Abstract
Abstract: Background
Cigarette smoking is the main cause of preventable death in developed countries. While the direct positive behavioral reinforcing effect of nicotine has historically been considered the primary mechanism driving the development of TUD, accumulating contemporary research suggests that the cognitive-enhancing effects of nicotine may also significantly contribute to the initiation and maintenance of TUD, especially in individuals with pre-existing cognitive deficits.
Methods
We provide a selective overview of recent advances in understanding nicotine’s effects on cognitive function, a discussion of the role of cognitive function in vulnerability to TUD, followed by an overview of the neurobiological mechanisms underlying the cognitive effects of nicotine.
Results
Preclinical models and human studies have demonstrated that nicotine has cognitive-enhancing effects. Attention, working memory, fine motor skills and episodic memory functions are particularly sensitive to nicotine’s effects. Recent studies have demonstrated that the α4, β2, and α7 subunits of the nicotinic acetylcholine receptor (nAChR) participate in the cognitive-enhancing effects of nicotine. Imaging studies have been instrumental in identifying brain regions where nicotine is active, and research on the dynamics of large-scale networks after activation by, or withdrawal from, nicotine hold promise for improved understanding of the complex actions of nicotine on human cognition.
Conclusion
Because poor cognitive performance at baseline predicts relapse among smokers who are attempting to quit smoking, studies examining the potential efficacy of cognitive-enhancement as strategy for the treatment of TUD may lead to the development of more efficacious interventions.
Keywords: Smoking, nicotine, cognition, smoking cessation, tobacco use disorder, nicotinic acetylcholine receptor, nAChR, behavioral pharmacology
1. INTRODUCTION
Cigarette smoking or tobacco use disorder (TUD) is the main cause of preventable death in developed countries, with an estimated number of 435,000 premature deaths in the U.S. and 5 million worldwide every year. Although 19.8% of US adults are currently smokers, the lowest rate ever recorded, cigarette smoking is disproportionately common among individuals with low socioeconomic status, low educational levels, and psychiatric comorbidities, including those with another substance use disorder (SUD) [1, 2]. Individuals with psychiatric comorbidities, compared to those without, begin smoking at an earlier age, consume more cigarettes, are more dependent on tobacco, and are less likely to quit smoking [2, 3]. Cigarette smoking is likely a major contributor to the reduced life expectancy of 20 to 25 years in smokers
with severe comorbid psychiatric disorders [4]. Given the low success rate of smoking cessation in this patient population, there is a need for more effective treatment approaches. The development of such treatments will benefit from a clearer understanding of the individual risk factors that contribute to TUD.
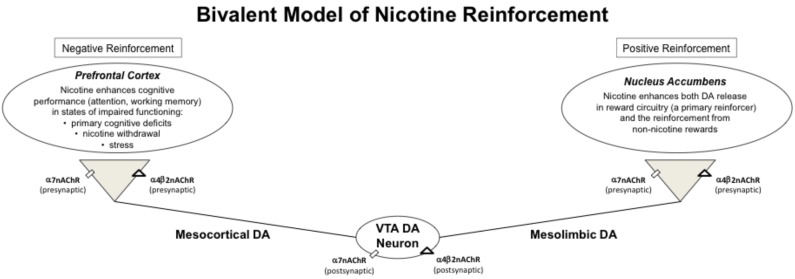
Although the positive reinforcing effects of nicotine are proposed to be the key mechanism for the initiation of maintenance of tobacco use disorder (TUD), a growing body of literature supports the importance of negative reinforcement in TUD as well [5]. For the purposes of this review, positive reinforcement reflects nicotine’s inherently rewarding and pleasant effects that increase the probability of continued self-administration, and negative reinforcement as nicotine’s effect on relieving the unpleasant affective state induced by cognitive deficits and other negative symptoms associated with nicotine withdrawal (Fig. 1). A primary reason smokers cite for continued smoking is to ‘stay focused’ [6-8], and this subjective experience is likely due to the difficulty concentrating, impaired attention, and impaired working memory functions that are core sequelae of smoking abstinence [9-14]. High rates of smoking are observed among individuals with psychiatric disorders including schizophrenia, bipolar disorder, major depression, attention deficit hyperactivity disorder (ADHD) and comorbid substance use disorders (SUD) [15, 16]. Because these psychiatric disorders are associated with various cognitive impairments, including deficits in attention, working memory, and response inhibition functions [17, 18], the cognitive enhancing effects of nicotine may be especially important determinants of the initation and maintenance of smoking in this comorbid population. Growing evidence suggest that cognitive enhancing effects of nicotine may also contribute to the difficulty in quitting smoking, especially in individuals with psychiatric disorders [19].
Fig. (1).
In this bivalent model, the behaviorally relevant effects of nicotine are driven by both negative and positive reinforcement through nicotine’s actions on α4β2 and α7nAChR. Nicotine’s cognitive effects in the prefrontal cortex provide negative reinforcement and nicotine’s effects on reward circuitry in the nucleus accumbens provide positive reinforcement. DA = dopamine. VTA = ventral tegmental area. nAChR = nicotinic acetylcholine receptors.
The effect of nicotine on cognitive function remains an active area of research. Although some controversy remains regarding nicotine’s effect on specific cognitive functions, and on individual differences in nicotine’s cognitive effects, the preponderance of evidence from animal and human studies has established cognitive-enhancing effects as a clinically relevant dimension of nicotine psychopharmacology. In addition, significant progress has been made in understanding the neurobiological mechanisms underlying these effects. These include improved knowledge about the role of the nicotinic acetylcholine receptor (nAChR) in cognitive function and reinforcement [20, 21], the localization of the brain regions that mediate nicotine’s effects on cognitive function [22], and the role of specific subtypes of nAChR in cognitive enhancement, most notably α7 and α4β2 nAChRs that represent viable targets for the pharmacological treatment of cognitive deficits in neuropsychiatric disorders, as well as TUD [23-25]. Finally, clinical approaches that target the cognitive deficits in individuals with TUD have been proposed as novel therapeutic strategies [26, 27].
Of necessity, this review will be highly selective given the vast body of work on the cognitive effects of nicotine. It will provide an overview of recent advances in understanding nicotine’s effects on cognitive function, a discussion of the role of cognitive function in vulnerability to TUD, followed by an overview of the neurobiological mechanisms underlying the cognitive effects of nicotine. Next, we review human data, including neuroimaging and behavioral studies, followed by an overview of interventions targeting cognitive functions as potential treatments for TUD. For more information about other aspects of this dynamic area of research, several excellent recent reviews focusing on basic neurobiology, genetics, behavioral pharmacology, neuroimaging, and clinical epidemiology of this topic are recommended [2, 24, 25, 27, 28].
2. Role of Cognitive Function in the Vulnerability to TUD
Cumulating evidence suggests that individuals with cognitive deficits may be both more vulnerable to developing TUD, and may have more difficulty in quitting smoking [19]. Cognitive deficits associated with TUD are well documented in both clinical and population-based studies. For example, compared to healthy controls, cigarette smokers have cognitive deficits in auditory–verbal and visuospatial learning, visuospatial memory, cognitive efficiency, executive skills, general intelligence, and processing speed (large effect sizes, d ≥ 0.8) [29]. Similar findings were also observed in previous studies with cigarette smokers and matched healthy controls [30, 31]. In a population-based study of 2163 participants, smokers demonstrated deficits in attention, working memory, and impulse control functions [32], although the severity of the deficits was not correlated with the duration of tobacco use. Furthermore, smokers with even low amounts of lifetime tobacco exposure display these deficits, suggesting that individuals with deficits in certain cognitive domains may be predisposed to developing TUD.
As previously mentioned, individuals with psychiatric disorders, including those with other addictions, are about twice as likely to be a smoker, have more severe dependence, and are less likely to quit smoking, as compared to those without these comorbidities [33]. The prevalence of smoking ranges from 44-88% in schizophrenia, 40-60% in major depression, 55-70% bipolar disorder, and 40-50% in PTSD [33, 34]. Similarly, smoking rates range from 50 to 70% for individuals with alcohol use disorder [35], 70 to 80% with cocaine, and to over 90% for opioid use disorder [36]. While the underlying mechanisms linking psychiatric disorders to the high rates of TUD remain to be elucidated, one likely contributor is the effect nicotine has on ameliorating the cognitive deficits commonly associated with psychiatric disorders. As summarized in Table 1, numerous meta-analyses have firmly established that, as compared to healthy controls, individuals with a variety of psychiatric diagnoses have significant cognitive deficits as a clinically meaningful manifestation of their condition. While it is important to note that these studies do not address whether cognitive deficits predated psychiatric disorders or if they are causally related, or the impact of concurrent tobacco product use on cognitive deficits, it is highly plausible that the cognitive enhancing effects of nicotine may be especially important in the initiation and maintenance of smoking in individuals with psychiatric disorders.
Table 1.
Cognitive deficits identified in meta-analyses across major psychiatric disorders.
| Schizophrenia [37] |
Bipolar (Euthymic) [38] |
MDD (Euthymic) [38] |
PTSD [39] |
ADHD [40] |
SUD-cocaine [41] |
SUD-opiate [42] |
|
|---|---|---|---|---|---|---|---|
| Smoking Prevalence | 44-88% | 55-70% | 40-60% | 40-50% | ~40% | 70-80% | >90% |
| Cognitive Domains | |||||||
| Executive functioning | L | S | M | M | M | ||
| Working memory | L | S | M | M | M | M | |
| Attention/vigilance | L | M | L | ||||
| Episodic memory | L | ||||||
| Visual memory | M | M | |||||
| Verbal memory | M | M | S | M | |||
| Language | L | M | M | L | |||
| Cognitive impulsivity | M | ||||||
| Sensory-perceptual | M | S | |||||
| Processing speed | S | M | |||||
L = large effect size (d > 0.8)
M = moderate effect size (0.8 > d ≥ 0.5)
S = small effect size (0.5 > d ≥ 0.2)
3. Neurobiology of Nicotine and Cognitive Function
3.1. Nicotine and nAChR
Nicotine is an addictive substance that plays a key role in initiating and maintaining tobacco use [43], and the rapid rate of delivery to the brain following inhalation of a cigarette puff (10 to 20 seconds) likely contributes to the rewarding properties of nicotine. Nicotine’s primary sites of drug action are at nicotinic acetylcholine receptors (nAChRs), ligand-gated ion channels consisting of various pentameric combinations of 9 α subunits (α2-α10) and 3 β subunits (β2-β4), arranged around a central pore that is permeable to sodium, potassium, and calcium ions [44, 45]. The majority of neuronal nAChRs in the CNS are excitatory and fast acting (millisecond range), modulate the release of other neurotransmitters including acetylcholine (ACh), dopamine (DA), serotonin, glutamate, GABA, and norepinephrine, and are located presynaptically [44, 45]. However, the nAChRs on DA neurons in the ventral tegmental area (VTA), a critical brain region for drug reinforcement, are located postsynaptically (Fig. 1). nAChRs are assembled as either homomeric receptors formed by a group of α subunits (e.g., α6 or α7), or heteromeric receptors that are formed by a combination of α and β subunits (e.g. α4β2, α3β4). The most prominent nAChRs in the brain are the α4β2 and α7 subtypes [46].
