Abstract
Stem cells are widely used for numerous clinical applications including limbal stem cell deficiency. Stem cell derived from the bulge region of the hair follicle have the ability to differentiate into a variety of cell types including interfollicular epidermis, hair follicle structures, sebaceous glands and corneal epithelial cells when provided the appropriate cues. Hair follicle stem cells are being studied as a valuable source of autologous stem cells to treat disease. The protocol described below details the isolation and expansion of these cells for eventual clinical application. We used a dual-reporter mouse model to visualize both isolation and eventual differentiation of these cells in a limbal stem cell-deficient mouse model.
Keywords: Holoclones, Clonal expansion, Hair follicle stem cells, Bulge, Stem cell isolation
Background
Stem cells are widely used for a multitude of translational and clinical applications. One such clinical application is for the treatment of limbal stem cell deficiency (LSCD). LSCD occurs when there is dysfunction or loss of the limbal stem cell population, which is critical for maintaining a healthy ocular surface, due to congenital or acquired pathologies. The primary treatment strategy for LSCD is cultivating autologous epithelial cell sheets from a limbal biopsy of the patient’s healthy eye ( Pellegrini et al., 1997 ; Shortt et al., 2007 ). The limitation of this strategy is that it is only applicable for patients that have unilateral LSCD. Those that have bilateral LSCD, must rely on an allogenic limbal biopsy from an immunologically related living donor or cadaveric tissue. Due to the need of systemic immunosuppressive therapy and the limited availability of donor tissue, the therapeutic success rate is decreased. Several research groups have been examining the use of cultivated oral mucosal cells for the treatment of LSCD and have achieved some success. However, these cells often fail to express the corneal epithelial differentiation marker, Keratin 12 ( Inatomi et al., 2006 ) and often result in the development of peripheral neovascularization ( Nakamura et al., 2004 ; Nishida et al., 2004 ; Ma et al., 2009 ). Due to these limitations, there was a need for an alternative source of autologous stem cells. Thus we focused on the use of hair follicle stem cells as they harbor multiple sources of stem cells that have been used in regenerative medicine ( Cotsarelis et al., 1990 ; Purba et al., 2014 ). The hair follicle contains mesenchymal stem cells in the dermal papilla and connective tissue sheath, which can give rise to several cell lineages ( Lako et al., 2002 ; Jahoda et al., 2003 ; Richardson et al., 2005 ). Additionally, the bulge region of the hair follicle contains stem cells, which can generate the interfollicular epidermis, hair follicle structures and sebaceous glands ( Cotsarelis et al., 1990 ; Taylor et al., 2000 ; Cotsarelis, 2006). The hair follicle stem cells (HFSC) derived from the bulge region express of variety of cytokeratins including cytokeratin 15 (Krt15) ( Tiede et al., 2007 ; Kloepper et al., 2008 ; Larouche et al., 2008 ), which has been successfully used for the purification and enrichment of HFSC ( Blazejewska et al., 2009 ). HFSC have been successfully used in the treatment of a mouse model of LSCD (Meyer- Blazejewska et al., 2011 ) and research continues to focus on other therapeutic applications and the eventual translation to humans ( Purba et al., 2014 ). Continued research efforts into these areas rely on a standard method for isolating and expanding the bugle-derived HFSC.
Materials and Reagents
Pipette tips (MidSci, Avant low binding tips)
35-mm cell culture dish (Thermo Fisher Scientific, catalog number: 153066)
6-well plates (Corning, Falcon®, catalog number: 353934)
100 mm cell culture dish (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 150464)
NIH-3T3 cells (ATCC, catalog number: CRL-1658)
3-5 week old K12rtTA/rtTA/TetO-Cre/RosamTmG (see Notes)
Ketamine/HCl 100 mg/ml (KetaJect; Henry Schein Animal Health, catalog number: 010177)
Xylazine AnaSed® 100 mg/ml (Santa Cruz Biotechnology, catalog number: sc-362949Rx)
Collagenase A (Sigma-Aldrich, Roche Diagnostics, catalog number: 10103578001)
-
Dispase II (Sigma-Aldrich, catalog number: 4942078001)
Manufacturer: Roche Diagnostics, catalog number: 04942078001.
