Abstract
Despite the technological improvements in orthopedic joint replacement implants, wear and corrosion products associated with the metal components of these implants may result in adverse local tissue and perhaps systemic reactions and toxicities. The current review encompasses a literature review of the local and systemic toxicity studies concerning the effect of CoCrMo wear debris released from wear and corrosion of orthopedic implants and prostheses. Release of metallic debris is mainly in the form of micro- and nano-particles, ions of different valences, and oxides composed of Co and Cr. Though these substances alter human biology, their direct effects of these substances on specific tissue types remain poorly understood. This may partially be the consequence of the multivariate research methodologies employed, leading to inconsistent reports. This review proposes the importance of developing new and more appropriate in-vitro methodologies to study the cellular responses and toxicity mediated by joint replacement wear debris in-vivo.
Keywords: Total joint replacement, Wear particles, CoCrMo, Toxicity, Systemic, Hip-simulator
Joint replacement surgery is the standard treatment option for patients with end-stage joint diseases.1–4 The most commonly replaced joint is the knee, followed by the hip. Currently, around 300,000 Total Hip Replacements (THRs) are annually performed in US, and this is expected to increase to 572,000 by 2030.5 Table S1 (see supplementary data) describes different joint arthroplasties and the materials used for manufacturing these implants.
A modern hip prosthesis is a modular system consisting of a femoral and acetabular component (similar to a ball (head) and socket (cup) joint). Subsequently, these can be assembled from two or more components each. Commonly, the femoral component is constructed of a stem and a head. The elongated part of the stem will be inserted in femoral stem and top part has a tapered neck that connects with the head (ball). The neck and head connections are known as modular taper junctions. Likewise, the acetabular component is modular (consisting of multiple interchangeable parts). A typical acetabular modular construct will include a metal shell perhaps fixed to the pelvis bone with screws, and a polyethylene liner that articulates with the femoral head. Currently, the components of artificial hip replacements include highly cross-linked ultra-high molecular weight polyethylene, cobalt chromium molybdenum alloys, titanium alloys, zirconium–niobium alloy, stainless steel, tantalum, ceramics, or metal–ceramic structures.6 The manufacturing methods and materials used to make these devices have been heterogeneous. The metals employed in these device components can be either cast, wrought, or 3D printed. Different combinations of materials have been employed at the articulation (head and cup), such as the metal-on-metal (MOM) hip implants introduced in 1950s, and metal-on-polyethylene (MOP) implants introduces during the 1960s. Many MOM devices were recalled due to a reported high rate of implant failures as indicated by revision surgery numbers.7,8 MOP implants are currently in use with uncertain limitations on their long-term survival. Ceramic on metal (COM), ceramic on ceramic (COC) and ceramic on polyethylene (COP) are other combinations available in the market. An emerging issue is the morbidity of biological and immunological responses9 caused by implant degradation products.
Irrespective of the nature of the bearing couple (MOP, MOM, COC or COP), the degradation products released from the implants, in the form of metal, polymer or ceramic nano- and micro-particles, and metal ions, are disseminated into the surrounding tissue as well as to remote locations in the body. The adverse effects of these processes are becoming apparent and were critical to the failure of many MOM designs.6,9–11 Substantial scientific evidence has been reported regarding the toxicity of these metal particles and ions by in vitro and in vivo studies.12–19 In-depth studies regarding the toxicity mechanisms of the independent metal particles with regard to fibroblasts,12,15 macrophages,20,21 lymphocytes,13 and osteoblasts22 have been reported. In addition, toxicity of the degradation products from different types of prosthetic component constructs, such as Co, Cr, Ti, Al, V, polymeric and ceramic debris has been studied by toxicologists, pathologists, and clinical researchers.17,23,24
Considering the increase in number of total joint replacement (TJR) surgeries to date, as well as thousands of new surgeries anticipated to be performed in future, the risk of toxicity from corrosion and wear particles is of great concern. Also, since there is a trend for younger patients to undergo joint replacement, this increases the expectation for implant manufacturers to develop implant components with enhanced performance and durability leading to a low risk of periprosthetic as well as systemic toxicity over the time.
From the robust evidence observed by different researchers, it was established that the degradation products (DPs) of the implants materials are complex, and they may present as metal–protein complexes, free metallic ions, inorganic metal salts or oxides and as organic storage form such as hemosiderin.11 In addition, there were substantial studies clearly depicting that the bioactivity of the DPs varies based on the physicochemical characteristics, which in turn vary depending on the technique adopted to generate the particles for particular studies.25–29 The particles characterized from the peri-prosthetic tissues of retrieved implants vary in their geometrical shapes and dimensions. These particles may be round, oval, needle, spike, etc., of varying sizes.26,30–33 However, there are disparities in the analysis of physicochemical characteristics of the degradation products from tissue samples due to the variation in isolation methodologies adopted by the researchers. Different isolation techniques have specific impact on the characteristics of the particles. Hence, the disparities on the reported physicochemical characteristics of the DPs and the toxicity associated with them are not consistent. It is important to consider the changes in characteristics of these products which occur during their transportation through the circulatory systems before reaching the site where they induce toxicity. Scharf et al demonstrated the macrophage mediated mechanism of formation of ions from Co and Cr nanoparticles. The study also suggested that several cellular pathways and functions could be compromised due to the interaction of metal ions with several important cellular proteins.34 Evaluation of such evolution of the wear products is highly complex. Moreover, unfortunately, some of the reported studies have simulated degradation products using commercially available materials, which may be very far from the chemical states of the in vivo DPs generated from joint replacement implants. This may lessen the relevance of such toxicology studies. Recently, in vitro hip-simulator studies provided substantial evidence to prove that the debris generated using serum (simulated synovial fluid) has similar characteristics to that of patient tissue samples.31
This review focuses on the overall evaluation of cobalt–chromium–molybdenum alloy (CoCrMo) implant degradation, the characteristics of the wear products, in vivo and in vitro studies concerning the interaction of CoCrMo wear debris with different cellular environments and the potential toxicity in the human body. The study also offers some understanding of the challenges and perspectives of implant wear debris-mediated toxicity studies.
Search strategy
A Medline bibliographical search (from 1988 up to 2017) was carried out. The following search items were explored: “metal implant” AND “wear”, “wear” AND “toxicity”, “CoCr wear particles” AND “toxicity”, “Co/Cr ions” AND “toxicity”, “Co/Cr particles” AND “cell toxicity”, “genotoxicity” AND “metal particles/ions”, “toxicity mechanism” AND “metal particles”, “clinical reports”, AND “hip implant”, “neurotoxicity”, “DNA damage AND metals”, “Cardiotoxicity AND metals” OR “renal toxicity” AND “metals”, liver toxicity” AND “metals”. The inclusion criteria were: in vitro studies; meta-analysis; randomized controlled trials; perspective cohort studies published in English. The search was limited to total hip replacements and cobalt CoCrMo-based orthopedic devices. Nano- and micro-particles composed of cobalt and chromium were also included although particles mixed with other metals or components were excluded in this study. Articles not involving of toxicity of cobalt–chromium debris were also excluded. The title and abstract of the identified articles were preliminarily evaluated based on the inclusion criteria. The evaluation of the appropriateness of articles was independently carried out by of the authors (Bijukumar and Segu). The search resulted in 220 papers; 37 did not fit the inclusion criteria resulting in 183 articles, which were selected for review for this report.
Degradation of structural materials
CoCrMo, Ti and its alloys and stainless steel (SS) are commonly used metals in implant design. Each of these materials has its advantages and disadvantages. CoCrMo alloys are known for their rigidity and long-term corrosion resistance, but one of the major disadvantages of this metal is the cost of fabrication because of its high rigidity.35 On the other hand, Ti alloys are of relatively low density and show excellent corrosion resistance and biocompatibility. However, Ti alloys have a relatively low shear strength, low wear resistance and high cost. Lastly, stainless steel has a relatively high elastic modulus and good corrosion and fatigue resistance in short term application, but tends to corrode in long-term application. In addition, it has a relatively high proportion of Ni, which is potentially immunogenic, thereby making it less desirable in permanent implant applications. An ideal implant material should have the modulus elasticity of bone, high corrosion and wear resistance and excellent biocompatibility. To attain the maximum efficiency and durability of an implant the manufacturers use different materials for different parts of an implant. For example, CoCrMo is used for the femoral head in total hip replacements and the femoral component of total knee replacements as it has excellent strength and wear resistance. The femoral stem of total hip replacements and the tibial tray of total knee replacements are typically fabricated from Ti-alloy (high strength with a modulus of elasticity closer to bone) and finally the acetabular cup and the tibial articulating surface are made of UHMWPE. Hence, CoCrMo alloy is widely used in both total hip and total knee replacments, which makes the research on toxicity of CoCrMo degradation products more imperative for patient safety and implant longevity.
Corrosion plays a major role in the release of metal ions,36 however, both wear (mechanical) and corrosion (chemical) act synergistically (tribocorrosion) in the presence of protein rich synovial fluid. This interaction results in the generation of complex degradation products. When two metals come in contact with each other and undergo tribological process (sliding or fretting), wear debris will be released from their interface. These are mechanical wear particles or corrosion products and/or metal ions. In general, tribocorrosion is an irreversible process resulting in transformation/degradation of the material with a resultant change in the mechanical function of the device.37 This is due to the synergistic interaction of sliding, abrasion, fretting, crevice and galvanic corrosion mechanisms leading to the mechanical alteration of the implant.38
Tribocorrosion behavior mainly depends on (i) the properties of the contacting materials, (ii) the mechanics of the tribological contact, and (iii) the physicochemical properties of the environment. These aspects are strongly interrelated—either synergistic or antagonistic, which can have beneficial or deleterious influence over the performance of the tribological system.38
In a femoral component of a hip implant, there are three interfaces; head–cup (sliding–corrosion),37–39 head–neck or trunnion (modular junction–galvanic corrosion, fretting– corrosion)40,41 and stem–bone (fretting–corrosion)42 (Figure 1). In an ideal implant, the head–cup interface should have smooth tribological motion, produce no debris and be biologically inert. The other two interfaces should not have any movement and should be biologically inert. However, these interfaces may experience micro-motion during the in vivo performance of the implant (physical activities under loading), which finally results in fretting–corrosion process. Fretting–corrosion is a type of tribocorrosion phenomenon that occurs during repeated cyclic micro-motion. This can lead to severe damage on the contacting surfaces.43 Recently, there have been concerning clinical reports regarding the performance of modular junctions.44–48 In fact, all component interfaces can lead to generation of wear particles and/ or metal ions. In certain cases local, and possibly systemic, biological reactions have occurred resulting in pain and local tissue damage that require revision surgery.9,11,16,49
Figure 1.
