Abstract
Occult hepatitis C virus (HCV) infection (OCI), first described in 2004, is defined as the presence of HCV RNA in hepatocytes or peripheral blood mononuclear cells without detectable HCV RNA in the serum. Here, we aimed to review the epidemiology, diagnostic methods, clinical implications and potential management recommendations currently described in the literature, as well as the future directions for investigation of this entity. PubMed and Cochrane databases were searched with combination of the following keywords: “occult”, “hepatitis C virus”, and “occult HCV infection”. There are data to support OCI as a potential culprit in cryptogenic liver disease. There are also consistent data demonstrating the existence of OCI in specific populations, such as dialysis, human immunodeficiency virus-infected and hepatitis B virus-infected patients, and also in the general population. While the gold standard for diagnosis is liver biopsy, examination of peripheral blood mononuclear cells may be a reliable, safer alternative method of diagnosis. Occult HCV infection is likely associated with liver fibrosis and progression of liver disease. Additional studies are required to determine the infectivity of OCI patients, as well as clarify the natural course and specific clinical implications of OCI. Lastly, studies are needed to determine whether treatment of OCI leads to decreased morbidity and/or mortality.
Keywords: Occult hepatitis C virus, OCI, HCV infection
Introduction
Occult hepatitis C virus (HCV) infection (OCI) is defined as the presence of HCV RNA in hepatocytes or peripheral blood mononuclear cells (PBMCs) with no detectable HCV RNA in the serum. The entity was first described by Castillo et al.1 in January 2004. In that study, the authors investigated 100 patients with long-standing abnormal liver function tests, the cause of which was unknown despite extensive work-up for all causes of liver disease. Of these 100 patients, 57 were found to have detectable HCV RNA in hepatocytes by reverse-transcription polymerase chain reaction (RT-PCR). This initial study prompted additional investigations to further elucidate the pathophysiology and clinical implications of OCI. At this time, there are two types of OCI that are recognized: seronegative OCI (antiHCV antibody-negative and serum HCV RNA-negative); and, seropositive OCI (antiHCV antibody-positive and serum HCV RNA-negative), also called secondary occult HCV infection. This review focuses on seronegative OCI.
It should be noted that the definition of occult HCV infection is different than that of occult hepatitis B virus (HBV) infection. This similar entity was first described in the late 1970s and is defined as the presence of detectable HBV DNA by PCR in the serum of patients who are negative for hepatitis B surface antigen.1 Occult HBV infection has well-described clinical implications in the literature. For example, transplantation of livers from patients with occult HBV can result in de novo hepatitis B infection in the recipient.2 Furthermore, occult HBV has been implicated in accelerated progression of chronic liver disease and hepatocellular carcinoma.3
Epidemiology
OCI is a relatively recently recognized entity, having been described only 13 years ago. While most studies of OCI have focused on patients with cryptogenic liver disease or specific coinfections or comorbidities, OCI has also been recognized in the general population as well. Cryptogenic liver disease is a diagnosis of exclusion after extensive evaluation, where recognizable causes of liver disease have been excluded. OCI has been detected in patients with cryptogenic liver disease at rates from 8.9% to 10%.4,5
One study performed by De Marco et al.6 in 2009 sought to evaluate the prevalence of OCI in the general population. The study used three series of subjects (n = 276) from other epidemiological studies, who had normal liver enzymes and tested negative for serum HCV antibodies and serum HCV RNA. All of these subjects had been chosen for the control arm of their respective trials. They found that 9 of the 276 subjects (3.3%) were positive for HCV RNA in their PBMCs, diagnostic of OCI in an otherwise unsuspecting cohort.
A similar study from China was published in 2017, which involved taking blood samples from blood donors who were seronegative for HCV and had PBMCs tested for HCV RNA.7 The prevalence of OCI in the blood donors was found to be 2.2%. It should be noted that of the patients who were found to have OCI, one was hepatitis B surface antigen-positive and the remainder had elevations in alanine aminotransferase (ALT).
