Abstract
Background:
Skull base is difficult to approach surgically due to its complex anatomy. A number of procedures that is endoscopic, microscopic, and open approaches have been used. The maxillary swing approach provides a wide exposure to the surgeon for better oncological clearance.
Patients and Methods:
A total of 62 patients with varied etiologies involving the skull base region were operated with maxillary swing procedure over a period of 15 years from 2001 to 2016 in plastic surgery department at a single institution.
Results:
There was no recurrence in the follow-up period. One patient had palatal fistula and one patient had mild nasal mucosal atrophy. None of the patients had malocclusion in the postoperative period. The minimum follow-up period was 24 months.
Conclusion:
Maxillary swing procedure provides excellent exposure to skull base, and most of the tumors involving this region can be effectively excised with minimal morbidity to the patient.
Keywords: Maxillary swing, skull base tumor, transfacial approach
BACKGROUND
Skull base is a region heralded by complex anatomy. The heterogeneity of tissues of the skull base makes it a bed for wide spectrum of benign and malignant lesions with variable prognoses.[1]
Anterior skull base is commonly involved with tumors due to its proximity to orbit, paranasal sinuses, and nasopharynx. Tumors arising from maxilla, nasopharynx, and ethmoid can easily involve the anterior skull base. In the middle skull base, tumors around the sphenoid are common. The main surgical methods of skull base access can be broadly described as pterional, frontolateral, transsphenoidal, and suboccipital lateral approaches. These surgical approaches mentioned here cover almost 95% of all skull base tumors.[2]
Combined craniofacial techniques for the resection of tumors of the anterior skull base was first described in 1963 by Ketcham et al.[3] As many of the skull base malignancies arise primarily from the nasal and paranasal regions, combined intracranial and extracranial access performed through transfacial incisions have gained popularity.[4]
Despite the development of lesser invasive endoscopic techniques,[5] traditional open methods continue to be the cornerstone strategy in the extirpation of anterior and anterolateral skull base malignancies.
This article describes our experience with the zygomatico-maxillary osteotomy, often referred to as the maxillary swing procedure in accessing lesions at various levels of the cranial base and the parapharyngeal regions.
PATIENTS AND METHODS
Between 2001 and 2016, 62 patients underwent resection of tumors located either in the cranial base or the parapharyngeal area through zygomatico-maxillary osteotomies, in the Department of Plastic Surgery, SMS Medical College and Hospital, Jaipur (Rajasthan, India). The surgical approach was decided after evaluation of tumor size, location, and the possibility of tumor resectability. The senior author (GSK) performed the transfacial approach in all cases, and the tumor resection was done.
Surgical technique
A representative case example of left sided nasopharyngeal angiofibroma is described to elaborate the surgical technique. [Figures 1–4]. A Weber–Ferguson incision is given. Incision is deepened up to bone, and no elevation of skin flap is done from anterior maxillary wall. In orbit, dissection is done up to the infraorbital rim, and the periorbita of the floor of the orbit is elevated. Medially, lateral wall of nose is separated up to the bone. Extension to the nasal cavity is done as per requirement. Laterally, zygomatic arch and lateral orbital wall are exposed with the orbital contents separated from orbital wall, medial, and lateral orbital wall. The incision is continued to the midline of the upper lip and then to the midline gingiva between the central incisors. The mucoperiosteal incision on the hard palate is then made either in the midline (as practiced by the senior author in the initial 10 cases) or along the inner alveolar ridge in the subsequent cases till date [Figure 5]. The incision is extended till the junction of the hard and soft palates then it is turns laterally to run behind the maxillary tuberosity.
Figure 1.
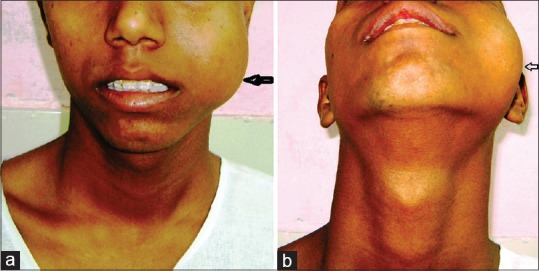
(a) Preoperative (Frontal view):- mass on the left cheek. (b) Preoperative (inferior view)
Figure 4.
