Abstract
Traumatic brain injury (TBI) is a major healthcare problem that affects millions of people worldwide. Despite advances in understanding and developing preventative and treatment strategies using preclinical animal models, clinical trials to date have failed, and a 'magic bullet’ for effectively treating TBI-induced damage does not exist. Thus, novel pharmacological strategies to effectively manipulate the complex and heterogeneous pathophysiology of secondary injury mechanisms are needed. Given that goal, this paper discusses the relevance and advantages of combination therapies (COMTs) for ‘multi-target manipulation’ of the secondary injury cascade by administering multiple drugs to achieve an optimal therapeutic window of opportunity (e.g., temporally broad window) and compares these regimens to monotherapies that manipulate a single target with a single drug at a given time. Furthermore, we posit that integrated mechanistic multiscale models that combine primary injury biomechanics, secondary injury mechanobiology/neurobiology, physiology, pharmacology and mathematical programming techniques could account for vast differences in the biological space and time scales and help to accelerate drug development, to optimize pharmacological COMT protocols and to improve treatment outcomes.
Keywords: Combination therapy, secondary injury, brain, multi-target, neurotherapeutic, model
1. Introduction
Traumatic brain injury (TBI) is a major cause of death and disability among both civilians and military personnel. In the United States, TBI is sustained by approximately two million people annually and is a major cause of injury-related deaths [1-5]. Although there are different approaches to classifying TBI, the types of TBI based on the mechanism of injury include closed, penetrating and blast-induced injuries. Closed TBI refers to head trauma resulting from impact or blunt force without any physical compromise to the head layers. Motor vehicle accidents, falls, and sports collisions are some examples of potential causes of closed injury. In penetrating TBI, an object physically penetrates the layers of tissue in the head, thereby causing a wound. The entry of foreign objects, such as low- or high-velocity projectiles from gunshots or fragments resulting from assaults and combat operations (including shrapnel) into the body, is an example of a penetrating injury. Finally, blast- induced TBI results from the explosion of roadside bombs such as improvised explosive devices, to which military personnel in combat-related operations are vulnerable [6-12].
Specifically, blast-induced TBI has become the signature casualty in the recent military operations in Afghanistan and Iraq and the primary reason for hospitalizations [13-18]. TBI thus presents a significant social burden and affects a large percentage of the population, including children and young adults [2, 5, 19, 20].
Clinically, the Glasgow Coma Scale (GCS) is used to assess the severity of brain injury. The GCS is a commonly used scoring system to measure neurological impairments and incorporates functions such as eye opening, verbal responses, and motor responses and also serves as a reliable neurological tool for assessing injury-induced loss of consciousness. GCS-based assessments of TBI severity encompass the following classifications: 3-8 for severe injuries, 9-12 for moderate injuries and 13-15 for mild injuries [21]. Statistics indicate that mild TBI accounts for approximately 75% of all reported TBI cases [22-26]. The diagnostic criteria for mild TBI, as defined by the American Congress of Rehabilitation Medicine, must include one of the following: loss of consciousness not exceeding 30 minutes and a GCS score of 13-15 after the loss of consciousness period; an altered mental state at the time of the accident; or memory loss of the event immediately before or after the accident, with post-traumatic amnesia not exceeding 24 hours [18, 27]. The post-concussive symptoms of mild TBI usually include headaches, sleep disturbances, nausea, impaired attention, and memory problems [28-32].
The pathophysiology associated with TBI is complex, heterogeneous and time-dependent and can be stratified into primary and secondary injury phases. Primary injury refers to the biomechanics of the initial mechanical insult to the head and the resulting effects of the forces on the brain generated by the insult. Depending on the nature of the mechanical insult (e.g., blunt impact or blast-induced), the primary injury can be focal or diffuse, i.e., localized or distributed throughout the brain parenchyma. Both linear and rotational forces resulting from acceleration/deceleration and twisting/shearing, respectively, can cause mechanical injury in the brain parenchyma [9, 11, 33]. Primary injury to the brain tissue microstructures, which results from rapid acceleration-deceleration and brain rotation, manifests as damaged axonal cytoskeletal neurofilaments, loss of membrane integrity and axonal injuries [34-37]. The physics of blast-induced brain injuries are relatively complicated because of the complex blast-wave propagation through the different layers and vascular spaces; the resulting interactions with different regions of the body; and the activation of systemic, local, and cerebral responses [6, 38-40]. Although blast injury is classified into multiple types [8, 40], the primary injury discussed in the context of blast in this article, refers to primary blast injury, i.e., the exposure of the head to a ‘blast’ wave itself and the direct effects of blast overpressure on the head and brain tissue layers.
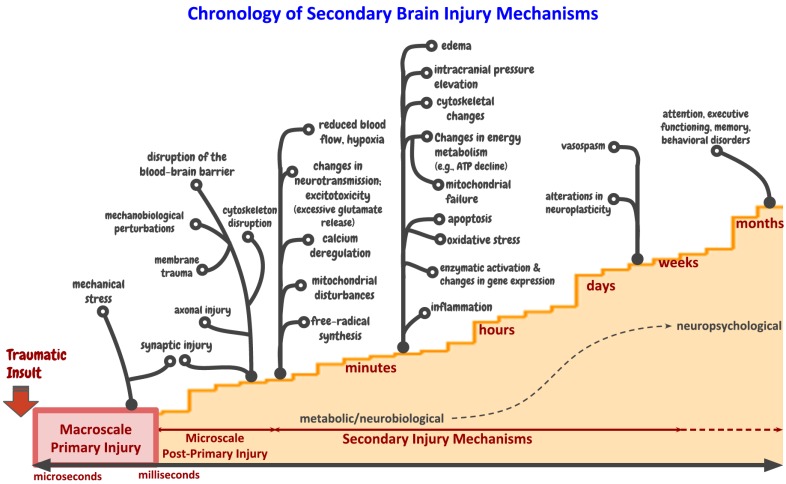
In TBI, the mechanical forces can cause axotomy, which is partial or complete severing of an axon from the neuronal cell body. This action compromises the physical integrity of the intra-axonal structures and transport processes. In the context of severe mechanical injury (e.g., rapid acceleration and deceleration insults), axonal severing that is thought to occur at the moment of the initial insult is directly attributed to the mechanical forces and is manifested by axonal disconnection and complete interruption of axonal transport processes. This sparse phenomenon, known as primary axotomy, manifests as single large axonal swelling called the “retraction ball” or terminal “axonal bulb”, which are thought to accumulate axonal transport proteins and organelles. Traumatic axonal injury can also cause partial interruption of axonal transport processes along the length of an intact axon, resulting in the formation of periodic axonal swellings called axonal varicosities. In minor forms of trauma, microtubule fragmentation, axonal disconnection and development of degeneration bulbs are attributed to a series of trauma-triggered pathological cascades spanning a large temporal window ranging from hours to days after the initial insult (a process known as secondary or delayed axotomy), although the structural continuity of the axon is conserved at the time of initial insult [34, 35, 37, 41-43]. Diffuse axonal injury (DAI), a hallmark of mild TBI, is associated with shear-induced damage to the white-matter tracts [44-48]. Although the primary micro-damage is brief and occurs within milliseconds to seconds, it initiates a slowly evolving cascade of secondary injury mechanisms comprising biophysical, vascular, neurobiological and neuropsychological deficits, as illustrated in Fig. (1) [41, 49-54]. The secondary injury cascade spans a broad time scale (from seconds to weeks) and eventually contributes to cell death and function. This complex, multiscale nature of secondary injury pathophysiology makes TBI less amenable to pharmacological treatments.
Fig. (1).
Pathogenesis of secondary brain injury mechanisms in response to primary injury (e.g., primary blast-induced or direct impact). This schematic illustrates the injury progression from primary (macroscale biomechanics) to post-primary injury (microscale mechanobiology) and culminates at the onset of time-dependent secondary injury mechanisms (biological); these pathways are largely based on preclinical models. The long duration and complexity of secondary injury provide opportunities for rational interventions, pharmacological or psychological protection, and treatment of the brain injury.
It is possible that the residual impairment following brain injury could trigger the pathogenesis of several cerebral pathologies [55-63], and several research groups have provided evidence to support this hypothesis. For example, damage to neuro-axonal structures resulting from brain injuries can trigger the onset of tauopathy, a pathological state in which irreversible phosphorylation of the tau protein causes tau to detach from the axonal site and subsequently migrate from the axon into the soma and toward the dendrites. Consequently, hyper-phosphorylated tau proteins form fibril-like aggregates called neurofibrillary tangles around the dendrites; these tangles then affect neurotransmission, synaptic integration, plasticity and other intra-neuronal/axonal processes [55, 58-60]. Although microglial activation can trigger defense mechanisms (such as neuroinflammation) to conserve physiological homeostasis and promote neuronal repair via a variety of molecular mechanisms following injury, uncontrolled and excessive neuroinflammation could act as a driver for chronic neuronal degeneration [64-66]. It is therefore not surprising that the incidence of post-traumatic neurodegenerative and neuropsychiatric diseases has risen in both civilian and military populations [7, 29, 30, 67]. Furthermore, given the heterogeneity of brain tissue, the diversity of affected neurons and glial cells (as well as their spatial variations across the brain volume) and the selective vulnerability of the neuro-axonal structures to distributed primary blast loads [61, 62, 68-73], the propensity of blast-induced TBI to cause comorbid neuropathologies (e.g., neuropsychiatric and neurodegenerative, overlapping or sequential) should not be overlooked. Thus, TBI presents a major public health challenge and is a significant burden to society, and extensive cross-disciplinary research efforts in the fields of science, biology, medicine and engineering are needed to discover effective and novel strategies that provide protective, preventative and therapeutic benefits to both civilians and military personnel as well as improve their quality of life.
1.1. The TBI Research Agenda
The ultimate goal of TBI research is to prevent, diagnose and treat TBI in both civilians and military personnel. Several important aspects need to be understood, including the mechanism by which primary injury initiates secondary injury and the sensitivity/specificity of different biomarkers to injury severity. In addition, identifying and evaluating novel targets for pharmacological protection and developing reliable extrapolation methods to predict pharmacological responses in humans are necessary. Toward that objective, U.S. research agencies have initiated several workshops and have funded research groups to understand primary and secondary injury mechanisms, to develop injury criteria and to identify and evaluate targets for pharmacological protection [74-77]. For example, the U.S. Department of Defense funds numerous TBI-related research projects (to the tune of tens of millions of dollars) that aim to prevent, protect against, diagnose and treat TBI and to improve the quality of life and psychological health of soldiers [3, 78]. Despite advances in research and development, healthcare costs associated with TBI are skyrocketing by billions of dollars [2, 4]; thus, identifying novel pharmacological strategies that effectively restrain and manipulate the evolution of injury pathways are a primary research focus to address the immediate need for better TBI treatments. We believe that computational models of neuro-axonal injury combined with complementary in vitro experiments can help to achieve TBI research objectives in the following ways: a) helping in the interpretation of experimental data, b) aiding in achieving a detailed understanding of injury mechanisms, c) determining mechanical injury thresholds, d) identifying targets for treatments, e) bridging in vitro and in vivo results, f) enabling animal-to-human scaling and g) aiding the development of optimized treatment strategies.
Given these goals, this article presents the relevance and merits of combination therapies (COMTs) for multi-target manipulation of complex secondary brain injury mechanisms by administering multiple drugs to achieve an optimal therapeutic window of opportunity (e.g., temporally broad window) and compares these merits with those of monotherapies (MONTs), which manipulate a single target with a single drug at a time. In the context of the COMTs described in the current article, the drugs are not physically combined; instead, they are administered separately to manipulate the desired targets (i.e., injury mechanisms) of the time-dependent secondary TBI cascade. Furthermore, to capture the vast differences in the mechanobiological space and time scales involved, we describe the need for a multiscale TBI model that combines primary biomechanics, secondary injury mechanobiology/neurobiology, pharmacology and mathematical programming techniques to help rationalize combination pharmacotherapy protocols and improve treatment outcomes.
1.2. Outline
The paper is organized as follows: Section two briefly reviews the different experimental models of neurotrauma that have been developed to study how primary injury triggers secondary mechanisms. Injury biomarkers, targets and drugs used for pharmacological manipulation (i.e., in MONTs) as well as the challenges inherent in TBI treatment are discussed here. The section transitions to a discussion of how COMTs utilize 'multi-target manipulation' of heterogeneous secondary injury mechanisms to mitigate TBI-induced damage. Section three describes the inherent challenges associated with combination pharmacotherapy and provides a basis for the rational design of optimal protocols to improve treatment outcomes. Section four presents the intellectual merits of model-guided design and optimization of combination pharmacotherapy for brain injury. The paper then closes with a brief overview of the current status, challenges and outlook regarding the use of modeling in future research and development efforts in this area.
2. Analysis of Secondary Brain Injury Mechanisms Toward Rational Pharmacolo-gical Manipulation of Injury Targets
Many research groups have pursued different approaches to model the effects of primary blast injury on the brain. These studies have aimed to understand how blast waves propagate through the different layers of tissue in the head and to determine the relationship between the intracranial dynamics and primary blast loads. For example, consider breacher studies, which are a type of human blast study involving a unique population among military and law enforcement personnel, known as “breachers”, who are repeatedly exposed to low-level blasts (which are considered “safe”) during training exercises and operations. The results of these studies have helped to identify not only the consequences of repeated blast injuries and related biomarkers but also the impact of blast exposures on neurocognitive performance [79, 80]. Human head surrogates, such as post-mortem human specimens, have also been used to study the effect of blast overpressure on intracranial responses [81]. Additionally, computational modeling groups have developed anatomically consistent head injury models that account for the blast-wave propagation through the tissue layers and vascular spaces of the head to predict the spatiotemporal distribution of stresses and intracranial pressures resulting from different blast overpressures [8, 82-84]. Although the data from these investigations have provided valuable insights into the biomechanical aspects of blast-wave propagation through different head layers and biological interfaces, the precise relationship between the primary and secondary injury is not straightforward (e.g., specificity of secondary injury mechanisms to primary blast loads). Thus, to systematically investigate TBI-induced effects, the etiology of secondary injury mechanisms, and the safety and efficacy of new compounds; to identify targets amenable for pharmacological manipulation; and to design optimal pharmacotherapy protocols and methods for human extrapolation, preclinical models of brain injury using well-defined protocols and procedures are often employed as a starting point. An overview of the preclinical models (both animal and in vitro) used to elucidate the primary and secondary brain injury mechanisms for the development of pharmacotherapies is provided below.
2.1. Preclinical Models of Brain Injury: Insights into Neuroprotective Mechanisms and Targets
In animal models of TBI, rodents are typically used as biological specimens to characterize experimental neurotrauma because of their small size, modest cost and ease of manipulation for experiments. Several excellent reviews of the different animal models of TBI (both blunt and blast-induced) have been previously reported [85-90]. These animal models have provided valuable insight into the pathophysiology of secondary injury and the extracellular and intracellular mechanisms that are involved as well as relevant biomarkers, as illustrated in Fig. (1) and briefly described below. Some of the critical mechanisms involve impairments to the following processes: neurophysiological processes, which result in elevated intracranial pressure; metabolic processes, which result in regional hypoperfusion (i.e., due to reduced cerebral blood flow) and impaired neurovascular coupling and tissue metabolism; abnormal neurotransmission, which results in excessive neurotransmitter spillover, unintended transport and excitotoxicity in the extracellular space; microglial activation; and mitochondrial processes, which result in oxidative stress, nitrosative stress and apoptosis [51, 91-94]. For example, Giza and Hovda investigated the neurochemical and metabolic cascades following brain injury and found that the onset of cerebral pathophysiology was characterized by an increase in the glycolytic rate [95-97]. Margulies et al. also provided an excellent overview of the different injury mechanisms occurring 72 hours after injury in rodent brain injury models [50]. Additionally, experimental models have helped to identify the increased presence and altered dynamics of autophagy after brain injury, although its mechanistic role in potentiating neuronal cell death and cross-talk with other secondary injury mechanisms is not readily apparent [98-102].
In addition to animal models, in vitro experiments have provided unique insights into the mechanobiological and intracellular aspects of neuro-axonal injury after a mechanical or blast-induced insult. A detailed description of the different in vitro approaches to neurotrauma is provided elsewhere [103-106]. Although the complexity of the in vivo brain environment cannot be replicated in vitro, elucidating injury mechanisms in vitro serves as a useful basis for identifying therapeutic targets and developing treatments. For example, in vitro models of neurotrauma using mechanical cell stretching or simulated blast protocols have provided valuable information on the electrophysiological and metabolic consequences of secondary injury that include membrane trauma-induced left shift of the voltage dependence of sodium channels [107, 108]; impairments to axonal microtubule-tau structures, transport processes, formation, axonal bulbs and varicosities [34-37, 109]; and impairments to cellular energy metabolism, mitochondrial energetics, calcium signaling, and apoptosis [110-112]. Furthermore, cell stretching experiments have identified the phenomenon of ‘molecular cross-talk’, in which injury mechanisms such as oxidative stress, nitrosative stress and caspase-mediated apoptosis compete to induce cell death. Of particular interest is the role of peroxynitrite, which acts as an effective switch between apoptotic and oxidant-mediated cell death by inhibiting caspase-3-mediated apoptosis via cysteine oxidation after neurotrauma [113]. Additionally, in vitro experiments have revealed that integrin pathways contribute to morphological and cellular changes associated with TBI [114, 115]. Injury-induced mechanical perturbations to the neuronal membrane can affect integrin transduction mechanisms and cause abnormal activation of Rho-associated protein kinase (ROCK) signaling pathways; the resulting dysregulation of ROCK activity can trigger the pathogenesis of several neurological disorders [116]. Likewise, complementary in silico models of experimental neurotrauma have shed light into how blast-induced stretching and shearing of the neuronal synapses (known as synaptic injury) may cause a provisional disconnect of the neural circuitry and a transient loss in neuronal communication [117].