There have been several studies investigating the presence of OCI in specific populations, which will be discussed later in this review. Table 1 outlines the various studies that have been completed and their respective rates of detection of OCI.
Table 1. Studies of OCI prevalence in various populations, in chronological order.
| Study | Year | Target Population | Prevalence | Source | |
| Castillo et al.1 | 2004 | Abnormal liver enzymes | 57% (57/100) | Hepatocytes | |
| Barril et al.20 | 2008 | Hemodialysis patients with abnormal liver enzymes | 45% (49/109) | PBMCs | |
| De Marco et al.8 | 2009 | General population | 3.3% (9/276) | PBMCs | |
| Bokharaei-Salim et al.5 | 2011 | Cryptogenic liver disease | 10% (7/69) | PBMCs | |
| De Marco et al.17 | 2012 | General population | 1.27% (4/314) | PBMCs | |
| Castillo et al.22 | 2013 | Hepatitis B patients | 40% (21/52) | Hepatocytes | |
| Keyvani et al.6 | 2013 | Cryptogenic cirrhosis | 8.9% (4/45) | PBMCs | |
| Gatserelia et al.24 | 2014 | HIV patients | No liver disease | 2% (2/98) | PBMCs |
| Cryptogenic liver disease | 12% (4/34) | ||||
| HIV/HBV coinfection | 31% (9/29) | ||||
| Castillo et al.25 | 2014 | Immune-mediated glomerulonephritis | 39% (34/87) | PBMCs and ultracentrifugated serum | |
| Bokharaei-Salim et al.26 | 2016 | HIV patients | 10.2% (6/59) | PBMCs | |
| Naghdi et al.21 | 2017 | Hemodialysis patients | 3% (6/198) | PBMCs | |
| Lin et al.9 | 2017 | General population (blood donors) | 2.2% (10/458) | PBMCs | |
Pathophysiology
HCV belongs to the Flaviviridae family, and is a single-stranded RNA molecule referred to as the positive strand or genomic HCV RNA. Virus replication requires the synthesis of a complementary strand of RNA, called the negative strand or antigenomic HCV RNA. It is presumed that the presence of the antigenomic HCV RNA strand suggests ongoing viral replication.
In the original study by Castillo et al.1 in 2004, 48 of 57 (84%) patients with OCI had antigenomic HCV RNA strands present in their hepatocytes. A follow-up study done by Castillo et al.8 in 2005 investigated the PBMCs as sites of HCV viral replication in 18 patients with OCI. While the liver is the main site of virus replication, it can also take place at extrahepatic sites, such as in the PBMCs. In that study, the antigenomic HCV RNA strand was present in 61% of the patients. This percentage of HCV replication in the PBMCs of patients with occult HCV infection was similar to that found in the PBMCs of patients with chronic hepatitis C. In addition, the ratio of HCV positive to negative strands in PBMCs was similar to the ratio for a virus replicating in vivo. These findings suggested that patients with OCI have ongoing viral replication.
One proposed hypothesis regarding the absence of antiHCV antibodies and HCV RNA in the serum is that a mutation impacts the virus encapsidation capacity or the formation and release of virions into blood circulation.9 This leads to low levels of viremia that are frequently below the detection rate of current assays.
