Abstract
Background: Acute leukemia is a common pediatric cancer. Novel strategies for treatment of acute leukemia have been developed, but treatment resistance is remained as the most problematic issue. It is hypothesized that the HO-1 gene up-regulation is responsible for tumor resistance to chemotherapy or radiotherapy-induced apoptosis. The levels of HO-1 expression are related to (GT)n microsatellite polymorphisms in the location of its promoter. This study designed to compare allelic frequencies of (GT)n microsatellite polymorphisms in HO-1 gene between acute leukemia patients and healthy controls. Indeed, 3-year disease-free survival was also evaluated.
Methods: Sixty-three patients with acute leukemia and seventy healthy infants were included in this study. We used the medical records of patients to collect information about survival after chemotherapy. The number of GT repeats in HO-1 promoter was determined by an ABI 3100 sequencer.
Results: The HO-1 GT repeats ranged from 14 to 34 with peaks at 27 repeats in both cases and controls. Children with longer alleles ((GT)n ≥ 27) had enhanced 3-year survival rate after treatment with chemotherapy or radiotherapy (P<0.05).
Conclusion: Although no significant differences were observed between leukemia patients and controls regarding allelic frequency, we found elevated frequency of “LL” genotype in leukemia patients with good prognosis and 3-year surveillance. Radiotherapy and chemotherapy might elevate the expression levels of HO-1 with subsequent increased resistance of leukemia patients to therapy.
Key Words: Acute leukemia, Survival, GT repeats, Heme-oxygenase-1 gene promoter
Introduction
Acute Leukemia is the most common pediatric cancer 1,2 . Despite numerous efforts focused on leukemia treatment, this type of disease remains one of the greatest challenges in pediatric oncology 3 . Current therapeutic strategies are unable to provide long-term remission for 20% of children with ALL (25-40% cure rate after relapse) 4 . Some subgroups of infants are likely to be over treated and might be healed using less intensive regimens which results in reduced toxicity and fewer long-term side effects 5-7 . Response to treatments is variable depending on different clinical, immunological, cytogenetic/genetic characteristics and environmental exposures, which are associated with increased risk of leukemia and their diverse response to treatment 8-12.
One of the mentioned genetic factors is Heme Oxygenase-1 gene (HO-1), located on chromosome 22. This gene produces an essential enzyme in heme catabolism. It has 2 isozymes, an inducible heme oxygenase-1 and a constitutive heme oxygenase-2 13 . HMOX1 and HMOX2 belong to the heme oxygenase family. Heme Oxygenase (HO) gene encodes an enzyme which catalyzes the first and rate-limiting steps in oxidative degradation of heme to the form of open-chain tetrapyrrole biliverdin-IX with final release of carbon dioxide, biliverdin and free iron . By now, three isozymes of mammalian HO have been identified, in which HO-1 variant is the only inducible isozyme with expression in different normal and neoplastic cells. HO-1 expression in malignant tissues is higher than its surrounding healthy tissues which could be up regulated by different stimuli and oxidative stresses such as radiotherapy, chemotherapy and photodynamic therapies 14-20 .
HO-1 is involved in pathogenesis of acute leukemia patients and their resistance to treatment 8-11 . Expression of HO-1 has the protective and anti-apoptotic effects through its active biological products. 21,22 . As a result, it's possible that the upregulation of HO-1 is responsible for increasing tumor resistance to chemotherapy or radiotherapy. 23, 24 . However, HO-1 expression level is dependent to its polymorphisms and fluctuates between individuals, quantitatively 25, 26 .
GT repeats in HO-1 gene promoter modulate transcription of HO-1 gene. The dinucleotide sequence can adversely affect the basal promoter activity 27-30 . GT repeats ranged between 12 - 40 base pairs. This range can be divided into short “S” and long “L” alleles although there is not an exact cut-off to separate two alleles16,31,32. Moreover, some surveys revealed higher prevalence of "S" allele in renal cell carcinoma, pancreatic cancer, gastric cancer and gastric adenocarcinoma 33 . Paradoxically, patients with squamous cell carcinoma and lung adenocarcinoma often carry “L” allele16,31,34-37. Some studies revealed that HO-1 targeting with either pegylated zinc protoporphyrin (PEG-ZnPP) or styrene maleicacid-micelle–encapsulated ZnPP (SMAZnPP) results in growth inhibition in cancer cells with subsequent increased sensitivity to radiotherapy and chemotherapy 22,38-40 .
