Abstract
Purpose
To compare the plan quality and organs at risk (OAR) sparing of auto-planned volumetric modulated art therapy (VMAT) and Gamma Knife (GK) for stereotactic radiosurgery of pituitary adenomas (PA) and vestibular schwannomas (VS).
Methods
VMAT radiosurgery plans were made using auto planning tool for eight vestibular schwannoma and eight pituitary adenoma patients previously treated with GK. VMAT plans were made with three non-coplanar arcs using 315, 0 and 45 degrees angles, 6MV FFF energy at 1400 MU/min dose rate and 2.5 mm thick MLC leaves. Both GK and VMAT plans were prescribed to similar isodose lines (50% – 60%).
Results
Respectively for GK and VMAT, the mean Paddick conformity index (PCI) was 0.62 ± 0.08 and 0.67 ± 0.10 (p > 0.05) for PA and 0.72 ± 0.09 and 0.660 ± 0.13 (p > 0.05) for VS; the mean gradient index (GI) was 2.76 ± 0.14 and 3.14 ± 0.40 Gy (p < 0.05) for PA and 3.71 ± 1.83 and 3.60 ± 0.84 Gy (p > 0.05) for VS; mean brainstem maximum dose was 9.13 ± 3.50 Gy and 7.31 ± 2.01 Gy (p > 0.05) for PA and 11.67 ± 4.56 Gy and 12.22 ± 4.55 Gy (p > 0.05) for VS; mean optic nerve maximum dose was 9.66 ± 1.0 Gy and 7.67 ± 2.58 Gy (p < 0.05); mean cochlea mean dose was 7.31 ± 2.7 Gy and 7.23 ± 3.13 Gy (p > 0.05); and mean treatment time was 68 min and 5 min for PA and 40 min and 3 min for VS.
Conclusions
Auto planning with standard template simplified the planning stage for VMAT and provided clinically acceptable plans. Comparison of GK and VMAT for plan quality and OAR sparing varied across patients but both were overall comparable.
Keywords: Linac SRS, Gamma Knife, acoustic schwannoma, vestibular schwannoma, pituitary adenoma, VMAT, auto planning, FFF, flattening filter free, SmartArc, radiosurgery, plan quality, OAR sparing
Introduction
Stereotactic radiosurgery (SRS) of intracranial tumors is a non-surgical procedure where in single fraction, high doses of radiation is delivered to the tumor while achieving steep dose fall off outside the target area. Vestibular schwannoma (VS) and pituitary adenoma (PA) are the two of benign tumors often treated by SRS with high local control rates and low radiation induced toxicities. Due to proximity of these tumors to critical structures and longer life expectancy of the patients, radiation delivery needs to be highly conformal to the target with steep dose fall off to protect the healthy brain tissue. SRS can be carried out via several different types of radiotherapy machines. Gamma Knife (GK) and linear accelerators (LINAC) are the most commonly used technologies for SRS, but CyberKnife, TomoTherapy and proton are also used and each of them has its own advantages and disadvantages(1).
GK delivers non-coplanar radiation from 192 cobalt-60 sources distributed over eight sectors (Perfexion system) and using three different collimator sizes. GK SRS, in general, involves attachment of a stereotactic head frame to the patient’s skull for immobilization and localization. Imaging, treatment planning and delivery are done on the same day, unlike LINAC SRS. GK SRS treatment times are typically longer especially compared to LINAC SRS. More so, GK treatment times gets longer as the source activity decreases and eventually requires replacement of sources. On the other hand, LINAC-based SRS has potential to reduce the treatment time significantly with the use of flattening filter free modes. For example, newer Varian LINACs has 6 MV FFF energy at maximum 1400 MU/min and 10 MV FFF energy at maximum 2400 MU/min. LINAC SRS can be delivered using thermoplastic masks instead of a rigid head frame (2–8). Treatment planning and delivery can be done on different days which allows much easier application of fractionated treatments if needed. All of these may increase the overall patient comfort, although the head frame is usually well tolerated and allow for reliable setup and decreased intrafractional motion.
Volumetric modulated arc (VMAT) therapy is an arc delivery technique used in LINACs where MLC positions, dose rate and gantry speed are simultaneously modulated to achieve highly conformal plans while reducing dose to organs at risk (OAR). Optimum VMAT planning is achieved via inverse planning process where the planner determines objectives for OARs and tuning structures to control dose fall off, hot spots and to limit dose to critical structures. Then the optimizer finds the best solution in an iterative way and the process is repeated until the desired clinical plan is achieved. This can be tedious and often times, the plan quality and planning time depends on the user experience. But with the advent of newly developed auto-planning algorithms, this process has become simpler and less user dependent. On the other hand, GK has not implemented a complete inverse planning algorithm in planning software that users can specify OAR objectives to date.
PA and VS cases were chosen due to proximity of these tumors to critical structures so that we can assess whether VMAT can produce excellent plan quality and/or OAR sparing compared to GK using treatment planning software’s auto planning feature.
