Abstract
Abstract: Background
The mean diffusivity (MD) parameter obtained by diffusion tensor imaging provides a measure of how freely water molecules move in brain tissue. Greater tissue density conferred by closely arrayed cellular structures is assumed to lower MD by inhibiting the free diffusion of water molecules.
Methods
In this paper, we review studies showing MD variation among regions of the brain dopaminergic system (MDDS), especially subcortical structures such as the putamen, caudate nucleus, and globus pallidus, in different conditions with known associations to dopaminergic system function or dysfunction. The methodologies and background related to MD and MDDS are also discussed.
Results
Past studies indicate that MDDS is sensitive to pathological derangement of dopaminergic activity, neural changes caused by cognitive and pharmacological interventions that are known to affect the dopaminergic system, and individual character traits related to dopaminergic function.
Conclusion
These results suggest that MDDS can be one useful tool to tap the neural differences related to the dopaminergic system.
Keywords: Mean diffusivity, dopaminergic system, dopamine, diffusion tensor imaging, basal ganglia, cognition
1. Introduction
Apparent diffusion coefficient (ADC) is a measure of the diffusion of water molecules within tissues calculated by diffusion-weighted magnetic resonance imaging (DW-MRI). Mean diffusivity (MD) refers to the average ADC measured in three orthogonal directions by diffusion tensor imaging (DTI) [1]. ADC measured in one direction tends to underestimate diffusion-related pathological changes because fiber tracts are not always oriented in the same direction [1]. Therefore, MD appears to be more sensitive than unidirectional ADC for detecting these changes. Traditionally, MD was often used to measure microstructural properties of white matter [2-4], but is now used with increasing frequency to investigate microstructural properties of gray matter as well [5, 6].
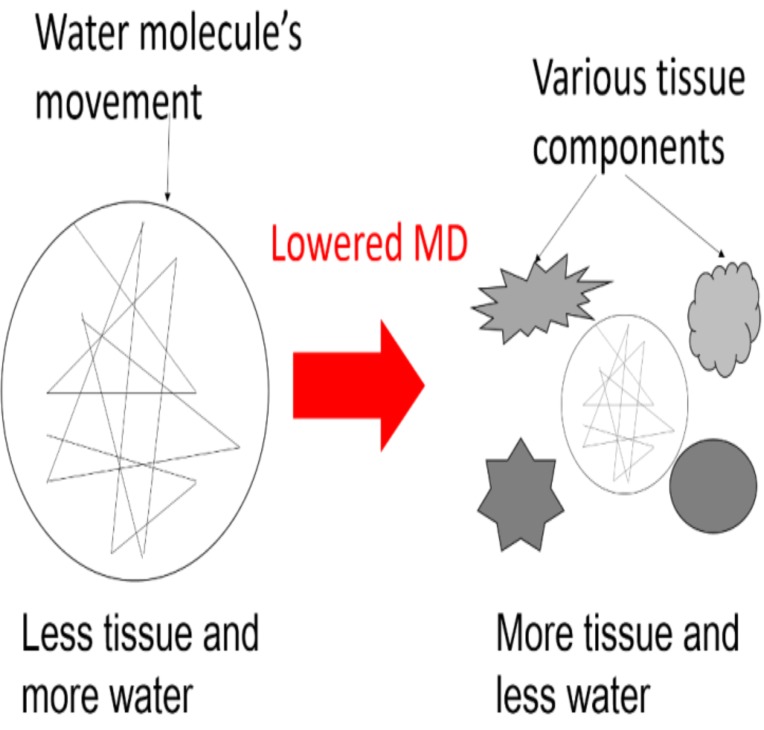
MD is positively correlated with the amount of water in tissues and is thought to reflect the overall tissue water content [7]. On the other hand, a greater density of cellular structures in tissues, such as capillaries, synapses, and macromolecular proteins, is expected to decrease MD (Fig. 1), as are changes in the shapes of neurons or glia and the directionality of tissue organization (e.g., by strengthening of the axonal or dendritic backbones and the surrounding tissues) [5, 8, 9]. However, MD is not specific to any one of these changes [10].
Fig. (1).
Schema of lower and higher mean diffusivity (MD) and association with water content in various tissue components.
Differences in MD are associated with individual cognitive, biological, and genetic characteristics as well as neurological diseases and psychiatric disorders [11-14]. In addition, Sagi et al. [5] showed that MD can detect rapid plasticity caused by cognitive intervention, suggesting its utility for in vivo studies of neural plasticity. Johansen-Berg et al. [15] proposed possible microlevel mechanisms that underlie these MD changes over different time frames. According to the authors, astrocyte swelling with increased neural activity affects MD over a period of seconds to minutes, synapse and dendritic spine formation over minutes to hours, and more elaborate structural changes such as dendritic sprouting, neurogenesis, and angiogenesis over days to weeks.
In this review, we focus on MD variation in areas of the dopaminergic system (MDDS), particularly subcortical areas such as the putamen, caudate nucleus, and globus pallidus, among healthy individuals and patients with various neurodegenerative and psychiatric diseases. As will be discussed in this article, MDDS is uniquely associated with several conditions related to differences or changes of the dopaminergic system.
2. Imaging methods
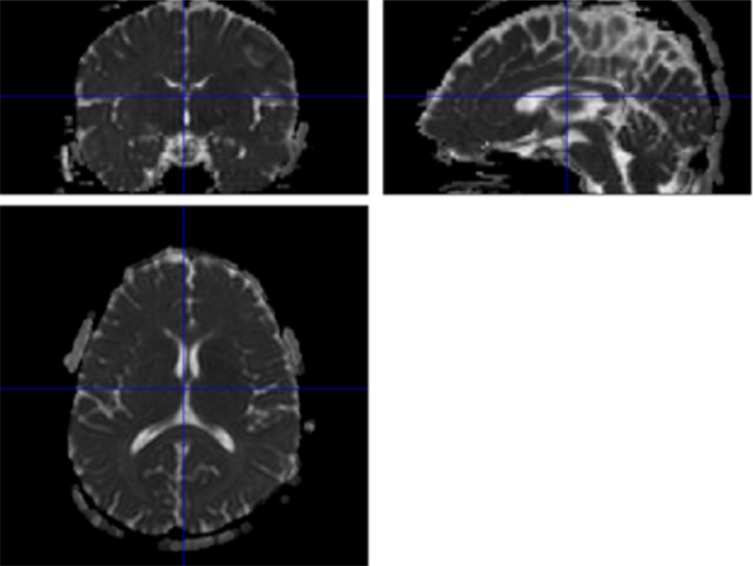
A number of methods have been used to investigate MDDS. One important issue in the preprocessing of MD maps is the exclusion of effects from cerebrospinal fluid (CSF) because voxels of brain parenchyma and CSF show markedly different MD values (Fig. 2). The traditional method to measure MDDS without CSF contamination is to manually trace anatomical areas by hand and use the mean MD values [16]. Another method involves exclusion of voxels with high signal intensity from the analysis, as voxels corresponding to CSF are likely to show much higher MD signals. However, the partial-volume effect at the brain parenchyma–CSF interface is still liable to affect the results. Yet another method is to coregister fractional anisotropy images (together with other DTI images) rich in signal differences among tissues with T1-weighted structural images that provide information for segmentation, and then normalize the coregistered images to a standard space [12]. Using this information for segmentation and normalization, the MD of gray matter in each anatomical area can be determined. While this may be a useful method under certain conditions, distortion induced by high-Tesla MRI may yield DTI images that are slightly different in shape than T1-weighted structural images. This is a potential pitfall when high-Tesla MRI is used.
Fig. (2).
One example MD image. Note the high signal intensity in areas of cerebral spinal fluid (CSF).
To circumvent these problems, we developed the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL)-based registration process, which utilizes the fractional anisotropy signal distribution in the white matter on DTI images [17]. In this method, both tissue probability distribution information within each tissue as well as fractional anisotropy signal distribution within the white matter are used for the registration of brain images for different individuals. In addition, we carefully remove the voxels that are unlikely to be gray or white matter in the template to exclude the effects of CSF on MD.
Finally, Biological Parametric Mapping [18] is a tool for correcting CSF effects on MD during statistical analysis based on the probability of CSF in each voxel. This is especially useful when contamination from CSF signals is still strongly suspected after preprocessing.
3. Imaging correlates of MDDS
Several studies have investigated the neuroimaging correlates of MDDS. Kawaguchi et al. [19] reported a significant negative correlation between MD and carbon-11-labeled levodopa, which reflects dopamine synthesis capacity, in the posterior caudate and putamen of 10 healthy young adults. Based on the assumption that MD in these regions reflects the density of wide-spreading axonal terminals in the striatum, the authors suggested that dopamine synthesis in the striatum may be related to the density of dopaminergic fibers. Scherfler et al. [20] reported a significant negative correlation between MD of the substantia nigra and putaminal 6-[18F] fluorolevodopa uptake, which reflects dopamine terminal activity from nigral projections in the striatum, in a study of 14 Parkinson’s disease (PD) patients. The results suggest that microstructural degeneration of the substantia nigra parallels the progression of putaminal dopaminergic dysfunction in PD. Baudrexel et al. [21] showed a significant negative correlation between MD in the putamen and signal strength on 18-fluorodeoxyglucose positron emission tomography, reflecting cerebellar metabolism, in the putamen of patients with the Parkinson variant multiple system atrophy (MSA-P). This suggests a close association between the extent of microstructural damage in the putamen and hypometabolism in MSA-P patients.
In short, studies using positron emission tomography have suggested associations between MDDS in relevant areas with both dopaminergic function and metabolism in health and disease.
4. Brain regions showing MD changes under conditions that involve altered dopaminergic system function
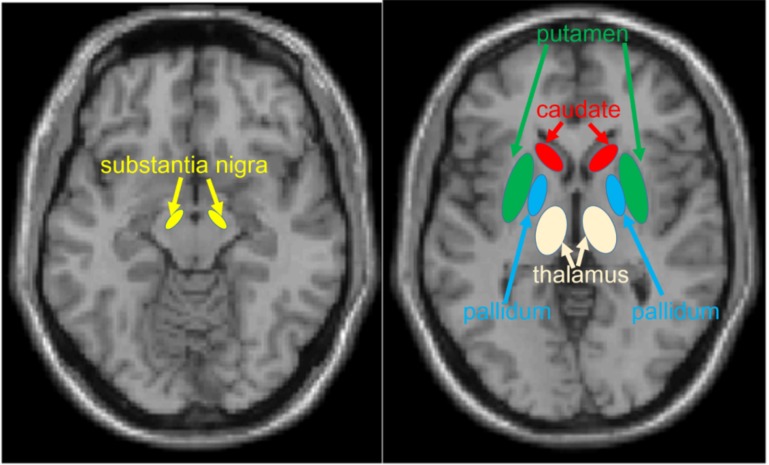
Brain areas where MD changes are frequently associated with alterations in dopaminergic system structure or function include the globus pallidus, putamen, caudate nucleus, thalamus, and substantia nigra (Fig. 3). Here, we introduce the basic characteristics of these regions in this subsection. All of these areas are involved in motor function [22]; however, in this subsection, we focus on regional functions related to cognition as they are more relevant to this review. The globus pallidus receives dopaminergic input from the substantia nigra [22, 23] and thus is an important part of the dopaminergic system. The globus pallidus plays a key role in motivation [6].
Fig. (3).
Areas where associations between MD and altered dopaminergic states are frequently observed.
