Abstract
Purpose
Ultra-hypofractionated radiotherapy with SBRT is an established technique for treating localized prostate cancer. CyberKnife based SBRT requires implantation of fiducial markers for soft tissue target tracking by the orthogonal KV X-ray imaging system. The spatial distribution of fiducial markers must allow accurate calculation of a 3D transformation that describes the position of the prostate within the reference frame of the planning CT scan. Accuray provides a fiducial implantation guideline for tracking soft tissue lesions. Despite using the guideline we experienced an unacceptably high rate of rotational tracking failure due to problems with fiducial placement. We adapted the Accuray guideline to prostate SBRT for improved fiducial placement and more reliable target tracking.
Methods and materials: 54 patients with prostate adenocarcinoma were treated with ultra-hypofractionated radiotherapy on CyberKnife. Patients had platinum fiducial markers implanted transrectally under ultrasound guidance by a Radiologist. For the first 26 patients, fiducial markers were positioned following the Accuray fiducial placement guidelines for soft tissue lesions (cohort 1). The initial rotational tracking error rate was unacceptably high (23%). On review, inappropriate fiducial placement was identified as the cause of error (especially insufficient spacing between seeds). In October 2016 we developed a seed placement protocol specifically for implanting fiducial markers within the prostate and a second cohort of patients was treated thereafter (cohort 2, 28 patients). The stipulations of the original guideline are maintained while the modified protocol requires that 4 fiducial markers be implanted in the postero-lateral peripheral zone in a single coronal plane.
Results
In cohort 1, patients had a median age of 64 years (50 - 74), PSA of 6.6mcg/L (1.1 – 14.7), and prostate volume of 56 cc (22 - 125), while in cohort 2 they had a mean age of 65 years (53 - 75), PSA of 6.2 mcg/L (1 - 12) and prostate volume of 47 cc (21 - 106). The fiducial markers were easily visualized and there were no cases of urosepsis related to fiducial implantation. In 6 of 26 patients (23%) from cohort 1, only translational mapping without accurate spatial rotations could be calculated. After adopting the prostate specific fiducial implantation protocol, rotational tracking error was eliminated. Accurate 6 degree tracking (accounting for translations and rotations) was achieved in all 28 patients from cohort 2. Using an in-house computer script we analyzed the dose distributions resulting from rotational misalignments of -10, -5, -3, 3, 5, and 10 degrees along all three rotational axes (pitch, roll and yaw). Rotational misalignments result in decreased minimum dose to the PTV and increased maximum dose to OARs.
Conclusion
Implementing a prostate specific fiducial placement protocol for SBRT significantly improved our ability to track prostate motion in 6 degrees 77% to 100% reliability. Failure to track rotations can potentially lead to underdosing and overdosing of portions of the prostate and OARs respectively.
Keywords: Prostate SBRT, Prostate Tracking, Fiducial Placement Protocol, CyberKnife, Ultra-hypofractionated prostate radiotherapy, tumor tracking, fiducial-based tracking for radiosurgery
Introduction
Radiation dose escalation has been shown to improve biochemical control in localized prostate cancer (1,2). Unlike most tumours, prostate cancer is associated with a low α/β ratio (3-6). Prostate cancer is therefore theoretically sensitive to high dose per fraction radiotherapy. According to analyses of tumour control data, the α/β ratio of prostate cancer is in the range of 1 to 3Gy. This value is lower than estimates of the α/β for the dose limiting organ at risk, namely, the rectum (6,7). This provides a rationale for hypofractionated radiotherapy (>2Gy per fraction) or ultra-hypofractionated radiotherapy (>5Gy per fraction) as a means of potentially improving the therapeutic ratio.
Moderately hypofractionated radiotherapy (e.g., 70Gy in 2.5Gy fractions) as a means of dose escalation has proven to be as effective as standard fractionation regimens in localized prostate cancer, and is now a standard of care (8-16). One phase III non-inferiority study reported a 5 year biochemical recurrence free survival (bRFS) for patients treated with conventional fractionation or moderately hypofractionated radiotherapy of 96.7%, 86.8% and 86.5% for low risk, intermediate risk and high risk disease respectively (8).There is a growing experience with ultra-hypofractionated radiotherapy via stereotactic body radiotherapy (SBRT) which is being increasingly used in routine clinical practice in patients with low or intermediate risk disease (17). In a pooled analysis of 1100 patients with localized prostate cancer treated and enrolled in 8 separate phase 2 clinical trials, the 5 year bRFS rate for prostate SBRT was 95%, 84%, and 81% for patients with low, intermediate, and high risk disease respectively (18). Loblaw et al reported acute and late grade 3 toxicities in 84 patients with low risk disease treated with accelerator based SBRT (35Gy in 5 fractions). Acute grade ≥3 gastrointestinal (GI) toxicity was 0% and genitourinary toxicity was 1%.At 5 years of follow-up late grade ≥ 3 GI toxicity was 1% GI, and GU toxicity was 1%. 5 year bPFS of 98% was reported (19). Phase III trials comparing prostate SBRT against standard treatments are underway. For example, PACE (NCT 01009008) is a phase III trial comparing surgery and conventional radiotherapy against ultra-hypofractionated radiotherapy in localized prostate cancer.