In addition to identifying the pathways involved in the injury, preclinical models have helped to identify biomarkers of brain injury and pharmacological targets for mitigating the effects of TBI. These biomarkers are usually components of the aforementioned secondary injury pathways and comprise proteins and fragments of cell signaling pathways, endogenously synthesized substances and physiological, biomechanical and electrophysiological quantities (variables/parameters) at different levels of the organism (i.e., from cell-level biology to organ-level physiology). Therefore, the relevant biomarkers objectively reflect the physiological state of the organism (i.e., healthy or diseased), and their concentrations are thought to vary when an underlying pathology initiates and develops over time (e.g., progressive neurological disorders). Therefore, a qualitative and quantitative understanding of biomarker dynamics in both healthy and injured states is an important step toward developing rational treatment protocols, elucidating dose-response relationships and improving outcomes. Biomarkers often serve as primary endpoints in basic and clinical research to predict outcomes and assess the safety and efficacy of drug compounds during the development of new therapies [118-121]. Accordingly, the identification of disease-specific biomarkers has become a primary focus of many research groups. An excellent review of brain injury biomarkers is discussed elsewhere [31, 122-130]. Although significant progress has been made in the context of injury pathways and biomarkers, determining the specificity and sensitivity of these biomarkers for assessing TBI severity (from primary blast or direct impact) and implementing these biomarkers as clinical diagnostic tools on a large scale remain challenging [131].
A comprehensive review of compounds known to exert neuroprotective effects after brain injury and their corresponding mechanisms of action are discussed in the literature. At least 80 different categories of neuroprotective agents have been tested in preclinical and clinical trials with varying degrees of success; Table 1 lists a few of them [3, 5, 76, 132-138]. These agents include ion channel blockers; calcium antagonists; mitochondrial protective agents; nanoparticles; glutamate transport promoters; NMDA (N-methyl-D-aspartic acid) antagonists (i.e., blocking the glycine and polyamine sites); NMDA receptor antagonists (competitive or non-competitive); AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptor antagonists; non-NMDA excitatory amino acid antagonists; cholinergic agents; endogenous gasotransmitters and neuromodulators (e.g., nitric oxide [NO], hydrogen [H2] and hydrogen sulfide [H2S]); apoptosis inhibitors; acetylcholinesterase inhibitors; nootropics; phosphodiesterase inhibitors; immunosuppressants; antioxidants, antibiotics; growth factors; statins; neuropeptides; hormones; glutamatergic agents; ROCK inhibitors; anti-inflammatory agents; corticotrophin; immune modulators; and nutraceuticals. Recently, the use of nucleic acid therapeutics to downregulate the undruggable targets within the TBI cascade has been explored in vitro [139]. Furthermore, non-pharmacological methods such as hypothermia induction have also been explored to treat brain injury [140, 141]; the hypothesis underlying this approach is that mild-to-moderate hypothermia may hinder the TBI-initiated metabolic processes that exacerbate the effects of injury. A detailed discussion of the different aspects and effects of hypothermia therapy is beyond the scope of this article.
Table 1.
Selected list of neurotherapeutics that have been evaluated for the treatment of brain injury in preclinical and clinical trials.
| Drug Category (Neurotherapeutic) | Mechanism of Action/Outcome(s) |
|---|---|
| Hormone therapy (17β estradiol, E2) |
• exerts neuroprotective effects via genomic mechanisms (delayed onset and prolonged duration via nuclear estrogen receptors) and non-genomic mechanisms (rapid onset and short duration via estrogen receptors in the plasma membrane resulting in the activation of signaling cascades such as kinase pathways—ERK/MAPK and PI3K/Akt, CREB, etc.) [207-209]; exerts neurotrophic effects [210] • regulates ion channels, second messengers, and kinase signaling pathways and reduces intracellular calcium overload to promote neuronal viability [207, 211] • promotes energy metabolism and mitochondrial function in metabolically compromised states to exert neuroprotection [212, 213] • attenuates glutamate-induced calcium overload in primary rat hippocampal neuron cultures [214] • attenuates abnormal excitation of neurons following perturbations in cerebral blood flow [215] • attenuates glutamate-induced calcium overload in primary rat hippocampal neuron cultures [214] and the accumulation of extracellular excitatory amino acids [216] • improves outcomes after cerebral ischemia and promotes cerebral blood flow recovery in experimental models [217-219] |
| Hormone therapy (estrogen sulfate, E2-SO4) |
• exerts both genomic and non-genomic effects; increases cerebral perfusion pressure; stabilizes the blood-brain barrier; decreases neuronal degeneration, apoptosis, and reactive astrogliosis; edema and intracranial pressure; increases cerebral glycolysis in a rat TBI model [220] |
| Hormone therapy (progesterone) |
• modulates excitotoxicity [160] • downregulates TBI-induced inflammation and cerebral edema [158, 160, 162, 221]; attenuates TBI-induced activation of the TLR/NF-κB signaling pathway to improve outcomes [222]; reconstitutes the blood-brain barrier [161] • clinical trials [153, 163, 223] |
| Tetracycline antibiotics (e.g., minocycline, doxycycline) |
• exhibit anti-inflammatory and anti-apoptotic properties; reduce TBI-mediated tissue injury and caspase-1 activity; improve spatial memory and neurological outcome after TBI [224, 225] |
| Acetylcholinesterase Inhibitors (e.g., donepezil, rivastigmine, galantamine) | • increase synaptic acetylcholine by inhibiting acetylcholinesterase breakdown in the synapse; reduce edema and improve cognitive outcomes [3, 19] |
| Immunosuppressant Cyclosporin A | • maintains the mitochondrial membrane homeostasis by inhibiting the opening of the mitochondrial permeability transition pore; maintains calcium homeostasis [3, 5, 226, 227] |
| Erythropoietin | • attenuates glutamate toxicity; have anti-apoptotic, antioxidant, and anti-inflammatory effects; increases hematocrit level; stimulates neurogenesis [3] |
| ROCK Inhibitors (e.g., fasudil [HA-1077]) |
• reduce neuronal focal swelling after neuronal injury in vitro [114]; improves neurological functions after ischemia stroke, subarachnoid hemorrhage and other central nervous system disorders, observed in clinical trials [228-230] |
| Antioxidants (e.g., Cu/Zn SOD, PEG-SOD, tirilazad, dexanabinol) |
• inhibit free radical-induced oxidative damage and lipid peroxidation and its effects in potentiating cellular injury [135, 136] |
| Antioxidant nanoparticles (e.g., ceria) | • reduces free radical damage, calcium dysregulation and neuronal death in vitro and in vivo [231, 232] • preserves the lifespan of mixed organotypic cultures of brain cells and pure neurons, while preserving normal calcium signaling during the extended lifespan [233, 234] • radical scavenging activity of ceria is regenerative under biological conditions permitting sustained activity [132, 233, 235-237] |
| Nootropics (e.g., BMY-21502, cerebrolysin, pyrrolidine derivatives) |
• known to improve cognitive functions in rat TBI models [26, 132, 138] |
| Drug Category (Neurotherapeutic) | Mechanism of Action/Outcome(s) |
| Nutraceuticals (e.g., vitamins, creatine, nicotinamide, resveratrol, curcumin) |
• nutritional agents/food supplements known to protect the brain [132, 238] |
| Gasotransmitters (e.g., NO therapeutics, molecular H2, H2S) |
• these gases are synthesized endogenously and act as key modulators on intracellular pathways to exert certain regulatory functions such as vasoactivity, signal transmission and neurotransmitter release [132, 239, 240]; and antioxidant, anti-inflammatory and anti-apoptotic effects [241-243]. The role of NO seems debatable as it can act as both a signaling molecule and a neurotoxin [244]. In particular, NO derived from endothelial nitric oxide synthase (eNOS) is thought to possess neuroprotective properties whereas NO derived from inducible nitric oxide synthase (iNOS) appears to have neurotoxic properties [240]. Accordingly, iNOS inhibitors have been evaluated for their neuroprotective properties [245, 246]. |
2.2. Why Have Current Therapies Failed Despite Increasing Knowledge of the Injury Mechanisms?
Although a myriad of experimental investigations has shed light on the pathophysiology of brain injury mechanisms and associated comorbidities, little is known regarding how macroscopic biomechanical forces cause cellular injury [5, 19, 142, 143]. Furthermore, the precise path of secondary injury progression, i.e., the trajectory of injury and its dynamic progression in the human brain, remains unknown. This non-deterministic nature of the injury trajectory limits the design of rational TBI pharmacotherapies; consequently, clinical trials have failed to demonstrate the efficacy of treatments. This lack of success is likely attributed to several factors, including the complexity and heterogeneity of TBI pathophysiology; the choice of drugs, dosages, delivery routes, dosing regimens, and/or treatment durations; and an incomplete understanding of the pharmacokinetics (PK), dose-response relationships and therapeutic windows [135, 143]. Therefore, there is currently no ‘magic bullet’ for delaying the progression of secondary injury.
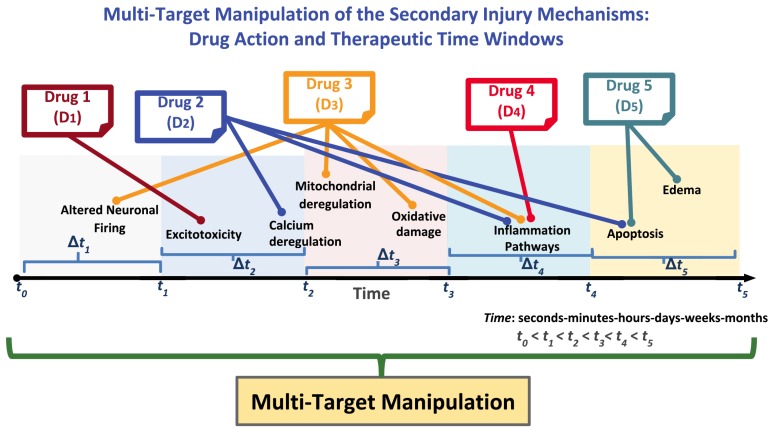
To overcome the abovementioned challenges, novel therapies that target multiple components of the secondary injury cascade need to be developed. Thus, instead of relying on a single drug and target at a time, the administration of multiple drugs may result in the multi-target manipulation as illustrated in Fig. (2). Such therapies in which multiple drugs are administered to achieve the desired pharmacodynamic (PD) response by broadening the therapeutic window of opportunity are known as COMTs or ‘polytherapies’. COMTs have shown promise in treating conditions such as tuberculosis, cancer, hypertension, rheumatoid arthritis, chronic hepatitis and blood pressure [144-147]. In COMTs, the actions of multiple drugs may lead to synergistic, additive, or antagonistic responses or, in certain cases, no effect. Antagonistic outcomes are not desirable for drug combinations, and synergistic outcomes may be more desirable than additive outcomes [3]. Regarding the toxicity and efficacy of COMTs, the criteria that these therapies should meet as suggested by Krainer include the following: (a) “each component should have single-agent activity with no cross-resistance”, (b) “there should be preclinical evidence of synergistic action” and (c) “the components should have non-overlapping safety profiles” [148].
Fig. (2).
Conceptual diagram of multi-target manipulation of the dynamically evolving secondary injury cascade using combination therapy as illustrated on the temporal axis (t1-t5). Different drugs (e.g., D1-D5) act on their respective targets, i.e., on the specific injury mechanism, within the time windows (Δt1-5). The combined drug actions aim to broaden the therapeutic window of opportunity to achieve better treatment outcomes.
3. COMT for Multi-Target Manipulation of Secondary Brain Injury Mechanisms
The starting point for the rational design of COMT protocols is the identification of MONTs that have demonstrated efficacy both in vitro and in animal models of TBI (e.g., the compounds listed in Table 1). Table 2 provides a list of COMTs that have been explored to treat preclinical models of brain injuries. Although preclinical MONTs have provided preliminary insights into specific drug combinations
Table 2.
Examples of COMTs that have been evaluated in preclinical and clinical trials for the treatment of brain injury.
| Combination Therapy | Experimental Model [Observation] |
|---|---|
|
#Progesterone (P4) and vitamin D hormone (VDH) [149] |
Neuronal cultures (oxygen glucose deprivation model in primary cortical neurons) and the ischemic transient middle cerebral artery occlusion model in rats [P4 and VDH demonstrated neuroprotection when administered individually; the effective concentration of VDH in COMT was lower than that observed in the MONT; the drug combination modulated neuroinflammation, oxidative damage and growth factors by triggering brain-derived neurotrophic factor (BDNF)/TRK-B/ERK1/2 signaling, resulting in a smaller infarct volume and improved functional recovery] |
| #Glypromate and minocycline [143] | Rat TBI model [synergistically decreased microglial reactivity and impaired axonal transport and caspase-3 activation] |
| Marrow stromal cells and simvastatin [247] | Rat TBI model [synergistic drug effect improved functional outcomes] |
| Dexamethasone and melatonin [248] | Mouse TBI model [synergistic drug effect reduced edema, oxidative stress, brain infarction and expression of apoptotic proteins] |
| Vitamin D3, progesterone, omega-3 fatty acids and glutamine [249] | Clinical trials [downregulated cytokine production, prevented oxidative stress (free radical oxygen formation), and reduced cerebral edema and inflammation] |
|
#Probenecid and N-acetyl cysteine (NAC) [133, 143, 250] |
Rat TBI model [probenecid increased the brain penetration/bioavailability and therapeutic potential of NAC] |
| Minocycline and glutathione precursor NAC [251, 252] |
Rat TBI model [drug combination reduced myelin loss, improved spaced learning, modulated inflammation and attenuated CD68-expressing phagocytic microglia without astrocyte activation at impact site; synergistic drug effect improved executive function and long-term memory] |
| Minocycline and botulinum toxin (Botox)-induced limb constraint [253] | Rat TBI model [synergistic drug effect reduced inflammation and spatial memory impairment] |
| E2 and memantine [254] | Organotypic hippocampal-slice cultures from Sprague-Dawley rats were subjected to TBI in vitro [synergistic drug effect attributed to memantine blocking the deleterious E2-mediated enhancement of NMDA receptors; drug combination significantly reduced cell death] |
|
#Small interfering RNA (siRNA) targeting aquaporin water channel (siAQP4) and c-Jun N-terminal kinase-1 inhibitor (D-JNKI-1) [143, 255, 256] |
Rat TBI model [the combination of siAQP4 and D-JNKI-1 improved spatial memory in comparison to nontreated juvenile rats two months after injury; improvement plateaued due to statistically indistinguishable outcomes between COMTs and MONTs (i.e., siAQP4 or D-JNKI-1 alone) for behavioral outcomes] |
| #Creatine and choline [143] | Rat TBI model [drug combination did not lead to additive or synergistic actions; no significant improvements over monotherapies [257-260]] |
| #Nicotinamide and progesterone [261] | Rat TBI model [drug combination offered improved neuroprotection and functional recovery in sensorimotor tasks; reduced neuronal degeneration and glial response after injury] |
#NIH-sponsored study.
and their dosages and neuroprotective effects, COMT experiments using MONT drugs can lead to different outcomes. For example, combined pre-injury creatine and post-injury choline administration did not improve the treatment outcomes compared to creatine and choline monotherapies [143]. In a preclinical COMT experiment evaluating the neuroprotective efficacy of the combination of progesterone and vitamin D against ischemic injury, Atif et al. showed that the concentration of vitamin D necessary to maximize the efficacy in COMT was different than that tested in MONT; the vitamin D concentration required to achieve a therapeutic effect in the COMT experiment was lower than that in the MONT experiment [149]. Furthermore, a recent article by Margulies et al. mentioned that among the six National Institutes of Health (NIH)-sponsored studies testing the efficacy of COMTs in preclinical TBI models, only one study demonstrated significant improvements in long-term behavioral outcomes [143]. We posit that an in silico modeling platform can help determine these uncertainties objectively a priori and help design pharmacotherapy protocols to maximize treatment outcomes in experimental trials.
The following subsections highlight the inherent challenges in (a) quantifying brain injury mechanisms via injury metrics, (b) designing optimal combination pharmacotherapies for brain injury and (c) extrapolating the MONT outcomes from specific preclinical TBI models to either preclinical COMTs or clinical MONTs.
3.1. Experimental Quantification of Brain Injury and Correlation with Human Injury
Preclinical TBI models have aided in enhancing our understanding of the effects of external forces on internal stress fields and assessing the safety and efficacy of neuroprotective compounds (partly discussed in Section Two). However, the applicability of animal experimental results to human predictions is not straightforward because of the inherent physiological differences between animals and humans and their dissimilar anthropometric and anatomical characteristics. The use of animal-to-human scaling laws that aim to translate the biomechanical quantities (variables/parameters) in conjunction with in silico brain injury models are reported elsewhere [87, 150]. Going forward, we anticipate that the development of new formulations will correlate primary injury quantities (variables/parameters) with secondary injury pathways, drug dosage and the desired pharmacodynamic response as well as provide strategies for in vitro to in vivo correlation.
