Abstract
Currently there are no effective preventive strategies for pancreatic cancer. Obesity has been increasingly recognized as a strong but modifiable risk factor of pancreatic cancer. In this article, we aim to review the literature regarding weight loss on prevention of pancreatic cancer. Epidemiological and laboratory studies have shown that obesity is associated with increased incidence of pancreatic cancer and potentially worse cancer outcome. Whereas the underlying pathomechanisms remain unclear, chronic inflammation, insulin resistance and altered intestinal microbiota are all implicated in the carcinogenic effect of obesity. Weight loss, especially the durable and significant weight loss after bariatric surgery, has been shown to reduce the risks of multiple cancers and may become a good intervention for pancreatic cancer prevention.
Keywords: obesity, pancreatic cancer, inflammation, insulin resistance, weight loss, bariatric surgery
INTRODUCTION
The incidence of obesity, defined as a body mass index (BMI: kg/m2) of 30 and above, has increased dramatically over the past decades in many countries and is an enormous health issue. In United States, most recent data indicate that more than two-thirds of adults were either overweight or obese (BMI ≥25), and 6.4% were extremely obese (BMI ≥40; class 3 obesity) in 2011–2012.1 Obesity is associated with a number of chronic medical conditions such as hypertension, hyperlipidemia, type 2 diabetes mellitus, cardiovascular disease, metabolic syndrome, and cancer. In 2010, obesity was estimated to cause 3.4 million deaths worldwide, and the global economic impact of obesity is now approximately $2 trillion. In United States, the per-person direct medical cost of overweight was $266 and of obesity was $1723 in 2008. The aggregate national cost of overweight and obesity patients was $113.9 billion.2
Importantly, obesity has also been recognized as a major risk factor of multiple solid cancers. Epidemiological studies have linked obesity to an increased incidence of endometrial cancer, post-menopausal breast cancer, esophageal adenocarcinoma, colon cancer, hepatic cellular carcinoma, renal cell carcinoma, and prostate cancer.3–8 In particular, obesity has been associated with an increased incidence of pancreatic cancer, an almost universally lethal disease.9–11 This review will discuss the link between obesity and pancreatic cancer and the benefits of weight loss in prevention of pancreatic cancer.
OBESITY AND PANCREATIC CANCER
Pancreatic cancer is currently the third leading cause of cancer death following lung and colorectal cancers. In United States, pancreatic cancer has an annual incidence of 48,960 and accounts for 40,560 cancer deaths in 2015.12 It accounts for 3% of newly diagnosed cancers per year but 7% of cancer death per year. The incidence is rapidly growing and it has been projected that by 2030, pancreatic cancer will surpass colorectal cancers to become the second leading cause of cancer death following lung cancer.13 Complete surgical resection significantly prolongs survival, however, most pancreatic cancer patients are diagnosed at late stage with locally advanced disease or distant metastasis and only a small percentage of patients are surgical candidates. With margin-negative resection and negative lymph nodes, the 5-year survival can reach 40% at best at some large tertiary centers.14–17 However, the overall prognosis of all pancreatic cancer patients is dismal with a 5-year survival rate of 7.2%.12