Prolonged activation of the nAChR leads to desensitization and upregulation of nAChR density [47, 48] and nicotine is more likely to induce these changes than the endogenous ligand acetylcholine (ACh). Following its release into the synapse, ACh is inactivated within milliseconds by the enzyme acetylcholinesterase. In contrast, nicotine is not a substrate for acetylcholinesterase and causes a prolonged activation of nAChRs [49] upon receptor binding, and with repeated nicotine exposure (i.e. from smoking cigarettes), nAChRs are readily desensitized diminishing nicotine’s effects.
The sensitivity of nAChRs to desensitization varies across subtypes. For example, the nAChR subtypes controlling glutamate release (primarily the α7 subtype) desensitize slower than those that control GABA release (mainly non-α7 subtypes) [50]. This differential sensitivity to desensitization may result in greater glutamate release relative to GABA release following prolonged nicotine exposure. A relative deficiency of GABA over glutamate may lead to an enhanced DA release in the nucleus accumbens, and this may be an important mechanism driving tobacco use [50]. As a result of nAChR desensitization, high nicotine doses, or prolonged exposure, results in antagonist-like activity of nicotine [51]. Although the effects of nicotine on desensitization and upregulation of nAChRs have been clearly established, the roles these processes play in the cognitive effects of nicotine are complex and incompletely characterized. Nicotine appears to exhibit an ‘inverted J dose-response’, with low doses or brief exposures improving cognitive function, while higher doses or prolonged exposure either do not improve, or impair cognitive functions [52]. The variable brain expression patterns of nAChRs, their differential sensitivities to nicotine, and the variable interactions nAChRs have with other neurotransmitter systems that mediate cognitive function underscore the challenge of clearly elucidating the mechanisms of nicotine’s effects on cognition.
3.2. nAChR and Cognitive Function
Although the neurobiological mechanisms underlying nicotine’s effects on cognitive function are not well characterized, informative data at different levels of analysis are continuing to emerge. Both the prefrontal cortex and hippocampal brain regions have been implicated in the cognitive effects of nicotine [53, 54] and cognitive enhancement may be due to improving signal-to-noise ratios, or facilitating synaptic plasticity, in specific neural circuits [53, 55]. The nAChR subunits that are likely to mediate the cognitive effects of nicotine include α2, α3, α4, α5, α7, β2, and β4 [20], and as discussed below, the strongest evidence is for involvement of α7 and β2 subunits [56].
3.3. α7nAChR
α7 nAChRs form ion channels that do not include other nAChR subunits (i.e., homomeric receptors), have a high permeability to calcium that allows them to control the release of other neurotransmitters (e.g., glutamate) [24] and are widely distributed in the mammalian brain [51]. Similar to the NMDA-type glutamate receptors, α7 nAChRs modulate synaptic plasticity, but have a lower affinity for nicotine than α4β2 nAChRs, and do not desensitize at low nicotine concentrations [48, 57]. These differences in desensitization allow α7 nAChRs to continue to be in active state after α4β2 nAChRs have desensitized, and this differential desensitization has implications for clinical therapeutics as discussed below [58].
Both preclinical and human data have shown that α7 nAChRs, which are especially dense in the hippocampus and prefrontal cortex, modulate a variety of cognitive functions [59, 60]. For example, several preclinical studies have demonstrated that α7 nAChR knock-out mice have more errors in sustained attention and working memory tasks than wild-types [61, 62]. However, it is important to note that interpretation of these data are confounded by the observation that these knock-outs differ from wild-type mice in the density and distribution of other nAChR subtypes due to compensatory changes in the expression of other nAChRs types during development [63].
At a clinical level, an accumulating body of evidence suggests that α7 nAChRs play a role in the cognitive deficits within numerous neuropsychiatric disorders including Alzheimer’s disease, Parkinson’s disease, autism spectrum disorders and schizophrenia [24]. For example, α7 nAChRs have been implicated in the sensory gating dysfunction observed in schizophrenia [64-66]. Sensory gating helps to separate irrelevant stimuli from meaningful ones, and it may underlie both sensory overload, and the cognitive deficits that are commonly observed in schizophrenic patients. The density of α7 nAChRs in the hippocampus, a region mediating sensory gating, is reduced in post-mortem brains of schizophrenic patients [67-70] and reductions in α7 nAChRs have been linked to this sensory gating dysfunction in schizophrenia [71]. Furthermore, in both animal models and schizophrenic patients, nicotine, or α7 nAChR agonists, have been shown to reverse sensory gating deficits [72]. Collectively, these results provide a highly plausible and testable biological mechanism driving the high rates of smoking among individuals with schizophrenia [73-77]. Consequently, an indirect benefit of targeting α7 nAChRs with pharmacological agonists for the treatment of cognitive deficits in schizophrenia may be the facilitation of smoking cessation [24, 25].
3.4. β2 nAChRs
The distribution of the β2 subunit is enriched in the basal ganglia, thalamus, and hippocampus, and accumulating evidence supports a primary role of the β2 subunit in the cognitive effects of nicotine [78]. β2 knock-out mice have deficits in cognitive processing including attention, working memory, inhibitory control, and behavioral flexibility [79-82]. In addition, as compared to wild-type mice, nicotine administration does not improve associative memory performance in β2 knock-out mice [83] and the deficits in exploratory behavior of β2 knock-outs are only partially mitigated with nicotine treatment [84]. Finally, medications that stimulate α4β2 nAChRs have cognitive enhancing effects. For example varenicline (trade name Chantix), which improves learning deficits induced by either alcohol administration [85], or nicotine withdrawal [86] in mice, also improves cognitive function in humans (see Section 5).
3.5. Other nAChR Subtypes
Data from preclinical models suggest that the α2, α5, α3, and β4 subunits may also play a role in the cognitive effects of nicotine [20]. For example, the α5 subunit is widely expressed as a component of α4β2, α3β2, and α3β4 receptors in the central and peripheral nervous system, and mice that lack the α5 subunit demonstrate increased nicotine reward, reduced aversion to high doses of nicotine [87] and reduced performance in a highly demanding attention task [88]. However, the finding that in this attentional task, nicotine treatment also reduced performance in wild-type, but not in the α5 knockouts, demonstrates the complex manifestations of nicotine’s effects on cognition and the limitations of preclinical knock-out models [89]. At a clinical level, the presence of the rs16969968 risk variant of α5 containing receptors in smokers is associated with heavy smoking and is predictive of reduced aversive effects from intravenous nicotine. Further, the finding that this risk allele is associated with greater improvement in response inhibition following nicotine [90] provides additional support for a role of the α5 subunit in the cognitive effects of nicotine.
3.6. Dopamine (DA) and other Neurotransmitters
In addition to its role in reinforcement, DA is a well-established modulator of various cognitive functions, including working memory, attention, and response inhibition [91-93]. The localization of nAChRs to DA cell bodies in the ventral tegmental area (VTA), a brain region with robust modulatory control over prefrontal activity, indicates another mechanism through which nicotine may influence cognitive processes. The D2–like receptor family (including D2, D3 and D4 receptors) serve as autoreceptors, and their activation reduces DA release in synaptic terminals [94]. In addition, the D2-like receptors likely mediate set shifting, cognitive flexibility and working memory functions [95, 96]. The activation of nAChRs on the VTA DA cell bodies, and presynaptic terminals, in the nucleus accumbens and the prefrontal cortex [53], contribute to the rewarding and cognitive-enhancing effects of nicotine, respectively (Fig. 1). In addition to ACh and DA, other neurotransmitters participating in the cognitive-enhancing effects of nicotine in both the hippocampus and the prefrontal cortex [21, 97, 98] include glutamate, norepinephrine, serotonin, and GABA. However, the precise role of these neurotransmitters in mediating the cognitive effects of nicotine have not yet been determined [53].
An additional system modulating nicotine’s effects on dopamine signaling is catechol-O-methyltransferase (COMT). COMT is a highly abundant enzyme in the brain that metabolizes DA and norepinephrine [99]. The activity of this enzyme regulates DA levels in the prefrontal cortex and striatum and therefore impacts cognitive functions. The COMT gene contains a well-studied single nucleotide polymorphism that results in a substitution of valine (Val) with methionine (Met) (val158met) [100]. The enzyme with the Met substitution is one-fourth as active as the enzyme that contains Val and results in higher basal synaptic DA levels, primarily in the PFC [101-103], and lower phasic dopamine release in ventral striatal regions [104, 105]. This genetic variation in COMT may account for the differences observed in working memory performance in various human cohorts and suggests that individuals with high Val expression (i.e. lower DA in the PFC) may be more responsive to the cognitive enhancing effects of nicotine. Indeed, in a meta-analysis examining the relationship between COMT polymorphisms and addiction, the strongest evidence for an association was found for higher levels of nicotine dependence in individuals with the high COMT enzyme activity Val allele [101]. In addition, recent studies have shown that the Val/Val genotype is associated with greater withdrawal severity, including difficult concentrating, in female smokers [106], as well as with an increased sensitivity to nicotine’s positive effects on abstinence-induced working memory impairment within healthy smokers [107], further illustrating the coupling of nAChR function and DA signaling in the cognitive and negative reinforcing effects of nicotine (Fig. 1). However, it should be noted that the relationship between COMT activity, cognition and smoking behavior is complex, with some studies demonstrating the absence of an association between COMT polymorphisms and working memory [108], other cognitive functions [109], as well with nicotine withdrawal severity [110]. Consequently, additional genetic studies of COMT, as well as other candidate neurochemical systems, will be needed to more fully characterize individual genetic risk factors for the development and maintenance of TUD.
4. COGNITIVE EFFECTS OF NICOTINE: HUMAN STUDIES
4.1. Behavioral Studies
A large number of laboratory-based studies have examined the effects of cigarette smoking, or of pure nicotine administration, on cognitive performance. However, the interpretation of findings after smoking cigarettes is confounded by the fact that cigarette smoke contains many other compounds in addition to nicotine that may have cognitive-enhancing effects [111, 112]. Furthermore, the amount of nicotine delivered via smoking is highly variable and dependent on the particular type of cigarette and individual smoking topography. To avoid these limitations, numerous studies on the modulation of cognition by nicotine have used pure nicotine administered via nasal spray [113], transdermal patch [114], subcutaneous injection [115], oral inhaler [116] or intravenous infusion [117]. A thorough review of studies conducted up to the 1980s concluded that cigarette smoking, or nicotine, had robust cognitive enhancing effects in abstinent smokers [118]. However, it is not clear whether these cognitive-enhancing effects were primary manifestations of relief from withdrawal states rather than direct cognitive enhancement [118, 119].