3.2. Design Challenges Associated with Combination Pharmacotherapy
3.2.1. Knowledge of the PK and PD of COMTs
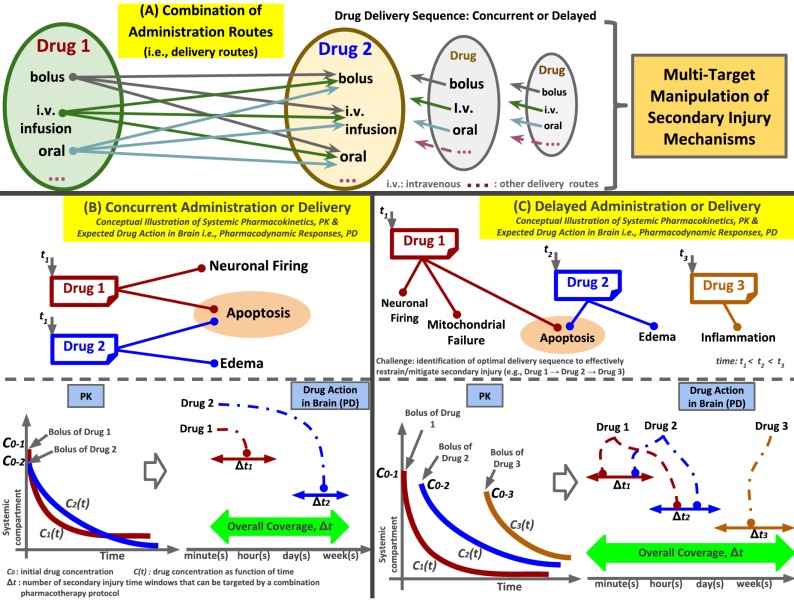
Although the concept of COMTs seems logical and promising, addressing the different design aspects that contribute to the pharmacokinetic outcomes of COMTs is a challenging endeavor. For example, the administration route (i.e., the delivery route), the drug delivery sequence, the ‘administration scenario’ (i.e., prophylaxis or treatment), the number of targets to manipulate, and the pharmacokinetic and pharmacodynamic outcomes of the combined administration should be considered, illustrated in Fig. (3A-C). Furthermore, the pharmacotherapy and resultant outcomes of a drug used in MONT when used in conjunction with another drug are not obvious (i.e., PK, PD and toxicodynamics [TD]). Thus, a rational choice of the pharmacotherapy variables should rely on the intended targets and the expected PK of the drug such that the drug can elicit the desired PD response when present at the anatomical target at a therapeutic concentration. Regarding sequencing, drugs can be administered (i.e., delivered) either concurrently (i.e., at the same time t1) via drug-specific delivery routes (i.e., intravenous infusion or oral) or sequentially (i.e., at different time points, such as t1, t2, and t3). For example, concurrent drug administration may be adopted to effectively target mechanisms that are proximal to each other on the temporal axis, which is conceptually illustrated in Fig. (3B) (e.g., injury mechanisms in time windows Δt1 and Δt2), whereas delayed administration can be utilized to manipulate mechanisms across different temporal intervals that are not proximal to each other, which is conceptually illustrated in Fig. (3C) (e.g., injury mechanisms in time windows, Δt1 and Δt3). In COMTs, when multiple drugs target a specific injury mechanism (e.g., apoptosis in Fig. 3B & C), it is conceivable that the progression of that mechanism is effectively restrained. Therefore, the therapeutic window of opportunity (also termed as ‘overall coverage’, Δt) of a combination pharmacotherapy protocol (i.e., the number of secondary injury time windows that a COMT protocol can target) will rely on the proper selection of drug candidates, administration routes (i.e., delivery routes), drug sequencing and administration scenarios.
Fig. (3).
TBI combination pharmacotherapy and multi-target manipulation of secondary injury pathways. (A) Combinations of drug administration routes (i.e., delivery routes such as intravenous bolus, intravenous infusion and oral) and (B-C) conceptual illustrations of expected pharmacological responses based on the drug sequencing (concurrent or delayed). In the context of the COMT described here, the drugs are not physically combined; instead, they are separately administered to effectively manipulate the desired targets (i.e., injury mechanisms) in the time-dependent secondary TBI cascade. In concurrent administration, the drugs are administered (i.e., delivered) at the same time point t1, whereas in delayed administration, the drugs are administered at different times (i.e., t1, t2, and t3). The pharmacokinetic profiles, C(t), the drug bioavailability, the pharmacodynamic responses in the brain and the therapeutic window of opportunity (i.e., the number of secondary injury time windows, Δt, that can be targeted by a combination pharmacotherapy protocol, termed ’overall coverage’ here) will rely on the proper selection of drug candidates, administration routes, drug sequencing and ‘administration scenarios’ (i.e., prophylaxis or treatment, not to be confused with administration routes). Model-guided simulations can help identify the optimal drugs and their sequence and can help design pharmacotherapy protocols to achieve the desired outcomes (i.e., safety and efficacy) by optimizing these therapeutic variables.
3.2.2. Information on the TD of COMTs
Another challenge is the potential for drug-drug interactions (DDIs) and the possible toxicity of the candidate drug combinations in an in vivo environment. Although DDIs of certain combinations (e.g., those between progesterone and other drugs) may not be clinically significant, the same may not hold true for other combinations, such as combinations of anticonvulsants and barbiturates, which can lead to adverse outcomes [151]. Furthermore, empirically designed COMT experiments could result in inexplicable pharmacokinetic and toxicity profiles that can convolute the extrapolation protocols.
3.3. Adaptation of Preclinical MONT for Preclinical COMT
3.3.1. Rigorous and Comprehensive Investigation of MONT
In preclinical studies, the safety and efficacy of MONTs must be evaluated for different injury severities (mild, moderate and severe) and in different species (rats, pigs and possibly primates) as well as account for population heterogeneity (e.g., the proportions of females and males). Reproducible, high-quality data from such experiments are required for reliable animal-to-human extrapolation [143].
3.3.2. Rationale for Assessing Treatment Outcomes for COMT
In addition to commonly used primary and secondary outcomes such as the Glasgow Outcome Scale, the disability rating scale, and the functional independence measure, the wealth of information on TBI biomarkers must be used to further define primary and secondary outcomes and to provide useful insights into the design of clinical trials [152, 153]. Furthermore, preclinical studies must consider neuropsychological outcome measures to evaluate the efficacy of COMTs and to assess the differences between MONTs and COMTs. Excellent discussions of the use of TBI outcome measures have been previously reported in the literature [154, 155].
3.3.3. Extrapolation of Preclinical MONTs to Design Clinical MONTs
Although MONTs have shown beneficial results in preclinical models (see Table 1), it is unclear whether the experimental outcomes can be scaled to reliably predict human responses. Some drugs that have worked well in animal models have performed poorly in human TBI trials. These discrepancies or failures to attain clinical success could be attributed to the inherent differences in organ physiology and cellular biology between animals and humans [156, 157] and the imprecise application of animal dose-response relationships for assessing safety and efficacy in humans during extrapolation. Specific examples of clinical therapeutic failures (i.e., with no clinical benefits or different clinical outcomes) are listed below:
Example 1: Consider the clinical performance of progesterone—although progesterone exerted pleiotropic effects and was associated with improved outcomes in preclinical animal TBI models [3, 158-162], the therapeutic potential of progesterone was not observed in multicenter Phase III clinical trials, such as the NIH-funded ProTECT trial (Progesterone for the Treatment of Traumatic Brain Injury III, ClinicalTrials.gov number NCT00822900) [163, 164] and the BHR Pharma-funded SYNAPSE trial (Study of the Neuroprotective Activity of Progesterone in Severe Traumatic Brain Injuries, ClinicalTrials.gov number NCT01143064) [152]. It has been postulated that the clinical failure of progesterone was due to the intrinsic complexity of TBI mechanisms acting in parallel, a lack of preclinical data from different animal models (rodents, higher animals and possibly primates) and reliable biomarkers and the absence of reliable multi-dimensional TBI characterization methods beyond the commonly used TBI measures [142, 152].
Example 2: Consider the efficacy of citicoline as a TBI treatment in Phase III clinical study called CORBIT (Citicoline Brain Injury Treatment Trial, ClinicalTrials.gov number NCT00545662): ninety days of citicoline administration did not improve the functional or cognitive states of TBI patients [165, 166].
Example 3: Although the combined estrogen/progestin formulation Prempro exhibited neuroprotective effects in animal models, no positive effects were observed in clinical trials; instead, Prempro increased the risk of dementia and stroke [167-169].
Considering the abovementioned challenges, a systematic approach to rationally design combination pharmacotherapy protocols is a necessary first step to improve treatment outcomes as well as facilitate and accelerate clinical testing. We posit that multiscale computational algorithms that integrate whole-body physiology/biology, drug pharmacology (i.e., PK, PD, and TD), primary and secondary injury mechanisms, and embedded mathematical programming techniques (optimization) can help accelerate the development of personalized therapies and improve treatment outcomes. To this end, the next section briefly presents the current status, challenges and outlook for future research and development in this area.
4. Modeling of Brain Injury Mechanisms: Current Status and Recommendations for Model-Guided Pharmacotherapy
Computational modeling of the different aspects of TBI has matured over the last few decades. Regarding primary injury biomechanics, several groups have developed analytical, spring-mass-damper and computational fluid dynamics (CFD) models to describe macroscopic brain biomechanics, accidental impacts and vehicle crashes [82-84, 170-175]. In particular, advanced three-dimensional CFD finite-element models of the cranial anatomy have gained popularity for simulating impact and blast biomechanics [8, 176, 177]. With steady refinements, these models have provided insight into how brain injury perturbs the distribution of stress/strain fields and intracranial pressure within the brain parenchyma. Furthermore, these models are being adapted to rationalize the design of protective equipment such as helmets. However, we suggest that the scope of the existing models of primary injury biomechanics can be further augmented by accounting for anatomically and physiologically consistent features (e.g., the elasticity of the skull, the flexibility of the neck and head, and the presence of cerebrospinal fluid), multiscale coupling of macroscale body/brain scale biomechanics with microscale mechanobiology to quantify the effects of primary micro-damage to neuro-axonal structures as well as by improving solvers to accommodate the spatiotemporal scales.
Regarding progress in neuronal electrophysiology, a myriad of in vitro and in vivo investigations from several experimental and modeling groups have shed light on the electrophysiological and biological aspects of both single neuron activity and large neuronal networks and have also provided neurobiological data supporting the development and validation of mathematical models [178-184]. While the outcomes have resulted in the development of simulators such as NEURON, GENESIS, MOOSE and LfPy [185-191], little progress has been made in modeling the microscale mechanobiology, electrophysiology and systems biology of damaged neuro-axonal structures. Regarding the secondary injury mechanisms, although individual models of the different injury pathways have been developed, a combined model linking the temporally distributed mechanisms has not been developed [192-198] partially because of a lack of precise information on the secondary injury trajectory in response to primary injury loads. Additionally, model-guided pharmacological manipulation of these pathways (supported by experimental data) has not been fully explored. Furthermore, the merits of using mathematical programming techniques for designing optimal pharmacotherapy protocols to achieve these outcomes have not yet been evaluated.
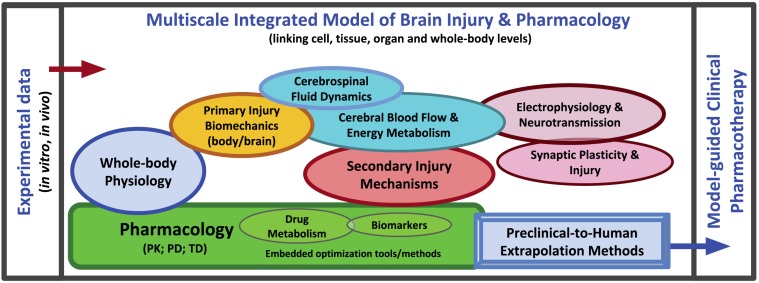
Going forward, we envision an integrated multiscale computational model of TBI that combines models of primary injury biomechanics, blood flow, cerebral hemodynamics, cerebrospinal fluid dynamics, secondary injury mechanisms, physiological and mechanobiological/neurobiological processes, and pharmacology (i.e., PK/PD/TD responses); a schematic of this integration is shown in Fig. (4). Once an effective multiscale computational model of brain injury pharmacology has been developed and validated with complementary experimental data, the integrated model will enable researchers to address the ultimate goals TBI research (as discussed in Section two), which are summarized below:
Fig. (4).
An integrated multiscale TBI model linking the model components across different spatial and temporal scales for the rational design and optimization of pharmacotherapies (i.e., MONTs and COMTs), drug evaluation and research. This article identifies the need to develop such models in the near future and supports the notion that model-guided approaches can help to accelerate drug development and rationalize treatment protocols to improve treatment outcomes.
understand how primary injury initiates the pathogenesis of secondary injury mechanisms
shed light on the injury trajectory, injury pathways and drug targets
provide a platform for conducting virtual experiments to explore the safety and efficacy of novel prophylactic and neurotherapeutic strategies in manipulating injury pathways and to facilitate the development of drug candidates
re-evaluate the clinical efficacy of drugs that have shown promise in preclinical trials but have failed in subsequent clinical trials
conduct dose-response studies to design reproducible experiments with scalable outcomes
extrapolate in vitro and animal data to predict human-relevant responses
Preclinical data from novel in vitro platforms such as human-on-chip platforms, which aim to integrate multiple organs on a chip to emulate the human responses to drugs, can also be explored to investigate the pharmacokinetic properties and PK/PD/TD responses of novel neurotherapeutics as well as potential synergistic effects when delivered in conjunction with other drugs [199-202]. In an ongoing U.S. Defense Advanced Research Projects Agency (DARPA)-funded project, computational approaches are being developed to rationalize the use of in vitro organ-on-chip technology to extrapolate on-chip data, with the goals of predicting human responses, accelerating drug testing [203, 204], and anticipating human responses using in vitro to in vivo extrapolation methods [205].
Regarding combination pharmacotherapies, the multiscale TBI model will seek to accomplish the following:
to help adapt preclinical MONT candidates for COMTs and to identify the optimal drug administration sequence to achieve a temporally broad therapeutic window of opportunity
to predict the PK, PD and TD of COMTs and to identify the synergistic, additive and antagonistic effects
to identify the design challenges associated with COMTs and to engineer methods to devise optimal pharmacotherapy protocols
to extrapolate preclinical COMT protocols to predict human-relevant responses
4.1. Example of Model-guided Optimization of COMT Protocols
Mathematically, the design of optimal COMT protocols can be considered a constrained optimization problem in which the objective is to effectively target multiple secondary injury mechanisms with drug safety and efficacy as regulatory constraints i.e., the combined therapy must be efficacious and should not cause toxicity due to potential DDIs, undesirable PK or anomalous drug behavior at either the anatomical target or other peripheral regions. Table 3 mathematically illustrates the constraints and optimization parameters necessary to achieve the proposed objective. A mathematical description of such optimization methods is discussed elsewhere [206].
Table 3.
Illustration of the mathematical optimization of COMT protocols to maximize efficacy without compromising the safety constraints (i.e., drug toxicity). Multiscale integrated models of brain injury linked to whole-body PK/PD/TD that are embedded with optimization techniques can accelerate drug development, experimental and regulatory testing, evaluation, and clinical translation.
|
Objective: To achieve the desired therapeutic window of opportunity (e.g., broad overall coverage) for the effective multi-target manipulation of secondary injury mechanisms Constraints: Regulatory constraints, i.e., safety and efficacy Safety (e.g., toxicity due to drug overload, washout to unintended areas, DDIs in the in vivo environment, antagonistic effects) Efficacy (e.g., primary and secondary biomarkers of treatment outcomes) Optimization parameters (i.e., the pharmacotherapy design variables/parameters): What to administer (drug candidates) How to administer (concurrent or delayed, inlet concentration, and other related factors) Delivery route (intravenous bolus or infusion, oral or other) When to administer (treatment or prophylaxis, i.e., pre- and/or post-injury administration times, termed as ‘administration scenarios’) |
| Expected outcome(s): In silico model-guided design of optimal pharmacotherapy protocol(s) can be implemented to effectively manipulate secondary brain injury mechanisms within the safety limits and to gain insights into the systemic pharmacokinetics, bioavailability and action of drug(s) in the brain microenvironment (pharmacodynamics). |
To the best of our knowledge, a multiscale data-driven computational model for predicting the extent of TBI-induced brain damage and identifying the pharmacological avenues for mitigating brain damage within a unified multiscale framework has not yet been reported in the published literature. In the near future, we envision that a model-guided approach will help to improve the development and performance of protective, preventative and treatment strategies. Model-guided COMTs can help to rationalize combination pharmacotherapy protocols by identifying the optimal candidates for COMTs and their ideal delivery sequences and doses to effectively contain and mitigate the progression of secondary injury mechanisms and improve treatment outcomes. Furthermore, a rigorously validated multiscale TBI model can help predict the safety and efficacy of new therapeutic compounds for experimental investigations a priori. In the long-term, the model-guided approach has the potential to revolutionize the management and treatment of head injuries resulting from sports, motor vehicle accidents and combat missions.
