.9615. This is the first large tandem mass spectrometry proteomics study that identified a four biomarker panel (ST2, CXCL9, MMP3, and OPN). The panel was measured in 2 independent cohorts of 211 and 180 cGVHD patients as a diagnostic marker for cGVHD. It was also found that this biomarker panel measured at +D100 post-HSCT could serve as a prognostic marker in a cohort of 172 patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46•.Hakim FT, Memon S, Jin P, et al. Upregulation of IFN-inducible and damage-response pathways in chronic graft-versus-host disease. J Immunol. 2016;197(9):3490–3503. doi: 10.4049/jimmunol.1601054. Comprehensive study that showed that circulating monocytes expressed an IFN type I and II transcripts signature, damage response transcripts signature. Further, these signatures were confirmed by high plasma levels of IFN-inducible chemokines (CXCL9 and CXCL10) both in lichenoid and sclerotic cGVHD patients, and decreased blood CXCR3+ T cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abu Zaid M, Wu J, Wu C, et al. Plasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCT. Blood. 2017;129(2):162–170. doi: 10.1182/blood-2016-08-735324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Croudace JE, Inman CF, Abbotts BE, et al. Chemokine-mediated tissue recruitment of CXCR3+ CD4+ T cells plays a major role in the pathogenesis of chronic GVHD. Blood. 2012;120(20):4246–4255. doi: 10.1182/blood-2012-02-413260. [DOI] [PubMed] [Google Scholar]
- 49.Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475. doi: 10.3389/fimmu.2017.00475. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Sullivan S, Gilmer JF, Medina C. Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm. 2015;2015:964131. doi: 10.1155/2015/964131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X, Yue Z, Yu J, et al. Proteomic characterization reveals that MMP-3 correlates with bronchiolitis obliterans syndrome following allogeneic hematopoietic cell and lung transplantation. Am Journal Transplantation: Official Journal Am Soc Transplant Am Soc Transpl Surgeons. 2016;16:2342–2351. doi: 10.1111/ajt.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inamoto Y, Martin PJ, Paczesny S, et al. Association of plasma CD163 concentration with de novo-onset chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017;23(8):1250–1256. doi: 10.1016/j.bbmt.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rice LM, Mantero JC, Stifano G, et al. A proteome-derived longitudinal pharmacodynamic biomarker for diffuse systemic sclerosis skin. J Invest Dermatol. 2017;137(1):62–70. doi: 10.1016/j.jid.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 54•.Du J, Flynn R, Paz K, et al. Murine chronic graft-versus-host disease proteome profiling discovers CCL15 as a novel biomarker in patients. Blood. 2018;131(15):1636–1743. doi: 10.1182/blood-2017-08-800623. The study conducted a tandem mass spectrometry proteomics analysis using a murine multiorgan cGVHD model to identify a cGVHD biomarker (CCL9) and a drug-targetable neutralizing antibody, then its human homolog (CCL15) which was elevated in a cohort of 211 patients, demonstrating that biomarkers discovered through murine proteomics can also serve for the identification of novel cGVHD biomarkers in patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pidala J, Sigdel TK, Wang A, et al. A combined biomarker and clinical panel for chronic graft versus host disease diagnosis. J Pathol Clin Res. 2017;3(1):3–16. doi: 10.1002/cjp2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holtan SG, Khera N, Levine JE, et al. Late acute graft-versus-host disease: a prospective analysis of clinical outcomes and circulating angiogenic factors. Blood. 2016;128(19):2350–2358. doi: 10.1182/blood-2015-09-669846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.She K, Gilman AL, Aslanian S, et al. Altered Toll-like receptor 9 responses in circulating B cells at the onset of extensive chronic graft-versus-host disease. Biol Blood Marrow Transpl. 2007;13(4):386–397. doi: 10.1016/j.bbmt.2006.12.441. [DOI] [PubMed] [Google Scholar]
- 58.Greinix HT, Pohlreich D, Kouba M, et al. Elevated numbers of immature/transitional CD21- B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14(2):208–219. doi: 10.1016/j.bbmt.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Kuzmina Z, Krenn K, Petkov V, et al. CD19(+)CD21(low) B cells and patients at risk for NIH-defined chronic graft-versus-host disease with bronchiolitis obliterans syndrome. Blood. 2013;121(10):1886–1895. doi: 10.1182/blood-2012-06-435008. [DOI] [PubMed] [Google Scholar]
- 60.D’Orsogna LJ, Wright MP, Krueger RG, et al. Allogeneic hematopoietic stem cell transplantation recipients have defects of both switched and IgM memory B cells. Biol Blood Marrow Transplant. 2009;15(7):795–803. doi: 10.1016/j.bbmt.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 61.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113(16):3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dander E, Balduzzi A, Zappa G, et al. Interleukin-17-producing T-helper cells as new potential player mediating graft-versus-host disease in patients undergoing allogeneic stem-cell transplantation. Transplantation. 2009;88(11):1261–1272. doi: 10.1097/TP.0b013e3181bc267e. [DOI] [PubMed] [Google Scholar]
