Abstract
Neuronal nicotinic acetylcholine receptors (nAChRs) belong to a super-family of Cys-loop ligand-gated ion chan-nels that respond to endogenous acetylcholine (ACh) or other cholinergic ligands. These receptors are also the targets of drugs such as nicotine (the main addictive agent delivered by cigarette smoke) and are involved in a variety of physiological and pathophysiological processes. Numerous studies have shown that the expression and/or function of nAChRs is com-promised in many neurological and psychiatric diseases.
Furthermore, recent studies have shown that neuronal nAChRs are found in a large number of non-neuronal cell types in-cluding endothelial cells, glia, immune cells, lung epithelia and cancer cells where they regulate cell differentiation, prolifera-tion and inflammatory responses.
The aim of this review is to describe the most recent findings concerning the structure and function of native nAChRs inside and outside the nervous system.
Keywords: Neuronal nicotinic acetylcholine receptor subtypes, subunit composition, ligand binding site, stochiometry, non-neuronal nicotinic acetylcholine receptors, knockout and knockout in mice
1. Introduction
The neurotransmitter acetylcholine (ACh) is synthesised, stored and released by cholinergic neurons, and exerts its effects on the central nervous system (CNS) and peripheral nervous system (PNS) through two distinct types of receptor: the muscarinic and nicotinic ACh receptors (mAChRs and nAChRs).
The ACh released by cholinergic neurons acts as a neurotransmitter, but ACh is also released by non-neuronal tissues where it is involved in cell-to-cell communication, and controls essential functions such as cell proliferation, adhesion, migration, secretion, survival and apoptosis, in an autocrine, paracrine or juxtacrine manner [1]. Together with that released by vagal nerve endings, ACh can also contribute to the cholinergic control of inflammation (reviewed in [2]). Accordingly, ACh and its synthesizing enzyme choline acetyltransferase (ChAT), are found in human and animal erythrocytes, immune cells, endothelial and epithelial cells (including airway epithelial cells) and placenta cells. Small amounts of ACh are even found in blood [1, 3-8].
In the brain, nAChRs are widely expressed, both presynaptically and postsynaptically, and are involved in several functions including learning and memory, arousal, reward, motor control, and analgesia. nAChRs are also the target of nicotine, the main addictive agent delivered by cigarette smoke [9].
A number of comprehensive reviews have previously described the structure and function of neuronal nAChRs [10-16]; the aim of this article is to provide a short overview of the structure and function of nAChR subtypes, particularly those expressed extraneuronally.
2. The structure of neuronal NICOTINIC acetylcholine receptors
Neuronal nAChRs belong to the super-family of homologous Cys-loop ion channel receptors, which include muscle-type nAChRs, GABAA, glycine and serotonin 5-HT3 receptors [16]. nAChRs are ACh-activated cationic channels consisting of nine α (α2 to α10) and three β subunits (β2-β4) (reviewed in [10-16]). The homomeric (α7 or α9) or heteromeric (α2-α6 with β2-β4) assembly of five subunits generates many distinctive subtypes that share a common basic structure, but have specific pharmacological and functional properties [10].
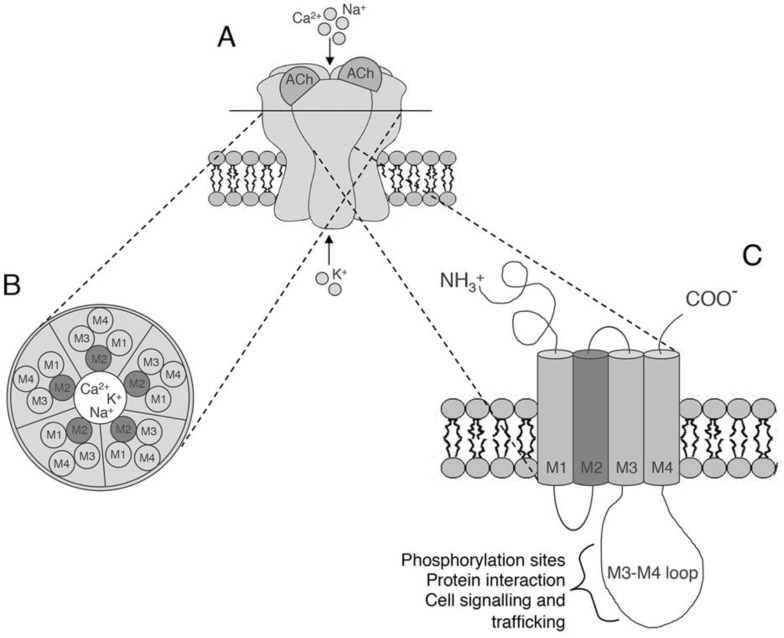
All of the subunits have a common architecture consisting of a large N-terminal extracellular domain followed by three hydrophobic transmembrane domains (M1-M3), a large cytoplasmic loop between M3 and M4, a fourth hydrophobic transmembrane domain (M4) and a short extracellular carboxyl domain (C-domain) (see Fig. 1) [15]. The M1–M4 transmembrane domains are arranged in concentric layers around the central pore: the M2 domain lines the pore membrane, M1 and M3 shield M2 from the surrounding lipid bilayer, and M4 is the most exposed to lipids (see Fig. 1) [15].
Fig. (1).
The structure of neuronal nicotinic acetylcholine receptors. A: Structure showing the arrangement of nAChR subunits and the location of the two ACh-binding sites. B) A section of the nAChR with the five subunits arranged around a central cation-conducting pore. C) A single nAChR subunit embedded in the membrane. The extracellular amino acid terminal portion is followed by three hydrophobic transmembrane domains (M1-M3), a large intracellular loop, and a fourth hydrophobic transmembrane domain (M4). The transmembrane M2 segments lining the ion path wall are shown in dark grey.
The antagonist αBungarotoxin binds and blocks some (but not all) nAChR subtypes, which have therefore been divided into two main classes: αBgtx-sensitive receptors, which may be homomeric (made up of the α7,α8 or α9) or heteromeric (α7α8, α9α10, α7β2), and αBgtx-insensitive receptors, which consist of α2-α6 and β2-β4 subunits, and bind nicotine and many nicotinic agonists with high affinity, but not αBgtx [15].
The N-terminal and transmembrane domains are well conserved among the different subunits, whereas the M3-M4 cytoplasmic loop is the most divergent and varies in length and amino acid composition [17]. This cytoplasmic loop contains multiple sequences that are important for receptor export from the endoplasmic reticulum (ER) and trafficking to the plasma membrane, sequences for post-synaptic scaffold protein interactions, and phosphorylation sites for various serine/threonine and tyrosine kinases [18-20]. Moreover, it has recently been shown that the intracellular loop of the α7 subunit contains a G protein binding cluster that promotes intracellular signalling [21, 22].
The functional properties of each subtype are unique, but overlap sufficiently to make them very difficult to distinguish using pharmacological agents, especially when the subtypes have subunits in common or contain different subunits with a high degree of homology (e.g., α3 and α6, or α2 and α4) [9].
nAChRs are not only permeable to monovalent Na+ and K+ ions, but also to Ca2+ ions. The heterologous expression of homomeric α7 and α9 nAChRs has revealed a fractional Ca2+ current comparable to that estimated for N-methyl-D-aspartate (NMDA) glutamate receptors, but much more permeable to Ca2+ ions than that of heteromeric nAChRs [23]. The ability of nAChRs to alter intracellular calcium levels leads to activation of different downstream intracellular pathways that can play a pivotal role in neuronal signalling and plasticity (reviewed in [24]).
The most widely expressed neuronal subtypes in the brain are heteromeric α4β2*(* means that additional subunits may be present) and homomeric α7 receptors, whereas α3β4* is the most widely expressed subtype in the PNS. In addition to those with different subunit compositions, some nAChRs have the same subunit composition but different subunit stoichiometry, as in the case of the α4β2 and α3β4 subtypes [9, 15]. The (α4β2)2α4 and (α4β2)2β2 stoichiometries are different in terms of calcium permeability and agonist or antagonist sensitivity, with the latter having a higher affinity for and greater sensitivity to ACh; in the case of the α3β4 subtype, (α3β4)2α3 and (α3β4)2β4 have markedly different single-channel conductance and kinetics, and differently sensitive to zinc enhancement [15, 25].
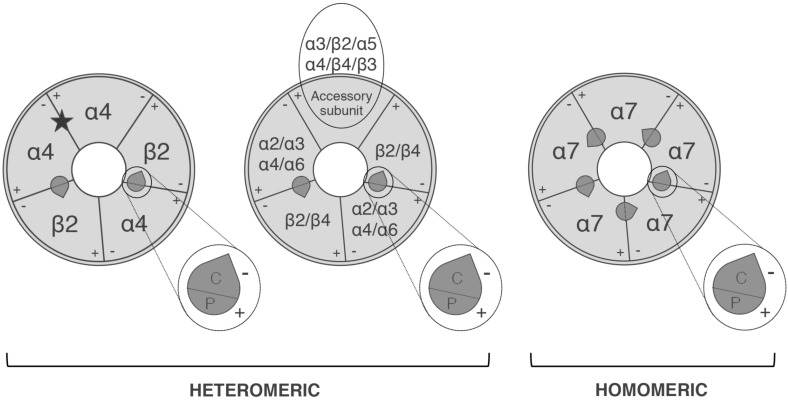
In addition to the two orthosteric binding sites at the α4/β2 interface, the (α4β2)2α4 subtype has, an additional unortodox binding site at the α4α4 interface (Fig. 2) that increases the activation of α4β2 sites [26, 27] and accelerates receptor desensitisation of (α4β2)2α4 [28].
Fig. (2).
The pentameric arrangement of nAChR subunits in an (α4)3(β2)2 subtype (left) and α7 homopentameric subtype (right). The localisations of the subunit interfaces of the orthosteric binding sites are indicated, together with the primary component P(+) carried by the α subunits and the complementary component C(-)carried by α or non-α subunit. The subunits that may occupy an accessory position are also indicated. In addition to the two orthosteric sites, the (α4)3(β2)2 subtype has a binding site at the α4α4 interface (star).