Acknowledgements
M.R.S. and A.J.P. acknowledge financial support from the U.S. Department of Defense Congressionally Directed Medical Research Programs (CDMRP) under contract W81XWH-11-2-0057 for this work. MRS and AJP would also like to thank Prof. Irshad Chaudry, PhD, and Dr. William Hubbard, PhD, at the University of Alabama at Birmingham for sharing their scientific insights on neuropharmacology from a related project.
list of Abbreviations
- Akt
Protein kinase B (PKB)
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CFD
Computational fluid dynamics
- CORBIT
Citicoline Brain Injury Treatment Trial
- COMT
Combination therapy or therapies
- CREB
cAMP response element binding protein, a cellular transcription factor
- DAI
Diffuse axonal injury
- DARPA
U.S. Defense Advanced Projects Research Agency
- DDIs
Drug-drug interactions
- E2
17β Estradiol
- eNOS
endothelial nitic oxide synthase
- ERK
Extracellular signal-regulated kinase
- GCS
Glasgow Coma Scale
- GENESIS
General Neural Simulation System
- IGF
Insulin-like growth factor
- iNOS
Inducible nitic oxide synthase
- MAPK
Mitogen-activated protein kinase
- MOOSE
Multiscale Object-Oriented Simulation Environment
- MPS
Maximum principal strain
- MONT
Monotherapy or monotherapies
- NMDA
N-methyl-D-aspartic acid or N-methyl-D-aspartate
- NF-κB
Nuclear factor κ-light-chain-enhancer of activated B cells
- NO
Nitric oxide
- PEG-SOD
Superoxide dismutase-polyethylene glycol
- PI3K
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PK
Pharmacokinetics
- PD
Pharmacodynamics
- ProTECT
Progesterone for the Treatment of Traumatic Brain Injury III
- TBI
Traumatic brain injury
- TD
Toxicodynamics
- TLR
Toll-like receptor
- ROCK
Rho-associated protein kinase
- SOD
Superoxide dismutase
- SYNAPSE
Study of the Neuroprotective Activity of Progesterone in Severe Traumatic Brain Injuries
Author Contributions
M.R.S: conceived and developed the ideas and concepts presented here and wrote the manuscript. A.J.P. and R.K.G: reviewed the manuscript and shared their technical insights.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Faul M., Xu L., Wald M.M., Coronado V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010. [http://dx.doi.org/10.15620/cdc.5571] [Google Scholar]
- 2.Coronado V.G., McGuire L.C., Sarmiento K., Bell J., Lionbarger M.R., Jones C.D., Geller A.I., Khoury N., Xu L. Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995-2009. J. Safety Res. 2012;43(4):299–307. doi: 10.1016/j.jsr.2012.08.011. [http://dx. doi.org/10.1016/j.jsr.2012.08.011]. [PMID: 23127680]. [DOI] [PubMed] [Google Scholar]
- 3.Diaz-Arrastia R., Kochanek P.M., Bergold P., Kenney K., Marx C.E., Grimes C.J., Loh L.T., Adam L.T., Oskvig D., Curley K.C., Salzer W. Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma. 2014;31(2):135–158. doi: 10.1089/neu.2013.3019. [http://dx.doi.org/10.1089/neu.2013.3019]. [PMID: 23968241]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelstein E. Corso, P.; Miller, T.; and associates. The Incidence and Economic Burden of Injuries in the United States. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 5.Hatton J. Pharmacological treatment of traumatic brain injury: a review of agents in development. CNS Drugs. 2001;15(7):553–581. doi: 10.2165/00023210-200115070-00005. [http://dx.doi.org/10.2165/00023210-200115070-00005]. [PMID: 11510625]. [DOI] [PubMed] [Google Scholar]
- 6.Cernak I., Noble-Haeusslein L.J. Traumatic brain injury: an overview of pathobiology with emphasis on military populations. J. Cereb. Blood Flow Metab. 2010;30(2):255–266. doi: 10.1038/jcbfm.2009.203. [http://dx.doi. org/10.1038/jcbfm.2009.203]. [PMID: 19809467]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risdall J.E., Menon D.K. Traumatic brain injury. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366(1562):241–250. doi: 10.1098/rstb.2010.0230. [http://dx.doi.org/ 10.1098/rstb.2010.0230]. [PMID: 21149359]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young L., Rule G.T., Bocchieri R.T., Walilko T.J., Burns J.M., Ling G. When physics meets biology: low and high-velocity penetration, blunt impact, and blast injuries to the brain. Front. Neurol. 2015;6:89. doi: 10.3389/fneur.2015.00089. [http://dx.doi.org/10.3389/fneur.2015.00089]. [PMID: 25999910]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agoston D.V., Sköld M.K. Editorial: When Physics Meets Biology; Biomechanics and Biology of Traumatic Brain Injury. Front. Neurol. 2016;7:91. doi: 10.3389/fneur.2016.00091. [http://dx.doi.org/10.3389/fneur.2016.00091]. [PMID: 27379010]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanielian T., Jaycox L.H., editors. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Santa Monica, CA: RAND Corporation; 2008. https://www.rand.org/pubs/monographs/MG720.html [Google Scholar]
- 11.Madikians A., Giza C.C. A clinician’s guide to the pathophysiology of traumatic brain injury. Indian J Neurotrauma. 2006;3(1):9–17. [http://dx.doi.org/10.1016/S0973-0508(06)80004-3]. [Google Scholar]
- 12.Eskridge S.L., Macera C.A., Galarneau M.R., Holbrook T.L., Woodruff S.I., MacGregor A.J., Morton D.J., Shaffer R.A. Injuries from combat explosions in Iraq: injury type, location, and severity. Injury. 2012;43(10):1678–1682. doi: 10.1016/j.injury.2012.05.027. [http://dx.doi.org/10.1016/ j.injury.2012.05.027]. [PMID: 22769977]. [DOI] [PubMed] [Google Scholar]
- 13.Armonda R.A., Bell R.S., Vo A.H., Ling G., DeGraba T.J., Crandall B., Ecklund J., Campbell W.W. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59(6):1215–1225. doi: 10.1227/01.NEU.0000249190.46033.94. [http://dx.doi.org/10.1227/01.NEU. 0000249190.46033.94]. [PMID: 17277684]. [DOI] [PubMed] [Google Scholar]
- 14.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., Castro C.A. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 2008;358(5):453–463. doi: 10.1056/NEJMoa072972. [http://dx.doi. org/10.1056/NEJMoa072972]. [PMID: 18234750]. [DOI] [PubMed] [Google Scholar]
- 15.Champion H.R., Holcomb J.B., Young L.A. Injuries from explosions: physics, biophysics, pathology, and required research focus. J. Trauma. 2009;66(5):1468–1477. doi: 10.1097/TA.0b013e3181a27e7f. [http://dx.doi.org/10.1097/ TA.0b013e3181a27e7f]. [PMID: 19430256]. [DOI] [PubMed] [Google Scholar]
- 16.Ling G., Bandak F., Armonda R., Grant G., Ecklund J. Explosive blast neurotrauma. J. Neurotrauma. 2009;26(6):815–825. doi: 10.1089/neu.2007.0484. [http://dx.doi.org/10.1089/neu.2007.0484]. [PMID: 19397423]. [DOI] [PubMed] [Google Scholar]
- 17.BrainLine Military http://www.brainlinemilitary.org/
- 18.Jagoda A.S., Bazarian J.J., Bruns J.J., Jr, Cantrill S.V., Gean A.D., Howard P.K., Ghajar J., Riggio S., Wright D.W., Wears R.L., Bakshy A., Burgess P., Wald M.M., Whitson R.R. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. J. Emerg. Nurs. 2009;35(2):e5–e40. doi: 10.1016/j.jen.2008.12.010. [http://dx.doi.org/10.1016/j.jen.2008.12.010]. [PMID: 19285163]. [DOI] [PubMed] [Google Scholar]
- 19.Tenovuo O.S. Cholinergic treatment of traumatic brain injury. Curr. Drug Ther. 2006;1(2):187–209. [http://dx.doi.org/10.2174/ 157488506776930932]. [Google Scholar]
- 20.Frieden T.R., Houry D., Baldwin G. Centers for Disease Control and Prevention. Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation. National Center for Injury Prevention and Control. Atlanta, GA: Division of Unintentional Injury Prevention; 2015. https://www.cdc.gov/ traumaticbraininjury/pdf/tbi_report_to_congress_epi_and_rehab-a.pdf [Google Scholar]
- 21.Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [http:// dx.doi.org/10.1016/S0140-6736(74)91639-0]. [PMID: 4136544]. [DOI] [PubMed] [Google Scholar]
- 22.Gerberding J.L., Binder S. National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Atlanta, GA: Centers for Disease Control and Prevention; 2003. https://www.cdc.gov/traumaticbraininjury/pdf/mtbireport-a.pdf [Google Scholar]
- 23.Heltemes K.J., Holbrook T.L., Macgregor A.J., Galarneau M.R. Blast-related mild traumatic brain injury is associated with a decline in self-rated health amongst US military personnel. Injury. 2012;43(12):1990–1995. doi: 10.1016/j.injury.2011.07.021. [http://dx.doi.org/10.1016/j.injury.2011. 07.021]. [PMID: 21855064]. [DOI] [PubMed] [Google Scholar]
- 24.Meaney D.F., Morrison B., Dale Bass C. The mechanics of traumatic brain injury: a review of what we know and what we need to know for reducing its societal burden. J. Biomech. Eng. 2014;136(2):021008. doi: 10.1115/1.4026364. [http://dx.doi.org/10.1115/1.4026364]. [PMID: 24384610]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskowski R.A., Creed J.A., Raghupathi R. Pathophysiology of Mild TBI: Implications for Altered Signaling Pathways. In: Kobeissy F.H., editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/ Taylor & Francis; 2015. [http://dx.doi.org/ 10.1201/b18126-6] [PMID: 26269903] [PubMed] [Google Scholar]
- 26.Chen C.C., Wei S.T., Tsaia S.C., Chen X.X., Cho D.Y. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. Br. J. Neurosurg. 2013;27(6):803–807. doi: 10.3109/02688697.2013.793287. [http://dx.doi.org/10.3109/ 02688697.2013.793287]. [PMID: 23656173]. [DOI] [PubMed] [Google Scholar]
- 27.Ruff R.M., Iverson G.L., Barth J.T., Bush S.S., Broshek D.K., NAN Policy and Planning Committee Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology education paper. Arch. Clin. Neuropsychol. 2009;24(1):3–10. doi: 10.1093/arclin/acp006. [http://dx.doi.org/10.1093/arclin/acp006]. [PMID: 19395352]. [DOI] [PubMed] [Google Scholar]
- 28.Belanger H.G., Curtiss G., Demery J.A., Lebowitz B.K., Vanderploeg R.D. Factors moderating neuropsychological outcomes following mild traumatic brain injury: a meta-analysis. J. Int. Neuropsychol. Soc. 2005;11(3):215–227. doi: 10.1017/S1355617705050277. [http://dx.doi.org/10.1017/ S1355617705050277]. [PMID: 15892898]. [DOI] [PubMed] [Google Scholar]
- 29.Verma A., Anand V., Verma N.P. Sleep disorders in chronic traumatic brain injury. J. Clin. Sleep Med. 2007;3(4):357–362. [PMID: 17694723]. [PMC free article] [PubMed] [Google Scholar]
- 30.Schreiber S., Barkai G., Gur-Hartman T., Peles E., Tov N., Dolberg O.T., Pick C.G. Long-lasting sleep patterns of adult patients with minor traumatic brain injury (mTBI) and non-mTBI subjects. Sleep Med. 2008;9(5):481–487. doi: 10.1016/j.sleep.2007.04.014. [http://dx.doi.org/10. 1016/j.sleep.2007.04.014]. [PMID: 17638592]. [DOI] [PubMed] [Google Scholar]
- 31.Dash P.K., Zhao J., Hergenroeder G., Moore A.N. Biomarkers for the diagnosis, prognosis, and evaluation of treatment efficacy for traumatic brain injury. Neurotherapeutics. 2010;7(1):100–114. doi: 10.1016/j.nurt.2009.10.019. [http://dx.doi.org/10.1016/j.nurt.2009.10.019]. [PMID: 20129502]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iverson G.L. Outcome from mild traumatic brain injury. Curr. Opin. Psychiatry. 2005;18(3):301–317. doi: 10.1097/01.yco.0000165601.29047.ae. [http://dx.doi.org/10.1097/ 01.yco.0000165601.29047.ae]. [PMID: 16639155]. [DOI] [PubMed] [Google Scholar]
- 33.Meaney D.F., Smith D.H. Biomechanics of concussion. Clin. Sports Med. 2011;30(1):19–31. doi: 10.1016/j.csm.2010.08.009. [http://dx.doi.org/10.1016/j.csm. 2010.08.009]. [PMID: 21074079]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith D.H., Meaney D.F. Axonal damage in traumatic brain injury. Neuroscientist. 2000;6(6):483–495. [http://dx.doi.org/10. 1177/107385840000600611]. [Google Scholar]
- 35.Johnson V.E., Stewart W., Smith D.H. Axonal pathology in traumatic brain injury. Exp. Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [http:// dx.doi.org/10.1016/j.expneurol.2012.01.013]. [PMID: 22285252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meythaler J.M., Peduzzi J.D., Eleftheriou E., Novack T.A. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch. Phys. Med. Rehabil. 2001;82(10):1461–1471. doi: 10.1053/apmr.2001.25137. [http:// dx.doi.org/10.1053/apmr.2001.25137]. [PMID: 11588754]. [DOI] [PubMed] [Google Scholar]
- 37.Tang-Schomer M.D., Johnson V.E., Baas P.W., Stewart W., Smith D.H. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp. Neurol. 2012;233(1):364–372. doi: 10.1016/j.expneurol.2011.10.030. [http:// dx.doi.org/10.1016/j.expneurol.2011.10.030]. [PMID: 22079153]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Front. Neurol. 2010;1:151. doi: 10.3389/fneur.2010.00151. [http://dx.doi.org/10.3389/fneur.2010.00151]. [PMID: 21206523]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risling M. Blast induced brain injuries–a grand challenge in TBI research. Front. Neurol. 2010;1:1. doi: 10.3389/fneur.2010.00001. [http://dx.doi.org/10.3389/ fneur.2010.00001]. [PMID: 21188247]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hicks R.R., Fertig S.J., Desrocher R.E., Koroshetz W.J., Pancrazio J.J. Neurological effects of blast injury. J. Trauma. 2010;68(5):1257–1263. doi: 10.1097/TA.0b013e3181d8956d. [http://dx.doi.org/10.1097/TA.0b013e3181d8956d]. [PMID: 20453776]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carron S.F., Alwis D.S., Rajan R. Traumatic brain injury and neuronal functionality changes in sensory cortex. Front. Syst. Neurosci. 2016;10:47. doi: 10.3389/fnsys.2016.00047. [http://dx.doi.org/10.3389/fnsys.2016. 00047]. [PMID: 27313514]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maxwell W.L. 2014. Secondary Axotomy. [Google Scholar]
- 43.Büki A., Povlishock J.T. All roads lead to disconnection?-- Traumatic axonal injury revisited. Acta Neurochir. (Wien) 2006;148(2):181–193. doi: 10.1007/s00701-005-0674-4. [Wien]. [DOI] [PubMed] [Google Scholar]
- 44.Taber K.H., Warden D.L., Hurley R.A. Blast-related traumatic brain injury: what is known? J. Neuropsychiatry Clin. Neurosci. 2006;18(2):141–145. doi: 10.1176/jnp.2006.18.2.141. [http://dx.doi.org/10.1176/jnp.2006.18.2.141]. [PMID: 16720789]. [DOI] [PubMed] [Google Scholar]
- 45.Taber K.H., Hurley R.A., Haswell C.C., Rowland J.A., Hurt S.D., Lamar C.D., Morey R.A. White matter compromise in veterans exposed to primary blast forces. J. Head Trauma Rehabil. 2015;30(1):E15–E25. doi: 10.1097/HTR.0000000000000030. [http://dx.doi.org/10.1097/HTR.0000000000000030]. [PMID: 24590156]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mac Donald C.L., Johnson A.M., Cooper D., Nelson E.C., Werner N.J., Shimony J.S., Snyder A.Z., Raichle M.E., Witherow J.R., Fang R., Flaherty S.F., Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364(22):2091–2100. doi: 10.1056/NEJMoa1008069. [http://dx.doi.org/10. 1056/NEJMoa1008069]. [PMID: 21631321]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jorge R.E., Acion L., White T., Tordesillas-Gutierrez D., Pierson R., Crespo-Facorro B., Magnotta V.A. White matter abnormalities in veterans with mild traumatic brain injury. [DOI] [PMC free article] [PubMed]
- 48.Sinclair M., Wittek A., Doyle B., Miller K., Joldes G.R. 2016. Modelling the Presence of Diffuse Axonal Injury in Primary Phase Blast-Induced Traumatic Brain Injury. [Google Scholar]
- 49.Adibhatla R.M., Hatcher J.F. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2010;12(1):125–169. doi: 10.1089/ars.2009.2668. [http://dx.doi.org/10.1089/ars.2009.2668]. [PMID: 19624272]. [DOI] [PubMed] [Google Scholar]
- 50.Margulies S., Hicks R. Combination Therapies for Traumatic Brain Injury Workshop Leaders. Combination therapies for traumatic brain injury: prospective considerations. J. Neurotrauma. 2009;26(6):925–939. doi: 10.1089/neu.2008.0794. [http://dx.doi.org/10.1089/neu.2008.0794]. [PMID: 19331514]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kochanek P.M., Clark R.S., Ruppel R.A., Adelson P.D., Bell M.J., Whalen M.J., Robertson C.L., Satchell M.A., Seidberg N.A., Marion D.W., Jenkins L.W. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatr. Crit. Care Med. 2000;1(1):4–19. doi: 10.1097/00130478-200007000-00003. [http://dx.doi.org/10.1097/00130478-200007000-00003]. [PMID: 12813280]. [DOI] [PubMed] [Google Scholar]