Mitomycin C (Sigma-Aldrich, catalog number: M7949-2MG)
Phosphate buffered saline (PBS)
Trypsin (2.5%) (Thermo Fisher Scientific, GibcoTM, catalog number: 15090046)
Versene (Thermo Fisher Scientific, GibcoTM, catalog number: 15040066)
Dulbecco’s modified Eagle medium (DMEM) without calcium and magnesium (Thermo Fisher Scientific, GibcoTM, catalog number: 21068028)
Ham’s F12 Nutrient Mix (Thermo Fisher Scientific, GibcoTM, catalog number: 11765047)
Fetal Bovine Serum (Thermo Fisher Scientific, GibcoTM, catalog number: 10082147)
Human recombinant epidermal growth factor (Merck, catalog number: GF144)
L-Glutamine (Thermo Fisher Scientific, GibcoTM, catalog number: 25030081)
Calcium Chloride solution 1 M (Sigma-Aldrich, catalog number: 21115)
Human corneal growth supplement (Thermo Fisher Scientific, GibcoTM, catalog number: S0095)
Penicillin-streptomycin (10,000 U/ml) (Thermo Fisher Scientific, GibcoTM, catalog number: 15140148)
Amphotericin B (Thermo Fisher Scientific, catalog number: 15290026)
Dulbecco’s modified Eagle medium (DMEM) high glucose (Thermo Fisher Scientific, GibcoTM, catalog number: 11960044)
Stem Cell Media (see Recipes)
3T3 media (see Recipes)
Equipment
Pipettes
Microdissection scissors (Fine Science Tools, catalog number: 15000-00)
Forceps (Fine Science Tools, catalog number: 11252-23)
Scissors (Fine Science Tools, catalog number: 14060-09)
Hemocytometer (Hausser Scientific, catalog number: 3200)
Dissecting Scope (ZEISS, model: Stemi DV4)
BSL2 Laminar flow hood (Thermo Fisher Scientific, Thermo ScientificTM, model: 1300 Series A2, catalog number: 1387)
CO2 incubator (Thermo Fisher Scientific, Thermo ScienticTM, model: NAPCO Series 8000 WJ)
Centrifuge (Hettich, model: Rotina 35)
Inverted fluorescent microscope (Zeiss Observer Z1 with an apotome attachment) (ZEISS, model: AxioObserver Z1)
Software
AxioVison 4.7
ImageJ
Procedure
-
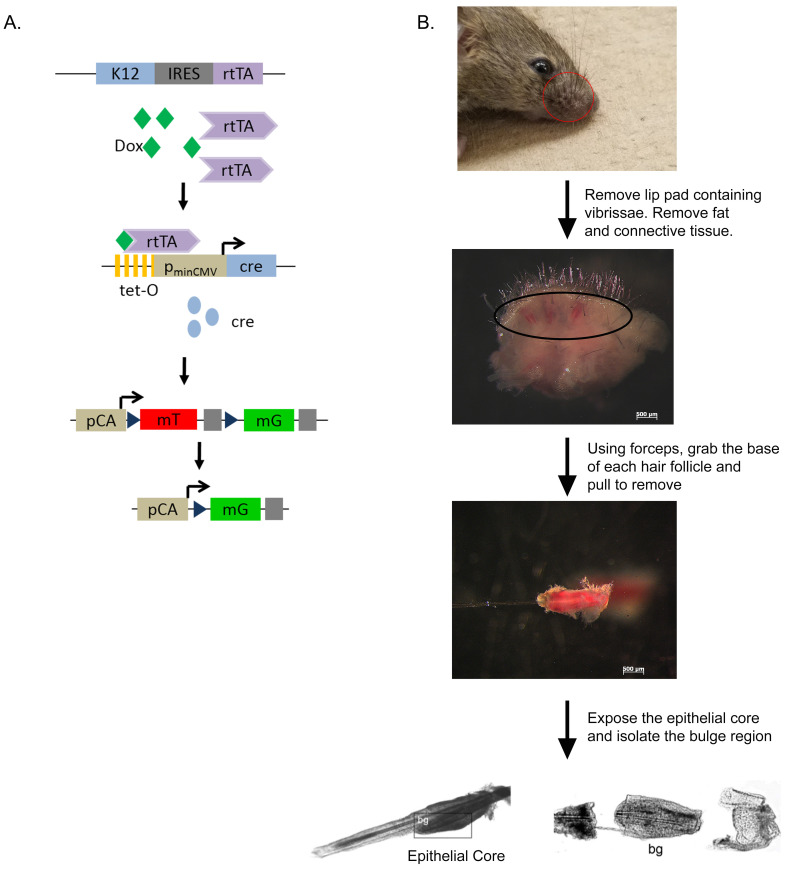
Removal of vibrissae (Figure 1)
Sacrifice 3-5 weeks old K12rtTA/rtTA/TetO-Cre/RosamTmG mice with ketamine/xylazine injection followed by cervical dislocation.