Despite advent of novel strategies for treatment of acute leukemia, resistance to treatment is the greatest concern until now. HO-1 over expression provides cytoprotective and antiapoptotic effects on malignant cells against radiotherapy and chemotherapy. Although the role of HO-1 in AML and CML cell lines has been studied, the distribution of allele frequency in leukemia and its association with resistance to treatment and survival remains unveiled 10 . This study was designed to compare the allelic frequencies of genetic variants in the promoter of HO-1 among patients suffering from acute leukemia compared with healthy controls. The relationship between GT repeats and patient’s 3-year and 5-year survival was also assessed.
MATERIALS AND METHODS
Patient selection
This is a controlled, cross-sectional study that included 63 patients with acute leukemia admitted to Seyed-al-Shohada Hospital, Isfahan from 2006 to 2009. The control group consisted of 54 gender and age-matched healthy children. Our data about survival after chemotherapy and bone graft were gathered from patients’ medical records. DNA was extracted from bone marrow samples of the patients. Genomic DNA was extracted through QIAamp DNA blood mini kit (QIAGEN, USA) according to the manufacturer’s instruction. The study protocol was approved by the Ethics Board of Isfahan University of Medical Sciences
Heme oxygenase-1 genotyping
The HO-1 microsatellite was amplified through PCR using a 5'-FAM-labeled forward primer (AGAGCCTGCAGCTTCTCAGA) and a 3'-unlabelled reverse primer (GTCCTATGGCCAGACTTTGT), in 30 cycles (94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds) and final extension at 72°C for 5 minutes by thermo cycler (Eppendorf Mastercycler EP Gradient, USA). Labeled PCR products were compared with a standard size marker GenoTypeTM TAMRA DNA ladder (size range 50–500 bp) (Gibco-BRL, Paisley, Scotland, UK). Number of GT repeats was determined with an ABI 3100 sequencer (Applied Biosystems, USA), using GeneMarker V1.97 software (Softgenetics, USA). Selected samples were sequenced by ABI 3100 sequencer (Applied Biosystems, USA).