- 52.Sidaros A., Skimminge A., Liptrot M.G., Sidaros K., Engberg A.W., Herning M., Paulson O.B., Jernigan T.L., Rostrup E. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. Neuroimage. 2009;44(1):1–8. doi: 10.1016/j.neuroimage.2008.08.030. [http://dx.doi.org/10.1016/ j.neuroimage.2008.08.030]. [PMID: 18804539]. [DOI] [PubMed] [Google Scholar]
- 53.Park E., Bell J.D., Baker A.J. Traumatic brain injury: can the consequences be stopped? CMAJ. 2008;178(9):1163–1170. doi: 10.1503/cmaj.080282. [http://dx.doi.org/10.1503/cmaj.080282]. [PMID: 18427091]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goutham S., Husni H., Vanchilingam S., Husain S. Neurovascular injuries in trauma: An under recognized entity. Indian. J. Neurotrauma. 2014;11(2):170–174. [http://dx.doi.org/10.1016/ j.ijnt.2014.12.009]. [Google Scholar]
- 55.Ballatore C., Lee V.M., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [http://dx.doi.org/10.1038/ nrn2194]. [PMID: 17684513]. [DOI] [PubMed] [Google Scholar]
- 56.Hay J., Johnson V.E., Smith D.H., Stewart W. Chronic traumatic encephalopathy: the neuropathological legacy of traumatic brain injury. Annu. Rev. Pathol. 2016;11:21–45. doi: 10.1146/annurev-pathol-012615-044116. [http://dx.doi.org/10. 1146/annurev-pathol-012615-044116]. [PMID: 26772317]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garga N., Lowenstein D.H. Posttraumatic epilepsy: a major problem in desperate need of major advances. Epilepsy Curr. 2006;6(1):1–5. doi: 10.1111/j.1535-7511.2005.00083.x. [http://dx.doi.org/10.1111/j.1535-7511.2005.00083.x]. [PMID: 16477313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin M., Shepardson N., Yang T., Chen G., Walsh D., Selkoe D.J. Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. USA. 2011;108(14):5819–5824. doi: 10.1073/pnas.1017033108. [http://dx.doi.org/10.1073/pnas.1017033108]. [PMID: 21421841]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blennow K., Hardy J., Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886–899. doi: 10.1016/j.neuron.2012.11.021. [http://dx.doi.org/10.1016/j.neuron.2012.11.021]. [PMID: 23217738]. [DOI] [PubMed] [Google Scholar]
- 60.Lucke-Wold B.P., Turner R.C., Logsdon A.F., Bailes J.E., Huber J.D., Rosen C.L. Linking traumatic brain injury to chronic traumatic encephalopathy: identification of potential mechanisms leading to neurofibrillary tangle development. J. Neurotrauma. 2014;31(13):1129–1138. doi: 10.1089/neu.2013.3303. [http://dx.doi.org/10.1089/neu.2013.3303]. [PMID: 24499307]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong J.C., Hazrati L.N. Parkinson’s disease, parkinsonism, and traumatic brain injury. Crit. Rev. Clin. Lab. Sci. 2013;50(4-5):103–106. doi: 10.3109/10408363.2013.844678. [http://dx.doi.org/10.3109/10408363.2013.844678]. [PMID: 24156652]. [DOI] [PubMed] [Google Scholar]
- 62.Sivanandam T.M., Thakur M.K. Traumatic brain injury: a risk factor for Alzheimer’s disease. Neurosci. Biobehav. Rev. 2012;36(5):1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [http://dx.doi.org/10.1016/j.neubiorev.2012.02. 013]. [PMID: 22390915]. [DOI] [PubMed] [Google Scholar]
- 63.Gardner R.C., Burke J.F., Nettiksimmons J., Goldman S., Tanner C.M., Yaffe K. Traumatic brain injury in later life increases risk for Parkinson disease. Ann. Neurol. 2015;77(6):987–995. doi: 10.1002/ana.24396. [http://dx.doi.org/10.1002/ana.24396]. [PMID: 25726936]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao H.M., Hong J.S. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. doi: 10.1016/j.it.2008.05.002. [http://dx.doi.org/10.1016/ j.it.2008.05.002]. [PMID: 18599350]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loane D.J., Byrnes K.R. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7(4):366–377. doi: 10.1016/j.nurt.2010.07.002. [http://dx.doi.org/10.1016/ j.nurt.2010.07.002]. [PMID: 20880501]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russo M.V., McGavern D.B. Inflammatory neuroprotection following traumatic brain injury. Science. 2016;353(6301):783–785. doi: 10.1126/science.aaf6260. [http://dx.doi.org/10.1126/science.aaf6260]. [PMID: 27540166]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pugh M.J., Orman J.A., Jaramillo C.A., Salinsky M.C., Eapen B.C., Towne A.R., Amuan M.E., Roman G., McNamee S.D., Kent T.A., McMillan K.K., Hamid H., Grafman J.H. The prevalence of epilepsy and association with traumatic brain injury in veterans of the Afghanistan and Iraq wars. J. Head Trauma Rehabil. 2015;30(1):29–37. doi: 10.1097/HTR.0000000000000045. [http://dx.doi.org/10.1097/HTR.0000000000000045]. [PMID: 24695268]. [DOI] [PubMed] [Google Scholar]
- 68.Pissadaki E.K., Bolam J.P. The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson’s disease. Front. Comput. Neurosci. 2013;7:13. doi: 10.3389/fncom.2013.00013. [http://dx. doi.org/10.3389/fncom.2013.00013]. [PMID: 23515615]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Breunig J.J., Guillot-Sestier M.V., Town T. Brain injury, neuroinflammation and Alzheimer’s disease. Front. Aging Neurosci. 2013;5:26. doi: 10.3389/fnagi.2013.00026. [http://dx.doi.org/10.3389/fnagi.2013.00026]. [PMID: 23874297]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masel B.E., DeWitt D.S. Traumatic brain injury: a disease process, not an event. J. Neurotrauma. 2010;27(8):1529–1540. doi: 10.1089/neu.2010.1358. [http://dx.doi.org/10.1089/neu.2010.1358]. [PMID: 20504161]. [DOI] [PubMed] [Google Scholar]
- 71.Grevesse T., Dabiri B.E., Parker K.K., Gabriele S. Opposite rheological properties of neuronal microcompartments predict axonal vulnerability in brain injury. Sci. Rep. 2015;5:9475. doi: 10.1038/srep09475. [http://dx.doi.org/10.1038/srep09475]. [PMID: 25820512]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saxena S., Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron. 2011;71(1):35–48. doi: 10.1016/j.neuron.2011.06.031. [http://dx.doi.org/10.1016/j.neuron.2011. 06.031]. [PMID: 21745636]. [DOI] [PubMed] [Google Scholar]
- 73.Roselli F., Caroni P. From intrinsic firing properties to selective neuronal vulnerability in neurodegenerative diseases. Neuron. 2015;85(5):901–910. doi: 10.1016/j.neuron.2014.12.063. [http://dx.doi.org/10.1016/j.neuron.2014. 12.063]. [PMID: 25741719]. [DOI] [PubMed] [Google Scholar]
- 74.Courtney A., Courtney M. The complexity of biomechanics causing primary blast-induced traumatic brain injury: a review of potential mechanisms. Front. Neurol. 2015;6:221. doi: 10.3389/fneur.2015.00221. [http://dx.doi.org/ 10.3389/fneur.2015.00221]. [PMID: 26539158]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tosetti P., Hicks R.R., Theriault E., Phillips A., Koroshetz W., Draghia-Akli R. Workshop Participants. Toward an international initiative for traumatic brain injury research. J. Neurotrauma. 2013;30(14):1211–1222. doi: 10.1089/neu.2013.2896. [http://dx.doi.org/10.1089/neu.2013.2896]. [PMID: 23731282]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith D.H., Hicks R., Povlishock J.T. Therapy development for diffuse axonal injury. J. Neurotrauma. 2013;30(5):307–323. doi: 10.1089/neu.2012.2825. [http://dx.doi.org/10.1089/neu.2012.2825]. [PMID: 23252624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.2008. http://www.ninds.nih.gov/news_and_events/proceedings/Neurological_Effects_of_Blast_Injury_Workshop.htm
- 78.U.S. Department of Defense - Congressionally Directed Medical Research Programs http://cdmrp.army.mil/about/ fundinghistory.shtml
- 79.St Onge P., McIlwain D.S., Hill M.E., Walilko T.J., Bardolf L.B. Marine Corps Breacher Training Study: auditory and vestibular findings. U.S. Army Med. Dep. J. 2011;•••:97–107. [PMID: 21805461]. [PubMed] [Google Scholar]
- 80.Tate C.M., Wang K.K., Eonta S., Zhang Y., Carr W., Tortella F.C., Hayes R.L., Kamimori G.H. Serum brain biomarker level, neurocognitive performance, and self-reported symptom changes in soldiers repeatedly exposed to low-level blast: a breacher pilot study. J. Neurotrauma. 2013;30(19):1620–1630. doi: 10.1089/neu.2012.2683. [http://dx.doi.org/ 10.1089/neu.2012.2683]. [PMID: 23687938]. [DOI] [PubMed] [Google Scholar]
- 81.Ganpule S., Salzar R., Chandra N. 2013 http://proceedings.asmedigitalcollection.asme.org/proceeding.aspx?articleid=1857856
- 82.Zhang L., Yang K.H., King A.I. Comparison of brain responses between frontal and lateral impacts by finite element modeling. J. Neurotrauma. 2001;18(1):21–30. doi: 10.1089/089771501750055749. [http://dx.doi.org/10.1089/ 089771501750055749]. [PMID: 11200247]. [DOI] [PubMed] [Google Scholar]
- 83.Zhang L., Makwana R., Sharma S. Brain response to primary blast wave using validated finite element models of human head and advanced combat helmet. Front. Neurol. 2013;4:88. doi: 10.3389/fneur.2013.00088. [http://dx.doi.org/10.3389/fneur.2013.00088]. [PMID: 23935591]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.El Sayed T., Mota A., Fraternali F., Ortiz M. Biomechanics of traumatic brain injury. Comput. Methods Appl. Mech. Eng. 2008;197(51-52):4692–4701. [http://dx.doi.org/10.1016/j.cma.2008.06. 006]. [Google Scholar]
- 85.Cernak I. Animal models of head trauma. NeuroRx. 2005;2(3):410–422. doi: 10.1602/neurorx.2.3.410. [http://dx.doi.org/10.1602/neurorx.2.3.410]. [PMID: 16389305]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xiong Y., Mahmood A., Chopp M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013;14(2):128–142. doi: 10.1038/nrn3407. [http://dx.doi.org/10.1038/nrn3407]. [PMID: 23329160]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bass C.R., Panzer M.B., Rafaels K.A., Wood G., Shridharani J., Capehart B. Brain injuries from blast. Ann. Biomed. Eng. 2012;40(1):185–202. doi: 10.1007/s10439-011-0424-0. [http://dx.doi.org/10.1007/s10439-011-0424-0]. [PMID: 22012085]. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y., Constantini S. Caveats for using shock tube in blast-induced traumatic brain injury research. Front. Neurol. 2013;4:117. doi: 10.3389/fneur.2013.00117. [http://dx.doi.org/10.3389/fneur.2013.00117]. [PMID: 23986741]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kabu S., Jaffer H., Petro M., Dudzinski D., Stewart D., Courtney A., Courtney M., Labhasetwar V. Blast-associated shock waves result in increased brain vascular leakage and elevated ROS levels in a rat model of traumatic brain injury. PLoS One. 2015;10(5):e0127971. doi: 10.1371/journal.pone.0127971. [http://dx.doi.org/10.1371/journal.pone.0127971]. [PMID: 26024446]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reneer D.V., Hisel R.D., Hoffman J.M., Kryscio R.J., Lusk B.T., Geddes J.W. A multi-mode shock tube for investigation of blast-induced traumatic brain injury. J. Neurotrauma. 2011;28(1):95–104. doi: 10.1089/neu.2010.1513. [http://dx.doi.org/10.1089/neu.2010.1513]. [PMID: 21083431]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wada K., Chatzipanteli K., Busto R., Dietrich W.D. Role of nitric oxide in traumatic brain injury in the rat. J. Neurosurg. 1998;89(5):807–818. doi: 10.3171/jns.1998.89.5.0807. [http://dx.doi.org/10.3171/jns.1998.89.5.0807]. [PMID: 9817419]. [DOI] [PubMed] [Google Scholar]
- 92.Readnower R.D., Chavko M., Adeeb S., Conroy M.D., Pauly J.R., McCarron R.M., Sullivan P.G. Increase in blood-brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast-induced traumatic brain injury. J. Neurosci. Res. 2010;88(16):3530–3539. doi: 10.1002/jnr.22510. [http://dx.doi.org/10.1002/jnr.22510]. [PMID: 20882564]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdul-Muneer P.M., Schuetz H., Wang F., Skotak M., Jones J., Gorantla S., Zimmerman M.C., Chandra N., Haorah J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Biol. Med. 2013;60:282–291. doi: 10.1016/j.freeradbiomed.2013.02.029. [http://dx.doi.org/10.1016/j.freeradbiomed.2013.02.029]. [PMID: 23466554]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mishra V., Skotak M., Schuetz H., Heller A., Haorah J., Chandra N. Primary blast causes mild, moderate, severe and lethal TBI with increasing blast overpressures: Experimental rat injury model. Sci. Rep. 2016;6:26992. doi: 10.1038/srep26992. [http://dx.doi.org/10.1038/ srep26992]. [PMID: 27270403]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hovda D.A., Lee S.M., Smith M.L., Von Stuck S., Bergsneider M., Kelly D., Shalmon E., Martin N., Caron M., Mazziotta J., Phelps M., Becker D.P. The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J. Neurotrauma. 1995;12(5):903–906. doi: 10.1089/neu.1995.12.903. [http://dx.doi.org/10.1089/neu. 1995.12.903]. [PMID: 8594218]. [DOI] [PubMed] [Google Scholar]
- 96.Giza C.C., Hovda D.A. The neurometabolic cascade of concussion. J. Athl. Train. 2001;36(3):228–235. [PMID: 12937489]. [PMC free article] [PubMed] [Google Scholar]
- 97.Giza C.C., Hovda D.A. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl. 4):S24–S33. doi: 10.1227/NEU.0000000000000505. [http://dx.doi. org/10.1227/NEU.0000000000000505]. [PMID: 25232881]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clark R.S., Bayir H., Chu C.T., Alber S.M., Kochanek P.M., Watkins S.C. Autophagy is increased in mice after traumatic brain injury and is detectable in human brain after trauma and critical illness. Autophagy. 2008;4(1):88–90. doi: 10.4161/auto.5173. [http://dx.doi.org/10.4161/ auto.5173]. [PMID: 17957135]. [DOI] [PubMed] [Google Scholar]
- 99.Au A.K., Bayir H., Kochanek P.M., Clark R.S. Evaluation of autophagy using mouse models of brain injury. Biochim. Biophys. Acta. 2010;1802(10):918–923. doi: 10.1016/j.bbadis.2009.10.010. [http://dx.doi.org/10.1016/j.bbadis. 2009.10.010]. [PMID: 19879944]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luo C.L., Li B.X., Li Q.Q., Chen X.P., Sun Y.X., Bao H.J., Dai D.K., Shen Y.W., Xu H.F., Ni H., Wan L., Qin Z.H., Tao L.Y., Zhao Z.Q. Autophagy is involved in traumatic brain injury induced cell death and contributes to functional outcome deficits in mice. Neuroscience. 2011;184:54–63. doi: 10.1016/j.neuroscience.2011.03.021. [http://dx.doi.org/10.1016/j. neuroscience.2011.03.021]. [PMID: 21463664]. [DOI] [PubMed] [Google Scholar]
- 101.Smith C.M., Chen Y., Sullivan M.L., Kochanek P.M., Clark R.S. Autophagy in acute brain injury: feast, famine, or folly? Neurobiol. Dis. 2011;43(1):52–59. doi: 10.1016/j.nbd.2010.09.014. [http://dx.doi.org/10.1016/j.nbd. 2010.09.014]. [PMID: 20883784]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sarkar C., Zhao Z., Aungst S., Sabirzhanov B., Faden A.I., Lipinski M.M. Impaired autophagy flux is associated with neuronal cell death after traumatic brain injury. Autophagy. 2014;10(12):2208–2222. doi: 10.4161/15548627.2014.981787. [http://dx.doi.org/10.4161/15548627.2014. 981787]. [PMID: 25484084]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y.C., Smith D.H., Meaney D.F. In-vitro approaches for studying blast-induced traumatic brain injury. J. Neurotrauma. 2009;26(6):861–876. doi: 10.1089/neu.2008.0645. [http://dx.doi.org/10.1089/neu.2008.0645]. [PMID: 19397424]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morrison B., III, Saatman K.E., Meaney D.F., McIntosh T.K. In vitro central nervous system models of mechanically induced trauma: a review. J. Neurotrauma. 1998;15(11):911–928. doi: 10.1089/neu.1998.15.911. [http:// dx.doi.org/10.1089/neu.1998.15.911]. [PMID: 9840765]. [DOI] [PubMed] [Google Scholar]
- 105.Morrison B., III, Elkin B.S., Dollé J.P., Yarmush M.L. In vitro models of traumatic brain injury. Annu. Rev. Biomed. Eng. 2011;13:91–126. doi: 10.1146/annurev-bioeng-071910-124706. [http://dx.doi.org/10.1146/annurev-bioeng-071910-124706]. [PMID: 21529164]. [DOI] [PubMed] [Google Scholar]
- 106.Kumaria A., Tolias C.M. In vitro models of neurotrauma. Br. J. Neurosurg. 2008;22(2):200–206. doi: 10.1080/02688690701772413. [http://dx.doi.org/10.1080/ 02688690701772413]. [PMID: 18348014]. [DOI] [PubMed] [Google Scholar]
- 107.Boucher P.A., Joós B., Morris C.E. Coupled left-shift of Nav channels: modeling the Na+-loading and dysfunctional excitability of damaged axons. J. Comput. Neurosci. 2012;33(2):301–319. doi: 10.1007/s10827-012-0387-7. [http://dx.doi.org/10.1007/s10827-012-0387-7]. [PMID: 22476614]. [DOI] [PubMed] [Google Scholar]