The CyberKnife (Accuray Inc., Sunnyvale, California) is a dynamic image-guided whole body robotic radiosurgery system developed by Accuray in 2001 (20). The machine consists of a robot arm mounted linear accelerator coupled to a stereoscopic kilovoltage (kV) X-ray imaging system. It is capable of non-coplanar, non-isocentric treatment delivery to complex targets with automatic intra-fraction image guidance and sub-millimeter accuracy. Multiple groups have reported on successful prostate SBRT using the CyberKnife (21-26). The CyberKnife system requires implantation of radio-opaque fiducial markers to track soft tissue targets (i.e., the prostate) with the stereoscopic kV X-ray imaging system. The spatial distribution of the fiducials must allow accurate calculation of a 3D transformation that describes the position of the prostate within the reference frame of the planning CT scan. The machine can thereby detect and automatically correct for target motion and setup error on the fly.
Accuray has a fiducial placement protocol for treating pancreas and liver lesions (27). The same protocol is applied for treating prostate cancer and other soft tissue lesions. The intent is to optimally place fiducials such that lesions can be accurately tracked in 6 degrees (accounting for translational and rotational movements). Despite following the Accuray fiducial placement guideline, when we began a prostate SBRT program at our institution, 6 degree tracking was not possible in one quarter of our patients. We present a modified fiducial placement protocol for prostate SBRT on CyberKnife which eliminated our tracking error.
Background
In fiducial-based tracking for radiosurgery, small markers are implanted at the treatment site with the expectation that they will remain in place through the course of planning and treatment. Ideally the markers should be small enough to approximate point landmarks and dense enough to be seen on X-ray, CT and portal imaging. During treatment on the CyberKnife, orthogonal radiographic images are taken from two perspectives. The position of the fiducials (a surrogate for the target position) is calculated through back-projection and the geometry of the X-ray imaging system. The fiducial coordinates in the planning CT and the fiducial coordinates during treatment are used to solve for the position (translation) and orientation (rotation) of the target during treatment: this is an example of the relative pose problem. The solution to the relative pose problem is a combination of physical translations and rotations about the x, y and z axes (6 degrees) mapping the pose of the target from the planning space to the pose of the target during treatment (28). With poor positioning of fiducial markers, it may not be possible to solve all parts of the relative pose problem.
The Accuray guideline is derived from a report on fiducial based tracking accuracy in the presence of uncertainties (noise or deformation) on CT and live radiographic images (27,28). A minimum of 3 fiducials are mathematically required for an analytic solution to the 6 degree prostate tracking relative pose problem (28). Accuray recommends implanting a minimum of 4 (1 more than required) and a maximum of 6 fiducials for CyberKnife tracking. There are a further 4 key principles from Accuray: 1) a minimum of 2.0cm between fiducials; 2) A maximum distance of 5-6cm from the lesion; 3) non-colinear placement (within the orthogonal imaging plane); 4) At least 15 degree angulation between any grouping of 3 fiducials. When we began using the CyberKnife for prostate SABR despite following the Accuray guideline we were unable track rotations in 23% of treatments (i.e., 6 of the first 26 patients).When tracking fails, the CyberKnife software generates a specific error message. Poor fiducial placement was the cause in an all 6 cases where rotational tracking failed. 4 error types were encountered, often simultaneously (see Table 1, Figure 1 - 3). A) Failure to meet the minimum distance between markers (66%); B) Failure to meet the minimum angle requirements between markers (17%); C) Not enough markers (17%); D) Markers are too closely spaced on digitally reconstructed radiograph (DRR) for accurate fiducial extraction (50%). Errors A –C are a direct result of failure to comply with the Accuray guidelines. Error D occurs when fiducials lay in the same 45 degree line from the transverse plane, which makes them appear too close together on DRR, or on kV orthogonal X-ray imaging. Fiducial markers are often implanted by other medical specialists (Radiologists or Urologists) who may not understand the importance of seed placement or the geometry of the kV X-ray imaging system. Even with the Accuray guideline in mind, failure to comply with its principles is possible. For example if the first marker is placed in the centre of the prostate (which is typically 3-4cm in diameter), all three subsequent markers will fail to meet the minimum distance requirement. Reliable rotational tracking requires a simple reproducible seed distribution and clear communication with other medical specialists.
Table 1.
Summary of Tracking Errors (cohort 1)
| Case # | Error | Translations Tracked | Rotations Tracked |
| 1 | A,B,D | Yes | No |
| 2 | D | Yes | No |
| 3 | A,D | Yes | No |
| 4 | C | Yes | No |
| 5 | A | Yes | No |
| 6 | A | Yes | No |
A. Failure to meet the minimum distance between markers
B. Failure to meet the minimum angle requirements between markers
C. Not enough markers
D. Markers are too closely spaced on DRR for accurate fiducial extraction
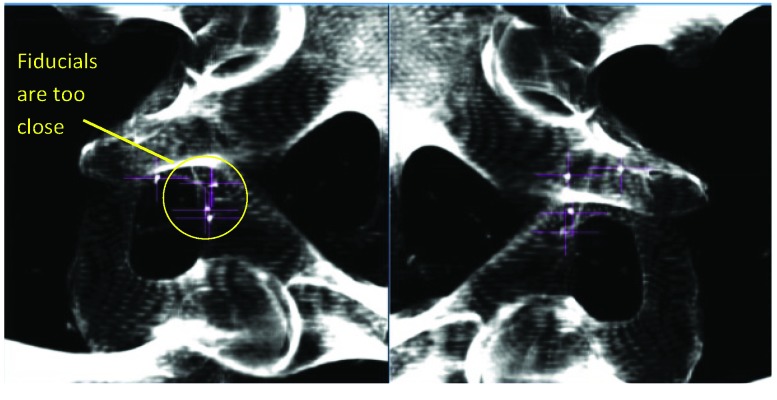
Figure 1.