Large epidemiological studies have shown the link between obesity and pancreatic cancer.11,18 A large population-based case-control study of pancreatic cancer demonstrated that obesity was associated with a statistically significant 50–60% increased risk of pancreatic cancer. There was also a statistically significant positive trend in risk with increasing caloric intake, with subjects in the highest quartile of caloric intake experiencing a 70% higher risk than those in the lowest quartile.19 The Metabolic Syndrome and Cancer Project was a large study with 577,315 individuals to investigate the components of metabolic syndrome on the association with overall and site-specific cancer risk. During the 12 years of follow-up, 315 women and 547 men were diagnosed with pancreatic cancer. Among women, obesity was found to increase the risk of pancreatic cancer (adjusted relative risk [RR], 1.54 (95% confidence interval [CI], 1.04–2.29)). However, this correlation was not seen in men.20 Pooled data from the National Cancer Institute Pancreatic Cancer Cohort Consortium also revealed a positive association between increasing BMI and risk of pancreatic cancer (adjusted odds ratio [OR] for the highest vs lowest BMI quartile, 1.33; 95% CI, 1.12–1.58; P <.001).18 A case control study of 841 patients with pancreatic adenocarcinoma and 754 healthy individuals showed that individuals who were overweight or obese from the ages of 20 to 49 years had an earlier onset of pancreatic cancer by 2 to 6 years (median age of onset was 64 years for patients with normal weight, 61 years for overweight patients [P = 0.02], and 59 years for obese patients [P < .001]). Overweight status or obesity during early adulthood is also associated with a greater risk of pancreatic cancer and obesity later in life and a lower overall survival in patients with pancreatic cancer.21 In a multicenter cohort study in Sweden, obesity was also found to be related to worse pancreatic cancer prognosis.22
Animal studies have provided strong evidence to link obesity to increased pancreatic cancer risk. A mouse model of pancreatic cancer by overexpressing constitutively active KrasG12D selectively using pancreatic pancreatic-specific Cre drivers led to PanIN development as early as two weeks.23 Fed with normal chow, only 10% of pancreatic ducts developed PanIN-1a lesions at three months of age. However, when these mice were fed with high fat high calorie diet and became obese, a significantly bigger portion (45%) of ductal cells developed PanINs, suggesting that obesity expedited the progression of pancreatic cancer.24
PUTATIVE MECHANISMS OF THE OBESITY-PANCREATIC CANCER LINK
Several mechanisms have been proposed to explain the increased cancer risk by obesity, including inflammation, insulin resistance, circulating lipids, cytokines, and changes in microbiome. Chronic inflammation is a known major risk factor for many cancers such as esophageal adenocarcinoma, gastric cancer, colorectal cancer, and hepatic cellular carcinoma.25–27 It is also well established that chronic pancreatitis is a major risk factor for pancreatic cancer.28,29 A hallmark of obesity is adipose tissue inflammation, which can promote cancer growth through the secretion of pro-inflammatory cytokines. Adipose tissue contains (pre-)adipocytes, immune cells, fibroblasts, endothelial cells, and stem/progenitor cells, many of which can release a variety of pro-inflammatory cytokines, e.g. TNF-α, TGF-β, IL-6, and leptin. Those factors can in turn stimulate cancer cell proliferation.30 More recently, the cancer-associated adipocyte has gained attention in the pathogenesis of breast cancer. Dirat et al. have shown that breast cancer induces peri-tumoral adipocytes, named cancer-associated adipocytes, to secret pro-inflammatory factors, which can reciprocally induce more aggressive breast cancer cells.31 Hertzer et al demonstrated that conditional KrasG12D mice fed a high fat, high calorie diet displayed a robust inflammation in the peri-pancreatic adipose tissue with an elevation of pro-inflammatory cytokines, suggesting an important role of obesity-induced adipose tissue inflammation in pancreatic cancer development.32
There is great interest in the role of intestinal microbes in obesity. It has been postulated that intestinal microbes contribute to the regulation of energy homeostasis, fat storage and may play a role in obesity.33,34 Obesity has been associated with altered gut microbiota composition, reduced microbial diversity, and reduced gene richness.35