- 108.Morris C.E., Boucher P.A., Joós B. Left-shifted Nav channels in injured bilayer: primary targets for neuroprotective Nav antagonists? Front. Pharmacol. 2012;3:19. doi: 10.3389/fphar.2012.00019. [PMID: 22375118]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Povlishock J.T., Christman C.W. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J. Neurotrauma. 1995;12(4):555–564. doi: 10.1089/neu.1995.12.555. [http:// dx.doi.org/10.1089/neu.1995.12.555]. [PMID: 8683606]. [DOI] [PubMed] [Google Scholar]
- 110.Ahmed S.M., Rzigalinski B.A., Willoughby K.A., Sitterding H.A., Ellis E.F. Stretch-induced injury alters mitochondrial membrane potential and cellular ATP in cultured astrocytes and neurons. J. Neurochem. 2000;74(5):1951–1960. [http://dx.doi.org/10. 1046/j.1471-4159.2000.0741951.x]. [PMID: 10800938]. [PubMed] [Google Scholar]
- 111.Ellis E.F., McKinney J.S., Willoughby K.A., Liang S., Povlishock J.T. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J. Neurotrauma. 1995;12(3):325–339. doi: 10.1089/neu.1995.12.325. [http://dx.doi.org/10.1089/neu.1995. 12.325]. [PMID: 7473807]. [DOI] [PubMed] [Google Scholar]
- 112.Arun P., Abu-Taleb R., Oguntayo S., Wang Y., Valiyaveettil M., Long J.B., Nambiar M.P. Acute mitochondrial dysfunction after blast exposure: potential role of mitochondrial glutamate oxaloacetate transaminase. J. Neurotrauma. 2013;30(19):1645–1651. doi: 10.1089/neu.2012.2834. [http://dx.doi.org/10.1089/neu.2012.2834]. [PMID: 23600763]. [DOI] [PubMed] [Google Scholar]
- 113.Bell J.D. Molecular cross talk in traumatic brain injury. J. Neurosci. 2007;27(9):2153–2154. doi: 10.1523/JNEUROSCI.4929-06.2007. [http://dx.doi.org/10.1523/JNEUROSCI. 4929-06.2007]. [PMID: 17329411]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hemphill M.A., Dabiri B.E., Gabriele S., Kerscher L., Franck C., Goss J.A., Alford P.W., Parker K.K. A possible role for integrin signaling in diffuse axonal injury. PLoS One. 2011;6(7):e22899. doi: 10.1371/journal.pone.0022899. [http://dx.doi.org/10.1371/journal.pone.0022899]. [PMID: 21799943]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hemphill M.A., Dauth S., Yu C.J., Dabiri B.E., Parker K.K. Traumatic brain injury and the neuronal microenvironment: a potential role for neuropathological mechanotransduction. Neuron. 2015;85(6):1177–1192. doi: 10.1016/j.neuron.2015.02.041. [http://dx.doi.org/10.1016/j.neuron.2015. 02.041]. [PMID: 25789754]. [DOI] [PubMed] [Google Scholar]
- 116.Mueller B.K., Mack H., Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat. Rev. Drug Discov. 2005;4(5):387–398. doi: 10.1038/nrd1719. [http://dx.doi.org/10.1038/nrd1719]. [PMID: 15864268]. [DOI] [PubMed] [Google Scholar]
- 117.Przekwas A., Somayaji M.R., Gupta R.K. Synaptic mechanisms of blast-induced brain injury. Front. Neurol. 2016;7:2. doi: 10.3389/fneur.2016.00002. [http://dx.doi.org/10.3389/fneur.2016.00002]. [PMID: 26834697]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Strimbu K., Tavel J.A. What are biomarkers? Curr. Opin. HIV AIDS. 2010;5(6):463–466. doi: 10.1097/COH.0b013e32833ed177. [http://dx.doi.org/10.1097/COH. 0b013e32833ed177]. [PMID: 20978388]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karsdal M.A., Henriksen K., Leeming D.J., Mitchell P., Duffin K., Barascuk N., Klickstein L., Aggarwal P., Nemirovskiy O., Byrjalsen I., Qvist P., Bay-Jensen A.C., Dam E.B., Madsen S.H., Christiansen C. Biochemical markers and the FDA Critical Path: how biomarkers may contribute to the understanding of pathophysiology and provide unique and necessary tools for drug development. Biomarkers. 2009;14(3):181–202. doi: 10.1080/13547500902777608. [http://dx.doi.org/ 10.1080/13547500902777608]. [PMID: 19399662]. [DOI] [PubMed] [Google Scholar]
- 120.Amur S., LaVange L., Zineh I., Buckman-Garner S. Woodcock, J. Biomarker qualification: toward a multiple stakeholder framework for biomarker development, regulatory acceptance, and utilization. Clin. Pharmacol. Ther. 2015;98(1):34–46. doi: 10.1002/cpt.136. [http://dx.doi. org/10.1002/cpt.136]. [PMID: 25868461]. [DOI] [PubMed] [Google Scholar]
- 121.Wagner J.A., Williams S.A., Webster C.J. Biomarkers and surrogate end points for fit-for-purpose development and regulatory evaluation of new drugs. Clin. Pharmacol. Ther. 2007;81(1):104–107. doi: 10.1038/sj.clpt.6100017. [http://dx.doi.org/10.1038/sj.clpt.6100017]. [PMID: 17186007]. [DOI] [PubMed] [Google Scholar]
- 122.Arun P., Oguntayo S., Alamneh Y., Honnold C., Wang Y., Valiyaveettil M., Long J.B., Nambiar M.P. Rapid release of tissue enzymes into blood after blast exposure: potential use as biological dosimeters. PLoS One. 2012;7(4):e33798. doi: 10.1371/journal.pone.0033798. [http://dx.doi. org/10.1371/journal.pone.0033798]. [PMID: 22493674]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shear D.A., Tortella F.C. A military-centered approach to neuroprotection for traumatic brain injury. Front. Neurol. 2013;4:73. doi: 10.3389/fneur.2013.00073. [http://dx.doi.org/10.3389/fneur.2013.00073]. [PMID: 23781213]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Diaz-Arrastia R., Wang K.K., Papa L., Sorani M.D., Yue J.K., Puccio A.M., McMahon P.J., Inoue T., Yuh E.L., Lingsma H.F., Maas A.I., Valadka A.B., Okonkwo D.O., Manley G.T. TRACK-TBI Investigators. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma. 2014;31(1):19–25. doi: 10.1089/neu.2013.3040. [http://dx.doi.org/10.1089/neu.2013.3040]. [PMID: 23865516]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yokobori S., Hosein K., Burks S., Sharma I., Gajavelli S., Bullock R. Biomarkers for the clinical differential diagnosis in traumatic brain injury--a systematic review. CNS Neurosci. Ther. 2013;19(8):556–565. doi: 10.1111/cns.12127. [http://dx.doi.org/10.1111/cns.12127]. [PMID: 23710877]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mondello S., Robicsek S.A., Gabrielli A., Brophy G.M., Papa L., Tepas J., III, Robertson C., Buki A., Scharf D., Jixiang M., Akinyi L., Muller U., Wang K.K., Hayes R.L. αII-spectrin breakdown products (SBDPs): diagnosis and outcome in severe traumatic brain injury patients. J. Neurotrauma. 2010;27(7):1203–1213. doi: 10.1089/neu.2010.1278. [http://dx.doi.org/10.1089/neu.2010.1278]. [PMID: 20408766]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zetterberg H., Smith D.H., Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat. Rev. Neurol. 2013;9(4):201–210. doi: 10.1038/nrneurol.2013.9. [http://dx.doi.org/10.1038/nrneurol. 2013.9]. [PMID: 23399646]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raheja A., Sinha S., Samson N., Bhoi S., Subramanian A., Sharma P., Sharma B.S. Serum biomarkers as predictors of long-term outcome in severe traumatic brain injury: analysis from a randomized placebo-controlled Phase II clinical trial. J. Neurosurg. 2016;125(3):631–641. doi: 10.3171/2015.6.JNS15674. [http://dx.doi.org/10.3171/2015.6.JNS15674]. [PMID: 26722854]. [DOI] [PubMed] [Google Scholar]
- 129.Sinha S., Raheja A., Samson N., Bhoi S., Selvi A., Sharma P., Sharma B.S. Blood mitochondrial enzymatic assay as a predictor of long-term outcome in severe traumatic brain injury. J. Clin. Neurosci. 2016;30:31–38. doi: 10.1016/j.jocn.2015.10.051. [http://dx.doi.org/10.1016/j.jocn.2015. 10.051]. [PMID: 27262871]. [DOI] [PubMed] [Google Scholar]
- 130.Dadas A., Washington J., Marchi N., Janigro D. Improving the clinical management of traumatic brain injury through the pharmacokinetic modeling of peripheral blood biomarkers. Fluids Barriers CNS. 2016;13(1):21. doi: 10.1186/s12987-016-0045-y. [http://dx.doi.org/10.1186/s12987-016-0045-y]. [PMID: 27903281]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Papa L., Edwards D., Ramia M. Exploring Serum Biomarkers for Mild Traumatic Brain Injury. In: Kobeissy F.H., editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton, FL: CRC Press/Taylor & Francis; 2015. [http://dx.doi.org/10.1201/b18126-27] [PMID: 26269900] [PubMed] [Google Scholar]
- 132.Jain K.K. The Handbook of Neuroprotection. 2011 http:// www.springer.com/us/book/9781617790485
- 133.Kochanek P.M., Jackson T.C., Ferguson N.M., Carlson S.W., Simon D.W., Brockman E.C., Ji J., Bayır H., Poloyac S.M., Wagner A.K., Kline A.E., Empey P.E. Clark, R.S.; Jackson, E.K.; Dixon, C.E. Emerging therapies in traumatic brain injury. Semin. Neurol. 2015;35(1):83–100. doi: 10.1055/s-0035-1544237. [http://dx.doi.org/10.1055/s-0035-1544237]. [PMID: 25714870]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marklund N., Bakshi A., Castelbuono D.J., Conte V., McIntosh T.K. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr. Pharm. Des. 2006;12(13):1645–1680. doi: 10.2174/138161206776843340. [http:// dx.doi.org/10.2174/138161206776843340]. [PMID: 16729876]. [DOI] [PubMed] [Google Scholar]
- 135.Hall E.D., Vaishnav R.A., Mustafa A.G. Antioxidant therapies for traumatic brain injury. Neurotherapeutics. 2010;7(1):51–61. doi: 10.1016/j.nurt.2009.10.021. [http://dx.doi.org/10.1016/j.nurt.2009.10.021]. [PMID: 20129497]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anthonymuthu T.S., Kenny E.M., Bayır H. Therapies targeting lipid peroxidation in traumatic brain injury. 2016. [DOI] [PMC free article] [PubMed]
- 137.Faden A.I., Knoblach S.M., Movsesyan V.A., Cernak I. Novel small peptides with neuroprotective and nootropic properties. J. Alzheimers Dis. 2004;6(S6):S93–S97. doi: 10.3233/jad-2004-6s603. [PMID: 15665420]. [DOI] [PubMed] [Google Scholar]
- 138.Pierce J.E., Smith D.H., Eison M.S., McIntosh T.K. The nootropic compound BMY-21502 improves spatial learning ability in brain injured rats. Brain Res. 1993;624(1-2):199–208. doi: 10.1016/0006-8993(93)90078-2. [http:// dx.doi.org/10.1016/0006-8993(93)90078-2]. [PMID: 8252392]. [DOI] [PubMed] [Google Scholar]
- 139.Kwon E.J., Skalak M., Lo Bu R., Bhatia S.N. Neuron-targeted nanoparticle for siRNA delivery to traumatic brain injuries. ACS Nano. 2016;10(8):7926–7933. doi: 10.1021/acsnano.6b03858. [http://dx.doi.org/10.1021/acsnano. 6b03858]. [PMID: 27429164]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Marion D.W., Penrod L.E., Kelsey S.F., Obrist W.D. ; Kochanek P.M., Palmer A.M., Wisniewski S.R., DeKosky S.T. Treatment of traumatic brain injury with moderate hypothermia. N. Engl. J. Med. 1997;336(8):540–546. doi: 10.1056/NEJM199702203360803. [http://dx.doi.org/10.1056/ NEJM199702203360803]. [PMID: 9023090]. [DOI] [PubMed] [Google Scholar]
- 141.Peterson K., Carson S., Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J. Neurotrauma. 2008;25(1):62–71. doi: 10.1089/neu.2007.0424. [http://dx.doi.org/10.1089/neu. 2007.0424]. [PMID: 18355159]. [DOI] [PubMed] [Google Scholar]
- 142.Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., Manley G.T., Workshop Scientific Team and Advisory Panel Members Classification of traumatic brain injury for targeted therapies. J. Neurotrauma. 2008;25(7):719–738. doi: 10.1089/neu.2008.0586. [http://dx.doi.org/ 10.1089/neu.2008.0586]. [PMID: 18627252]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Margulies S., Anderson G., Atif F., Badaut J., Clark R., Empey P., Guseva M., Hoane M., Huh J., Pauly J., Raghupathi R., Scheff S., Stein D., Tang H., Hicks M. Combination therapies for traumatic brain injury: retrospective considerations. J. Neurotrauma. 2016;33(1):101–112. doi: 10.1089/neu.2014.3855. [http://dx.doi.org/10.1089/neu.2014. 3855]. [PMID: 25970337]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bozic I., Reiter J.G., Allen B., Antal T., Chatterjee K., Shah P., Moon Y.S., Yaqubie A., Kelly N., Le D.T., Lipson E.J., Chapman P.B., Diaz L.A., Jr, Vogelstein B., Nowak M.A. Evolutionary dynamics of cancer in response to targeted combination therapy. eLife. 2013;2:e00747. doi: 10.7554/eLife.00747. [http://dx.doi.org/10.7554/eLife.00747]. [PMID: 23805382]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Calgüneri M., Pay S., Calişkaner Z., Apraş S., Kiraz S., Ertenli I., Cobankara V. Combination therapy versus monotherapy for the treatment of patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 1999;17(6):699–704. [PMID: 10609068]. [PubMed] [Google Scholar]
- 146.Wald D.S., Law M., Morris J.K., Bestwick J.P., Wald N.J. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am. J. Med. 2009;122(3):290–300. doi: 10.1016/j.amjmed.2008.09.038. [http://dx.doi.org/10.1016/j.amjmed. 2008.09.038]. [PMID: 19272490]. [DOI] [PubMed] [Google Scholar]
- 147.Carey I., Harrison P.M. Monotherapy versus combination therapy for the treatment of chronic hepatitis B. Expert Opin. Investig. Drugs. 2009;18(11):1655–1666. doi: 10.1517/13543780903241599. [http://dx.doi.org/10.1517/ 13543780903241599]. [PMID: 19852566]. [DOI] [PubMed] [Google Scholar]
- 148.Krainer M. Efficacy of combination therapy versus monotherapy. Breast Cancer Res. Treat. 2003;81(Suppl. 1):S11–S15. [http://dx. doi.org/10.1023/A:1026352303876]. [Google Scholar]
- 149.Atif F., Yousuf S., Sayeed I., Ishrat T., Hua F., Stein D.G. Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: the role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacology. 2013;67:78–87. doi: 10.1016/j.neuropharm.2012.10.004. [http://dx.doi.org/10.1016/j.neuropharm. 2012.10.004]. [PMID: 23154302]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jean A., Nyein M.K., Zheng J.Q., Moore D.F., Joannopoulos J.D., Radovitzky R. An animal-to-human scaling law for blast-induced traumatic brain injury risk assessment. Proc. Natl. Acad. Sci. USA. 2014;111(43):15310–15315. doi: 10.1073/pnas.1415743111. [http://dx.doi.org/10.1073/ Pnas.1415743111]. [PMID: 25267617]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wright D.W., Ritchie J.C., Mullins R.E., Kellermann A.L., Denson D.D. Steady-state serum concentrations of progesterone following continuous intravenous infusion in patients with acute moderate to severe traumatic brain injury. J. Clin. Pharmacol. 2005;45(6):640–648. doi: 10.1177/0091270005276201. [http://dx.doi.org/10.1177/0091270005276201]. [PMID: 15901745]. [DOI] [PubMed] [Google Scholar]
- 152.Skolnick B.E., Maas A.I., Narayan R.K., van der Hoop R.G., MacAllister T., Ward J.D., Nelson N.R., Stocchetti N., SYNAPSE Trial Investigators A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 2014;371(26):2467–2476. doi: 10.1056/NEJMoa1411090. [http://dx.doi.org/10.1056/NEJMoa1411090]. [PMID: 25493978]. [DOI] [PubMed] [Google Scholar]
- 153.Wright D.W., Kellermann A.L., Hertzberg V.S., Clark P.L., Frankel M., Goldstein F.C., Salomone J.P., Dent L.L., Harris O.A., Ander D.S., Lowery D.W., Patel M.M., Denson D.D., Gordon A.B., Wald M.M., Gupta S., Hoffman S.W., Stein D.G. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann. Emerg. Med. 2007;49(4):391–402. doi: 10.1016/j.annemergmed.2006.07.932. [http://dx.doi.org/10.1016/j.annemergmed.2006.07.932]. [PMID: 17011666]. [DOI] [PubMed] [Google Scholar]
- 154.Shukla D., Devi B.I., Agrawal A. Outcome measures for traumatic brain injury. Clin. Neurol. Neurosurg. 2011;113(6):435–441. doi: 10.1016/j.clineuro.2011.02.013. [http://dx.doi.org/10.1016/j.clineuro.2011.02.013]. [PMID: 21440363]. [DOI] [PubMed] [Google Scholar]
- 155.Wilde E.A., Whiteneck G.G., Bogner J., Bushnik T., Cifu D.X., Dikmen S., French L., Giacino J.T., Hart T., Malec J.F., Millis S.R., Novack T.A., Sherer M., Tulsky D.S., Vanderploeg R.D., von Steinbuechel N. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 2010;91(11):1650–1660. doi: 10.1016/j.apmr.2010.06.033. [http://dx.doi.org/10.1016/j. apmr.2010.06.033]. [PMID: 21044708]. [DOI] [PubMed] [Google Scholar]
- 156.Kramer J.A., Sagartz J.E., Morris D.L. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat. Rev. Drug Discov. 2007;6(8):636–649. doi: 10.1038/nrd2378. [http://dx.doi.org/10.1038/nrd2378]. [PMID: 17643090]. [DOI] [PubMed] [Google Scholar]