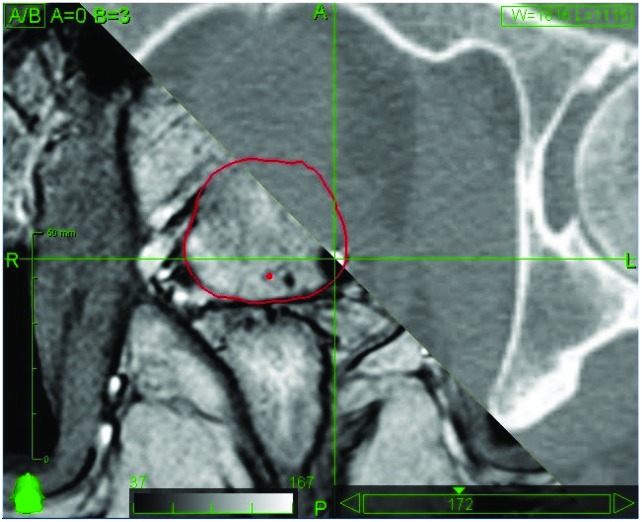
MultiPlan (Accuray Inc., Sunnyvale, California) screenshot showing fiducial placement on orthogonal kV X-ray images. In this case the fiducial markers are too close together for accurate calculation of 3D rotations.
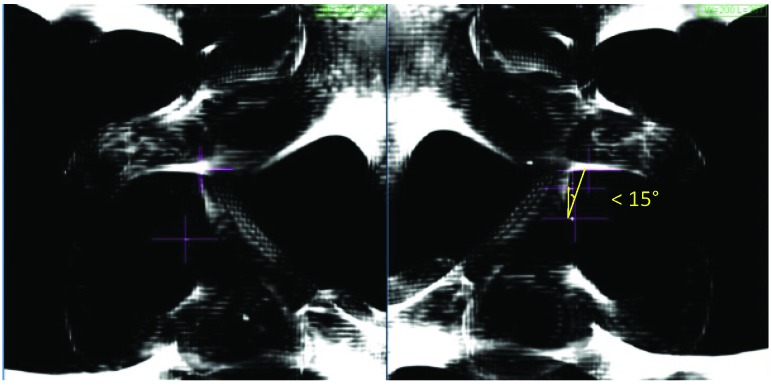
Figure 3.
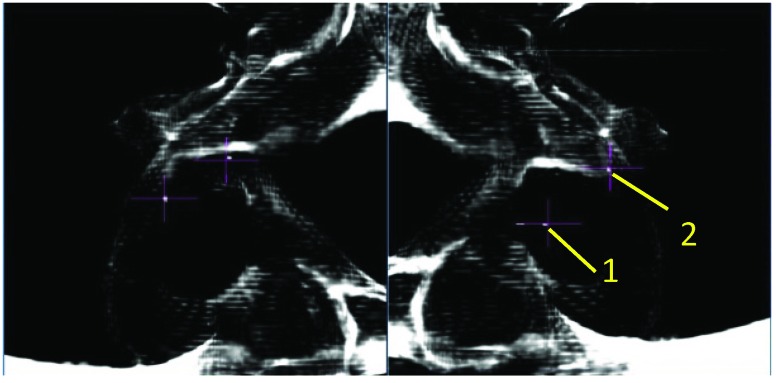
MultiPlan (Accuray Inc., Sunnyvale, California) screenshot showing fiducial placement on orthogonal kV X-ray images. In this case, the fiducials fail to produce a triangle where each internal angle is ≥15°.
Figure 2.
MultiPlan (Accuray Inc., Sunnyvale, California) screenshot showing fiducial placement on orthogonal kV X-ray images. In this case the position of the fiducial markers on DRR is too close to allow accurate fiducial extraction. This is usually caused by colinear placement of fiducial markers with respect to the orthogonal kV X-ray system.