The putamen receives the dopaminergic input from the substantia nigra and a major part of the nigrostriatal pathway of the dopaminergic system [22]. The putamen is involved in various aspects of reward such as encoding reward magnitude [24], reward expectation [25], and reward predictability [26]. Activation of the putamen is associated with motivation regardless of whether the motivation is intrinsic or extrinsic (e.g., driven by the prospect of a monetary reward) [27]. Furthermore, the putamen is involved in various distressing emotions such as hatred [28], disgust [29], and anger [30, 31] as well as with emotions associated with romantic love [32]. In addition, the putamen is also thought to be involved in action or action planning driven by distressing emotions and rewards [28, 33].
The caudate nucleus also receives dopaminergic input from the substantia nigra and a major part of the nigrostriatal pathway of the dopaminergic system [22]. This area is involved in reward and expectation of reward [34] as well as with learning through positive feedback [35].
The thalamus receives inputs from the substantia nigra and relays this information to widespread cortical areas [22]. The thalamus contains the highest level of dopamine D2 receptors outside the caudate and putamen [36]. In the thalamus, dopamine is involved in changing the threshold of information gating [37] thereby influencing the excitation of cortical regions [38]. We believe that these mechanisms may be associated with a greater concentration in the motivational state, which is related to dopaminergic functions [39].
Finally, dopaminergic neurons from the substantia nigra project to other regions. Degeneration of the substantia nigra leads to PD, which impairs motor function as well as a wide range of cognitive functions [22].
5. Association between MDDS and individual traits and states
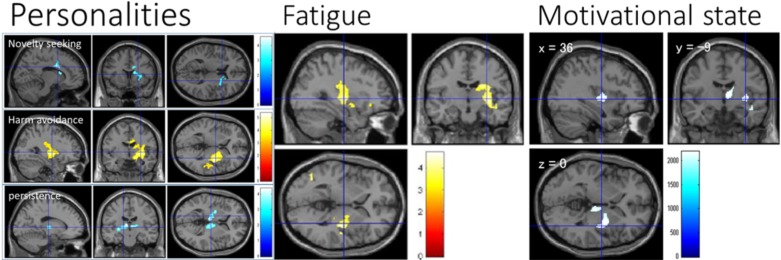
Several studies have revealed associations between MD (or MDDS) and individual traits linked to dopaminergic function. For instance, Yokoyama et al. [40] reported an negative association between extraversion and right putamen MD among 884 young healthy subjects. In this study, the NEOFFI scale [41] was used to measure extraversion, a stable personality trait strongly dependent on dopaminergic function [42, 43]. Nakagawa et al. [44] reported an association between fatigue and MDDS in an anatomical cluster including, right globus pallidus, and right putamen in 883 young normal subjects (Fig. 4). In this study, the Checklist Individual Strength Questionnaire [45] was used to measure individual fatigue over a period of a few weeks. Disruption within the dopaminergic system is considered a common mechanism underlying fatigue [46]. Takeuchi et al. [39] reported an association between the motivated state (vigor) and MD in several areas, including right globus pallidus, right putamen, right posterior insula, right caudate nucleus, right thalamus, and right superior temporal gyrus, in 776 healthy young subjects (Fig. 4). The motivational state was measured by the vigor/activity subscale of the Profile of Mood States (POMS) [47]. In contrast, there was little correlation between MDDS and other mood subscales of the POMS, including tension/anxiety, depression/dejection, anger/hostility, fatigue/inertia, and confusion/bewilderment. Increased motivation is known to be associated with upregulated dopaminergic activity [48]. Collectively, these studies substantiate the association between region-specific MDDS and individual trait/state differences related to dopaminergic activity.
Fig. (4).
Associations of MDDS with traits and states. Red color indicates positive associations between MDDS and altered cognitive states; blue color indicates negative associations. Left row: Associations between MDDS and the personality types novelty seeking, harm avoidance, and persistence [adapted from Takeuchi et al. [6]]. The color bar indicates the t scores for the respective associations. Middle row: Association between MDDS and fatigue [adapted from Nakagawa et al. [44]]. The color bar indicates the t scores for the respective associations. Right row: Association between MDDS and motivational state [adapted from Takeuchi et al. [39]]. The color bar indicates the value of threshold-free cluster enhancement [114], which reflects the strength and extent of the effects at each voxel. (The color version of the figure is available in the electronic copy of the article).
Takeuchi et al. [6] investigated the associations between MD and personality type as measured by the Temperament and Character Inventory in 776 healthy subjects (Fig. 4). Interestingly, a negative correlation between novelty seeking and MD, a positive correlation between harm avoidance and MD, and a negative correlation between self-directedness and MD overlapped in areas of the right caudate nucleus, right putamen, and right globus pallidus together with contiguous regions. Harm avoidance also showed a negative correlation with MD in the putamen, and self-transcendence showed a negative correlation with MD in the right globus pallidus. In addition, persistence (perseverance despite frustration and fatigue as well as a tendency toward a persistent pursuit of desired goals) showed a negative association with MD in the thalamus, left putamen, and left globus pallidus. The personality types showing negative associations with MDDS (high novelty seeking, low harm avoidance, high self-directedness, high self-transcendence, and high persistence) showed robust positive associations with self-fulfillment, achievement motivation, and state of vigor (motivational state) [6], which in turn are believed to be strongly dependent on dopaminergic functions as described above. Therefore, these results are consistent with the notion that individual character differences related to dopaminergic function are associated with variation in MDDS.
In addition, Laricchiuta et al. [12] reported a positive association between MD in the bilateral putamen and harm avoidance among 125 healthy adults, but in this study, no significant association was observed between novelty seeking and MDDS, possibly due to smaller sample size and a wider age range in the subject population.
For a summary of these findings, see Table 1.
Table 1.
Association of MDDS with individual trait and state differences in cross-sectional studies.
| Study | Independent Variable | Subjects | Putamen | Caudate Nucleus | Globus Pallidus | Thalamus | Other Areas |
|---|---|---|---|---|---|---|---|
| Yokoyama, Nozawa, Takeuchi, Taki, Sekiguchi, Nouchi, Kotozaki, Nakagawa, Miyauchi, Iizuka, Shinada, Yamamoto, Hanawa, Araki, Hashizume, Kunitoki, Uehara, Sassa and Kawashima [40] | Extraversion | 884 young (age:-) |
R (−) (ROI) | n.s. (ROI) |
n.s. (ROI) |
n.s. (ROI) |
n.s.4 |
| Nakagawa, Takeuchi, Taki, Nouchi, Kotozaki, Shinada, Maruyama, Sekiguchi, Iizuka and Yokoyama [44] | Fatigue | 883 young (20.7 ± 1.81 years) |
R (+) (WB) |
R (+) (WB) |
R (+) (WB) |
n.s. (WB) |
n.s. (WB) |
| Takeuchi, Taki, Sekiguchi, Nouchi, Kotozaki, Nakagawa, Miyauchi, Iizuka, Yokoyama and Shinada [39]1 | Motivational State (state of Vigor) | 766 young (20.7 ± 1.8 years) |
R (−) (WB) |
R (−) (WB) |
R (−) (WB) |
R(−) (WB) |
Right Posterior insula, right superior temporal gyrus (−) (WB) |
| Takeuchi, Taki, Sekuguchi, Hashizume, Nouchi, Sassa, Kotozaki, Miyauchi, Yokoyama, Iizuka, Nakagawa, Nagase, Kunitoki and Kawashima [6]2 | Novelty seeking | 766 young (age:-) |
R (−) (WB) |
R (−) (WB) |
R (−) (WB, ROI) |
n.s. (WB) |
Right anterior and middle cingulate, right corpus callus, right insula (−) (WB) |
| Takeuchi, Taki, Sekuguchi, Hashizume, Nouchi, Sassa, Kotozaki, Miyauchi, Yokoyama, Iizuka, Nakagawa, Nagase, Kunitoki and Kawashima [6] | Harm avoidance | 766 young (age:-) |
R (+) (WB) |
R (+) (WB) |
R (+) (WB, ROI) |
R(+) (WB) |
Right insula (+), right middle cingulate cortex, right corpus callosum, right superior temporal gyrus (+) (WB) |
| Takeuchi, Taki, Sekuguchi, Hashizume, Nouchi, Sassa, Kotozaki, Miyauchi, Yokoyama, Iizuka, Nakagawa, Nagase, Kunitoki and Kawashima [6] | Persistence | 766 young (age:-) |
L (−) (WB) |
n.s. (WB) |
L (−) (WB, ROI) |
B(−) (WB) |
n.s. (WB) |
| Takeuchi, Taki, Sekuguchi, Hashizume, Nouchi, Sassa, Kotozaki, Miyauchi, Yokoyama, Iizuka, Nakagawa, Nagase, Kunitoki and Kawashima [6] | Self-directedness | 766 young (age:-) |
R (−) (WB) |
R (−) (WB) |
R (−) (WB, ROI) |
n.s. (WB) |
Right insula (−) (WB) |
| Takeuchi, Taki, Sekuguchi, Hashizume, Nouchi, Sassa, Kotozaki, Miyauchi, Yokoyama, Iizuka, Nakagawa, Nagase, Kunitoki and Kawashima [6] | Self-transcendence | 766 young (age:-) |
n.s. (WB) |
n.s. (WB) |
R (−) (ROI) |
n.s. (WB) |
n.s. (WB) |
| Laricchiuta, Petrosini, Piras, Cutuli, Macci, Picerni, Chiapponi, Caltagirone and Spalletta [12] 3 | Harm avoidance | 125 adults (34.9 ± 12.4 years) | R (+) (ROI) |
n.s. (ROI) |
n.s. (ROI) |
n.a. | n.a. |
“R,” “L,” and “B” indicate findings in the right hemisphere, left hemisphere, and bilateral hemispheres, respectively. (+) indicates positive correlation and (–) indicates negative correlation. (WB) indicates results of whole brain analyses. (ROI) indicates the results of region of interest analyses. n.s. represents “not significant”. n.a. represents ”not analyzed”.
1 There were only weak correlations of MDDS with other mood subscales of the POMS, including tension/anxiety, depression/dejection, anger/hostility, fatigue/inertia, and confusion/bewilderment.
2 Reward dependence and cooperativeness was not associated with MDDS.
3 In this study, an association between MDDS and novelty seeking was not observed.
4 In this study, only subcortical areas were analyzed.
6. Studies of cognitive function
In addition to associations of traits and states with MDDS, a number of studies have also shown associations of MDDS with cognitive functioning. In our previous study of 895 healthy young adults, MD in the bilateral globus pallidus showed a significant negative correlation with creativity as measured by divergent thinking [6]. Further, the associations appeared to be mediated by personality traits such as persistence (left globus pallidus) and harm avoidance, novelty seeking, self-transcendence, and self-directedness (right globus pallidus). In that study, we also summarized previous studies that suggested an association between creativity as measured by divergent thinking and the dopaminergic system, including studies implicating the dopaminergic system in the promotion of creativity [49, 50]. Such evidence includes a positive association of creativity with novelty seeking [51] and extroversion [42, 43, 52], traits strongly associated with dopaminergic function [53-57], and motivation not caused by external incentives [58], which is also strongly dependent on dopaminergic function [59]. Furthermore, dopamine antagonists suppress creativity [49] and decreased creativity is well-documented in conditions with pathological impairment of the dopaminergic system [60]. Finally, creativity can be rescued by dopaminergic agonist therapy [61].
In another study [62], we observed a negative association between MD and psychometric intelligence as measured by the Wechsler IQ test in a large cohort (N = 253) of children. Significant negative correlations between MD and full scale IQ, verbal IQ, and performance IQ were observed in partially overlapping areas around the left thalamus, left hippocampus, left putamen, left Heschl’s gyrus, and the adjacent white matter bundles. In addition, a significant negative correlation of MD with performance IQ was seen in widespread areas around the entire brain. We suggest that this broadly distributed MD correlation with IQ test performance could reflect the critical involvement of motivation processes (which are strongly associated with the dopaminergic system) in IQ test performance among children [63].