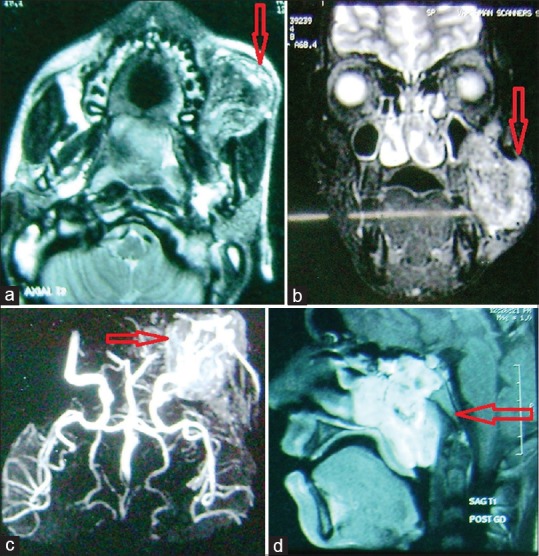
(a) Magnetic resonance imaging scan (axial section). (b) Magnetic resonance imaging scan (coronal section). (c) Magnetic resonance imaging angiography showing tumor blush. (d) Magnetic resonance imaging scan (sagittal section)
Figure 5.
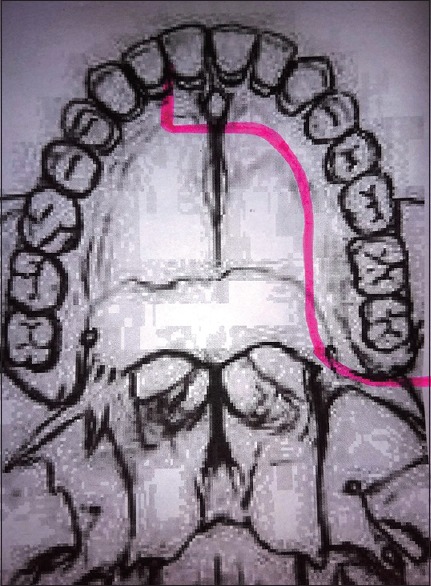
Palatal mucoperiosteal incision
Figure 2.
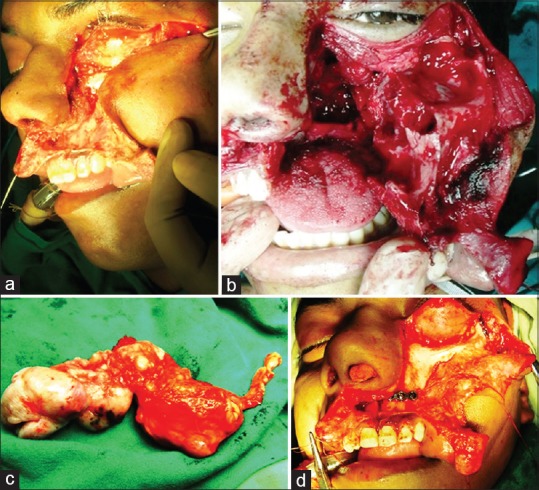
(a) Skin incision and soft-tissue dissection. (b) The complete maxillary swing. (c) Specimen. (d) Facial bone approximation with miniplates after completion of procedure
Figure 3.
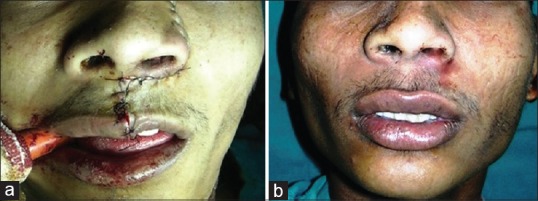
(a) Postoperative with skin sutures in situ. (b) Follow-up with nicely healed suture line
In the author's technique, drill holes are made, and preplating is done before doing the osteotomy to make refixation quick and accurate.
The osteotomies are planned so that:
Osteotomy cuts do not damage vital structures
Blood supply to the soft tissues remains intact
Postoperative occlusion is maintained
Technique is safe and repeatable and
Refixation is stable.