To tease apart the direct cognitive effects of nicotine from withdrawal alleviation, Heishman et al. conducted a meta-analysis of 41 placebo-controlled studies that included nicotine administration to either non-smokers, or satiated smokers [120]. They found that nicotine had significant positive effects on fine motor, short-term episodic memory, and working memory performance. In addition, ‘alerting attention’ (maintenance of an alert state) and ‘orienting attention’ (directing attention to sensory events) were also positively impacted [121]. However, nicotine’s effect on cognitive performance, either within or across domains of cognitive function, was not dose dependent again indicating the heterogeneous nature of nicotine pharmacodynamics.
In addition to these findings in satiated smokers, studies have also revealed differences in nicotine’s effects on cognitive functions between abstinent smokers and non-smokers. While nicotine administration improved working memory performance in abstinent smokers, improvements in working memory function of non-smokers were not observed [122], and in a more recent study of non-smokers, nicotine improved basic attentional functions, but did not improve response inhibition or top-down executive attentional function [123]. In another study of overnight abstinent smokers, overall accuracy in an attentional processing task improved after smoking nicotine-yielding vs. placebo cigarettes, providing additional evidence that nicotine improves cognitive deficits induced by nicotine deprivation [124].
Finally, it has been suggested that the cognitive enhancing effects of nicotine may contribute to its mood-enhancing or mood-stabilizing effects [125]. For example, nicotine may alleviate the negative consequences of a stressor by improving attentional focus on a benign distractor stimulus [126]. Collectively, these studies demonstrate that nicotine has differential effects on cognition in humans that depends on smoking history and nicotine withdrawal status at the time of testing, and that cognitive enhancement from smoking may have downstream effects on mood by shifting the attentional bias of smokers away from negative stimuli.
4.2. Neuroimaging Studies
Recent advances in neuroimaging techniques, including positron emission tomography (PET), single photon emission computed tomography (SPECT), and functional magnetic resonance imaging (fMRI), have allowed for more precise localization of nicotine’s activity in brain circuits with an established role in cognitive functions [22]. In particular, the development of radiotracers selective for a4β2 or β2-containing nAChRs have made it possible to examine receptor occupancy following smoking or nicotine administration using PET and SPECT. For example, in a pioneering PET study, a nicotine dose response for a4β2 nAChR occupancy was identified following varying amounts of cigarette smoking, with 3 puffs or one full cigarette resulting in 88% and 95% receptor occupancy, respectively [127]. These findings suggest that nicotine intake achieved by regular smoking results in almost complete occupancy of a4β2 nAChRs. A SPECT study using a radiotracer selective for β2-containing nAChRs found 67% receptor occupancy following smoking to satiety (average 2.4 cigarettes) consistent with the findings by Brody et al. [128]. SPECT and PET imaging studies have also demonstrated the upregulation of a4β2 or β2-containing nAChRs in smokers, as compared to non-smokers [129, 130], a finding consistent with results from preclinical models and post-mortem human studies [131, 132].
SPECT studies have also provided data about the persistence of changes in the upregulation of nAChRs after smoking abstinence. In two different studies, the recovery of the nAChR upregulation following smoking abstinence demonstrated a prolonged recovery of nAChRs ranging from 3 to 12 weeks. The slow return of nAChRs to baseline levels may contribute to the symptoms of nicotine withdrawal, including cognitive difficulties, as the delayed nAChR recovery was identified in the cortex and cerebellum, brain regions with important roles in human cognition [133, 134].
Brain regions activated by nicotine administration that mediate cognitive processing have also been identified in functional neuroimaging studies in humans. In one of the first fMRI studies to examine brain activation patterns in response to nicotine, Stein et al. [135] administered nicotine intravenously in a cumulative manner (0.75, 1.50, and 2.25 mg/70 kg) in non-deprived smokers. Nicotine increased fMRI blood oxygenation level dependent (bold) signal in a number of subcortical and cortical regions associated with reward and cognitive functions. Additional fMRI studies that used nicotine gum administration reported increased bold signal in prefrontal and parietal brain regions [136-138]. And in a recent meta-analysis of fMRI studies examining the brain regions activated by nicotine (either by pure nicotine or by cigarette smoking) in smokers or non-smokers [28], increased activity was confirmed in multiple brain regions including the anterior cingulate cortex, frontoparietal cortices, thalamus, and cuneus. In contrast, ventromedial prefrontal cortex, posterior cingulate cortex, parahippocampus, insula, and the parietal and precentral cortices showed reduced activation in response to nicotine.
These results provide support for a reciprocal model of nicotine’s effects on large-scale brain networks: nicotine reduces activity in default-mode network regions and increases activity in executive control regions. These effects on large-scale networks may contribute to a more comprehensive understanding of the mechanisms by which nicotine enhances cognition [28]. For example, in a fMRI study examining the strength of coupling among three well characterized large-scale networks during smoking satiety and withdrawal, the strength of interactions among salience, executive control, and default mode networks was significantly lower during abstinence than smoking satiety [139]. The reduced strength of association among these networks predicted both cravings to smoke, and less suppression of default mode activity during a working memory task. These imaging findings illustrate how advances in computational techniques can reveal the changes in distributed brain networks that mediate the deficits in cognitive processing associated with abstinence from nicotine, and further studies in this dynamic area of research may guide the development of improved therapeutic approaches for smoking cessation [139].
5. TARGETING COGNITIVE FUCNTION IN THE TREATMENT OF TUD
Among smokers attempting to quit smoking, cognitive function may be an important predictor of smoking relapse. In human laboratory studies, worse performance on a working memory task, impaired response inhibition, and motor impulsivity have been shown to predict relapse to smoking [140, 141]. In addition, attentional biases to smoking cues, an index of automatic cognitive processes, are also predictive of relapse [141-143]. These findings suggest that cognitive-enhancement strategies may assist in interventions targeting smoking cessation. Such an approach was recently evaluated in a randomized clinical trial of 12 weeks of computer-based cognitive exercise training in smokers intending to quit smoking [144]. Although there was no difference between the active and control (relaxation) conditions on quit rates, it is important to note that the cognitive intervention was given as an adjunct to nicotine replacement therapy, and was conducted in healthy subjects. Consequently, it is possible that the efficacy of adjunctive cognitive-enhancing strategies may be greater in smokers with cognitive deficits (e.g., those with neuropsychiatric disorders or during acute nicotine withdrawal).
Alternatively, pharmacological approaches using drugs that enhance cognition may also be effective. Varenicline, a partial agonist at 4β2 nAChRs, is approved for smoking cessation and also improves cognitive function in smokers [145-148]. However, it is not clear whether the efficacy of varenicline for TUD is mediated by cognitive enhancement. In addition, the cholinesterase inhibitors galantamine and donepezil that are used to treat Alzheimer's disease and other neuropsychiatric disorders with cognitive deficits [149], may be helpful in the treatment of TUD as indicated by preliminary findings with galantamine [150]. However, randomized, clinical trials with cognitive-enhancing medications for the treatment of TUD have not been performed, although the scientific rationale for such approaches remains compelling [150, 151].
An alternative pharmacological approach is to use medications that selectively activate nAChR subtypes. While the use of nicotine itself as a cognitive-enhancer is limited by the rapid desensitization of α4β2 nAChRs, the use of selective α7 nAChR agonists is a viable strategy. These selective medications are undergoing clinical trials as cognitive-enhancers for patients with schizophrenia, Alzheimer’s disease and ADHD. The recent advances in understanding the relationship between TUD-related cognitive deficits, and difficulty in smoking cessation, suggest that TUD should also be a target indication in clinical trials with selective α7 nAChR agonists [25].
Transcranial Direct Current Stimulation (tDCS), represents an additional, albeit more exploratory, cognitive-enhancement strategy for treating TUD. For example, compared to sham treatment, a single session of active tDCS over the left dorsolateral prefrontal cortex (DLPFC) increased the latency to smoke following overnight abstinence, and reduced cigarette consumption during an ad libitum smoking session [152]. In addition, five sessions of tDCS over the DLPFC reduced craving for cigarettes and improved performance on decision-making tasks [153], however, the absence of an effect of tDCS on an attention task and craving has also been observed [154], so the utility of this approach clearly requires further study. Finally, evidence-based behavioral approaches that improve various cognitive functions, such as working memory, attention, and attentional bias to smoking cues, have also shown some promise, but negative findings indicate that this area of research needs further development to optimize these approaches that have such high theoretical appeal [145, 155, 156].
CONCLUSIONS
Preclinical models and human studies have demonstrated that nicotine has cognitive-enhancing effects, including improvement of fine motor functions, attention, working memory, and episodic memory. These cognitive-enhancing effects of nicotine may be an important factor in vulnerability to TUD, especially in individuals with cognitive deficits, including a majority of individuals with primary psychiatric disorders. Accumulating evidence suggest that both α7 and β2 nAChRs participate in the cognitive effects of nicotine. The α7 subunit may modulate a sensory filtering function associated with schizophrenia, and the β2 subunit appears to mediate attention, working memory, and behavioral flexibility functions. The neurotransmitters that contribute to nicotine’s cognitive effects include DA, glutamate, serotonin, norepinephrine, GABA, and ACh. Imaging studies have been instrumental in identifying brain regions where nicotine is active, and research on the dynamics of large-scale networks after activation by, or withdrawal from, nicotine hold promise for improved understanding of the complex actions of nicotine on human cognition.
Consent for Publication
Not applicable.