Protocol
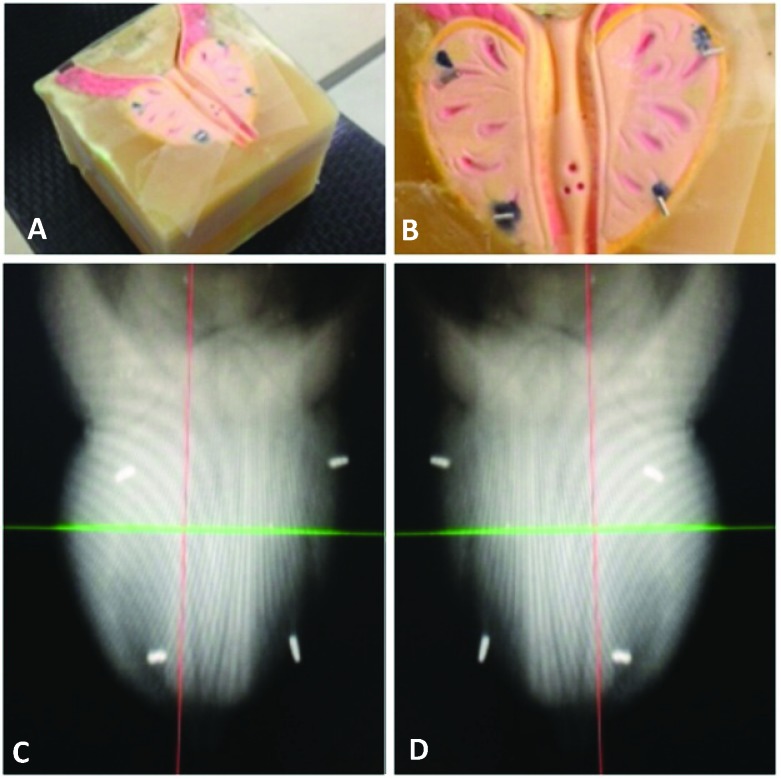
Our modified fiducial placement protocol for prostate SBRT consists of a simple and easily reproduced seed distribution that maintains the original principles of the generalized Accuray guideline with some considerations specific to prostate tracking. 1) To limit seed migration and to ensure their distribution is a reasonable surrogate for prostate position/motion, fiducial markers should be implanted within the prostate gland (inside the prostatic capsule). 2) In order to achieve a minimum distance of 2.0cm between all seeds, they should be implanted in the periphery of the prostate. 3) Implanting the seeds in a single coronal plane is simple to visualize, and it ensures non-colinear placement in the orthogonal imaging plane. 4) In order to accurately track the posterior aspect of the prostate (and thereby avoid overdosing the rectal wall) we suggest implanting all 4 fiducial markers in the postero-lateral prostate in a single coronal plane. In this way, optimal fiducial placement can be achieved through either the transrectal or transperineal route. A schematic figure outlining the idealized anatomical fiducial placement was provided to our ultrasound radiologists performing transrectal fiducial implantation (Figure 5). The idealized scenario is demonstrated using a wax prostate phantom with platinum fiducials imaged by the CyberKnife stereoscopic kV X-ray system (Figure 6, Figure 7).
Figure 5.
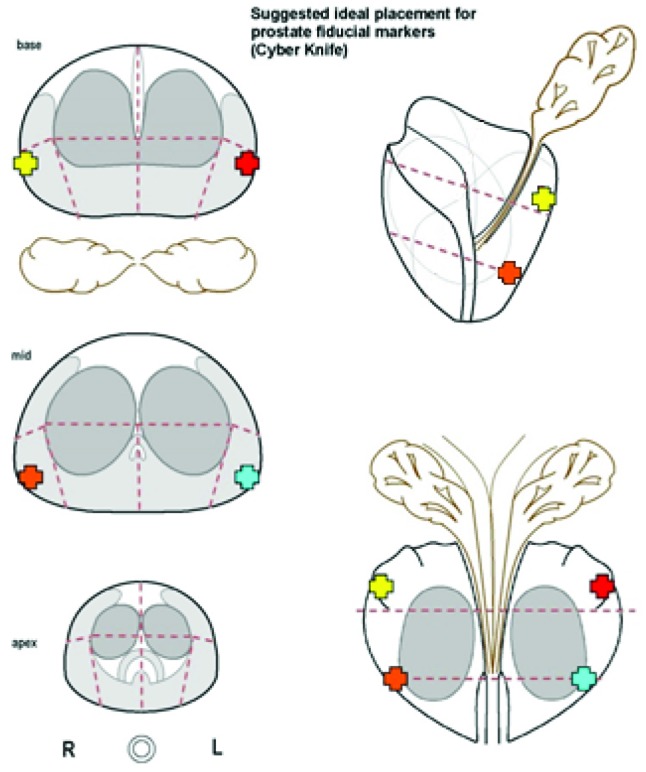
Schematic given to ultrasound radiologists at our institution showing idealized locations for fiducial implantation. 4 markers are placed in the postero-lateral prostate in a single coronal plane otherwise in accordance with Accuray’s original fiducial placement guideline.
Figure 6.
A,B. Wax prostate phantom with platinum fiducials placed in a single coronal plane in the postero-lateral prostate. C,D. Paired CyberKnife orthogonal kV X-ray images showing optimal platinum fiducial placement in the wax phantom
Figure 7.
MultiPlan (Accuray Inc., Sunnyvale, California) screenshot showing a T2-weighted turbo spin echo MRI sequence co-registered with the CT simulation scan using platinum fiducial markers imbedded in the postero-lateral prostate.
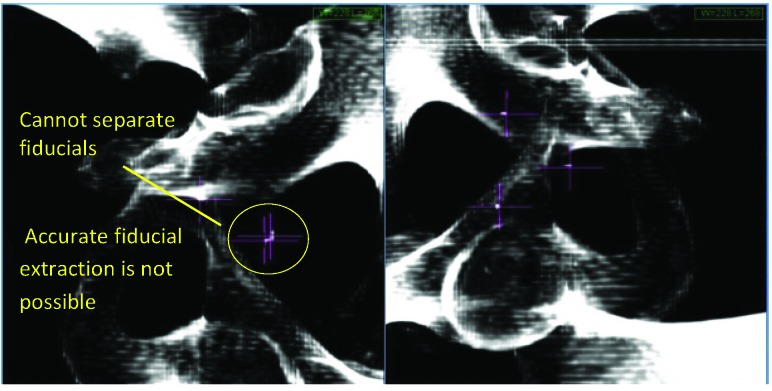
Figure 4.