In the small intestine of genetically susceptible KrasG12D mice, a high-fat diet (HFD) promoted tumor growth, independently of obesity.36 The transfer of fecal samples from HFD-fed mice with intestinal tumors to healthy adult KrasG12D mice was sufficient to transmit disease in the absence of an HFD. Furthermore, treatment with antibiotics completely blocked HFD-induced tumor progression. These findings suggested that changes in the microbiota plays a pivotal role in carcinogenesis and tumorigenesis may be transmissible among genetically predisposed individuals.36
Obesity is also often associated with insulin resistance and type 2 diabetes mellitus, with elevated levels of insulin and insulin-like growth factor 1 (IGF-1). Diabetes has been associated with increased cancer risk.37–39 Epidemiology studies have shown that every 10 mg/ml increase in fasting blood glucose is associated with 14% increase in pancreatic cancer.39 Increased insulin/IGF-1 signaling is also known to contribute to cancers such as Ewing sarcoma, breast, ovarian and lung cancers.40–42 Expression levels of both insulin receptor (IR) and insulin-like growth factor receptor (IGF-1R) are increased in many cancers.43 IR and IGF-1R share a significant sequence homology and can function as heterodimers which bind both IGF-1 and IGF-2. Overactivation of these pathways can activate the Ras/ERK pathway and cause increased cell division. IGF-1 pathway can also stimulate the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathways which stimulate proliferation and inhibit apoptosis.44–46 On the other hand, overactivation of IGF-1 can further downregulate cell-cycle tumor suppressors such as PTEN.47
Anti-diabetes medications have great potential in decreasing risks of pancreatic cancer and prolonging pancreatic cancer patient survival.48,49 Metformin, a widely used oral diabetic medication, decreases the serum insulin and IGF-1 level.50,51 At the molecular level, it inhibits the AMP-directed Protein Kinease (AMPK) pathway indirectly by inhibiting the Complex I of the mitochondrial electron transport chain during oxidative phosphorylation.46,52 In vitro studies have shown that metformin inhibits pancreatic cancer cell growth and downregulates mTOR/Sp6 expression level.53
ROLE OF WEIGHT LOSS IN PANCREATIC CANCER PREVENTION
Intentional weight loss has been shown to reduce cancer incidence in women, particularly post-menopausal breast cancer and endometrial cancer.54,55 However, most of these studies were observational. Calorie restriction in animal models has been shown to slow pancreatic cancer growth and development. In a pancreatic cancer xenograft model, calorie restricted (CR) C57BL/6 mice weighed less and had smaller tumors compared with those mice fed with control diet. Similar CR effects on tumor growth were observed in nude mice transplanted with MiaPaCa-2 human pancreatic tumor cell line.56 In another study using conditional KrasG12D mice, both intermittent calorie restriction and chronic calorie restriction reduced the percentage of PanIN-3 lesions compared to the ad libitum group.57
Calorie restriction can lead to short term weight loss for obese patients. However, the vast majorities of those patients are not able to keep the calorie restriction in the long term and usually gained the lost weight back. Many experts advocate biologically based interventions with new anti-obesity drugs and bariatric surgery to treat obesity.58 Obese patients frequently have comorbidities such as hypertension, hyperlipidemia, type 2 diabetes mellitus, and cardiovascular disease. Bariatric surgery offers long term and durable weight loss as well as comorbidity resolution.59 There are several different bariatric surgery techniques, such as Roux-en-Y gastric bypass, sleeve gastrectomy, and laparoscopic gastric banding. Substantial weight loss (roughly 25 % from initial body weight for Roux-en-Y gastric bypass) has been reported with up to 20-year follow-up.60 Roux-en-Y gastric bypass is the gold standard for modern bariatric surgery and can lead to sustained long-term weight loss. A meta-analysis showed that the mean sample-size-weighted percentage of excess weight loss for gastric bypass was 65.7% (n = 3544) vs 45.0% (n = 4109) for gastric band.61 Due to its high long term complication rate and inferior weight loss, the use of gastric band has significantly decreased in recent years.62 Recently, laparoscopic sleeve gastrectomy has been widely accepted as the most popular and definitive bariatric procedure in United States because of the technical simplicity, low complication rate, and excellent weight loss.62,63