Given the associations of MDDS with a wide range of conditions linked to altered dopaminergic activity, these findings reinforce the notion that creativity as measured by divergent thinking and suggest psychometric intelligence in children are also dependent on dopaminergic activity. For a summary of these findings, see Tables 2 and 3.
Table 2.
Associations of MDDS with cognitive function, cognitive intervention, daily habits, and genotype.
| Study | Variables of Interest | Design | Subjects | Putamen | Caudate Nucleus | Globus Pallidus | Thalamus | Other Areas |
|---|---|---|---|---|---|---|---|---|
| Takeuchi, Taki, Sekuguchi, Hashizume, Nouchi, Sassa, Kotozaki, Miyauchi, Yokoyama, Iizuka, Nakagawa, Nagase, Kunitoki and Kawashima [6] | Verbal creativity of divergent thinking | Cross-sectional Correlation |
895 young (20.8 ± 1.8 years) |
n.s. (WB, ROI) |
n.s. (WB, ROI) |
B (−) (WB, ROI) |
n.s. (WB,ROI) |
n.s. (WB) |
| Takeuchi, Taki, Hashizume, Asano, Asano, Sassa, Yokota, Kotozaki, Nouchi and Kawashima [62] | Full scale IQ | Cross-sectional Correlation |
253 children (age:-) | L (−) (WB) |
n.s. (WB) |
n.s. (WB) |
(−) (WB) |
Left hippocampus, left insula, left Heschl gyrus, contingent white-matter areas (−)(WB) |
| Takeuchi, Taki, Hashizume, Asano, Asano, Sassa, Yokota, Kotozaki, Nouchi and Kawashima [62] | Verbal IQ | Cross-sectional Correlation |
253 children (age:-) | L (−) (WB) |
n.s. (WB) |
n.s. (WB) |
(−) (WB) |
Left hippocampus, left insula, left Heschl gyrus, contingent white-matter areas (−)(WB) |
| Takeuchi, Taki, Hashizume, Asano, Asano, Sassa, Yokota, Kotozaki, Nouchi and Kawashima [62] | Performance IQ | Cross-sectional Correlation |
253 children (age:-) | L (−) (WB) |
L (−) (WB) |
L (−) (WB) |
(−) (WB) |
Widespread gray and white-matter areas across the wide range of areas in the whole brain (−)(WB) |
| Takeuchi, Taki, Nouchi, Hashizume, Sekiguchi, Kotozaki, Nakagawa, Miyauchi, Sassa and Kawashima [67] | Working memory training | Intervention | 34 young of the intervention (21.0 ± 1.6 years) vs. 17 young of the passive control (21.2 ± 2.4 years) | R (+) (WB, ROI) |
B (+) (WB, ROI)* |
n.s. (WB, ROI) |
n.s. (WB, ROI) |
Left dorsolateral prefrontal cortex, right substantia nigra, ventral tegmental area right anterior cingulate cortex (+)(ROI) |
| Takeuchi, Taki, Hashizume, Asano, Asano, Sassa, Yokota, Kotozaki, Nouchi and Kawashima [62] | Length of videogame play | Cross-sectional Correlation |
240 children (11.5 ± 3.1 years) |
B (+) (WB) |
B (+) (WB) |
B (+) (WB) |
L(+) (WB) |
Extensive gray and white-matter areas that mainly spread around the anterior parts of the brain (+)(WB) |
| Takeuchi, Taki, Hashizume, Asano, Asano, Sassa, Yokota, Kotozaki, Nouchi and Kawashima [62] | Length of videogame play | Longitudinal predictive analysis | 189 children (14.5 ± 3.0 years) |
B (+) (WB) |
B (+) (WB) |
L (+) (WB) |
B(+) (WB) |
Sporadic gray and white matter around Left frontal, occipital, temporal, fusiform, Right temporal, Heschl gyrus, right insula. (+)(WB) |
| Takeuchi, Tomita, Taki, Kikuchi, Ono, Yu, Sekiguchi, Nouchi, Kotozaki and Nakagawa [14] | 5-repeat allele of dopamine receptor D4 | Carriers vs. non-carriers Cross-sectional |
756 subjects (age:-) | R (+) (WB) |
R (+) (WB) |
R (+) (WB) |
R(+) (WB) |
Widespread areas of the gray and white-matter areas of the whole brain, particularly dorsal part (+)(WB) |
“R,” “L,” “B” indicate findings in the right hemisphere, left hemisphere, and bilateral hemispheres, respectively. (+) indicates positive correlations or increased MD and (–) indicates negative correlations or decreased MD. (WB) indicates results of whole brain analyses. (ROI) indicates the results of ROI analyses. n.s. represents “not significant”. n.a. represents ”not analyzed”.
• Significant results were found only in the right caudate by whole brain analyses. In ROI analysis, significant results were found in the bilateral caudate.
Table 3.
Associations of MDDS (or ADC in the dopaminergic system) with psychiatric and neurological disorders.
| Study, (MD or ADC) | Groups of Interest | Control Subjects | Putamen | Caudate Nucleus | Globus Pallidus | Thalamus | Other Areas |
|---|---|---|---|---|---|---|---|
| Péran, Cherubini, Assogna, Piras, Quattrocchi, Peppe, Celsis, Rascol, Démonet and Stefani [80], MD | 30 PD patients (61.9 ± 11.1 years) |
22 control subjects (57.4 ± 9.7 years) |
R (+) (WB) |
R (+) (WB) |
n.s. (WB,ROI) |
B,C(+) (WB, ROI) |
Left substantia nigra (+)(WB) |
| Razek, Elmongy, Hazem, Zakareyia and Gabr [81], ADC | 25 PD patients (66.33 ± 6.22 years) receiving levodopa | 25 PD patients (65.44 ± 5.22 years) without treatment |
C (+) (ROI) |
n.s. (ROI) |
n.a. | n.s. (ROI) |
n.s. (ROI) |
| Baudrexel, Seifried, Penndorf, Klein, Middendorp, Steinmetz, Grünwald and Hilker [21], MD | 11 MSA-P patients (66.1 ± 11.7 years) | 13 PD patients (66.8 ± 8.0 years) 8 PSP patients (73.9 ± 3.6 years) 6 control subjects (65.3 ± 10.8 years) |
C (+)1 (ROI) |
n.a. | n.a. | n.a. | n.a. |
| Seppi, Schocke, Donnemiller, Esterhammer, Kremser, Scherfler, Diem, Jaschke, Wenning and Poewe [85], ADC | 15 MSA-P patients (63.9 ± 5.6 years) | 17 PD patients (60.1 ± 10.6 years) 8 control subjects (59.3 ± 5.7 years) |
C (+)1 (ROI) |
C (+)1 (ROI) |
n.a. | n.a. | n.a. |
| Spoletini, Cherubini, Banfi, Rubino, Peran, Caltagirone and Spalletta [87], MD | 45 patients with schizophrenia (39.8 ± 11.02 years) |
45 control subjects (39.47 ± 17.10 years) |
n.s. (ROI) |
n.s. (ROI) |
n.s. (ROI) |
B(+) (ROI) |
Bilateral hippocampus, left nucleus accumbens (+)(ROI) |
| Rose, Chalk, Janke, Strudwick, Windus, Hannah, McGrath, Pantelis, Wood and Mowry [88], MD | 12 patients with schizophrenia (31.4 ± 10.7 years) |
12 control subjects (32.2 ± 11.7 years years) |
n.s. (WB) |
B (+) (WB) |
n.s. (WB) |
B(+) (WB) |
Right parahippocampal gyrus, anterior cingulate gyrus, bilateral temporal, prefrontal, right precentral (+)(WB) |
| Makki, Behen, Bhatt, Wilson and Chugani [90], MD | 23 children with Tourette syndrome (11.75 ± 3.25 years) | 35 control children (13.10 ± 3.17 years) |
B (+) (ROI) |
n.s. (ROI) |
n.s. (ROI) |
n.s. (ROI) |
n.a. |
| Alicata, Chang, Cloak, Abe and Ernst [93]2, ADC | 30 methamphetamine users (33.3 ± 8.6 years) |
30 control subjects (32.7 ± 9.5 years) |
B (+) (ROI) |
L (+) (ROI) |
n.s. (ROI) |
n.s. (ROI) |
n.s. (ROI) |
| Lebel, Rasmussen, Wyper, Walker, Andrew, Yager and Beaulieu [16], MD | 24 children with FASD (9.1 ± 2.2 years) |
95 control children (9.8 ± 2.2 years) |
R (+) (ROI) |
n.s. (ROI) |
B (+) (ROI) |
R(+) (ROI) |
Corpus callosum, cingulum, corticospinal tracts, inferior fronto-occipital fasciculus and inferior and superior longitudinal fasciculi together (+)(ROI) |
| Study, (MD or ADC) | Groups of Interest | Control Subjects | Putamen | Caudate Nucleus | Globus Pallidus | Thalamus | Other Areas |
| Sánchez-Castañeda, Cherubini, Elifani, Peran, Orobello, Capelli, Sabatini and Squitieri [96], MD | 29 HD middle-aged patients (age:-) | 29 control middle-aged subjects (age:-) | C (+) (ROI) |
C (+) (ROI) |
n.s. (ROI) |
C (+) (ROI) |
Combined accumbens, combined hippocampus (+)(ROI) |
| Douaud, Behrens, Poupon, Cointepas, Jbabdi, Gaura, Golestani, Krystkowiak, Verny and Damier [97], MD | 14 HD patients (42 ± 8 years) | 10 control subjects (37 ± 12 years) |
B (+) (WB, ROI) |
B (+) (WB, ROI) |
B (+) (WB, ROI) |
B(+) (WB, ROI4) |
Bilateral ventral striatum (+)(WB, ROI) |
“R,” “L,” “B,” indicate findings in the right hemisphere, left hemisphere, and bilateral hemispheres, respectively. “C” indicates that bilateral regions of interest were combined and used in the analysis. (+) indicates the increase of MD and (–) indicates decrease of MD. (WB) indicates results of whole brain analyses. (ROI) indicates the results of ROI analyses.
PD = Parkinson’s disease. MSA-P = Parkinson variant of multiple system atrophy. PSP = Progressive supranuclear palsy. FASD = fetal alcohol spectrum disorder. HD = Huntington’s disease
1Patients of MSA-P > other groups. Striatal ADC was investigated in this study.
2Lin et al. [94] investigated MD of methamphetamine users (ROI analyses of right caudate and right putamen) with smaller sample size and could not find significant associations.
3Whole brain analysis showed significant results in the bilateral thalamus, while ROI analyses showed a significant result only in the right thalamus.
7. Studies of neural plasticity
A few studies have applied the analyses of MDDS to investigate the effects of intervention and daily habits. These studies suggest that environmental factors known to alter dopaminergic activity are associated with MDDS alterations.
7.1. Working Memory Training
Working memory is a limited capacity storage system involved in the maintenance and manipulation of information
over a short time period [64]. The capacity of working memory is thought to be important for higher-order cognitive functions [64]. Cortical dopamine release, particularly in the prefrontal cortex, is thought to be critical for working memory [65] while dopamine release in the striatum is critical for executive processing (updating) of working memory [66]. It was also shown that a working memory training regimen involving updating leads to increased dopamine release during the task [66].
In our previous study [67], a 4-week working memory training protocol enhanced working memory capacity for new material as well as executive function and non-verbal reasoning. Subjects who completed the training (N = 34) showed increased MDDS compared to the passive control group (N = 17). Affected regions included bilateral caudate nucleus, right putamen, left dorsolateral prefrontal cortex, anterior cingulate cortex, substantia nigra, and ventral tegmental area, all areas belonging to major dopaminergic systems (Fig. 5). The underlying mechanisms by which working memory training increases MD are not clear, but our study using an overlapping subject sample found increased cerebral blood flow and regional gray matter volume [68]. One possibility, therefore, is that increased water content (MDDS) in these areas resulted from increased cerebral blood flow.