Homomeric α7 receptors are one of the two most abundant nAChR subtypes in the CNS, where they are mainly localised in the cortex, hippocampus, hypothalamus and some brain stem nuclei. However, emerging evidence demonstrates the presence of heteromeric α7 nAChRs in heterologous systems and native neurons. In heterologous systems, α7 subunits form functional channels with the β2 [29], β3 [30], β4 [31], or α5 subunit [32]. Moreover, our group has recently biochemically identified a native α7β2 subtype expressed in rodent and human basal forebrain that has different functional and pharmacological properties from those of homomeric α7 nAChRs [15, 33].
The CHRNA7 gene that codes for the α7 subunit is partially duplicated (α7dup) in humans and forms a hybrid gene with the novel FAM7A gene (CHRFAM7A), whose transcript codes for a α7dup protein that lacks the signal peptide and the ligand-binding domain for ACh, and has a lower molecular weight than the α7 subunit [34].
CHRFAM7A mRNA expression is low in human brain, but abundant in peripheral lymphocytes and tissues [35]. Functional studies have shown that α7dup, expressed in oocytes, acts as a dominant negative regulator of α7 nAChR activity by means of a mechanism involving a reduction in the number of functional α7 nAChRs incorporated into the oocyte surface (reviewed in [36]). Use of the Forster resonance energy transfer (FRET) technique has confirmed that the α7dup subunits are assembled and transported to the cell membrane together with full-length α7 subunits, and that these α7- α7dup receptors show functional alterations [37].
3. Neuronal NICOTINIC acetylcholine receptor binding sites
3.1. Ligand Binding Sites
Information about the interactions between agonists and nAChRs at atomic scale was initially provided by studies of the crystal structures of Lymnaea stagnalis ACh-binding proteins (L-AChBPs) complexed with nicotine or carbamylcholine, and Aplysia californica AChBPs (A-AChBPs) complexed with lobeline or epibatidine [38, 39]. However, homology between AChBP and nAChR extracellular domains is less than 30%, and AChBPs lack transmembrane domains. More recently, the structures of nAChRs and related receptors with both extracellular and transmembrane domains have been determined in resting, open or closed conformations [40, 41].
Homomeric and heteromeric nAChRs both made up of five subunits organised around a central channel: the homomeric receptors have five identical (orthosteric) ACh-binding sites per receptor molecule [42] located at the interface between two adjacent subunits, whereas heteromeric receptors contain at least two orthosteric ACh-binding sites at the interface between the primary (+) side of an α subunit and the complementary (−) side of a β2 or β4 subunit] (see Fig. 2) [43]. Studies of AChBPs have shown that many amino acid residues contribute to ACh binding sites. They are grouped into short sequences that form loops A, B and C (the primary component) and D, E and F (the complementary component). Loops A, B, D and F, in the middle of the interface of two adjacent subunits provide a hydrophobic pocket mainly consist of aromatic residues to which the tertiary or quaternary ammonium of nicotinic ligands bind. In the absence of an agonist or presence of an antagonist, loop C does not cover the hydrophobic pocket whereas, in the presence of an agonist, the binding site has a closed conformation and is covered by loop C [43].
The accessory subunits are those that do not directly participate in forming the orthosteric binding site. It has so far been shown that the α5, β3, α3, α4, β2 and β4 subunits can occupy the accessory position in functional receptors. The role of accessory subunits has been investigated in α4β2* subtypes, in which the presence of different accessory subunits changes their pharmacological and biophysical properties, and their sensitivity to allosteric modulators and up-regulation by nicotine [26, 44-46].
Until recently, it was thought that the α5 and β3 subunits only assembled in the accessory position, but Jin et al. [47] used the concatamer approach to express dimeric constructs of α4 and β2 subunits with a free α5 subunit, or concatameric pentameric receptors incorporating a single copy of α5 in different positions, and found that the α5 subunit can occupy the position of a non-binding subunit, or replace a β2 subunit participating in an orthosteric binding site. Moreover, Jain et al. [48] used concatamer experiments to show that α5 and β3 act as α or β subunits to form functional ACh-binding sites with an α4 subunit [49].
However, it still needs to be demonstrated that the α5 subunit can participate in the formation of an orthosteric binding site in native receptors.
In order to elicit maximal nAChR activation, ACh needs to bind two binding sites in heteromeric receptors and, in native α7 receptors, the occupancy of a single site is sufficient to give maximal activation [50]. In homomeric α7–5HT3A (lab-generated) chimeric receptors maximal activation is obtained when three agonist molecules are bound in a non-consecutive array. The binding of ACh to more than three binding sites in the α7–5HT3A receptors increases receptor desensitisation [51, 52].
3.2. Allosteric Binding Sites
Apart from the ACh-binding site (which is also called the nAChR orthosteric binding site), allosteric sites have been identified on nAChRs that modulate nAChR function (reviewed in [53]). These allosteric binding sites are located in the ion pore or the extracellular, cytoplasmic, and transmembrane domains. Allosteric modulators are a heterogeneous class of compounds that include positive allosteric modulators (PAMs), negative allosteric modulators (NAMs), and silent allosteric modulators (SAMs). Allosteric modulators often have no intrinsic activity and only modulate the effects of an agonist. PAMs typically have low intrinsic activity (although, a few can act as full agonists [54] and reduce the concentration of agonist required to achieve channel opening, enhance channel opening or decrease nAChR desensitisation. When they are administered alone, they usually do not activate nAChRs, but increase the activation elicited by endogenous nicotinic agonists, such as choline or ACh. NAMs are compounds that reduce the response to orthosteric agonists and normally include competitive and non-competitive nAChR antagonists. The non-competitive antagonists also include antagonists that bind in the channel pore and occlude ion flux (open-channel blockers) [53]. SAMs are compounds that do not potentiate or inhibit responses to orthosteric agonists, but can influence allosteric modulation by blocking the effects of other allosteric modulators.
4. Neuronal nicotinic acetylcholine receptor functions
Neuronal nAChRs in the brain are preferentially localised at presynaptic and/or preterminal sites, where they regulate the release of several excitatory or inhibitory neurotransmitters [55]. Consequently they can have opposite modulatory effects on the same circuit, depending on the inhibitory or excitatory nature of the stimulated neurons. nAChRs are expressed also at the somatodendritic postsynaptic site, where they regulate neuron depolarisation, firing and long-term potentiation [9]. Moreover these receptors are also involved in proliferation, differentiation and migration of neural progenitors [56, 57].
The development of genetically engineered mice with the targeted deletion of specific subunits (knockout, Ko) or mutations in critical receptor domains (knock-in, Kin), and the use of lentiviral vectors to re-express nAChR subunits in selected brain regions of Ko mice, has led to the in vivo identification of complex subtypes, and allowed the study of individual subtypes in specific cells and complex neurobiological systems (reviewed in [58, 59]). These studies have provided important information concerning the physiological role of different nAChR subtypes. nAChRs contribute to cognitive function, and changes in their number and/or function are associated with various pathological conditions such as cognitive disorders, anxiety, depression, Alzheimer’s and Parkinson’s disease, pain and epilepsy [12, 60-63].
nAChRs are particularly important in two critical periods of brain life: early pre- and post-natal circuit formation, and age-related cell degeneration. They are involved in neuronal survival, as it has been shown that nicotinic agonists are neuroprotective in in vivo and in vitro models [64, 65].
The use of these animal models has provided insights into the cellular and molecular mechanisms of nicotine addiction and withdrawal. Once in the bloodstream, nicotine, rapidly crosses the blood/brain barrier, and accumulates and exerts its pharmacological effects [9, 58] (including psychostimulation, reward and the reduction of stress and anxiety) in the brain by binding to nAChRs. Chronic nicotine exposure induces neural adaptations that change cell physiology and behaviour mainly as a result of activation and/or desensitisation of nAChRs. Studies of the brains of animals and smokers chronically exposed to nicotine have shown an increase in the number of nAChRs (up-regulation). The up-regulation of nAChRs has also been obtained using nicotinic agonists (cytisine, carbamylcholine and varenicline) [66, 67], antagonists (dihydro-β-erythroidine, mecamylamine) [68-70] and a partial agonist (CC4) [71]. The concentration dependence of up-regulation does not match that of other known receptor processes, such as activation, competitive inhibition, or desensitisation. There is evidence indicating that key steps in nicotine-induced up-regulation are receptor assembly [72, 73], decreased proteasomal degradation [74], trafficking [75] and cell surface expression [69]. It was once assumed that nicotine-induced up-regulation is caused by a single process, but it now seems to be the consequence of changes in various pathways and processes that have different time courses and are quantitatively different among receptor subtypes (reviewed in [19]).
The mesocorticolimbic sy stem is the central mediator of nicotine reward and reinforcement, and this connects the neurons present in the ventral tegmental area (VTA) with two principal targets: the nucleus accumbens (NAc) and the prefrontal cortex (PFC) [76]. Dopamine (DA) neurons, which project to the NAc receive both excitatory glutamatergic and cholinergic afferents that mediate nicotine reward, and inhibitory GABAergic afferents, that mediate aversion [77]. The release of these neurotransmitters is modulated by the nAChRs expressed in cholinergic, glutamatergic and GABAergic terminals [78]. By acting on the α7 receptors in glutamate terminals, acutely administrated nicotine stimulates the release of glutamate, which facilitates the burst firing of VTA DA neurons and eventually leads to LTP [79], and increases the firing rate of the GABAergic neurons of the rostromedial tegmental nucleus [80, 81]. Although nicotine facilitates GABA release, this does not elicit DA cell inhibition, probably because of the simultaneous nicotine-induced increase in glutamate release [80, 81]. By activating the α4β2 receptors on inhibitory GABAergic inputs to the VTA or GABAergic interneurons, smoked concentrations of nicotine transiently increase the release of GABA and subsequently depress it for about one hour [82]. Finally, by binding to the nAChR subtypes expressed by DA neuron cell bodies, nicotine modulates the shift towards burst firing and increases DA release in the NAc [76, 78].