Acknowledgements
Declared none.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Jamal A., King B.A., Neff L.J., Whitmill J., Babb S.D. ; Graffunder C.M. Current cigarette smoking among adults-united states, 2005-2015. MMWR Morb. Mortal. Wkly. Rep. 2016;65(44):1205–1211. doi: 10.15585/mmwr.mm6544a2. [http://dx.doi.org/10.15585/mmwr.mm6544a2]. [PMID: 27832052]. [DOI] [PubMed] [Google Scholar]
- 2.Prochaska J.J., Das S., Young-Wolff K.C. Smoking, Mental Illness, and Public Health. Annu. Rev. Public Health. 2017;38:165–185. doi: 10.1146/annurev-publhealth-031816-044618. [http://dx.doi.org/10.1146/annurev-publhealth-031816-044618]. [PMID: 27992725]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vital signs: current cigarette smoking among adults aged ≥18 years with mental illness - United States, 2009-2011. MMWR Morb. Mortal. Wkly. Rep. 2013;62(5):81–87. [PMID: 23388551]. [PMC free article] [PubMed] [Google Scholar]
- 4.Holford T.R., Meza R., Warner K.E., Meernik C., Jeon J., Moolgavkar S.H., Levy D.T. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964-2012. JAMA. 2014;311(2):164–171. doi: 10.1001/jama.2013.285112. [http://dx.doi.org/10.1001/jama. 2013.285112]. [PMID: 24399555]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall F.S., Der-Avakian A., Gould T.J., Markou A., Shoaib M., Young J.W. Negative affective states and cognitive impairments in nicotine dependence. Neurosci. Biobehav. Rev. 2015;58:168–185. doi: 10.1016/j.neubiorev.2015.06.004. [http://dx.doi.org/10.1016/j.neubiorev.2015.06.004]. [PMID: 26054790]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell M.A.H., Peto J., Patel U.A. Classification of smoking by factorial structure of motives. J. R. Stat. Soc. Ser. A Stat. Soc. 1974;137:313–346. [http://dx.doi.org/10.2307/ 2344953]. [Google Scholar]
- 7.Wesnes K., Warburton D.M. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9(4):223–229. doi: 10.1159/000117969. [http://dx.doi.org/10.1159/000117969]. [PMID: 6646394]. [DOI] [PubMed] [Google Scholar]
- 8.Piper M.E., Piasecki T.M., Federman E.B., Bolt D.M., Smith S.S., Fiore M.C., Baker T.B. A multiple motives approach to tobacco dependence: The wisconsin inventory of smoking dependence motives (WISDM-68). J. Consult. Clin. Psychol. 2004;72(2):139–154. doi: 10.1037/0022-006X.72.2.139. [http://dx.doi.org/10.1037/0022-006X.72.2.139]. [PMID: 15065950]. [DOI] [PubMed] [Google Scholar]
- 9.Hatsukami D.K., Hughes J.R., Pickens R.W., Svikis D. Tobacco withdrawal symptoms: an experimental analysis. Psychopharmacology (Berl.) 1984;84(2):231–236. doi: 10.1007/BF00427451. [http://dx.doi.org/10.1007/ BF00427451]. [PMID: 6438682]. [DOI] [PubMed] [Google Scholar]
- 10.Hughes J.R., Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch. Gen. Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [http://dx.doi. org/10.1001/archpsyc.1986.01800030107013]. [PMID: 3954551]. [DOI] [PubMed] [Google Scholar]
- 11.Jacobsen L.K., Krystal J.H., Mencl W.E., Westerveld M., Frost S.J., Pugh K.R. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol. Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [http://dx.doi.org/10.1016/j.biopsych.2004.10.022]. [PMID: 15607301]. [DOI] [PubMed] [Google Scholar]
- 12.Xu J., Mendrek A., Cohen M.S., Monterosso J., Rodriguez P., Simon S.L., Brody A., Jarvik M., Domier C.P., Olmstead R., Ernst M., London E.D. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol. Psychiatry. 2005;58(2):143–150. doi: 10.1016/j.biopsych.2005.03.028. [http://dx.doi.org/10.1016/ j.biopsych.2005.03.028]. [PMID: 16038685]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison E.L., Coppola S., McKee S.A. Nicotine deprivation and trait impulsivity affect smokers’ performance on cognitive tasks of inhibition and attention. Exp. Clin. Psychopharmacol. 2009;17(2):91–98. doi: 10.1037/a0015657. [http://dx.doi.org/10.1037/a0015657]. [PMID: 19331485]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClernon F.J., Kollins S.H., Lutz A.M., Fitzgerald D.P., Murray D.W., Redman C., Rose J.E. Effects of smoking abstinence on adult smokers with and without attention deficit hyperactivity disorder: results of a preliminary study. Psychopharmacology (Berl.) 2008;197(1):95–105. doi: 10.1007/s00213-007-1009-3. [http://dx.doi.org/10.1007/s00213-007-1009-3]. [PMID: 18038223]. [DOI] [PubMed] [Google Scholar]
- 15.de Leon J., Diaz F.J. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr. Res. 2005;76(2-3):135–157. doi: 10.1016/j.schres.2005.02.010. [http://dx. doi.org/10.1016/j.schres.2005.02.010]. [PMID: 15949648]. [DOI] [PubMed] [Google Scholar]
- 16.Milberger S., Biederman J., Faraone S.V., Chen L., Jones J. Further evidence of an association between attention-deficit/ hyperactivity disorder and cigarette smoking. Findings from a high-risk sample of siblings. Am. J. Addict. 1997;6(3):205–217. [PMID: 9256986]. [PubMed] [Google Scholar]
- 17.Millan M.J., Agid Y., Brüne M., Bullmore E.T., Carter C.S., Clayton N.S., Connor R., Davis S., Deakin B., DeRubeis R.J., Dubois B., Geyer M.A., Goodwin G.M., Gorwood P., Jay T.M., Joëls M., Mansuy I.M., Meyer-Lindenberg A., Murphy D., Rolls E., Saletu B., Spedding M., Sweeney J., Whittington M., Young L.J. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [http://dx.doi.org/10.1038/nrd3628]. [PMID: 22293568]. [DOI] [PubMed] [Google Scholar]
- 18.Sofuoglu M., DeVito E.E., Waters A.J., Carroll K.M. Cognitive function as a transdiagnostic treatment target in stimulant use disorders. J. Dual Diagn. 2016;12(1):90–106. doi: 10.1080/15504263.2016.1146383. [http://dx.doi.org/ 10.1080/15504263.2016.1146383]. [PMID: 26828702]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Besson M., Forget B. Cognitive dysfunction, affective states, and vulnerability to nicotine addiction: A multifactorial perspective. Front. Psychiatry. 2016;7:160. doi: 10.3389/fpsyt.2016.00160. [http://dx.doi.org/10.3389/fpsyt. 2016.00160]. [PMID: 27708591]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Changeux J.P. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat. Rev. Neurosci. 2010;11(6):389–401. doi: 10.1038/nrn2849. [http://dx.doi.org/10.1038/nrn2849]. [PMID: 20485364]. [DOI] [PubMed] [Google Scholar]
- 21.dos Santos Coura R., Granon S. Prefrontal neuromodulation by nicotinic receptors for cognitive processes. Psychopharmacology (Berl.) 2012;221(1):1–18. doi: 10.1007/s00213-011-2596-6. [http://dx.doi.org/10.1007/s00213-011-2596-6]. [PMID: 22249358]. [DOI] [PubMed] [Google Scholar]
- 22.Jasinska A.J., Zorick T., Brody A.L., Stein E.A. Dual role of nicotine in addiction and cognition: a review of neuroimaging studies in humans. Neuropharmacology. 2014;84:111–122. doi: 10.1016/j.neuropharm.2013.02.015. [http://dx. doi.org/10.1016/j.neuropharm.2013.02.015]. [PMID: 23474015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace T.L., Porter R.H. Targeting the nicotinic alpha7 acetylcholine receptor to enhance cognition in disease. Biochem. Pharmacol. 2011;82(8):891–903. doi: 10.1016/j.bcp.2011.06.034. [http://dx.doi.org/10.1016/j.bcp. 2011.06.034]. [PMID: 21741954]. [DOI] [PubMed] [Google Scholar]
- 24.Bertrand D., Lee C.H., Flood D., Marger F., Donnelly-Roberts D. Therapeutic potential of α7 nicotinic acetylcholine receptors. Pharmacol. Rev. 2015;67(4):1025–1073. doi: 10.1124/pr.113.008581. [http://dx.doi.org/10. 1124/pr.113.008581]. [PMID: 26419447]. [DOI] [PubMed] [Google Scholar]
- 25.Hurst R., Rollema H., Bertrand D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol. Ther. 2013;137(1):22–54. doi: 10.1016/j.pharmthera.2012.08.012. [http://dx.doi.org/10.1016/j.pharmthera.2012.08.012]. [PMID: 22925690]. [DOI] [PubMed] [Google Scholar]
- 26.Ashare R.L., Schmidt H.D. Optimizing treatments for nicotine dependence by increasing cognitive performance during withdrawal. Expert Opin. Drug Discov. 2014;9(6):579–594. doi: 10.1517/17460441.2014.908180. [http:// dx.doi.org/10.1517/17460441.2014.908180]. [PMID: 24707983]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson C.D. Neurocognitive Function as a Treatment Target for Tobacco Use Disorder. Curr. Behav. Neurosci. Rep. 2017;4(1):10–20. [http://dx.doi.org/10.1007/s40473-017-0105-x]. [Google Scholar]
- 28.Sutherland M.T., Ray K.L., Riedel M.C., Yanes J.A., Stein E.A., Laird A.R. Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies. Biol. Psychiatry. 2015;78(10):711–720. doi: 10.1016/j.biopsych.2014.12.021. [http://dx.doi.org/10.1016/j.biopsych.2014.12.021]. [PMID: 25662104]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durazzo T.C., Meyerhoff D.J., Nixon S.J. A comprehensive assessment of neurocognition in middle-aged chronic cigarette smokers. Drug Alcohol Depend. 2012;122(1-2):105–111. doi: 10.1016/j.drugalcdep.2011.09.019. [http:// dx.doi.org/10.1016/j.drugalcdep.2011.09.019]. [PMID: 21992872]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nooyens A.C., van Gelder B.M., Verschuren W.M. Smoking and cognitive decline among middle-aged men and women: the Doetinchem Cohort Study. Am. J. Public Health. 2008;98(12):2244–2250. doi: 10.2105/AJPH.2007.130294. [http://dx.doi.org/10.2105/AJPH.2007.130294]. [PMID: 18923116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paul R.H., Brickman A.M., Cohen R.A., Williams L.M., Niaura R., Pogun S., Clark C.R., Gunstad J., Gordon E. Cognitive status of young and older cigarette smokers: data from the international brain database. J. Clin. Neurosci. 2006;13(4):457–465. doi: 10.1016/j.jocn.2005.04.012. [http://dx. doi.org/10.1016/j.jocn.2005.04.012]. [PMID: 16678725]. [DOI] [PubMed] [Google Scholar]