Interestingly, a few retrospective clinical studies have shown that bariatric surgery reduced the incidence of multiple cancers including breast, endometrial, colorectal, melanoma and non-Hodgkin lymphoma. McCawley et al. examined women who underwent bariatric surgery at a single university hospital in Virginia from 1990–2006 and found that the incidence of breast, endometrial and cervical cancers were significantly lower in the bariatric surgery group than the control group (3.6% vs. 5.8% in overall cancer incidence).64 Christou et al. analyzed 1035 bariatric surgery (mostly gastric bypass) patients from 1986 to 2002 in Canada and found that bariatric surgery reduced the incidence of many cancers but only to a significant level for breast cancer.65 Adams et al. analyzed 6956 gastric bypass patients in the State of Utah over a 24-year follow-up period. The total cancer incidence was significantly lower in the surgical group compared to controls (hazard ratio [HR], 0.76; 95% CI, 0.65–0.89). Cancer mortality was 46% lower in the surgery group compared to controls (HR, 0.54; 95% CI, 0.37–0.78).66 The Swedish Obesity Subjects Study (SOS) also examined the risks of cancer reduction after bariatric surgery and found that the number of first-time cancers was lower in the surgery group (n=117) than in the control group (n = 169; HR, 0.67; 95% CI, 0.53–0.85). In women, the number of first-time cancers was lower in the surgery group (n = 79) than in the control group (n = 130; HR, 0.58; 95% CI, 0.44–0.77), whereas there was no effect of surgery in men (38 in the surgery group vs 39 in the control group; HR, 0.97; 95% CI, 0.62–1.52).67
Although those studies have consistently showed a decreased cancer risks and mortality after bariatric surgery, whether or not weight loss caused by bariatric surgery can decrease the risks of pancreatic cancer is still unclear. In the Virginia study, no pancreatic cancer occurred in the bariatric surgery group patients and the study didn’t mention the incidence of pancreatic cancer in the control group.64 In the Canadian study, pancreatic cancer was found in 1 out of 1035 surgery patient (0.1%) and 19 out of 5746 control patients (0.33%). The relative risk was 0.29 (95% CI, 0.039–2.175) and P value was 0.1666.65 In the Utah study, there were 9 pancreatic cancer cases in 6596 surgical patients and 8 pancreatic cancer cases in 9442 control group patients and no difference was found.66 The SOS study did not list each cancer type and it was impossible to know the incidence of pancreatic cancer in that study.45 Multi-center studies with longer follow-up are needed to investigate whether or not there is a decreased incidence of pancreatic cancer after bariatric surgery.
MECHANISMS OF BARIATRIC SURGERY ON CANCER RISK REDUCTION
There are several possible mechanisms whereby bariatric surgery may reduce the cancer risk. Bariatric surgery can reduce secretion of inflammatory cytokines. In obese diabetic patients, bariatric surgery caused a significant decrease of inflammatory markers including C-reactive protein (CRP), interleukin-6 (IL-6) and tartrate-resistant acid phosphatase 5a (TRACP 5a).68 Bariatric surgery also has been shown to reduce tissue inflammation69,70. Schneck et al. performed sleeve gastrectomy on 33-week old diet-induced obese C57BI/6 J mice. There were significantly decreased activated T cells ratio and increased anti-inflammatory regulatory T cells in epididymal adipose tissue 3 weeks after surgery.69