Chronic nicotine treatment also activates the α7 receptors expressed on glutamatergic terminals, increases the release of glutamate (which facilitates the burst firing of VTA DA neurons), increases NMDA receptor activity, and LTP [79], but simultaneosusly induces the desensitisation of the α4β2 receptors on GABAergic terminals. Overall, these effects decrease the inhibition onto DA neurons, and increase DA release in the NAc [82].
The habenulo-interpeduncular (Hb-IPN) pathway has recently received much attention as a key pathway mediating natural and drug reinforcement [83]. The Hb-IPN consists of the medial habenula (MHb), which is composed of ventrally located cholinergic neurons and dorsally located substance P neurons, and the lateral habenula (LHb). The MHb, which co-releases glutamate (Glu), projects almost exclusively to the IPN through the fasciculus retroflexus, whereas the LHb projects directly or indirectly to midbrain areas and transmits an inhibitory signal to VTA DA neurons [84].
The Hb-IPN system expresses the highest levels and variety of nAChR subunits and subtypes in mammalian brain [85], and is the only central system expressing high levels of α3, β4 and α5 subunits. This finding has recently attracted increasing scientific attention because human genetics studies have shown a highly significant association between a number of single nucleotide polymorphisms (SNPs) in the gene cluster that codes for α3, α5 and β4 subunits and tobacco dependence and dependence-related diseases. The coding SNP α5 D398N is closely associated with nicotine consumption [86].
Animal studies have shown that α5 and β4-containing receptors in the Hb-IPN play an important role in for nicotine dependence. α5 subunit Ko mice or mice with selective knock down of the α5 subunits in the Hb develop increased nicotine intake in a self-administration paradigm that is blocked by the selective re-expression of the α5 subunit within the MHb; thus indicating that the α5-containing nAChRs located in this brain area also play an important role in regulating the negative effects of nicotine. On the contrary the overexpression of β4* nAChRs in mice decreases nicotine reinforcing properties and consumption [87]. Moreover, α5 or α2 subunit Ko mice chronically treated with nicotine show fewer somatic signs after nAChR antagonist mecamylamine-elicited withdrawal [84]. Finally, a study has shown that α2 Ko mice have enhanced nicotine self-administration behaviour [88]. These findings suggest that α5* nAChRs in the MHb, and α2* nAChRs in the IPN may underlie aversive responses to nicotine.
5. Neuronal NICOTINIC acetylcholine receptors expressed in non-neuronal cells
The mRNAs for nAChR subunits are present in skin [8], bronchial, oral and gastrointestinal epithelial cells [89], lymphocytes, macrophages, vascular endothelium and muscle fibers (together with muscle-type subunits) [1, 12]. Moreover, brain endothelial cells, which are essential components of the blood/brain barrier, express nicotinic α5, α7, β2 and β3 subunits [90], and hippocampal astrocytes [91] express α3, α4, α7, β3 and β4 subunits [92-94]. Nicotinic subunit mRNAs are also expressed by oligodendrocyte progenitor cells [84]. In addition, nAChR subunit transcripts have been detected in many cancer cell types. However it must be underlined that interpreting these data is not easy as there is not always a correlation between mRNA and expressed subunit levels. Moreover, many of the immunolocalisation and Western blot studies have used anti-receptor subunit antibodies whose specificity has been questioned [95].
The most convincing evidence of nAChRs in non-neuronal cells has been obtained pharmacologically using subtype-specific antagonists that block many cancer-promoting processes [96] and/or by comparing the effects of antagonists on Wt and Ko mice. Another successful approach is the in vivo silencing of specific subunits by means of RNA interference, a very powerful technique that has provided important information concerning the in vivo involvement of neuronal nAChRs containing particular subunits in the effects of nicotine [97, 98].
The binding of ACh or nicotine activates neuronal nAChRs thus leading to the influx of Na+ and Ca2+ and efflux of K+. This depolarises the plasma membrane and opens voltage-operated calcium channels (VGCCs), with a further influx of Ca2+ that may induce calcium-induced calcium release from the endoplasmic reticulum through the activation of IP3 and ryanodine receptors [99]. The cytoplasmic increase in calcium triggers the secretion of mitogenic factors and activates the signalling cascades involved in cell proliferation, migration and angiogenesis and the inhibition of apoptosis [100, 101].
In non-excitable cells, ionic influx through nAChRs seems to play a major role because VGCCs are poorly expressed. For this reason, Ca2+ ions flowing inside the cell through nAChRs raise the concentration of free intracellular Ca2+ [102]. In astrocytes, which have a membrane resting potential that is critically lower than that measured in neurons, ionic influx through ligand-gated channels could be mainly responsible for increasing intracellular Ca2+ levels in order to modulate Ca2+-gated channels locally [91] and activate various Ca2+-triggered signalling mechanisms globally [102].
In non-neuronal cells, signalling cascades downstream of nAChR activation may involve both ionic and non-ionic mechanisms [8] particularly the phospho/dephosphorylation of intracellular proteins [8]. Depending on the type of cell and expressed nAChR subtypes, the binding of ACh or nicotine can induce conformational changes in the nAChRs and/or associated proteins that can activate different intracellular signalling pathways and regulate gene expression [101]. In keratinocytes, the activation of nAChRs causes the inhibition of genes encoding the proteins involved in signal transduction, cell cycle regulation, apoptosis, cell-to-cell and cell-to-substrate adhesion [8, 103].
Non-neuronal cholinergic signalling uses the same nAChRs as neuronal cholinergic signalling and the nAChRs in both neuronal and non-neuronal networks are modulated by members of the ly-6 family of small proteins related to snake α-neurotoxins such as the α7 nAChR antagonist αBgtx [104-106]. These proteins include Lynx1, a glycophosphatidylinositol- anchored membrane protein that can form a stable complex, negatively regulates the responses of α4β2 and α7 nAChRs in heterologous systems and enhances the rate and extent of desensitisation of α4β2 nAChRs, thus acting as a molecular brake on nAChR function [107]. Lynx1 is expressed in normal and neoplastic lung tissue, where it limits the ability of chronic nicotine exposure to increase nAChR levels, but its levels are lower in lung cancers than in the adjacent normal lung. The knockdown of Lynx1 by siRNAs increases the growth of lung cancer cells, whereas the expression of Lynx1 in lung cancer cells decreases cell proliferation, thus suggesting that it may regulate lung cancer growth [108].
The most widely expressed non-neuronal nAChR subtypes are those containing the α5, α7, and α9 and α10 subunits. We briefly review the most important findings concerning the extraneuronal nAChRs containing these subunits.
5.1. α5-containing Receptors
The α5 subunit, which is the product of the CHRNA5 gene, has been shown to form functional channels when it is associated with the α4 and β2 or α3 and β4 subunits [11].
Human genetic studies have shown that the non-synonymous coding SNP D398N is associated with lung cancer and nicotine dependence [109]. However, as non-smokers bearing this polymorphism also are at increased risk of lung cancer, the disease may be directly caused by the polymorphism [109].
In order to investigate the role of the α5 subunit in normal bronchiolar epithelial cells and A549 lung adenocarcinoma cells, Krais et al. [97] silenced its expression and found that this significantly increased the migration of normal and tumour cells. They also found that silencing the α5 subunit increased cell capacity to invade extracellular matrix, thus indicating that the signalling of α5-containing receptors can alter the expression of the components of cell–cell and/or cell–matrix adhesion complexes. On the contrary, Sun et al. [98] found that treating the same adenocarcinoma A549 cells with α5 subunit-specific siRNA blocks the nicotine-stimulated activation of α5-containing nAChRs, and suppresses A549 cell migration and invasion. These findings suggest that, by activating α5-containing nAChRs, nicotine affects migration and invasion, but the role of these receptors in tumours is still unclear.
5.2. α7-containing Receptors
α7-containing receptors are expressed in neurons and non-excitable cells in order to mediate pro-proliferative, survival and anti-inflammatory signalling. In addition, various studies have shown the expression of α7 nAChR (as mRNA and protein), in many different cancer cells obtained from human tumours. One important characteristic of α7 is its ability to activate different downstream pathways, that stimulate the proliferation and migration of cancer cells after agonist (i.e ACh, choline, nicotine) binding, (reviewed in [101, 109].
The presence of α7 receptors in non-neuronal immune cells, in which no ACh-dependent currents can be recorded at the plasma membrane, indicates that they can also activate metabotropic-like second messenger signalling (reviewed in [110]. Accordingly, it has recently been shown that the intracellular loop of the α7 subunit contains a G protein binding cluster that promotes intracellular signalling [22]. α7-containing nAChRs on neurons and astrocytes function as ligand-gated ion channels and their calcium permeability is relatively high [23], whereas those on microglia increase intracellular calcium levels and signalling cascades without using channel function, and those on macrophages and other immunological cells signal through the JAK2/STAT3 transcription factor pathway [111].
Airway epithelium cells synthesise, store, process, secrete and reabsorb ACh, which acts as an autocrine and paracrine growth factor (reviewed in [1, 112]). In normal respiratory tissue, ACh is secreted by large and small airway epithelial and pulmonary neuroendrocrine cells but, unlike neurons, which have the high affinity transporter CHT1, some lung cells use transporter-like proteins [113]. Moreover, ACh is stored in vesicles via the vesicular ACh transporter (VACh) in neurons, whereas the secretion of ACh is not necessarily vesicular in lung cells [114]. The basal cells normally localised at the basement membrane are enriched in α7 receptors, which play a role in limiting basal cell proliferation [115]. α7 nAChRs are essential for the plasticity of the airway epithelium as α7 Ko mice show altered basal cell layer formation, hyperplasia, and uncontrolled growth [116]. These alterations are very similar to those observed in cultured human airway cells or in ex vivo human lung explants treated with the selective α7 antagonist αBgtx, or epithelial cell cultures chronically exposed to nicotine in which nicotine-induced desensitisation of α7 receptors mimics the absence of α7 nAChR [1].