- 32.Wagner M., Schulze-Rauschenbach S., Petrovsky N., Brinkmeyer J., von der Goltz C., Gründer G., Spreckelmeyer K.N., Wienker T., Diaz-Lacava A., Mobascher A., Dahmen N., Clepce M., Thuerauf N., Kiefer F., de Millas J.W., Gallinat J., Winterer G. Neurocognitive impairments in non-deprived smokers--results from a population-based multi-center study on smoking-related behavior. Addict. Biol. 2013;18(4):752–761. doi: 10.1111/j.1369-1600.2011.00429.x. [http://dx. doi.org/10.1111/j.1369-1600.2011.00429.x]. [PMID: 22339903]. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence D., Mitrou F., Zubrick S.R. Smoking and mental illness: results from population surveys in Australia and the United States. BMC Public Health. 2009;9:285. doi: 10.1186/1471-2458-9-285. [http://dx.doi.org/10. 1186/1471-2458-9-285]. [PMID: 19664203]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall S.M., Prochaska J.J. Treatment of smokers with co-occurring disorders: emphasis on integration in mental health and addiction treatment settings. Annu. Rev. Clin. Psychol. 2009;5:409–431. doi: 10.1146/annurev.clinpsy.032408.153614. [http://dx.doi.org/10.1146/annurev.clinpsy.032408.153614]. [PMID: 19327035]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant B.F., Hasin D.S., Chou S.P., Stinson F.S., Dawson D.A. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch. Gen. Psychiatry. 2004;61(11):1107–1115. doi: 10.1001/archpsyc.61.11.1107. [http://dx.doi.org/10.1001/archpsyc.61.11.1107]. [PMID: 15520358]. [DOI] [PubMed] [Google Scholar]
- 36.Stark M.J., Campbell B.K. Cigarette smoking and methadone dose levels. Am. J. Drug Alcohol Abuse. 1993;19(2):209–217. doi: 10.3109/00952999309002681. [http://dx.doi.org/10.3109/00952999309002681]. [PMID: 8484357]. [DOI] [PubMed] [Google Scholar]
- 37.Schaefer J., Giangrande E., Weinberger D.R., Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr. Res. 2013;150(1):42–50. doi: 10.1016/j.schres.2013.07.009. [http://dx.doi.org/10.1016/j.schres.2013.07.009]. [PMID: 23911259]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bourne C., Aydemir Ö., Balanzá-Martínez V., Bora E., Brissos S., Cavanagh J.T., Clark L., Cubukcuoglu Z., Dias V.V. ; Dittmann S., Ferrier I.N., Fleck D.E., Frangou S., Gallagher P. ; Jones L., Kieseppä T., Martínez-Aran A., Melle I., Moore P.B. ; Mur M., Pfennig A., Raust A., Senturk V., Simonsen C., Smith D.J., Bio D.S., Soeiro-de-Souza M.G., Stoddart S.D., Sundet K. ; Szöke A., Thompson J.M., Torrent C., Zalla T., Craddock N. ; Andreassen O.A., Leboyer M., Vieta E., Bauer M., Worhunsky P.D., Tzagarakis C., Rogers R.D., Geddes J.R., Goodwin G.M. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr. Scand. 2013;128(3):149–162. doi: 10.1111/acps.12133. [http://dx.doi.org/10.1111/ acps.12133]. [PMID: 23617548]. [DOI] [PubMed] [Google Scholar]
- 39.Scott J.C., Matt G.E., Wrocklage K.M., Crnich C., Jordan J., Southwick S.M., Krystal J.H., Schweinsburg B.C. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull. 2015;141(1):105–140. doi: 10.1037/a0038039. [http://dx.doi.org/10.1037/a0038039]. [PMID: 25365762]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoechlin C., Engel R.R. Neuropsychological performance in adult attention-deficit hyperactivity disorder: meta-analysis of empirical data. Arch. Clin. Neuropsychol. 2005;20(6):727–744. doi: 10.1016/j.acn.2005.04.005. [http://dx.doi.org/10.1016/j.acn.2005.04.005]. [PMID: 15953706]. [DOI] [PubMed] [Google Scholar]
- 41.Jovanovski D., Erb S., Zakzanis K.K. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J. Clin. Exp. Neuropsychol. 2005;27(2):189–204. doi: 10.1080/13803390490515694. [http://dx.doi.org/10.1080/ 13803390490515694]. [PMID: 15903150]. [DOI] [PubMed] [Google Scholar]
- 42.Baldacchino A., Balfour D.J., Passetti F., Humphris G., Matthews K. Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci. Biobehav. Rev. 2012;36(9):2056–2068. doi: 10.1016/j.neubiorev.2012.06.006. [http://dx.doi.org/10.1016/j.neubiorev. 2012.06.006]. [PMID: 22771335]. [DOI] [PubMed] [Google Scholar]
- 43.Benowitz N.L. Pharmacology of nicotine: addiction, smoking-induced disease, and therapeutics. Annu. Rev. Pharmacol. Toxicol. 2009;49:57–71. doi: 10.1146/annurev.pharmtox.48.113006.094742. [http://dx.doi.org/10.1146/annurev.pharmtox.48. 113006.094742]. [PMID: 18834313]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clader J.W., Wang Y. Muscarinic receptor agonists and antagonists in the treatment of Alzheimer’s disease. Curr. Pharm. Des. 2005;11(26):3353–3361. doi: 10.2174/138161205774370762. [http://dx.doi.org/10.2174/138161205774370762]. [PMID: 16250841]. [DOI] [PubMed] [Google Scholar]
- 45.Dani J.A., Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [http://dx.doi.org/10.1146/annurev.pharmtox.47.120505.105214]. [PMID: 17009926]. [DOI] [PubMed] [Google Scholar]
- 46.Zoli M., Pistillo F., Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. 2015. [DOI] [PubMed]
- 47.Picciotto M.R., Addy N.A., Mineur Y.S., Brunzell D.H. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog. Neurobiol. 2008;84(4):329–342. doi: 10.1016/j.pneurobio.2007.12.005. [http://dx.doi.org/10.1016/j.pneurobio.2007.12.005]. [PMID: 18242816]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quick M.W., Lester R.A. Desensitization of neuronal nicotinic receptors. J. Neurobiol. 2002;53(4):457–478. doi: 10.1002/neu.10109. [http://dx.doi.org/10.1002/neu.10109]. [PMID: 12436413]. [DOI] [PubMed] [Google Scholar]
- 49.Penton R.E., Lester R.A. Cellular events in nicotine addiction. Semin. Cell Dev. Biol. 2009;20(4):418–431. doi: 10.1016/j.semcdb.2009.01.001. [http://dx.doi.org/10. 1016/j.semcdb.2009.01.001]. [PMID: 19560047]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansvelder H.D., Mertz M., Role L.W. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin. Cell Dev. Biol. 2009;20(4):432–440. doi: 10.1016/j.semcdb.2009.01.007. [http://dx.doi.org/10.1016/j.semcdb.2009.01.007]. [PMID: 19560048]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buccafusco J.J., Beach J.W., Terry A.V., Jr Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J. Pharmacol. Exp. Ther. 2009;328(2):364–370. doi: 10.1124/jpet.108.145292. [http://dx. doi.org/10.1124/jpet.108.145292]. [PMID: 19023041]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levin E.D. Complex relationships of nicotinic receptor actions and cognitive functions. Biochem. Pharmacol. 2013;86(8):1145–1152. doi: 10.1016/j.bcp.2013.07.021. [http://dx.doi.org/10.1016/j.bcp.2013.07.021]. [PMID: 23928190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace T.L., Bertrand D. Importance of the nicotinic acetylcholine receptor system in the prefrontal cortex. Biochem. Pharmacol. 2013;85(12):1713–1720. doi: 10.1016/j.bcp.2013.04.001. [http://dx.doi.org/10.1016/j.bcp.2013. 04.001]. [PMID: 23628449]. [DOI] [PubMed] [Google Scholar]
- 54.Kutlu M.G., Gould T.J. Nicotinic receptors, memory, and hippocampus. Curr. Top. Behav. Neurosci. 2015;23:137–163. doi: 10.1007/978-3-319-13665-3_6. [http://dx.doi.org/10.1007/978-3-319-13665-3_6]. [PMID: 25655890]. [DOI] [PubMed] [Google Scholar]
- 55.Couey J.J., Meredith R.M., Spijker S., Poorthuis R.B., Smit A.B., Brussaard A.B., Mansvelder H.D. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54(1):73–87. doi: 10.1016/j.neuron.2007.03.006. [http://dx.doi.org/10.1016/j.neuron.2007.03.006]. [PMID: 17408579]. [DOI] [PubMed] [Google Scholar]
- 56.Kenney J.W., Gould T.J. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol. Neurobiol. 2008;38(1):101–121. doi: 10.1007/s12035-008-8037-9. [http://dx.doi.org/10.1007/s12035-008-8037-9]. [PMID: 18690555]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wooltorton J.R., Pidoplichko V.I., Broide R.S., Dani J.A. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 2003;23(8):3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [PMID: 12716925]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giniatullin R., Nistri A., Yakel J.L. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28(7):371–378. doi: 10.1016/j.tins.2005.04.009. [http://dx.doi.org/10.1016/j.tins.2005.04.009]. [PMID: 15979501]. [DOI] [PubMed] [Google Scholar]
- 59.Gotti C., Moretti M., Gaimarri A., Zanardi A., Clementi F., Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem. Pharmacol. 2007;74(8):1102–1111. doi: 10.1016/j.bcp.2007.05.023. [http://dx.doi.org/10.1016/j.bcp.2007.05.023]. [PMID: 17597586]. [DOI] [PubMed] [Google Scholar]
- 60.Leiser S.C., Bowlby M.R., Comery T.A., Dunlop J. A cog in cognition: how the alpha 7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol. Ther. 2009;122(3):302–311. doi: 10.1016/j.pharmthera.2009.03.009. [http://dx.doi.org/10.1016/j.pharmthera. 2009.03.009]. [PMID: 19351547]. [DOI] [PubMed] [Google Scholar]
- 61.Hoyle E., Genn R.F., Fernandes C., Stolerman I.P. Impaired performance of alpha7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology (Berl.) 2006;189(2):211–223. doi: 10.1007/s00213-006-0549-2. [http://dx.doi.org/10.1007/s00213-006-0549-2]. [PMID: 17019565]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernandes C., Hoyle E., Dempster E., Schalkwyk L.C., Collier D.A. Performance deficit of alpha7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes Brain Behav. 2006;5(6):433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [http://dx.doi.org/10.1111/j.1601-183X.2005.00176.x]. [PMID: 16923147]. [DOI] [PubMed] [Google Scholar]
- 63.Young J.W., Finlayson K., Spratt C., Marston H.M., Crawford N., Kelly J.S., Sharkey J. Nicotine improves sustained attention in mice: evidence for involvement of the alpha7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29(5):891–900. doi: 10.1038/sj.npp.1300393. [http://dx.doi.org/10.1038/sj.npp.1300393]. [PMID: 14970827]. [DOI] [PubMed] [Google Scholar]