Statistical analysis
Statistical analyses were performed using SPSS 16.0. Chi-square test was used for categorical variables. Three-year survival was compared with allelic frequencies of HO-1 polymorphism. Differences in alleli c frequency of HO-1 promoter were compared between leukemia patients and controls. Difference of data with P< 0.05 was considered as a significant.
Results
Demographic characteristics of patients including age and sex are shown in Table 1. Frequency distribution of age and sex was not significantly different between studied groups. Patients whose data were incomplete were excluded from the study.
Table1.
Demographic characteristics of patients (age and sex)
| Group | Case | control | P-value | ||
|---|---|---|---|---|---|
| Age (years) Mean ±SD | 5.93 ±0.63 | 4.87±0.26 | >0.05 | ||
| Gender (%) | F | 59.5 | 46.2 | >0.05 | |
| M | 40.5 | 53.8 | |||
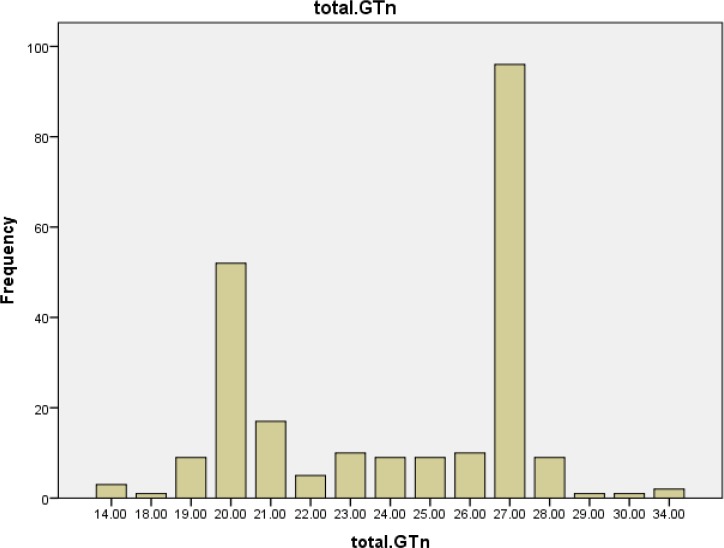
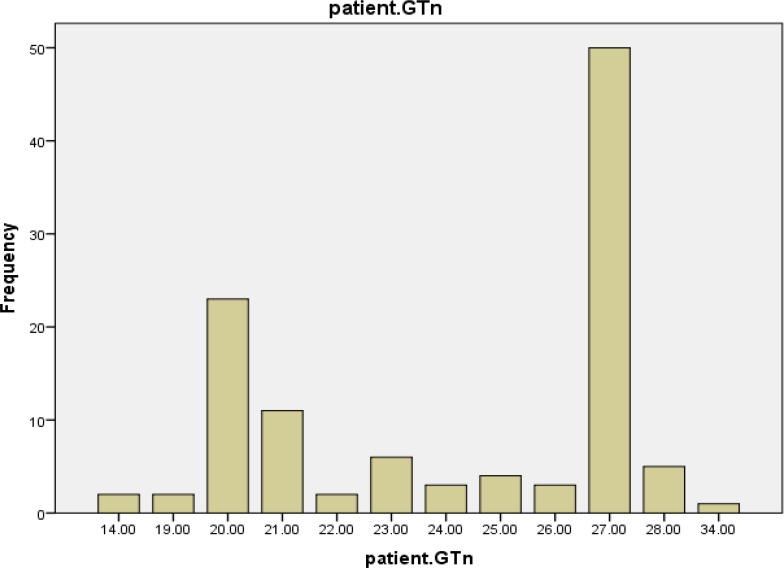
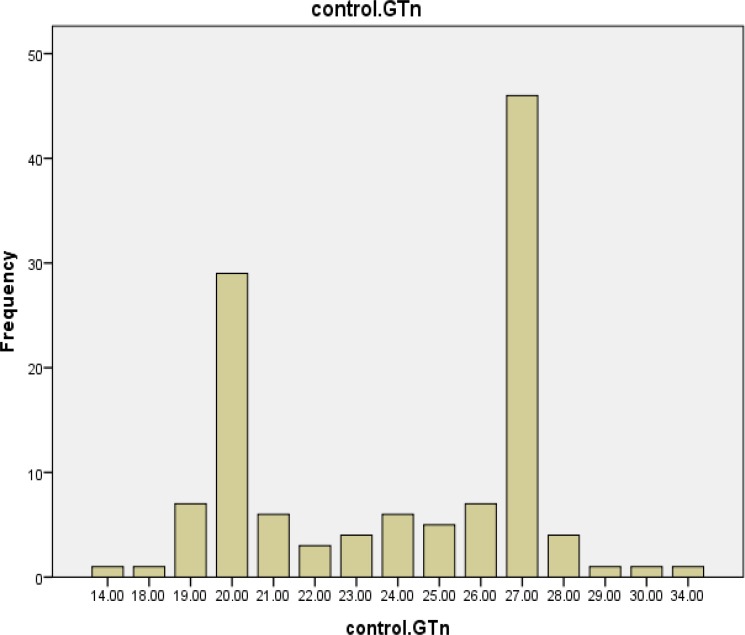
The HO-1 genotyping process for 63 cases and 54 healthy controls was performed successfully. The alleles frequency of (GT)n microsatellite polymorphism of HO -1 gene promoter was found in both case and control groups (Figures 1, 2 and 3). The GT repeat numbers ranged from 14 to 34, and the most prevalent allele in our population was (GT)27. Therefore, we divided these alleles into two subgroups. (GT)n : short (S) alleles <27 repeats and long (L) alleles ≥27 repeats. Three genotypes (SS, SL and LL) were created for the study population. The subjects with genotype SS and SL were placed in a group identified as "short carrier genotype" and those with LL genotype were placed in a group identified as "long genotype".