When lung cancer arises from the airway epithelium, cell growth is stimulated by ACh or nicotine, and this growth loop may provide endogenous mitogenic signalling without any further activation [117]. The antimitogenic effects of α7 nAChR activity in airway epithelia are the opposite to the mitogenic effects observed in cultured lung cancer cells thus indicating that the α7 regulation of cell proliferation is different in normal epithelium and lung cancer cells.
5.3. α9 and α9-α10-containing Receptors
Normal brain does not express α9 or α9-α10 mRNAs, which are only found in cochlear and vestibular hair cells in which α9 and α10-containing receptors are involved in cochlea hair cell development [118, 119]. The CHRNA9 gene encodes a plasma membrane protein that forms homo- (α9) or hetero- (α9-α10) oligomeric cation channels that have an atypical, mixed nicotinic–muscarinic pharmacological profile [120]. Unlike the α9 subunit, the α10 subunit is only functional when it is co-expressed with an α9 subunit. In Xenopus oocytes, the co-injection of α9 and α10 subunits increases functional nAChR expression at least 100 times more than the injection of α9 alone. α9 and α9-α10 nAChRs have a number of interesting characteristics: they are activated by ACh but not by the classical agonist nicotine. Choline is also a potent selective agonist of the α9 subtype [121], whereas phosphocholine (PC) does not evoke ion current responses in Xenopus oocytes expressing functional homomeric α9 or heteromeric α9-α10 nAChRs [121]. However, preincubation with PC attenuates choline-induced ion current changes, thus suggesting that PC is a silent agonist of these two subtypes [121].
α9 or a9 and α10 subunits are expressed in most immune cells, dorsal root ganglion cells, human keratinocytes and colon and breast cancer cells. Lee et al. [122] have found that α9 nAChRs are ubiquitously expressed in many epithelial, lung and breast cancer cell lines, most of which also express α5 and α10 nAChR subunits. α9 nAChRs are also present in primary tumour and non-malignant breast tissue obtained from patients, but their expression is higher in breast cancer cells than the surrounding normal tissue. Silencing α9 nAChR expression in the tumour cells reduces their proliferation and tumorigenic potential in in vitro and in vivo assays [122].
Among all nAChR subtypes the homomeric channels consisting of α7 or α9 subunits, as well as the heteromeric nAChRs containing α9 and α10, have the greatest Ca2+ permeability. The Ca2+ ions that enter cells through nAChRs increase the concentration of intracellular free Ca2+, but experiments using various types of non-neuronal cells have shown that nicotinic effects can also be elicited in the absence of Na+ or Ca2+ entry. This suggests that downstream signalling from nAChRs expressed in non-neuronal cells may use both ionic and non-ionic pathways, and different types of signalling may be required to elicit specific biological responses to stimulation by the nAChR cellular signalling network [8, 110].
Conclusion
Endogenous cholinergic ligands, acetylcholine and choline, are widespread informational molecules used by neuronal and non-neuronal cells throughout tissues and organs for a number of essential cell functions. Accordingly, nAChRs are increasingly acknowledged as mediators of cholinergic ligands not only in neural tissues, but also in many peripheral tissues where “neuronal” nAChRs may play a major role.
This review briefly describes recent findings concerning nAChR subtype composition, stoichiometry, structure and non-ionic signalling, and their localisation and putative functions in non-neuronal tissues. One future challenge will be to use the accurate and sophisticated approaches set-up for studying the structure and functions of nAChR subtypes expressed by neuronal circuits in order to establish their functional role in non-neuronal cell types.
Acknowledgements
This work was supported by the CNR Research Project on Aging, the CNR project PRONAT and Interomics and by grants from the Fondazione Monzino (Milano), from the Fondazione Vollaro (Milano) and from UNIMORE FAR2014.
List of abbreviations
- αBgtx
αBungarotoxin
- A-AChBPs
Aplysia californica acetylcholine-binding proteins
- ACh
acetylcholine
- ChAT
choline acetyltransferase
- CNS
central nervous system
- DA
dopamine
- GABA
γ-aminobutyric acid
- Glu
glutamate
- Hb-IPN
habenulo-interpeduncular
- Kin
knock-in
- Ko
knockout
- L-AChBPs
Lymnaea stagnalis acetylcholine-binding proteins
- LHb
lateral habenula
- LTP
long-term potentiation
- mAChRs
muscarinic acetylcholine receptors
- MHb
medial habenula
- NAc
nucleus accumbens
- nAChRs
nicotinic acetylcholine receptors
- NMDA
N-methyl-D-aspartate
- PBMCs
peripheral blood-derived monocytes
- PC
phosphocholine
- PFC
prefrontal cortex
- PNS
peripheral nervous system
- SNPs
single nucleotide polymorphisms
- TM
transmembrane
- VACh
vesicular acetylcholine transporter
- VGCCs
voltage-gated calcium channels
- VTA
ventral tegmental area
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Wessler I., Kirkpatrick C.J. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 2008;154(8):1558–1571. doi: 10.1038/bjp.2008.185. [http://dx.doi.org/10.1038/bjp.2008.185]. [PMID: 18500366]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex-linking immunity and metabolism. Nat. Rev. Endocrinol. 2012;8(12):743–754. doi: 10.1038/nrendo.2012.189. [http://dx.doi.org/10.1038/nrendo.2012.189]. [PMID: 23169440]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawashima K., Watanabe N., Oohata H., Fujimoto K., Suzuki T., Ishizaki Y., Morita I., Murota S. Synthesis and release of acetylcholine by cultured bovine arterial endothelial cells. Neurosci. Lett. 1990;119(2):156–158. doi: 10.1016/0304-3940(90)90822-q. [http://dx.doi.org/10.1016/0304-3940(90)90822-Q]. [PMID: 2280888]. [DOI] [PubMed] [Google Scholar]
- 4.Kawashima K., Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol. Ther. 2000;86(1):29–48. doi: 10.1016/s0163-7258(99)00071-6. [http://dx.doi.org/ 10.1016/S0163-7258(99)00071-6]. [PMID: 10760545]. [DOI] [PubMed] [Google Scholar]
- 5.Wessler I., Kirkpatrick C.J., Racké K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol. Ther. 1998;77(1):59–79. doi: 10.1016/s0163-7258(97)00085-5. [http://dx.doi.org/10.1016/S0163-7258(97)00085-5]. [PMID: 9500159]. [DOI] [PubMed] [Google Scholar]
- 6.Klapproth H., Reinheimer T., Metzen J., Münch M., Bittinger F., Kirkpatrick C.J., Höhle K.D., Schemann M., Racké K., Wessler I. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch. Pharmacol. 1997;355(4):515–523. doi: 10.1007/pl00004977. [http://dx.doi.org/10. 1007/PL00004977]. [PMID: 9109369]. [DOI] [PubMed] [Google Scholar]
- 7.Zia S., Ndoye A., Nguyen V.T., Grando S.A. Nicotine enhances expression of the alpha 3, alpha 4, alpha 5, and alpha 7 nicotinic receptors modulating calcium metabolism and regulating adhesion and motility of respiratory epithelial cells. Res. Commun. Mol. Pathol. Pharmacol. 1997;97(3):243–262. [PMID: 9387186]. [PubMed] [Google Scholar]
- 8.Grando S.A. Connections of nicotine to cancer. Nat. Rev. Cancer. 2014;14(6):419–429. doi: 10.1038/nrc3725. [http://dx.doi.org/10.1038/nrc3725]. [PMID: 24827506]. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz N.L. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [http://dx.doi.org/10.1146/annurev.pharmtox.48. 113006.094742]. [PMID: 18834313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotti C., Clementi F., Fornari A., Gaimarri A., Guiducci S., Manfredi I., Moretti M., Pedrazzi P., Pucci L., Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 2009;78(7):703–711. doi: 10.1016/j.bcp.2009.05.024. [http://dx.doi. org/10.1016/j.bcp.2009.05.024]. [PMID: 19481063]. [DOI] [PubMed] [Google Scholar]
- 11.Hurst R., Rollema H., Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol. Ther. 2013;137(1):22–54. doi: 10.1016/j.pharmthera.2012.08.012. [http://dx.doi.org/10.1016/j.pharmthera.2012.08.012]. [PMID: 22925690]. [DOI] [PubMed] [Google Scholar]
- 12.Gotti C., Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog. Neurobiol. 2004;74(6):363–396. doi: 10.1016/j.pneurobio.2004.09.006. [http://dx. doi.org/10.1016/j.pneurobio.2004.09.006]. [PMID: 15649582]. [DOI] [PubMed] [Google Scholar]
- 13.Dani J.A., Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [http://dx.doi. org/10.1146/annurev.pharmtox.47.120505.105214]. [PMID: 17009926]. [DOI] [PubMed] [Google Scholar]