MultiPlan (Accuray Inc., Sunnyvale, California) screenshot showing fiducial placement on orthogonal kV X-ray images. In this case, only two fiducials have been implanted, and a minimum of three are required to track rotations.
Methods
Between 2012 and 2017 54 patients with prostate adenocarcinoma were treated using CyberKnife SBRT. For the first 26 patients, fiducials were implanted transrectally under ultrasound guidance according to the Accuray fiducial placement guidelines (cohort 1, 26 patients). Due to unacceptably high tracking error rate, in October 2015 the fiducial placement guidelines were amended –as previously described- for all future implantations (cohort 2, 28 patients). Transrectal implantations were performed by an experienced radiologist using local anesthesia and ultrasound guidance. The patients were given prophylactic antibiotics at the time of seed implantation to reduce the risk of iatrogenic urosepsis.
Fiducials for prostate cancer should be biologically inert and easily ‘seen’ on imaging. Commercially available fiducial markers are typically made of 24-carat gold. Polymer-based seeds, and seeds containing steel or electromagnetic transponders (for Calypso) are also available (29). Platinum is a biologically inert metal with higher magnetic susceptibility than gold. Platinum fiducial seeds are well visualized on MRI, CT, and with the stereoscopic kV X-ray system, and they have been shown to outperform gold fiducials in the context of CyberKnife tracking (30). At our institution, we use platinum fiducials made in-house. Platinum fiducials are prepared using a process accredited by a third party that involves cutting high-grade platinum wire, measuring individual fiducials and sterilizing them according to our institutional protocol prior to implantation (30). Our in-house platinum seeds were used for all patients in this study. The seeds are cylindrical 0.92mm in diameter and 3.0mm in length.
All radiation plans were created using CyberKnife Multiplan software. CT simulations were done with a full bladder and an empty rectum. A second CT simulator scan was done for each patient, with a urinary catheter in place in order to visualize the urethra. T1-weighted gradient echo and T2-weighted turbo spin echo MRI sequences were co-registered with CT scans in Multiplan using the implanted platinum fiducial markers (see Figure 7). The prostate and surrounding avoidance structures (urethra, bladder, rectum, bowel, penile bulb, and testis) were contoured using planning MRI and CT scans as a reference. The PTV was defined as an anisotropic expansion around the prostate (5mm in all directions except 3mm posteriorly). A PRV urethra was created using an isotropic 2mm expansion around the urethra. Each patient received 3625cGy in 5 fractions, prescribed at the 80% isodose over a 12-day course. Patients were treated with an empty rectum prior to receiving each fraction.
Results
All 54 patients were successfully treated with CyberKnife based prostate SBRT. There were no cases of urosepsis. There were no major acute toxicities. The platinum fiducials markers were well visualized, on CT, MRI and by the stereoscopic KV X-ray tracking system, in all 54 patients. Patient age, prostatic volume and initial PSA were similar between the two groups (Table 2). Accurate 6-degree tracking (accounting for translations and rotations) was possible in all 28 patients from the protocol group (cohort 2). In 6 of 26 patients (23%) from cohort 1, only translational mapping could be calculated; potential rotational misalignments could not be observed or accounted for. A Pearson’s Chi-squared test of independence was used to test the relationship between rotational tracking and the fiducial implantation protocol used. Tracking prostate rotations was significantly more likely in patients with fiducials implanted under the modified protocol (cohort 2), X2 = 7.27, p = 0.007.
Table 2.
Patient characteristics
| Cohort 1 -Accuray protocol (n=26) |
Cohort 2 -Modified protocol (n = 28) |
|
| Median Age (range), years | 64 (50 – 74) | 65 (53 – 75) |
| Mean Prostate Volume (range),cc | 56 (22 – 125) | 47 (21 – 106) |
| Mean PSA(range), µg/L | 6.6 (1.1 – 14.7) | 6.2 (1 – 12) |
In order to assess the potential consequences of uncorrected rotations on target coverage and OARs we ran an in-house computer script to rotate the dose distributions about their align centres and recalculate the DVHs. For the 6 patients where rotations could not be tracked, the ratio of maximum dose (OARs) and minimum dose (targets) before and after rotations was calculated for 10, 5, 3, 3, 5 and 10 degree rotations along all three rotational axes. The resulting deviations in target coverage and OAR dose are summarized in Table 3 and Table 4, respectively. A 3 degree rotation could cause a drop in minimum PTV dose up to 9%, or increase in maximal rectal dose of up to 4%, these effects are exaggerated for larger rotational misalignments.