Figure 1.
Frequency distributions of (GT) repeats in all participants (n=117)
Figure 2.
Frequency distributions of (GT) repeats in leukemia patient (n=56)
Figure 3.
Frequency distributions of (GT) repeats in control group (n=61)
There was no significant difference in the frequency of genotypes between leukemia patients and controls (P= 0.629) (Table 2). The "long genotype" was more frequent in leukemia patients with better vs. worse 3-year surveillance (P< 0.05)(Table 3). Furthermore, there was no significant difference in hepatomegaly, splenomegaly and CSF metastasis in two genotype groups (P≥0.05) (Table 4).
Table 2.
Genotype* distribution in case and control groups
| Genotypes | P-value | |||
|---|---|---|---|---|
| LL | LS | SS | 0.629 | |
| Case | 33.3% | 47.6% | 19% | |
| Control | 33.3% | 40.7% | 25.9% | |
(GT)n < 27 Repeats: S allele, (GT)n ≥ 27 Repeats: L allele, SS: Homozygote for Short Allele, LL: Homozygote for Long Allele, LS: Heterozygote Person
Table 3.
Genotype* distribution for leukemia patients according to 3-and 5-year survival, respectively
| Genotype | P-value | |||
|---|---|---|---|---|
| S carrier | LL | |||
| 3-year survival rate | ||||
| Yes | 38.8% | 30.5% | 0.043 | |
| No | 27.7% | 2.7% | ||
| 5-year survival rate | ||||
| Yes | 39.3% | 21.2% | 0.371 | |
| No | 30.3% | 9.0% |
(GT)n < 27 Repeats: S allele, (GT)n ≥ 27 Repeats: L allele, SS: Homozygote for Short Allele, LL: Homozygote for Long Allele, LS: Heterozygote Person
Table 4.
Genotype* distribution of leukemic regarding hepatomegaly, splenomegaly and CSF metastasis
| Genotypes | P-value | |||
|---|---|---|---|---|
| S carrier | LL | |||
| Hepatomegaly | (+) (-) |
21.0% 50.0% |
15.85 13.1% |
0.142 |
| Splenomegaly | (+) (-) |
40.5% 29.7% |
16.2% 13.5% |
0.571 |
| CSF Metastasis | (+) (-) |
2.9% 67.6% |
0.0% 29.4% |
0.706 |
S carrier: a person who is carrier for S allele, LL: Homozygote for L allele, CSF Metastasis: Metastasis to Cerebrospinal fluids (CSF)
Discussion
In this study, we investigated polymorphic (GT)n repeats in HO-1 promoter region in patients suffering from leukemia. In addition, we investigated the association between allelic frequency and patients’ 3-year surveillance. We found no relationship between frequency distribution of (GT)n repeats and leukemia incidence. We have observed that patients carrying “LL” allele had significantly better 3-year survival. Five-year survival was slightly better in carriers of “LL” allele although this difference was not statistically significant. Moreover, we studied genotype distribution among patients considering hepatomegaly, splenomegaly and CSF metastasis (Table 4), which revealed no relationship between (GT)n repeats in HO-1 promoter and clinical features of patients.
HO-1 gene is induced in response to oxidative stress such as chemotherapy and radiotherapy41. The basal and induced levels of HO-1 gene expression are different in each individual. The polymorphism length of HO-1, influences the level of gene transcription. Excessive GT repeats reduced promoter activity, while short GT alleles led to higher levels of HO-1 expression with further anti-apoptotic effects. Our results suggest that “SS” and “SL” genotypes make tumors more resistant to anticancer therapy in acute leukemia.
HO-1 considered as a “friend” which supports normal tissues against carcinogen-induced invasion 42,43 Nevertheless, during the neoplastic development, HO-1 turns into a “false friend” and facilitate tumor development. HO-1 over expression in CML tumor cells increases its viability through apoptosis inhibition.
Meyerhof et al. have treated CML cells by zinc-(II)-deuteroporphyrin-IX, inhibitor of HO-1 and hemin, inducer of an HO-1. Parallel usage of HO-1 inhibitors and inducers, decreases and increases CML cells viability in stress oxidative environment, respectively. Also, it has been apparent that HO-1 reduces sensitivity of cancer cells to chemotherapy and radiotherapy22,44.