- 63.Zhao XY, Lv M, Xu LL, et al. Donor Th17 cells and IL-21 may contribute to the development of chronic graft-versus-host disease after allogeneic transplantation. Eur J Immunol. 2013;43(3):838–850. doi: 10.1002/eji.201242816. [DOI] [PubMed] [Google Scholar]
- 64.Malard F, Bossard C, Brissot E, et al. Increased Th17/Treg ratio in chronic liver GVHD. Bone Marrow Transplant. 2014;49(4):539–544. doi: 10.1038/bmt.2013.215. [DOI] [PubMed] [Google Scholar]
- 65.Forcade E, Paz K, Flynn R, et al. An activated Th17-prone T cell subset involved in chronic graft-versus-host disease sensitive to pharmacological inhibition. JCI Insight. 2017;2(12) doi: 10.1172/jci.insight.92111. pii: 92111 Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greinix HT, Kuzmina Z, Weigl R, et al. CD19+CD21low B cells and CD4+CD45RA+CD31+ T cells correlate with first diagnosis of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2015;21(2):250–258. doi: 10.1016/j.bbmt.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4 +CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matsuoka K, Kim HT, McDonough S, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479–1493. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark FJ, Gregg R, Piper K, et al. Chronic graft-versus-host disease is associated with increased numbers of peripheral blood CD4 +CD25high regulatory T cells. Blood. 2004;103(6):2410–2416. doi: 10.1182/blood-2003-06-2073. [DOI] [PubMed] [Google Scholar]
- 70.Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127(5):646–657. doi: 10.1182/blood-2015-10-672345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forcade E, Kim HT, Cutler C, et al. Circulating T follicular helper cells with increased function during chronic graft-versus-host disease. Blood. 2016;127(20):2489–2497. doi: 10.1182/blood-2015-12-688895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirakawa M, Matos TR, Liu H, et al. Low-dose IL-2 selectively activates subsets of CD4+ Tregs and NK cells. JCI Insight. 2016;1(18):e89278. doi: 10.1172/jci.insight.89278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huenecke S, Cappel C, Esser R, et al. Development of three different NK cell subpopulations during immune reconstitution after pediatric allogeneic hematopoietic stem cell transplantation: prognostic markers in GVHD and viral infections. Front Immunol. 2017;8:109. doi: 10.3389/fimmu.2017.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kariminia A, Ivison S, Ng B, et al. CD56(bright) natural killer regulatory cells in filgrastim primed donor blood or marrow products regulate chronic graft-versus-host disease: the Canadian Blood and Marrow Transplant Group randomized 0601 study results. Haematologica. 2017;102(11):1936–1946. doi: 10.3324/haematol.2017.170928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skert C, Damiani D, Michelutti A, et al. Kinetics of Th1/Th2 cytokines and lymphocyte subsets to predict chronic GVHD after allo-SCT: results of a prospective study. Bone Marrow Transplant. 2009;44(11):729–737. doi: 10.1038/bmt.2009.80. [DOI] [PubMed] [Google Scholar]
- 76.Stikvoort A, Chen Y, Radestad E, et al. Combining flow and mass cytometry in the search for biomarkers in chronic graft-versus-host disease. Front Immunol. 2017;8:717. doi: 10.3389/fimmu.2017.00717. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Botari CME, Nunes AJF, De Souza MP, et al. Oral chronic graft-versus-host disease: analysis of dendritic cells subpopulations. An Bras Dermatol. 2014;89(4):632–637. doi: 10.1590/abd1806-4841.20142464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waller EK, Rosenthal H, Sagar L. DC2 effect on survival following allogeneic bone marrow transplantation. Oncology (Williston Park) 2002;16(1 Suppl 1):19–26. [PubMed] [Google Scholar]
- 79.Chan GW, Gorgun G, Miller KB, et al. Persistence of host dendritic cells after transplantation is associated with graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(3):170–176. doi: 10.1053/bbmt.2003.50006. [DOI] [PubMed] [Google Scholar]
- 80.Arpinati M, Chirumbolo G, Marzocchi G, et al. Increased donor CD86+CD14+ cells in the bone marrow and peripheral blood of patients with chronic graft-versus-host disease. Transplantation. 2008;85(12):1826–1832. doi: 10.1097/TP.0b013e3181788a84. [DOI] [PubMed] [Google Scholar]
- 81.Khleif SN, Doroshow JH, Hait WN. AACR-FDA-NCI Cancer Biomarkers Collaborative consensus report: advancing the use of biomarkers in cancer drug development. Clin Cancer Res. 2010;16(13):3299–3318. doi: 10.1158/1078-0432.CCR-10-0880. [DOI] [PubMed] [Google Scholar]