Fig. (5).
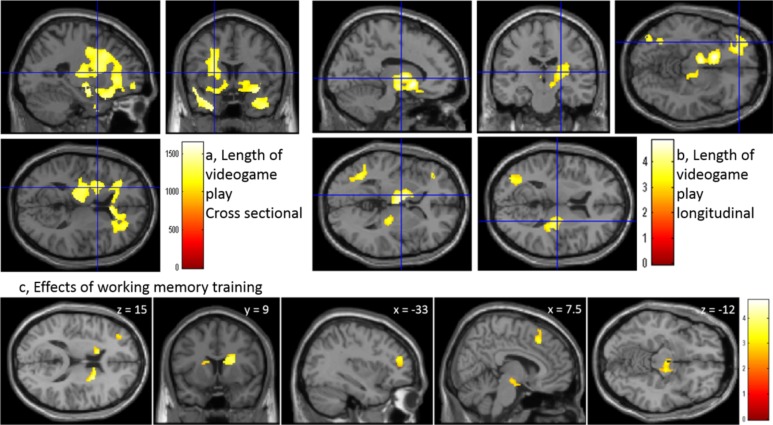
Plasticity of MDDS. Red color indicates increased MDDS associated with daily habits and interventions. Upper Left: Areas of positive associations between MDDS and length of videogame play based on a cross-sectional study in children [adapted from Takeuchi et al. [62]]. The color bar indicates the value of threshold-free cluster enhancement. Upper right: Areas of positive association between length of videogame play in the pre-experiment and changes of MD over 3 years [adapted from Takeuchi et al. [62]. The color bar indicates the t scores for the respective associations. Lower panels: Increased MDDS in response to working memory training for 1 month [adapted from Takeuchi et al., [67]. The color bar indicates the t value. (The color version of the figure is available in the electronic copy of the article).
7.2. Videogame Habits
Videogame play triggers substantial dopamine release in the dopaminergic system [69], which in turn may lead to videogame addiction [70]. Videogame play has been shown to have a number of beneficial effects such as facilitation of certain types of visual cognition [71] as well as a number of non-preferred effects such as reduced verbal memory and certain types of attention, learning, and knowledge deficits [71-73].
We investigated the effects of videogame play habits in a two-part experiment consisting of a cross-sectional study with 240 children and a longitudinal study including 189 of these same children conducted at an average interval of 3 years. In the cross-sectional analysis, the duration of daily videogame play showed a significant positive correlation with MD over a widespread area, particularly in the anterior part of the brain, including the bilateral globus pallidus, caudate nucleus, putamen, and thalamus (Fig. 5). In the longitudinal analysis as well, the duration of videogame play in the first experiment showed a positive correlation with MD changes from baseline (pre-experiment level) in several areas, such as the left basal ganglia, right putamen, thalamus, ventral parts of the prefrontal cortex, and left medial temporal lobe (Fig. 5). Further, the duration of videogame play showed a significant positive correlation with verbal intelligence in the cross-sectional analysis, and with the change in verbal intelligence from pre-experiment level in the longitudinal analysis. The areas in which the effects of videogame play duration were commonly observed included the left middle and inferior prefrontal cortex (involved in attention and working memory) [64], left hippocampus (associated with memory) [74], and orbital frontal cortex, left globus pallidus, left putamen, left caudate nucleus, right putamen, and right insula (involved in reward, motivation, and emotion) [75]. The neural plasticity in these regions may underlie the above-mentioned effects of videogame play on cognition and addiction.
Dopamine is known to exhibit neurotoxic properties, even without induction of excessive release by psychostimulants [76]. Thus, some of the deleterious effects of videogame play on neural systems may be attributable at least in part to excessive dopamine release [69]. For a summary of these findings, see Table 2.
8. Genetic studies
We have also investigated the association of MD with dopamine-related genetic polymorphisms, and the results are also at least partially consistent with the aforementioned association between MDDS and dopaminergic system function.
In one such study, we investigated the potential association between a dopamine receptor D4 (DRD4) polymorphism and MD in a cohort of 756 healthy young adults [14]. This 5-repeat allele of the DRD4 gene was previously shown to be a risk allele for attention deficit hyperactivity disorder [77] and our study showed increased MD in DRD 5-repeat allele carriers over widespread areas of the entire brain not limited to the dopaminergic system. Nonetheless, affected areas included the right putamen, right caudate nucleus, and right globus pallidus. These MD changes may reflect neurodegeneration in widespread areas, including degeneration of dopaminergic neurons, in DRD 5-repeat allele carriers. The mechanism for this association is not clear. It appears that carriers of DRD4 risk alleles may be less sensitive to dopamine stimulation [78]. Further, deficient dopaminergic transmission in these carriers may lead to increased MD from brain atrophy concomitant with chronically reduced neural activity.
These findings are consistent with an association between MDDS and dopaminergic function. However, because the effects were widespread, this association may not be limited to impaired dopaminergic activity. For a summary of these findings, see Table 2.
9. MDDS in neurological diseases and psychiatric disorders
Here we introduce findings of MD or ADC changes in various diseases. As there are voluminous studies in this field, only representative findings for each disease are presented.
9.1. PD
A number of studies have investigated associations between MD (ADC) or MDDS (ADCDS) and PD, which is characterized by degeneration of dopamine-producing neurons in the substantia nigra and resultant striatal dopamine depletion [79]. Péran et al. [80] reported increased regional MDDS in PD patients (N = 30) compared to the control group (N = 22), including in the thalamus, right putamen, right caudate nucleus, and left substantia nigra. In contrast, these changes were not detected by analyses of gray matter volume. Therefore, this result constitutes evidence for an association between altered dopaminergic system function and MDDS changes prior to detectable brain atrophy. In a study by Scherfler et al. [20], 16 PD patients showed significantly greater MD in the substantia nigra and olfactory bulb compared to 14 healthy controls. A prospective study [81] revealed increased ADC of the putamen in PD patients (N = 25) receiving levodopa, a dopamine-agonist used for treatment [22], compared to untreated patients (N = 25). The mechanisms underlying this association are not clear. One possibility is simply that patients with advanced pathology (reflected by increased ADC in the dopaminergic system) are more likely to receive levodopa treatment.
Other studies investigated MD in patients with the Parkinson variant multiple system atrophy (MSA-P), a progressive neurodegenerative disorder characterized by parkinsonism with poor response to levodopa [82]. MAS-P and PD are both characterized by degeneration of neurons that project from the substantia nigra; however, unlike Parkinson’s disease, MAS-P is also characterized by prominent degeneration of neurons that project from the striatum [83, 84]. Baudrexel et al. [21] compared MD among 11 MSA-P patients, 13 PD patients, eight patients with progressive supranuclear palsy (another neurodegenerative disease), and six control subjects and found higher putaminal MD in MSA-P compared to all other groups. On the other hand, Seppi et al. [85] compared ADC in 15 patients with MSA-P to 17 PD patients and 10 control subjects and found significantly greater ADC in the striatum of MSA-P patients compared to both groups. In addition, patients with MSA-P showed a significantly lower ratio of striatal to frontal cortex dopamine D2 receptor binding by single photon emission computed tomography (SPECT) with [123I] iodobenzamide (a semi-quantitative measure of the relative density of basal ganglia dopamine receptors). However, ADC in the striatum showed a significantly higher overall predictive accuracy for distinguishing patients from controls than dopamine D2 receptor binding with [123I]iodobenzamide. MDDS changes in MSA-P patients are not surprising given the additional degeneration of neurons that project from the striatum. In both of these MSA-P studies, however, no clear difference in striatal ADC was found between PD patients and the control group (in contrast to many of the aforementioned studies), possibly due to lower sample size, effect size, or publication bias. Based on these findings, Seppi et al. (2004) suggested the advantage of ADCDS (or MDDS) over SPECT using dopamine receptor binding agents for investigation of diseases involving the dopaminergic system.
Overall, studies that including both PD and MSA-P patients showed higher MD in MSA-P compared to controls and PD patients, while studies including only PD patients showed increased MD (or ADC) in the putamen compared to healthy controls. These studies including PD and MSA-P also failed to show a clear difference between PD and controls. This could reflect smaller sample and effect sizes, but could also reflect publication bias for studies with significant results for MSA-P.
9.2. Schizophrenia
Excessive presynaptic dopaminergic activity is a cardinal feature of schizophrenia [86]. Spolentini et al. [87] reported increased MD in the bilateral thalamus and hippocampus as well as in the left nucleus accumbens of middle-aged schizophrenia patients (N = 45) compared to the control group (N = 45). No significant between-group difference was observed with respect to volume of these structures, which suggests a greater sensitivity of MD for detection of pathological changes compared to morphometrics [87]. In a study by Rose et al. [88], patients with schizophrenia (N = 12) showed elevated MD of the thalamus and bilateral caudate nucleus as well as of areas in the medial frontal gyrus and anterior cingulate, other prefrontal areas, and temporal and parietal areas compared to the control group (N =12). The inconsistency in the affected areas between these two studies may be due to small sample size or widespread but weak effects. In addition, the possibility of publication bias without true effects cannot be eliminated for this and the other diseases discussed below.
9.3. Tourette Syndrome
Tourette syndrome is a childhood-onset neuropsychiatric disorder characterized by motor and vocal tics [89]. Evidence suggests that this disorder is associated with overactivity or hypersensitivity of the dopaminergic system [89]. Makki et al. [90] reported a significantly higher MD in the bilateral putamen of children with Tourette syndrome (N = 23) compared to a control group (N = 35), consistent with increased dopamine release in the putamen [91]. Therefore, this finding provides additional evidence linking individual differences in dopaminergic function with variation in MDDS.
9.4. Methamphetamine Users
Methamphetamine is a psychostimulant that induces excessive release of dopamine in the brain [92]. Alicata et al. [93] reported significantly higher MD in the bilateral putamen and left caudate nucleus of methamphetamine users (N = 30) than in healthy controls (N = 30). Methamphetamine induces hyperthermia, aberrant dopamine or glutamate transmission, and/or mitochondrial dysfunction, resulting in generation of reactive species and consequent toxic damage of dopaminergic neurons [92]. Damage to cellular structures may lead to increased MDDS. However, a subsequent study including a slightly smaller sample of methamphetamine users (N = 18) and healthy controls (N = 22) failed to corroborate the associations between MD and amphetamine abuse [94].
9.5. Subjects with other Brain Atrophies
It should be noted, however, that patients with other forms of neural atrophy not limited to the dopaminergic system may also show increased MD in regions of the dopaminergic systems. For instance, Lebel et al. [16] reported increased MD of the corpus callosum, cingulum, corticospinal tracts, inferior fronto-occipital fasciculus, and inferior and superior longitudinal fasciculi as well as subcortical structures (globus pallidus, putamen, and thalamus) in children with fetal alcohol spectrum disorder (N = 24) compared to healthy children (control group, N = 95). In addition, atrophy of non-dopaminergic neurons in brain regions containing dopaminergic neurons or inputs also increases MD, as expected given that MD does not distinguish among factors that impede water diffusion. For example, Huntington’s disease (HD) is characterized by progressive degeneration of cholinergic neurons and GABAergic neurons in the striatum, while dopaminergic neurons are relatively unaffected. While symptoms of HD reflect the consequences of relatively facilitated dopaminergic transmission [95], multiple studies have showed increased MDDS in HD patients. For example, Sánchez-Castañeda et al. [96] reported higher MD in the caudate nucleus, putamen, thalamus, and hippocampus of HD patients (N = 29) than the control group (N = 29). Douaud et al. [97] also reported increased MD in the putamen, globus pallidus, ventral striatum, caudate nucleus, and thalamus as well as in the corona radiata of HD patients (N = 14) compared to the control group (N = 10).