Methods and Materials
Patient Data
Eight PA and eight VS patients that were previously treated with Gamma Knife Perfection were randomly selected with varying tumor volume and proximity to critical structures. To immobilize and define a stereotactic frame of reference, a head frame was physically attached to the skull of every patient. All patients went through T1-weighted MRI imaging with 1 mm slice thickness for target delineation and CT imaging with 1 mm slice thickness for skull definition and dose calculation. At least one imaging study was performed using head frame and localizer box. Clinical target volumes (CTV) and OAR volumes were contoured by a neurosurgeon. There was no CTV to planning target volume (PTV) expansion.
GK Planning
GK planning was performed by experienced medical physicists or neurosurgeons using Gamma Plan treatment planning system version 10. PAs and VSs were prescribed to 12-20 Gy and 12-13 Gy, respectively. Shots with collimator sizes 4, 8 and 16 mm were placed inside the target and manipulated to achieve 100 % coverage with the prescription dose to the target while manually optimizing conformity and gradient index and minimizing the dose to nearby organs at risk (OAR) structures until a clinically acceptable plan was achieved. Often mixed collimator sizes and sector blocking was used to achieve acceptable plan quality and OAR sparing. To minimize low dose spillage, targets were prescribed to ~50% isodose line for most patients. Two VS patients with very small target volumes were prescribed to ~ 80% isodose line. Planners used 90 degrees gamma angle if no frame collision was detected. Total treatment time was not the main criteria during planning, but a treatment plan with a shorter treatment time was preferred over another plan with similar plan quality. Dose calculation was performed with TMR 10 algorithm and using dose grid ranging 1 mm – 2 mm depending on the size of the target (9). For pituitary adenomas, the maximum accepted dose to the optic nerve and chiasm was 10 Gy. The radiation oncologist finalized the dose used, and reviewed the contours and the plan for quality and adherence to dose homogeneity, dose conformity and OAR dose criteria. These plans were used clinically for treatment. CT images, target and OAR contours were transferred from GK planning software to Pinnacle (version 9.10, Phillips) for VMAT planning.
VMAT Planning
VMAT radiosurgery plans (SmartArc optimization algorithm) were made using Pinnacle’s (version 9.10, Phillips) auto-planning tool using three non-coplanar arcs at 0 (full arc), 315 and 45 (half arcs) degrees couch angles (10). All plans were made for delivery on EDGE linear accelerator (Varian, Palo Alto, CA) with 6MV energy, flattening filter free (FFF) mode, at 1400 MU/min dose rate and equipped with HD120 MLC (2.5 mm leaf width). All plans were normalized to deliver the prescription dose to 99.5% of the target volume. The following advanced auto-planning settings were found to be optimum for most cases; the tuning balance was 0%; dose fall-off margin was 1 cm; hot spot maximum was 200%-250% and use cold-spot ROIs was selected “no”. Tuning balance is a priory adjustment between conformality and OAR sparing ranging between 0% (conformality) and 100% (OAR sparing). Dose fall-off margin is used to set the level of dose fall off. Lowest (steepest dose fall off) is 1 cm. Hot spot maximum is used to limit dose heterogeneity within the target. Targets were prescribed to similar isodose lines as GK plans, i.e. ~50%. Auto-planning took about 30 minutes to 1 hour depending on the load of the server during planning, dose grid size and resolution (2 mm). There was no time required to generate tuning structures as they were generated by the auto-planning system. Even though the dose and volume constraints for critical structures as shown in Table 1 were used for VMAT optimization, the treatment planning system’s auto-planning algorithm is designed to seek for a lower dose or volume. The final dose calculation was performed with collapsed cone convolution algorithm, with heterogeneity correction and using dose grid ranging 1 mm – 1.5 mm depending on the size of the target.
Table 1.
DVH Parameters and OAR Constraints
| Structure | DVH Parameter | Planning Constrainta |
| Optic Apparatus | Dmax | 10 Gyb |
| Optic Apparatus | V8Gy | - |
| Optic Apparatus | V6Gy | - |
| Brainstem | Dmax | - |
| Brainstem | D0.5cc | - |
| Brainstem | D1cc | 12 Gy |
| Brainstem | V12Gy | - |
| Brainstem | V10Gy | - |
| Cochlea | Dmax | 12 Gy |
| Cochlea | Dmean | - |
| Brain | V25%Rx | - |
Abbreviations: DVH = dose volume histogram; OAR = organs at risk; VXGy = volume of OAR receiving X Gy; Dmax = maximum point dose; Dmean = mean dose; DXcc = dose received by X cc volume of OAR; V25%Rx = volume receive 25% of prescription dose. a not used for VMAT if the risk structure was not too close and possible to achieve with the natural dose fall off. b 12 Gy was used for one patient.
Plan Quality Evaluation
Both GK and VMAT plans were exported to a third party independent software (MIM, Version 6.5, Cleveland, OH) for plan quality evaluation to eliminate possible bias that may be introduced by the differences in the treatment planning systems.