Bariatric surgery leads to significantly improved insulin resistance. Taylor and colleagues found that among patients with T2DM who underwent a very low calorie diet or gastric bypass surgery, the hepatic fat content decreased rapidly in parallel with improved hepatic insulin sensitivity and normalization of fasting plasma glucose levels within 7 days.71 Bariatric surgery also improves intestinal microbiota profile. It has been postulated that intestinal microbes contribute to the regulation of energy homeostasis and fat storage and may play a role in obesity.33,34 Obesity has been associated with altered gut microbiota composition, reduced microbial diversity, and reduced gene richness.35 Both Roux-en-Y gastric bypass and vertical banded gastroplasty induced similar and durable changes on the gut microbiome that were not dependent on body mass index and resulted in altered levels of fecal and circulating metabolites compared with obese controls.72 Fecal transplantation from these bariatric surgery patients also altered microbiota deposition in germ-free recipient mice.72
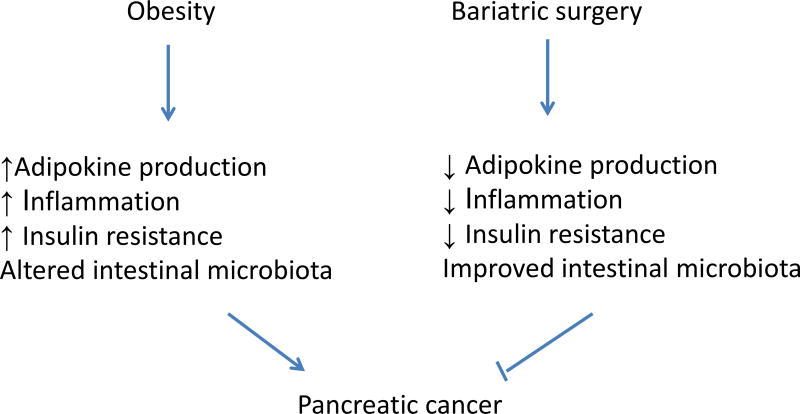
In summary, studies have consistently demonstrated a significantly reduced cancer incidence and/or mortality after bariatric surgery. The possible mechanisms are outlined in Figure 1. However, due to the relatively small sample size and short follow-up duration, published retrospective studies were not able to detect a difference in pancreatic cancer risk. Increased inflammation, cytokine secretion, altered microbiome and insulin resistance have all been implicated in the development of obesity related pancreatic cancer.28–30,32–36 Clinical and experimental data showed clearly that bariatric surgery can lead to decreased tissue inflammation and adipokin secretion, improved microbiome and decreased insulin resistance.69,70,72–74 Although no epidemiology studies have ever shown a definitive decrease of pancreatic cancer incidence after bariatric surgery due to the small sample size, we speculate that bariatric surgery could lead to a significant decrease of pancreatic cancer incidence (Figure 1). Large, multi-center studies with longer follow-up time may help us to answer this important question in the next 5–10 years. Well-designed animal studies may help us to explore the role and mechanisms of bariatric surgery in pancreatic cancer prevention.
Figure 1.
Potential mechanisms of pancreatic cancer risk reduction after bariatric surgery.
FUTURE RESEARCH DIRECTIONS
More studies are greatly needed to prove that weight loss and/or bariatric surgery can reduce the risk of developing pancreatic cancer. Animal bariatric surgery models have been established and they can be used to test if bariatric surgery can reverse PanIN progression. Calorie restriction and/or bariatric surgery in animal pancreatic cancer models are also very useful to elucidate the pancreatic cancer prevention mechanisms by weight loss. A few large HMO organizations in United States such as Kaiser Permanente have large number of obese patients undergoing bariatric surgery every year and a significant portion of those patients will have follow-up with the same HMO organization. It is possible that the bariatric databases from those HMO organizations will have more than 10,000 bariatric patients who have been followed for more than 10 years in the near future. That data will be very valuable to assess if bariatric surgery can decrease pancreatic cancer and other cancer risk. Eventually, prospective multi-center studies with large number of bariatric patients and long-term follow-up will definitively establish the relationship between weight loss and pancreatic cancer prevention.
CONCLUSIONS
Obesity has become an endemic in the past few decades and is associated with increased cancer incidence, mortality and health-cost. In particular, obesity increases the risk of pancreatic cancer, possibly through multiple mechanisms such as inflammation, insulin resistance, and altered intestinal microbiota. As a modifiable risk factor, obesity can be managed with a multidisciplinary approach. Bariatric surgery has been shown to lead to durable weight loss in the long term and reduce risks of many cancers. It is important to further study the effect of weight loss on the risk reduction of pancreatic cancer, one of the most deadly solid cancers.