Remove the lip pad containing vibrissae with scissors and place in Stem Cell Media.
Under a dissecting microscope, remove the subcutaneous fat and connective tissue with forceps and scissors to expose the rows of vibrissae.
Remove individual vibrissae by pulling away from the pad using fine forceps.
-
Isolation of hair follicle-derived stem cells
Expose the epithelial cores of the vibrissae by cutting the collagen capsule, loosening it from the core and pulling it down along the hair shaft.
Section the epithelial cores into three portions (Figure 2). Place the middle portion containing the hair follicle bulge region into a 35-mm dish containing 2 ml of collagenase (2 mg/ml) and incubate at 37 °C with 5% CO2 for 1 h in order to remove any residual mesenchymal remnants of the capsule.
Transfer the partially digested hair follicle bulge region to another 35-mm dish containing 5 ml dispase/trypsin (2.4 U/0.05%) solution and digest for 1.5 h at 37 °C with 5% CO2 in a humidified chamber to obtain a single-cell suspension of epithelial cells.
-
Preparing feeder layer
Add Mitomycin C (40 μg/ml) to a 70% confluent dish of NIH3T3 cells and incubate in a humidified chamber at 37 °C with 5% CO2 for 2 h.
Replace the Mitomycin C with 3T3 media.
Trypsinize and seed at 2 x 105 cells per well of a 6-well culture plate.
-
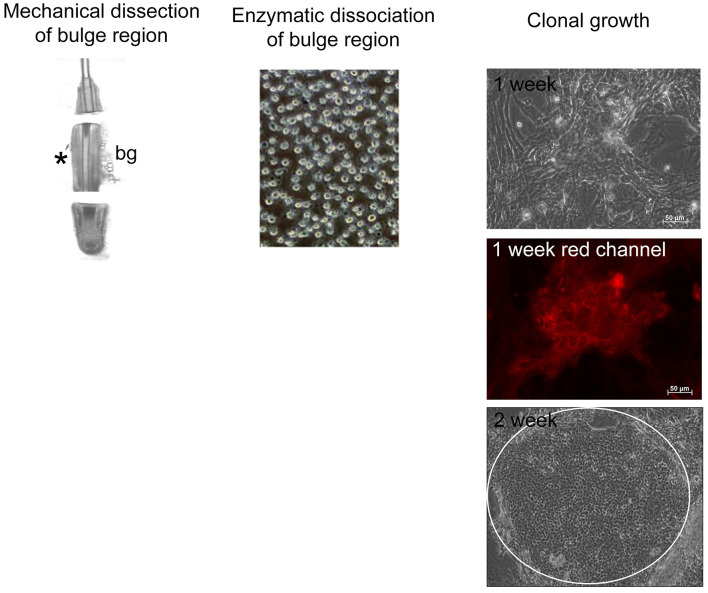
Expansion/clonal growth assay
Enrich stem and progenitor cells by seeding at 1 x 103 cells/cm2 onto a Mitomycin C inactivated NIH 3T3 feeder layer in a 6-well culture dish (see above).
Cultivate for 14-21 days in a humidified chamber at 37 °C with 5% CO2 to obtain holoclones (Figure 2). Change the medium every 2 days. Cultivate until holoclones are obtained. These will be large colonies containing tightly packed cells.
-
Subcultivation of hair follicle stem cells
Remove the 3T3 feeder layer with Versene for 60 sec at room temperature.
Wash 2 times with phosphate buffered saline (PBS).
Remove the attached holoclones with trypsin (0.25% Trypsin-EDTA) for 15 min at 37 °C with 5% CO2.
Centrifuge at 170 × g for 5 min.
Cells are ready for application of choice (Note 6).
Figure 1. Isolation of the hair follicle bulge region from K12rtTA/rtTA/TetO-Cre/RosamTmG reporter mice.