Plan quality was assessed using the International Commission on Radiation Measurements (ICRU) 62 definition of Conformity Index (CI) (11),

Paddick Conformity Index (PCI) (12),
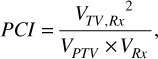
and Paddick Gradient Index (GI) (13),

where VTV,Rx is the volume of target covered by the prescription isodose line, VRx is the volume of the prescription isodose line, V50% Rx is the volume of the 50% of prescription isodose line and VPTV is the volume of the PTV (since there was no PTV margin, PTV = CTV in this study).
For a perfectly conformal plan, where the prescription isodose line exactly matches the PTV contour (i.e. VRx = VPTV), there is no over- or under-coverage. Since the plans in this study were made to cover 100% of PTV, CI values were expected to be always greater than 1 (as CI increases, the plan quality gets worse). Paddick’s conformity index formula checks coverage whereas ICRU definition doesn’t. We can easily rewrite the formula as PCI = (coverage)2 ÷ which shows that PCI penalizes the conformity index with the coverage square. Even though this should not have been an issue for this study with 100% coverage, after transferring to MIM (Cleveland, OH; Version 6.5) software for consistent analysis, we realized the target coverage varied across patients. Because of this, PCI values were slightly different than 1/CI. Since several previous studies used Paddick’s formula to calculate conformity, we also reported for reference to previous and future studies. GI is a measure to evaluate the dose gradient outside the PTV. It is desirable to have a treatment plan with lower GI values to achieve lower low dose spread outside of the target volume.
For both GK and VMAT planning, CI < 1.5 and < 2.0 was used for AS and PA cases, respectively as acceptable plan quality metric. In general GI < 3.0 was desired, but was not strictly enforced during planning.
OAR Sparing Evaluation
Brainstem (BS), optic apparatus (OA) and cochlea were the critical structures depending on the treatment site. The entire OA was contoured for PA cases. Planning OAR constraints (if used) and dose volume histogram (DVH) parameters that were used to compare the OAR sparing of GK and VMAT plans are shown in Table 1. For VMAT, planning OAR constraint not used if the risk structure was not too close or if the plan quality was much worse when used (if OAR objectives easily met with dose fall off). Low dose spread outside the target volume into the healthy brain tissue (V25%Rx) was also analyzed as part of OAR sparing evaluation of GK and VMAT plans. Plan quality and OAR sparing results from VMAT and GK plans were compared using Wilcoxon signed rank test and p values less than 0.05 was considered statistically significant.
Results
Both GK and VMAT plans were prescribed to similar isodose lines (50% – 60%) and had overall similar plan quality as shown in Table 2a and 2b. The difference between VMAT and GK plan conformality was not statistically significant (p > 0.05 for PCI and for CI). On the other hand, GK plans had lower GI for pituitary cases (p < 0.05) and similar GI for vestibular cases (P > 0.05). Two of the VS cases had larger GI values compared to the rest of the cases. For example, case 5 had a GI of 8.03 for GK and 5.08 for VMAT. We reviewed these cases again. We believe the reason for large GI for these cases were due to the shape and smaller volume of the tumors. For example, the volume of the case 5 tumor is 0.37 cc, one of the smallest tumor volumes in the study. The length (maximum dimension) of tumor is 2.24 cm, one of the longest in the study. The dependence of gradient index with tumor volume is known and varying GI requirements with tumor volumes are already used in several protocols (14). The effect of shape on plan quality needs to be further evaluated.
Table 2a.
Plan quality indices for Pituitary Adenoma cases
| Patient | Volume (cc) | GK Tx Time (min) | Prescription Isodose Line (%) | Conformity Index (CI) | Paddick Conformity Index (PCI) | Gradient Index (GI) | ||||
| Gamma Knife | VMAT | Gamma Knife | VMAT | Gamma Knife | VMAT | Gamma Knife | VMAT | |||
| 1 | 7.98 | 52 | 53 | 50 | 1.53 | 1.31 | 0.65 | 0.76 | 2.72 | 2.92 |
| 2 | 2.27 | 43 | 50 | 56 | 2.03 | 1.70 | 0.49 | 0.59 | 2.80 | 3.45 |
| 3 | 1.07 | 69 | 54 | 50 | 1.57 | 1.60 | 0.63 | 0.62 | 2.92 | 3.11 |
| 4 | 2.34 | 75 | 51 | 55 | 1.80 | 1.71 | 0.55 | 0.58 | 2.75 | 3.46 |
| 5 | 9.57 | 80 | 51 | 58 | 1.32 | 1.30 | 0.75 | 0.77 | 2.65 | 2.93 |
| 6 | 1.27 | 42 | 52 | 52 | 1.72 | 1.80 | 0.58 | 0.55 | 3.00 | 3.80 |
| 7 | 20.71 | 140 | 51 | 56 | 1.40 | 1.23 | 0.71 | 0.81 | 2.67 | 2.55 |
| 8 | 3.41 | 45 | 50 | 54 | 1.56 | 1.48 | 0.64 | 0.67 | 2.61 | 2.89 |
| Mean ± SD | 6.08 ± 6.69 | 68 ± 33 | 52 ± 1 | 54 ± 3 | 1.62 ± 0.23 | 1.52 ± 0.22 | 0.62 ± 0.08 | 0.67 ± 0.10 | 2.76 ± 0.14 | 3.14 ± 0.40 |
| P | <0.05 | > 0.05 | > 0.05 | > 0.05 | < 0.05 | |||||
VMAT plans had slightly more target coverage, 99.74% (range: 99.41% – 100%) vs. 99.49% (range: 99.34% - 99.64%) p < 0.05, as calculated by the third party independent software. This difference was mainly due to differences in dose grid and the software’s resolution and interpolation.