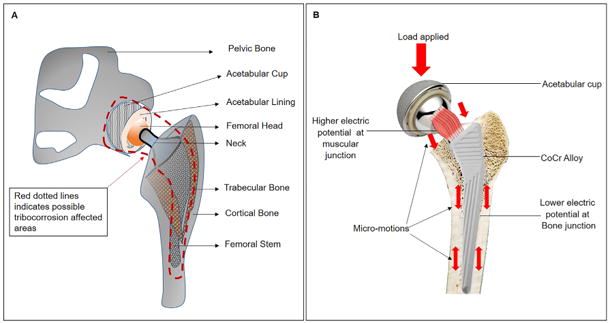
(A) Components of a total hip implant. Red dotted line indicating the area around the implant with possibilities of tribocorrosion. (B) Showing the sites of micromotion on a total hip implant during physical activity. The load generated at the head region will transferred to the stem through neck and possible site of micromotion during physical activity are clearly spotted with direction of micromotion (red arrows).
When sliding/fretting occurs in the setting of a crevice geometry (as is present at the head/neck junction of a femoral component of a total hip replacement), a synergistic degradation by wear and corrosion takes place to cause metal ion and particle release at an unexpected high level.39 Wear process can be accelerated based on the corrosion processes such as pitting50 or intergranular corrosion.47,51–53 This is directly related to the properties of the material and the alloy microstructure.6,37,50 Crevice corrosion can occur at any implant interface where the gap between the contacting surface is very minimum, leading to oxygen depletion and low pH levels creating a highly acidic environment.54,55
Degradation products (DPs) are generated and released from orthopedic implants and prostheses due to wear and corrosion during daily functional performance.56 Major components of the complex degradation products (DPs) are wear particles composed of polymers, ceramics or metals modified by the environment.11 The release of metallic debris from CoCrMo-based prostheses (weight percentage compositions are approximately 68% Co, 28% Cr and 7% Mo) is mainly in the form of micro/nanoparticles and metal ions of different valences and reactivities. These particles can cause adverse local tissue reactions (ALTR, which includes aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL), necrosis, osteolysis and so-called pseudotumors) and systemic toxicity (including cardiomyopathy, polycythemia, hypothyroidism, and neurological disorders).57–64 The bioactivity of the nano-scale metal particles is higher than that for micro-sized due to the large surface area per mass.24,65 According to Doorn et al, the wear rate of MOM articulation is approximately 20 times lower than that of MOP articulation. Also, the released metal particles are significantly smaller than polyethylene particles.30
Toxicity to peri-prosthetic tissues
After THR surgery using CoCrMo-based orthopedic prosthesis, an increase in the level of Co and Cr ions in serum and synovial fluid66 has been reported. Periprosthetic tissues become potential sites of local toxicity due in part to the presence of wear particles released from the prosthetic materials. The degradation products (DPs) generated from the implant due to wear and corrosion cause an ALTR mediated by monocytes, macrophages, and lymphocytes as illustrated in Figure 2. Ricciardi et al described macrophage predominant, mixed lymphocytic–macrophagic cell infiltrates with or without hypersensitivity reactions, and with sarcoid-like granulomas.67 The wear debris activate endothelial cells at the implant–tissue interfaces and in turn express the adhesion molecules such as P-selectin, ICAM-1, VCAM and CD44.68–70 Granulocytes, macrophages, monocytes and lymphocytes are attracted by these adhesion molecules towards the tissue interfaces. Macrophages activated by the phagocytosis of the wear particles trigger the secretion of pro- inflammatory cytokines (IL-8, IL1-β, IL-6), chemokines, growth factors, prostaglandins, degradative enzymes, reactive oxygen species and other factors.71–75 Additionally, multinucleated giant cells and osteoclasts generated by the fusion of the macrophages, adhere to the metal surface of the bone-metal interface, triggering osteolysis which may lead to aseptic loosening of the implant.76–79
Figure 2.
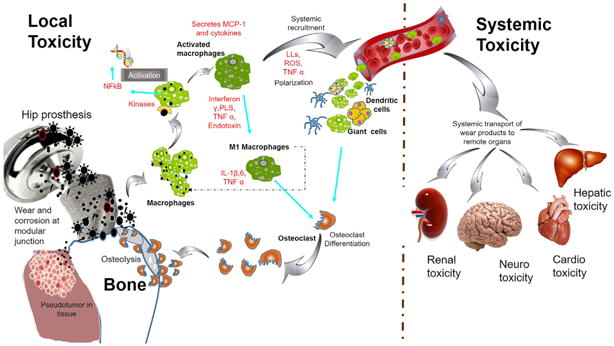
Schematic of adverse tissue reaction cascade at peri-prosthetic tissue region and systemic toxicity leading to osteolysis. Wear particles generated from implant, particularly from the modular junctions induce macrophages mediated inflammatory reactions. The wear debris uptaken by the macrophages via phagocytosis, diffusion and membrane mediated transport mechanism will activate periprosthetic macrophages and lead to systemic recruitment and polarization of systemic macrophages, giant cells and dendritic cells towards periprosthetic region. Particulate debris via circulatory system can cause toxicity to distal organs.
Debris induced aseptic loosening and osteolysis depends on the physico-chemical characteristics of the tribocorrosion debris, which in turn influenced by the chemical composition of the prosthesis.80 According to Doorn et al, the size of the CoCr wear particles isolated from surrounding tissues of the failed implant is in the range of 6-834 nm in the case of MOM implant.30 However, the size CoCr wear particles may vary from 0.07 to 12.2 μm in the case of MOP implants.31,33 Moreover, Davies et al reported that the inflammation pattern exhibited by the anatomical layers of neocapsule formed around the implant varies with type of articulation.81 More ulcerated tissue samples were obtained from MOM hip prosthesis than from other types. Far less surface ulceration was observed on MOP prostheses. Those implants exhibited aseptic loosening with no clear evidence of ulceration and lymphocytic and plasma cells infiltrations and the loosening is mainly attributed to the involvement of histiocytes (a stationary phagocytic cell present in the tissue/organ).81
Another ALTR associated with wear debris is so-called pseudotumor formation. The prevalence of pseudotunor following metal-on- metal THA is 1%-39%.82–85 However, asymptomatic pseudotumor was a supplementary finding in 57%-78% of cases83,86–88 even though THA revision surgery due to symptomatic psuedotumor was only 1.7%-5.6%.86,89 Characteristic features of pseudotumors include lymphocytic infiltration, lymphoid aggregates and prominent necrotic pattern with macrophages. The condition can be destructive and sometimes painful, and patients may require revision surgery. Though the etiology of this abnormal condition is not yet clearly understood, serum levels of Co, and Cr in patients with pseudotumors are elevated in comparison to patients without pseudotumors.90 In turn, elevated metal levels correlate with excessive wear debris generation.
Systemic toxicity of cobalt and chromium
Systemic toxicity of the CoCrMo wear debris is also of conern in orthopedic surgery. Table S2 (See supplementary data) summarizes relevant toxicity studies reported previously. Several clinical reports reveal a clear correlation between CoCr-based hip prosthesis and systemic cobalt toxicity.91–96 In addition, the deposition of the metal wear debris found in distant organs has also been reported.23,92,95,96
Nano-scale metal and polymeric particles or metal ions can circulate systemically via lymphatics to lymph nodes, bone marrow, liver, and spleen. In addition, the metal particles are reported to enter the bloodstream, and concentrated inside the erythrocytes.97 Erythrocytes containing metal then circulate throughout the body and further enhance the cytotoxic, genotoxic and immunological effects.30,95,98
Intracellular transport of metal particles mainly occurs by diffusion or endocytosis through the plasma membrane of the cell and receptor-mediated mechanisms.26 Large sized particles are taken up by the cells via the pinocytosis and phagocytosis processes of macrophages.15 Hence, the debris released by the implants, regardless of their composition, are capable of disseminating into the bloodstream and entering into different tissue compartment depending on the size regime.