10. Comprehensive view of neuroimaging findings and possible physiological mechanisms
As described in this review, decreased MDDS appears to be associated with facilitated dopaminergic function in healthy subjects. However, studies of neural plasticity and other environmental factors that lead to increased dopamine release appear to be associated with increased MDDS. In clinical samples, on the other hand, elevated MDDS is associated with pathology regardless of whether the pathological condition results from increased or decreased dopaminergic activity. In many instances, the dopamine dose-response relationship exhibits an inverted U pattern [98]. One interesting speculation is that the relationships between various dopaminergic functions and MDDS also show such a pattern, although empirical evidence is yet to be obtained.
It is also tempting to associate findings of MDDS with “Reward Deficiency Syndrome” [99]. This condition involves deficits in mesocorticolimbic function. Genetic, molecular, and neuronal alterations in key components of this circuitry contribute to a reward deficit state [100]. Functionally, this state appears to be associated with altered corticostriatal functional connectivity, which in turn is associated with a number of cognitive/behavioral functions such as computing reward, decision making, motivation, and habit learning [100-104]. This results in lack of pleasure and reward from activities that would provide others with pleasure (ahedonia) and displaces the sense of well-being with negative feelings. The need to mask these feelings may lead to the use of substances of abuse, as well as impulsive, compulsive, and addictive behaviors [99]. This state and accompanying increased MDDS appears to be associated with a number of conditions summarized in this review, namely the state and trait personalities, lower creativity, low motivation, a more severe gaming habit, and substance abuse. Thus, greater MDDS appears to be associated with these intrinsic hypodopaminergic states. However, still the increased MDDS exist in some of the hyperdopaminergic conditions (such as Tourette syndrome).
It is still unknown why MDDS appears so strongly associated with such a wide range of conditions involve alterations of dopaminergic function. Given the assumed association between higher tissue density and lower MD, it is logical that facilitated functions are associated with lower MD in healthy subjects. This relationship should then be generally applicable to all parts of the brain. However, the associations between MDDS and altered dopaminergic states appear outstanding in many instances and we can only speculate on the underlying reasons. One possibility is that unlike cortex, the putamen, globus pallidus, and caudate nucleus are free from the partial-volume effects of CSF. In addition, it is easy to effectively eliminate the effects of CSF because these areas do not have the convoluted morphology that typifies areas with greater exposure to CSF such as the cortex and medial temporal structures. Another distinct possibility is that the observed association of excessive dopamine release with increased MD (e.g., in patients with schizophrenia, methamphetamine users, and habitual videogame players) reflects reduced tissue component density due to dopamine-induced neurotoxicity. Our final speculation is related to metals. MDDS may also be related to the functioning of the dopaminergic system owing to the strong association between metals and a number of dopaminergic functions [105]. MD values in gray matter are known to be lowered by metals that have paramagnetic properties [106]; moreover, accumulated iron without toxicity appears to lower MD, while neurodegeneration from excessively elevated iron elevates MD [107]. Furthermore, some metals such as iron and copper are believed to play an essential role in the dopaminergic system [108, 109]. Indeed, iron was shown to accumulate in dopamine neurovesicles [108]. In addition, inhibition of dopamine synthesis results in decreased vesicular storage of iron [108]. Thus, more dopamine in tissues may well lower MD signals. However, these are pure speculations, and any combination of these factors may affect MD.
10.1. Other Measures
In this review, we focused on MDDS. However, there may be other diffusion measures for detection of altered dopaminergic states. At least in the case of PD, fractional anisotropy (degree of directionality) of the substantia nigra was shown to have a robust association with disease severity in a meta-analysis [110]. Also, one study investigated the mean kurtosis measure from diffusion kurtosis imaging, together with MD and FA in patients with PD [111]. Water in biological structures often displays non-Gaussian diffusion behavior because of hindrance by interactions with other molecules and cell membranes. Diffusion kurtosis imaging is a means to quantify non-Gaussian diffusion [112]. In this study, mean kurtosis of the dopaminergic system was shown to clearly distinguish patients with PD from healthy controls. In addition, free water diffusion MRI analysis using a bi-tensor model explicitly estimates the fractional volume of freely diffusing water molecules. Fractional volume of free water increases mean diffusivity (MD) and decreases FA. Using this method, Ofori et al. [113] reported elevated free water content in the substantia nigra of PD patients [113]. Further, there were no significant differences in free water corrected fractional anisotropy and free water corrected MD in the substantia nigra between PD patients and healthy controls.
Summary and conclusion
MDDS is associated with a wide range of conditions marked by altered dopaminergic activity. In healthy individuals, MDDS is associated with dopamine synthesis capacity. Lower MDDS is associated with certain traits or states with facilitated dopaminergic function, such as motivation, lower fatigue, and specific personality types (e.g., extroversion, novelty seeking, and harm avoidance), as well as with genotypes that show greater sensitivity to dopamine. Lower MDDS is associated with greater creativity (as measured by divergent thinking) and psychometric intelligence in children, consistent with reported associations of these cognitive functions with dopaminergic function and motivation.
On the other hand, videogame habit and working memory training, both of which are associated with release of dopamine, are associated with elevated MDDS. Furthermore, both diseases associated with dopaminergic overdrive and diseases associated with degeneration of dopaminergic systems show elevated MDDS. Similarly, disorders associated with global deficits as well as diseases associated with neurodegeneration in basal ganglia, but not with dopaminergic functions, also exhibit elevated MDDS.
Results accrued to date suggest that MDDS is a valuable metric to evaluate dopaminergic function and dysfunction during induced plasticity and disease progression.
Consent for Publication
Not applicable.
Acknowledgements
This study was supported by JST/RISTEX, JST/CREST. The authors would like to thank Enago (www.enago.jp) for the English language review.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Pierpaoli C., Jezzard P., Basser P.J., Barnett A., Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201(3):637–648. doi: 10.1148/radiology.201.3.8939209. [http://dx.doi.org/10.1148/radiology.201.3. 8939209]. [PMID: 8939209]. [DOI] [PubMed] [Google Scholar]
- 2.Madden D.J., Whiting W.L., Huettel S.A., White L.E., MacFall J.R., Provenzale J.M. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21(3):1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [http://dx.doi.org/10.1016/j. neuroimage.2003.11.004]. [PMID: 15006684]. [DOI] [PubMed] [Google Scholar]
- 3.Werring D.J., Clark C.A., Barker G.J., Thompson A.J., Miller D.H. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52(8):1626–1632. doi: 10.1212/wnl.52.8.1626. [http://dx.doi.org/10.1212/WNL.52.8.1626]. [PMID: 10331689]. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi H., Taki Y., Sassa Y., Hashizume H., Sekiguchi A., Fukushima A., Kawashima R. Verbal working memory performance correlates with regional white matter structures in the frontoparietal regions. Neuropsychologia. 2011;49(12):3466–3473. doi: 10.1016/j.neuropsychologia.2011.08.022. [http://dx.doi.org/10.1016/j.neuropsychologia.2011.08.022]. [PMID: 21906608]. [DOI] [PubMed] [Google Scholar]
- 5.Sagi Y., Tavor I., Hofstetter S., Tzur-Moryosef S., Blumenfeld-Katzir T., Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73(6):1195–1203. doi: 10.1016/j.neuron.2012.01.025. [http://dx.doi.org/ 10.1016/j.neuron.2012.01.025]. [PMID: 22445346]. [DOI] [PubMed] [Google Scholar]
- 6.Takeuchi H., Taki Y., Sekiguchi A., Hashizume H., Nouchi R., Sassa Y., Kotozaki Y., Miyauchi C.M., Yokoyama R., Iizuka K., Nakagawa S., Nagase T., Kunitoki K., Kawashima R. Mean diffusivity of globus pallidus associated with verbal creativity measured by divergent thinking and creativity-related temperaments in young healthy adults. Hum. Brain Mapp. 2015;36(5):1808–1827. doi: 10.1002/hbm.22739. [http://dx.doi.org/10.1002/hbm.22739]. [PMID: 25627674]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley M., Bammer R., Illes J. Diffusion-tensor imaging of cognitive performance. Brain Cogn. 2002;50(3):396–413. doi: 10.1016/s0278-2626(02)00524-9. [http://dx.doi.org/10.1016/S0278-2626(02)00524-9]. [PMID: 12480486]. [DOI] [PubMed] [Google Scholar]
- 8.Assaf Y., Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J. Mol. Neurosci. 2008;34(1):51–61. doi: 10.1007/s12031-007-0029-0. [http://dx.doi.org/10.1007/s12031-007-0029-0]. [PMID: 18157658]. [DOI] [PubMed] [Google Scholar]
- 9.Ni J.M., Chen S., Liu J.J., Huang G., Shen T.Z., Chen X.R. Regional diffusion changes of cerebral grey matter during normal aging--a fluid-inversion prepared diffusion imaging study. Eur. J. Radiol. 2010;75(2):134–138. doi: 10.1016/j.ejrad.2009.04.028. [http://dx.doi.org/10.1016/j.ejrad. 2009.04.028]. [PMID: 19443158]. [DOI] [PubMed] [Google Scholar]
- 10.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7-8):435–455. doi: 10.1002/nbm.782. [http://dx.doi.org/10.1002/nbm.782]. [PMID: 12489094]. [DOI] [PubMed] [Google Scholar]
- 11.Piras F., Caltagirone C., Spalletta G. Working memory performance and thalamus microstructure in healthy subjects. Neuroscience. 2010;171(2):496–505. doi: 10.1016/j.neuroscience.2010.09.006. [http://dx.doi.org/10.1016/j.neuroscience. 2010.09.006]. [PMID: 20850507]. [DOI] [PubMed] [Google Scholar]
