Abstract
Malignant lymphomas are about 5% of all malignant tumors. Extranodal non-Hodgkin's lymphomas (NHLs) are found in 26% of these cases. Lymphomas of the head-and-neck area occur in 2%–3% of all malignancies, with 28% with an extranodal manifestation. Extranodal NHLs in the oral cavity are usually found in the maxilla, and rarely in the mandible. Their symptoms and clinical manifestation have no pathognomonic features; therefore, the expression of this uncommon entity can be diagnosed with an odontogenic inflammatory process, leading to a misdiagnosis. Delay in the decision for a biopsy, and adequate treatment for the patient directly impairs the prognosis of this neoplasm. This study reports a case of a patient with discomfort in the right mandible and paresthesia of the right lower lip and chin without any dental focus. After performing further diagnostic examinations including a subsequent biopsy, the final diagnosis was a diffuse large B-cell lymphoma (DLBCL). Intraosseous DLBCLs are uncommon in the daily clinical routine, but emphasize the need for careful examination by the clinicians also considering the differential diagnosis of sensory neuropathy. Neurological symptoms with no apparent cause should raise the suspicion of malignancy until the opposite is proven.
Keywords: Mandible, non-Hodgkin's lymphoma, oral cancer, oral cavity, periapical lesion, swelling
INTRODUCTION
Non-Hodgkin's lymphomas (NHLs) are a heterogeneous group of cancer that arises from lymphoreticular histogenesis and differentiation. The progeny of the affected cell usually carries the phenotype of a B, T, or natural killer cell, as determined by immunophenotyping and/or gene rearrangement studies.[1] According to the type of lymphoid cell and its behavior, over 20 different subtypes of NHL have been classified. For the definition of each subtype, analysis of the morphological, immunophenotypic, and cytogenetic characteristics is essential.[2]
These malignancies may develop in lymph nodes or other organs, either by spread from a lymphatic site or as a manifestation of primary extranodal disease.[3] NHL may occur in extranodal sites at least at 40%–45%. These cases are commonly found in the gastrointestinal tract, followed by the head and neck, predominantly in the lymphoid tissue of Waldeyer's ring (nasopharynx, palatine tonsils, base of the tongue, and oropharynx), the oral mucosa, salivary glands, paranasal sinuses, and less often in the osseous structures, with the ones arising from the mandible being rarer at about 0.6%[4] than those arising from the maxillary bone.[5] Analyzing all NHL subtypes, the most common phenotype developing in extranodal sites is the diffuse large B-cell lymphoma (DLBCL).[6]
As described, an extranodal manifestation of an NHL as a primary intraosseous lesion in the mandible is an uncommon entity, but it is still the second most common malignant tumor in the head-and-neck area.[1] Clinical symptoms can be similar to an odontogenic process.[7] In rare cases, a numb chin syndrome can be the only symptom, which should always be considered as a sign of malignancy.[8] Radiological signs are expansion of the bone, enlargement of the mandibular canal and mental foramen, and alveolar bone loss. In advanced stages, bone destruction can occur, appearing as a solitary defect or resorption of the alveolar bone margin, which resembles periodontitis or a periodontal abscess.[9,10] In addition, signs such as swelling, pain, paresthesia, tooth mobility, or pathologic fracture can be strikingly diffuse. Therefore, an NHL can mimic other pathologic lesions, presenting clinical behavior in early stages and radiological signs of periodontal disease, pericoronitis, apical radiolucency, or dental abscesses.
The purpose of this report is to describe a rare case of a DLBCL arising as a primary intraosseous lesion in the mandible without clinical signs except pain and paresthesia. There was no clinical evidence of a dental inflammatory entity causing the symptoms in the oral cavity as described. Furthermore, the importance of an exhaustive clinical assessment is outlined, which enables an accurate diagnose and thus early treatment, improving the prognosis of this malignant neoplasm.
CASE REPORT
A 40-year-old female patient was referred to the Department of Oral and Maxillofacial Surgery for a clinical evaluation. She had a history of consulting her general dental practitioner 1 month before due to numbness and discomforting sensation related to the right side of the chin and mandible. The general medical condition of the patient did not contribute to defining a possible cause of the indicated symptoms. There was no history of preexisting health conditions or other symptoms, like B symptoms (fever > 38°C, night sweat, and weight loss).
Intraorally, slightly enhanced probing depths could be found around tooth 42. The other soft tissues showed no apparent abnormalities. No features of periapical or other odontogenic disease were visible in the panoramic radiography [Figure 1]. Consequently, a periodontal treatment was indicated in this situation. The patient reported that, in the following weeks, the feeling of pressure and paresthesia in the region of the right mandible and chin had increased.