The role of cobalt and chromium particles and /or ions in inducing cytotoxicity has been studied using different cell types99–101 as shown in Table S2 (See supplementary data). In vitro studies also demonstrated that Co2+ and Cr3+ ions and nano-particles can induce apoptosis and necrosis and inflammatory responses in macrophages and pneumocytes.14,102 In addition, the Co2+ can influence iron metabolism by binding with apotransferrin, which might affect normal hematopoietic tissue metabolism.103 However, few studies have been reported in relation to implant wear debris and iron metabolism. One study revealed that Co nano-particle at a concentration of 25 and 100 ppm had toxic effect on the human hematopoietic progenitor cells derived colonies.104
Dose-dependent toxicity of cobalt and chromium nano-particles of size at 30-60 nm and their respective ions was investigated for macrophages by Kwon et al. It was found that cobalt nanoparticles at 1 × 1012 particles/ml and Co2+ ions at 1000 μM (589 ppm) concentration exhibited various levels of toxicity to the macrophages whereas Cr3+ failed to produce any toxicity at this concentration. Nevertheless, Catelas and coworkers reported Co2+ and Cr3+ at 8-10 μg/ml (8-10 ppm) and 350-500 μg/ml (350-500 ppm) respectively as toxic concentrations.14,20,105 A significant level of toxicity has also been demonstrated by studies using several different sizes of cobalt and chromium particles as well as ions with different valences (Table S2, See supplementary data). Using commercially available cobalt–chromium wear debris (DePuy international, Leeds, UK), a significant increase in uptake of ions and apoptosis gene up-regulation was demonstrated at concentrations of 0-5 mg/106 in monocyte-like U937 cells.106 More importantly, the study demonstrated clear evidence of an increase in wear particle-mediated toxicity after revision surgery by comparing wear particle toxicity of ion pretreated cells versus that of non-pretreated cells.106,107
Apart from in vitro studies, clinical reports analyzed the relationship between serum metal concentration and circulating immune cells counts and suggested that released Cr3+ ions may cause changes in lymphocyte subpopulations in THR patients.108
Deposition of wear debris in distant organs is another concern. Even though cobalt–chromium alloy particles accumulated within the liver macrophages and kidney,23,109 toxic effect was not detected by histologic analysis.110 However, there is a correlation between wear debris accumulation and upregulation of metalloprotein I/II, which can result in the alteration of xenobiotic metabolism by liver. Despite the low level of immediate toxicity of metal particles in the liver,95,96 in the long-term, there is still a possibility of diffusion of ions and transferring of metal particles to other organs similar to iron deposition. An in-vivo study demonstrated that an intramuscular CoCrMo device, implanted for a period of nine months, resulted in metal accumulation in liver and kidney tissue. This indicated that after long term exposure metal particle and ions are released from an implant even under non-functional conditions.109
Concerning clinical complications, involvement of metals such as Ni, Al, and Co in parkinsonian dementia, dialysis encephalopathy and Alzhemeir's disease111–113 etc. has been reported. Oxidative stress-mediated toxicity of cobalt can be reversible by pretreatment of cells with α-estradiol.114 However, the correlation with patients who had undergone joint replacement was limited. Recently, there have been clinical reports of visual and hearing impairments as well as numbness of the feet.63 For instance, retinal pathology has been linked to a high level of serum cobalt and chromium by several clinical reports.57,62,63,115,116 Clinical studies have also reported polyneuropathy with progressive sensory disturbance and hearing loss with sural nerve biopsy indicating axonopathy and cardiomyopathy.59,64,93,94 Mortality due to cardiac failure with clear evidence of Co deposition into the patient's heart tissue (cardiomyocytes) was reported recently,136 highlighting the need for research in this field.
Genotoxic effect of nano-debris
Genotoxicity as the result of metal debris has also been extensively investigated. Nanoparticles are major constituents of wear debris and hence the understanding of the effect of nanoparticles at the molecular level is very important. Through the evolution of nano-toxicology, several investigators have studied the cellular response to metal particles at the nano-scale, starting from uptake to molecular activity and genotoxicity. Humans have a basic tolerance to various nano-particles at a certain concentration. However, the quantum properties of nano-materials make them unpredictable in developing carcinogenicity via genotoxicity. The major reason for the toxicity is their small size, allowing them to penetrate the cells and different organelles and the capability to generate an excess amount of reactive oxygen species (ROS). The physical and chemical properties of nano-materials cannot be predicted based on the bulk composition and their physicochemical properties will vary according to the surroundings. The consequences of interaction between metallic nano- or micro-particles with intracellular biological components cause oxidative stress, protein conformational changes, mutations and alterations in signaling pathways (Figure 3).117–119
Figure 3.
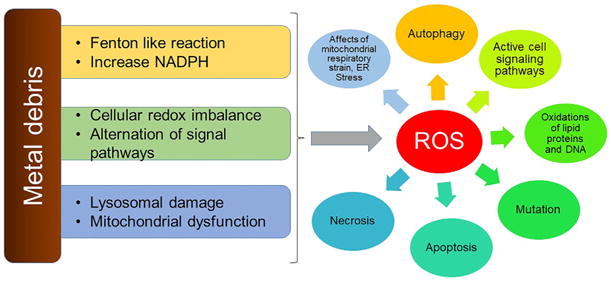
Depicting the ROS mediated cellular toxic mechanism due to metal particles and ions. Metal particles can cause Fenton-like reaction as wells as cellular and molecular changes. Most of the cellular damage is due to ROS species.
In vitro studies have demonstrated that cobalt nano-particles have well-known genotoxic effect. In comparison to chromium, cobalt is considered less toxic, and the mechanism of action of each is critically different. Carcinogenicity was also detected in their ionic form. Several previous studies have shown a strong interest in the mutagenic and genotoxic effects of Co2+ and Cr3+ (Table S2, See supplementary data). The major mechanism of genotoxicity of cobalt includes single strand breaks (Fenton-type reaction),120 cross-linking (topoisomerase poison),121 sister chromatid exchanges, aneuploidy and ineffective DNA repair properties (damage to the zinc-finger domains of repair proteins)122–124 (Figure 4). Colognato et al studied the genotoxicity of cobalt micro-particle at a size scale at 0.1-0.5 μm and showed increases in the tailing of DNA.125 Moreover, single and double stranded breaks mediated by cobalt nano-particles have also been reported in further studies.125,126 In fact, the role of chromium in inducing DNA damage is mainly attributed to the reactive oxygen species once Cr4+ undergoes reduction to Cr3+. Another peculiarity of Cr3+ is its ability to form stable Cr-DNA adducts.127 Also, it can cause DNA damage through single and double stranded breaks as well as cross-linking.128 A comparative investigation of the distinct toxic response of mouse fibroblast to cobalt particles and CoCl2 solution showed that CoCl2 primary caused DNA damage while cobalt particles induced neoplastic transformation and genotoxicity129 (Table S2, See supplementary data). In addition, it was concluded that cobalt particles are more toxic than CoCl2.126,129
Figure 4.
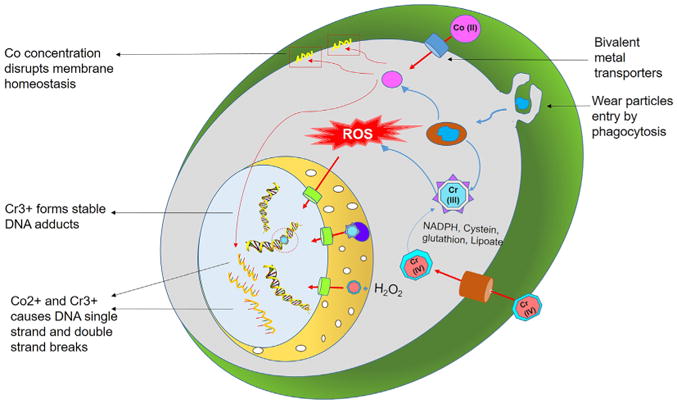
Schematic representation of transport of Co and Cr ions inside the cells and nucleus leads to genotoxicity. The metal ions can cause direct DNA damage by causing single and double stranded breaks in DNA as well as indirect damage via ROS mediated genetic alterations leading to abnormal gene expression.
Peripheral lymphocyte analysis of patients with MOM implants, showed increased lethal and non-lethal aneuploidy and chromosomal translocations130 that correlated with metal ion levels; detectable genetic damage was reduced following revision to a MOP device.131,132 A recent study on the toxicity of soluble (cobalt chloride hexahydrate) and particulate cobalt metal to the lung cells showed that soluble cobalt was more genotoxic than particulate cobalt, even though the isolated intracellular ionic concentrations using ICP-MS were higher in particulate than the soluble form.133 Co2+ and Cr3+ions have the ability to break DNA as reported in several studies (Table S2, See supplementary data).101,134,135
While short-term in vitro investigations into exposure of cells to individual particles or ions have been reported, the long-term effect of particles and ions on cells has not been elucidated. Moreover, apart from ions or particles, evaluation on the toxicity of the metal– protein complexes or with phosphates and sulfates formed during exposure into the in vivo environment needs to be considered.
Current limitations in implant-based toxicity evaluation: challenges and perspectives
The outcome of the toxicity studies of metal debris reported in this review demonstrates the importance and clinical significance of research into the development of new prosthetic devices. Notwithstanding, several aspects related the methodology used to assess degradation products (DPs) of orthopedic implants and prostheses should be meticulously evaluated in order to determine clinical failures pathways. The objective should be the development of standardized physical–chemical characterization techniques for evaluation of the toxicity of prosthetic functional wear debris based on morphology and material composition.
Physicochemical properties of the wear particles will vary with the composition of the implant materials, the extant of degradation processes and possibly the patient's state of health (which may affect the surrounding fluid properties). Attention should be paid to each of these variables in evaluating the toxicity of wear debris (Figure 5). However, elucidation of precise in vivo physicochemical properties of wear debris is difficult due to the limitations in methodologies for isolation and characterization (such as enzymatic, alkaline digestion for LC-MS analysis).
Figure 5.
Summary of different variables influencing the property of wear particles in vivo.
Morphology and content of wear debris
Characterization of metallic wear particulates in the periprosthetic tissues of retrieved MOP and MOM implants demonstrated varying morphologies: round, oval, needle and spike-like.31,32 Moreover, the size of the particles varies significantly based on the type of implant. As reported by Doorn et al, the particles from MOM implants ranged from 6 nm up to 0.83 μm.30 Campbell et al also reported a similar size range, varying from 18 nm up to 0.47 μm. For MOP prostheses, the particle size reported ranged from 7 nm up to 6.3 μm, which is significantly larger than those recorded for MOM.26,33 These investigators emphasized the limitations in the characterization of particles smaller than 0.4 μm, which indicates the apparent inconsistency in observations available in the literature. In addition, the concentration of Co and Cr species in the samples were dissimilar in crystalline and amorphous areas of the particle.30 A high Co content was detected in crystalline areas; high Cr and oxygen content was detected in the amorphous areas. According to Campbell et al, the periprosthetic tissue analysis demonstrated a higher content of CoCr based particles and a lesser amount of Cr oxide particles. There are very few reports addressing the properties of wear particles from synovial fluid. The available reports using different isolation and characterization techniques137,138 show dissimilar material properties. In addition, there are no studies reported clearly depicting the surface area of the wear particles generated in vivo, other than the size measurements described above. Ogunwale et al showed the surface area of 4 nm sized CoCr particles generated by spark discharge method as 185 m2g-1.139 Again, one may question the clinical relevance of such particles. Surface charge of the CoCr wear particles has significant influence on their bioactivity. CoCr particles of 30 nm (generated by pin-on-plate tribometer) and 80 nm (generated by thermal plasma techniques) showed a surface charge of −14 mV and −12 mV respectively. The study also showed differences in metal ion release by particles prepared by these two different methods. Specifically, 80 nm particles generated by thermal spray technique released 7 times more Co and Cr ions in comparison to thermal plasma, even though there was no significant difference in their surface charge. It might be difficult to measure the surface charge of particles from the periprosthetic milieu due to binding of proteins on the surface. It was also speculated by Simoes et al that the strong dissolution of Mo from CoCrMo alloy nanoparticles in the presence of bovine serum albumin (BSA) is due to the similarity of their surface charge to that of the isoelectric point of BSA.140,141 Overall, these studies provide very little and inconclusive information about the surface characteristics of the CoCr wear particles in vivo, leaving a large gap in this research field.