- 14.Molas S., Dierssen M. The role of nicotinic receptors in shaping and functioning of the glutamatergic system: a window into cognitive pathology. Neurosci. Biobehav. Rev. 2014;46(Pt 2):315–325. doi: 10.1016/j.neubiorev.2014.05.012. [http://dx.doi.org/10.1016/j.neubiorev.2014.05.012]. [PMID: 24879992]. [DOI] [PubMed] [Google Scholar]
- 15.Zoli M., Pistillo F., Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. 2015. [DOI] [PubMed]
- 16.Picciotto M.R., Caldarone B.J., Brunzell D.H., Zachariou V., Stevens T.R., King S.L. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol. Ther. 2001;92(2-3):89–108. doi: 10.1016/s0163-7258(01)00161-9. [http://dx.doi.org/10.1016/S0163-7258(01)00161-9]. [PMID: 11916531]. [DOI] [PubMed] [Google Scholar]
- 17.Stokes C., Treinin M., Papke R.L. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2015;36(8):514–523. doi: 10.1016/j.tips.2015.05.002. [http://dx.doi.org/10.1016/j.tips.2015.05.002]. [PMID: 26067101]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millar N.S., Harkness P.C. Assembly and trafficking of nicotinic acetylcholine receptors. Mol. Membr. Biol. 2008;25(4):279–292. doi: 10.1080/09687680802035675. [Review]. [Review]. [http://dx.doi.org/10.1080/09687680802035675]. [PMID: 18446614]. [DOI] [PubMed] [Google Scholar]
- 19.Colombo S.F., Mazzo F., Pistillo F., Gotti C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem. Pharmacol. 2013;86(8):1063–1073. doi: 10.1016/j.bcp.2013.06.023. [http://dx.doi.org/10.1016/ j.bcp.2013.06.023]. [PMID: 23830821]. [DOI] [PubMed] [Google Scholar]
- 20.Crespi A., Colombo S.F., Gotti C. Proteins and chemical chaperones involved in neuronal nicotinic receptor expression and function: an update. Br. J. Pharmacol. 2017 doi: 10.1111/bph.13777. [http://dx.doi.org/10. 1111/bph.13777]. [PMID: 28294298]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King J.R., Kabbani N. Alpha 7 nicotinic receptor coupling to heterotrimeric G proteins modulates RhoA activation, cytoskeletal motility, and structural growth. J. Neurochem. 2016;138(4):532–545. doi: 10.1111/jnc.13660. [http://dx.doi.org/10.1111/jnc.13660]. [PMID: 27167578]. [DOI] [PubMed] [Google Scholar]
- 22.King J.R., Nordman J.C., Bridges S.P., Lin M.K., Kabbani N. Identification and characterization of a G protein-binding cluster in α7 nicotinic acetylcholine receptors. J. Biol. Chem. 2015;290(33):20060–20070. doi: 10.1074/jbc.M115.647040. [http://dx.doi.org/10.1074/jbc.M115.647040]. [PMID: 26088141]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35(1):1–8. doi: 10.1016/j.ceca.2003.08.006. [http://dx.doi.org/10.1016/j.ceca. 2003.08.006]. [PMID: 14670366]. [DOI] [PubMed] [Google Scholar]
- 24.Dajas-Bailador F., Wonnacott S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004;25(6):317–324. doi: 10.1016/j.tips.2004.04.006. [http://dx.doi.org/10.1016/j.tips.2004.04. 006]. [PMID: 15165747]. [DOI] [PubMed] [Google Scholar]
- 25.Krashia P., Moroni M., Broadbent S., Hofmann G., Kracun S., Beato M., Groot-Kormelink P.J., Sivilotti L.G. Human α3β4 neuronal nicotinic receptors show different stoichiometry if they are expressed in Xenopus oocytes or mammalian HEK293 cells. PLoS One. 2010;5(10):e13611. doi: 10.1371/journal.pone.0013611. [http://dx.doi.org/10.1371/journal.pone. 0013611]. [PMID: 21049012]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J., Kuryatov A., Sriram A., Jin Z., Kamenecka T.M., Kenny P.J., Lindstrom J. An accessory agonist binding site promotes activation of α4β2* nicotinic acetylcholine receptors. J. Biol. Chem. 2015;290(22):13907–13918. doi: 10.1074/jbc.M115.646786. [http://dx.doi.org/10. 1074/jbc.M115.646786]. [PMID: 25869137]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harpsøe K., Ahring P.K., Christensen J.K., Jensen M.L., Peters D., Balle T. Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J. Neurosci. 2011;31(30):10759–10766. doi: 10.1523/JNEUROSCI.1509-11.2011. [http://dx.doi.org/10.1523/JNEUROSCI. 1509-11.2011]. [PMID: 21795528]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benallegue N., Mazzaferro S., Alcaino C., Bermudez I. The additional ACh binding site at the α4(+)/α4(-) interface of the (α4β2)2α4 nicotinic ACh receptor contributes to desensitization. Br. J. Pharmacol. 2013;170(2):304–316. doi: 10.1111/bph.12268. [http://dx.doi.org/10. 1111/bph.12268]. [PMID: 23742319]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khiroug S.S., Harkness P.C., Lamb P.W., Sudweeks S.N., Khiroug L., Millar N.S., Yakel J.L. Rat nicotinic ACh receptor alpha7 and beta2 subunits co-assemble to form functional heteromeric nicotinic receptor channels. J. Physiol. 2002;540(Pt 2):425–434. doi: 10.1113/jphysiol.2001.013847. [http://dx.doi.org/10.1113/jphysiol.2001.013847]. [PMID: 11956333]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palma E., Maggi L., Barabino B., Eusebi F., Ballivet M. Nicotinic acetylcholine receptors assembled from the alpha7 and beta3 subunits. J. Biol. Chem. 1999;274(26):18335–18340. doi: 10.1074/jbc.274.26.18335. [http://dx. doi.org/10.1074/jbc.274.26.18335]. [PMID: 10373437]. [DOI] [PubMed] [Google Scholar]
- 31.Criado M., Valor L.M., Mulet J., Gerber S., Sala S., Sala F. Expression and functional properties of α7 acetylcholine nicotinic receptors are modified in the presence of other receptor subunits. J. Neurochem. 2012;123(4):504–514. doi: 10.1111/j.1471-4159.2012.07931.x. [http://dx.doi.org/10.1111/ j.1471-4159.2012.07931.x]. [PMID: 22913551]. [DOI] [PubMed] [Google Scholar]
- 32.Girod R., Crabtree G., Ernstrom G., Ramirez-Latorre J., McGehee D., Turner J., Role L. Heteromeric complexes of alpha 5 and/or alpha 7 subunits. Effects of calcium and potential role in nicotine-induced presynaptic facilitation. Ann. N. Y. Acad. Sci. 1999;868:578–590. doi: 10.1111/j.1749-6632.1999.tb11331.x. [http://dx.doi.org/10.1111/j.1749-6632.1999. tb11331.x]. [PMID: 10414339]. [DOI] [PubMed] [Google Scholar]
- 33.Wu J., Liu Q., Tang P., Mikkelsen J.D., Shen J., Whiteaker P., Yakel J.L. Heteromeric α7β2 Nicotinic Acetylcholine Receptors in the Brain. Trends Pharmacol. Sci. 2016;37(7):562–574. doi: 10.1016/j.tips.2016.03.005. [http:// dx.doi.org/10.1016/j.tips.2016.03.005]. [PMID: 27179601]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gault J., Robinson M., Berger R., Drebing C., Logel J., Hopkins J., Moore T., Jacobs S., Meriwether J., Choi M.J., Kim E.J., Walton K., Buiting K., Davis A., Breese C., Freedman R., Leonard S. Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7). Genomics. 1998;52(2):173–185. doi: 10.1006/geno.1998.5363. [http://dx.doi.org/10. 1006/geno.1998.5363]. [PMID: 9782083]. [DOI] [PubMed] [Google Scholar]
- 35.Araud T., Graw S., Berger R., Lee M., Neveu E., Bertrand D., Leonard S. The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of α7*nAChR function. Biochem. Pharmacol. 2011;82(8):904–914. doi: 10.1016/j.bcp.2011.06.018. [http://dx.doi.org/10.1016/j.bcp.2011.06.018]. [PMID: 21718690]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinkus M.L., Graw S., Freedman R., Ross R.G., Lester H.A., Leonard S. The human CHRNA7 and CHRFAM7A genes: A review of the genetics, regulation, and function. 2015. [DOI] [PMC free article] [PubMed]
- 37.Wang Y., Xiao C., Indersmitten T., Freedman R., Leonard S., Lester H.A. The duplicated α7 subunits assemble and form functional nicotinic receptors with the full-length α7. J. Biol. Chem. 2014;289(38):26451–26463. doi: 10.1074/jbc.M114.582858. [http://dx.doi.org/10.1074/jbc.M114. 582858]. [PMID: 25056953]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celie P.H., Klaassen R.V., van Rossum-Fikkert S.E., van Elk R., van Nierop P., Smit A.B., Sixma T.K. Crystal structure of acetylcholine-binding protein from Bulinus truncatus reveals the conserved structural scaffold and sites of variation in nicotinic acetylcholine receptors. J. Biol. Chem. 2005;280(28):26457–26466. doi: 10.1074/jbc.M414476200. [http://dx.doi.org/10.1074/jbc.M414476200]. [PMID: 15899893]. [DOI] [PubMed] [Google Scholar]
- 39.Rucktooa P., Smit A.B., Sixma T.K. Insight in nAChR subtype selectivity from AChBP crystal structures. Biochem. Pharmacol. 2009;78(7):777–787. doi: 10.1016/j.bcp.2009.06.098. [http://dx.doi.org/10.1016/j.bcp.2009.06. 098]. [PMID: 19576182]. [DOI] [PubMed] [Google Scholar]
- 40.Changeux J.P., Christopoulos A. Allosteric modulation as a unifying mechanism for receptor function and regulation. Cell. 2016;166(5):1084–1102. doi: 10.1016/j.cell.2016.08.015. [http://dx.doi.org/10.1016/j.cell.2016.08.015]. [PMID: 27565340]. [DOI] [PubMed] [Google Scholar]