Diagnosis
Liver biopsy remains the gold standard for diagnosis of OCI by identifying HCV RNA in hepatocytes. However, liver biopsy is not always readily available and it carries risks, such as bleeding or inadvertent puncture of other organs. Most studies have, therefore, made the diagnosis of OCI by testing PBMCs. Testing of PBMCs likely underestimates the true prevalence of OCI, as PBMCs will detect only approximately 70% of cases when compared with hepatocyte testing.1 Serial testing of PBMCs may increase that detection rate if there is high suspicion for OCI.10
One hypothesis regarding the absence of detectable HCV RNA in sera is that the number of circulating viral particles is too low to be detected by RT-PCR. To test this hypothesis, Quiroga et al.11 investigated the use of ultracentrifugation combined with sensitive RT-PCR, and they effectively demonstrated that patients with occult HCV infection may have low amounts of viral particles in their sera. By concentrating 2 mL of serum by ultracentrifugation then using HCV RNA detection by RT-PCR, the sensitivity of HCV RNA detection in serum was increased 8-fold. This is in contrast with the standard methods for HCV RNA detection, in which a maximum of 250 μL of serum is used for isolation of RNA.12 Another published method is detection of IgG antibodies to an HCV core-derived peptide.13 Combination of these two methods, in addition to testing for HCV RNA in PBMCs, can detect up to 91% of patients that have had proven OCI by liver biopsy.14
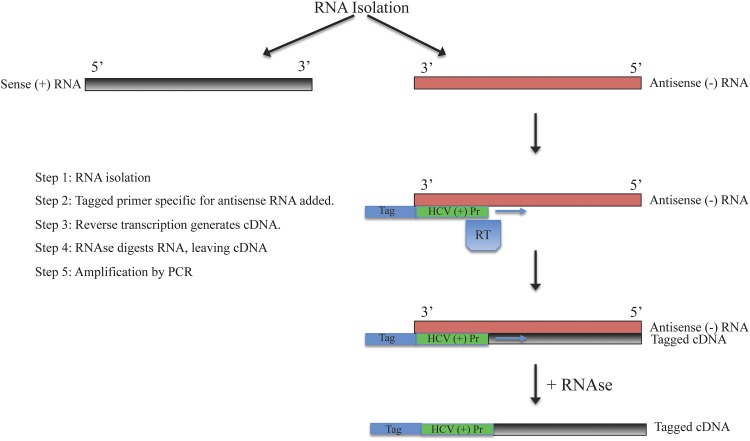
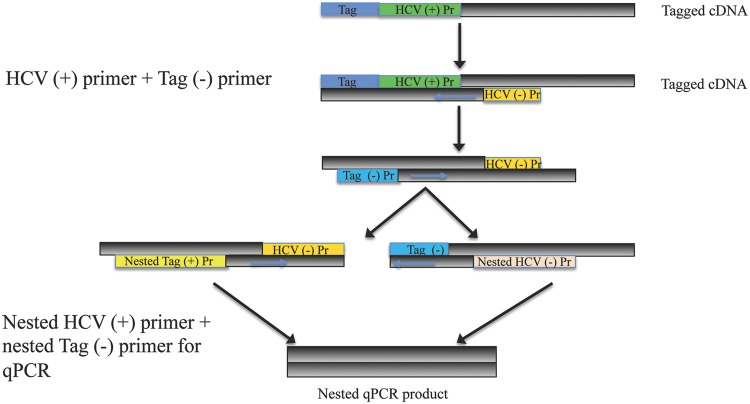
As mentioned earlier, the detection of antisense RNA is frequently used to demonstrate active HCV replication since it is the replicative intermediate. The ability to detect the presence of the antisense RNA was an important part of clarifying the natural course of chronic hepatitis C, and it is also a useful marker for the same reason in OCI. Regular RT-PCR is unable to differentiate between positive and negative HCV RNA strands. Therefore, strand-specific RT-PCR was developed to aid in identification and quantification of the negative strand. In strand-specific RT-PCR, a tagged primer specific to the antisense RNA is used, along with other methods meant to increase specificity, such as the use of high temperatures during cDNA synthesis.13 Nested PCR involves using two sets of primers in succession with the second set targeting a sequence within the first set, which increases the sensitivity of PCR. Refer to Figs. 1 and 2 for depictions of strand-specific RT-PCR and nested PCR, and their applications in testing for HCV.
Fig. 1. Strand specific RT-PCR for amplification of antisense RNA strand.
Abbreviations: HCV, hepatitis C virus; Pr, primer; RT, reverse transcriptase; cDNA, complementary DNA.
Fig. 2. Nested qPCR for quantitation of tagged HCV cDNA.