The length polymorphism of GT repeats in HO-1 gene promoter had various impacts in different disease states. Chang et al. reported an increased risk of oral squamous cell carcinoma in patients with more than 31 GT-repeats 45 . Moreover, some surveys revealed a higher frequency of "S" allele in renal cell carcinoma, pancreatic cancer, gastric cancer, gastric adenocarcinoma, lymphoma and Kaposi sarcoma. In this study, the frequency of “S” alleles and “L” alleles were equal in both leukemia patients and control groups.
This study is the first to investigate HO-1 gene promoter polymorphism and surveillance (3 years) in leukemia patients. Although we found no significant differences between leukemia patients and control groups regarding short (GT) n repeat alleles in HO-1 promoter, higher frequency of “LL” genotype in leukemia patients with positive 3-year surveillance was found. Indeed, LL genotype in leukemia patients was associated with slightly better 5-year surveillance.
Oxidative stress conditions such as radiotherapy and chemotherapy lead to additional expression of HO-1 gene 41 . These data supports the idea that higher levels of HO-1 gene expression, associated with “SS” and “SL” genotype, might play an important role in elevated resistance of leukemia patients to chemotherapy or radiotherapy.
CONCLUSION
Modification of HO-1 expression level might have positive effect on the prognosis of leukemia patients under treatment. Further studies are required to determine the impact of HO-1 genotype in patients with acute leukemia.
Acknowledgment
The authors appreciate the patients and their families for participation in this study. This work was supported by a grant from the Isfahan University of Medical Sciences.
CONFLICT OF INTEREST
Authors declare any conflict of interest.
References
- 1.Lo Nigro L. Biology of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35(4):245–52. doi: 10.1097/MPH.0b013e31828f8746. [DOI] [PubMed] [Google Scholar]
- 2.Arora RS, Arora B. Acute leukemia in children: A review of the current Indian data. South Asian J Cancer. 2016;5(3):155–60. doi: 10.4103/2278-330X.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunger SP, Raetz EA, Loh ML, et al. Improving outcomes for high‐risk ALL: Translating new discoveries into clinical care. Pediatr Blood Cancer. 2011;56(6):984–93. doi: 10.1002/pbc.22996. [DOI] [PubMed] [Google Scholar]
- 4.Conter V, Arico M, Basso G, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) Studies 82, 87, 88, 91 and 95 for childhood acute lymphoblastic leukemia. Leukemia. 2010;24(2):255–64. doi: 10.1038/leu.2009.250. [DOI] [PubMed] [Google Scholar]
- 5.Hunger SP, Winick NJ, Sather HN, et al. Therapy of low-risk subsets of childhood acute lymphoblastic leukemia: when do we say enough? Pediatr Blood Cancer. 2005;45(7):876–80. doi: 10.1002/pbc.20501. [DOI] [PubMed] [Google Scholar]
- 6.Arico M, Conter V, Valsecchi MG, et al. Treatment reduction in highly selected standard-risk childhood acute lymphoblastic leukemia. The AIEOP ALL-9501 study. Haematologica. 2005;90(9):1186–91. [PubMed] [Google Scholar]
- 7.Van Dongen-Melman JE, De Groot A, Van Dongen JJ, et al. Cranial irradiation is the major cause of learning problems in children treated for leukemia and lymphoma: a comparative study. Leukemia. 1997;11(8):1197–200. doi: 10.1038/sj.leu.2400702. [DOI] [PubMed] [Google Scholar]