Discussion
Ultra-hypofractionation is intended to reduce toxicity and improve disease outcomes by exploiting the relatively low α/β ratio characteristic of prostate adenocarcinoma. Care must be taken to avoid increased toxicity in neighboring sensitive tissues (rectum, bladder, urethra, and bowel). CyberKnife based prostate SBRT combines an X-band linear accelerator with industrial robotics and stereoscopic kV X-ray imaging to provide sub-millimeter accuracy and intra-fraction target tracking. Accurate 6 degree tumour tracking requires implantation of radio-opaque fiducial markers identifiable by the X-ray imaging system as a surrogate for target motion. The fiducial markers must be placed properly in order to track prostate motion accounting for translations and rotations about the x y and z axes. To this end, Accuray provides a fiducial placement guideline intended for all soft tissue targets. We have built upon this guideline to improve 6 degree tracking specifically for prostate SBRT.
Despite non-optimally placed seeds in 6 cases, translations were tracked for all 54 patients. We observed increased sensitivity to seed placement in rotational tracking. In our first cohort of 26 patients, for 6 patients (23%) the so-called “relative pose problem” was only partially solved; target tracking accounted only for translations and not rotations. In this series, placing the seeds too closely was the most common cause of rotational tracking failure. By definition, close placement of fiducials leads to an increase in the ratio of positional uncertainty to interfiducial separation and has been shown to increase rotational error (28). It follows that rotational error can be reduced by decreasing the positional uncertainty of the seeds or increasing the interfiducial separation (Accuray recommends ≥2.0cm). Even when the seed spacing is at ≥2.0cm they may appear too close together on DRR; when the fiducial markers are in the same colinear plane (i.e., one behind the other from the perspective of the orthogonal kV X-ray imaging system). Following Accuray’s guideline for treating soft tissue tumours should avoid these types of tracking problems. Yet, problems may still arise without a clear plan for fiducial placement up front. Placing the fiducial markers in single coronal plane in the postero-lateral prostate (Figure 5) is easy to communicate and it helps to avoid common tracking problems. We achieved reliable seed separation and accurate rotational tracking in the entire second cohort of 28 patients.
Late rectal toxicity represents one of the most clinically significant complications of prostate SBRT –and indeed- any external beam prostate radiotherapy technique for prostate cancer. Physiologic prostate motion is greatest in the anterior-posterior and superior-inferior axes compared to medial to lateral (31). Achieving a high dose gradient is most important at the posterior (rectal) margin. We chose the posterior aspect of the prostate for fiducial implantation because it most closely approximates the rectal margin. While some degree of deformation of the prostate and rectum are expected (31,32) during and between treatments, it is unlikely that the rectal wall will bulge through the coronal plane defined by fiducial markers. Even if the prostate changes shape, the seeds will remain firmly fixed next to the high dose gradient area ensuring it is accurately tracked, potentially avoiding excess dose to the rectum and subsequent late tissue complications. Alternatively if the seeds are placed elsewhere within the prostate (e.g., centrally) deformation can occur such that the high dose area falls within the rectum.
From a technical standpoint, the modified protocol improved our CyberKnife prostate SBRT treatments by allowing consistent rotational tracking. The dosimetric significance of correcting for rotational errors is controversial (36-40). Using an in-house developed computer script we tested the dosimetric consequences of rotations up to 10 degrees along any axis of rotation (roll, tilt, and yaw). For rotations as small as 3 degrees we observed a drop in minimum PTV dose up to 9%, or increase in maximal rectal dose of up to 4%. This effect was exaggerated for larger rotational misalignments. Previous fiducial based studies on intra-fraction prostate motion have shown minimal (<2°) rotations around the S-I and A-P axes (roll and yaw, respectively) (references). However, rotations around the lateral axis (pitch), are associated with physiologic rectal and bladder filling and range between 7° to 27° with a mean of 8° (41). Our limited data suggests on average this could lead to a 13% under dosing and 7% overdosing to portions of the PTV and rectum respectively. When the target volume includes the seminal vesicles rotational corrections may be more important for improved target coverage and reduced rectal toxicity (43). Long-term objective data supporting this hypothesis are lacking and beyond the scope of this project.
Conclusion
After implementation of a modified fiducial implantation protocol a significant increase in the ability to accurately track prostate motion was achieved. This technical improvement represents improved treatment accuracy and may lead to improved disease outcomes (improved bRFS and reduced toxicity). It is recommended that any group using a stereoscopic kV X-ray imaging system for intrafraction target tracking consider adopting the fiducial placement protocol described herein.
Acknowledgments
Authors’ disclosure of potential conflicts of interest
Eric Vandervoort is the principal investigator for a research grant, unrelated to the manuscript topic, funded by Accuray Incorporated, which holds research agreements with the Ottawa Hospital Cancer Centre. Janice Doody, Julie Gratton, Elizabeth. Henderson, Oliver Holmes, Shawn Malone, Scott Morgan, Joseph O’Sullivan and Janos Szanto reported no conflicts of interest.