Abbreviations: HCV, hepatitis C virus; Pr, primer; cDNA, complementary DNA.
Natural history and clinical importance
There are few studies that have focused on long-term follow-up of patients with OCI. One prospective study published in 2011 focused on a small sample of 37 patients with OCI diagnosed by liver biopsy, and viral markers were monitored for an average of 55.7 months, testing for antiHCV antibodies once per year as well as serum and PBMC HCV RNA every 3–4 months.12 For all patients, the two serum viral markers remained undetectable for the length of time they were monitored. The study found that HCV RNA in PBMCs was intermittently detectable in 23 patients (74%). For the patients with HCV RNA in PBMCs, they had significantly (p = 0.028) higher genomic HCV RNA levels in the liver. In addition, ultracentrifugation allowed detection of HCV RNA in the serum of 33/37 (89%) patients, intermittently in 29 of those and only once in 4 of the patients. This illustrates the dynamic nature of OCI with fluctuating levels of viremia and possibly variability in the assay. Interestingly, graphs plotting levels of detectable viremia by ultracentrifugation and ALT values over time did find that the detectable viremia coincided with ALT flares. The findings of that study, thus, suggest a persistent infection.
Another study followed six patients with OCI for 2 years, diagnosed as part of an investigation looking at the prevalence of OCI in a population unselected for liver disease.15 At the end of the 2-year timeframe, all of the patients tested negative for OCI (negative for HCV RNA in serum and PBMCs), even when using ultracentrifugation. There was no seroconversion, alteration of liver enzymes, or effects on liver synthesis; although, it should be noted that at baseline, five of the subjects had normal liver enzymes. In comparison with the aforementioned study, the sample size here was much smaller and follow-up was significantly shorter. The disappearance of HCV RNA from PBMCs in that study suggests clearance of infection rather than persistent infection. However, longer follow-up would be helpful to support this.
There is concern as to whether a patient with OCI can transmit the infection to others, especially given fluctuating levels of viremia detected by serum ultracentrifugation and evidence of active replication in PBMCs. One study in 2009 studied family members of patients with OCI and found that family members were frequently seropositive for HCV.16 Furthermore, a greater proportion of family members of OCI patients were seropositive for HCV antibodies compared with family members of patients with chronic hepatitis C. However, caution must be employed in using these results as an indicator of infectivity. The converse may have been true in the study, in that the seropositive family member may have transmitted HCV leading to the occult infection. Phylogenetic analysis of the HCV sequences showed a common source of HCV infection within each family, making it unlikely that the infections were independently contracted. Additional investigations are warranted to determine the infectivity of patients with OCI.
Special populations
Dialysis patients
Hepatitis C is a major cause of chronic liver disease in end-stage renal disease (ESRD) patients. Hepatitis C is more prevalent in dialysis patients, with various studies showing rates ranging from less than 5 up to 60% depending on geographical location.17 With the implementation of routine screening for HCV as well as measures designed to prevent its spread, the prevalence has decreased. However, OCI may be under-recognized and could be the culprit for continued spread of HCV as OCI is not detected by current screening methods.
Barril et al.18 looked at 109 hemodialysis patients with abnormal levels of liver enzymes who were seronegative for HCV antibodies and HCV RNA. They tested quantitative genomic and antigenomic HCV RNA in PBMCs, and found that 45% of the patients had OCI, with 53% of those demonstrating ongoing HCV replication based on the presence of antigenomic HCV RNA. To ensure specificity of their results, the investigators employed two different operators to perform HCV RNA detection on different days, and they also used PBMCs from healthy patients as negative controls. A significant finding was that during the surveillance period of the patients, 39% of the OCI patients died during the follow-up period compared with 20% without OCI (p = 0.031); however, none of the deaths were directly related to liver disease. Another important finding in the study was that the length of time on dialysis was found to correlate with OCI infection, raising the question of whether there was nosocomial transmission.