- 82.Dubovsky JA, Flynn R, Du J, et al. Ibrutinib treatment ameliorates murine chronic graft-versus-host disease. J Clin Invest. 2014;124(11):4867–4876. doi: 10.1172/JCI75328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schutt SD, Fu J, Nguyen H, et al. Inhibition of BTK and ITK with ibrutinib is effective in the prevention of chronic graft-versus-host disease in mice. PloS One. 2015;10(9):e0137641. doi: 10.1371/journal.pone.0137641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miklos D, Cutler CS, Arora M, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243–2250. doi: 10.1182/blood-2017-07-793786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flynn R, Du J, Veenstra RG, et al. Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood. 2014;123(25):3988–3998. doi: 10.1182/blood-2014-03-562231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Allen JL, Tata PV, Fore MS, et al. Increased BCR responsiveness in B cells from patients with chronic GVHD. Blood. 2014;123(13):2108–2115. doi: 10.1182/blood-2013-10-533562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leonhardt F, Zirlik K, Buchner M, et al. Spleen tyrosine kinase (Syk) is a potent target for GvHD prevention at different cellular levels. Leukemia. 2012;26(7):1617–1629. doi: 10.1038/leu.2012.10. [DOI] [PubMed] [Google Scholar]
- 88.Le Huu D, Kimura H, Date M, et al. Blockade of Syk ameliorates the development of murine sclerodermatous chronic graft-versus-host disease. J Dermatol Sci. 2014;74(3):214–221. doi: 10.1016/j.jdermsci.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 89.Flynn R, Allen JL, Luznik L, et al. Targeting Syk-activated B cells in murine and human chronic graft-versus-host disease. Blood. 2015;125(26):4085–4094. doi: 10.1182/blood-2014-08-595470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, et al. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114(24):4919–4927. doi: 10.1182/blood-2008-10-161638. [DOI] [PubMed] [Google Scholar]
- 91.Teshima T. JAK inhibitors: a home run for GVHD patients? Blood. 2014;123(24):3691–3693. doi: 10.1182/blood-2014-04-570325. [DOI] [PubMed] [Google Scholar]
- 92.Zeiser R, Burchert A, Lengerke C, et al. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Herrera AF, Kim HT, Bindra B, et al. A phase II study of bortezomib plus prednisone for initial therapy of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20(11):1737–1743. doi: 10.1016/j.bbmt.2014.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pai CC, Chen M, Mirsoian A, et al. Treatment of chronic graft-versus-host disease with bortezomib. Blood. 2014;124(10):1677–1688. doi: 10.1182/blood-2014-02-554279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rimkus TK, Carpenter RL, Qasem S, et al. Targeting the Sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel) 2016;8:2. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zerr P, Palumbo-Zerr K, Distler A, et al. Inhibition of hedgehog signaling for the treatment of murine sclerodermatous chronic graft-versus-host disease. Blood. 2012;120(14):2909–2917. doi: 10.1182/blood-2012-01-403428. [DOI] [PubMed] [Google Scholar]
- 97.Furlan SN, Watkins BK, Mortari A, et al. Defining the primate t cell transcriptome during GVHD: new data implicating Sonic hedgehog and aurora kinase pathways in GVHD pathogenesis and prevention. Biol Blood Marrow Transplant. 2015;21(2):S56. [Google Scholar]
- 98.DeFilipp Z, Nazarian RM, El-Jawahri A, et al. Phase 1 study of the Hedgehog pathway inhibitor sonidegib for steroid-refractory chronic graft-versus-host disease. Blood Advances. 2017;1(22):1919–1922. doi: 10.1182/bloodadvances.2017011239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 100.Chinen T, Kannan AK, Levine AG, et al. An essential role for the IL-2 receptor in Treg cell function. Nat Immunol. 2016;17(11):1322–1333. doi: 10.1038/ni.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beres AJ, Drobyski WR. The role of regulatory T cells in the biology of graft versus host disease. Front Immunol. 2013;4:163. doi: 10.3389/fimmu.2013.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Koreth J, Kim HT, Jones KT, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128(1):130–137. doi: 10.1182/blood-2016-02-702852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra143. doi: 10.1126/scitranslmed.3005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kuna P, Jenkins M, O’Brien CD, et al. AZD9668, a neutrophil elastase inhibitor, plus ongoing budesonide/formoterol in patients with COPD. Respir Med. 2012;106(4):531–539. doi: 10.1016/j.rmed.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 106.Stockley R, De Soyza A, Gunawardena K, et al. Phase II study of a neutrophil elastase inhibitor (AZD9668) in patients with bronchiectasis. Respir Med. 2013;107(4):524–533. doi: 10.1016/j.rmed.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 107.Elborn JS, Perrett J, Forsman-Semb K, et al. Efficacy, safety and effect on biomarkers of AZD9668 in cystic fibrosis. Eur Respir J. 2012;40(4):969–976. doi: 10.1183/09031936.00194611. [DOI] [PubMed] [Google Scholar]
- 108.Im A, Hakim FT, Pavletic SZ. Novel targets in the treatment of chronic graft-versus-host disease. Leukemia. 2017;31(3):543–554. doi: 10.1038/leu.2016.367. [DOI] [PubMed] [Google Scholar]
- 109.Flynn R, Paz K, Du J, et al. Targeted Rho-associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3-dependent mechanism. Blood. 2016;127(17):2144–2154. doi: 10.1182/blood-2015-10-678706. [DOI] [PMC free article] [PubMed] [Google Scholar]