Limitations in the evaluation of physico-chemical properties
There are disparities in reports of the physico-chemical characteristics of the wear particles from patient samples. This is primarily due to methodology chosen for evaluation of the samples. Metal particle analysis is a complex process, with different steps including digestion, isolation, morphological characterization and chemical analysis. Investigators can choose different protocols for each step based on expediency.142 Previous studies have reported different methods to separate and isolate particles for physical–chemical characterization.30,143–146 For example, enzymatic digestion can be accomplished in three different ways, namely: (1) Papain and Proteinase K, (2) Papain and Proteinase K with NaOH wash, (3) Papain and Proteinase K with yeast lytic enzyme and zymolase. Each technique has its own effect on defining the characteristic properties of the material and might lead to disparate results.
Materials commonly used for the some of the previous in vitro studies are summarized in Table S2 (See supplementary data). The majority of studies tested CoCl2, CrCl3, Cr6O2, Cr3O4 or K2Cr2O7 (potassium dichromate) as the source of Co and Cr ions. In addition, commercially available CoCr-based nano- and micro-particles were also used to evaluate the cytotoxicity of wear particles from orthopedic implants and prostheses. The above-mentioned particles from different sources have varying degrees of clinical relevance. There is no standard method for generating particles for in-vitro and in-vivo studies. To accurately model the biological impact of wear debris, it is important to simulate the in vivo degradation products, both particles and ions, as closely as possible. Moreover, it is understood that wear debris in vivo may consist of metal–protein complexes, free metallic ions, inorganic metal salts or oxides and as organic storage form (e.g. hemosiderin11). In fact, the bioactivity of the degradation products varies significantly based on their physicochemical properties and biological environment.25–29 However, previous reports have not considered these facts when performing toxicity evaluation within their experimental designs. Instead, commercially available metal particles were used to study the toxic effect of wear debris. For example, some reported studies addressed the toxicity of CoCr alloy particles (metal ion valence: Co2+, Cr3+, Cr6+) and Ti ions in vitro on fibroblasts,12,147 macrophages,20,21 lymphocytes,13 osteoblasts22 and osteoclasts.22 However, the particles used in these studies may have very different surface properties than the wear particles generated in vivo.148–153 Nonetheless, those studies are a helpful first-order approximation. The toxicity of wear debris in vivo is a cumulative effect of all the different forms of debris that are generated. In addition, the toxicity of the organometallic species154 generated in the synovial fluid may be unique (and possibly less toxic) than its ionic form. It was also reported that the albumin can enhance the dissolution of Co ions from wear debris.155 These findings suggest a fruitful area of investigation that is currently poorly understood.
Second generation of toxicity evaluation
To potentially mimic the in vivo joint conditions, 2nd generation toxicity evaluation researchers used a hip wear simulator for generating wear debris. Many studies have shown that in vitro simulator testing is an accurate methodology to predict the performance of joint replacements.154,156–158 Moreover, Catelas et al carried out a comparative evaluation of the physicochemical properties of wear particulate extracted from tissue samples of patients who underwent CoCrMo implant revision surgery in comparison to wear particles generated from the hip simulator. It was revealed that the particles generated in the simulator were comparable to those found in the periprosthetic patient samples with regards to the chemical composition, size and shape.159 However, the characterstics of wear particles generarated from pin-on-disk/plate may be influenced by the contact bearing surfaces and the testing lubricant solution.31 Researchers in this field are now aware of the pitfalls in the physical–chemical characterization methodologies used to isolate and quantify particles from the simulator as well as from patient tissue samples. In addition, there are very in limited in vivo studies utilizing particles from hip simulators with clinically relevant joint fluid for toxicity evaluation.160 Therefore, the reported studies on the wear debris generated from hip simulator may have limited applicability to the actual in vivo scenario.
The type of cells used for toxicity evaluation is equally important. Generally, immortalized cells are considered for such evaluations due to their unlimited number of cell division to grow in unlimited quantities. However, the use of immortalized cells for such investigations has been questioned due to their genetic modification, which alters their morphological, molecular and phenotypic characteristics in comparison to native cells.161 In short, the major challenges in the current cytotoxicity studies of CoCrMo wear particles include i) particle source, ii) physico-chemical properties, iii) dosage, iv) static nature of the in vitro cell culture studies compared to the dynamic nature of the in vivo scenario, and iv) type of cells used for toxicity studies. In order to address these challenges, a uniform in vitro methodology, optimally simulating in vivo conditions, should be established to study the cellular responses and toxicity caused by wear debris.
Important points that need to be considered before undertaking toxicity studies of wear debris include: 1) using clinically relevant dosing, preferably with wear debris generated in a hip simulator with simulated joint fluids; 2) using wear debris with clinically relevant physico-chemical properties; and 3) using clinically relevant primary tissue specific cells.
Concluding remarks
The purpose of this review was to summarize the present status of the systemic and local biological response to implant wear debris, with special emphasis on the toxicology of CoCr alloy wear debris. In vitro studies addressing the effect of metal particles and ions on different cells/cell lines are numerous, although the experimental methodology varies substantially in the extant literature. While previous studies have provided a good basis for the understanding of the mechanism of action for various degradation products, due to the non-uniformity of the methodologies used in these studies there are many gaps in our knowledge. In addition, the cumulative effects of the products of wear and corrosion may be different from their individual components in terms of both the mechanism of action and the intensity of the response. To develop an accurate risk assessment of wear debris in implant patients, experimental models should be developed using wear debris, cells, culture media and environmental milieu which more closely simulate in vivo conditions.
Supplementary Material
Acknowledgments
The authors acknowledge support from Blazer foundation for the Regenerative Medicine and Disability lab at Department of Biomedical Sciences, UIC College of Medicine at Rockford and National Institutes of Health R01 grant (R01 AR070180 (M.T. Mathew, R. Pourzal, H. Lundberg PIs)).
Footnotes
Conflict of interests: The authors confirm that there is no conflict of interest
Appendix A. Supplementary data: Supplementary data to this article can be found online at https://doi.org/10.1016/j.nano.2018.01.001.
References
- 1.Jones CA, Beaupre LA, Johnston DWC, Suarez-Almazor ME. Total joint arthroplasties: Current concepts of patient outcomes after surgery. Clin Geriatr Med. 2005;21(3 SPEC ISS):527–541. doi: 10.1016/j.cger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Harding P, Holland AE, Delany C, Hinman RS. Do activity levels increase after total hip and knee arthroplasty? Clin Orthop Relat Res. 2014;472(5):1502–1511. doi: 10.1007/s11999-013-3427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzgerald JD, Orav EJ, Lee TH, Marcantonio ER, Poss R, Goldman L, et al. Patient quality of life during the 12 months following joint replacement surgery. Arthritis Care Res. 2004;51(1):100–109. doi: 10.1002/art.20090. [DOI] [PubMed] [Google Scholar]
- 4.Wanderling C, Liles J, Finkler E, Carlsgaard P, Hopkinson W, Guler N, et al. Dysregulation of tissue factor, thrombin-activatable fibrinolysis inhibitor, and fibrinogen in patients undergoing total joint arthroplasty. Clin Appl Thromb Hemost. 2017;23(8):967–972. doi: 10.1177/1076029617700998. 1076029617700998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maradit Kremers H, Larson DR, Crowson CS, Kremers WK, Washington RE, Steiner CA, et al. Prevalence of total hip and knee replacement in the United States. J Bone Joint Surg Am. 2015;97(17):1386–1397. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao Y, Hoffman E, Wimmer M, Fischer A, Jacobs J, Marks L. CoCrMo metal-on-metal hip replacements. Phys Chem Chem Phys. 2013;15(3):746–756. doi: 10.1039/c2cp42968c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 8.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(7):1614–1620. doi: 10.2106/JBJS.H.01220. [DOI] [PubMed] [Google Scholar]
- 9.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67(2):182–188. [PubMed] [Google Scholar]