A more recent study by Naghdi et al.19 in 2017 looked at a general hemodialysis patient population in Iran, regardless of liver function tests, and found that 3% of the patients screened were positive for OCI. Their method for isolation of HCV RNA used reverse transcriptase-nested PCR, which theoretically increases specificity for detection of HCV RNA. There was no correlation between length of time and dialysis and OCI in that study. The lower incidence of OCI in that study could be attributed to the fact that the study did not select for subjects with abnormal liver enzyme levels. As it was a cross-sectional study, PBMCs were only tested once, and ultracentrifugation was not performed.
HBV and human immunodeficiency virus (HIV) coinfection
In two small studies, OCI was detected at rates between 28% and 40% in patients with hepatitis B.11,20 The levels of liver enzymes were found to be higher in patients with OCI than without, and histological assessment of liver biopsies showed that liver fibrosis was significantly higher in the hepatitis B patients with OCI, suggesting that coinfection had an impact on the severity of liver disease. However, of the patients in the study receiving antiviral treatment for chronic hepatitis B, half of them had OCI. Response rates to antiviral treatment were unaffected by the OCI status, suggesting that coinfection does not interfere with antiviral therapy. Furthermore, serum and intrahepatic HBV DNA levels were found to be decreased in patients with OCI compared to those without. This is consistent with clinical observations showing a viral interaction in patients coinfected with HBV/HCV, in which HBV replication is inhibited in the presence of HCV.21
HIV patients are another population of interest, given the similar risk factors and routes of transmission with HCV. A recent study from 2014 studied OCI in HIV patients from Georgia, where there was a high rate of HCV (6.7%).22 HIV patients were divided into the following three groups: (1) no evidence of liver disease; (2) cryptogenic liver disease; and, (3) HIV/HBV coinfection. All groups had patients with OCI, with the highest being in the HIV/HBV coinfected group (31%), followed by the cryptogenic liver disease group (12%) and then the group without evidence of liver disease (2%). Evaluation with transient elastography found that liver fibrosis was present more frequently and the fibrosis score was significantly higher in the patients with OCI than in those without, supporting the notion that OCI has an impact on liver disease.
Prospective studies with surveillance of HBV and HIV patients with OCI are needed. Data on the development and progression of liver disease stratified by CD4 counts and severity of immune compromise would help to clarify the role of immunosuppression, if any, in the natural course of OCI.
Glomerular nephropathies
A large spectrum of renal diseases is associated with HCV, and there are data to suggest that occult HCV could be related to the pathogenesis of immune-mediated glomerulonephritis. A 2014 study published by Castillo et al.23 found that 34 of 87 (39%) patients with immune-mediated glomerulonephritis were positive for OCI. Furthermore, the patients with occult HCV had significantly worse renal function as well as more frequent progression to ESRD than those without occult HCV.
Classical HCV-associated glomerular disease is thought to be due to deposition of HCV immune complexes in glomeruli, which would not necessarily be the mechanism of pathogenesis in OCI.24 Rather, occult HCV may have a direct pathogenic role. There are studies that support virus-induced renal injury in patients who have no detectable serum HCV RNA, with HCV-NS3 antigen being found in renal tissue.25
Treatment
With the aforementioned clinical implications of occult hepatitis C demonstrated in studies, a major question is whether there would be benefit to treating OCI.
One study performed by Pardo et al.26 in 2006 sought to assess the effect of pegylated-interferon plus ribavirin therapy in 10 patients with OCI. The patients underwent treatment for 24 weeks and were followed up for 24 weeks after therapy. All patients had abnormal ALT and were HCV RNA-positive in PBMCs, with liver biopsy demonstrating necroinflammation. At the end of treatment, 80% of the patients had normal ALT, and HCV RNA in PBMCs was not detectable in 80% of the subjects. However, at follow-up 24 weeks after therapy, sustained response was observed in only 30% of the cases. The percentage of infected PBMCs was not recorded; rather, the authors documented whether the patient was (+) or (−) for HCV RNA in PBMCs. Quantitation of HCV RNA in PBMCs would have been helpful to further analyze the effects of treatment. Five patients did undergo repeat biopsy, and HCV RNA persisted; although the viral loads were significantly lower in the posttreatment biopsy, the percentage of infected hepatocytes was lower, and necroinflammation and fibrosis had decreased in three cases.