Figure 1.
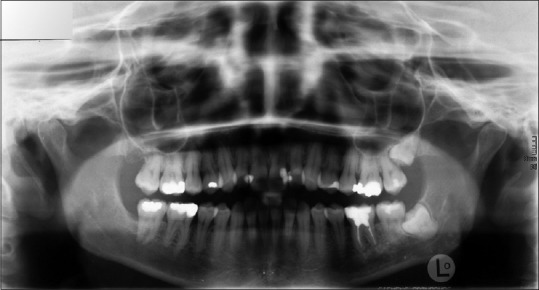
Panoramic X-ray: Diffuse limited borders in the region of the right mandible with bone sclerosis and narrowing of the mandibular canal
A computed tomography (CT) scan [Figure 2] and magnetic resonance imaging (MRI) [Figure 3] were performed as complementary procedures, which revealed a suspicious intraosseous lesion of the right mandibular angle area next to the course of the inferior alveolar nerve and its bony limitation. The differential diagnosis based on the clinical and radiographic findings was either an intraosseous schwannoma or a primary lymphoma of the bone. A biopsy of the region was taken under the local anesthesia. Immunohistochemical and histological examination showed fragments of connective and adipose tissue, which were infiltrated by a dense lymphoid infiltrate. The cells were pleomorphic, with small lymphoid cells, dense chromatin with variably prominent nucleoli, and abundant mitotic figures throughout the lesion. Various immunostaining cells showed positivity for CD20 and CD79a. Isolated cells could be marked with CD138. There was no association with Epstein–Barr virus (EBV) and no reaction to AE1/3, PanCK, HMB45, S100, and CD56. In the chromogenic in situ hybridization and fluorescence in situ hybridization assays, no breakpoints for the Bcl2, Bcl6, and Myc genes were found. Based on the histopathology and immunohistochemical findings, the lesion was diagnosed with high-grade B-cell lymphoma with a high rate of proliferation.
Figure 2.
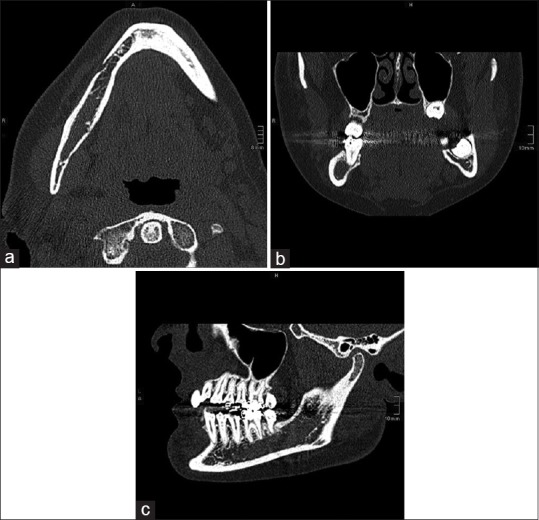
(a-c) Computed tomography: Axial, coronal, and sagittal view: Diffuse limited borders with slight erosion of the right cortical mandible and reduction of trabecular bone microstructure. The mandibular canal was breached
Figure 3.
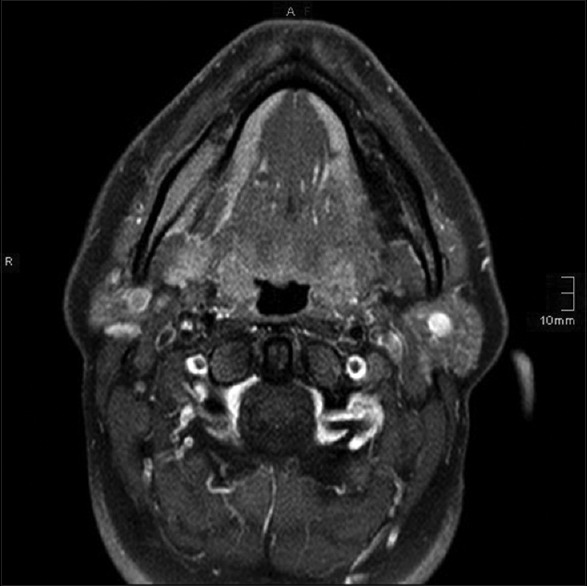
Magnetic resonance imaging: Axial view, T1: Enlarged, contrast-enhancing mass of the right mandible
Therapy was continued at the Department of Oncology. Subsequently, positron emission tomography (PET) and CT scans were performed covering the head and neck, chest, abdomen, and pelvis, to detect further lesions in the body [Figure 4]. The PET scan showed activity only in the right mandible. In this particular case, the manifestation of the malignant B-cell lymphoma was extranodal and affected the mandible. The patient was in stage IVa according to the Ann Arbor classification.