- 41.Morales-Perez C.L., Noviello C.M., Hibbs R.E. X-ray structure of the human α4β2 nicotinic receptor. Nature. 2016;538(7625):411–415. doi: 10.1038/nature19785. [http://dx.doi.org/10.1038/nature19785]. [PMID: 27698419]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palma E., Bertrand S., Binzoni T., Bertrand D. Neuronal nicotinic alpha 7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. J. Physiol. 1996;491(Pt 1):151–161. doi: 10.1113/jphysiol.1996.sp021203. [http://dx.doi.org/10.1113/jphysiol.1996.sp021203]. [PMID: 9011607]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taly A., Corringer P.J., Guedin D., Lestage P., Changeux J.P. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009;8(9):733–750. doi: 10.1038/nrd2927. [http://dx.doi.org/10.1038/nrd2927]. [PMID: 19721446]. [DOI] [PubMed] [Google Scholar]
- 44.Moroni M., Zwart R., Sher E., Cassels B.K., Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol. Pharmacol. 2006;70(2):755–768. doi: 10.1124/mol.106.023044. [http://dx.doi.org/10.1124/mol.106.023044]. [PMID: 16720757]. [DOI] [PubMed] [Google Scholar]
- 45.Tapia L., Kuryatov A., Lindstrom J. Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol. Pharmacol. 2007;71(3):769–776. doi: 10.1124/mol.106.030445. [http://dx.doi.org/10.1124/mol.106.030445]. [PMID: 17132685]. [DOI] [PubMed] [Google Scholar]
- 46.Kuryatov A., Onksen J., Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol. Pharmacol. 2008;74(1):132–143. doi: 10.1124/mol.108.046789. [http://dx.doi.org/10.1124/mol.108.046789]. [PMID: 18381563]. [DOI] [PubMed] [Google Scholar]
- 47.Jin X., Bermudez I., Steinbach J.H. The nicotinic α5 subunit can replace either an acetylcholine-binding or nonbinding subunit in the α4β2* neuronal nicotinic receptor. Mol. Pharmacol. 2014;85(1):11–17. doi: 10.1124/mol.113.089979. [http://dx.doi.org/10.1124/mol.113.089979]. [PMID: 24184962]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain A., Kuryatov A., Wang J., Kamenecka T.M., Lindstrom J. Unorthodox Acetylcholine Binding Sites Formed by α5 and β3 Accessory Subunits in α4β2* Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2016;291(45):23452–23463. doi: 10.1074/jbc.M116.749150. [http://dx.doi.org/10. 1074/jbc.M116.749150]. [PMID: 27645992]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J., Lindstrom J. Orthosteric and allosteric potentiation of heteromeric neuronal nicotinic acetylcholine receptors. Br. J. Pharmacol. 2017 doi: 10.1111/bph.13745. [http://dx.doi.org/10.1111/bph.13745]. [PMID: 28199738]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen N., Corradi J., Sine S.M., Bouzat C. Stoichiometry for activation of neuronal α7 nicotinic receptors. Proc. Natl. Acad. Sci. USA. 2013;110(51):20819–20824. doi: 10.1073/pnas.1315775110. [http://dx.doi.org/10.1073/pnas. 1315775110]. [PMID: 24297903]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rayes D., De Rosa M.J., Sine S.M., Bouzat C. Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J. Neurosci. 2009;29(18):6022–6032. doi: 10.1523/JNEUROSCI.0627-09.2009. [http://dx.doi.org/10.1523/JNEUROSCI.0627-09.2009]. [PMID: 19420269]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouzat C., Sine S.M. Nicotinic acetylcholine receptors at the single-channel level. Br. J. Pharmacol. 2017 doi: 10.1111/bph.13770. [http://dx.doi.org/ 10.1111/bph.13770]. [PMID: 28261794]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chatzidaki A., Millar N.S. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015;97(4):408–417. doi: 10.1016/j.bcp.2015.07.028. [http://dx.doi.org/10.1016/j.bcp.2015.07.028]. [PMID: 26231943]. [DOI] [PubMed] [Google Scholar]
- 54.Gill J.K., Savolainen M., Young G.T., Zwart R., Sher E., Millar N.S. Agonist activation of alpha7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc. Natl. Acad. Sci. USA. 2011;108(14):5867–5872. doi: 10.1073/pnas.1017975108. [http://dx.doi.org/10.1073/pnas. 1017975108]. [PMID: 21436053]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gotti C., Zoli M., Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci. 2006;27(9):482–491. doi: 10.1016/j.tips.2006.07.004. [http://dx.doi.org/10.1016/j.tips.2006. 07.004]. [PMID: 16876883]. [DOI] [PubMed] [Google Scholar]
- 56.Liu Z., Zhang J., Berg D.K. Role of endogenous nicotinic signaling in guiding neuronal development. Biochem. Pharmacol. 2007;74(8):1112–1119. doi: 10.1016/j.bcp.2007.05.022. [http://dx.doi.org/10.1016/j.bcp.2007.05.022]. [PMID: 17603025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell N.R., Fernandes C.C., Halff A.W., Berg D.K. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J. Neurosci. 2010;30(26):8734–8744. doi: 10.1523/JNEUROSCI.0931-10.2010. [http://dx.doi. org/10.1523/JNEUROSCI.0931-10.2010]. [PMID: 20592195]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Changeux J.P. Allosteric receptors: from electric organ to cognition. Annu. Rev. Pharmacol. Toxicol. 2010;50:1–38. doi: 10.1146/annurev.pharmtox.010909.105741. [http://dx.doi. org/10.1146/annurev.pharmtox.010909.105741]. [PMID: 20055696]. [DOI] [PubMed] [Google Scholar]
- 59.Stoker A.K., Markou A. Unraveling the neurobiology of nicotine dependence using genetically engineered mice. Curr. Opin. Neurobiol. 2013;23(4):493–499. doi: 10.1016/j.conb.2013.02.013. [http://dx.doi.org/10.1016/j.conb.2013. 02.013]. [PMID: 23545467]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Picciotto M.R., Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front. Biosci. 2008;13:492–504. doi: 10.2741/2695. [http://dx. doi.org/10.2741/2695]. [PMID: 17981563]. [DOI] [PubMed] [Google Scholar]
- 61.Higley M.J., Picciotto M.R. Neuromodulation by acetylcholine: examples from schizophrenia and depression. Curr. Opin. Neurobiol. 2014;29:88–95. doi: 10.1016/j.conb.2014.06.004. [http://dx.doi.org/10.1016/j.conb.2014. 06.004]. [PMID: 24983212]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lombardo S., Maskos U. Role of the nicotinic acetylcholine receptor in Alzheimer's disease pathology and treatment. 2015. [DOI] [PubMed]
- 63.Kutlu M.G., Gould T.J. Nicotine modulation of fear memories and anxiety: Implications for learning and anxiety disorders. Biochem. Pharmacol. 2015;97(4):498–511. doi: 10.1016/j.bcp.2015.07.029. [http://dx.doi.org/10. 1016/j.bcp.2015.07.029]. [PMID: 26231942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quik M., Zhang D., McGregor M., Bordia T. Alpha7 nicotinic receptors as therapeutic targets for Parkinson’s disease. Biochem. Pharmacol. 2015;97(4):399–407. doi: 10.1016/j.bcp.2015.06.014. [http://dx.doi.org/10.1016/j.bcp. 2015.06.014]. [PMID: 26093062]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalappa B.I., Sun F., Johnson S.R., Jin K., Uteshev V.V. A positive allosteric modulator of α7 nAChRs augments neuroprotective effects of endogenous nicotinic agonists in cerebral ischaemia. Br. J. Pharmacol. 2013;169(8):1862–1878. doi: 10.1111/bph.12247. [http://dx.doi.org/10. 1111/bph.12247]. [PMID: 23713819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riganti L., Matteoni C., Di Angelantonio S., Nistri A., Gaimarri A., Sparatore F., Canu-Boido C., Clementi F., Gotti C. Long-term exposure to the new nicotinic antagonist 1,2-bisN-cytisinylethane upregulates nicotinic receptor subtypes of SH-SY5Y human neuroblastoma cells. Br. J. Pharmacol. 2005;146(8):1096–1109. doi: 10.1038/sj.bjp.0706434. [http://dx.doi.org/10.1038/sj.bjp.0706434]. [PMID: 16273122]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazzo F., Pistillo F., Grazioso G., Clementi F., Borgese N., Gotti C., Colombo S.F. Nicotine-modulated subunit stoichiometry affects stability and trafficking of α3β4 nicotinic receptor. J. Neurosci. 2013;33(30):12316–12328. doi: 10.1523/JNEUROSCI.2393-13.2013. [http://dx.doi.org/10.1523/ JNEUROSCI.2393-13.2013]. [PMID: 23884938]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kishi M., Steinbach J.H. Role of the agonist binding site in up-regulation of neuronal nicotinic alpha4beta2 receptors. Mol. Pharmacol. 2006;70(6):2037–2044. doi: 10.1124/mol.106.029298. [http://dx.doi.org/10.1124/mol. 106.029298]. [PMID: 16966476]. [DOI] [PubMed] [Google Scholar]
- 69.Henderson B.J., Lester H.A. Inside-out neuropharmacology of nicotinic drugs. 2015. [DOI] [PMC free article] [PubMed]