- 10.Hallab NJ. A review of the biologic effects of spine implant debris: Fact from fiction. SAS J. 2009;3(4):143–160. doi: 10.1016/j.esas.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs JJ, Hallab NJ, Skipor AK, Urban RM. Metal degradation products: a cause for concern in metal-metal bearings? Clin Orthop. 2003;(417):139–147. doi: 10.1097/01.blo.0000096810.78689.62. [DOI] [PubMed] [Google Scholar]
- 12.Madathil BK, Lin Q, Hew CL, Mohanty M. Hypoxia-like effect of cobalt chromium alloy micro particles on fibroblasts in vitro. J Orthop Res. 2010;28(10):1360–1367. doi: 10.1002/jor.21133. [DOI] [PubMed] [Google Scholar]
- 13.Akbar M, Brewer JM, Grant MH. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J Immunotoxicol. 2011;8(2):140–149. doi: 10.3109/1547691X.2011.553845. [DOI] [PubMed] [Google Scholar]
- 14.Catelas I, Petit A, Vali H, Fragiskatos C, Meilleur R, Zukor DJ, et al. Quantitative analysis of macrophage apoptosis vs necrosis induced by cobalt and chromium ions in vitro. Biomaterials. 2005;26(15):2441–2453. doi: 10.1016/j.biomaterials.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Papageorgiou I, Yin Z, Ladon D, Baird D, Lewis AC, Sood A, et al. Genotoxic effects of particles of surgical cobalt chrome alloy on human cells of different age in vitro. Mutat Res. 2007;619(1–2):45–58. doi: 10.1016/j.mrfmmm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Samelko L, Landgraeber S, McAllister K, Jacobs J, Hallab NJ. Cobalt alloy implant debris induces inflammation and bone loss primarily through danger signaling, not TLR4 activation: implications for DAMP-ening implant related inflammation. PLoS One. 2016;11(7):e0160141. doi: 10.1371/journal.pone.0160141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zijlstra WP, Bulstra SK, van Raay JJAM, van Leeuwen BM, Kuijer R. Cobalt and chromium ions reduce human osteoblast-like cell activity in vitro, reduce the OPG to RANKL ratio, and induce oxidative stress. J Orthop Res. 2012;30(5):740–747. doi: 10.1002/jor.21581. [DOI] [PubMed] [Google Scholar]
- 18.Abudayyak M, Altincekic Gurkaynak T, Özhan G. In vitro toxicological assessment of cobalt ferrite nanoparticles in several mammalian cell types. Biol Trace Elem Res. 2016;175(2):458–465. doi: 10.1007/s12011-016-0803-3. [DOI] [PubMed] [Google Scholar]
- 19.VanOs R, Lildhar LL, Lehoux EA, Beaulé PE, Catelas I. In vitro macrophage response to nanometer-size chromium oxide particles. J Biomed Mater Res B Appl Biomater. 2014;102(1):149–159. doi: 10.1002/jbm.b.32991. [DOI] [PubMed] [Google Scholar]
- 20.Catelas I, Petit A, Zukor DJ, Antoniou J, Huk OL. TNF-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro-qualitative analysis of apoptosis. Biomaterials. 2003;24(3):383–391. doi: 10.1016/s0142-9612(02)00351-4. [DOI] [PubMed] [Google Scholar]
- 21.Kwon YM, Xia Z, Glyn-Jones S, Beard D, Gill HS, Murray DW. Dose-dependent cytotoxicity of clinically relevant cobalt nanoparticles and ions on macrophages in vitro. Biomed Mater. 2009;4(2):025018. doi: 10.1088/1748-6041/4/2/025018. [DOI] [PubMed] [Google Scholar]
- 22.Andrews RE, Shah KM, Wilkinson JM, Gartland A. Effects of cobalt and chromium ions at clinically equivalent concentrations after metal-on-metal hip replacement on human osteoblasts and osteoclasts: implications for skeletal health. Bone. 2011;49(4):717–723. doi: 10.1016/j.bone.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Urban RM, Tomlinson MJ, Hall DJ, Jacobs JJ. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty. J Arthroplast. 2004;19(8):94–101. doi: 10.1016/j.arth.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Caicedo MS, Samelko L, McAllister K, Jacobs JJ, Hallab NJ. Increasing both CoCrMo-alloy particle size and surface irregularity induces increased macrophage inflammasome activation in vitro potentially through lysosomal destabilization mechanisms. J Orthop Res. 2013;31(10):1633–1642. doi: 10.1002/jor.22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prokopovich P. Interactions between mammalian cells and nano- or micro-sized wear particles: physico-chemical views against biological approaches. Adv Colloid Interf Sci. 2014;213:36–47. doi: 10.1016/j.cis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Billi F, Campbell P. Nanotoxicology of metal wear particles in total joint arthroplasty: a review of current concepts. J Appl Biomater Biomech. 2010;8(1):1–6. [PubMed] [Google Scholar]
- 27.Bowsher JG, Hussain A, Williams PA, Shelton JC. Metal-on-metal hip simulator study of increased wear particle surface area due to “severe” patient activity. Proc Inst Mech Eng H. 2006;220(2):279–287. doi: 10.1243/09544119JEIM93. [DOI] [PubMed] [Google Scholar]
- 28.Pajarinen J, Mackiewicz Z, Pöllänen R, Takagi M, Epstein NJ, Ma T, et al. Titanium particles modulate expression of Toll-like receptor proteins. J Biomed Mater Res A. 2009;92(4):1528–1537. doi: 10.1002/jbm.a.32495. [DOI] [PubMed] [Google Scholar]
- 29.Mirshafiee V, Kim R, Park S, Mahmoudi M, Kraft ML. Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials. 2016;75:295–304. doi: 10.1016/j.biomaterials.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 30.Doorn PF, Campbell PA, Worrall J, Benya PD, McKellop HA, Amstutz HC. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res. 1998;42(1):103–111. doi: 10.1002/(sici)1097-4636(199810)42:1<103::aid-jbm13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Madl AK, Liong M, Kovochich M, Finley BL, Paustenbach DJ, Oberdörster G. Toxicology of wear particles of cobalt–chromium alloy metal-on-metal hip implants. Part I: physicochemical properties in patient and simulator studies. Nanomedicine. 2015;11(5):1201–1215. doi: 10.1016/j.nano.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Madl AK, Kovochich M, Liong M, Finley BL, Paustenbach DJ, Oberdörster G. Toxicology of wear particles of cobalt–chromium alloy metal-on-metal hip implants. Part II: importance of physicochemical properties and dose in animal and in vitro studies as a basis for risk assessment. Nanomedicine. 2015;11(5):1285–1298. doi: 10.1016/j.nano.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Campbell P, Ma S, Yeom B, McKellop H, Schmalzried TP, Amstutz HC. Isolation of predominantly submicron-sized UHMWPE wear particles from periprosthetic tissues. J Biomed Mater Res. 1995;29(1):127–131. doi: 10.1002/jbm.820290118. [DOI] [PubMed] [Google Scholar]
- 34.Scharf B, Clement CC, Zolla V, Perino G, Yan B, Elci SG, et al. Molecular analysis of chromium and cobalt-related toxicity. Sci Rep. 2014;4 doi: 10.1038/srep05729. srep05729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayan R, editor. Biomedical materials [;Internet]; Boston, MA: Springer US; 2009. [cited 2017 Nov 23]. Available from: http://link.springer.com/10.1007/978-0-387-84872-3. [Google Scholar]
- 36.Udofia IJ, Yew A, Jin ZM. Contact mechanics analysis of metal-on-metal hip resurfacing prostheses. Proc Inst Mech Eng H. 2004;218(5):293–305. doi: 10.1243/0954411041932854. [DOI] [PubMed] [Google Scholar]
- 37.Wimmer MA, Fischer A, Büscher R, Pourzal R, Sprecher C, Hauert R, et al. Wear mechanisms in metal-on-metal bearings: the importance of tribochemical reaction layers. J Orthop Res. 2010;28(4):436–443. doi: 10.1002/jor.21020. [DOI] [PubMed] [Google Scholar]
- 38.Mathew MT, Srinivasa Pai P, Pourzal R, Fischer A, Wimmer MA. Significance of tribocorrosion in biomedical applications: overview and current status. Adv Tribol. 2009;2009:1–12. [Google Scholar]
- 39.Mathew MT, Jacobs JJ, Wimmer MA. Wear-corrosion synergism in a CoCrMo hip bearing alloy is influenced by proteins. Clin Orthop. 2012;470(11):3109–3117. doi: 10.1007/s11999-012-2563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Royhman D, Patel M, Runa MJ, Wimmer MA, Jacobs JJ, Hallab NJ, et al. Fretting-corrosion behavior in hip implant modular junctions: The influence of friction energy and pH variation. J Mech Behav Biomed Mater. 2016;62:570–587. doi: 10.1016/j.jmbbm.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Swaminathan V, Gilbert JL. Fretting corrosion of CoCrMo and Ti6Al4V interfaces. Biomaterials. 2012;33(22):5487–5503. doi: 10.1016/j.biomaterials.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 42.Runa MJ, Mathew MT, Fernandes MH, Rocha LA. First insight on the impact of an osteoblastic layer on the bio-tribocorrosion performance of Ti6Al4V hip implants. Acta Biomater. 2015;12:341–351. doi: 10.1016/j.actbio.2014.10.032. [DOI] [PubMed] [Google Scholar]
- 43.Oladokun A, Pettersson M, Bryant M, Engqvist H, Persson C, Hall R, et al. Fretting of CoCrMo and Ti6Al4V alloys in modular prostheses. Tribol Mater Surf Interfaces. 2015;9(4):165–173. [Google Scholar]
- 44.Kwon YM. Evaluation of the painful dual taper modular neck stem total hip arthroplasty: do they all require revision? J Arthroplast. 2016;31(7):1385–1389. doi: 10.1016/j.arth.2016.01.074. [DOI] [PubMed] [Google Scholar]
- 45.Dimitriou D, Liow MHL, Tsai TY, Leone WA, Li G, Kwon YM. Early outcomes of revision surgery for taper corrosion of dual taper total hip arthroplasty in 187 patients. J Arthroplast. 2016;31(7):1549–1554. doi: 10.1016/j.arth.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Del Balso C, Teeter MG, Tan SC, Lanting BA, Howard JL. Taperosis: does head length affect fretting and corrosion in total hip arthroplasty? Bone Joint J. 2015;97–B(7):911–916. doi: 10.1302/0301-620X.97B7.35149. [DOI] [PubMed] [Google Scholar]