- 157.Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G., Lilly P., Sanders J., Sipes G., Bracken W., Dorato M., Van Deun K., Smith P., Berger B., Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [http://dx.doi.org/10.1006/ rtph.2000.1399]. [PMID: 11029269]. [DOI] [PubMed] [Google Scholar]
- 158.Wright D.W., Bauer M.E., Hoffman S.W., Stein D.G. Serum progesterone levels correlate with decreased cerebral edema after traumatic brain injury in male rats. J. Neurotrauma. 2001;18(9):901–909. doi: 10.1089/089771501750451820. [http://dx.doi.org/10.1089/089771501750451820]. [PMID: 11565602]. [DOI] [PubMed] [Google Scholar]
- 159.Pan D.S., Liu W.G., Yang X.F., Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed. Environ. Sci. 2007;20(5):432–438. [PMID: 18188998]. [PubMed] [Google Scholar]
- 160.Pettus E.H., Wright D.W., Stein D.G., Hoffman S.W. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049(1):112–119. doi: 10.1016/j.brainres.2005.05.004. [http:// dx.doi.org/10.1016/j.brainres.2005.05.004]. [PMID: 15932748]. [DOI] [PubMed] [Google Scholar]
- 161.Duvdevani R., Roof R.L., Fülöp Z., Hoffman S.W., Stein D.G. Blood-brain barrier breakdown and edema formation following frontal cortical contusion: does hormonal status play a role? J. Neurotrauma. 1995;12(1):65–75. doi: 10.1089/neu.1995.12.65. [http://dx.doi.org/10.1089/neu. 1995.12.65]. [PMID: 7783233]. [DOI] [PubMed] [Google Scholar]
- 162.Roof R.L., Duvdevani R., Heyburn J.W., Stein D.G. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp. Neurol. 1996;138(2):246–251. doi: 10.1006/exnr.1996.0063. [http://dx.doi.org/10.1006/exnr.1996.0063]. [PMID: 8620923]. [DOI] [PubMed] [Google Scholar]
- 163.Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., Manley G.T., Merck L.H., Janis L.S., Barsan W.G. NETT Investigators. Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 2014;371(26):2457–2466. doi: 10.1056/NEJMoa1404304. [http://dx.doi.org/10.1056/NEJMoa1404304]. [PMID: 25493974]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Goldstein F.C., Caveney A.F., Hertzberg V.S., Silbergleit R., Yeatts S.D., Palesch Y.Y., Levin H.S., Wright D.W. Very early administration of progesterone does not improve neuropsychological outcomes in subjects with moderate to severe traumatic brain injury. J. Neurotrauma. 2017;34(1):115–120. doi: 10.1089/neu.2015.4313. [http://dx.doi.org/ 10.1089/neu.2015.4313]. [PMID: 26973025]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zafonte R., Friedewald W.T., Lee S.M., Levin B., Diaz-Arrastia R., Ansel B., Eisenberg H., Timmons S.D., Temkin N., Novack T., Ricker J., Merchant R., Jallo J. The citicoline brain injury treatment (COBRIT) trial: design and methods. J. Neurotrauma. 2009;26(12):2207–2216. doi: 10.1089/neu.2009.1015. [http://dx.doi.org/10.1089/neu.2009. 1015]. [PMID: 19803786]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zafonte R.D., Bagiella E., Ansel B.M., Novack T.A., Friedewald W.T., Hesdorffer D.C., Timmons S.D., Jallo J., Eisenberg H., Hart T., Ricker J.H., Diaz-Arrastia R., Merchant R.E., Temkin N.R., Melton S., Dikmen S.S. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA. 2012;308(19):1993–2000. doi: 10.1001/jama.2012.13256. [http://dx.doi.org/10.1001/jama.2012. 13256]. [PMID: 23168823]. [DOI] [PubMed] [Google Scholar]
- 167.Shumaker S.A., Legault C., Rapp S.R., Thal L., Wallace R.B., Ockene J.K., Hendrix S.L., Jones B.N., III, Assaf A.R., Jackson R.D., Kotchen J.M., Wassertheil-Smoller S., Wactawski-Wende J., WHIMS Investigators Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [http://dx. doi.org/10.1001/jama.289.20.2651]. [PMID: 12771112]. [DOI] [PubMed] [Google Scholar]
- 168.Singh M., Dykens J.A., Simpkins J.W. Novel mechanisms for estrogen-induced neuroprotection. Exp. Biol. Med. (Maywood) 2006;231(5):514–521. doi: 10.1177/153537020623100505. [http://dx.doi.org/10.1177/153537020623100505]. [PMID: 16636299]. [DOI] [PubMed] [Google Scholar]
- 169.Singh M., Sumien N., Kyser C., Simpkins J.W. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front. Biosci. 2008;13:1083–1089. doi: 10.2741/2746. [PMID: 17981614]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Takhounts E.G., Eppinger R.H., Campbell J.Q., Tannous R.E., Power E.D., Shook L.S. On the development of the SIMon finite element head model. Stapp Car Crash J. 2003;47:107–133. doi: 10.4271/2003-22-0007. [PMID: 17096247]. [DOI] [PubMed] [Google Scholar]
- 171.King A.I., Ruan J.S., Zhou C., Hardy W.N., Khalil T.B. Recent advances in biomechanics of brain injury research: a review. J. Neurotrauma. 1995;12(4):651–658. doi: 10.1089/neu.1995.12.651. [http://dx.doi.org/10.1089/ neu.1995.12.651]. [PMID: 8683616]. [DOI] [PubMed] [Google Scholar]
- 172.Brands D.W., Peters G.W., Bovendeerd P.H. Design and numerical implementation of a 3-D non-linear viscoelastic constitutive model for brain tissue during impact. J. Biomech. 2004;37(1):127–134. doi: 10.1016/s0021-9290(03)00243-4. [http://dx.doi.org/10.1016/S0021-9290(03)00243-4]. [PMID: 14672576]. [DOI] [PubMed] [Google Scholar]
- 173.Kleiven S. Predictors for traumatic brain injuries evaluated through accident reconstructions. Stapp Car Crash J. 2007;51:81–114. doi: 10.4271/2007-22-0003. [PMID: 18278592]. [DOI] [PubMed] [Google Scholar]
- 174.Antona-Makoshi J., Davidsson J., Ejima S., Ono K., Brolin K., Anata K. Correlation of global head and brain tissue injury criteria to experimental concussion derived from monkey head trauma experiments.; 2013 IRCOBI Conference Proceedings - International Research Council on the Biomechanics of Injury.; 2013. pp. 509–522. [Google Scholar]
- 175.Marjoux D., Baumgartner D., Deck C., Willinger R. Head injury prediction capability of the HIC, HIP, SIMon and ULP criteria. Accid. Anal. Prev. 2008;40(3):1135–1148. doi: 10.1016/j.aap.2007.12.006. [http://dx.doi.org/10. 1016/j.aap.2007.12.006]. [PMID: 18460382]. [DOI] [PubMed] [Google Scholar]
- 176.Gupta R.K., Przekwas A. Mathematical models of blast-induced TBI: current status, challenges, and prospects. Front. Neurol. 2013;4:59. doi: 10.3389/fneur.2013.00059. [http://dx.doi.org/10.3389/fneur.2013.00059]. [PMID: 23755039]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kraft R.H., McKee P.J., Dagro A.M., Grafton S.T. Combining the finite element method with structural connectome-based analysis for modeling neurotrauma: connectome neurotrauma mechanics. PLOS Comput. Biol. 2012;8(8):e1002619. doi: 10.1371/journal.pcbi.1002619. [http://dx.doi.org/ 10.1371/journal.pcbi.1002619]. [PMID: 22915997]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ascoli G.A. Mobilizing the base of neuroscience data: the case of neuronal morphologies. Nat. Rev. Neurosci. 2006;7(4):318–324. doi: 10.1038/nrn1885. [http://dx.doi.org/10.1038/nrn1885]. [PMID: 16552417]. [DOI] [PubMed] [Google Scholar]
- 179.Gleeson P., Steuber V., Silver R.A. Neuro construct: a tool for modeling networks of neurons in 3D space. Neuron. 2007;54(2):219–235. doi: 10.1016/j.neuron.2007.03.025. [http://dx.doi.org/10.1016/j.neuron.2007.03.025]. [PMID: 17442244]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Martone M.E., Tran J., Wong W.W., Sargis J., Fong L., Larson S., Lamont S.P., Gupta A., Ellisman M.H. The cell centered database project: an update on building community resources for managing and sharing 3D imaging data. J. Struct. Biol. 2008;161(3):220–231. doi: 10.1016/j.jsb.2007.10.003. [http://dx.doi.org/10.1016/j.jsb.2007.10.003]. [PMID: 18054501]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.He H.Y., Cline H.T., Diadem X. automated 4 dimensional analysis of morphological data. Neuroinformatics. 2011;9(2-3):107–112. doi: 10.1007/s12021-011-9098-x. [http://dx.doi.org/10.1007/s12021-011-9098-x]. [PMID: 21271361]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Halchenko Y.O., Hanke M. Open is not enough. Let’s take the next step: an integrated, community-driven computing platform for neuroscience. Front. Neuroinform. 2012;6:22. doi: 10.3389/fninf.2012.00022. [http://dx.doi.org/ 10.3389/fninf.2012.00022]. [PMID: 23055966]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Leergaard T.B., Hilgetag C.C., Sporns O. Mapping the connectome: multi-level analysis of brain connectivity. Front. Neuroinform. 2012;6:14. doi: 10.3389/fninf.2012.00014. [http://dx.doi.org/10.3389/fninf.2012.00014/ full]. [PMID: 22557964]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Churchland P.S., Sejnowski T.J. The computational brain. Cambridge, Massachusetts; London, England: MIT press; 2017. http://www.jstor.org/stable/j.ctt1h4mjjp [Google Scholar]
- 185.Carnevale N.T., Hines M.L. The NEURON book. Cambridge University Press; 2006. [http://dx.doi.org/10.1017/CBO9780511541612] [Google Scholar]
- 186.Kager H., Wadman W.J., Somjen G.G. Simulated seizures and spreading depression in a neuron model incorporating interstitial space and ion concentrations. J. Neurophysiol. 2000;84(1):495–512. doi: 10.1152/jn.2000.84.1.495. [http://dx.doi.org/10.1152/jn.2000.84.1.495]. [PMID: 10899222]. [DOI] [PubMed] [Google Scholar]
- 187.Lindén H., Hagen E., Lęski S., Norheim E.S., Pettersen K.H., Einevoll G.T. LFPy: a tool for biophysical simulation of extracellular potentials generated by detailed model neurons. Front. Neuroinform. 2014;7:41. doi: 10.3389/fninf.2013.00041. [http://dx.doi.org/10.3389/fninf.2013.00041]. [PMID: 24474916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Calvetti D., Somersalo E. Dynamic activation model for a glutamatergic neurovascular unit. J. Theor. Biol. 2011;274(1):12–29. doi: 10.1016/j.jtbi.2010.12.007. [http://dx.doi.org/10.1016/j.jtbi.2010.12.007]. [PMID: 21176783]. [DOI] [PubMed] [Google Scholar]
- 189.Kozloski J., Wagner J. An ultrascalable solution to large-scale neural tissue simulation. Front. Neuroinform. 2011;5:15. doi: 10.3389/fninf.2011.00015. [http://dx.doi.org/10.3389/fninf.2011.00015]. [PMID: 21954383]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bower J.M., Cornelis H., Beeman D. 2013. [Google Scholar]
- 191.Dudani N., Ray S., George S., Bhalla U.S. Multiscale modeling and interoperability in MOOSE. In: Eighteenth Annual Computational Neuroscience Meeting: CNS*2009. BMC Neurosci. 2009;10(Suppl. 1):54. [https://doi.org/10.1186/1471-2202-10-S1-P54]. [Google Scholar]
- 192.Aubert A., Costalat R. Interaction between astrocytes and neurons studied using a mathematical model of compartmentalized energy metabolism. J. Cereb. Blood Flow Metab. 2005;25(11):1476–1490. doi: 10.1038/sj.jcbfm.9600144. [http://dx.doi.org/10.1038/sj.jcbfm.9600144]. [PMID: 15931164]. [DOI] [PubMed] [Google Scholar]
- 193.Dronne M.A., Boissel J.P., Grenier E. A mathematical model of ion movements in grey matter during a stroke. J. Theor. Biol. 2006;240(4):599–615. doi: 10.1016/j.jtbi.2005.10.023. [http://dx.doi.org/10.1016/j.jtbi.2005.10. 023]. [PMID: 16368113]. [DOI] [PubMed] [Google Scholar]
- 194.Dronne M.A., Grenier E., Chapuisat G., Hommel M., Boissel J.P. A modelling approach to explore some hypotheses of the failure of neuroprotective trials in ischemic stroke patients. Prog. Biophys. Mol. Biol. 2008;97(1):60–78. doi: 10.1016/j.pbiomolbio.2007.10.001. [http://dx.doi.org/10.1016/j. pbiomolbio.2007.10.001]. [PMID: 18076975]. [DOI] [PubMed] [Google Scholar]
- 195.Fussenegger M., Bailey J.E., Varner J. A mathematical model of caspase function in apoptosis. Nat. Biotechnol. 2000;18(7):768–774. doi: 10.1038/77589. [http://dx.doi.org/10.1038/77589]. [PMID: 10888847]. [DOI] [PubMed] [Google Scholar]
- 196.Gaohua L., Kimura H. A mathematical model of intracranial pressure dynamics for brain hypothermia treatment. J. Theor. Biol. 2006;238(4):882–900. doi: 10.1016/j.jtbi.2005.06.036. [http://dx.doi.org/10.1016/j.jtbi.2005.06.036]. [PMID: 16098539]. [DOI] [PubMed] [Google Scholar]
- 197.Østby I., Øyehaug L., Einevoll G.T., Nagelhus E.A., Plahte E., Zeuthen T., Lloyd C.M., Ottersen O.P., Omholt S.W. Astrocytic mechanisms explaining neural-activity-induced shrinkage of extraneuronal space. PLOS Comput. Biol. 2009;5(1):e1000272. doi: 10.1371/journal.pcbi.1000272. [http:// dx.doi.org/10.1371/journal.pcbi.1000272]. [PMID: 19165313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cortassa S., Aon M.A., Winslow R.L., O’Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys. J. 2004;87(3):2060–2073. doi: 10.1529/biophysj.104.041749. [http://dx.doi.org/10.1529/ biophysj.104.041749]. [PMID: 15345581]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sutherland M.L., Fabre K.M., Tagle D.A. The National Institutes of Health Microphysiological Systems Program focuses on a critical challenge in the drug discovery pipeline. Stem Cell Res. Ther. 2013;4(Suppl. 1):I1. doi: 10.1186/scrt361. [http://dx.doi.org/10.1186/scrt361]. [PMID: 24565163]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Schwartz M.P., Hou Z., Propson N.E., Zhang J., Engstrom C.J., Santos Costa V., Jiang P., Nguyen B.K., Bolin J.M., Daly W., Wang Y., Stewart R., Page C.D., Murphy W.L., Thomson J.A. Human pluripotent stem cell-derived neural constructs for predicting neural toxicity. Proc. Natl. Acad. Sci. USA. 2015;112(40):12516–12521. doi: 10.1073/pnas.1516645112. [http://dx.doi.org/10.1073/pnas.1516645112]. [PMID: 26392547]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pamies D., Hartung T., Hogberg H.T. Biological and medical applications of a brain-on-a-chip. Exp. Biol. Med. (Maywood) 2014;239(9):1096–1107. doi: 10.1177/1535370214537738. [http://dx.doi.org/10.1177/1535370214537738]. [PMID: 24912505]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cho H., Seo J.H., Wong K.H., Terasaki Y., Park J., Bong K., Arai K., Lo E.H., Irimia D. Three-dimensional blood-brain barrier model for in vitro studies of neurovascular pathology. Sci. Rep. 2015;5:15222. doi: 10.1038/srep15222. [http://dx.doi.org/10.1038/srep15222]. [PMID: 26503597]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hartung T., Zurlo J. Alternative approaches for medical countermeasures to biological and chemical terrorism and warfare. ALTEX. 2012;29(3):251–260. doi: 10.14573/altex.2012.3.251. [http://dx.doi.org/10.14573/altex. 2012.3.251]. [PMID: 22847253]. [DOI] [PubMed] [Google Scholar]
- 204.Microphysiological Systems DARPA. http://www.darpa.mil/program/microphysiological-systems
- 205.Somayaji M.R., Das D., Przekwas A. Computational approaches for modeling and analysis of human-on-chip systems for drug testing and characterization. Drug Discov. Today. 2016;21(12):1859–1862. doi: 10.1016/j.drudis.2016.11.002. [http://dx.doi.org/10.1016/j.drudis.2016.11.002]. [PMID: 27871942]. [DOI] [PubMed] [Google Scholar]
- 206.Somayaji M.R., Xenos M., Zhang L., Mekarski M., Linninger A.A. Systematic design of drug delivery therapies. Comput. Chem. Eng. 2008;32(1):89–98. [http://dx.doi.org/10.1016/j. compchemeng.2007.06.014]. [Google Scholar]
- 207.Raz L., Khan M.M., Mahesh V.B., Vadlamudi R.K., Brann D.W. Rapid estrogen signaling in the brain. Neurosignals. 2008;16(2-3):140–153. doi: 10.1159/000111559. [http://dx.doi.org/10.1159/000111559]. [PMID: 18253054]. [DOI] [PubMed] [Google Scholar]
- 208.McEwen B.S., Alves S.E. Estrogen actions in the central nervous system. Endocr. Rev. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [http://dx.doi.org/ 10.1210/edrv.20.3.0365]. [PMID: 10368772]. [DOI] [PubMed] [Google Scholar]
- 209.Segars J.H., Driggers P.H. Estrogen action and cytoplasmic signaling cascades. Part I: membrane-associated signaling complexes. Trends Endocrinol. Metab. 2002;13(8):349–354. doi: 10.1016/s1043-2760(02)00633-1. [http://dx.doi. org/10.1016/S1043-2760(02)00633-1]. [PMID: 12217492]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee S.J., McEwen B.S. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [http://dx.doi.org/10.1146/annurev. pharmtox.41.1.569]. [PMID: 11264469]. [DOI] [PubMed] [Google Scholar]
- 211.Lapanantasin S., Chongthammakun S., Floyd C.L., Berman R.F. Effects of 17β-estradiol on intracellular calcium changes and neuronal survival after mechanical strain injury in neuronal-glial cultures. Synapse. 2006;60(5):406–410. doi: 10.1002/syn.20308. [http://dx.doi.org/10.1002/ syn.20308]. [PMID: 16856173]. [DOI] [PubMed] [Google Scholar]