Osteotomy sites
Using an oscillating or reciprocating saw osteotomy is done at the following sites: [Figure 6]
Figure 6.
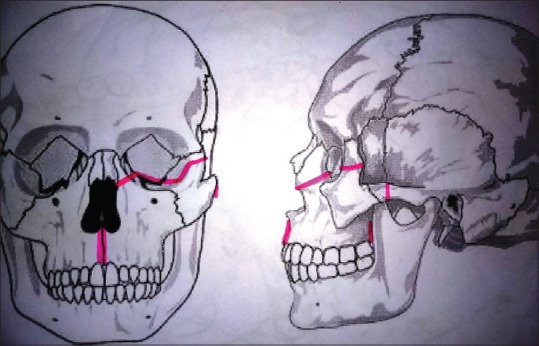
Osteotomy cuts
Just below the zygomatic frontal suture
Zygomatic arch near the zygoma
In the floor of the orbit extending to lateral orbital wall similar to Le-Fort III osteotomy
Frontal process of maxilla below the lacrimal sac
Upper alveolus between the central incisors/lateral and central incisor
Palate in the center or paramedian, at a distance of 1 cm from the alveolar margin.
Bony cuts are made, and maxilla is separated from the pterygoid plates. Hard palate is cut in the center or along the alveolar margin [Figure 7]. It is to be emphasized that the mucoperiosteal incision on the hard palate and the osteotomy cut on the bony hard palate do not coincide for better flap approximation and augment wound healing.
Figure 7.
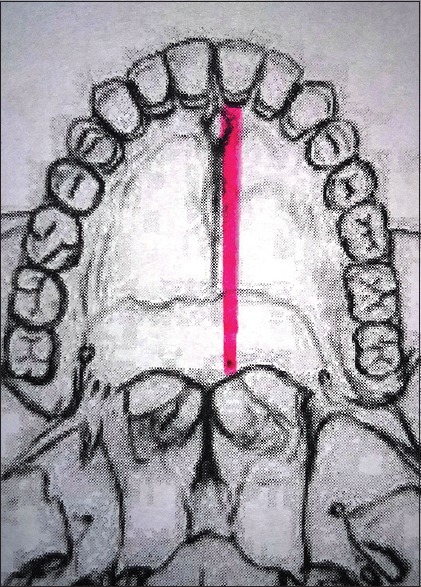
Palatal osteotomy site
Now, maxilla and zygoma can be mobilized laterally keeping skin and muscle attachments intact. This approach gives a good visualization of any pathology in the paranasal sinuses, ethmoid and sphenoid sinuses, and the nasopharynx. This approach enables the highly vascular tumors in this region to be excised with minimal blood loss due to excellent access.
After removal of the tumor, maxilla and zygoma are replaced to their original position and fixed with miniplates in the predrilled holes. The palatal incision is closed with absorbable sutures.
Fixation of osteotomy
Fixation is done using 2 mm titanium miniplates. Miniplates are used on.
Lateral wall of the orbit
Zygomatic arch
Upper alveolus
Nasomaxillary buttress.
Soft palate is repaired if divided and the skin and soft tissue incisions are closed in layers.
RESULTS
A total of 62 patients were operated for various etiologies [Table 1] over a period of 15 years from 2001 to 2016. There were 54 male and 8 female patients in the age range of 15–49 years. The follow-up period was 24 months.
Table 1.
Tumor etiology

Postoperative complications included mild atrophy of nasal mucosa in one patient and one patient developed palatal fistula. None of the patients had any postoperative occlusal disturbance as per the Angle's classification of malocclusion criteria [Table 2].
Table 2.