- 47.Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: a retrieval study of 16 cases. J Arthroplast. 2009;24(7):1019–1023. doi: 10.1016/j.arth.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Nassif NA, Nawabi DH, Stoner K, Elpers M, Wright T, Padgett DE. Taper design affects failure of large-head metal-on-metal total hip replacements. Clin Orthop. 2013;472(2):564–571. doi: 10.1007/s11999-013-3115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burton L, Paget D, Binder NB, Bohnert K, Nestor BJ, Sculco TP, et al. Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. J Orthop Res. 2013;31(1):73–80. doi: 10.1002/jor.22190. [DOI] [PubMed] [Google Scholar]
- 50.Panigrahi P, Liao Y, Mathew MT, Fischer A, Wimmer MA, Jacobs JJ, et al. Intergranular pitting corrosion of CoCrMo biomedical implant alloy. J Biomed Mater Res B Appl Biomater. 2014;102(4):850–859. doi: 10.1002/jbm.b.33067. [DOI] [PubMed] [Google Scholar]
- 51.Jennings JM, Dennis DA, Yang CC. Corrosion of the head–neck junction after total hip arthroplasty. J Am Acad Orthop Surg. 2016;24(6):349–356. doi: 10.5435/JAAOS-D-15-00111. [DOI] [PubMed] [Google Scholar]
- 52.Cooper HJ. The local effects of metal corrosion in total hip arthroplasty. Orthop Clin North Am. 2014;45(1):9–18. doi: 10.1016/j.ocl.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Mischler S, Muñoz AI. Wear of CoCrMo alloys used in metal-on-metal hip joints: a tribocorrosion appraisal. Wear. 2013;297(1–2):1081–1094. [Google Scholar]
- 54.Bryant M, Hu X, Farrar R, Brummitt K, Freeman R, Neville A. Crevice corrosion of biomedical alloys: a novel method of assessing the effects of bone cement and its chemistry. J Biomed Mater Res B Appl Biomater. 2013;101(5):792–803. doi: 10.1002/jbm.b.32883. [DOI] [PubMed] [Google Scholar]
- 55.Reclaru L, Brooks RA, Zuberbühler M, Eschler PY, Constantin F, Tomoaia G. Evaluation of taper joints with combined fatigue and crevice corrosion testing: Comparison to human explanted modular prostheses. Mater Sci Eng C. 2014;34(0):69–77. doi: 10.1016/j.msec.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Lee RK, Longaray J, Essner A, Wang A. Metal-on-metal bearings: the problem is edge-loading wear. Surg Technol Int. 2010;20:303–308. [PubMed] [Google Scholar]
- 57.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847–2851. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 58.Tower SS. Arthroprosthetic cobaltism associated with metal on metal hip implants. BMJ. 2012;344:e430. doi: 10.1136/bmj.e430. [DOI] [PubMed] [Google Scholar]
- 59.Ikeda T, Takahashi K, Kabata T, Sakagoshi D, Tomita K, Yamada M. Polyneuropathy caused by cobalt–chromium metallosis after total hip replacement. Muscle Nerve. 2010;42(1):140–143. doi: 10.1002/mus.21638. [DOI] [PubMed] [Google Scholar]
- 60.Machado C, Appelbe A, Wood R. Arthroprosthetic cobaltism and cardiomyopathy. Heart Lung Circ. 2012;21(11):759–760. doi: 10.1016/j.hlc.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Pelclova D, Sklensky M, Janicek P, Lach K. Severe cobalt intoxication following hip replacement revision: clinical features and outcome. Clin Toxicol (Phila) 2012;50(4):262–265. doi: 10.3109/15563650.2012.670244. [DOI] [PubMed] [Google Scholar]
- 62.Rizzetti MC, Liberini P, Zarattini G, Catalani S, Pazzaglia U, Apostoli P, et al. Loss of sight and sound. Could it be the hip? Lancet. 2009;373(9668):1052. doi: 10.1016/S0140-6736(09)60490-6. [DOI] [PubMed] [Google Scholar]
- 63.Steens W, Foerster GV, Katzer A. Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip—a case report. Acta Orthop. 2006;77(5):830–832. doi: 10.1080/17453670610013079. [DOI] [PubMed] [Google Scholar]
- 64.Oldenburg M, Wegner R, Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J Arthroplast. 2009;24(5):825.e15–825.e20. doi: 10.1016/j.arth.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Firkins PJ, Tipper JL, Saadatzadeh MR, Ingham E, Stone MH, Farrar R, et al. Quantitative analysis of wear and wear debris from metal-on-metal hip prostheses tested in a physiological hip joint simulator. Biomed Mater Eng. 2001;11(2):143–157. [PubMed] [Google Scholar]
- 66.Dumbleton JH, Manley MT. Metal-on-metal total hip replacement: what does the literature say? J Arthroplast. 2005;20(2):174–188. doi: 10.1016/j.arth.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Ricciardi BF, Nocon AA, Jerabek SA, Wilner G, Kaplowitz E, Goldring SR, et al. Histopathological characterization of corrosion product associated adverse local tissue reaction in hip implants: a study of 285 cases. BMC Clin Pathol. 2016;16(3):1–17. doi: 10.1186/s12907-016-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke SA, Revell PA. Integrin expression at the bone/biomaterial interface. J Biomed Mater Res. 2001;57(1):84–91. doi: 10.1002/1097-4636(200110)57:1<84::aid-jbm1145>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 69.McFarlane T, Revell PA. The expression of CD44 in archival paraffin embedded interface tissues of failed orthopaedic implants. J Mater Sci Mater Med. 2004;15(4):315–319. doi: 10.1023/b:jmsm.0000021094.50889.5c. [DOI] [PubMed] [Google Scholar]
- 70.Dutta DK, Potnis PA, Rhodes K, Wood SC. Wear particles derived from metal hip implants induce the generation of multinucleated giant cells in a 3-dimensional peripheral tissue-equivalent model. In: Rameshwar P, editor. PLoS One. 4. Vol. 10. 2015. p. e0124389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodman SB, Gibon E, Pajarinen J, Lin TH, Keeney M, Ren PG, et al. Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. Soc Interface. 2014;11(93):1–12. doi: 10.1098/rsif.2013.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuan RS, Tuan FYI, Konttinen Y, Wilkinson JM, Smith RL. What are the local and systemic biological reactions and mediators to wear debris and what host factors determine or modulate the biological response to wear particles? J Am Acad Orthop Surg. 2008;16(Suppl 1):S42–8. doi: 10.5435/00124635-200800001-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiba J, Rubash HE, Kim KJ, Iwaki Y. The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop. 1994;300:304–312. [PubMed] [Google Scholar]
- 74.Konttinen YT, Zhao D, Beklen A, Ma G, Takagi M, Kivelä-Rajamäki M, et al. The microenvironment around total hip replacement prostheses. Clin Orthop. 2005;430:28–38. doi: 10.1097/01.blo.0000150451.50452.da. [DOI] [PubMed] [Google Scholar]
- 75.Wang CT, Lin YT, Chiang BL, Lee SS, Hou SM. Over-expression of receptor activator of nuclear factor-κB ligand (RANKL), inflammatory cytokines, and chemokines in periprosthetic osteolysis of loosened total hip arthroplasty. Biomaterials. 2010;31(1):77–82. doi: 10.1016/j.biomaterials.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 76.Kondo Y, Yasui K, Yashiro M, Tsuge M, Kotani N, Morishima T. Multi-nucleated giant cell formation from human cord blood monocytes in vitro, in comparison with adult peripheral blood monocytes. Clin Exp Immunol. 2009;158(1):84–90. doi: 10.1111/j.1365-2249.2009.03990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kadoya Y, Revell PA, Al-Saffar N, Kobayashi A, Scott G, Freeman MA. Bone formation and bone resorption in failed total joint arthroplasties: histomorphometric analysis with histochemical and immunohistochemical technique. J Orthop Res. 1996;14(3):473–482. doi: 10.1002/jor.1100140318. [DOI] [PubMed] [Google Scholar]
- 78.Chiu R, Ma T, Smith RL, Goodman SB. Polymethylmethacrylate particles inhibit osteoblastic differentiation of bone marrow osteopro-genitor cells. J Biomed Mater Res A. 2006;77(4):850–856. doi: 10.1002/jbm.a.30697. [DOI] [PubMed] [Google Scholar]
- 79.Chiu R, Ma T, Smith RL, Goodman SB. Ultrahigh molecular weight polyethylene wear debris inhibits osteoprogenitor proliferation and differentiation in vitro. J Biomed Mater Res A. 2009;89A(1):242–247. doi: 10.1002/jbm.a.32001. [DOI] [PubMed] [Google Scholar]
- 80.Hallab NJ, Anderson S, Caicedo M, Skipor A, Campbell P, Jacobs JJ. Immune responses correlate with serum-metal in metal-on-metal hip arthroplasty. J Arthroplast. 2004;19(8 Suppl 3):88–93. doi: 10.1016/j.arth.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87(1):18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 82.Davis DL, Morrison JJ. Hip arthroplasty pseudotumors: pathogenesis, imaging, and clinical decision making. J Clin Imaging Sci. 2016;6:1–17. doi: 10.4103/2156-7514.181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Weegen W, Sijbesma T, Hoekstra HJ, Brakel K, Pilot P, Nelissen RGHH. Treatment of pseudotumors after metal-on-metal hip resurfacing based on magnetic resonance imaging, metal ion levels and symptoms. J Arthroplast. 2014;29(2):416–421. doi: 10.1016/j.arth.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 84.Bosker BH, Ettema HB, van Rossum M, Boomsma MF, Kollen BJ, Maas M, et al. Pseudotumor formation and serum ions after large head metal-on-metal stemmed total hip replacement. Risk factors, time course and revisions in 706 hips. Arch Orthop Trauma Surg. 2015;135(3):417–425. doi: 10.1007/s00402-015-2165-2. [DOI] [PubMed] [Google Scholar]
- 85.Murray DW, Grammatopoulos G, Gundle R, Gibbons CLMH, Whitwell D, Taylor A, et al. Hip resurfacing and pseudotumour. Clin Exp Res Hip Pathol Ther. 2011;21(3):279–283. doi: 10.5301/HIP.2011.8405. [DOI] [PubMed] [Google Scholar]
- 86.Bisschop R, Boomsma MF, Van Raay JJ, Tiebosch AT, Maas M, Gerritsma CL. High prevalence of pseudotumors in patients with a Birmingham Hip Resurfacing prosthesis: a prospective cohort study of one hundred and twenty-nine patients. J Bone Joint Surg Am. 2013;95(17):1554–1560. doi: 10.2106/JBJS.L.00716. [DOI] [PubMed] [Google Scholar]