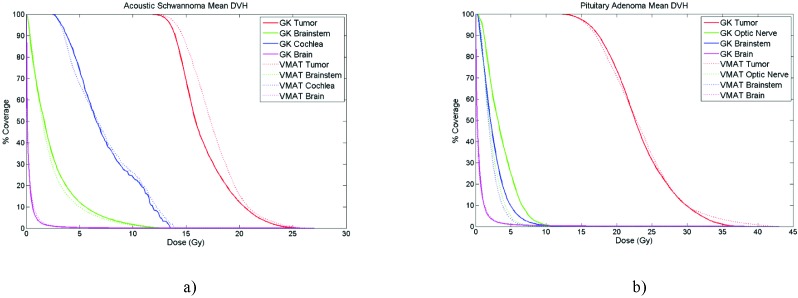
The main advantage of VMAT plans were the shorter beam on time of 3 - 4 minutes for vestibular cases and 3 - 7 minutes for pituitary cases (1400 MU/min dose rate) compared to GK treatment times of 68 and 40 minutes respectively for pituitary and vestibular cases. The GK source activity was ~80% of initial activity and the reported treatment times were the actual times without normalizing to a specific source activity. The mean values of DVH parameters showing OAR sparing of GK and VMAT plans for PA and VS cases are shown in Tables 3a and 3b, respectively. For PA cases, VMAT plans were significantly better for sparing OA (Dmax, V8Gy (cc), and V6Gy (cc), p < 0.05). The difference in brainstem dose was not statistically significant. For VS cases, the difference between VMAT and GK was not statistically significant for sparing brainstem and cochlea. Low dose spread outside the target volume into the healthy brain tissue (Brain V25%Rx) was similar for both VMAT and GK for both sites. Mean DVH plot of both sites clearly shows the similarities and differences of GK and VMAT plans in terms of plan quality and OAR sparing (Figure 2a and 2b).
Table 3a.
OAR Sparing for Pituitary Adenoma cases
| OAR | Mean ± SD | p | |
| Gamma Knife | VMAT | ||
| Optic Apparatus Dmax (Gy) | 9.66 ± 1.0 | 7.67 ± 2.58 | p < 0.05 |
| Optic Apparatus V8Gy (cc) | 0.06 ± 0.10 | 0.01 ± 0.01 | p < 0.05 |
| Optic Apparatus V6Gy (cc) | 0.25 ± 0.17 | 0.03 ± 0.04 | p < 0.05 |
| Brainstem Dmax (Gy) | 9.13 ± 3.50 | 7.31 ± 2.01 | > 0.05 |
| Brainstem D0.5cc (Gy) | 5.51 ± 2.78 | 4.32 ± 1.62 | > 0.05 |
| Brainstem D1cc (Gy) | 4.70 ± 2.53 | 3.61 ± 1.68 | > 0.05 |
| Brain V25%Rx (cc) | 68.70 ± 71.13 | 67.31 ± 57.10 | > 0.05 |
Figure 2.
a) Mean DVH of VS cases. b) Mean DVH of PA cases.
Discussion
For an SRS plan to be clinically acceptable, clinicians check the plan quality and the dose to the OAR. Majority of previous LINAC SRS of intracranial tumors studies focused on the plan quality aspect and OAR sparing aspect had limited attention (2,7,15–17). Moreover, most of these studies investigated the feasibility of single isocenter VMAT treatment for multiple brain metastases and results were compared against multi-isocenter VMAT or other treatment modalities like GK, TomoTherapy, CyberKnife and LINAC IMRT (3,7,18–21). There exist several VMAT optimization platforms that varies with vendors and RapidArc of Eclipse treatment planning software (Varian, Palo Alto) was used in most of these investigations (2,3,18,20–22).
Table 2b.