- 8.Dorantes-Acosta E, Pelayo R. Lineage Switching in Acute Leukemias: A Consequence of Stem Cell Plasticity? Bone Marrow Res. 2012;2012:406796. doi: 10.1155/2012/406796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belson M, Kingsley B, Holmes A. Risk factors for acute leukemia in children: a review. Environ Health Perspect. 2007;115(1):138–145. doi: 10.1289/ehp.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heasman SA, Zaitseva L, Bowles KM, et al. Protection of acute myeloid leukaemia cells from apoptosis induced by front-line chemotherapeutics is mediated by haem oxygenase-1. Oncotarget. 2011;2(9):658–68. doi: 10.18632/oncotarget.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 12.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–43. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 13.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86(2):583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 14.Kiemer AK, Bildner N, Weber NC, et al. Characterization of heme oxygenase 1 (heat shock protein 32) induction by atrial natriuretic peptide in human endothelial cells. Endocrinology. 2003;144(3):802–12. doi: 10.1210/en.2002-220610. [DOI] [PubMed] [Google Scholar]
- 15.Immenschuh S, Ramadori G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem Pharmacol. 2000;60(8):1121–8. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- 16.Exner M, Minar E, Wagner O, et al. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37(8):1097–104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Salinas M, Diaz R, Abraham NG, et al. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278(16):13898–904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 18.Martin D, Rojo AI, Salinas M, et al. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J Biol Chem. 2004;279(10):8919–29. doi: 10.1074/jbc.M309660200. [DOI] [PubMed] [Google Scholar]
- 19.Malaguarnera L, Imbesi RM, Scuto A, et al. Prolactin increases HO-1 expression and induces VEGF production in human macrophages. J Cell Biochem. 2004;93(1):197–206. doi: 10.1002/jcb.20167. [DOI] [PubMed] [Google Scholar]
- 20.Lam CW, Getting SJ, Perretti M. In vitro and in vivo induction of heme oxygenase 1 in mouse macrophages following melanocortin receptor activation. J Immunol. 2005;174(4):2297–304. doi: 10.4049/jimmunol.174.4.2297. [DOI] [PubMed] [Google Scholar]
- 21.Fang J, Akaike T, Maeda H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004;9(1):27–35. doi: 10.1023/B:APPT.0000012119.83734.4e. [DOI] [PubMed] [Google Scholar]
- 22.Mayerhofer M, Florian S, Krauth MT, et al. Identification of heme oxygenase-1 as a novel BCR/ABL-dependent survival factor in chronic myeloid leukemia. Cancer Res. 2004;64(9):3148–54. doi: 10.1158/0008-5472.can-03-1200. [DOI] [PubMed] [Google Scholar]
- 23.Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox signal. 2007;9(12):2099–117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yajima T, Ochiai H, Uchiyama T, et al. Resistance to cytotoxic chemotherapy-induced apoptosis in side population cells of human oral squamous cell carcinoma cell line Ho-1-N-1. Int J Oncol. 2009;35(2):273–80. [PubMed] [Google Scholar]
- 25.Tang D, Tang W-J, Shi X-L, et al. Association of the microsatellite (GT) n repeat polymorphisms of the HO-1 gene promoter and corresponding serum levels with the risk of laryngeal squamous cell carcinoma. Acta Oto-Laryngologica. 2016;136(8):806–11. doi: 10.3109/00016489.2016.1157265. [DOI] [PubMed] [Google Scholar]
- 26.Køllgaard T, Kornblit B, Petersen J, et al. (GT)n Repeat Polymorphism in Heme Oxygenase-1 (HO-1) Correlates with Clinical Outcome after Myeloablative or Nonmyeloablative Allogeneic Hematopoietic Cell Transplantation. PloS one. 2016;11(12):e0168210. doi: 10.1371/journal.pone.0168210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rich A, Nordheim A, Wang AH. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- 28.Naylor LH, Clark EM. d(TG)n.d(CA)n sequences upstream of the rat prolactin gene form Z-DNA and inhibit gene transcription. Nucleic Acids Res. 1990;18(6):1595–601. doi: 10.1093/nar/18.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delic J, Onclercq R, Moisan-Coppey M. Inhibition and enhancement of eukaryotic gene expression by potential non-B DNA sequences. Biochem Biophys Res Commun. 1991;181(2):818–26. doi: 10.1016/0006-291x(91)91263-c. [DOI] [PubMed] [Google Scholar]