Postoperative complications

DISCUSSION
The location and nature of the tumor are the most important determinants in deciding the surgical approach to the tumor. The tumors involving the anterior skull base region could be intracranial or extracranial with intracranial extension.[5,6] Despite the significant advances in both radiation and oncology, resection remains the mainstay of effective curative or palliative treatment of many of these tumors.[6]
The documented origin of Skull base surgery was in the late 19th century when Harvey Cushing and his contemporaries popularized the transnasal approaches to the pituitary gland..[7,8]
A number of approaches have been described for approach to the skull base. Broadly, approaches can be open or endoscopic. Although technological advances have expanded the use of endoscopic techniques to include endonasal approaches to skull base lesions, its scope has been limited.[9]
The regular transfacial approaches include transnasal, transeptal, transsphenoidal, and intraoral (Le Fort I) approaches. These approaches have limitations because the access here is through a small window with limited surgical field, which may preclude the wide exposure required for complete clearance of oncological lesions.[9]
In selecting a complex approach for tumor removal, it is important to consider both the degree and quality of exposure to know the anatomical location of the tumor and its extension, and then to obtain vascular access both proximally and distally.[10,11]
Transfacial swing approaches maximize access with minimal morbidity and add a great degree of flexibility of tissue dissection. They provide the possibility of extension of dissection superiorly and inferiorly and thus can be considered for extradural and intradural lesions in this compartment.[11,12] They also allow excellent exposure of the midline compartment of the cranial base, with a short distance to the target, which facilitates precise tumor resection.[12]
Based on the path chosen and bones mobilized, transfacial swing osteotomies can be transmandibular, transmaxillary, zygomaticotemporal, fronto-orbital (glabellar) approach, and combination approaches including zygomaticomaxillary, nasomaxillary, and nasozygomaticomaxillary.[9] The approach may be tailored according to the requirements of the lesion in the individual patient.
Wei et al. first described the maxillary swing as an approach to the surgical management of postradiotherapy recurrent nasopharyngeal and paranasopharyngeal carcinomas.[13] In postradiotherapy cases in this region, bone healing is hampered by the damage to the bone morphogenetic proteins and decreased microcirculation in the bone.[14] This approach is more advantageous than other anterior approaches to the central skull base lesions, especially in postradiotherapy cases due to markedly reduced incidences of bone flap necrosis.[15]
Wei et al. in their original description had placed the hard palatal mucosal incision in the midline,[14] but the incidence of postoperative palatal fistula ranged from 20% to 25% in these cases, especially in cases in which the patients had radiotherapy before the surgery.[16,17]
To overcome this problem, Ng and Wei modified their incision by placing the incision in the hard palate mucosa from the opposite lateral incisor and continuing along the inner margin of the upper alveolus on the side of the maxilla that was to be swung, keeping 3 mm intact mucosa from the inner border of the gingival and elevating a mucoperiosteal flap 1 cm across the midline.[18] The authors reported an uneventful healing of the palatal wound in 14 of 15 consecutive patients with the modified technique.
The senior author (GSK) in the initial 10 cases followed the midline approach, and palatal fistula was formed in one of these. In the subsequent cases, the lateral approach (along the alveolus) was followed, and none of the patients had any palatal complication.
Although a number of complications have been described in literature associated with the procedure,[19,20] it is imperative to comprehend that the patient's outcome is more significantly related to complete resection of the lesion, and this should not be compromised by inappropriate exposure.[12]
CONCLUSION
The maxillary swing is an excellent procedure providing wide exposure for complete oncological clearance of lesions. With a combined approach of a craniofacial and skull base surgeon, the procedure provides good long-term functional and esthetic results with low tumor recurrence rates.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Richardson MS. Pathology of skull base tumors. Otolaryngol Clin North Am. 2001;34:1025–42. doi: 10.1016/s0030-6665(05)70363-7. vii. [DOI] [PubMed] [Google Scholar]