Plan quality indices for Vestibular Schwannoma cases
| Patient | Volume (cc) | GK Tx Time (min) | Prescription Isodose Line (%) | Conformity Index (CI) | Paddick Conformity Index (PCI) | Gradient Index (GI) | ||||
| Gamma Knife | VMAT | Gamma Knife | VMAT | Gamma Knife | VMAT | Gamma Knife | VMAT | |||
| 1 | 4.43 | 47 | 50 | 62 | 1.30 | 1.27 | 0.76 | 0.79 | 2.58 | 2.89 |
| 2 | 5.26 | 47 | 60 | 56 | 1.27 | 1.26 | 0.78 | 0.78 | 3.19 | 2.71 |
| 3 | 0.47 | 44 | 50 | 55 | 1.36 | 1.49 | 0.72 | 0.67 | 2.94 | 4.01 |
| 4 | 5.39 | 58 | 50 | 57 | 1.23 | 1.20 | 0.80 | 0.83 | 2.77 | 2.69 |
| 5 | 0.37 | 16 | 80 | 73 | 1.95 | 2.08 | 0.51 | 0.48 | 8.03 | 5.08 |
| 6 | 0.21 | 19 | 76 | 65 | 1.29 | 1.57 | 0.77 | 0.63 | 4.30 | 4.42 |
| 7 | 0.72 | 37 | 52 | 50 | 1.35 | 1.74 | 0.74 | 0.57 | 2.71 | 3.10 |
| 8 | 1.11 | 54 | 56 | 62 | 1.41 | 1.77 | 0.70 | 0.56 | 3.13 | 3.65 |
| Mean ± SD | 2.25 ± 2.34 | 40 ± 15 | 59 ± 12 | 60 ± 7 | 1.39 ± 0.23 | 1.57 ± 0.28 | 0.72 ± 0.09 | 0.66 ± 0.13 | 3.71 ± 1.83 | 3.60 ±0.84 |
| P | <0.05 | > 0.05 | > 0.05 | > 0.05 | > 0.05 | |||||
Abbreviations: VMAT = Volumetric modulated arc therapy; SD = standard deviation; GK Tx = Gamma Knife Treatment; p =Wilcoxon signed rank test, comparison of Gamma Knife and VMAT, p < 0.05 considered to be statistically significant.
Table 3b. OAR Sparing for Vestibular Schwannoma cases
| OAR | Mean ± SD | p | |
| Gamma Knife | VMAT | ||
| Brainstem Dmax (Gy) | 11.67 ± 4.56 | 12.22 ± 4.55 | > 0.05 |
| Brainstem D0.5cc (Gy) | 6.12 ± 3.56 | 6.08 ± 3.22 | > 0.05 |
| Brainstem D1cc (Gy) | 4.90 ± 3.22 | 4.23 ± 2.58 | > 0.05 |
| Cochlea Dmax (Gy) | 11.53 ± 3.24 | 10.29 ± 3.36 | > 0.05 |
| Cochlea Dmean (Gy) | 7.31 ± 2.7 | 7.23 ± 3.13 | > 0.05 |
| Brain V25%Rx (cc) | 24.38 ± 22.41 | 26.57 ± 21.80 | > 0.05 |
Abbreviations: VMAT = Volumetric modulated arc therapy; SD = standard deviation; p = Wilcoxon signed rank test, comparison of Gamma Knife and VMAT, p < 0.05 considered to be statistically significant.
Plan quality and OAR sparing of VMAT planning was compared to GK planning in this study which differs from previous studies mainly in the following aspects: 1) VMAT plans were done using SmartArc optimization of Pinnacle treatment planning software (Version 9.10, Philips); 2) Auto planning feature of Pinnacle was used. 3) Both GK (Perfection, Elekta) and VMAT (Varian Edge with HD MLC and 6 MV FFF mode) plans were done using best available technology of the respective vendors at the time of study (The newest GK (Icon, Elekta) has same delivery infrastructure as Perfexion but with added CBCT imaging capabilities allowing frameless fractionated radiosurgery); 4) VS and PA cases selected to study OAR sparing of VMAT; 5) VMAT plans were normalized to similar isodose lines (~50%) as GK.
The results show that in general, VMAT plan quality and OAR sparing including the low dose spread to healthy brain was similar to GK (Tables 2, 3 and Figure 2). VMAT was able to reduce dose to OA (p < 0.05) but cochlea and BS dose was similar to GK.
It is difficult to make a comparison of these results with the existing literature due to the differences in the methods mentioned before. Thomas et al. (20) showed that for multiple metastasis single isocenter VMAT (4 non-coplanar arcs, 10 MV FFF, RapidArc) radiosurgery plan quality and low dose spillage was similar to GK (C/4C model). Huss et al. (23) compared GK (Perfection) and VMAT (RapidArc) for fractionated radiotherapy of multiple metastases and gliomas and found that GK produced better plan quality for smaller volumes (<15 cm3) and consistently lower low dose spillage whereas VMAT was better for large targets and sparing of OARs. In another study, Abacioglu et al. (22) compared VMAT (4-8 non-coplanar arcs, 10 MV FFF, RapidArc) and GK (Perfection) for radiosurgery of benign lesions. Their results show that VMAT and GK plans had similar conformity and OAR sparing but GK had sharper dose fall off. In general our results agrees with their findings except that we were able to achieve similar dose fall off with VMAT as GK. The reason could be their selection of the relatively higher prescription isodose line for dose normalization (70-85%). In a similar study, Kim et al. (3), VMAT (5 non-colanar arcs, 6 MV, 5mm MLC, RapidArc) achieved better conformity but worse gradient index and similar OAR sparing including low dose spillage.
Figure 1.
Three non-coplanar arcs at couch angles 0 (full arc), 315 and 45 (half arcs) used for VMAT planning.
Low dose spillage to healthy brain tissue is a concern for causing radiation induced malignancy and radiation necrosis, especially patients with benign lesions. We were able to achieve similar dose fall off with VMAT compared to GK as opposed to several studies reported in the literature (16,22–24) by prescribing to similar isodose lines as GK (i.e. ~50%) (5).