A. Diagram of the triple transgenic mouse model showing the inducible, tissue-specific nature of the system. B. Scheme showing isolation of the hair follicle bulge region. bg–bulge region. Parts Reprinted with permission from Blazejewska et al., 2009 .
Figure 2. Scheme for the clonal growth assay.
The bulge region of the hair follicle is separated via mechanical dissection and enzymatically dissociated. The cells are plated on a 3T3 feeder layer and allowed to form holoclones (white circle at 2 weeks). The red channel depicts the membrane-bound tomato red fluorescence as these cells were derived from the reporter mice described in Figure 1.
Data analysis
The conditions provided in this protocol have been optimized to obtain holoclones, which were assessed based on the size of the colony and colony forming efficiency. A detailed analysis of the isolation and clonal expansion of the hair follicle stem cells can be found at Blazejewska et al., 2009 . (Stem Cells 2009 27(3):642-652)
Notes
Perform all cell culture work in a class II biological safety cabinet.
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. Genetically modified mouse lines Krt12rtTA ( Chikama et al., 2005 ), TetO-cre ( Perl et al., 2002 ) and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J(ROSAmTmG) ( Muzumdar et al., 2007 ) have been previously described. Compound transgenic mice were generated by breeding individual mouse lines to create K12rtTA/rtTA/TetO-Cre/RosamTmG. This dual reporter mouse model uses the keratin 12 promoter (corneal epithelium specific) to drive the expression of the Tet-on system. In conjunction with doxycycline and cre, the membrane tomato red hair follicle stem cells will turn green if they have differentiated into corneal epithelial cells.
Take care when removing the hair follicle and exposing the epithelial cores as to not damage the bulge region stem cells with the forceps.
All cell counts were performed using a hemocytometer.
The purity of the hair follicle stem cell cultures could be assessed in a parallel experiment by examining the expression of Krt15.
Bio-protocol title “Murine Hair Follicle Derived Stem Cell Transplantation onto the Cornea Using a Fibrin Carrier” ( Call et al., 2018 ) demonstrates the use of the bulge derived hair follicle stem cells to treat a mouse model of limbal stem cell deficiency.
Recipes
-
Stem cell media
3 parts DMEM/High glucose without Ca2+ or Mg2+
1 part Ham’s F12
10% FBS
10 ng/ml EGF
500 mg/L L-glutamine
0.4 mM calcium chloride
1x human corneal growth supplement
10,000 U/ml penicillin
10,000 μg/ml streptomycin
25 μg/ml amphotericin B
-
3T3 media
DMEM-high glucose
10% FBS
10,000 U/ml penicillin
10,000 U/ml streptomycin
25 μg/ml amphotericin B
Acknowledgments
This study was supported in part by grants from the NIH/NEI EY011845, Ohio Lions Eye Research Foundation to W.W.K. This work was adapted from Blazejewska et al., 2009 in Stem Cells. Authors do not have any conflicts of interest.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1. Blazejewska E. A., Schlotzer-Schrehardt U., Zenkel M., Bachmann B., Chankiewitz E., Jacobi C. and Kruse F. E.(2009). Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells 27(3): 642-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Call M., Meyer A. E., Kao W., Kruse F. and Schlӧtzer-Schrehardt U.(2018). Murine hair follicle derived stem cell Transplantation onto the cornea using a fibrin carrier. Bio-protocol 8(10) e2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chikama T., Hayashi Y., Liu C. Y., Terai N., Terai K., Kao C. W., Wang L., Hayashi M., Nishida T., Sanford P., Doestchman T. and Kao W. W.(2005). Characterization of tetracycline-inducible bitransgenic Krt12rtTA/+/tet-O-LacZ mice . Invest Ophthalmol Vis Sci 46(6): 1966-1972. [DOI] [PubMed] [Google Scholar]
- 4. Cotsarelis G.(2006). Epithelial stem cells: a folliculocentric view. J Invest Dermatol 126(7): 1459-1468. [DOI] [PubMed] [Google Scholar]
- 5. Cotsarelis G., Sun T. T. and Lavker R. M.(1990). Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61(7): 1329-1337. [DOI] [PubMed] [Google Scholar]