- 30.Waring GO 3rd, Laibson PR. Keratoplasty in infants and children. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(2):283–96. [PubMed] [Google Scholar]
- 31.Sawa T, Mounawar M, Tatemichi M, et al. Increased risk of gastric cancer in Japanese subjects is associated with microsatellite polymorphisms in the heme oxygenase-1 and the inducible nitric oxide synthase gene promoters. Cancer Lett. 2008;269(1):78–84. doi: 10.1016/j.canlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 32.Javanmard SH, Keyhanian K, Loghmani P, et al. Association between heme oxygenase-1 gene promoter polymorphisms and metabolic syndrome in Iranians. Mol Biol Rep. 2012;39(3):3355–60. doi: 10.1007/s11033-011-1105-7. [DOI] [PubMed] [Google Scholar]
- 33.Lo SS, Lin SC, Wu CW, et al. Heme oxygenase-1 gene promoter polymorphism is associated with risk of gastric adenocarcinoma and lymphovascular tumor invasion. Ann Surg Oncol. 2007;14(8):2250–6. doi: 10.1245/s10434-006-9290-7. [DOI] [PubMed] [Google Scholar]
- 34.Berberat PO, Dambrauskas Z, Gulbinas A, et al. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res. 2005;11(10):3790–8. doi: 10.1158/1078-0432.CCR-04-2159. [DOI] [PubMed] [Google Scholar]
- 35.Marinissen MJ, Tanos T, Bolós M, et al. Inhibition of heme oxygenase-1 interferes with the transforming activity of the Kaposi sarcoma herpesvirus-encoded G protein-coupled receptor. J Biol Chem. 2006;281(16):11332–46. doi: 10.1074/jbc.M512199200. [DOI] [PubMed] [Google Scholar]
- 36.Sunamura M, Duda DG, Ghattas MH, et al. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis. 2003;6(1):15–24. doi: 10.1023/a:1025803600840. [DOI] [PubMed] [Google Scholar]
- 37.Yanagawa T, Omura K, Harada H, et al. Heme oxygenase-1 expression predicts cervical lymph node metastasis of tongue squamous cell carcinomas. Oral Oncol. 2004;40(1):21–7. doi: 10.1016/s1368-8375(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 38.Mayerhofer M, Gleixner KV, Mayerhofer J, et al. Targeting of heat shock protein 32 (Hsp32)/heme oxygenase-1 (HO-1) in leukemic cells in chronic myeloid leukemia: a novel approach to overcome resistance against imatinib. Blood. 2008;111(4):2200–10. doi: 10.1182/blood-2006-11-055723. [DOI] [PubMed] [Google Scholar]
- 39.Fang J, Sawa T, Akaike T, et al. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer. 2004;109(1):1–8. doi: 10.1002/ijc.11644. [DOI] [PubMed] [Google Scholar]
- 40.Nowis D, Legat M, Grzela T, et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene. 2006;25(24):3365–74. doi: 10.1038/sj.onc.1209378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Was H, Sokolowska M, Sierpniowska A, et al. Effects of heme oxygenase-1 on induction and development of chemically induced squamous cell carcinoma in mice. Free Radic Biol Med. 2011;51(9):1717–26. doi: 10.1016/j.freeradbiomed.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrando M, Wan X, Meiss R, et al. Heme oxygenase-1 (HO-1) expression in prostate cancer cells modulates the oxidative response in bone cells. PloS one. 2013;8(11):e80315. doi: 10.1371/journal.pone.0080315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Labanca E, De Luca P, Gueron G, et al. Association of HO-1 and BRCA1 is critical for the maintenance of cellular homeostasis in prostate cancer. Mol Cancer Res. 2015;13(11):1455–64. doi: 10.1158/1541-7786.MCR-15-0150-T. [DOI] [PubMed] [Google Scholar]
- 44.Traverso N, Ricciarelli R, Nitti M, et al. Role of glutathione in cancer progression and chemoresistance. Oxid Med Cell Longev. 2013;2013:972913. doi: 10.1155/2013/972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang KW, Lee TC, Yeh WI, et al. Polymorphism in heme oxygenase-1 (HO-1) promoter is related to the risk of oral squamous cell carcinoma occurring on male areca chewers. Br J Cancer. 2004;91(8):1551–5. doi: 10.1038/sj.bjc.6602186. [DOI] [PMC free article] [PubMed] [Google Scholar]