Often times there is a tradeoff between plan quality (conformity, gradient, heterogeneity) and OAR sparing. One good example to this tradeoff was the cochlea dose in this study. The reason for hearing worsening after radiosurgery of VS has not been understood clearly. Previous studies reported that hearing loss is strongly related with the cochlea dose. Even though no clear threshold dose that is safe for cochlea was determined, a mean dose >4 Gy was associated with increased risk of hearing loss. But this is not easy to achieve with GK planning for many patients without sacrificing coverage or plan quality due to close proximity of the cochlea as shown in Figure 4. For GK planning, usually meeting the dose constraint for OAR along with meeting the conformity requirements is sufficient and planners usually do not pay too much attention to lower isodose lines near critical structures unless enforced by OAR constraints as shown in Figures 3a, 3c and 4a. On the other hand, VMAT has the ability to craft high dose gradients near critical structures using inverse planning techniques as shown in Figure 3b and 3d for a PA case near the optic nerve and Figure 4b for a VS case near cochlea. The auto-planning algorithm used in this study tries to achieve lowest possible dose for the critical structure without sacrificing tumor coverage. This desirable feature was worsening the conformity index for some cases as shown in Figure 4. In this example, an added cochlea objective in VMAT inverse planning optimization allowed reducing the mean cochlea dose from 3.9 Gy (4c) to 1.0 Gy (4b) but also increased the CI to 1.35 from 1.20.
Figure 4.
Axial view of comparison of a) GK, b) VMAT with cochlea planning objective and c) VMAT without cochlea objective. An added cochlea objective in VMAT inverse planning optimization allowed sparing of cochlea by reducing the mean dose from 4.1 Gy (a, GK) and 3.9 Gy (c, VMAT) to 1.0 Gy (b, VMAT). But this also increased the CI from 1.20 to 1.35. d) DVH of all three plans shows the reduction in cochlea dose and changes in other structures. Isodose lines: blue is Rx, orange is 50% of Rx and yellow is 25% of Rx.
Figure 3.
Sagittal (a,b) and coronal (c,d) views of a sample pituitary patient’s plans with GK (left: a and c) and VMAT (right, b and d). VMAT was able to form sharp dose gradient near optic nerve. Isodose lines: blue is Rx, orange is 50% of Rx and yellow is 25% of Rx.
Reduction in treatment time is important for overall patient comfort as well as reduction in intrafraction motion, which is critical in LINAC SRS more so than GK SRS. The short delivery time of LINAC radiosurgery may also have radiobiological advantage over GK resulting in reduced survival fraction(3). Others have reported 15 minutes average time for VMAT radiosurgery including setup which is a significant advantage over GK(7). The VMAT plans in this study had total beam on time of ranging 3 - 7 minutes with 6 MV FFF beam (1400 MU/min dose rate) compared to 40 – 68 for GK. We did not have 10 MV FFF energy (2400 MU/min) available clinically for comparison which would have reduced treatment times further.
On the contrary, the advantage of GK is the quick turnaround time for treatment plans and simple QA process such that treatment can be done the same day as treatment planning. Also, by using a head frame, there is little need for PTV. Some practitioners of LINAC SRS use a 1 mm PTV margin given potential movement within the mask. This has the potential of eliminating some of the dosimetric advantages of LINAC over GK(3,6). We did not use PTV margin mainly to have consistent comparison between GK and VMAT plans. Added margin would have resulted better plan quality but worse OAR sparing for VMAT. But frameless LINAC SRS with no added PTV margin is not uncommon(2,20,22). Others used 1-2 mm PTV margin but with reduced PTV coverage (98%) that reduces the real margin as much as the distance between 98% and 100% isodose lines non-uniformly which maybe significant depending on the size, shape and location of target(3). Currently, there is no consensus about necessity of adding PTV margin for frameless SRS. Several studies show that frameless LINAC SRS with image guidance has sub-millimeter accuracy(4–6,8). Ramakrishna et al. compared patient setup and intrafraction motion using frameless and frame based SRS. They concluded that overall accuracy of frameless SRS can be considered similar to frame based SRSwith slightly increased intrafraction motion with mask (0.7 mm vs 0.4 mm) but within a range appropriate for SRS (4). Moreover, intrafractional errors increase with total treatment time which means less need for margin for frameless LINAC SRS than frameless GK SRS(8).
One limitation of this study is that the GK plans (used clinically) were available before starting VMAT plans. So the GK plan quality and OAR sparing values were available and the GK plans were not optimized as a competitor. We decided to use clinical GK plans and generate VMAT plans with similar mindset of the GK planning. Therefore VMAT plans were optimized to achieve similar or better plan quality as GK while meeting mainly the optic nerve and brainstem constraints. Another limitation is, as is the case for all planning studies, results depend on planner skills and selected techniques (number of beams, allowed couch angles etc.). We used auto planning and a standard template for all the VMAT plans to eliminate this bias but GK planning is user dependent at this point. On the other hand, having simple predefined planning parameters allows application of similar approach elsewhere easily. The VMAT plans were done using 3 arcs at fixed couch and gantry angles. But it is possible to tweak these parameters easily (adding more arcs) and possibly achieve a better plan quality and OAR sparing.