- 64.Taiminen T.J., Salokangas R.K., Saarijärvi S., Niemi H., Lehto H., Ahola V., Syvälahti E. Smoking and cognitive deficits in schizophrenia: a pilot study. Addict. Behav. 1998;23(2):263–266. doi: 10.1016/s0306-4603(97)00028-2. [http://dx.doi.org/10.1016/S0306-4603(97)00028-2]. [PMID: 9573430]. [DOI] [PubMed] [Google Scholar]
- 65.Nomikos G.G., Schilström B., Hildebrand B.E., Panagis G., Grenhoff J., Svensson T.H. Role of alpha7 nicotinic receptors in nicotine dependence and implications for psychiatric illness. Behav. Brain Res. 2000;113(1-2):97–103. doi: 10.1016/s0166-4328(00)00204-7. [http://dx.doi.org/10.1016/ S0166-4328(00)00204-7]. [PMID: 10942036]. [DOI] [PubMed] [Google Scholar]
- 66.Adler L.E., Hoffer L.D., Wiser A., Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am. J. Psychiatry. 1993;150(12):1856–1861. doi: 10.1176/ajp.150.12.1856. [http://dx.doi.org/10.1176/ajp.150.12.1856]. [PMID: 8238642]. [DOI] [PubMed] [Google Scholar]
- 67.Martin-Ruiz C.M., Haroutunian V.H., Long P., Young A.H., Davis K.L., Perry E.K., Court J.A. Dementia rating and nicotinic receptor expression in the prefrontal cortex in schizophrenia. Biol. Psychiatry. 2003;54(11):1222–1233. doi: 10.1016/s0006-3223(03)00348-2. [http://dx.doi.org/10.1016/ S0006-3223(03)00348-2]. [PMID: 14643090]. [DOI] [PubMed] [Google Scholar]
- 68.Freedman R., Hall M., Adler L.E., Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry. 1995;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. [http://dx.doi.org/10.1016/0006-3223(94)00252-X]. [PMID: 7548469]. [DOI] [PubMed] [Google Scholar]
- 69.Breese C.R., Lee M.J., Adams C.E., Sullivan B., Logel J., Gillen K.M., Marks M.J., Collins A.C., Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23(4):351–364. doi: 10.1016/S0893-133X(00)00121-4. [http://dx.doi.org/10.1016/S0893-133X(00)00121-4]. [PMID: 10989262]. [DOI] [PubMed] [Google Scholar]
- 70.Guan Z.Z., Zhang X., Blennow K., Nordberg A. Decreased protein level of nicotinic receptor alpha7 subunit in the frontal cortex from schizophrenic brain. Neuroreport. 1999;10(8):1779–1782. doi: 10.1097/00001756-199906030-00028. [http://dx.doi.org/10.1097/00001756-199906030-00028]. [PMID: 10501574]. [DOI] [PubMed] [Google Scholar]
- 71.Potter D., Summerfelt A., Gold J., Buchanan R.W. Review of clinical correlates of P50 sensory gating abnormalities in patients with schizophrenia. Schizophr. Bull. 2006;32(4):692–700. doi: 10.1093/schbul/sbj050. [http://dx.doi.org/10.1093/schbul/sbj050]. [PMID: 16469942]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martin L.F., Freedman R. Schizophrenia and the alpha7 nicotinic acetylcholine receptor. Int. Rev. Neurobiol. 2007;78:225–246. doi: 10.1016/S0074-7742(06)78008-4. [http://dx.doi.org/10.1016/S0074-7742(06)78008-4]. [PMID: 17349863]. [DOI] [PubMed] [Google Scholar]
- 73.Palmer B.W., Heaton R.K., Paulsen J.S., Kuck J., Braff D., Harris M.J., Zisook S., Jeste D.V. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11(3):437–446. doi: 10.1037//0894-4105.11.3.437. [http://dx.doi.org/10.1037/0894-4105.11.3.437]. [PMID: 9223148]. [DOI] [PubMed] [Google Scholar]
- 74.Reichenberg A., Weiser M., Caspi A., Knobler H.Y., Lubin G., Harvey P.D., Rabinowitz J., Davidson M. Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. J. Clin. Exp. Neuropsychol. 2006;28(2):193–207. doi: 10.1080/13803390500360372. [http://dx.doi.org/10.1080/13803390500360372]. [PMID: 16484093]. [DOI] [PubMed] [Google Scholar]
- 75.Medalia A., Thysen J., Freilich B. Do people with schizophrenia who have objective cognitive impairment identify cognitive deficits on a self report measure? Schizophr. Res. 2008;105(1-3):156–164. doi: 10.1016/j.schres.2008.07.007. [http://dx.doi.org/10.1016/j.schres.2008.07.007]. [PMID: 18718740]. [DOI] [PubMed] [Google Scholar]
- 76.Leonard S., Adler L.E., Benhammou K., Berger R., Breese C.R., Drebing C., Gault J., Lee M.J., Logel J., Olincy A., Ross R.G., Stevens K., Sullivan B., Vianzon R., Virnich D.E., Waldo M., Walton K., Freedman R. Smoking and mental illness. Pharmacol. Biochem. Behav. 2001;70(4):561–570. doi: 10.1016/s0091-3057(01)00677-3. [http://dx.doi.org/10.1016/S0091-3057(01)00677-3]. [PMID: 11796154]. [DOI] [PubMed] [Google Scholar]
- 77.Poirier M.F., Canceil O., Baylé F., Millet B., Bourdel M.C., Moatti C., Olié J.P., Attar-Lévy D. Prevalence of smoking in psychiatric patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26(3):529–537. doi: 10.1016/s0278-5846(01)00304-9. [http://dx.doi.org/10.1016/S0278-5846(01)00304-9]. [PMID: 11999904]. [DOI] [PubMed] [Google Scholar]
- 78.Feduccia A.A., Chatterjee S., Bartlett S.E. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Front. Mol. Neurosci. 2012;5:83. doi: 10.3389/fnmol.2012.00083. [http://dx.doi.org/10.3389/fnmol.2012.00083]. [PMID: 22876217]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Granon S., Changeux J.P. Attention-deficit/hyperactivity disorder: a plausible mouse model? Acta Paediatr. 2006;95(6):645–649. doi: 10.1080/08035250600719747. [http://dx.doi.org/10.1080/08035250600719747]. [PMID: 16754543]. [DOI] [PubMed] [Google Scholar]
- 80.Granon S., Faure P., Changeux J.P. Executive and social behaviors under nicotinic receptor regulation. Proc. Natl. Acad. Sci. USA. 2003;100(16):9596–9601. doi: 10.1073/pnas.1533498100. [http://dx.doi.org/10.1073/pnas. 1533498100]. [PMID: 12876201]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guillem K., Bloem B., Poorthuis R.B., Loos M., Smit A.B., Maskos U., Spijker S., Mansvelder H.D. Nicotinic acetylcholine receptor β2 subunits in the medial prefrontal cortex control attention. Science. 2011;333(6044):888–891. doi: 10.1126/science.1207079. [http://dx.doi.org/10. 1126/science.1207079]. [PMID: 21836018]. [DOI] [PubMed] [Google Scholar]
- 82.Cole R.D., Poole R.L., Guzman D.M., Gould T.J., Parikh V. Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology (Berl.) 2015;232(7):1207–1217. doi: 10.1007/s00213-014-3754-4. [http://dx.doi.org/10.1007/s00213-014-3754-4]. [PMID: 25281224]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Picciotto M.R., Zoli M., Léna C., Bessis A., Lallemand Y., Le Novère N., Vincent P., Pich E.M., Brûlet P., Changeux J.P. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374(6517):65–67. doi: 10.1038/374065a0. [http://dx.doi.org/10.1038/374065a0]. [PMID: 7870173]. [DOI] [PubMed] [Google Scholar]
- 84.Besson M., Suarez S., Cormier A., Changeux J.P., Granon S. Chronic nicotine exposure has dissociable behavioural effects on control and beta2-/- mice. Behav. Genet. 2008;38(5):503–514. doi: 10.1007/s10519-008-9216-1. [http://dx.doi.org/10.1007/s10519-008-9216-1]. [PMID: 18607712]. [DOI] [PubMed] [Google Scholar]
- 85.Gulick D., Gould T.J. Varenicline ameliorates ethanol-induced deficits in learning in C57BL/6 mice. Neurobiol. Learn. Mem. 2008;90(1):230–236. doi: 10.1016/j.nlm.2008.03.002. [http://dx.doi.org/10.1016/j.nlm.2008.03.002]. [PMID: 18411066]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raybuck J.D., Portugal G.S., Lerman C., Gould T.J. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav. Neurosci. 2008;122(5):1166–1171. doi: 10.1037/a0012601. [http://dx.doi.org/10.1037/a0012601]. [PMID: 18823172]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jackson K.J., Marks M.J., Vann R.E., Chen X., Gamage T.F., Warner J.A., Damaj M.I. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J. Pharmacol. Exp. Ther. 2010;334(1):137–146. doi: 10.1124/jpet.110.165738. [http://dx. doi.org/10.1124/jpet.110.165738]. [PMID: 20400469]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bailey C.D., De Biasi M., Fletcher P.J., Lambe E.K. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J. Neurosci. 2010;30(27):9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.2258-10.2010]. [PMID: 20610759]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bailey C.D., De Biasi M., Fletcher P.J., Lambe E.K. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J. Neurosci. 2010;30(27):9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [http://dx.doi.org/10.1523/JNEUROSCI.2258-10.2010]. [PMID: 20610759]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jensen K.P., DeVito E.E., Herman A.I., Valentine G.W., Gelernter J., Sofuoglu M.A. CHRNA5 smoking risk variant decreases the aversive effects of nicotine in humans. Neuropsychopharmacology. 2015;40(12):2813–2821. doi: 10.1038/npp.2015.131. [http://dx.doi.org/10.1038/npp. 2015.131]. [PMID: 25948103]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nieoullon A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [http://dx.doi.org/10.1016/S0301-0082(02)00011-4]. [PMID: 12126656]. [DOI] [PubMed] [Google Scholar]
- 92.Tanila H., Björklund M., Riekkinen P., Jr Cognitive changes in mice following moderate MPTP exposure. Brain Res. Bull. 1998;45(6):577–582. doi: 10.1016/s0361-9230(97)00452-8. [http://dx.doi.org/10.1016/S0361-9230(97)00452-8]. [PMID: 9566501]. [DOI] [PubMed] [Google Scholar]
- 93.Colzato L.S., van den Wildenberg W.P., van Wouwe N.C., Pannebakker M.M., Hommel B. Dopamine and inhibitory action control: evidence from spontaneous eye blink rates. Exp. Brain Res. 2009;196(3):467–474. doi: 10.1007/s00221-009-1862-x. [http://dx.doi.org/10.1007/s00221-009-1862-x]. [PMID: 19484465]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Missale C., Nash S.R., Robinson S.W., Jaber M., Caron M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [http://dx.doi.org/10.1152/physrev.1998.78. 1.189]. [PMID: 9457173]. [DOI] [PubMed] [Google Scholar]