- 12.Laricchiuta D., Petrosini L., Piras F., Cutuli D., Macci E., Picerni E., Chiapponi C., Caltagirone C., Spalletta G. Linking novelty seeking and harm avoidance personality traits to basal ganglia: volumetry and mean diffusivity. Brain Struct. Funct. 2014;219(3):793–803. doi: 10.1007/s00429-013-0535-5. [PMID: 23494736]. [DOI] [PubMed] [Google Scholar]
- 13.Taki Y., Thyreau B., Hashizume H., Sassa Y., Takeuchi H., Wu K., Kotozaki Y., Nouchi R., Asano M., Asano K., Fukuda H., Kawashima R. Linear and curvilinear correlations of brain white matter volume, fractional anisotropy, and mean diffusivity with age using voxel-based and region-of-interest analyses in 246 healthy children. Hum. Brain Mapp. 2013;34(8):1842–1856. doi: 10.1002/hbm.22027. [http://dx.doi.org/10.1002/hbm.22027]. [PMID: 22438164]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeuchi H., Tomita H., Taki Y., Kikuchi Y., Ono C., Yu Z., Sekiguchi A., Nouchi R., Kotozaki Y., Nakagawa S., Miyauchi C.M., Iizuka K., Yokoyama R., Shinada T., Yamamoto Y., Hanawa S., Araki T., Hashizume H., Kunitoki K., Sassa Y., Kawashima R. Cognitive and neural correlates of the 5-repeat allele of the dopamine D4 receptor gene in a population lacking the 7-repeat allele. Neuroimage. 2015;110:124–135. doi: 10.1016/j.neuroimage.2015.01.053. [http://dx.doi.org/10.1016/ j.neuroimage.2015.01.053]. [PMID: 25659462]. [DOI] [PubMed] [Google Scholar]
- 15.Johansen-Berg H., Baptista C.S., Thomas A.G. Human structural plasticity at record speed. Neuron. 2012;73(6):1058–1060. doi: 10.1016/j.neuron.2012.03.001. [http:// dx.doi.org/10.1016/j.neuron.2012.03.001]. [PMID: 22445333]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebel C., Rasmussen C., Wyper K., Walker L., Andrew G., Yager J., Beaulieu C. Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol. Clin. Exp. Res. 2008;32(10):1732–1740. doi: 10.1111/j.1530-0277.2008.00750.x. [http://dx.doi.org/10.1111/j.1530-0277. 2008.00750.x]. [PMID: 18671811]. [DOI] [PubMed] [Google Scholar]
- 17.Takeuchi H., Taki Y., Thyreau B., Sassa Y., Hashizume H., Sekiguchi A., Nagase T., Nouchi R., Fukushima A., Kawashima R. White matter structures associated with empathizing and systemizing in young adults. Neuroimage. 2013;77(15):222–236. doi: 10.1016/j.neuroimage.2013.04.004. [http://dx.doi.org/10.1016/j.neuroimage.2013.04.004]. [PMID: 23578577]. [DOI] [PubMed] [Google Scholar]
- 18.Casanova R., Srikanth R., Baer A., Laurienti P.J., Burdette J.H., Hayasaka S., Flowers L., Wood F., Maldjian J.A. Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34(1):137–143. doi: 10.1016/j.neuroimage.2006.09.011. [http://dx.doi.org/ 10.1016/j.neuroimage.2006.09.011]. [PMID: 17070709]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawaguchi H., Obata T., Takano H., Nogami T., Suhara T., Ito H. Relation between dopamine synthesis capacity and cell-level structure in human striatum: a multi-modal study with positron emission tomography and diffusion tensor imaging. PLoS One. 2014;9(1):e87886. doi: 10.1371/journal.pone.0087886. [http://dx.doi.org/10.1371/journal.pone.0087886]. [PMID: 24498218]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scherfler C., Esterhammer R., Nocker M., Mahlknecht P., Stockner H., Warwitz B., Spielberger S., Pinter B., Donnemiller E., Decristoforo C., Virgolini I., Schocke M., Poewe W., Seppi K. Correlation of dopaminergic terminal dysfunction and microstructural abnormalities of the basal ganglia and the olfactory tract in Parkinson’s disease. Brain. 2013;136(Pt 10):3028–3037. doi: 10.1093/brain/awt234. [http://dx.doi.org/10.1093/brain/awt234]. [PMID: 24014521]. [DOI] [PubMed] [Google Scholar]
- 21.Baudrexel S., Seifried C., Penndorf B., Klein J.C., Middendorp M., Steinmetz H., Grünwald F., Hilker R. The value of putaminal diffusion imaging versus 18-fluorodeoxyglucose positron emission tomography for the differential diagnosis of the Parkinson variant of multiple system atrophy. Mov. Disord. 2014;29(3):380–387. doi: 10.1002/mds.25749. [http://dx.doi.org/10.1002/mds.25749]. [PMID: 24243813]. [DOI] [PubMed] [Google Scholar]
- 22.Greenstein B., Greenstein A. Color Atlas of Neuroscience: Neuroanatomy and Neurophysiology. New York: George Thieme Verlag; 2000. [Google Scholar]
- 23.Lindvall O., Björklund A. Dopaminergic innervation of the globus pallidus by collaterals from the nigrostriatal pathway. Brain Res. 1979;172(1):169–173. doi: 10.1016/0006-8993(79)90907-7. [http://dx.doi.org/10.1016/0006-8993 (79)90907-7]. [PMID: 466461]. [DOI] [PubMed] [Google Scholar]
- 24.Cromwell H.C., Schultz W. Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J. Neurophysiol. 2003;89(5):2823–2838. doi: 10.1152/jn.01014.2002. [http://dx.doi.org/10.1152/ jn.01014.2002]. [PMID: 12611937]. [DOI] [PubMed] [Google Scholar]
- 25.O’Doherty J.P., Deichmann R., Critchley H.D., Dolan R.J. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33(5):815–826. doi: 10.1016/s0896-6273(02)00603-7. [http://dx.doi.org/10.1016/S0896-6273(02) 00603-7]. [PMID: 11879657]. [DOI] [PubMed] [Google Scholar]
- 26.O’Doherty J.P., Dayan P., Friston K., Critchley H., Dolan R.J. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [http://dx.doi.org/10. 1016/S0896-6273(03)00169-7]. [PMID: 12718865]. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno K., Tanaka M., Ishii A., Tanabe H.C., Onoe H., Sadato N., Watanabe Y. The neural basis of academic achievement motivation. Neuroimage. 2008;42(1):369–378. doi: 10.1016/j.neuroimage.2008.04.253. [http://dx.doi.org/ 10.1016/j.neuroimage.2008.04.253]. [PMID: 18550387]. [DOI] [PubMed] [Google Scholar]
- 28.Zeki S., Romaya J.P. Neural correlates of hate. PLoS One. 2008;3(10):e3556. doi: 10.1371/journal.pone.0003556. [http://dx.doi.org/10.1371/journal.pone.0003556]. [PMID: 18958169]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips M.L., Young A.W., Senior C., Brammer M., Andrew C., Calder A.J., Bullmore E.T., Perrett D.I., Rowland D., Williams S.C., Gray J.A., David A.S. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389(6650):495–498. doi: 10.1038/39051. [http://dx.doi.org/10.1038/39051]. [PMID: 9333238]. [DOI] [PubMed] [Google Scholar]
- 30.Paulus M.P., Simmons A.N., Fitzpatrick S.N., Potterat E.G., Van Orden K.F., Bauman J., Swain J.L. Differential brain activation to angry faces by elite warfighters: neural processing evidence for enhanced threat detection. PLoS One. 2010;5(4):e10096. doi: 10.1371/journal.pone.0010096. [http:// dx.doi.org/10.1371/journal.pone.0010096]. [PMID: 20418943]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultheiss O.C., Wirth M.M., Waugh C.E., Stanton S.J., Meier E.A., Reuter-Lorenz P. Exploring the motivational brain: effects of implicit power motivation on brain activation in response to facial expressions of emotion. Soc. Cogn. Affect. Neurosci. 2008;3(4):333–343. doi: 10.1093/scan/nsn030. [http://dx.doi.org/10.1093/scan/nsn030]. [PMID: 19015083]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartels A., Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [http://dx.doi.org/ 10.1016/j.neuroimage.2003.11.003]. [PMID: 15006682]. [DOI] [PubMed] [Google Scholar]
- 33.Haruno M., Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J. Neurophysiol. 2006;95(2):948–959. doi: 10.1152/jn.00382.2005. [http://dx.doi.org/10.1152/ jn.00382.2005]. [PMID: 16192338]. [DOI] [PubMed] [Google Scholar]
- 34.Delgado M.R. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104(1):70–88. doi: 10.1196/annals.1390.002. [http://dx.doi.org/10. 1196/annals.1390.002]. [PMID: 17344522]. [DOI] [PubMed] [Google Scholar]
- 35.van Duijvenvoorde A.C., Zanolie K., Rombouts S.A., Raijmakers M.E., Crone E.A. Evaluating the negative or valuing the positive? Neural mechanisms supporting feedback-based learning across development. J. Neurosci. 2008;28(38):9495–9503. doi: 10.1523/JNEUROSCI.1485-08.2008. [http:// dx.doi.org/10.1523/JNEUROSCI.1485-08.2008]. [PMID: 18799681]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall H., Farde L., Halldin C., Hurd Y.L., Pauli S., Sedvall G. Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using [125I]epidepride. Synapse. 1996;23(2):115–123. doi: 10.1002/(SICI)1098-2396(199606)23:2<115::AID-SYN7>3.0.CO;2-C. [http://dx.doi.org/10.1002/(SICI)1098-2396(199606) 23:2<115:AID-SYN7>3.0.CO;2-C]. [PMID: 8723716]. [DOI] [PubMed] [Google Scholar]
- 37.Yasuno F., Suhara T., Okubo Y., Sudo Y., Inoue M., Ichimiya T., Takano A., Nakayama K., Halldin C., Farde L. Low dopamine d(2) receptor binding in subregions of the thalamus in schizophrenia. Am. J. Psychiatry. 2004;161(6):1016–1022. doi: 10.1176/appi.ajp.161.6.1016. [http://dx.doi. org/10.1176/appi.ajp.161.6.1016]. [PMID: 15169689]. [DOI] [PubMed] [Google Scholar]
- 38.Seamans J.K., Gorelova N., Durstewitz D., Yang C.R. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J. Neurosci. 2001;21(10):3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [PMID: 11331392]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeuchi H., Taki Y., Sekiguchi A., Nouchi R., Kotozaki Y., Nakagawa S., Miyauchi C.M., Iizuka K., Yokoyama R., Shinada T. Mean diffusivity of basal ganglia and thalamus specifically associated with motivational states among mood states. Brain Struct. Funct. 2017;222(2):1027–1037. doi: 10.1007/s00429-016-1262-5. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama R., Nozawa T., Takeuchi H., Taki Y., Sekiguchi A., Nouchi R., Kotozaki Y., Nakagawa S., Miyauchi C.M., Iizuka K., Shinada T., Yamamoto Y., Hanawa S., Araki T., Hashizume H., Kunitoki K., Uehara M., Sassa Y., Kawashima R. 2014. [Google Scholar]
- 41.Yoshimura K. Reliability and validity of the Japanese version of the NEO Five-Factor Inventory (NEO-FFI): A population-based survey in Aomori prefecture. Jap. J. Stress Sci. 1998;13:45–53. [Google Scholar]
- 42.Ashby F.G., Isen A.M., Turken A.U. A neuropsychological theory of positive affect and its influence on cognition. Psychol. Rev. 1999;106(3):529–550. doi: 10.1037/0033-295x.106.3.529. [http://dx.doi.org/10.1037/0033-295X.106. 3.529]. [PMID: 10467897]. [DOI] [PubMed] [Google Scholar]