Figure 4.
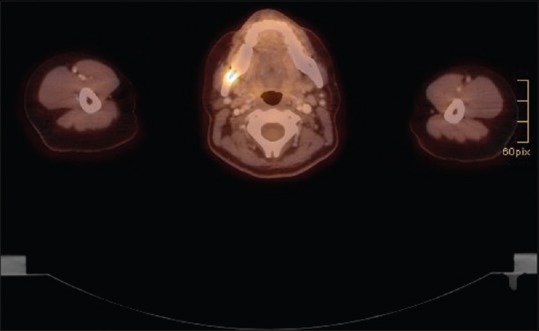
Positron emission tomography/computed tomography: Axial view: Uptake of 18F-fluorodesoxyglucose of the right mandible
DISCUSSION
In the group of intraoral malignancies, 3.5% are lymphomas.[11,12] Malignant lymphomas are divided into Hodgkin's lymphomas and NHLs and are the most common nonepithelial tumors of the oral cavity.[13,14,15,16] The disease manifests at a median age of 42 years, with increasing incidence with advancing age.[17] Within the group of NHLs, 30%–40% are DLBCLs.[15] With a frequency of 30%, the described DLBCL is the most common entity.[18] There is limited knowledge about the causes for the development of NHLs. Immunodeficiency is discussed as a significant risk factor, and an NHL can be triggered by an immune response to EBV.[19,20] Environmental toxins as an explanation for the growing incidence are discussed as well.[16] In 28% of the NHLs of the head-and-neck area, the lymphomas are located extranodal, and in the case of DLBCL, the mandible bone is commonly affected.[21,22] In most cases, teeth are directly associated with the tumor. Symptoms such as tooth mobility, patient discomfort, and bone pain are regularly seen. In our case, there was only the bone pain, without affection or clinical difficulties of the periodontium. For classification, the Ann Arbor staging system was used.[13] Nearly, 40% of the patients can be classified as Stage I (single lymph node) or in Stage II (2 lymph nodes on the same side of the diaphragm), and 40% show extranodal manifestation.
As described in the literature, radiolucency of extranodal NHL similar to that in dental abscesses is obvious.[1] The extent of radiolucency and bone involvement corresponds to the time point of diagnosis. A delayed treatment leads to cortical bone perforation and bone expansion, shown as ill-defined irregular margins within the radiographic examination.[14] In the three-dimensional images generated using CT and MRI, the boarders are typically faintly defined due to the invasive growth of the tumor. The cancellous bone is completely radiolucent, associated with some single parts of radiopacity together with the reactive bone formation, resulting in a “moth-eaten” appearance.[6] Sometimes soft-tissue mass with the lamellar periosteal new bone formation is also present.[6] In our case, the panoramic radiograph showed no apical radiolucency in the right mandible. The following CT imaging shows the described characteristics but without lamellar bone formation. The mandible was not widened, and no erosion of the cortical bone could be seen. In the MRI scan, the right mandible shows a diffuse hyperintensity in the T1-weighted image. Proof of the diagnosis could be provided by taking biopsies. The following treatment used multi-agent chemotherapy together with an anti-CD20 antibody (rituximab) as the therapy of choice.[23]
As described in this case report, the awareness of a possible differential diagnosis of diffuse bone pain can help to avoid treatment delay. The slight clinical symptoms together with clinical and radiological findings may not be clear enough to provide the dentist with specific indications. In most of the cases, periapical lesions as radiological findings are the basis for the first attempt to treat the pain by root channel or periodontal treatment. Persisting pain, discomfort, and local destruction of bone structures without odontogenic infection should be seen as a red flag to rethink the diagnosis of a dental abscess or periapical granuloma.[6] The clinician should be aware of the possibility of jaw tumors and should not delay the decision in favor of further radiographic and histological examination to avoid tumor progression.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 3rd ed. St Louis, MO: Saunders Elsevier; 2009. [Google Scholar]
- 2.Silva TD, Ferreira CB, Leite GB, de Menezes Pontes JR, Antunes HS. Oral manifestations of lymphoma: A systematic review. Ecancermedicalscience. 2016;10:665. doi: 10.3332/ecancer.2016.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kipps TJ. Chronic lymphocytic leukemia and related diseases. Williams Hematol. 2001;6:1163–94. [Google Scholar]