- 70.Marks M.J., O’Neill H.C., Wynalda-Camozzi K.M., Ortiz N.C., Simmons E.E., Short C.A., Butt C.M., McIntosh J.M., Grady S.R. Chronic treatment with varenicline changes expression of four nAChR binding sites in mice. Neuropharmacology. 2015;99:142–155. doi: 10.1016/j.neuropharm.2015.07.019. [http://dx.doi.org/10.1016/j.neuropharm.2015.07.019]. [PMID: 26192545]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sala M., Braida D., Pucci L., Manfredi I., Marks M.J., Wageman C.R., Grady S.R., Loi B., Fucile S., Fasoli F., Zoli M., Tasso B., Sparatore F., Clementi F., Gotti C. CC4, a dimer of cytisine, is a selective partial agonist at α4β2/α6β2 nAChR with improved selectivity for tobacco smoking cessation. Br. J. Pharmacol. 2013;168(4):835–849. doi: 10.1111/j.1476-5381.2012.02204.x. [http://dx.doi.org/10.1111/j.1476-5381.2012.02204.x]. [PMID: 22957729]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kuryatov A., Luo J., Cooper J., Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol. Pharmacol. 2005;68(6):1839–1851. doi: 10.1124/mol.105.012419. [PMID: 16183856]. [DOI] [PubMed] [Google Scholar]
- 73.Sallette J., Pons S., Devillers-Thiery A., Soudant M., Prado de Carvalho L., Changeux J.P., Corringer P.J. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46(4):595–607. doi: 10.1016/j.neuron.2005.03.029. [http://dx.doi.org/10.1016/j.neuron. 2005.03.029]. [PMID: 15944128]. [DOI] [PubMed] [Google Scholar]
- 74.Govind A.P., Walsh H., Green W.N. Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J. Neurosci. 2012;32(6):2227–2238. doi: 10.1523/JNEUROSCI.5438-11.2012. [http://dx.doi. org/10.1523/JNEUROSCI.5438-11.2012]. [PMID: 22323734]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Darsow T., Booker T.K., Piña-Crespo J.C., Heinemann S.F. Exocytic trafficking is required for nicotine-induced up-regulation of alpha 4 beta 2 nicotinic acetylcholine receptors. J. Biol. Chem. 2005;280(18):18311–18320. doi: 10.1074/jbc.M501157200. [http://dx.doi.org/10.1074/jbc. M501157200]. [PMID: 15741168]. [DOI] [PubMed] [Google Scholar]
- 76.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69(4):628–649. doi: 10.1016/j.neuron.2011.02.010. [http://dx.doi.org/ 10.1016/j.neuron.2011.02.010]. [PMID: 21338876]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lammel S., Ion D.I., Roeper J., Malenka R.C. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70(5):855–862. doi: 10.1016/j.neuron.2011.03.025. [http://dx.doi. org/10.1016/j.neuron.2011.03.025]. [PMID: 21658580]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faure P., Tolu S., Valverde S., Naudé J. Role of nicotinic acetylcholine receptors in regulating dopamine neuron activity. Neuroscience. 2014;282:86–100. doi: 10.1016/j.neuroscience.2014.05.040. [http://dx.doi.org/10.1016/ j.neuroscience.2014.05.040]. [PMID: 24881574]. [DOI] [PubMed] [Google Scholar]
- 79.Zhao-Shea R., Liu L., Soll L.G., Improgo M.R., Meyers E.E., McIntosh J.M., Grady S.R., Marks M.J., Gardner P.D., Tapper A.R. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36(5):1021–1032. doi: 10.1038/npp.2010.240. [http://dx.doi.org/10.1038/npp. 2010.240]. [PMID: 21289604]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lecca S., Melis M., Luchicchi A., Ennas M.G., Castelli M.P., Muntoni A.L., Pistis M. Effects of drugs of abuse on putative rostromedial tegmental neurons, inhibitory afferents to midbrain dopamine cells. Neuropsychopharmacology. 2011;36(3):589–602. doi: 10.1038/npp.2010.190. [http://dx.doi.org/10.1038/npp.2010.190]. [PMID: 21048703]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lecca S., Melis M., Luchicchi A., Muntoni A.L., Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37(5):1164–1176. doi: 10.1038/npp.2011.302. [http://dx.doi.org/10.1038/npp.2011.302]. [PMID: 22169942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mansvelder H.D., Keath J.R., McGehee D.S. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [http://dx.doi.org/10.1016/S0896-6273(02)00625-6]. [PMID: 11906697]. [DOI] [PubMed] [Google Scholar]
- 83.Pistillo F., Clementi F., Zoli M., Gotti C. Nicotinic, glutamatergic and dopaminergic synaptic transmission and plasticity in the mesocorticolimbic system: focus on nicotine effects. Prog. Neurobiol. 2015;124:1–27. doi: 10.1016/j.pneurobio.2014.10.002. [http://dx.doi.org/10.1016/j.pneurobio. 2014.10.002]. [PMID: 25447802]. [DOI] [PubMed] [Google Scholar]
- 84.Picciotto M.R., Kenny P.J. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb. Perspect. Med. 2013;3(1):a012112. doi: 10.1101/cshperspect.a012112. [http://dx.doi.org/10.1101/ cshperspect.a012112]. [PMID: 23143843]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grady S.R., Moretti M., Zoli M., Marks M.J., Zanardi A., Pucci L., Clementi F., Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J. Neurosci. 2009;29(7):2272–2282. doi: 10.1523/JNEUROSCI.5121-08.2009. [http:// dx.doi.org/10.1523/JNEUROSCI.5121-08.2009]. [PMID: 19228980]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berrettini W.H., Doyle G.A. The CHRNA5-A3-B4 gene cluster in nicotine addiction. Mol. Psychiatry. 2012;17(9):856–866. doi: 10.1038/mp.2011.122. [http://dx.doi.org/10.1038/mp.2011.122]. [PMID: 21968931]. [DOI] [PubMed] [Google Scholar]
- 87.Slimak M.A., Ables J.L., Frahm S., Antolin-Fontes B., Santos-Torres J., Moretti M., Gotti C., Ibañez-Tallon I. Habenular expression of rare missense variants of the β4 nicotinic receptor subunit alters nicotine consumption. Front. Hum. Neurosci. 2014;8:12. doi: 10.3389/fnhum.2014.00012. [http://dx.doi.org/10.3389/fnhum.2014.00012]. [PMID: 24478678]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lotfipour S., Byun J.S., Leach P., Fowler C.D., Murphy N.P., Kenny P.J., Gould T.J., Boulter J. Targeted deletion of the mouse α2 nicotinic acetylcholine receptor subunit gene (Chrna2) potentiates nicotine-modulated behaviors. J. Neurosci. 2013;33(18):7728–7741. doi: 10.1523/JNEUROSCI.4731-12.2013. [http://dx.doi.org/10.1523/JNEUROSCI.4731-12.2013]. [PMID: 23637165]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arredondo J., Chernyavsky A.I., Grando S.A. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol. Ther. 2006;5(5):511–517. doi: 10.4161/cbt.5.5.2601. [http://dx.doi.org/10.4161/cbt.5.5.2601]. [PMID: 16582591]. [DOI] [PubMed] [Google Scholar]
- 90.Abbruscato T.J., Lopez S.P., Mark K.S., Hawkins B.T., Davis T.P. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J. Pharm. Sci. 2002;91(12):2525–2538. doi: 10.1002/jps.10256. [http://dx.doi.org/10.1002/jps.10256]. [PMID: 12434396]. [DOI] [PubMed] [Google Scholar]
- 91.Hösli L., Hösli E., Della B.G., Quadri L., Heuss L. Action of acetylcholine, muscarine, nicotine and antagonists on the membrane potential of astrocytes in cultured rat brainstem and spinal cord. Neurosci. Lett. 1988;92(2):165–170. doi: 10.1016/0304-3940(88)90054-7. [http://dx.doi.org/10. 1016/0304-3940(88)90054-7]. [PMID: 3185987]. [DOI] [PubMed] [Google Scholar]
- 92.Graham A.J., Ray M.A., Perry E.K., Jaros E., Perry R.H., Volsen S.G., Bose S., Evans N., Lindstrom J., Court J.A. Differential nicotinic acetylcholine receptor subunit expression in the human hippocampus. J. Chem. Neuroanat. 2003;25(2):97–113. doi: 10.1016/s0891-0618(02)00100-x. [http://dx.doi.org/10.1016/S0891-0618(02)00100-X]. [PMID: 12663058]. [DOI] [PubMed] [Google Scholar]
- 93.Sharma G., Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc. Natl. Acad. Sci. USA. 2001;98(7):4148–4153. doi: 10.1073/pnas.071540198. [http://dx.doi.org/10.1073/pnas.071540198]. [PMID: 11259680]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu W.F., Guan Z.Z., Bogdanovic N., Nordberg A. High selective expression of alpha7 nicotinic receptors on astrocytes in the brains of patients with sporadic Alzheimer’s disease and patients carrying Swedish APP 670/671 mutation: a possible association with neuritic plaques. Exp. Neurol. 2005;192(1):215–225. doi: 10.1016/j.expneurol.2004.12.015. [http:// dx.doi.org/10.1016/j.expneurol.2004.12.015]. [PMID: 15698636]. [DOI] [PubMed] [Google Scholar]
- 95.Moser N., Mechawar N., Jones I., Gochberg-Sarver A., Orr-Urtreger A., Plomann M., Salas R., Molles B., Marubio L., Roth U., Maskos U., Winzer-Serhan U., Bourgeois J.P., Le Sourd A.M., De Biasi M., Schröder H., Lindstrom J., Maelicke A., Changeux J.P., Wevers A. Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J. Neurochem. 2007;102(2):479–492. doi: 10.1111/j.1471-4159.2007.04498.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.04498.x]. [PMID: 17419810]. [DOI] [PubMed] [Google Scholar]
- 96.Wu C.H., Lee C.H., Ho Y.S. Nicotinic acetylcholine receptor-based blockade: applications of molecular targets for cancer therapy. Clin. Cancer Res. 2011;17(11):3533–3541. doi: 10.1158/1078-0432.CCR-10-2434. [http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2434]. [PMID: 21444681]. [DOI] [PubMed] [Google Scholar]