- 43.Depue R.A., Collins P.F. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav. Brain Sci. 1999;22(3):491–517. doi: 10.1017/s0140525x99002046. [http://dx.doi.org/10. 1017/S0140525X99002046]. [PMID: 11301519]. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa S., Takeuchi H., Taki Y., Nouchi R., Kotozaki Y., Shinada T., Maruyama T., Sekiguchi A., Iizuka K., Yokoyama R., Yamamoto Y., Hanawa S., Araki T., Miyauchi C.M., Magistro D., Sakaki K., Jeong H., Sasaki Y., Kawashima R. Basal ganglia correlates of fatigue in young adults. Sci. Rep. 2016;6:21386. doi: 10.1038/srep21386. [http://dx.doi.org/10.1038/srep21386]. [PMID: 26893077]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aratake Y., Tanaka K., Wada K., Watanabe M., Katoh N., Sakata Y., Aizawa Y. Development of Japanese version of the checklist individual strength questionnaire in a working population. J. Occup. Health. 2007;49(6):453–460. doi: 10.1539/joh.49.453. [http://dx.doi.org/10.1539/ joh.49.453]. [PMID: 18075205]. [DOI] [PubMed] [Google Scholar]
- 46.Lorist M.M., Bezdan E., ten Caat M., Span M.M., Roerdink J.B., Maurits N.M. The influence of mental fatigue and motivation on neural network dynamics; an EEG coherence study. Brain Res. 2009;1270:95–106. doi: 10.1016/j.brainres.2009.03.015. [http://dx.doi.org/10.1016/j.brainres.2009. 03.015]. [PMID: 19306850]. [DOI] [PubMed] [Google Scholar]
- 47.Yokoyama K., Araki S., Kawakami N., Tkakeshita T. 1990. [PubMed] [Google Scholar]
- 48.Wise R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [http://dx.doi.org/10.1038/nrn1406]. [PMID: 15152198]. [DOI] [PubMed] [Google Scholar]
- 49.Flaherty A.W. Frontotemporal and dopaminergic control of idea generation and creative drive. J. Comp. Neurol. 2005;493(1):147–153. doi: 10.1002/cne.20768. [http://dx.doi.org/10.1002/cne.20768]. [PMID: 16254989]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heilman K.M., Nadeau S.E., Beversdorf D.O. Creative innovation: possible brain mechanisms. Neurocase. 2003;9(5):369–379. doi: 10.1076/neur.9.5.369.16553. [http://dx.doi.org/10.1076/neur.9.5.369.16553]. [PMID: 14972752]. [DOI] [PubMed] [Google Scholar]
- 51.Chavez-Eakle R.A., del Carmen Lara M., Cruz-Fuentes C. Personality: A possible bridge between creativity and psychopathology? Creat. Res. J. 2006;18(1):27–38. [http://dx.doi.org/10. 1207/s15326934crj1801_4]. [Google Scholar]
- 52.King L.A., Walker L.M., Broyles S.J. Creativity and the five-factor model. J. Res. Pers. 1996;30(2):189–203. [http://dx.doi.org/ 10.1006/jrpe.1996.0013]. [Google Scholar]
- 53.Suhara T., Yasuno F., Sudo Y., Yamamoto M., Inoue M., Okubo Y., Suzuki K. Dopamine D2 receptors in the insular cortex and the personality trait of novelty seeking. Neuroimage. 2001;13(5):891–895. doi: 10.1006/nimg.2001.0761. [http://dx.doi.org/10.1006/nimg.2001.0761]. [PMID: 11304084]. [DOI] [PubMed] [Google Scholar]
- 54.Kaasinen V., Aalto S., Någren K., Rinne J.O. Insular dopamine D2 receptors and novelty seeking personality in Parkinson’s disease. Mov. Disord. 2004;19(11):1348–1351. doi: 10.1002/mds.20191. [http://dx.doi.org/10. 1002/mds.20191]. [PMID: 15389994]. [DOI] [PubMed] [Google Scholar]
- 55.Bódi N., Kéri S., Nagy H., Moustafa A., Myers C.E., Daw N., Dibó G., Takáts A., Bereczki D., Gluck M.A. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain. 2009;132(Pt 9):2385–2395. doi: 10.1093/brain/awp094. [http://dx.doi. org/10.1093/brain/awp094]. [PMID: 19416950]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tomer R., Aharon-Peretz J. Novelty seeking and harm avoidance in Parkinson’s disease: effects of asymmetric dopamine deficiency. J. Neurol. Neurosurg. Psychiatry. 2004;75(7):972–975. doi: 10.1136/jnnp.2003.024885. [http://dx. doi.org/10.1136/jnnp.2003.024885]. [PMID: 15201352]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schinka J.A., Letsch E.A., Crawford F.C. DRD4 and novelty seeking: results of meta-analyses. Am. J. Med. Genet. 2002;114(6):643–648. doi: 10.1002/ajmg.10649. [http://dx.doi.org/10.1002/ajmg.10649]. [PMID: 12210280]. [DOI] [PubMed] [Google Scholar]
- 58.Prabhu V., Sutton C., Sauser W. Creativity and certain personality traits: Understanding the mediating effect of intrinsic motivation. Creat. Res. J. 2008;20(1):53–66. [http://dx.doi.org/10.1080/ 10400410701841955]. [Google Scholar]
- 59.Kaplan F., Oudeyer P-Y. In search of the neural circuits of intrinsic motivation. Front. Neurosci. 2007;1(1):225–236. doi: 10.3389/neuro.01.1.1.017.2007. [http://dx. doi.org/10.3389/neuro.01.1.1.017.2007]. [PMID: 18982131]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drago V., Foster P.S., Skidmore F.M., Heilman K.M. Creativity in Parkinson’s disease as a function of right versus left hemibody onset. J. Neurol. Sci. 2009;276(1-2):179–183. doi: 10.1016/j.jns.2008.09.026. [http://dx.doi.org/ 10.1016/j.jns.2008.09.026]. [PMID: 18952243]. [DOI] [PubMed] [Google Scholar]
- 61.Kulisevsky J., Pagonabarraga J., Martinez-Corral M. Changes in artistic style and behaviour in Parkinson’s disease: dopamine and creativity. J. Neurol. 2009;256(5):816–819. doi: 10.1007/s00415-009-5001-1. [http://dx.doi.org/10. 1007/s00415-009-5001-1]. [PMID: 19240966]. [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi H., Taki Y., Hashizume H., Asano K., Asano M., Sassa Y., Yokota S., Kotozaki Y., Nouchi R., Kawashima R. Impact of videogame play on the brain’s microstructural properties: cross-sectional and longitudinal analyses. Mol. Psychiatry. 2016;21(12):1781–1789. doi: 10.1038/mp.2015.193. [http://dx.doi.org/10.1038/mp.2015.193]. [PMID: 26728566]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duckworth A.L., Quinn P.D., Lynam D.R., Loeber R., Stouthamer-Loeber M. Role of test motivation in intelligence testing. Proc. Natl. Acad. Sci. USA. 2011;108(19):7716–7720. doi: 10.1073/pnas.1018601108. [http:// dx.doi.org/10.1073/pnas.1018601108]. [PMID: 21518867]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baddeley A. Working memory: looking back and looking forward. Nat. Rev. Neurosci. 2003;4(10):829–839. doi: 10.1038/nrn1201. [http://dx.doi.org/10. 1038/nrn1201]. [PMID: 14523382]. [DOI] [PubMed] [Google Scholar]
- 65.Aalto S., Brück A., Laine M., Någren K., Rinne J.O. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J. Neurosci. 2005;25(10):2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [http:// dx.doi.org/10.1523/JNEUROSCI.2097-04.2005]. [PMID: 15758155]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bäckman L., Nyberg L., Soveri A., Johansson J., Andersson M., Dahlin E., Neely A.S., Virta J., Laine M., Rinne J.O. Effects of working-memory training on striatal dopamine release. Science. 2011;333(6043):718–718. doi: 10.1126/science.1204978. [http://dx.doi.org/10.1126/science. 1204978]. [PMID: 21817043]. [DOI] [PubMed] [Google Scholar]
- 67.Takeuchi H., Taki Y., Nouchi R., Hashizume H., Sekiguchi A., Kotozaki Y., Nakagawa S., Miyauchi C.M., Sassa Y., Kawashima R. Working memory training impacts the mean diffusivity in the dopaminergic system. Brain Struct. Funct. 2015;220(6):3101–3111. doi: 10.1007/s00429-014-0845-2. [http://dx.doi.org/10.1007/s00429-014-0845-2]. [PMID: 25023736]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeuchi H., Taki Y., Nouchi R., Hashizume H., Sekiguchi A., Kotozaki Y., Nakagawa S., Miyauchi C.M., Sassa Y., Kawashima R. Effects of working memory training on functional connectivity and cerebral blood flow during rest. Cortex. 2013;49(8):2106–2125. doi: 10.1016/j.cortex.2012.09.007. [http://dx.doi.org/10.1016/j.cortex.2012.09.007]. [PMID: 23079491]. [DOI] [PubMed] [Google Scholar]
- 69.Koepp M.J., Gunn R.N., Lawrence A.D., Cunningham V.J., Dagher A., Jones T., Brooks D.J., Bench C.J., Grasby P.M. Evidence for striatal dopamine release during a video game. Nature. 1998;393(6682):266–268. doi: 10.1038/30498. [http://dx.doi.org/10.1038/30498]. [PMID: 9607763]. [DOI] [PubMed] [Google Scholar]
- 70.Weinstein A.M. Computer and video game addiction-a comparison between game users and non-game users. Am. J. Drug Alcohol Abuse. 2010;36(5):268–276. doi: 10.3109/00952990.2010.491879. [http://dx.doi.org/10.3109/00952990. 2010.491879]. [PMID: 20545602]. [DOI] [PubMed] [Google Scholar]
- 71.Barlett C.P., Anderson C.A., Swing E.L. Video game effects—confirmed, suspected, and speculative: A review of the evidence. Simul. Gaming. 2008;40(3):377–403. [http://dx.doi.org/10.1177/ 1046878108327539]. [Google Scholar]
- 72.Anand V. A study of time management: the correlation between video game usage and academic performance markers. Cyberpsychol. Behav. 2007;10(4):552–559. doi: 10.1089/cpb.2007.9991. [http://dx.doi.org/10.1089/cpb. 2007.9991]. [PMID: 17711364]. [DOI] [PubMed] [Google Scholar]
- 73.Dworak M., Schierl T., Bruns T., Strüder H.K. Impact of singular excessive computer game and television exposure on sleep patterns and memory performance of school-aged children. Pediatrics. 2007;120(5):978–985. doi: 10.1542/peds.2007-0476. [http://dx.doi.org/10.1542/peds.2007-0476]. [PMID: 17974734]. [DOI] [PubMed] [Google Scholar]
- 74.Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L., Kim J.S., Heo S., Alves H., White S.M., Wojcicki T.R., Mailey E., Vieira V.J., Martin S.A., Pence B.D., Woods J.A., McAuley E., Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [http://dx.doi.org/ 10.1073/pnas.1015950108]. [PMID: 21282661]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeuchi H., Taki Y., Nouchi R., Sekiguchi A., Kotozaki Y., Miyauchi C.M., Yokoyama R., Iizuka K., Hashizume H., Nakagawa S., Kunitoki K., Sassa Y., Kawashima R. Regional gray matter density is associated with achievement motivation: evidence from voxel-based morphometry. Brain Struct. Funct. 2014;219(1):71–83. doi: 10.1007/s00429-012-0485-3. [http://dx.doi.org/10.1007/s00429-012-0485-3]. [PMID: 23212300]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng N., Maeda T., Kume T., Kaneko S., Kochiyama H., Akaike A., Goshima Y., Misu Y. Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons. Brain Res. 1996;743(1-2):278–283. doi: 10.1016/s0006-8993(96)01056-6. [http://dx.doi.org/10.1016/S0006-8993(96)01056-6]. [PMID: 9017256]. [DOI] [PubMed] [Google Scholar]
- 77.Li D., Sham P.C., Owen M.J., He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum. Mol. Genet. 2006;15(14):2276–2284. doi: 10.1093/hmg/ddl152. [http://dx.doi.org/10.1093/hmg/ddl152]. [PMID: 16774975]. [DOI] [PubMed] [Google Scholar]
- 78.Asghari V., Sanyal S., Buchwaldt S., Paterson A., Jovanovic V., Van Tol H.H. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J. Neurochem. 1995;65(3):1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [http://dx.doi.org/10.1046/j.1471-4159. 1995.65031157.x]. [PMID: 7643093]. [DOI] [PubMed] [Google Scholar]
