Abstract
Relapsed Philadelphia chromosome (Ph) positive Acute Lymphoblastic Leukemia (ALL) is an aggressive lymphoid malignancy with a poor prognosis and no randomized studies demonstrating superiority of any single salvage regimen. We present the case of a 33-year-old woman with relapsed Ph positive precursor (pre) B-cell ALL with rapidly rising peripheral blasts while on blinatumomab monotherapy initially, but ultimately responded with the addition of Vincristine Sulfate Liposome Injection (VSLI). Ponatinib was added later when it became available for the patient, and she ultimately achieved a complete remission. Further study is warranted to explore mechanisms of potential synergy, and the safety and efficacy of the combination of blinatumomab and VSLI.
Introduction
Approximately 25% of adult patients with precursor B-cell acute lymphoblastic leukemia present with a reciprocal translation between chromosomes 9 and 22, commonly known as the Philadelphia chromosome (Ph) [1,2]. This results in activation of multiple signaling pathways that contribute to tumor growth and proliferation. The advent of Tyrosine Kinase Inhibitors (TKIs) marked a major therapeutic advance, resulting in improved outcomes when combined with multi-agent chemotherapy in the frontline setting [3–5]. Complete Response (CR) rates are high (80-90%) with multidrug TKI-based induction and consolidation therapy, but only 20-40% of patients have long-term disease-free survival [6–9]. For patients who relapse, prognosis is abysmal with 5-year survival of only 6% [10], with no randomized data on optimal second-line therapy.
CD19 is expressed in nearly all patients with pre B-cell ALL [11], and is an attractive therapeutic target. Blinatumomab, a bispecific T-cell-engaging (BiTE) antibody against CD19 and CD3 [12] was approved by the U.S. Food and Drug Administration (FDA) for the treatment of relapsed or refractory Ph negative pre B-cell ALL in 2014. As a single agent, blinatumomab produced a CR/CR with partial hematological recovery (CRh) of 41.6% in an open-label, multicenter, single arm study [13,14]. A subsequent confirmatory phase III study resulted in improved CR+CRh rate (43% vs. 20%) and overall survival (median 7.7 months vs. 4.0 months) with blinatumomab when compared to conventional chemotherapy [15]. The FDA recently extended the label of blinatumomab to include patients with Ph positive disease based on results of a phase II study demonstrating a CR+CRh rate of 36% in this population [16].
Similarly, Vincristine Sulfate Liposome Injection (VSLI) was granted accelerated approval in 2012 for relapsed Ph-negative pre B-cell ALL based on an international, open-label, multi-center, single-arm trial. CR was achieved in 3 of 65 patients (4.6%) and CR with incomplete blood count recovery (CRi) was achieved in 7 of 65 patients (10.8%) [17], but there is little data on the use of this agent in Ph-positive ALL. We present the case of a young woman with relapsed Ph positive pre B-cell ALL treated safely and effectively with a novel combination of blinatumomab and VSLI.
Case Report
The patient is a 33-year-old woman who initially presented with painful cervical adenopathy and leukocytosis to 263,000/μL. Peripheral blood flow cytometry was positive for CD19, CD10, CD22, CD20, CD11b (partial), CD34 (partial), cCD79a, and cTdT, consistent with pre-B cell ALL. There was also aberrant expression of CD33 (dim) and CD13, but the remaining myeloid and T-cell markers were negative. Fluorescence In Situ Hybridization (FISH) was positive for the Ph chromosome in 90.5% of interphase cells, and peripheral blood karyotype was 46,XX,t(9;22)(q34;q11.2)[7]/46,XX[1]. Initial bone marrow biopsy was deferred due to critical illness, as the patient required intubation and continuous renal replacement therapy shortly after arrival. The patient was started on dasatinib 70 mg twice daily with prednisone and rituximab. She had full recovery of her medical issues and bone marrow biopsy after full recovery of blood counts on day 55 was normocellular with no morphologic or immunophenotypic evidence of ALL, consistent with complete remission. Karyotype was normal, FISH for BCR-ABL was negative in 200 interphase cells, and PCR for BCR-ABL was undetectable (<0.001%). She was evaluated for allogeneic stem cell transplantation, but there were multiple delays in finding a donor. She subsequently received 8 courses of fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (hyper-CVAD) alternating with high-dose methotrexate and cytarabine (MA) therapy with rituximab and dasatinib.