- 212.Brinton R.D. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer’s disease. Adv. Drug Deliv. Rev. 2008;60(13-14):1504–1511. doi: 10.1016/j.addr.2008.06.003. [http://dx.doi.org/10.1016/j.addr.2008.06.003]. [PMID: 18647624]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Simpkins J.W., Yi K.D., Yang S.H., Dykens J.A. Mitochondrial mechanisms of estrogen neuroprotection. Biochim. Biophys. Acta. 2010;1800(10):1113–1120. doi: 10.1016/j.bbagen.2009.11.013. [http://dx.doi.org/10.1016/j.bbagen. 2009.11.013]. [PMID: 19931595]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Huang Y., Huang Y.L., Zhang S., Zhu Y.C., Yao T. Estradiol acutely attenuates glutamate-induced calcium overload in primarily cultured rat hippocampal neurons through a membrane receptor-dependent mechanism. Brain Res. 2004;1026(2):254–260. doi: 10.1016/j.brainres.2004.08.038. [http:// dx.doi.org/10.1016/j.brainres.2004.08.038]. [PMID: 15488487]. [DOI] [PubMed] [Google Scholar]
- 215.Saleh T.M., Connell B.J., Legge C., Cribb A.E. Estrogen attenuates neuronal excitability in the insular cortex following middle cerebral artery occlusion. Brain Res. 2004;1018(1):119–129. doi: 10.1016/j.brainres.2004.05.074. [http:// dx.doi.org/10.1016/j.brainres.2004.05.074]. [PMID: 15262213]. [DOI] [PubMed] [Google Scholar]
- 216.Chen J., Xu W., Jiang H. 17 β-estradiol protects neurons from ischemic damage and attenuates accumulation of extracellular excitatory amino acids. Anesth. Analg. 2001;92(6):1520–1523. doi: 10.1097/00000539-200106000-00033. [http://dx.doi.org/10.1097/00000539-200106000-00033]. [PMID: 11375837]. [DOI] [PubMed] [Google Scholar]
- 217.Pelligrino D.A., Santizo R., Baughman V.L., Wang Q. Cerebral vasodilating capacity during forebrain ischemia: effects of chronic estrogen depletion and repletion and the role of neuronal nitric oxide synthase. Neuroreport. 1998;9(14):3285–3291. doi: 10.1097/00001756-199810050-00026. [http://dx.doi. org/10.1097/00001756-199810050-00026]. [PMID: 9831465]. [DOI] [PubMed] [Google Scholar]
- 218.Roof R.L., Hall E.D. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J. Neurotrauma. 2000;17(5):367–388. doi: 10.1089/neu.2000.17.367. [http://dx.doi.org/10.1089/ neu.2000.17.367]. [PMID: 10833057]. [DOI] [PubMed] [Google Scholar]
- 219.Lebesgue D., Chevaleyre V., Zukin R.S., Etgen A.M. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74(7):555–561. doi: 10.1016/j.steroids.2009.01.003. [http://dx.doi.org/10.1016/j.steroids.2009.01.003]. [PMID: 19428444]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kim H., Cam-Etoz B., Zhai G., Hubbard W.J., Zinn K.R., Chaudry I.H. Salutary effects of estrogen sulfate for traumatic brain injury. J. Neurotrauma. 2015;32(16):1210–1216. doi: 10.1089/neu.2014.3771. [http://dx. doi.org/10.1089/neu.2014.3771]. [PMID: 25646701]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stein D.G. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24(7):386–391. doi: 10.1016/s0166-2236(00)01821-x. [http://dx.doi.org/10.1016/S0166-2236(00)01821-X]. [PMID: 11410269]. [DOI] [PubMed] [Google Scholar]
- 222.Chen G., Shi J., Jin W., Wang L., Xie W., Sun J., Hang C. Progesterone administration modulates TLRs/NF-kappaB signaling pathway in rat brain after cortical contusion. Ann. Clin. Lab. Sci. 2008;38(1):65–74. [PMID: 18316784]. [PubMed] [Google Scholar]
- 223.Xiao G., Wei J., Yan W., Wang W., Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit. Care. 2008;12(2):R61. doi: 10.1186/cc6887. [http://dx.doi.org/10.1186/cc6887]. [PMID: 18447940]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sanchez M.R.O., Ona V.O., Li M., Friedlander R.M. Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction. Neurosurgery. 2001;48(6):1393–1399. doi: 10.1097/00006123-200106000-00051. [PMID: 11383749]. [DOI] [PubMed] [Google Scholar]
- 225.Kovesdi E., Kamnaksh A., Wingo D., Ahmed F., Grunberg N.E., Long J.B., Kasper C.E., Agoston D.V. Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front. Neurol. 2012;3:111. doi: 10.3389/fneur.2012.00111. [http://dx.doi.org/ 10.3389/fneur.2012.00111]. [PMID: 22811676]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sullivan P.G., Thompson M., Scheff S.W. Continuous infusion of cyclosporin A postinjury significantly ameliorates cortical damage following traumatic brain injury. Exp. Neurol. 2000;161(2):631–637. doi: 10.1006/exnr.1999.7282. [http://dx.doi.org/10.1006/exnr.1999.7282]. [PMID: 10686082]. [DOI] [PubMed] [Google Scholar]
- 227.Margulies S.S., Kilbaugh T., Sullivan S., Smith C., Propert K., Byro M., Saliga K., Costine B.A., Duhaime A.C. Establishing a clinically relevant large animal model platform for TBI therapy development: using cyclosporin a as a case study. Brain Pathol. 2015;25(3):289–303. doi: 10.1111/bpa.12247. [http://dx.doi.org/10.1111/bpa.12247]. [PMID: 25904045]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhao J., Zhou D., Guo J., Ren Z., Zhou L., Wang S., Zhang Y., Xu B., Zhao K., Wang R., Mao Y., Xu B., Zhang X., Fasudil Aneurysmal Subarachnoid Hemorrhage Study Group Efficacy and safety of fasudil in patients with subarachnoid hemorrhage: final results of a randomized trial of fasudil versus nimodipine. Neurol. Med. Chir. (Tokyo) 2011;51(10):679–683. doi: 10.2176/nmc.51.679. [http:// dx.doi.org/10.2176/nmc.51.679]. [PMID: 22027241]. [DOI] [PubMed] [Google Scholar]
- 229.Shibuya M., Hirai S., Seto M., Satoh S., Ohtomo E., Fasudil Ischemic Stroke Study Group Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J. Neurol. Sci. 2005;238(1-2):31–39. doi: 10.1016/j.jns.2005.06.003. [http://dx.doi.org/10. 1016/j.jns.2005.06.003]. [PMID: 16005902]. [DOI] [PubMed] [Google Scholar]
- 230.Kubo T., Yamaguchi A., Iwata N., Yamashita T. The therapeutic effects of Rho-ROCK inhibitors on CNS disorders. Ther. Clin. Risk Manag. 2008;4(3):605–615. doi: 10.2147/tcrm.s2907. [http://dx.doi.org/10.2147/TCRM. S2907]. [PMID: 18827856]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bailey Z.S., Nilson E., Bates J.A., Oyalowo A., Hockey K.S., Sajja V.S.S.S., Thorpe C., Rogers H., Dunn B., Frey A.S., Billings M.J., Sholar C.A., Hermundstad A., Kumar C., VandeVord P.J., Rzigalinski B.A. 2016.
- 232.Rzigalinski B.A., Carfagna C.S., Ehrich M. Cerium oxide nanoparticles in neuroprotection and considerations for efficacy and safety. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9(4):e1444. doi: 10.1002/wnan.1444. [http://dx.doi.org/10.1002/wnan.1444]. [PMID: 27860449]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rzigalinski B.A., Meehan K., Davis R.M., Xu Y., Miles W.C., Cohen C.A. Radical nanomedicine. Nanomedicine (Lond.) 2006;1(4):399–412. doi: 10.2217/17435889.1.4.399. [http://dx.doi.org/10.2217/17435889.1.4.399]. [PMID: 17716143]. [DOI] [PubMed] [Google Scholar]
- 234.Singh N., Cohen C.A., Rzigalinski B.A. Treatment of neurodegenerative disorders with radical nanomedicine. Ann. N. Y. Acad. Sci. 2007;1122(1):219–230. doi: 10.1196/annals.1403.015. [http://dx.doi.org/10.1196/annals. 1403.015]. [PMID: 18077575]. [DOI] [PubMed] [Google Scholar]
- 235.Estevez A.Y., Erlichman J.S. Cerium oxide nanoparticles for the treatment of neurological oxidative stress diseases. In: Andreescu S., Hepel M., editors. ACS Symposium Series. Vol. 1083. American Chemical Society; 2011. [Google Scholar]
- 236.Estevez A.Y., Pritchard S., Harper K., Aston J.W., Lynch A., Lucky J.J., Ludington J.S., Chatani P., Mosenthal W.P., Leiter J.C., Andreescu S., Erlichman J.S. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Radic. Biol. Med. 2011;51(6):1155–1163. doi: 10.1016/j.freeradbiomed.2011.06.006. [http://dx.doi.org/10.1016/j.freeradbiomed.2011.06.006]. [PMID: 21704154]. [DOI] [PubMed] [Google Scholar]
- 237.Schubert D., Dargusch R., Raitano J., Chan S.W. Cerium and yttrium oxide nanoparticles are neuroprotective. Biochem. Biophys. Res. Commun. 2006;342(1):86–91. doi: 10.1016/j.bbrc.2006.01.129. [http://dx.doi.org/10.1016/ j.bbrc.2006.01.129]. [PMID: 16480682]. [DOI] [PubMed] [Google Scholar]
- 238.Vonder Haar C., Peterson T.C., Martens K.M., Hoane M.R. 2016. [DOI] [PMC free article] [PubMed]
- 239.Cherian L., Hlatky R., Robertson C.S. Nitric oxide in traumatic brain injury. Brain Pathol. 2004;14(2):195–201. doi: 10.1111/j.1750-3639.2004.tb00053.x. [http://dx.doi. org/10.1111/j.1750-3639.2004.tb00053.x]. [PMID: 15193032]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Garry P.S., Ezra M., Rowland M.J., Westbrook J., Pattinson K.T. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp. Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [http://dx.doi.org/10.1016/j.expneurol.2014.10.017]. [PMID: 25447937]. [DOI] [PubMed] [Google Scholar]
- 241.Huang C.S., Kawamura T., Toyoda Y., Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic. Res. 2010;44(9):971–982. doi: 10.3109/10715762.2010.500328. [http://dx.doi.org/10.3109/ 10715762.2010.500328]. [PMID: 20815764]. [DOI] [PubMed] [Google Scholar]
- 242.Ohno K., Ito M., Ichihara M., Ito M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. 2012. [DOI] [PMC free article] [PubMed]
- 243.Wang J.F., Li Y., Song J.N., Pang H.G. Role of hydrogen sulfide in secondary neuronal injury. Neurochem. Int. 2014;64:37–47. doi: 10.1016/j.neuint.2013.11.002. [http://dx.doi.org/10.1016/j.neuint.2013.11.002]. [PMID: 24239876]. [DOI] [PubMed] [Google Scholar]
- 244.Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20(3):132–139. doi: 10.1016/s0166-2236(96)10074-6. [http://dx.doi. org/10.1016/S0166-2236(96)10074-6]. [PMID: 9061868]. [DOI] [PubMed] [Google Scholar]
- 245.Jafarian-Tehrani M., Louin G., Royo N.C., Besson V.C., Bohme G.A., Plotkine M., Marchand-Verrecchia C. 1400W, a potent selective inducible NOS inhibitor, improves histopathological outcome following traumatic brain injury in rats. Nitric Oxide. 2005;12(2):61–69. doi: 10.1016/j.niox.2004.12.001. [http://dx.doi.org/10.1016/j.niox.2004.12.001]. [PMID: 15740979]. [DOI] [PubMed] [Google Scholar]
- 246.Louin G., Marchand-Verrecchia C., Palmier B., Plotkine M., Jafarian-Tehrani M. Selective inhibition of inducible nitric oxide synthase reduces neurological deficit but not cerebral edema following traumatic brain injury. Neuropharmacology. 2006;50(2):182–190. doi: 10.1016/j.neuropharm.2005.08.020. [http://dx.doi.org/10.1016/j.neuropharm.2005.08.020]. [PMID: 16242164]. [DOI] [PubMed] [Google Scholar]
- 247.Mahmood A., Goussev A., Lu D., Qu C., Xiong Y., Kazmi H., Chopp M. Long-lasting benefits after treatment of traumatic brain injury (TBI) in rats with combination therapy of marrow stromal cells (MSCs) and simvastatin. J. Neurotrauma. 2008;25(12):1441–1447. doi: 10.1089/neu.2007.0495. [http://dx.doi.org/10.1089/neu.2007.0495]. [PMID: 19072586]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Campolo M., Ahmad A., Crupi R., Impellizzeri D., Morabito R., Esposito E., Cuzzocrea S. Combination therapy with melatonin and dexamethasone in a mouse model of traumatic brain injury. J. Endocrinol. 2013;217(3):291–301. doi: 10.1530/JOE-13-0022. [http://dx.doi.org/10. 1530/JOE-13-0022]. [PMID: 23532863]. [DOI] [PubMed] [Google Scholar]
- 249.Matthews L.R., Danner O.K., Ahmed Y., Dennis-Griggs D.M., Frederick A., Clark C., Moore R., DuMornay W., Childs E.W., Wilson K.L. Combination therapy with vitamin D3, progesterone, omega-3 fatty acids and glutamine reverses coma and improves clinical outcomes in patients with severe traumatic brain injuries: A case series. Int. J. Case Rep. Imag. 2012;4(3):143–149. [http://www.ijcasereportsandimages.com/archive/2013/ 003-2013-ijcri/002-03-2013-matthews/ijcri-00203201322-matthews-full-text.php]. [Google Scholar]
- 250.Hagos F.T., Daood M.J., Ocque J.A., Nolin T.D., Bayir H., Poloyac S.M., Kochanek P.M., Clark R.S., Empey P.E. Probenecid, an organic anion transporter 1 and 3 inhibitor, increases plasma and brain exposure of N-acetylcysteine. Xenobiotica. 2017;47(4):346–353. doi: 10.1080/00498254.2016.1187777. [http://dx.doi.org/10.1080/00498254.2016.1187777]. [PMID: 27278858]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Abdel Baki S.G., Schwab B., Haber M., Fenton A.A., Bergold P.J. Minocycline synergizes with N-acetylcysteine and improves cognition and memory following traumatic brain injury in rats. PLoS One. 2010;5(8):e12490. doi: 10.1371/journal.pone.0012490. [http://dx.doi.org/10.1371/journal. pone.0012490]. [PMID: 20824218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Haber M., Abdel Baki S.G., Grin’kina N.M., Irizarry R., Ershova A., Orsi S., Grill R.J., Dash P., Bergold P.J. Minocycline plus N-acetylcysteine synergize to modulate inflammation and prevent cognitive and memory deficits in a rat model of mild traumatic brain injury. Exp. Neurol. 2013;249:169–177. doi: 10.1016/j.expneurol.2013.09.002. [http://dx.doi.org/ 10.1016/j.expneurol.2013.09.002]. [PMID: 24036416]. [DOI] [PubMed] [Google Scholar]
- 253.Lam T.I., Bingham D., Chang T.J., Lee C.C., Shi J., Wang D., Massa S., Swanson R.A., Liu J. Beneficial effects of minocycline and botulinum toxin-induced constraint physical therapy following experimental traumatic brain injury. Neurorehabil. Neural Repair. 2013;27(9):889–899. doi: 10.1177/1545968313491003. [http://dx.doi.org/10.1177/1545968313491003]. [PMID: 23778701]. [DOI] [PubMed] [Google Scholar]
- 254.Lamprecht M.R., Morrison B., III A Combination therapy of 17β-Estradiol and memantine is more neuroprotective than monotherapies in an organotypic brain slice culture model of traumatic brain injury. J. Neurotrauma. 2015;32(17):1361–1368. doi: 10.1089/neu.2015.3912. [http://dx.doi. org/10.1089/neu.2015.3912]. [PMID: 25752651]. [DOI] [PubMed] [Google Scholar]
- 255.Badaut J., Ashwal S., Adami A., Tone B., Recker R., Spagnoli D., Ternon B., Obenaus A. Brain water mobility decreases after astrocytic aquaporin-4 inhibition using RNA interference. J. Cereb. Blood Flow Metab. 2011;31(3):819–831. doi: 10.1038/jcbfm.2010.163. [http://dx.doi.org/10. 1038/jcbfm.2010.163]. [PMID: 20877385]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Fukuda A.M., Adami A., Pop V., Bellone J.A., Coats J.S., Hartman R.E., Ashwal S., Obenaus A., Badaut J. Posttraumatic reduction of edema with aquaporin-4 RNA interference improves acute and chronic functional recovery. J. Cereb. Blood Flow Metab. 2013;33(10):1621–1632. doi: 10.1038/jcbfm.2013.118. [http://dx.doi.org/10.1038/jcbfm. 2013.118]. [PMID: 23899928]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tarnopolsky M.A., Beal M.F. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann. Neurol. 2001;49(5):561–574. [http://dx.doi.org/10. 1002/ana.1028]. [PMID: 11357946]. [PubMed] [Google Scholar]
- 258.Sullivan P.G., Geiger J.D., Mattson M.P., Scheff S.W. Dietary supplement creatine protects against traumatic brain injury. Ann. Neurol. 2000;48(5):723–729. [PMID: 11079535]. [PubMed] [Google Scholar]
- 259.Scheff S.W., Dhillon H.S. Creatine-enhanced diet alters levels of lactate and free fatty acids after experimental brain injury. Neurochem. Res. 2004;29(2):469–479. doi: 10.1023/b:nere.0000013753.22615.59. [http://dx.doi.org/10.1023/ B:NERE.0000013753.22615.59]. [PMID: 15002746]. [DOI] [PubMed] [Google Scholar]
- 260.Guseva M.V., Hopkins D.M., Scheff S.W., Pauly J.R. Dietary choline supplementation improves behavioral, histological, and neurochemical outcomes in a rat model of traumatic brain injury. J. Neurotrauma. 2008;25(8):975–983. doi: 10.1089/neu.2008.0516. [http://dx.doi.org/10.1089/ neu.2008.0516]. [PMID: 18665805]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Peterson T.C., Anderson G.D., Kantor E.D., Hoane M.R. A comparison of the effects of nicotinamide and progesterone on functional recovery of cognitive behavior following cortical contusion injury in the rat. J. Neurotrauma. 2012;29(18):2823–2830. doi: 10.1089/neu.2012.2471. [http://dx.doi.org/10.1089/neu.2012.2471]. [PMID: 23016598]. [DOI] [PMC free article] [PubMed] [Google Scholar]