Author contributions
Conception and design: Oliver Holmes, Janos Szanto, Joseph O’Sullivan, Scott Morgan, Julie Gratton, Shawn Malone
Data collection: Julie Gratton, Janos Szanto, Janice Doody, Eric Vandervoort, Elizabeth Henderson, Joseph O’Sullivan, Oliver Holmes
Data analysis and interpretation: Oliver Holmes, Janos Szanto, Julie Gratton, Eric Vandervoort, Shawn Malone, Scott Morgan
Manuscript writing: Oliver Holmes, Scott Morgan, Shawn Malone, Julie Gratton
Final approval of manuscript: Oliver Holmes, Scott Morgan, Shawn Malone, Julie Gratton, Eric Vandervoort, Janice Doody, Elizabeth Henderson, Joseph O’Sullivan
References
- 1. Zelefsky MJ, Fuks Z, Hunt M, Lee HJ, Lombardi D, Ling CC, Reuter VE, Venkatraman ES, Leibel SA. High dose radiation delivered by intensity modulated conformal radiotherapy improves the outcome of localized prostate cancer. J Urol. 2001;166:876-81. [PubMed] [Google Scholar]
- 2. Viani GA, Stefano EJ, Afonso SL. Higher-than conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009. Aug 1;75(5): 1405-18. [DOI] [PubMed] [Google Scholar]
- 3. Duchesne GM, Peters LJ. What is the alpha/beta ratio for prostate cancer? Rationale for hypofractionated high-dose-rate brachytherapy [editorial]. Int J Radiat Oncol Biol Phys. 1999. Jul 1;44:747-8. [DOI] [PubMed] [Google Scholar]
- 4. Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001. Jul 15;50:1021-31. [DOI] [PubMed] [Google Scholar]
- 5. Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour HP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002. Jan 1;52:6-13. [DOI] [PubMed] [Google Scholar]
- 6. Ritter M. Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin Radiat Oncol. 2008;18(4):249-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dasu A. Is the alpha/beta value for prostate tumours low enough to be safely used in clinical trials? Clin Oncol (R Coll Radiol). 2007;19(5):289-301. [DOI] [PubMed] [Google Scholar]
- 8. Dearnaley D, Syndikus I, Mossop H, Vincent K, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet. 2016;17(8):1047-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31(31):3860-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aluwini S, Pos F, Schimmel E, van der Toorn PP, de Jager H, Alemayehu WG, Heemsbergen W, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. Lancet 2015;16:274-83. [DOI] [PubMed] [Google Scholar]
- 11. Hoffman KE, Voong KR, Pugh TJ, Skinnner H, Levy LB, Takiar V, Choi S, et al. Risk of late toxicity in men receiving dose-escalated hypofractionated intensity modulated prostate radiation therapy: Results from a randomized trial. Int J Radiat Oncol Biol Phys. 2014. Apr 1;88(5):1074-84. [DOI] [PubMed] [Google Scholar]
- 12. Lukka H, Hayter C, Jullian JA, Warde P, Morris WJ, Gospodarowicz M, Levine M, et al. Randomized trial comparing two fractionation schemes for patients with localized prostate cancer. J Clin Oncol. 2005. Sep 1;23(25) 6132-8. [DOI] [PubMed] [Google Scholar]
- 13. Yeoh EE, Botten RJ, Butters J, Di Matteo AC, Holloway RH, Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: Final results of Phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81(5):1271-8. [DOI] [PubMed] [Google Scholar]
- 14. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized Phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol. 2016;34(20):2325-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norkus D, Karklelyte A, Engels B, Vorsmessen H, Griskevicus H, De Ridder M, Storme G, et al. A randomized hypofractionation dose escalation trial for high risk prostate cancer patients: interim analysis of acute toxicity and quality of life in 124 patients. Radiat Oncol. 2013. Sep 4;8(206):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, Krol S, van der Toorn PP, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised multicentre, open-label, phase 3 trial. Lancet. 2016;17(8):1061-9. [DOI] [PubMed] [Google Scholar]
- 17. Musunuru HB, Cheung P, Loblaw A. Evolution of hypofractionated accelerated radiotherapy for prostate cancer – the Sunnybrook experience. Front Oncol. 2014;4:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. King CR, Freeman D, Kaplan I, Fuller D, Bolzicco G, Collins S, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol. 2013;109:217-21. [DOI] [PubMed] [Google Scholar]
- 19. Loblaw A, Cheung P, D’Alimonte L, Deabreu A, Mamedov A, Zhang L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: Toxicity, biochemical, and pathological outcomes. Radiother Oncol. 2013;107:153-8. [DOI] [PubMed] [Google Scholar]
- 20. Adler JR, Chang SD, Jr, Murphy MJ, Doty J, Geis P, Hancock SL. The CyberKnife: a frameless robotic system for radiosurgery. Stereotact Funct Neurosurg. 1997;69:124–8. [DOI] [PubMed] [Google Scholar]
- 21. Friedland JL, Freeman DE, Masterson-McGary ME, Spellberg DM. Steretoactic body radiotherapy: An emerging treatment approach for localized prostate cancer. Technol Canc Res Treat. 2009;8(5):387-91. [DOI] [PubMed] [Google Scholar]
- 22. McBride SM, Wong DS, Dombrowski JJ, Harkins B, Tapella P, Hanscom HN, et al. Hypofractionated stereotactic body radiotherapy in low-risk prostate adenocarcinoma. Cancer. 2012;15:3681-90 [DOI] [PubMed] [Google Scholar]