The above-mentioned study was performed at a time when pegylated-interferon plus ribavirin was the standard of care, and the results were consistent with treatment results for chronic hepatitis C. With the advent of newer, more effective direct-acting treatments for hepatitis C, updated studies using these for OCI are needed.
Conclusions and future directions
The existence of occult HCV infection has been well described in the literature, for the general population and specific populations, such as patients on dialysis, those coinfected with HBV or HIV, and those with immune-mediated glomerulonephritis. There is a geographical distribution, likely related to endemic rates of HCV. It has been shown to be a dynamic entity, with low fluctuating levels of PBMC viremia. There is some evidence at this time to suggest that OCI is related to progression of liver disease, as many studies not only demonstrate higher rates of liver fibrosis in patients with infection, but also improvement in fibrosis with treatment of it.
Careful attention must be paid to the methods used for diagnosis and surveillance of OCI. While liver biopsy remains the gold standard, it is not always feasible for diagnosis, and less so for monitoring. The current literature supports serial testing of PBMCs with quantitative RT-PCR in combination with ultracentrifugation. Standardization of protocols, including specific temperatures and lengths of time, along with methods and length of storage will help to validate findings of studies across different populations, as variability in these affect yield of the assays.
OCI likely has different implications in various clinical scenarios, but further studies are needed to delineate these, particularly in patients that already have abnormalities in their liver enzyme levels or other signs of liver disease. Although there is evidence that the virus is actively replicating in these patients, there is not enough data to declare whether OCI is a transmissible disease. If it is indeed transmissible, this would have important implications regarding how patients are screened for hepatitis C, and for the safety of the blood supply. Additional data are needed regarding the potential beneficial effects of treatment of OCI. The main barriers to treatment are the lack of approved PBMC screening methods, the cost of novel hepatitis C treatment, and the lack of published clinical sequelae of OCI. Future studies should focus on surveillance of patients that have been diagnosed with OCI, particularly if the diagnosis is made in the setting of cryptogenic liver disease.
Acknowledgments
Support from the Herman Lopata Chair in Hepatitis Research and a grant from Alexion Corp. are gratefully acknowledged (GYW).
Abbreviations
- ALT
alanine aminotransferase
- ESRD
end-stage renal disease
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- OCI
occult HCV infection
- PBMC
peripheral blood mononuclear cell
- RT-PCR
reverse-transcription polymerase chain reaction
References
- 1.Ocana S, Casas ML, Buhigas I, Lledo JL. Diagnostic strategy for occult hepatitis B virus infection. World J Gastroenterol. 2011;17:1553–1557. doi: 10.3748/wjg.v17.i12.1553. doi: 10.3748/wjg.v17.i12.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chazouillères O, Mamish D, Kim M, Carey K, Ferrell L, Roberts JP, et al. “Occult” hepatitis B virus as source of infection in liver transplant recipients. Lancet. 1994;343:142–146. doi: 10.1016/s0140-6736(94)90934-2. [DOI] [PubMed] [Google Scholar]