Shortly after completion of cycle 8, bone marrow biopsy and aspirate showed 79% blasts, consistent with relapse. Karyotype was positive for Ph chromosome in 2 out of 20 metaphases, FISH was positive in 32.5% of interphase cells analyzed, and PCR was positive for e1a2 at a level of 0.101% and b2a2 at 0.147%. The patient was admitted with an initial White Blood Cell count (WBC) of 5100/ μL. She was continued on dasatinib daily with the plan to change to ponatinib therapy based on medication procurement. Due to rapidly rising WBC and peripheral blasts, the patient was initiated on blinatumomab at the standard dose of 9 mcg/day on Days 1-7 along with dexamethasone. There was an initial decrease in WBC (from a peak of 80,500/μL to a nadir 25,400/μL); however, WBC again began to climb on day 4 of blinatumomab and ultimately reached 94,500/ μL. Ponatinib was still not available, thus weekly VSLI 2.25 mg/m2 was started on day 8. Blinatumomab continuous infusion was also increased on day 8 to 28 mcg/day based on the approved dosing schedule. WBC peaked at 145,000/μL the following day, then rapidly declined (Figure 1). On day 10 dasatinib was discontinued and she was started on ponatinib 45 mg daily. Clinically, the patient experienced cytokine release syndrome with pain and fevers that was controlled with hydration, intravenous pain medications, dexamethasone, and tocilizumab. Symptoms resolved within 12 hours of the initial dose of tocilizumab. She continued on blinatumomab as a continuous infusion, weekly VSLI, and ponatinib daily. The remainder of her hospitalization was uneventful, and repeat bone marrow biopsy on day 42 of blinatumomab therapy was consistent with complete morphologic and cytogenetic remission. PCR from the bone marrow was positive for the e1a2 transcript at a level of 0.047. She did develop worsening peripheral neuropathy around this time, but otherwise the patient is now clinically doing well and she continues to undergo evaluation for allogeneic hematopoietic stem cell transplantation.
Figure 1.
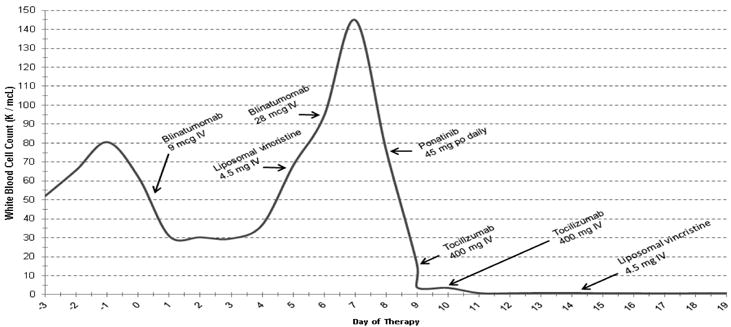
Trend in WBC during course of therapy.
*VSLI, Vincristine sulfate liposome injection.
Discussion
We present a case of a young woman with relapsed Ph+ ALL treated successfully with blinatumomab, VSLI, and subsequently ponatinib. At the time treatment was received, neither the blinatumomab nor VSLI had received FDA approval for the treatment of relapsed or refractory Ph positive pre B-cell ALL. While giving an alternative TKI with or without chemotherapy is recommended by current National Comprehensive Cancer Network guidelines [18], there is often a delay in securing second- or third-generation TKIs, during which time clinical deterioration and rapidly increasing peripheral blasts can be seen. In this case, blinatumomab with dexamethasone was started according to the recommended dose and schedule but despite 7 days of blinatumomab therapy, exponential progression of disease was life threatening and urgent additional therapy was needed [19].
VSLI was specifically chosen based on its single-agent activity in relapsed/refractory ALL, and due to the minimal hematologic toxicity [20]. In this circumstance, the concern with administering more traditional cytotoxic chemotherapy regimens was the depletion of CD3+ T-cells, on which blinatumomab is dependent for its therapeutic activity. Results were dramatic as shown by the significant drop in peripheral blasts and acute cytokine storm, which has been correlated with favorable response [21,22]. These events both occurred prior to administration of ponatinib, demonstrating the efficacy of the blinatumomab and VSLI combination.