Conclusion
We demonstrated that VMAT (SmartArc) can achieve comparable plan quality and OAR sparing to GK with significantly shorter delivery time. This may to be counterbalanced with treatment planning time and need for plan QA with VMAT SRS. Further reduction in OAR dose was possible for VMAT without significant tradeoff in plan quality, but this advantage may be lost with the possible need for PTV margin. Despite possible tradeoffs, auto planning with standard template does simplify the planning stage for VMAT and allows for excellent dosimetric characteristics for those who use LINAC SRS for benign tumors.
Acknowledgments
Authors’ disclosure of potential conflicts of interest
Dr. Chao reports receipt of personal fees from: Varian Medical Systems; Novocure; Zeiss; and Abbvie, outside the submitted work; Dr. Neyman reports receipt of consultancy fees from Elekta AB, outside the submitted work. Dr Balik reported no conflict of interest.
Author contributions
Conception and design: Salim Balik, Gennady Neyman, Samuel Chao
Data collection: Salim Balik, Gennady Neyman
Data analysis and interpretation: Salim Balik, Gennady Neyman, Samuel Chao
Manuscript writing: Salim Balik, Gennady Neyman, Samuel Chao
Final approval of manuscript: Salim Balik, Gennady Neyman, Samuel Chao
Nomenclature
Stereotactic radiosurgery (SRS), vestibular schwannoma (VS), pituitary adenoma (PA), Gamma Knife (GK), linear accelerator (LINAC), megavolt (MV), flattening filter free (FFF), volumetric modulated arc therapy (VMAT), intensity modulated radiation therapy (IMRT), organs at risk (OAR), International Commission on Radiation Measurements (ICRU), conformity index (CI), Paddick conformity index (PCI), Paddick gradient index (GI), prescription (Rx), the volume of the prescription isodose line (VRx), the volume of target covered by the prescription isodose line (VTV, Rx), the volume of the 50% of prescription isodose line (V50% Rx), clinical target volume (CVT), planning target volume (PTV), the volume of the PTV (VPTV), brainstem (BS), optic apparatus (OA), dose volume histogram (DVH), 25% of prescription isodose line volume (V25%Rx).
References
- 1. Amichetti M, Amelio D, Minniti G. Radiosurgery with photons or protons for benign and malignant tumours of the skull base: A review. Radiat Oncol [Internet]. Radiation Oncology; 2012;7(1):1. Available from: Radiation Oncology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim H, Potrebko P, Rivera A, Liu H, Eldredge-Hindy HB, Gunn V, et al. Tumor volume threshold for achieving improved conformity in VMAT and Gamma Knife stereotactic radiosurgery for vestibular schwannoma. Radiother Oncol [Internet]. Elsevier Ireland Ltd; 2015;115(2):229–34. Available from: http://dx.doi.org/10.1016/j.radonc.2015.03.031 [DOI] [PubMed] [Google Scholar]
- 3. Mayo CS, Ding L, Addesa A, Kadish S, Fitzgerald TJ, Moser R. Initial experience with volumetric IMRT (RapidArc) for intracranial stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;78(5):1457–66. [DOI] [PubMed] [Google Scholar]
- 4. Ramakrishna N, Rosca F, Friesen S, Tezcanli E, Zygmanszki P, Hacker F. A clinical comparison of patient setup and intra-fraction motion using frame-based radiosurgery versus a frameless image-guided radiosurgery system for intracranial lesions. Radiother Oncol [Internet]. Elsevier Ireland Ltd; 2010;95(1):109–15. Available from: http://dx.doi.org/10.1016/j.radonc.2009.12.030 [DOI] [PubMed] [Google Scholar]
- 5. Minniti G, Scaringi C, Clarke E, Valeriani M, Osti M, Enrici RM. Frameless linac-based stereotactic radiosurgery (SRS) for brain metastases: analysis of patient repositioning using a mask fixation system and clinical outcomes. Radiat Oncol. 2011;6(1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calvo Ortega JF, Wunderink W, Delgado D, Moragues S, Pozo M, Casals J. Evaluation of the setup margins for cone beam computed tomography–guided cranial radiosurgery: A phantom study. Med Dosim [Internet]. Elsevier; 2016;41(3):199–204. Available from: http://dx.doi.org/10.1016/j.meddos.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 7. Clark GM, Popple RA, Prendergast BM, Spencer SA, Thomas EM, Stewart JG, et al. Plan quality and treatment planning technique for single isocenter cranial radiosurgery with volumetric modulated arc therapy. Pract Radiat Oncol [Internet]. American Society for Radiation Oncology; 2012;2(4):306–13. Available from: http://dx.doi.org/10.1016/j.prro.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 8. Guckenberger M, Roesch J, Baier K, Sweeney RA, Flentje M. Dosimetric consequences of translational and rotational errors in frame-less image-guided radiosurgery. Radiat Oncol. 