- 6. Inatomi T., Nakamura T., Koizumi N., Sotozono C., Yokoi N. and Kinoshita S.(2006). Midterm results on ocular surface reconstruction using cultivated autologous oral mucosal epithelial transplantation. Am J Ophthalmol 141(2): 267-275. [DOI] [PubMed] [Google Scholar]
- 7. Jahoda C. A., Whitehouse J., Reynolds A. J. and Hole N.(2003). Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol 12(6): 849-859. [DOI] [PubMed] [Google Scholar]
- 8. Kloepper J. E., Tiede S., Brinckmann J., Reinhardt D. P., Meyer W., Faessler R. and Paus R.(2008). Immunophenotyping of the human bulge region: the quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp Dermatol 17(7): 592-609. [DOI] [PubMed] [Google Scholar]
- 9. Lako M., Armstrong L., Cairns P. M., Harris S., Hole N. and Jahoda C. A.(2002). Hair follicle dermal cells repopulate the mouse haematopoietic system. J Cell Sci 20): 3967-3974. [DOI] [PubMed] [Google Scholar]
- 10. Larouche D., Tong X., Fradette J., Coulombe P. A. and Germain L.(2008). Vibrissa hair bulge houses two populations of skin epithelial stem cells distinct by their keratin profile. FASEB J 22(5): 1404-1415. [DOI] [PubMed] [Google Scholar]
- 11. Ma D. H., Kuo M. T., Tsai Y. J., Chen H. C., Chen X. L., Wang S. F., Li L., Hsiao C. H. and Lin K. K.(2009). Transplantation of cultivated oral mucosal epithelial cells for severe corneal burn. Eye(Lond) 23(6): 1442-1450. [DOI] [PubMed] [Google Scholar]
- 12. Meyer-Blazejewska E. A., Call M. K., Yamanaka O., Liu H., Schlotzer-Schrehardt U., Kruse F. E. and Kao W. W.(2011). From hair to cornea: toward the therapeutic use of hair follicle-derived stem cells in the treatment of limbal stem cell deficiency. Stem Cells 29(1): 57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muzumdar M. D., Tasic B., Miyamichi K., Li L. and Luo L.(2007). A global double-fluorescent Cre reporter mouse. Genesis 45(9): 593-605. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura T., Inatomi T., Sotozono C., Amemiya T., Kanamura N. and Kinoshita S.(2004). Transplantation of cultivated autologous oral mucosal epithelial cells in patients with severe ocular surface disorders. Br J Ophthalmol 88(10): 1280-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishida K., Yamato M., Hayashida Y., Watanabe K., Yamamoto K., Adachi E., Nagai S., Kikuchi A., Maeda N., Watanabe H., Okano T. and Tano Y.(2004). Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med 351(12): 1187-1196. [DOI] [PubMed] [Google Scholar]
- 16. Pellegrini G., Traverso C. E., Franzi A. T., Zingirian M., Cancedda R. and De Luca M.(1997). Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349(9057): 990-993. [DOI] [PubMed] [Google Scholar]
- 17. Perl A. K., Wert S. E., Nagy A., Lobe C. G. and Whitsett J. A.(2002). Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A 99(16): 10482-10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Purba T. S., Haslam I. S., Poblet E., Jimenez F., Gandarillas A., Izeta A. and Paus R.(2014). Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioessays 36(5): 513-525. [DOI] [PubMed] [Google Scholar]
- 19. Richardson G. D., Arnott E. C., Whitehouse C. J., Lawrence C. M., Reynolds A. J., Hole N. and Jahoda C. A.(2005). Plasticity of rodent and human hair follicle dermal cells: implications for cell therapy and tissue engineering. J Investig Dermatol Symp Proc 10(3): 180-183. [DOI] [PubMed] [Google Scholar]
- 20. Shortt A. J., Secker G. A., Notara M. D., Limb G. A., Khaw P. T., Tuft S. J. and Daniels J. T.(2007). Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results . Surv Ophthalmol 52(5): 483-502. [DOI] [PubMed] [Google Scholar]
- 21. Taylor G., Lehrer M. S., Jensen P. J., Sun T. T. and Lavker R. M.(2000). Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102(4): 451-461. [DOI] [PubMed] [Google Scholar]
- 22. Tiede S., Kloepper J. E., Bodo E., Tiwari S., Kruse C. and Paus R.(2007). Hair follicle stem cells: walking the maze. Eur J Cell Biol 86(7): 355-376. [DOI] [PubMed] [Google Scholar]