- 87.Hart AJ, Satchithananda K, Liddle AD, Sabah SA, McRobbie D, Henckel J, et al. Pseudotumors in association with well-functioning metal-on-metal hip prostheses: a case-control study using three-dimensional computed tomography and magnetic resonance imaging. J Bone Joint Surg Am. 2012;94(4):317–325. doi: 10.2106/JBJS.J.01508. [DOI] [PubMed] [Google Scholar]
- 88.Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg Am. 2011;93(23):2164–2171. doi: 10.2106/JBJS.J.01884. [DOI] [PubMed] [Google Scholar]
- 89.Berend KR, Morris MJ, Adams JB, Lombardi AV. Metal-on-metal hip arthroplasty: going, going, gone … - affirms. J Bone Joint Surg Br. 2012;94(11 Suppl A):75–77. doi: 10.1302/0301-620X.94B11.30745. [DOI] [PubMed] [Google Scholar]
- 90.Back DL, Young DA, Shimmin AJ. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin Orthop. 2005;438:177–181. doi: 10.1097/01.blo.0000166901.84323.5d. [DOI] [PubMed] [Google Scholar]
- 91.Mistretta V, Kurth W, Charlier C. Are the hip prostheses dangerous? Science. 2016;32(8–9):732–738. doi: 10.1051/medsci/20163208021. [DOI] [PubMed] [Google Scholar]
- 92.Zywiel MG, Cherian JJ, Banerjee S, Cheung AC, Wong F, Butany J, et al. Systemic cobalt toxicity from total hip arthroplasties. Bone Joint J. 2016;98–B(1):14–20. doi: 10.1302/0301-620X.98B1.36712. [DOI] [PubMed] [Google Scholar]
- 93.Charette RS, Neuwirth AL, Nelson CL. Arthroprosthetic cobaltism associated with cardiomyopathy. Arthroplasty Today. 2017;3(3):151–153. doi: 10.1016/j.artd.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moniz S, Hodgkinson S, Yates P. Cardiac transplant due to metal toxicity associated with hip arthroplasty. Arthroplasty Today. 2017;3:225–228. doi: 10.1016/j.artd.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement*. J Bone Joint Surg Am. 2000;82(4):457. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Abdel-Gadir A, Berber R, Porter JB, Quinn PD, Suri D, Kellman P, et al. Detection of metallic cobalt and chromium liver deposition following failed hip replacement using T2* and R2 magnetic resonance. J Cardiovasc Magn Reson. 2016;18:1–29. doi: 10.1186/s12968-016-0248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rothen-Rutishauser BM, Schürch S, Haenni B, Kapp N, Gehr P. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environ Sci Technol. 2006;40(14):4353–4359. doi: 10.1021/es0522635. [DOI] [PubMed] [Google Scholar]
- 98.Sansone V, Pagani D, Melato M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin Cases Miner Bone Metab. 2013;10(1):34–40. doi: 10.11138/ccmbm/2013.10.1.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nyga A, Hart A, Tetley TD. Importance of the HIF pathway in cobalt nanoparticle-induced cytotoxicity and inflammation in human macro-phages. Nanotoxicology. 2015;9(7):905–917. doi: 10.3109/17435390.2014.991430. [DOI] [PubMed] [Google Scholar]
- 100.Wang Z, Yan Y, Su Y, Qiao L. Effect of proteins on the surface microstructure evolution of a CoCrMo alloy in bio-tribocorrosion processes. Biointerfaces. 2016;145:176–184. doi: 10.1016/j.colsurfb.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Alarifi S, Ali D, AOS Y, Ahamed M, Siddiqui MA, Al-Khedhairy AA. Oxidative stress contributes to cobalt oxide nanoparticles-induced cytotoxicity and DNA damage in human hepatocarcinoma cells. Nanomedicine. 2013;8:189–199. doi: 10.2147/IJN.S37924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huk OL, Catelas I, Mwale F, Antoniou J, Zukor DJ, Petit A. Induction of apoptosis and necrosis by metal ions in vitro. J Arthroplast. 2004;19(8 Suppl 3):84–87. doi: 10.1016/j.arth.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 103.Ani M, Moshtaghie AA. The effect of chromium on parameters related to iron metabolism. Biol Trace Elem Res. 1992;32:57–64. doi: 10.1007/BF02784588. [DOI] [PubMed] [Google Scholar]
- 104.Bregoli L, Chiarini F, Gambarelli A, Sighinolfi G, Gatti AM, Santi P, et al. Toxicity of antimony trioxide nanoparticles on human hematopoietic progenitor cells and comparison to cell lines. Toxicology. 2009;262(2):121–129. doi: 10.1016/j.tox.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 105.Catelas I, Wimmer MA. New Insights into Wear and Biological Effects of Metal-on-Metal Bearings. J Bone Joint Surg Am. 2011;93(Supplement 2):76–83. doi: 10.2106/JBJS.J.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Posada OM, Gilmour D, Tate RJ, Grant MH. CoCr wear particles generated from CoCr alloy metal-on-metal hip replacements, and cobalt ions stimulate apoptosis and expression of general toxicology-related genes in monocyte-like U937 cells. Toxicol Appl Pharmacol. 2014;281(1):125–135. doi: 10.1016/j.taap.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 107.Posada O, Tate R, Meek RM, Grant M. In vitro analyses of the toxicity, immunological, and gene expression effects of cobalt–chromium alloy wear debris and co ions derived from metal-on-metal hip implants. Lubricants. 2015;3(3):539–568. [Google Scholar]
- 108.Savarino L, Granchi D, Ciapetti G, Stea S, Donati ME, Zinghi G, et al. Effects of metal ions on white blood cells of patients with failed total joint arthroplasties. JBiomed Mater Res. 1999;47(4):543–550. doi: 10.1002/(sici)1097-4636(19991215)47:4<543::aid-jbm11>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 109.Jakobsen SS, Danscher G, Stoltenberg M, Larsen A, Bruun JM, Mygind T, et al. Cobalt–chromium–molybdenum alloy causes metal accumulation and metallothionein up-regulation in rat liver and kidney. Basic Clin Pharmacol Toxicol. 2007;101(6):441–446. doi: 10.1111/j.1742-7843.2007.00137.x. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y, Xu H, Liu F, Tao R, Yin J. Effects of serum cobalt ion concentration on the liver, kidney and heart in mice. Orthop Surg. 2010;2(2):134–140. doi: 10.1111/j.1757-7861.2010.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yokel RA. The toxicology of aluminum in the brain: a review. Neurotoxicology. 2000;21(5):813–828. [PubMed] [Google Scholar]
- 112.Savory J, Herman MM, Ghribi O. Mechanisms of aluminum-induced neurodegeneration in animals: implications for Alzheimer's disease. J Alzheimers Dis. 2006;10(2–3):135–144. doi: 10.3233/jad-2006-102-302. [DOI] [PubMed] [Google Scholar]
- 113.Savory J, Ghribi O. Can studies of aluminum toxicity in vivo and in vitro provide relevant information on the pathogenesis and etiology of Alzheimer's disease? J Alzheimers Dis. 2007;11(4):429–430. doi: 10.3233/jad-2007-11402. discussion 431-432. [DOI] [PubMed] [Google Scholar]
- 114.Olivieri G, Novakovic M, Savaskan E, Meier F, Baysang G, Brockhaus M, et al. The effects of beta-estradiol on SHSY5Y neuroblastoma cells during heavy metal induced oxidative stress, neurotoxicity and beta-amyloid secretion. Neuroscience. 2002;113(4):849–855. doi: 10.1016/s0306-4522(02)00211-7. [DOI] [PubMed] [Google Scholar]
- 115.Rizzetti MC, Catalani S, Apostoli P, Padovani A. Cobalt toxicity after total hip replacement: a neglected adverse effect? Muscle Nerve. 2011;43(1):146–147. doi: 10.1002/mus.21902. author reply 147. [DOI] [PubMed] [Google Scholar]
- 116.Apel W, Stark D, Stark A, O'Hagan S, Ling J. Cobalt–chromium toxic retinopathy case study. Doc Ophthalmol. 2013;126(1):69–78. doi: 10.1007/s10633-012-9356-8. [DOI] [PubMed] [Google Scholar]
- 117.Chakraborti S, Chatterjee T, Joshi P, Poddar A, Bhattacharyya B, Singh SP, et al. Structure and activity of lysozyme on binding to ZnO nanoparticles. Langmuir. 2010;26(5):3506–3513. doi: 10.1021/la903118c. [DOI] [PubMed] [Google Scholar]
- 118.Gheshlaghi ZN, Riazi GH, Ahmadian S, Ghafari M, Mahinpour R. Toxicity and interaction of titanium dioxide nanoparticles with microtubule protein. Acta Biochim Biophys Sin. 2008;40(9):777–782. [PubMed] [Google Scholar]
- 119.Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnol. 2013;11(26):1–12. doi: 10.1186/1477-3155-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lloyd DR, Carmichael PL, Phillips DH. Comparison of the formation of 8-hydroxy-2′-deoxyguanosine and single- and double-strand breaks in DNA mediated by fenton reactions. Chem Res Toxicol. 1998;11(5):420–427. doi: 10.1021/tx970156l. [DOI] [PubMed] [Google Scholar]
- 121.Baldwin EL, Byl JAW, Osheroff N. Cobalt enhances DNA cleavage mediated by human topoisomerase II alpha in vitro and in cultured cells. Biochemistry (Mosc) 2004;43(3):728–735. doi: 10.1021/bi035472f. [DOI] [PubMed] [Google Scholar]
- 122.Witkiewicz-Kucharczyk A, Bal W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol Lett. 2006;162(1):29–42. doi: 10.1016/j.toxlet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 123.Lison D, De Boeck M, Verougstraete V, Kirsch-Volders M. Update on the genotoxicity and carcinogenicity of cobalt compounds. Occup Environ Med. 2001;58(10):619–625. doi: 10.1136/oem.58.10.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.De Boeck M, Kirsch-Volders M, Lison D. Cobalt and antimony: genotoxicity and carcinogenicity. Mutat Res. 2003;533(1-2):135–152. doi: 10.1016/j.mrfmmm.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 125.Colognato R, Bonelli A, Ponti J, Farina M, Bergamaschi E, Sabbioni E, et al. Comparative genotoxicity of cobalt nanoparticles and ions on human peripheral leukocytes in vitro. Mutagenesis. 2008;23(5):377–382. doi: 10.1093/mutage/gen024. [DOI] [PubMed] [Google Scholar]
- 126.Ponti J, Sabbioni E, Munaro B, Broggi F, Marmorato P, Franchini F, et al. Genotoxicity and morphological transformation induced by cobalt nanoparticles and cobalt chloride: an in vitro study in Balb/3T3 mouse fibroblasts. Mutagenesis. 2009;24(5):439–445. doi: 10.1093/mutage/gep027. [DOI] [PubMed] [Google Scholar]