- 23. Bolzicco G, Favretto MS, Scremin E, Tambone C, Tasca A, Guglielmi R. Image-guided stereotactic body radiation therapy for clinically localized prostate cancer: preliminary clinical results. Technol Canc Res Treat. 2010. Oct;9(5):473-7. [DOI] [PubMed] [Google Scholar]
- 24. Katz AJ, Santoro M, Ashley R, Diblasio F, Witten M. Stereotactic body radiotherapy for organ-confined prostate cancer. BMC Urol. 2010;10:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fuller DB, Naitoh J, Lee C, Hardy S, Jin H. Virtual HDRSM CyberKnife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys. 2008;70(5):1588-97. [DOI] [PubMed] [Google Scholar]
- 26. King CR, Brooks JD, Gill H, Presti JC. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;82(2):877-82. [DOI] [PubMed] [Google Scholar]
- 27. Kee ST. Fiducial placement to facilitate the treatment of pancreas and liver lesions with the CyberKnife system. Accuray Incorporated 2005. [Google Scholar]
- 28. Murphy MJ. Fiducial-based targeting accuracy for external-beam radiotherapy. Med Phys. 2002;29(3):334-44. [DOI] [PubMed] [Google Scholar]
- 29. Ng M, Brown E, Williams A, Chao M, Lawrentschuk N, Chee R. Fiducial markers and spacers in prostate radiotherapy: current applications. BJU Int. 2014;113(S2):13-20. [DOI] [PubMed] [Google Scholar]
- 30. Nair VJ, Szanto J, Vandervoort E, Henderson E, Avruch L, Malone S, Pantarotto J. Feasibility, detectability and clnical experience with platinum fiducial seeds for MRI/CT fusion and real-time tumor tracking during CyberKnife stereotactic ablative radiotherapy. J Radiosurg SBRT. 2015;3:315-23. [PMC free article] [PubMed] [Google Scholar]
- 31. Schiffner DC, Gottschalk AR, Lometti M, Aubin M, Pouliot J, Speight J, et al. Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007. Feb 1;67:610-9. [DOI] [PubMed] [Google Scholar]
- 32. Wu J, Haycocks T, Alasti H, Ottewell G, Middlemiss N, Abdolell M, et al. Positioning errors and prostate motion during conformal prostate radiotherapy using on-line isocentre set-up verification and implanted prostate markers. Radiother Oncol. 2001;61:127-33. [DOI] [PubMed] [Google Scholar]
- 33. Crook JM, Raymond Y, Salhani D, Yang H, Esche B. Prostate motion during standard radiotherapy as assessed by fiducial markers. Radiother Oncol 1995;37:37-42. [DOI] [PubMed] [Google Scholar]
- 34. Malone S, Crook JM, Kendal WS, Szanto J. Respiratory-induced prostate motion: Quantification and characterization. Int J Radiat Oncol Biol Phys. 2000;48(1):105-9. [DOI] [PubMed] [Google Scholar]
- 35. Ten Haken RK, Forman JD, Heimburger DK, Gerhardsson A, McShan DL, Perez-Tamayo C, et al. Treatment planning issues related to prostate movement in response to differential filling of the rectum and bladder. Int J Radiat Oncol Biol Phys. 1991;20:1317-24. [DOI] [PubMed] [Google Scholar]
- 36. Chiesa S, Placidi L, Azario L, Mattiucci GC, Greco F, Damiani A, et al. Adaptive optimization by 6 DOF robotic couch in prostate volumetric IMRT treatment: rototranslational shift and dosimetric consequences. J Appl Clin Med Phys. 2015. Sep 8;16(5):35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amro H, Hamstra DA, McShan DL, Sadler H, Vineberg K, Hadley S, Litzenberg D. The dosimetric impact of prostate rotations during electromagnetically guided external-beam radiation therapy. Int J Rad Oncol Biol Phys. 2013;85(1):230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Haaren PMA, Bel A, Hofman P, van Vulpen M, Kotte AN, van der Heide UA. Influence of daily setup measurements and corrections on the estimated delivered dose during IMRT treatment of prostate cancer patients. Radiother Oncol. 2009;90:291-8. [DOI] [PubMed] [Google Scholar]
- 39. Guckenberger M, Roesch J, Baier K, Sweeney RA, Flentje M. Dosimetric consequences of translational and rotational errors in frame-less image-guided radiosurgery. Radiat Oncol. 2012. Apr 24;7(63):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu X, Fu D, La De, Zerda A, Bossart E, Shao H, Both J, et al. Patient alignment and target tracking in radiosurgery of soft-tissue tumors using combined fiducial and skeletal structures tracking techniques In: Urschel HC, Kresl JJ, Luketich JD, Papiez L, Timmerman RD, Schulz RA, editors. Treating Tumors that Move with Respiration. Springer, Berlin, Heidelberg; 2007. P 31-6. [Google Scholar]
- 41. Noel CE, Santanam L, Olsen JR, Baker KW, Parikh PJ. An automated method for adaptive radiation therapy for prostate cancer patients using continuous fiducial-based tracking. Phys Med Biol. 2010 Jan 7;55:65-82. [DOI] [PubMed] [Google Scholar]
- 42. Van Der Heide UA, Kotte ANTJ, Dehnad H, Hofman P, Lagenijk JJ, van Vulpen M. Analysis of fiducial marker-based position verification in the external beam radiotherapy of patients with prostate cancer. Radiother Oncol. 2007;82:38-45. [DOI] [PubMed] [Google Scholar]
- 43. Lei S, Piel N, Oermann EK, Chen V, Ju AW, Dahal KN, et al. Six-dimensional correction of intra-fractional prostate motion with CyberKnife stereotactic body radiation therapy. Front Oncol. 2011;1(48):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]