- 95.van Holstein M., Aarts E., van der Schaaf M.E., Geurts D.E., Verkes R.J., Franke B., van Schouwenburg M.R., Cools R. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (Berl.) 2011;218(3):567–578. doi: 10.1007/s00213-011-2340-2. [http://dx.doi.org/10.1007/s00213-011-2340-2]. [PMID: 21611724]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Floresco S.B. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31(2):297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 97.Parikh V., Man K., Decker M.W., Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J. Neurosci. 2008;28(14):3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [http://dx.doi.org/10.1523/JNEUROSCI.5251-07.2008]. [PMID: 18385335]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sarter M., Parikh V., Howe W.M. nAChR agonist-induced cognition enhancement: integration of cognitive and neuronal mechanisms. Biochem. Pharmacol. 2009;78(7):658–667. doi: 10.1016/j.bcp.2009.04.019. [http://dx. doi.org/10.1016/j.bcp.2009.04.019]. [PMID: 19406107]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Männistö P.T., Tuomainen P., Tuominen R.K. Different in vivo properties of three new inhibitors of catechol O-methyltransferase in the rat. Br. J. Pharmacol. 1992;105(3):569–574. doi: 10.1111/j.1476-5381.1992.tb09020.x. [http://dx. doi.org/10.1111/j.1476-5381.1992.tb09020.x]. [PMID: 1628144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sengupta S., Grizenko N., Schmitz N., Schwartz G., Bellingham J., Polotskaia A., Stepanian M.T., Goto Y., Grace A.A., Joober R. COMT Val108/158Met polymorphism and the modulation of task-oriented behavior in children with ADHD. Neuropsychopharmacology. 2008;33(13):3069–3077. doi: 10.1038/npp.2008.85. [http://dx.doi.org/10.1038/npp.2008.85]. [PMID: 18580877]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tammimäki A.E., Männistö P.T. Are genetic variants of COMT associated with addiction? Pharmacogenet. Genomics. 2010;20(12):717–741. doi: 10.1097/FPC.0b013e328340bdf2. [PMID: 20975619]. [DOI] [PubMed] [Google Scholar]
- 102.Gogos J.A., Morgan M., Luine V., Santha M., Ogawa S., Pfaff D., Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl. Acad. Sci. USA. 1998;95(17):9991–9996. doi: 10.1073/pnas.95.17.9991. [http://dx.doi.org/10.1073/pnas.95.17.9991]. [PMID: 9707588]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yavich L., Forsberg M.M., Karayiorgou M., Gogos J.A., Männistö P.T. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J. Neurosci. 2007;27(38):10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [http://dx.doi.org/10.1523/ JNEUROSCI.0665-07.2007]. [PMID: 17881525]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brody A.L., Mandelkern M.A., Olmstead R.E., Scheibal D., Hahn E., Shiraga S., Zamora-Paja E., Farahi J., Saxena S., London E.D., McCracken J.T. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch. Gen. Psychiatry. 2006;63(7):808–816. doi: 10.1001/archpsyc.63.7.808. [http://dx.doi.org/10.1001/archpsyc.63.7.808]. [PMID: 16818870]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo S., Chen D.F., Zhou D.F., Sun H.Q., Wu G.Y., Haile C.N., Kosten T.A., Kosten T.R., Zhang X.Y. Association of functional catechol O-methyl transferase (COMT) Val108Met polymorphism with smoking severity and age of smoking initiation in Chinese male smokers. Psychopharmacology (Berl.) 2007;190(4):449–456. doi: 10.1007/s00213-006-0628-4. [http://dx.doi.org/10.1007/s00213-006-0628-4]. [PMID: 17206495]. [DOI] [PubMed] [Google Scholar]
- 106.Herman A.I., Jatlow P.I., Gelernter J., Listman J.B., Sofuoglu M. COMT Val158Met modulates subjective responses to intravenous nicotine and cognitive performance in abstinent smokers. Pharmacogenomics J. 2013;13(6):490–497. doi: 10.1038/tpj.2013.1. [http://dx.doi.org/10. 1038/tpj.2013.1]. [PMID: 23459442]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Loughead J., Wileyto E.P., Valdez J.N., Sanborn P., Tang K., Strasser A.A., Ruparel K., Ray R., Gur R.C., Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol. Psychiatry. 2009;14(8):820–826. doi: 10.1038/mp.2008.132. [http://dx.doi.org/10.1038/mp.2008.132]. [PMID: 19065145]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wardle M.C., de Wit H., Penton-Voak I., Lewis G., Munafò M.R. Lack of association between COMT and working memory in a population-based cohort of healthy young adults. Neuropsychopharmacology. 2013;38(7):1253–1263. doi: 10.1038/npp.2013.24. [http://dx.doi.org/10.1038/ npp.2013.24]. [PMID: 23337869]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barnett J.H., Scoriels L., Munafò M.R. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol. Psychiatry. 2008;64(2):137–144. doi: 10.1016/j.biopsych.2008.01.005. [http://dx.doi.org/10.1016/j.biopsych.2008.01.005]. [PMID: 18339359]. [DOI] [PubMed] [Google Scholar]
- 110.Ashare R.L., Valdez J.N., Ruparel K., Albelda B., Hopson R.D., Keefe J.R., Loughead J., Lerman C. Association of abstinence-induced alterations in working memory function and COMT genotype in smokers. Psychopharmacology (Berl.) 2013;230(4):653–662. doi: 10.1007/s00213-013-3197-3. [http://dx.doi.org/10.1007/s00213-013-3197-3]. [PMID: 23828159]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoffmann D., Wynder E.L. Chemical constituents and bioactivity of tobacco smoke. 1986. [PubMed] [Google Scholar]
- 112.Hatsukami D.K. Biomarkers to assess the utility of potential reduced exposure tobacco products. Nicotine Tob. Res. 2006;8(4):600–622. doi: 10.1080/14622200600858166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Myers C.S., Taylor R.C., Moolchan E.T., Heishman S.J. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33(3):588–598. doi: 10.1038/sj.npp.1301425. [http://dx.doi.org/10.1038/sj.npp.1301425]. [PMID: 17443125]. [DOI] [PubMed] [Google Scholar]
- 114.Poltavski D.V., Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiol. Behav. 2006;87(3):614–624. doi: 10.1016/j.physbeh.2005.12.011. [http://dx.doi.org/10.1016/ j.physbeh.2005.12.011]. [PMID: 16466655]. [DOI] [PubMed] [Google Scholar]
- 115.Foulds J., Stapleton J., Swettenham J., Bell N., McSorley K., Russell M.A. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology (Berl.) 1996;127(1):31–38. doi: 10.1007/BF02805972. [http://dx.doi.org/10.1007/BF02805972]. [PMID: 8880941]. [DOI] [PubMed] [Google Scholar]
- 116.Kleykamp B.A., Jennings J.M., Blank M.D., Eissenberg T. The effects of nicotine on attention and working memory in never-smokers. Psychol. Addict. Behav. 2005;19(4):433–438. doi: 10.1037/0893-164X.19.4.433. [http://dx. doi.org/10.1037/0893-164X.19.4.433]. [PMID: 16366815]. [DOI] [PubMed] [Google Scholar]
- 117.DeVito E.E., Herman A.I., Waters A.J., Valentine G.W., Sofuoglu M. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39(6):1431–1440. doi: 10.1038/npp.2013.339. [http://dx.doi.org/10.1038/npp.2013.339]. [PMID: 24345818]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Heishman S.J., Taylor R.C., Henningfield J.E. Nicotine and smoking:a review of effects on human performance. Exp. Clin. Psychopharmacol. 1994;2:345–395. [http://dx.doi.org/10.1037/ 1064-1297.2.4.345]. [Google Scholar]
- 119.Heishman S.J. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93(3):317–320. doi: 10.1080/09652149835864. [http://dx. doi.org/10.1080/09652149835864]. [PMID: 10328040]. [DOI] [PubMed] [Google Scholar]
- 120.Heishman S.J., Kleykamp B.A., Singleton E.G. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl.) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [http://dx. doi.org/10.1007/s00213-010-1848-1]. [PMID: 20414766]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Posner M.I., Rothbart M.K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 2007;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [http://dx.doi.org/10.1146/annurev.psych.58. 110405.085516]. [PMID: 17029565]. [DOI] [PubMed] [Google Scholar]
- 122.Grundey J., Amu R., Ambrus G.G., Batsikadze G., Paulus W., Nitsche M.A. Double dissociation of working memory and attentional processes in smokers and non-smokers with and without nicotine. Psychopharmacology (Berl.) 2015;232(14):2491–2501. doi: 10.1007/s00213-015-3880-7. [http://dx.doi.org/10.1007/s00213-015-3880-7]. [PMID: 25721074]. [DOI] [PubMed] [Google Scholar]
- 123.Ettinger U., Faiola E., Kasparbauer A.M., Petrovsky N., Chan R.C., Liepelt R., Kumari V. Effects of nicotine on response inhibition and interference control. Psychopharmacology (Berl.) 2017;234(7):1093–1111. doi: 10.1007/s00213-017-4542-8. [http://dx.doi.org/10.1007/s00213-017-4542-8]. [PMID: 28150023]. [DOI] [PubMed] [Google Scholar]
- 124.Evans D.E., Maxfield N.D., Van Rensburg K.J., Oliver J.A., Jentink K.G., Drobes D.J. Nicotine deprivation influences P300 markers of cognitive control. Neuropsychopharmacology. 2013;38(12):2525–2531. doi: 10.1038/npp.2013.159. [http://dx.doi.org/10.1038/npp.2013.159]. [PMID: 23807239]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Waters A.J., Sutton S.R. Direct and indirect effects of nicotine/smoking on cognition in humans. Addict. Behav. 2000;25(1):29–43. doi: 10.1016/s0306-4603(99)00023-4. [http://dx.doi.org/10.1016/S0306-4603(99)00023-4]. [PMID: 10708317]. [DOI] [PubMed] [Google Scholar]
- 126.Kassel J.D., Shiffman S. Attentional mediation of cigarette smoking’s effect on anxiety. Health Psychol. 1997;16(4):359–368. doi: 10.1037//0278-6133.16.4.359. [http://dx.doi.org/10.1037/0278-6133.16.4.359]. [PMID: 9237088]. [DOI] [PubMed] [Google Scholar]