We cannot exclude the possibility that the fall in peripheral blasts on day 9 was due to the scheduled increase in the dose of blinatumomab on day 7, or that CR could have been attained with blinatumomab as monotherapy. Similarly, cytokine storm generally correlates with disease burden and is typically seen between days 1 to 14 of administration, thus we also cannot exclude the possibility that the cytokine release syndrome observed was also a direct result of the increased blinatumomab dose. However, our patient’s peripheral blasts decreased by a relatively small proportion while blinatumomab was administered at 9 mcg daily, then proceeded to rise again very rapidly, arguing that there was a synergistic effect of VSLI in overcoming initial blinatumomab resistance. Finally, ponatinib also has known activity in Ph+ ALL [23]; thus it likely also contributed to the deeper response to therapy seen on the bone marrow biopsy obtained on day 42, but was not administered until peripheral blasts had decreased significantly.
Both blinatumomab and vincristine sulfate are associated with significant neurologic adverse events, thus there is potential for overlapping toxicity. The neurotoxicity associated with two highly neurotoxic chemotherapy regimens of blinatumomab and vincristine sulfate must be accounted for during induction. In the phase III TOWER trial, severe adverse events were reported in 65% of patients in the blinatumomab group with a 9.4% incidence of neurologic events including encephalopathy, seizures, and cerebellar dysfunction, compared to an 8.3% incidence in the conventional chemotherapy group. The majority of neurologic events were controlled with brief discontinuation of therapy, anti-epileptic treatment, and dexamethasone prophylaxis and/or treatment [15]. In contrast, VSLI neurotoxicity most commonly manifests as peripheral neuropathy. In the phase II registration trial, grade 3 peripheral neuropathy-related events occurred in 23% of patients, and 1 patient experienced a grade 4 event. Higher VSLI exposure appeared to be associated with higher-grade peripheral neuropathy, but also with increased response [18]. Similarly, in a phase I dose-escalation study in children, adolescents, and young adults with refractory solid tumors or leukemias, continued exposure to VSLI was associated with increased peripheral neuropathy [24]. Central nervous system events are much rarer, however altered mental status, seizures, leukoencephalopathy, Posterior Reversible Encephalopathy Syndrome (PRES), and cerebellar ataxia, have been reported [25,26] and would be more difficult to distinguish clinically from blinatumomab neurologic toxicity. Our patient underwent neurologic exams every 12 hours with particular focus on cerebellar function. She had existing, mild peripheral neuropathy likely secondary to prior chemotherapy exposure, but did experience increased symptoms while on the combination of blinatumomab and VSLI; however, this was controlled with gabapentin and did not reach grade 3.
Conclusion
We presented the case of a 33 year old woman with relapsed Ph chromosome positive pre B-cell ALL successfully treated with blinatumomab and weekly liposomal vincristine sulfate with limited side effects. Based on our experience, further study may be warranted to explore mechanisms of potential synergy, and the safety and efficacy of this combination.
Acknowledgments
The manuscript was partially funded by NCI grant, and grant number: P30CA134274.
References
- 1.Schlieben S, Borkhardt A, Reinisch I, Ritterbach J, Janssen JW, Ratei R, et al. Incidence and clinical outcome of children with BCR/ABL-positive acute lymboblastic leukemia (ALL): A prospective RT-PCR study based on 673 patients enrolled in the German pediatric multicenter therapy trials ALL-BFM-90 and CoALL-05-92. Leukemia. 1996;10(6):957–63. [PubMed] [Google Scholar]
- 2.Melo JV. The diversity of BCR-ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88(7):2375–84. [PubMed] [Google Scholar]
- 3.Fielding AK, Rowe JM, Buck G, Foroni L, Gerrard G, Litzow MR, et al. UKALLXII/ECOG2993: Addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123(6):843–50. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Labarthe A, Rousselot P, Huguet-Rigal F, Delabesse E, Witz F, Maury S, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: Results of the GRAAPH-2003 study. Blood. 2007;109(4):1408–13. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- 5.Thomas DA, Faderl S, Cortes J, Obrien S, Giles FJ, Kornblau SM, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103(12):4396–407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 6.Rinera JM, Oriol A, Gonzalez M, Vidriales B, Brunet S, Esteve J, et al. Concurrent intensive chemotherapy and imatinib before and after stem cell transplantation in newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Final results of the CSTIBES02 trial. Haematologica. 