Acknowledgments
This study was supported by the National Institutes of Health (P01CA163200 and T32 Gastroenterology Training Grant DK07180-40), the SSAT Mentored Research Award, and the Hirshberg Foundation for Pancreatic Cancer Research.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai J, Ford ES, Zhao G, et al. Multiple health behaviors and serum hepatic enzymes among US adults with obesity. Prev Med. 2011;53:278–283. doi: 10.1016/j.ypmed.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11(Suppl 2):S94–S100. [PubMed] [Google Scholar]
- 4.Kredel LI, Siegmund B. Adipose-tissue and intestinal inflammation - visceral obesity and creeping fat. Front Immunol. 2014;5:462. doi: 10.3389/fimmu.2014.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Kvapil P, Hacken-Bitar J, et al. Abdominal obesity and the risk of Barrett's esophagus. Am J Gastroenterol. 2005;100:2151–2156. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 7.Steffen A, Schulze MB, Pischon T, et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18:2079–2089. doi: 10.1158/1055-9965.EPI-09-0265. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger S, Aleksandrova K, Pischon T, et al. Abdominal obesity, weight gain during adulthood and risk of liver and biliary tract cancer in a European cohort. International journal of cancer. Int J Cancer. 2013;132:645–657. doi: 10.1002/ijc.27645. [DOI] [PubMed] [Google Scholar]
- 9.Berrington de Gonzalez A, Sweetland S, Spencer E. A meta-analysis of obesity and the risk of pancreatic cancer. Br J Cancer. 2003;89:519–523. doi: 10.1038/sj.bjc.6601140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer. 2007;120:1993–1998. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 11.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 13.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 14.Donahue TR, Reber HA. Pancreatic surgery. Curr Opin Gastroenterol. 2013;29:552–558. doi: 10.1097/MOG.0b013e3283639359. [DOI] [PubMed] [Google Scholar]
- 15.Ansari D, Williamsson C, Tingstedt B, et al. Pancreaticoduodenectomy--the transition from a low- to a high-volume center. Scand J Gastroenterol. 2014;49:481–484. doi: 10.3109/00365521.2013.847116. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a "true" R0 resection? Ann Surg. 2013;257:731–736. doi: 10.1097/SLA.0b013e318263da2f. [DOI] [PubMed] [Google Scholar]
- 17.Rochefort MM, Ankeny JS, Kadera BE, et al. Impact of tumor grade on pancreatic cancer prognosis: validation of a novel TNMG staging system. Ann Surg Oncol. 2013;20:4322–4329. doi: 10.1245/s10434-013-3159-3. [DOI] [PubMed] [Google Scholar]
- 18.Bracci PM. Obesity and pancreatic cancer: overview of epidemiologic evidence and biologic mechanisms. Mol Carcinog. 2012;51:53–63. doi: 10.1002/mc.20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman DT, Swanson CA, Gridley G, et al. Dietary and nutritional factors and pancreatic cancer: a case-control study based on direct interviews. J Natl Cancer Inst. 1998;90:1710–1719. doi: 10.1093/jnci/90.22.1710. [DOI] [PubMed] [Google Scholar]
- 20.Johansen D, Stocks T, Jonsson H, et al. Metabolic factors and the risk of pancreatic cancer: a prospective analysis of almost 580,000 men and women in the Metabolic Syndrome and Cancer Project. Cancer Epidemiol Biomarkers Prev. 2010;19:2307–2317. doi: 10.1158/1055-9965.EPI-10-0234. [DOI] [PubMed] [Google Scholar]
- 21.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasenda B, Bass A, Koeberle D, et al. Survival in overweight patients with advanced pancreatic carcinoma: a multicentre cohort study. BMC Cancer. 2014;14:728. doi: 10.1186/1471-2407-14-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 24.Dawson DW, Hertzer K, Moro A, et al. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev Res (Phila) 2013;6:1064–1073. doi: 10.1158/1940-6207.CAPR-13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos-Nino ME. The role of chronic inflammation in obesity-associated cancers. ISRN Oncol. 2013;2013:697521. doi: 10.1155/2013/697521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicholson A, Jankowski J. Acid reflux and oesophageal cancer. Recent Results. Cancer Res. 2011;185:65–82. doi: 10.1007/978-3-642-03503-6_4. [DOI] [PubMed] [Google Scholar]