- 97.Krais A.M., Hautefeuille A.H., Cros M.P., Krutovskikh V., Tournier J.M., Birembaut P., Thépot A., Paliwal A., Herceg Z., Boffetta P., Brennan P., Hainaut P.L. CHRNA5 as negative regulator of nicotine signaling in normal and cancer bronchial cells: effects on motility, migration and p63 expression. Carcinogenesis. 2011;32(9):1388–1395. doi: 10.1093/carcin/bgr090. [http://dx.doi.org/10.1093/carcin/bgr090]. [PMID: 21586512]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sun H., Ma X. α5-nAChR modulates nicotine-induced cell migration and invasion in A549 lung cancer cells. Exp. Toxicol. Pathol. 2015;67(9):477–482. doi: 10.1016/j.etp.2015.07.001. [http://dx.doi.org/10.1016/j.etp.2015.07.001]. [PMID: 26205096]. [DOI] [PubMed] [Google Scholar]
- 99.Schaal C., Padmanabhan J., Chellappan S. The Role of nAChR and Calcium Signaling in Pancreatic Cancer Initiation and Progression. Cancers (Basel) 2015;7(3):1447–1471. doi: 10.3390/cancers7030845. [http://dx.doi.org/ 10.3390/cancers7030845]. [PMID: 26264026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ambrosi P., Becchetti A. Targeting neuronal nicotinic receptors in cancer: new ligands and potential side-effects. Recent Patents Anticancer Drug Discov. 2013;8(1):38–52. doi: 10.2174/15748928130105. [http://dx.doi.org/10. 2174/1574892811308010038]. [PMID: 22537644]. [DOI] [PubMed] [Google Scholar]
- 101.Schaal C., Chellappan S.P. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res. 2014;12(1):14–23. doi: 10.1158/1541-7786.MCR-13-0541. [http://dx.doi.org/10.1158/1541-7786.MCR-13-0541]. [PMID: 24398389]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zia S., Ndoye A., Lee T.X., Webber R.J., Grando S.A. Receptor-mediated inhibition of keratinocyte migration by nicotine involves modulations of calcium influx and intracellular concentration. J. Pharmacol. Exp. Ther. 2000;293(3):973–981. [PMID: 10869400]. [PubMed] [Google Scholar]
- 103.Grando S.A. Cholinergic control of epidermal cohesion. Exp. Dermatol. 2006;15(4):265–282. doi: 10.1111/j.0906-6705.2006.00410.x. [http://dx.doi.org/10.1111/j.0906-6705.2006.00410.x]. [PMID: 16512874]. [DOI] [PubMed] [Google Scholar]
- 104.Lyukmanova E.N., Shulepko M.A., Buldakova S.L., Kasheverov I.E., Shenkarev Z.O., Reshetnikov R.V., Filkin S.Y., Kudryavtsev D.S., Ojomoko L.O., Kryukova E.V., Dolgikh D.A., Kirpichnikov M.P., Bregestovski P.D., Tsetlin V.I. Water-soluble LYNX1 residues important for interaction with muscle-type and/or neuronal nicotinic receptors. J. Biol. Chem. 2013;288(22):15888–15899. doi: 10.1074/jbc.M112.436576. [http://dx.doi.org/10.1074/jbc.M112.436576]. [PMID: 23585571]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miwa J.M., Lester H.A., Walz A. Optimizing cholinergic tone through lynx modulators of nicotinic receptors: implications for plasticity and nicotine addiction. Physiology (Bethesda) 2012;27(4):187–199. doi: 10.1152/physiol.00002.2012. [http://dx.doi.org/10.1152/physiol.00002.2012]. [PMID: 22875450]. [DOI] [PubMed] [Google Scholar]
- 106.Tsetlin V.I. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 2015;36(2):109–123. doi: 10.1016/j.tips.2014.11.003. [http://dx.doi.org/10.1016/j.tips.2014.11.003]. [PMID: 25528970]. [DOI] [PubMed] [Google Scholar]
- 107.Ibañez-Tallon I., Miwa J.M., Wang H.L., Adams N.C., Crabtree G.W., Sine S.M., Heintz N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33(6):893–903. doi: 10.1016/s0896-6273(02)00632-3. [http://dx.doi.org/ 10.1016/S0896-6273(02)00632-3]. [PMID: 11906696]. [DOI] [PubMed] [Google Scholar]
- 108.Fu X.W., Song P.F., Spindel E.R. Role of Lynx1 and related Ly6 proteins as modulators of cholinergic signaling in normal and neoplastic bronchial epithelium. Int. Immunopharmacol. 2015;29(1):93–98. doi: 10.1016/j.intimp.2015.05.022. [http://dx.doi.org/10.1016/j.intimp.2015.05.022]. [PMID: 26025503]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mucchietto V., Crespi A., Fasoli F., Clementi F., Gotti C. Neuronal acetylcholine nicotinic receptors as new targets for lung cancer treatment. Curr. Pharm. Des. 2016;22(14):2160–2169. doi: 10.2174/1381612822666160203144114. [http://dx.doi.org/10.2174/1381612822666160203144114]. [PMID: 26845123]. [DOI] [PubMed] [Google Scholar]
- 110.Treinin M., Papke R.L., Nizri E., Ben-David Y., Mizrachi T., Brenner T. Role of the alpha7 nicotinic acetylcholine receptor and RIC-3 in the cholinergic anti-inflammatory pathway. Cent. Nerv. Syst. Agents Med. Chem. 2016 doi: 10.2174/1871524916666160829114533. [DOI] [PubMed] [Google Scholar]
- 111.de Jonge W.J., van der Zanden E.P., The F.O., Bijlsma M.F., van Westerloo D.J., Bennink R.J., Berthoud H.R., Uematsu S., Akira S., van den Wijngaard R.M., Boeckxstaens G.E. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005;6(8):844–851. doi: 10.1038/ni1229. [http://dx.doi.org/10.1038/ni1229]. [PMID: 16025117]. [DOI] [PubMed] [Google Scholar]
- 112.Spindel E.R. Cholinergic targets in lung cancer. Curr. Pharm. Des. 2016;22(14):2152–2159. doi: 10.2174/1381612822666160127114237. [http://dx.doi.org/10.2174/ 1381612822666160127114237]. [PMID: 26818857]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song P., Spindel E.R. Basic and clinical aspects of non-neuronal acetylcholine: expression of non-neuronal acetylcholine in lung cancer provides a new target for cancer therapy. J. Pharmacol. Sci. 2008;106(2):180–185. doi: 10.1254/jphs.fm0070091. [http://dx.doi.org/10.1254/jphs.FM0070091]. [PMID: 18285655]. [DOI] [PubMed] [Google Scholar]
- 114.Traiffort E., Ruat M., O’Regan S., Meunier F.M. Molecular characterization of the family of choline transporter-like proteins and their splice variants. J. Neurochem. 2005;92(5):1116–1125. doi: 10.1111/j.1471-4159.2004.02962.x. [http://dx.doi.org/10.1111/j.1471-4159.2004.02962.x]. [PMID: 15715662]. [DOI] [PubMed] [Google Scholar]
- 115.Maouche K., Polette M., Jolly T., Medjber K., Cloëz-Tayarani I., Changeux J.P., Burlet H., Terryn C., Coraux C., Zahm J.M., Birembaut P., Tournier J.M. alpha7 nicotinic acetylcholine receptor regulates airway epithelium differentiation by controlling basal cell proliferation. Am. J. Pathol. 2009;175(5):1868–1882. doi: 10.2353/ajpath.2009.090212. [http://dx.doi.org/10.2353/ajpath.2009.090212]. [PMID: 19808646]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maouche K., Medjber K., Zahm J.M., Delavoie F., Terryn C., Coraux C., Pons S., Cloëz-Tayarani I., Maskos U., Birembaut P., Tournier J.M. Contribution of α7 nicotinic receptor to airway epithelium dysfunction under nicotine exposure. Proc. Natl. Acad. Sci. USA. 2013;110(10):4099–4104. doi: 10.1073/pnas.1216939110. [http://dx.doi.org/10.1073/ pnas.1216939110]. [PMID: 23431157]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schuller H.M. Is cancer triggered by altered signalling of nicotinic acetylcholine receptors? Nat. Rev. Cancer. 2009;9(3):195–205. doi: 10.1038/nrc2590. [http://dx.doi.org/10.1038/nrc2590]. [PMID: 19194381]. [DOI] [PubMed] [Google Scholar]
- 118.Elgoyhen A.B., Johnson D.S., Boulter J., Vetter D.E., Heinemann S. Alpha 9: an acetylcholine receptor with novel pharmacological properties expressed in rat cochlear hair cells. Cell. 1994;79(4):705–715. doi: 10.1016/0092-8674(94)90555-x. [http://dx.doi.org/10.1016/0092-8674(94)90555-X]. [PMID: 7954834]. [DOI] [PubMed] [Google Scholar]
- 119.Sgard F., Charpantier E., Bertrand S., Walker N., Caput D., Graham D., Bertrand D., Besnard F. A novel human nicotinic receptor subunit, alpha10, that confers functionality to the alpha9-subunit. Mol. Pharmacol. 2002;61(1):150–159. doi: 10.1124/mol.61.1.150. [http://dx.doi.org/ 10.1124/mol.61.1.150]. [PMID: 11752216]. [DOI] [PubMed] [Google Scholar]
- 120.Verbitsky M., Rothlin C.V., Katz E., Elgoyhen A.B. Mixed nicotinic-muscarinic properties of the alpha9 nicotinic cholinergic receptor. Neuropharmacology. 2000;39(13):2515–2524. doi: 10.1016/s0028-3908(00)00124-6. [http:// dx.doi.org/10.1016/S0028-3908(00)00124-6]. [PMID: 11044723]. [DOI] [PubMed] [Google Scholar]
- 121.Richter K., Mathes V., Fronius M., Althaus M., Hecker A., Krasteva-Christ G., Padberg W., Hone A.J., McIntosh J.M., Zakrzewicz A., Grau V. Phosphocholine - an agonist of metabotropic but not of ionotropic functions of α9-containing nicotinic acetylcholine receptors. Sci. Rep. 2016;6:28660. doi: 10.1038/srep28660. [http://dx.doi.org/ 10.1038/srep28660]. [PMID: 27349288]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee C.H., Huang C.S., Chen C.S., Tu S.H., Wang Y.J., Chang Y.J., Tam K.W., Wei P.L., Cheng T.C., Chu J.S., Chen L.C., Wu C.H., Ho Y.S. Overexpression and activation of the alpha9-nicotinic receptor during tumorigenesis in human breast epithelial cells. J. Natl. Cancer Inst. 2010;102(17):1322–1335. doi: 10.1093/jnci/djq300. [http://dx. doi.org/10.1093/jnci/djq300]. [PMID: 20733118]. [DOI] [PubMed] [Google Scholar]