2010;95(1):87–95. doi: 10.3324/haematol.2009.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassan R, Rossi G, Pogliani EM, Di Bona E, Angelucci E, Cavattoni I, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28(22):3644–52. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 8.Stock W, Johnson JL, Stone RM, Kolitz JE, Powell BL, Wetzler M, et al. Dose intensification of daunorubicin and cytarabine during treatment of adult acute lymphoblastic leukemia: Results of cancer and leukemia group B study 19802. Cancer. 2013;119(1):90–8. doi: 10.1002/cncr.27617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DA, O’Brien S, Faderl S, Gercia-Mandero G, Ferrajoli A, Wierda W, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28(24):3880–9. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL):An MRC UKALL12/ECOG 2993 study. Blood. 2007;109(3):944–50. doi: 10.1182/blood-2006-05-018192. [DOI] [PubMed] [Google Scholar]
- 11.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. Blintumomab: A historical perspective. Pharmacol Ther. 2012;136(3):334–42. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Portell CA, Wenzell CM, Advani AS. Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia. Clin Pharmacol. 2013;5(Supp 1):5–11. doi: 10.2147/CPAA.S42689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raponi S, De Propris MS, Intoppa S, Milani ML, Vitale A, Elia L, et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: Analysis of 552 cases. Leuk Lymphoma. 2011;52(6):1098–107. doi: 10.3109/10428194.2011.559668. [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Ko CW, Deisseroth A, Yancey CL, Candau-Chacon R, Chiu HJ, et al. FDA approval: Blinatumomab. Clin Cancer Res. 2015;21(18):4035–9. doi: 10.1158/1078-0432.CCR-15-0612. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Stein A, Gokbuget N, Fielding A, Schuh A, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376:836–47. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinelli G, Boissel N, Chevallier P, Ottmann O, Gokuget N, Topp M, et al. Complete hematologic and molecular response in adult patients with relapsed/refractory Philadelphia chromosome–positive b-precursor acute lymphoblastic leukemia following treatment with blinatumomab: Results from a phase II, single-arm, multicenter study. J Clin Oncol. 2017;35(16):1795–1802. doi: 10.1200/JCO.2016.69.3531. [DOI] [PubMed] [Google Scholar]
- 17.Marqibo® [package insert] San South San Francisco, CA: Talon Therapeutics; Jul, 2015. [Google Scholar]
- 18.O’Brien SM, Aulitzky W, Yehuda B, Lister J, Schiller GJ, Seiter K, et al. Phase II study of Marqibo in adult patients with refractory or relapsed Philadelphia chromosome negative (Ph-) acute lymphoblastic leukemia (ALL) J Clin Oncol Abstrac t 6507, ASCO Annual Meeting. 2010 [Google Scholar]
- 19.National Comprehensive Cancer Network. Acute Lymphoblastic Leukemia. http://www.nccn.org/professionals/physician_gls/pdf/bone.pdf. Accessed July 18, 2017.
- 20.Deitcher O, Glaspy J, Gonzalez R, Sato R, Bedikian A, Segarini K, et al. High-dose vincristine sulfate liposome injection (Marqibo) is not associated with clinically meaningful hematologic toxicity. Clin Lymphoma Myeloma Leuk. 2014;14(3):197–202. doi: 10.1016/j.clml.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Topp MS, Gokbuget N, Zugmaier G, Klappers P, Stelljes M, Neumann S, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(26):4134–40. doi: 10.1200/JCO.2014.56.3247. [DOI] [PubMed] [Google Scholar]
- 22.Topp MS, Gokuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: Amulticentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 23.Cortes J, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, Bicolini F, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med. 2013;369:1783–96. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah NN, Merchant MS, Cole DE, Jayaprakash N, Bernstein D, Delbrook C, et al. Vincristine sulfate liposomes injection (VSLI, Marqibo®): Results from a phase I study in children, adolescents, and young adults with refractory solid tumors or leukemias. Pediatr Blood Cancer. 2016;63(6):997–1005. doi: 10.1002/pbc.25937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas DA, Kantarjian HM, Tock W, Heffner, Faderl S, Garcia-Manero G, Ferrajoli A, et al. Phase 1 multicenter study of vincristine sulfate liposomes injection and dexamethasone in adults with relapsed or refractory acute lymphoblastic leukemia. Cancer. 2009;115(23):5490–8. doi: 10.1002/cncr.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone JB, DeAngelis LM. Cancer treatment-induced neurotoxicity: A focus on newer treatments. Nat Rev Clin Oncol. 2016;13(2):92–105. doi: 10.1038/nrclinonc.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]