- 3.Squadrito G, Cacciola I, Alibrandi A, Pollicino T, Raimondo G. Impact of occult hepatitis B virus infection on the outcome of chronic hepatitis C. J Hepatol. 2013;59:696–700. doi: 10.1016/j.jhep.2013.05.043. doi: 10.1016/j.jhep.2013.05.043. [DOI] [PubMed] [Google Scholar]
- 4.Bokharaei-Salim F, Keyvani H, Monavari SH, Alavian SM, Madjd Z, Toosi MN, et al. Occult hepatitis C virus infection in Iranian patients with cryptogenic liver disease. J Med Virol. 2011;83:989–995. doi: 10.1002/jmv.22044. doi: 10.1002/jmv.22044. [DOI] [PubMed] [Google Scholar]
- 5.Keyvani H, Bokharaei-Salim F, Monavari SH, Esghaei M, Nassiri Toosi M, Fakhim S, et al. Occult hepatitis C virus infection in candidates for liver transplant with cryptogenic cirrhosis. Hepat Mon. 2013;13:e11290. doi: 10.5812/hepatmon.11290. doi: 10.5812/hepatmon.11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Marco L, Gillio-Tos A, Fiano V, Ronco G, Krogh V, Palli D, et al. Occult HCV infection: an unexpected finding in a population unselected for hepatic disease. PLoS One. 2009;4:e8128. doi: 10.1371/journal.pone.0008128. doi: 10.1371/journal.pone.0008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin H, Chen X, Zhu S, Mao P, Zhu S, Liu Y, et al. Prevalence of occult hepatitis C virus infection among blood donors in Jiangsu, China. Intervirology. 2016;59:204–210. doi: 10.1159/000455854. doi: 10.1159/000455854. [DOI] [PubMed] [Google Scholar]
- 8.Castillo I, Rodríguez-Iñigo E, Bartolomé J, de Lucas S, Ortíz-Movilla N, López-Alcorocho JM, et al. Hepatitis C virus replicates in peripheral blood mononuclear cells of patients with occult hepatitis C virus infection. Gut. 2005;54:682–685. doi: 10.1136/gut.2004.057281. doi: 10.1136/gut.2004.057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreño V. Occult hepatitis C virus infection: a new form of hepatitis C. World J Gastroenterol. 2006;12:6922–6925. doi: 10.3748/wjg.v12.i43.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo I, Bartolomé J, Quiroga JA, Barril G, Carreño V. Long-term virological follow up of patients with occult hepatitis C virus infection. Liver Int. 2011;31:1519–1524. doi: 10.1111/j.1478-3231.2011.02613.x. doi: 10.1111/j.1478-3231.2011.02613.x. [DOI] [PubMed] [Google Scholar]
- 11.Bartolomé J, López-Alcorocho JM, Castillo I, Rodríguez-Iñigo E, Quiroga JA, Palacios R, et al. Ultracentrifugation of serum samples allows detection of hepatitis C virus RNA in patients with occult hepatitis C. J Virol. 2007;81:7710–7715. doi: 10.1128/JVI.02750-06. doi: 10.1128/JVI.02750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desombere I, Van Vlierberghe H, Couvent S, Clinckspoor F, Leroux-Roels G. Comparison of qualitative (COBAS AMPLICOR HCV 2.0 versus VERSANT HCV RNA) and quantitative (COBAS AMPLICOR HCV monitor 2.0 versus VERSANT HCV RNA 3.0) assays for hepatitis C virus (HCV) RNA detection and quantification: impact on diagnosis and treatment of HCV infections. J Clin Microbiol. 2005;43:2590–2597. doi: 10.1128/JCM.43.6.2590-2597.2005. doi: 10.1128/JCM.43.6.2590-2597.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quiroga JA, Castillo I, Llorente S, Bartolomé J, Barril G, Carreño V. Identification of serologically silent occult hepatitis C virus infection by detecting immunoglobulin G antibody to a dominant HCV core peptide epitope. J Hepatol. 2009;50:256–263. doi: 10.1016/j.jhep.2008.08.021. doi: 10.1016/j.jhep.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Castillo I, Bartolomé J, Quiroga JA, Barril G, Carreño V. Diagnosis of occult hepatitis C without the need for a liver biopsy. J Med Virol. 2010;82:1554–1559. doi: 10.1002/jmv.21866. doi: 10.1002/jmv.21866. [DOI] [PubMed] [Google Scholar]