- 27.Brawner KM, Morrow CD, Smith PD. Gastric microbiome and gastric cancer. Cancer J. 2014;20:211–216. doi: 10.1097/PPO.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tong GX, Geng QQ, Chai J, et al. Association between pancreatitis and subsequent risk of pancreatic cancer: a systematic review of epidemiological studies. Asian Pac J Cancer Prev. 2014;15:5029–5034. doi: 10.7314/apjcp.2014.15.12.5029. [DOI] [PubMed] [Google Scholar]
- 30.Kwon H, Pessin JE. Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne) 2013;4:71. doi: 10.3389/fendo.2013.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dirat B, Bochet L, Dabek M, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 32.Hertzer KM, Xu M, Moro A, et al. Robust Early Inflammation of the Peripancreatic Visceral Adipose Tissue During Diet-Induced Obesity in the KrasG12D Model of Pancreatic Cancer. Pancreas. 2016;45:458–465. doi: 10.1097/MPA.0000000000000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 34.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 35.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 36.Schulz MD, Atay C, Heringer J, et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514:508–512. doi: 10.1038/nature13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Forsmark CE. Incretins, Diabetes, Pancreatitis and Pancreatic Cancer: What the GI specialist needs to know. Pancreatology. 2016;16:10–13. doi: 10.1016/j.pan.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294:2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 39.Liao WC, Tu YK, Wu MS, et al. Blood glucose concentration and risk of pancreatic cancer: systematic review and dose-response meta-analysis. BMJ. 2015;349:g7371. doi: 10.1136/bmj.g7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werner H. Tumor suppressors govern insulin-like growth factor signaling pathways: implications in metabolism and cancer. Oncogene. 2012;31:2703–2714. doi: 10.1038/onc.2011.447. [DOI] [PubMed] [Google Scholar]
- 41.Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim WY, Jin Q, Oh SH, et al. Elevated epithelial insulin-like growth factor expression is a risk factor for lung cancer development. Cancer Res. 2009;69:7439–7448. doi: 10.1158/0008-5472.CAN-08-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis. Trends Endocrinol Metab. 2006;17:328–336. doi: 10.1016/j.tem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 45.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozengurt E. Mechanistic target of rapamycin (mTOR): a point of convergence in the action of insulin/IGF-1 and G protein-coupled receptor agonists in pancreatic cancer cells. Front Physiol. 2014;5:357. doi: 10.3389/fphys.2014.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma J, Sawai H, Matsuo Y, et al. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J Surg Res. 2010;160:90–101. doi: 10.1016/j.jss.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z, Lai ST, Xie L, et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2014;106:19–26. doi: 10.1016/j.diabres.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Choi Y, Kim TY, Oh DY, et al. The Impact of Diabetes Mellitus and Metformin Treatment on Survival of Patients with Advanced Pancreatic Cancer Undergoing Chemotherapy. Cancer Res Treat. 2016;48:171–179. doi: 10.4143/crt.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berker B, Emral R, Demirel C, et al. Increased insulin-like growth factor-I levels in women with polycystic ovary syndrome, and beneficial effects of metformin therapy. Gynecol Endocrinol. 2004;19:125–133. doi: 10.1080/09513590400007309. [DOI] [PubMed] [Google Scholar]
- 51.Goodwin PJ, Pritchard KI, Ennis M, et al. Insulin-lowering effects of metformin in women with early breast cancer. Clin Breast Cancer. 2008;8:501–505. doi: 10.3816/CBC.2008.n.060. [DOI] [PubMed] [Google Scholar]
- 52.Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair V, Pathi S, Jutooru I, et al. Metformin inhibits pancreatic cancer cell and tumor growth and downregulates Sp transcription factors. Carcinogenesis. 2013;34:2870–2879. doi: 10.1093/carcin/bgt231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williamson DF, Pamuk E, Thun M, et al. Prospective study of intentional weight loss and mortality in never-smoking overweight US white women aged 40–64 years. Am J Epidemiol. 1995;141:1128–1141. doi: 10.1093/oxfordjournals.aje.a117386. [DOI] [PubMed] [Google Scholar]
- 55.Parker ED, Folsom AR. Intentional weight loss and incidence of obesity-related cancers: the Iowa Women's Health Study. Int J Obes Relat Metab Disord. 2003;27:1447–1452. doi: 10.1038/sj.ijo.0802437. [DOI] [PubMed] [Google Scholar]