- 127.Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem Res Toxicol. 2005;18(1):3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- 128.Reynolds MF, Peterson-Roth EC, Bespalov IA, Johnston T, Gurel VM, Menard HL, et al. Rapid DNA double-strand breaks resulting from processing of Cr-DNA cross-links by both MutS dimers. Cancer Res. 2009;69(3):1071–1079. doi: 10.1158/0008-5472.CAN-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uboldi C, Orsière T, Darolles C, Aloin V, Tassistro V, George I, et al. Poorly soluble cobalt oxide particles trigger genotoxicity via multiple pathways. Part Fibre Toxicol. 2016;13(5):1–15. doi: 10.1186/s12989-016-0118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ladon D, Doherty A, Newson R, Turner J, Bhamra M, Case CP. Changes in metal levels and chromosome aberrations in the peripheral blood of patients after metal-on-metal hip arthroplasty. J Arthroplast. 2004;19(8 Suppl 3):78–83. doi: 10.1016/j.arth.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 131.Dunstan E, Ladon D, Whittingham-Jones P, Carrington R, Briggs TWR. Chromosomal aberrations in the peripheral blood of patients with metal-on-metal hip bearings. J Bone Joint Surg Am. 2008;90(3):517–522. doi: 10.2106/JBJS.F.01435. [DOI] [PubMed] [Google Scholar]
- 132.Gill HS, Grammatopoulos G, Adshead S, Tsialogiannis E, Tsiridis E. Molecular and immune toxicity of CoCr nanoparticles in MoM hip arthroplasty. Trends Mol Med. 2012;18(3):145–155. doi: 10.1016/j.molmed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 133.Xie H, Smith LJ, Holmes AL, Zheng T, Pierce Wise J. The cytotoxicity and genotoxicity of soluble and particulate cobalt in human lung epithelial cells. Environ Mol Mutagen. 2016;57(4):282–287. doi: 10.1002/em.22009. [DOI] [PubMed] [Google Scholar]
- 134.Horev-Azaria L, Kirkpatrick CJ, Korenstein R, Marche PN, Maimon O, Ponti J, et al. Predictive toxicology of cobalt nanoparticles and ions: comparative in vitro study of different cellular models using methods of knowledge discovery from data. Toxicol Sci. 2011;122(2):489–501. doi: 10.1093/toxsci/kfr124. [DOI] [PubMed] [Google Scholar]
- 135.Afolaranmi GA, Henderson C, Grant MH. Effect of chromium and cobalt ions on phase I and phase II enzymatic activities in vitro in freshly isolated rat hepatocytes. Toxicol In Vitro. 2011;25(1):125–130. doi: 10.1016/j.tiv.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 136.Martin JR, Spencer-Gardner L, Camp CL, Stulak JM, Sierra RJ. Cardiac cobaltism: a rare complication after bilateral metal-on-metal total hip arthroplasty. Arthroplasty Today. 2015;1(4):99–102. doi: 10.1016/j.artd.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Davda K, Lali FV, Sampson B, Skinner JA, Hart AJ. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-on-metal hip replacements. J Bone Joint Surg Br. 2011;93(6):738–745. doi: 10.1302/0301-620X.93B6.25804. [DOI] [PubMed] [Google Scholar]
- 138.De Pasquale D, Stea S, Squarzoni S, Bordini B, Amabile M, Catalani S, et al. Metal-on-metal hip prostheses: correlation between debris in the synovial fluid and levels of cobalt and chromium ions in the bloodstream. Int Orthop. 2014;38(3):469–475. doi: 10.1007/s00264-013-2137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ogunwale B, Schmidt-Ott A, Meek RMD, Brewer JM. Investigating the immunologic effects of CoCr nanoparticles. Clin Orthop Relat Res. 2009;467(11):3010. doi: 10.1007/s11999-009-0949-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Simoes TA, Brown AP, Milne SJ, Brydson RMD. Bovine serum albumin binding to CoCrMo nanoparticles and the influence on dissolution. J Phys Conf Ser. 2015;644(1):012039. [Google Scholar]
- 141.Simoes TA, Bryant MG, Brown AP, Milne SJ, Ryan M, Neville A, et al. Evidence for the dissolution of molybdenum during tribocorrosion of CoCrMo hip implants in the presence of serum protein. Acta Biomater. 2016;45:410–418. doi: 10.1016/j.actbio.2016.08.051. [DOI] [PubMed] [Google Scholar]
- 142.Billi Fabrizio, Benya Paul, Ebramzadeh Edward, Campbell Pat, Chan Frank, McKellop Harry A. [cited 2017 Feb 27];Metal wear particles: What we know, what we do not know, and why [Internet] 2013 doi: 10.1016/j.esas.2009.11.006. Available from: http://ijssurgery.com/10.1016/j.esas.2009.11.006. [DOI] [PMC free article] [PubMed]
- 143.Tipper JL, Firkins PJ, Ingham E, Fisher J, Stone MH, Farrar R. Quantitative analysis of the wear and wear debris from low and high carbon content cobalt chrome alloys used in metal on metal total hip replacements. J Mater Sci Mater Med. 1999;10(6):353–362. doi: 10.1023/a:1026473723777. [DOI] [PubMed] [Google Scholar]
- 144.Catelas I, Bobyn JD, Medley JB, Krygier JJ, Zukor DJ, Petit A, et al. Effects of digestion protocols on the isolation and characterization of metal–metal wear particles. I. Analysis of particle size and shape J Biomed Mater Res. 2001;55(3):320–329. doi: 10.1002/1097-4636(20010605)55:3<320::aid-jbm1020>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 145.Schmiedberg SK, Chang DH, Frondoza CG, Valdevit ADC, Kostuik JP. Isolation and characterization of metallic wear debris from a dynamic intervertebral disc prosthesis. J Biomed Mater Res. 1994;28(11):1277–1288. doi: 10.1002/jbm.820281105. [DOI] [PubMed] [Google Scholar]
- 146.Brown C, Williams S, Tipper JL, Fisher J, Ingham E. Characterisation of wear particles produced by metal on metal and ceramic on metal hip prostheses under standard and microseparation simulation. J Mater Sci Mater Med. 2007;18(5):819–827. doi: 10.1007/s10856-006-0015-z. [DOI] [PubMed] [Google Scholar]
- 147.Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, et al. The effect of nano- and micron-sized particles of cobalt–chromium alloy on human fibroblasts in vitro. Biomaterials. 2007;28(19):2946–2958. doi: 10.1016/j.biomaterials.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 148.Collins SJ. Varied storage conditions on the cytotoxic potential of cobalt chrome nanoparticles when cultured with L929 fibroblasts. Biosci Horiz. 2012;5(0):2946–2958. [Google Scholar]
- 149.Zhao J. Genotoxicity and carcinogenicity of cobalt-, nickel- and copper-based nanoparticles (Review) Exp Ther Med. 2012;4(4):551–561. doi: 10.3892/etm.2012.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rae T. The haemolytic action of particulate metals (Cd, Cr, Co, Fe, Mo, Ni, Ta, Ti, Zn, Co-Cr alloy) J Pathol. 1978;125(2):81–89. doi: 10.1002/path.1711250203. [DOI] [PubMed] [Google Scholar]
- 151.Raghunathan VK, Devey M, Hawkins S, Hails L, Davis SA, Mann S, et al. Influence of particle size and reactive oxygen species on cobalt chrome nanoparticle-mediated genotoxicity. Biomaterials. 2013;34(14):3559–3570. doi: 10.1016/j.biomaterials.2013.01.085. [DOI] [PubMed] [Google Scholar]
- 152.Ribeiro AR, Gemini-Piperni S, Travassos R, Lemgruber L, Silva RC, Rossi AL, et al. Trojan-like internalization of anatase titanium dioxide nanoparticles by human osteoblast cells. Sci Rep. 2016;6:23615. doi: 10.1038/srep23615. 1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shah KM, Wilkinson JM, Gartland A. Cobalt and chromium exposure affects osteoblast function and impairs the mineralization of prosthesis surfaces in vitro. J Orthop Res. 2015;33(11):1663–1670. doi: 10.1002/jor.22932. [DOI] [PubMed] [Google Scholar]
- 154.Wang Y, Yan Y, Su Y, Qiao L. Release of metal ions from nano CoCrMo wear debris generated from tribo-corrosion processes in artificial hip implants. J Mech Behav Biomed Mater. 2017;68:124–133. doi: 10.1016/j.jmbbm.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 155.Yan Y, Dowson D, Neville A. In-situ electrochemical study of interaction of tribology and corrosion in artificial hip prosthesis simulators. J Mech Behav Biomed Mater. 2013;18:191–199. doi: 10.1016/j.jmbbm.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 156.Leslie IJ, Williams S, Isaac G, Ingham E, Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop. 2009;467(9):2259–2265. doi: 10.1007/s11999-009-0830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hadley M, Hadley M, Hardaker C, Williams S, Jin Z, Isaac G, et al. [cited 2017 Jan 4];Development of a stop-dwell-start (SDS) protocol for in vitro wear testing of metal-on-metal total hip replacements. 2013 Available from: http://www.astm.org/DIGITAL_LIBRARY/STP/PAGES/STP156020120032.htm.
- 158.Barbour PS, Stone MH, Fisher J. A hip joint simulator study using simplified loading and motion cycles generating physiological wear paths and rates. Proc Inst Mech Eng H. 1999;213(6):455–467. doi: 10.1243/0954411991535068. [DOI] [PubMed] [Google Scholar]
- 159.Catelas I, Medley JB, Campbell PA, Huk OL, Bobyn JD. Comparison of in vitro with in vivo characteristics of wear particles from metal-metal hip implants. Appl Biomater. 2004;70(2):167–178. doi: 10.1002/jbm.b.20036. [DOI] [PubMed] [Google Scholar]
- 160.Heath JC, Freeman MAR, Swanson SAV. Carcinogenic properties of wear particles from prostheses made in cobalt–chromium alloy. Lancet. 1971;297(7699):564–566. doi: 10.1016/s0140-6736(71)91162-7. [DOI] [PubMed] [Google Scholar]
- 161.Caballero MV, Espinoza-Lewis RA, Candiracci M. Application of stem cells and iPS cells in toxicology In: Sahu SC, editor Stem Cells in Toxicology and Medicine [Internet] John Wiley & Sons, Ltd; 2016. [cited 2017 Apr 16]. pp. 5–25. Available from: http://onlinelibrarywiley.com/doi/10.1002/9781119135449.ch2/summary. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