- 15.De Marco L, Manzini P, Trevisan M, Gillio-Tos A, Danielle F, Balloco C, et al. Prevalence and follow-up of occult HCV infection in an Italian population free of clinically detectable infectious liver disease. PLoS One. 2012;7:e43541. doi: 10.1371/journal.pone.0043541. doi: 10.1371/journal.pone.0043541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo I, Bartolomé J, Quiroga JA, Barril G, Carreño V. Hepatitis C virus infection in the family setting of patients with occult hepatitis C. J Med Virol. 2009;81:1198–1203. doi: 10.1002/jmv.21483. doi: 10.1002/jmv.21483. [DOI] [PubMed] [Google Scholar]
- 17.Perico N, Cattaneo D, Bikbov B, Remuzzi G. Hepatitis C infection and chronic renal diseases. Clin J Am Soc Nephrol. 2009;4:207–220. doi: 10.2215/CJN.03710708. doi: 10.2215/CJN.03710708. [DOI] [PubMed] [Google Scholar]
- 18.Barril G, Castillo I, Arenas MD, Espinosa M, Garcia-Valdecasas J, Garcia-Fernández N, et al. Occult hepatitis C virus infection among hemodialysis patients. J Am Soc Nephrol. 2008;19:2288–2292. doi: 10.1681/ASN.2008030293. doi: 10.1681/ASN.2008030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naghdi R, Ranjbar M, Bokharaei-Salim F, Keyvani H, Savaj S, Ossareh S, et al. Occult hepatitis C infection among hemodialysis patients: a prevalence study. Ann Hepatol. 2017;16:510–513. doi: 10.5604/01.3001.0010.0277. doi: 10.5604/01.3001.0010.0277. [DOI] [PubMed] [Google Scholar]
- 20.Castillo I, Bartolomé J, Quiroga JA, Carreño V. High prevalence of occult hepatitis C virus infection in patients with chronic hepatitis B virus infection. J Med Microbiol. 2013;62:1235–1238. doi: 10.1099/jmm.0.058297-0. doi: 10.1099/jmm.0.058297-0. [DOI] [PubMed] [Google Scholar]
- 21.Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, et al. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27–33. doi: 10.1016/s0168-8278(98)80198-0. [DOI] [PubMed] [Google Scholar]
- 22.Gatserelia L, Sharvadze L, Karchava M, Dolmazashvili E, Tsertsvadze T. Occurrence of occult HCV infection among Hiv infected patients in Georgia. Georgian Med News. 2014;226:37–41. [PubMed] [Google Scholar]
- 23.Castillo I, Martinez-Ara J, Olea T, Bartolomé J, Madero R, Hernández E, et al. High prevalence of occult hepatitis C virus infection in patients with primary and secondary glomerular nephropathies. Kidney Int. 2014;86:619–624. doi: 10.1038/ki.2014.68. doi: 10.1038/ki.2014.68. [DOI] [PubMed] [Google Scholar]
- 24.Barsoum RS. Hepatitis C virus: from entry to renal injury–facts and potentials. Nephrol Dial Transplant. 2007;22:1840–1848. doi: 10.1093/ndt/gfm205. doi: 10.1093/ndt/gfm205. [DOI] [PubMed] [Google Scholar]
- 25.Bataille S, Kaplanski G, Boucraut J, Halfon P, Camus C, Daniel L, et al. Membranoproliferative glomerulonephritis and mixed cryoglobulinemia after hepatitis C virus infection secondary to glomerular NS3 viral antigen deposits. Am J Nephrol. 2012;35:134–140. doi: 10.1159/000335375. doi: 10.1159/000335375. [DOI] [PubMed] [Google Scholar]
- 26.Pardo M, López-Alcorocho JM, Castillo I, Rodríguez-Iñigo E, Perez-Mota A, Carreño V. Effect of anti-viral therapy for occult hepatitis C virus infection. Aliment Pharmacol Ther. 2006;23:1153–1159. doi: 10.1111/j.1365-2036.2006.02886.x. doi: 10.1111/j.1365-2036.2006.02886.x. [DOI] [PubMed] [Google Scholar]