- 56.Harvey AE, Lashinger LM, Hays D, et al. Calorie restriction decreases murine and human pancreatic tumor cell growth, nuclear factor-kappaB activation, and inflammation-related gene expression in an insulin-like growth factor-1-dependent manner. PLoS One. 2014;9:e94151. doi: 10.1371/journal.pone.0094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanza-Jacoby S, Yan G, Radice G, et al. Calorie restriction delays the progression of lesions to pancreatic cancer in the LSL-KrasG12D; Pdx-1/Cre mouse model of pancreatic cancer. Exp Biol Med (Maywood) 2013;238:787–797. doi: 10.1177/1535370213493727. [DOI] [PubMed] [Google Scholar]
- 58.Ochner CN, Tsai AG, Kushner RF, et al. Treating obesity seriously: when recommendations for lifestyle change confront biological adaptations. Lancet Diabetes Endocrinol. 2015;3:232–234. doi: 10.1016/S2213-8587(15)00009-1. [DOI] [PubMed] [Google Scholar]
- 59.Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA. 2012;308:1122–1131. doi: 10.1001/2012.jama.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–234. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 61.Puzziferri N, Roshek TB, 3rd, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312:934–942. doi: 10.1001/jama.2014.10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spaniolas K, Kasten KR, Brinkley J, et al. The Changing Bariatric Surgery Landscape in the USA. Obes Surg. 2015;25:1544–1546. doi: 10.1007/s11695-015-1764-x. [DOI] [PubMed] [Google Scholar]
- 63.Alvarenga ES, Lo Menzo E, Szomstein S, et al. Safety and efficacy of 1020 consecutive laparoscopic sleeve gastrectomies performed as a primary treatment modality for morbid obesity. A single-center experience from the metabolic and bariatric surgical accreditation quality and improvement program. Surg Endosc. 2016;30:2673–2678. doi: 10.1007/s00464-015-4548-4. [DOI] [PubMed] [Google Scholar]
- 64.McCawley GM, Ferriss JS, Geffel D, et al. Cancer in obese women: potential protective impact of bariatric surgery. J Am Coll Surg. 2009;208:1093–1098. doi: 10.1016/j.jamcollsurg.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 65.Christou NV, Lieberman M, Sampalis F, et al. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4:691–695. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 66.Adams TD, Stroup AM, Gress RE, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity (Silver Spring) 2009;17:796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sjostrom L, Gummesson A, Sjostrom CD, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol. 2009;10:653–662. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 68.Shih KC, Janckila AJ, Lee WJ, et al. Effects of bariatric weight loss surgery on glucose metabolism, inflammatory cytokines, and serum tartrate-resistant acid phosphatase 5a in obese Chinese adults. Clin Chim Acta. 2016;453:197–202. doi: 10.1016/j.cca.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Schneck AS, Iannelli A, Patouraux S, et al. Effects of sleeve gastrectomy in high fat diet-induced obese mice: respective role of reduced caloric intake, white adipose tissue inflammation and changes in adipose tissue and ectopic fat depots. Surg Endosc. 2014;28:592–602. doi: 10.1007/s00464-013-3211-1. [DOI] [PubMed] [Google Scholar]
- 70.Montecucco F, Lenglet S, Quercioli A, et al. Gastric bypass in morbid obese patients is associated with reduction in adipose tissue inflammation via N-oleoylethanolamide (OEA)-mediated pathways. Thromb Haemost. 2015;113:838–850. doi: 10.1160/TH14-06-0506. [DOI] [PubMed] [Google Scholar]
- 71.Lim EL, Hollingsworth KG, Aribisala BS, et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia. 2011;54:2506–2514. doi: 10.1007/s00125-011-2204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015;22:228–238. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Minervino D, Gumiero D, Nicolazzi MA, et al. Leukocyte Activation in Obese Patients: Effect of Bariatric Surgery. Medicine (Baltimore) 2015;94:e1382. doi: 10.1097/MD.0000000000001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu XJ, Apovian C, Hess D, et al. Improved Insulin Sensitivity 3 Months After RYGB Surgery Is Associated With Increased Subcutaneous Adipose Tissue AMPK Activity and Decreased Oxidative Stress. Diabetes. 2015;64:3155–3159. doi: 10.2337/db14-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]