2012;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu Y, Bhatnagar JP, Bednarz G, Niranjan A, Flickinger J, Lunsford LD, et al. Dose Differences Between the Three Dose Calculation Algorithms in Leksell GammaPlan. J Appl Clin Med PhysicsMedical Phys. 2014;15(5):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krayenbuehl J, Norton I, Studer G, Guckenberger M. Evaluation of an automated knowledge based treatment planning system for head and neck. Radiat Oncol [Internet]. Radiation Oncology; 2015;10(1):4–11. Available from: http://dx.doi.org/10.1186/s13014-015-0533-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Commission on Radiation Units and Measurements. ICRU Report 62. Prescribing, Recording, and Reporting Photon Beam Therapy (Supplement to ICRU Report 50). J ICRU. 1999;(November):Ix +52. [Google Scholar]
- 12. Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg. 2000;93 Suppl 3(Suppl 3):219–22. [DOI] [PubMed] [Google Scholar]
- 13. Paddick I, Lippitz B. A simple dose gradient measurement tool to complement the conformity index. J Neurosurg. 2006;105 Suppl:194–201. [DOI] [PubMed] [Google Scholar]
- 14. Bezjak A. RTOG 0813: Seamless Phase I/Ii Study of Stereotactic Lung Radiotherapy (SBRT) for Early Stage, Centrally Located, Non-Small Cell Lung Cancer (NSCLC) in Medically Inoperable Patients. Rtog 0813 [Internet]. 2011;1–80. Available from: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0813%5Cnpapers3://publication/uuid/C4EA7F55-8443-4EAC-B628-796EE0D54040 [Google Scholar]
- 15. Balagamwala EH, Suh JH, Barnett GH, Khan MK, Neyman G, Cai RS, et al. The importance of the conformality, heterogeneity, and gradient indices in evaluating gamma knife radiosurgery treatment plans for intracranial meningiomas. Int J Radiat Oncol Biol Phys [Internet]. Elsevier Inc; 2012;83(5):1406–13. Available from: http://dx.doi.org/10.1016/j.ijrobp.2011.10.024 [DOI] [PubMed] [Google Scholar]
- 16. Mcdonald D, Schuler J, Takacs I, Peng J, Jenrette J, Vanek K. Comparison of radiation dose spillage from the Gamma Knife Perfexion with that from volumetric modulated arc radiosurgery during treatment of multiple brain metastases in a single fraction. J Neurosurg [Internet]. 2014;121(121):51–9. Available from: http://thejns.org/doi/abs/10.3171/2014.7.GKS141358 [DOI] [PubMed] [Google Scholar]
- 17. Ohtakara K, Hayashi S, Hoshi H. Dose Gradient Analyses in Linac-based Intracranial Stereotactic Radiosurgery Using Paddick’s Gradient Index: Consideration of the Optimal Method for Plan Evaluation. J Radiat Res [Internet]. 2011;52(5):592–9. Available from: https://academic.oup.com/jrr/article-lookup/doi/10.1269/jrr.11005 [DOI] [PubMed] [Google Scholar]
- 18. Clark GM, Popple RA, Young PE, Fiveash JB. Feasibility of Single-Isocenter Volumetric Modulated Arc Radiosurgery for Treatment of Multiple Brain Metastases. Int J Radiat Oncol Biol Phys. 2010;76(1):296–302. [DOI] [PubMed] [Google Scholar]
- 19. Lau SKM, Zakeri K, Zhao X, Carmona R, Knipprath E, Simpson DR, et al. Single-Isocenter Frameless Volumetric Modulated Arc Radiosurgery for Multiple Intracranial Metastases. Neurosurgery. 2015;77(2):233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas EM, Popple RA, Wu X, Clark GM, Markert JM, Guthrie BL, et al. Comparison of plan quality and delivery time between volumetric arc therapy (rapidarc) and gamma knife radiosurgery for multiple cranial metastases. Neurosurgery. 2014;75(4):409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Audet C, Poffenbarger BA, Chang P, Jackson PS, Lundahl RE, Ryu SI, et al. Evaluation of volumetric modulated arc therapy for cranial radiosurgery using multiple noncoplanar arcs. Med Phys. 2011;38(11):5863–72. [DOI] [PubMed] [Google Scholar]
- 22. Abacioglu U, Ozen Z, Yilmaz M, Arifoglu A, Gunhan B, Kayalilar N, et al. Critical appraisal of RapidArc radiosurgery with flattening filter free photon beams for benign brain lesions in comparison to GammaKnife: A treatment planning study. Radiat Oncol. 2014;9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huss M, Barsoum P, Dodoo E, Sinclair G, Toma-Dasu I. Fractionated SRT using VMAT and Gamma Knife for brain metastases and gliomas - A planning study. J Appl Clin Med Phys. 2015;16(6):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma L, Hossain S, Nichol A, Higby C, Ahmad S, Petti P, et al. Normal brain tissue dose following stereotactic radiosurgery ( SRS ) of multiple brain metastases : variation across modern SRS treatment platforms. J Radiosurgery \& SBRT. 2013;2:18–20. [Google Scholar]