- 127.Brody A.L., Mandelkern M.A., London E.D., Olmstead R.E., Farahi J., Scheibal D., Jou J., Allen V., Tiongson E., Chefer S.I., Koren A.O., Mukhin A.G. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Arch. Gen. Psychiatry. 2006;63(8):907–915. doi: 10.1001/archpsyc.63.8.907. [http://dx.doi.org/10.1001/archpsyc. 63.8.907]. [PMID: 16894067]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Esterlis I., Cosgrove K.P., Batis J.C., Bois F., Stiklus S.M., Perkins E., Seibyl J.P., Carson R.E., Staley J.K. Quantification of smoking-induced occupancy of beta2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. J. Nucl. Med. 2010;51(8):1226–1233. doi: 10.2967/jnumed.109.072447. [http://dx.doi.org/10.2967/jnumed.109. 072447]. [PMID: 20660383]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brody A.L., Mukhin A.G., La Charite J., Ta K., Farahi J., Sugar C.A., Mamoun M.S., Vellios E., Archie M., Kozman M., Phuong J., Arlorio F., Mandelkern M.A. Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int. J. Neuropsychopharmacol. 2013;16(5):957–966. doi: 10.1017/S1461145712001022. [http://dx.doi.org/10.1017/S1461145712001022]. [PMID: 23171716]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cosgrove K.P., Esterlis I., McKee S.A., Bois F., Seibyl J.P., Mazure C.M., Krishnan-Sarin S., Staley J.K., Picciotto M.R., O’Malley S.S. Sex differences in availability of β2*-nicotinic acetylcholine receptors in recently abstinent tobacco smokers. Arch. Gen. Psychiatry. 2012;69(4):418–427. doi: 10.1001/archgenpsychiatry.2011.1465. [http://dx.doi.org/10.1001/ archgenpsychiatry.2011.1465]. [PMID: 22474108]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eaton J.B., Lucero L.M., Stratton H., Chang Y., Cooper J.F., Lindstrom J.M., Lukas R.J., Whiteaker P. The unique α4+/-α4 agonist binding site in (α4)3(β2)2 subtype nicotinic acetylcholine receptors permits differential agonist desensitization pharmacology versus the (α4)2(β2)3 subtype. J. Pharmacol. Exp. Ther. 2014;348(1):46–58. doi: 10.1124/jpet.113.208389. [http://dx.doi.org/10.1124/jpet.113.208389]. [PMID: 24190916]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Breese C.R., Marks M.J., Logel J., Adams C.E., Sullivan B., Collins A.C., Leonard S. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J. Pharmacol. Exp. Ther. 1997;282(1):7–13. [PMID: 9223534]. [PubMed] [Google Scholar]
- 133.Cosgrove K.P., Batis J., Bois F., Maciejewski P.K., Esterlis I., Kloczynski T., Stiklus S., Krishnan-Sarin S., O’Malley S., Perry E., Tamagnan G., Seibyl J.P., Staley J.K. beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch. Gen. Psychiatry. 2009;66(6):666–676. doi: 10.1001/archgenpsychiatry.2009.41. [http://dx.doi.org/10.1001/archgenpsychiatry.2009.41]. [PMID: 19487632]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mamede M., Ishizu K., Ueda M., Mukai T., Iida Y., Kawashima H., Fukuyama H., Togashi K., Saji H. Temporal change in human nicotinic acetylcholine receptor after smoking cessation: 5IA SPECT study. J. Nucl. Med. 2007;48(11):1829–1835. doi: 10.2967/jnumed.107.043471. [http://dx.doi.org/10.2967/jnumed.107.043471]. [PMID: 17942810]. [DOI] [PubMed] [Google Scholar]
- 135.Stein E.A., Pankiewicz J., Harsch H.H., Cho J.K., Fuller S.A., Hoffmann R.G., Hawkins M., Rao S.M., Bandettini P.A., Bloom A.S. Nicotine-induced limbic cortical activation in the human brain: a functional MRI study. Am. J. Psychiatry. 1998;155(8):1009–1015. doi: 10.1176/ajp.155.8.1009. [http://dx.doi.org/10.1176/ajp.155.8.1009]. [PMID: 9699686]. [DOI] [PubMed] [Google Scholar]
- 136.Giessing C., Thiel C.M., Rösler F., Fink G.R. The modulatory effects of nicotine on parietal cortex activity in a cued target detection task depend on cue reliability. Neuroscience. 2006;137(3):853–864. doi: 10.1016/j.neuroscience.2005.10.005. [http://dx.doi.org/10.1016/j.neuroscience.2005.10.005]. [PMID: 16309846]. [DOI] [PubMed] [Google Scholar]
- 137.Thiel C.M., Fink G.R. Effects of the cholinergic agonist nicotine on reorienting of visual spatial attention and top-down attentional control. Neuroscience. 2008;152(2):381–390. doi: 10.1016/j.neuroscience.2007.10.061. [http://dx.doi.org/10.1016/j.neuroscience.2007.10.061]. [PMID: 18272290]. [DOI] [PubMed] [Google Scholar]
- 138.Vossel S., Thiel C.M., Fink G.R. Behavioral and neural effects of nicotine on visuospatial at-tentional reorienting in non-smoking subjects. Neuropsychopharmacology. 2008;33(4):731–738. doi: 10.1038/sj.npp.1301469. [http://dx.doi.org/10.1038/sj.npp.1301469]. [DOI] [PubMed] [Google Scholar]
- 139.Lerman C., Gu H., Loughead J., Ruparel K., Yang Y., Stein E.A. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523–530. doi: 10.1001/jamapsychiatry.2013.4091. [http://dx.doi.org/10.1001/jamapsychiatry. 2013.4091]. [PMID: 24622915]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Patterson F. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106(1):61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [PMID: 19733449]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Powell J., Dawkins L., West R., Powell J., Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology (Berl.) 2010;212(4):537–549. doi: 10.1007/s00213-010-1975-8. [http://dx.doi.org/10.1007/s00213-010-1975-8]. [PMID: 20703450]. [DOI] [PubMed] [Google Scholar]
- 142.Waters A.J., Shiffman S., Sayette M.A., Paty J.A., Gwaltney C.J., Balabanis M.H. Attentional bias predicts outcome in smoking cessation. Health Psychol. 2003;22(4):378–387. doi: 10.1037/0278-6133.22.4.378. [http://dx.doi.org/10.1037/0278-6133.22.4.378]. [PMID: 12940394]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Janes A.C., Pizzagalli D.A., Richardt S. deB Frederick, B.; Chuzi, S.; Pachas, G.; Culhane, M.A.; Holmes, A.J.; Fava, M.; Evins, A.E.; Kaufman, M.J. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol. Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [http://dx.doi.org/10.1016/j.biopsych.2009.12.034]. [PMID: 20172508]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Loughead J., Falcone M., Wileyto E.P., Albelda B., Audrain-McGovern J., Cao W., Kurtz M.M., Gur R.C., Lerman C. Can brain games help smokers quit?: Results of a randomized clinical trial. Drug Alcohol Depend. 2016;168:112–118. doi: 10.1016/j.drugalcdep.2016.08.621. [http://dx.doi.org/10.1016/j.drugalcdep.2016.08.621]. [PMID: 27635998]. [DOI] [PubMed] [Google Scholar]
- 145.Patterson F., Jepson C., Strasser A.A., Loughead J., Perkins K.A., Gur R.C., Frey J.M., Siegel S., Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol. Psychiatry. 2009;65(2):144–149. doi: 10.1016/j.biopsych.2008.08.028. [http://dx.doi.org/10.1016/j.biopsych. 2008.08.028]. [PMID: 18842256]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ashare R.L., McKee S.A. Effects of varenicline and bupropion on cognitive processes among nicotine-deprived smokers. Exp. Clin. Psychopharmacol. 2012;20(1):63–70. doi: 10.1037/a0025594. [http://dx.doi.org/10.1037/ a0025594]. [PMID: 21942262]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rhodes J.D., Hawk L.W., Jr, Ashare R.L., Schlienz N.J., Mahoney M.C. The effects of varenicline on attention and inhibitory control among treatment-seeking smokers. Psychopharmacology (Berl.) 2012;223(2):131–138. doi: 10.1007/s00213-012-2700-6. [http://dx.doi.org/10.1007/s00213-012-2700-6]. [PMID: 22526531]. [DOI] [PubMed] [Google Scholar]
- 148.Austin A.J., Duka T., Rusted J., Jackson A. Effect of varenicline on aspects of inhibitory control in smokers. Psychopharmacology (Berl.) 2014;231(18):3771–3785. doi: 10.1007/s00213-014-3512-7. [http://dx.doi.org/10.1007/ s00213-014-3512-7]. [PMID: 24652107]. [DOI] [PubMed] [Google Scholar]
- 149.Sofuoglu M., Mooney M. Cholinergic functioning in stimulant addiction: implications for medications development. CNS Drugs. 2009;23(11):939–952. doi: 10.2165/11310920-000000000-00000. [http://dx.doi.org/10.2165/11310920-000000000-00000]. [PMID: 19845415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sofuoglu M., Herman A.I., Li Y., Waters A.J. Galantamine attenuates some of the subjective effects of intravenous nicotine and improves performance on a Go No-Go task in abstinent cigarette smokers: a preliminary report. Psychopharmacology (Berl.) 2012;224(3):413–420. doi: 10.1007/s00213-012-2763-4. [http://dx.doi.org/10.1007/s00213-012-2763-4]. [PMID: 22700039]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ashare R.L., Ray R., Lerman C., Strasser A.A. Cognitive effects of the acetylcholinesterase inhibitor, donepezil, in healthy, non-treatment seeking smokers: a pilot feasibility study. Drug Alcohol Depend. 2012;126(1-2):263–267. doi: 10.1016/j.drugalcdep.2012.04.019. [http://dx.doi.org/10.1016/ j.drugalcdep.2012.04.019]. [PMID: 22595038]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Falcone M., Bernardo L., Ashare R.L., Hamilton R., Faseyitan O., McKee S.A., Loughead J., Lerman C. Transcranial direct current brain stimulation increases ability to resist smoking. Brain Stimul. 2016;9(2):191–196. doi: 10.1016/j.brs.2015.10.004. [http://dx.doi.org/10.1016/j.brs.2015. 10.004]. [PMID: 26572280]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fecteau S., Agosta S., Hone-Blanchet A., Fregni F., Boggio P., Ciraulo D., Pascual-Leone A. Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend. 2014;140:78–84. doi: 10.1016/j.drugalcdep.2014.03.036. [http://dx.doi.org/10.1016/j.drugalcdep.2014.03. 036]. [PMID: 24814566]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu J., Fregni F., Brody A.L., Rahman A.S. Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front. Psychiatry. 2013;4(112):112. doi: 10.3389/fpsyt.2013.00112. [PMID: 24065930]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Field M., Duka T., Tyler E., Schoenmakers T. Attentional bias modification in tobacco smokers. Nicotine Tob. Res. 2009;11(7):812–822. doi: 10.1093/ntr/ntp067. [http://dx.doi.org/10.1093/ntr/ntp067]. [PMID: 19474181]. [DOI] [PubMed] [Google Scholar]
- 156.Lopes F.M., Pires A.V., Bizarro L. Attentional bias modification in smokers trying to quit: a longitudinal study about the effects of number of sessions. J. Subst. Abuse Treat. 2014;47(1):50–57. doi: 10.1016/j.jsat.2014.03.002. [http://dx.doi.org/10.1016/j.jsat.2014.03.002]. [PMID: 24666812]. [DOI] [PubMed] [Google Scholar]