- 79.Höglinger G.U., Rizk P., Muriel M.P., Duyckaerts C., Oertel W.H., Caille I., Hirsch E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004;7(7):726–735. doi: 10.1038/nn1265. [http://dx.doi.org/10.1038/nn1265]. [PMID: 15195095]. [DOI] [PubMed] [Google Scholar]
- 80.Péran P., Cherubini A., Assogna F., Piras F., Quattrocchi C., Peppe A., Celsis P., Rascol O., Démonet J-F., Stefani A., Pierantozzi M., Pontieri F.E., Caltagirone C., Spalletta G., Sabatini U. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010;133(11):3423–3433. doi: 10.1093/brain/awq212. [http://dx. doi.org/10.1093/brain/awq212]. [PMID: 20736190]. [DOI] [PubMed] [Google Scholar]
- 81.Razek A.A., Elmongy A., Hazem M., Zakareyia S., Gabr W. Idiopathic Parkinson disease effect of levodopa on apparent diffusion coefficient value of the brain. Acad. Radiol. 2011;18(1):70–73. doi: 10.1016/j.acra.2010.08.023. [PMID: 21145029]. [DOI] [PubMed] [Google Scholar]
- 82.Wenning G.K., Colosimo C., Geser F., Poewe W. Multiple system atrophy. Lancet Neurol. 2004;3(2):93–103. doi: 10.1016/s1474-4422(03)00662-8. [http://dx. doi.org/10.1016/S1474-4422(03)00662-8]. [PMID: 14747001]. [DOI] [PubMed] [Google Scholar]
- 83.Ozawa T., Paviour D., Quinn N.P., Josephs K.A., Sangha H., Kilford L., Healy D.G., Wood N.W., Lees A.J., Holton J.L., Revesz T. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127(Pt 12):2657–2671. doi: 10.1093/brain/awh303. [http://dx.doi.org/10.1093/brain/awh303]. [PMID: 15509623]. [DOI] [PubMed] [Google Scholar]
- 84.Wenning G.K., Tison F., Ben S.Y., Daniel S.E., Quinn N.P. Multiple system atrophy: a review of 203 pathologically proven cases. Mov. Disord. 1997;12(2):133–147. doi: 10.1002/mds.870120203. [http://dx.doi.org/10. 1002/mds.870120203]. [PMID: 9087971]. [DOI] [PubMed] [Google Scholar]
- 85.Seppi K., Schocke M.F., Donnemiller E., Esterhammer R., Kremser C., Scherfler C., Diem A., Jaschke W., Wenning G.K., Poewe W. Comparison of diffusion-weighted imaging and [123I] IBZM-SPECT for the differentiation of patients with the Parkinson variant of multiple system atrophy from those with Parkinson’s disease. Mov. Disord. 2004;19(12):1438–1445. doi: 10.1002/mds.20229. [http://dx.doi.org/ 10.1002/mds.20229]. [PMID: 15390073]. [DOI] [PubMed] [Google Scholar]
- 86.Howes O.D., Kambeitz J., Kim E., Stahl D., Slifstein M., Abi-Dargham A., Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169. [http://dx.doi.org/10.1001/ archgenpsychiatry.2012.169]. [PMID: 22474070]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spoletini I., Cherubini A., Banfi G., Rubino I.A., Peran P., Caltagirone C., Spalletta G. Hippocampi, thalami, and accumbens microstructural damage in schizophrenia: a volumetry, diffusivity, and neuropsychological study. Schizophr. Bull. 2011;37(1):118–130. doi: 10.1093/schbul/sbp058. [http://dx.doi.org/10.1093/schbul/sbp058]. [PMID: 19542526]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rose S.E., Chalk J.B., Janke A.L., Strudwick M.W., Windus L.C., Hannah D.E., McGrath J.J., Pantelis C., Wood S.J., Mowry B.J. Evidence of altered prefrontal-thalamic circuitry in schizophrenia: an optimized diffusion MRI study. Neuroimage. 2006;32(1):16–22. doi: 10.1016/j.neuroimage.2006.03.003. [http://dx.doi.org/10.1016/j.neuroimage.2006. 03.003]. [PMID: 16626974]. [DOI] [PubMed] [Google Scholar]
- 89.Leckman J.F. Tourette’s syndrome. Lancet. 2002;360(9345):1577–1586. doi: 10.1016/S0140-6736(02)11526-1. [http://dx.doi.org/10.1016/S0140-6736(02)11526-1]. [PMID: 12443611]. [DOI] [PubMed] [Google Scholar]
- 90.Makki M.I., Behen M., Bhatt A., Wilson B., Chugani H.T. Microstructural abnormalities of striatum and thalamus in children with Tourette syndrome. Mov. Disord. 2008;23(16):2349–2356. doi: 10.1002/mds.22264. [http://dx.doi.org/10.1002/mds.22264]. [PMID: 18759338]. [DOI] [PubMed] [Google Scholar]
- 91.Singer H.S., Szymanski S., Giuliano J., Yokoi F., Dogan A.S., Brasic J.R., Zhou Y., Grace A.A., Wong D.F. Elevated intrasynaptic dopamine release in Tourette’s syndrome measured by PET. Am. J. Psychiatry. 2002;159(8):1329–1336. doi: 10.1176/appi.ajp.159.8.1329. [http://dx.doi.org/10. 1176/appi.ajp.159.8.1329]. [PMID: 12153825]. [DOI] [PubMed] [Google Scholar]
- 92.Riddle E.L., Fleckenstein A.E., Hanson G.R. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8(2):E413–E418. doi: 10.1007/BF02854914. [http://dx.doi.org/10.1007/BF02854914]. [PMID: 16808044]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alicata D., Chang L., Cloak C., Abe K., Ernst T. Higher diffusion in striatum and lower fractional anisotropy in white matter of methamphetamine users. Psychiatry Res. 2009;174(1):1–8. doi: 10.1016/j.pscychresns.2009.03.011. [http:// dx.doi.org/10.1016/j.pscychresns.2009.03.011]. [PMID: 19782540]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin J.C., Jan R.K., Kydd R.R., Russell B.R. Investigating the microstructural and neurochemical environment within the basal ganglia of current methamphetamine abusers. Drug Alcohol Depend. 2015;149:122–127. doi: 10.1016/j.drugalcdep.2015.01.026. [http://dx.doi.org/10.1016/j.drugalcdep. 2015.01.026]. [PMID: 25700612]. [DOI] [PubMed] [Google Scholar]
- 95.Reynolds G.P., Garrett N.J. Striatal dopamine and homovanillic acid in Huntington’s disease. J. Neural Transm. (Vienna) 1986;65(2):151–155. doi: 10.1007/BF01256491. [http://dx.doi.org/10.1007/BF01256491]. [PMID: 2939198]. [DOI] [PubMed] [Google Scholar]
- 96.Sánchez-Castañeda C., Cherubini A., Elifani F., Péran P., Orobello S., Capelli G., Sabatini U., Squitieri F. Seeking Huntington disease biomarkers by multimodal, cross-sectional basal ganglia imaging. Hum. Brain Mapp. 2013;34(7):1625–1635. doi: 10.1002/hbm.22019. [http://dx.doi.org/10.1002/hbm.22019]. [PMID: 22359398]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Douaud G., Behrens T.E., Poupon C., Cointepas Y., Jbabdi S., Gaura V., Golestani N., Krystkowiak P., Verny C., Damier P., Bachoud-Lévi A.C., Hantraye P., Remy P. In vivo evidence for the selective subcortical degeneration in Huntington’s disease. Neuroimage. 2009;46(4):958–966. doi: 10.1016/j.neuroimage.2009.03.044. [http://dx.doi.org/10.1016/j. neuroimage.2009.03.044]. [PMID: 19332141]. [DOI] [PubMed] [Google Scholar]
- 98.Gjedde A., Kumakura Y., Cumming P., Linnet J., Møller A. Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc. Natl. Acad. Sci. USA. 2010;107(8):3870–3875. doi: 10.1073/pnas.0912319107. [http://dx.doi.org/10.1073/pnas. 0912319107]. [PMID: 20133675]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blum K., Oscar-Berman M., Demetrovics Z., Barh D., Gold M.S. Genetic addiction risk score (GARS): molecular neurogenetic evidence for predisposition to reward deficiency syndrome (RDS). Mol. Neurobiol. 2014;50(3):765–796. doi: 10.1007/s12035-014-8726-5. [http://dx.doi.org/10.1007/ s12035-014-8726-5]. [PMID: 24878765]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Febo M., Blum K., Badgaiyan R.D., Baron D., Thanos P.K., Colon-Perez L.M., Demortrovics Z., Gold M.S. Dopamine homeostasis: brain functional connectivity in reward deficiency syndrome. Front. Biosci. 2017;22:669–691. doi: 10.2741/4509. [http://dx.doi.org/10.2741/4509]. [PMID: 27814639]. [DOI] [PubMed] [Google Scholar]
- 101.Hu Y., Salmeron B.J., Gu H., Stein E.A., Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72(6):584–592. doi: 10.1001/jamapsychiatry.2015.1. [http:// dx.doi.org/10.1001/jamapsychiatry.2015.1]. [PMID: 25853901]. [DOI] [PubMed] [Google Scholar]
- 102.Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8(11):1481–1489. doi: 10.1038/nn1579. [http://dx.doi.org/10.1038/nn1579]. [PMID: 16251991]. [DOI] [PubMed] [Google Scholar]
- 103.Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [http://dx.doi.org/10.1038/npp.2009.129]. [PMID: 19812543]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yin H.H., Knowlton B.J. The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [http://dx. doi.org/10.1038/nrn1919]. [PMID: 16715055]. [DOI] [PubMed] [Google Scholar]
- 105.McCann J.C., Ames B.N. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am. J. Clin. Nutr. 2007;85(4):931–945. doi: 10.1093/ajcn/85.4.931. [PMID: 17413089]. [DOI] [PubMed] [Google Scholar]
- 106.Sener R.N. Echo-planar and gradient-echo diffusion MRI of normal brain iron in the globus pallidus. Clin. Imaging. 2002;26(6):371–374. doi: 10.1016/s0899-7071(02)00487-4. [http://dx.doi.org/10.1016/S0899-7071(02)00487-4]. [PMID: 12427429]. [DOI] [PubMed] [Google Scholar]
- 107.Pfefferbaum A., Adalsteinsson E., Rohlfing T., Sullivan E.V. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol. Aging. 2010;31(3):482–493. doi: 10.1016/j.neurobiolaging.2008.04.013. [http://dx.doi.org/10.1016/j.neurobiolaging.2008.04.013]. [PMID: 18513834]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ortega R., Cloetens P., Devès G., Carmona A., Bohic S. Iron storage within dopamine neurovesicles revealed by chemical nano-imaging. PLoS One. 2007;2(9):e925. doi: 10.1371/journal.pone.0000925. [http://dx.doi.org/10.1371/ journal.pone.0000925]. [PMID: 17895967]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pfeiffer C.C., Mailloux R. Excess copper as a factor in human diseases. J. Orthomol. Med. 1987;2(3):171–182. [Google Scholar]
- 110.Cochrane C.J., Ebmeier K.P. Diffusion tensor imaging in parkinsonian syndromes: a systematic review and meta-analysis. Neurology. 2013;80(9):857–864. doi: 10.1212/WNL.0b013e318284070c. [http://dx.doi.org/10.1212/WNL.0b013 e318284070c]. [PMID: 23439701]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J-J., Lin W-Y., Lu C-S., Weng Y-H., Ng S-H., Wang C-H., Liu H-L., Hsieh R-H., Wan Y-L., Wai Y-Y. Parkinson disease: diagnostic utility of diffusion kurtosis imaging. Radiology. 2011;261(1):210–217. doi: 10.1148/radiol.11102277. [http://dx.doi.org/10.1148/radiol.11102277]. [PMID: 21771952]. [DOI] [PubMed] [Google Scholar]
- 112.Jensen J.H., Helpern J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23(7):698–710. doi: 10.1002/nbm.1518. [http://dx.doi.org/10.1002/nbm.1518]. [PMID: 20632416]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ofori E., Pasternak O., Planetta P.J., Burciu R., Snyder A., Febo M., Golde T.E., Okun M.S., Vaillancourt D.E. Increased free water in the substantia nigra of Parkinson’s disease: a single-site and multi-site study. Neurobiol. Aging. 2015;36(2):1097–1104. doi: 10.1016/j.neurobiolaging.2014.10.029. [http://dx.doi.org/10.1016/j.neurobiolaging.2014.10.029]. [PMID: 25467638]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [http://dx.doi.org/10.1016/j.neuroimage.2008.03.061]. [PMID: 18501637]. [DOI] [PubMed] [Google Scholar]