- 2.Scholz M, Parvin R, Thissen J, Löhnert C, Harders A, Blaeser K, et al. Skull base approaches in neurosurgery. Head Neck Oncol. 2010;2:16. doi: 10.1186/1758-3284-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketcham AS, Wilkins RH, Vanburen JM, Smith RR. A combined intracranial facial approach to the paranasal sinuses. Am J Surg. 1963;106:698–703. doi: 10.1016/0002-9610(63)90387-8. [DOI] [PubMed] [Google Scholar]
- 4.Jackson IT, Hide TA. A systematic approach to tumours of the base of the skull. J Maxillofac Surg. 1982;10:92–8. doi: 10.1016/s0301-0503(82)80019-2. [DOI] [PubMed] [Google Scholar]
- 5.Krischek B, Carvalho FG, Godoy BL, Kiehl R, Zadeh G, Gentili F, et al. From craniofacial resection to endonasal endoscopic removal of malignant tumors of the anterior skull base. World Neurosurg. 2014;82:S59–65. doi: 10.1016/j.wneu.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Pieper DR, LaRouere M, Jackson IT. Operative management of skull base malignancies: Choosing the appropriate approach. Neurosurg Focus. 2002;12:e6. doi: 10.3171/foc.2002.12.5.7. [DOI] [PubMed] [Google Scholar]
- 7.Donald PJ. History of skull base surgery. Skull Base Surg. 1991;1:1–3. doi: 10.1055/s-2008-1056983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pendleton C, Adams H, Salvatori R, Wand G, Quiñones-Hinojosa A. On the shoulders of giants: Harvey Cushing's experience with acromegaly and gigantism at the johns Hopkins hospital, 1896-1912. Pituitary. 2011;14:53–60. doi: 10.1007/s11102-010-0258-z. [DOI] [PubMed] [Google Scholar]
- 9.Khanna JN, Natrajan S, Galinde J. Skull base tumors: A kaleidoscope of challenge. J Neurol Surg Rep. 2014;75:e11–21. doi: 10.1055/s-0033-1358381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammirati M, Bernardo A. Analytical evaluation of complex anterior approaches to the cranial base: An anatomic study. Neurosurgery. 1998;43:1398–407. doi: 10.1097/00006123-199812000-00081. [DOI] [PubMed] [Google Scholar]
- 11.Ammirati M, Ma J, Cheatham ML, Mei ZT, Bloch J, Becker DP, et al. The mandibular swing-transcervical approach to the skull base: Anatomical study. Technical note. J Neurosurg. 1993;78:673–81. doi: 10.3171/jns.1993.78.4.0673. [DOI] [PubMed] [Google Scholar]
- 12.Moreira-Gonzalez A, Pieper DR, Cambra JB, Simman R, Jackson IT. Skull base tumors: A comprehensive review of transfacial swing osteotomy approaches. Plast Reconstr Surg. 2005;115:711–20. doi: 10.1097/01.prs.0000152437.71574.4f. [DOI] [PubMed] [Google Scholar]
- 13.Wei WI, Ho CM, Yuen PW, Fung CF, Sham JS, Lam KH, et al. Maxillary swing approach for resection of tumors in and around the nasopharynx. Arch Otolaryngol Head Neck Surg. 1995;121:638–42. doi: 10.1001/archotol.1995.01890060036007. [DOI] [PubMed] [Google Scholar]
- 14.Wei WI, Lam KH, Sham JS. New approach to the nasopharynx: The maxillary swing approach. Head Neck. 1991;13:200–7. doi: 10.1002/hed.2880130306. [DOI] [PubMed] [Google Scholar]
- 15.Suárez C, Llorente JL, Muñoz C, García LA, Rodrigo JP. Facial translocation approach in the management of central skull base and infratemporal tumors. Laryngoscope. 2004;114:1047–51. doi: 10.1097/00005537-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 16.King WW, Ku PK, Mok CO, Teo PM. Nasopharyngectomy in the treatment of recurrent nasopharyngeal carcinoma: A twelve-year experience. Head Neck. 2000;22:215–22. doi: 10.1002/(sici)1097-0347(200005)22:3<215::aid-hed2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Hao SP, Tsang NM, Chang CN. Salvage surgery for recurrent nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2002;128:63–7. doi: 10.1001/archotol.128.1.63. [DOI] [PubMed] [Google Scholar]
- 18.Ng RW, Wei WI. Elimination of palatal fistula after the maxillary swing procedure. Head Neck. 2005;27:608–12. doi: 10.1002/hed.20220. [DOI] [PubMed] [Google Scholar]
- 19.Chan JY, Tsang RK, Wei WI. Morbidities after maxillary swing nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2015;37:487–92. doi: 10.1002/hed.23633. [DOI] [PubMed] [Google Scholar]
- 20.Roy Chowdhury S, Rajkumar K, Deshmukh T. Complications of midface swing for management of juvenile nasopharyngeal angiofibroma. J Maxillofac Oral Surg. 2017;16:96–100. doi: 10.1007/s12663-016-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