- 4.Longo F, De Maria G, Esposito P, Califano L. Primary non-Hodgkin's lymphoma of the mandible. Report of a case. Int J Oral Maxillofac Surg. 2004;33:801–3. doi: 10.1016/j.ijom.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Kemp S, Gallagher G, Kabani S, Noonan V, O’Hara C. Oral non-Hodgkin's lymphoma: Review of the literature and World Health Organization classification with reference to 40 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:194–201. doi: 10.1016/j.tripleo.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Regezi JA, Sciubba JJ, Jordan RC. USA: Health Sciences; 2016. Oral Pathology: Clinical Pathologic Correlations. [Google Scholar]
- 7.Vega F, Lin P, Medeiros LJ. Extranodal lymphomas of the head and neck. Ann Diagn Pathol. 2005;9:340–50. doi: 10.1016/j.anndiagpath.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 8.Fan Y, Luka R, Noronha A. Non-Hodgkin lymphoma presenting with numb chin syndrome. BMJ Case Rep 2011. 2011 doi: 10.1136/bcr.01.2011.3712. pii: bcr0120113712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robledo J, Roach B. Diffuse large B-cell lymphoma of the oral cavity. Tex Dent J. 2010;127:317–322-3. [PubMed] [Google Scholar]
- 10.Kini R, Saha A, Naik V. Diffuse large B-cell lymphoma of mandible: A case report. Med Oral Patol Oral Cir Bucal. 2009;14:e421–4. [PubMed] [Google Scholar]
- 11.Epstein JB, Epstein JD, Le ND, Gorsky M. Characteristics of oral and paraoral malignant lymphoma: A population-based review of 361 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:519–25. doi: 10.1067/moe.2001.116062. [DOI] [PubMed] [Google Scholar]
- 12.Manjunatha BS, Gowramma R, Nagarajappa D, Tanveer A. Extranodal non-Hodgkin's lymphoma presenting as gingival mass. J Indian Soc Periodontol. 2011;15:418–20. doi: 10.4103/0972-124X.92584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaikh AB, Waghmare S, Koshti-Khude S, Koshy AV. Unusual presentation of non-Hodgkin's lymphoma: Case report and review of literature. J Oral Maxillofac Pathol. 2016;20:510–7. doi: 10.4103/0973-029X.190956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bugshan A, Kassolis J, Basile J. Primary diffuse large B-cell lymphoma of the mandible: Case report and review of the literature. Case Rep Oncol. 2015;8:451–5. doi: 10.1159/000441469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szumera-Ciećkiewicz A, Gałązka K, Szpor J, Rymkiewicz G, Jesionek-Kupnicka D, Gruchała A, et al. Distribution of lymphomas in poland according to World Health Organization classification: Analysis of 11718 cases from national histopathological lymphoma register project – The polish lymphoma research group study. Int J Clin Exp Pathol. 2014;7:3280–6. [PMC free article] [PubMed] [Google Scholar]
- 16.Heuberger BM, Weiler D, Bussmann C, Kuttenberger JJ. Non-Hodgkin lymphoma of the mandible – A case report with differential diagnostic considerations. Schweiz Monatsschr Zahnmed. 2011;121:449–60. [PubMed] [Google Scholar]
- 17.Czuczman MS, Grillo-López AJ, White CA, Saleh M, Gordon L, LoBuglio AF, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–76. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- 18.Rüdiger T, Müller-Hermelink HK. Die WHO-Klassifikation maligner Lymphome. Radiologe. 2002;42:936–42. doi: 10.1007/s00117-002-0832-0. [DOI] [PubMed] [Google Scholar]
- 19.Myriam BD, Sonia Z, Hanene S, Teheni L, Mounir T. Prognostic significance of Epstein-Barr virus (EBV) infection in Hodgkin lymphoma patients. J Infect Chemother. 2017;23:121–30. doi: 10.1016/j.jiac.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Zhou XG, Zhang YL, Xie JL, Huang YH, Zheng YY, Li WS, et al. The understanding of Epstein-Barr virus associated lymphoproliferative disorder. Zhonghua Bing Li Xue Za Zhi. 2016;45:817–21. doi: 10.3760/cma.j.issn.0529-5807.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Etemad-Moghadam S, Tirgary F, Keshavarz S, Alaeddini M. Head and neck non-Hodgkin's lymphoma: A 20-year demographic study of 381 cases. Int J Oral Maxillofac Surg. 2010;39:869–72. doi: 10.1016/j.ijom.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–60. doi: 10.1002/1097-0142(197201)29:1<252::aid-cncr2820290138>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Barnes JA, Lacasce AS, Feng Y, Toomey CE, Neuberg D, Michaelson JS, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for burkitt's lymphoma: A retrospective analysis. Ann Oncol. 2011;22:1859–64. doi: 10.1093/annonc/mdq677. [DOI] [PubMed] [Google Scholar]


