ABSTRACT
The QuantiFERON-TB Gold Plus (QFT-Plus; Qiagen, Germantown, MD) interferon gamma release assay (IGRA) received FDA clearance in 2017 and will replace the prior version of the assay, the QFT-Gold In-Tube (QFT-GIT). Here, we compared performances of the QFT-Plus assay and the QFT-GIT version in a diverse patient population, including patients undergoing evaluation for or follow-up of latent tuberculosis infection (LTBI; n = 39) or active TB infection (n = 3), and in health care workers (HCWs; n = 119) at Mayo Clinic (Rochester, MN). Compared to the QFT-GIT, the QFT-Plus assay showed 91.2% (31/34) positive, 98.4% (124/126) negative, and 96.6% (156/161) overall qualitative agreement among the 161 enrolled subjects, with a Cohen's kappa value of 0.91 (excellent interrater agreement). Among the 28 patients diagnosed with LTBI at the time of enrollment, the QFT-GIT and QFT-Plus assays agreed in 24 (85.7%) patients; in all four discordant patients, the positivity of the QFT-GIT or QFT-Plus IGRA was associated with low-level interferon gamma (IFN-γ) reactivity, ranging from 0.36 IU/ml to 0.66 IU/ml. Additionally, we document a high degree of correlation between IFN-γ levels in the QFT-GIT TB antigen tube and each of the two QFT-Plus TB antigen tubes, as well as between the QFT-Plus TB1 and TB2 tubes (Pearson's correlation coefficients [R] > 0.95). Overall, we show comparable results between the QFT-GIT and QFT-Plus assays in our study population composed of subjects presenting with a diverse spectrum of TB infections. Our findings suggest that the necessary transition to the QFT-Plus assay will be associated with a minimal difference in assay performance characteristics.
KEYWORDS: IGRA, LTBI, QuantiFERON-TB Gold In-Tube, QuantiFERON-TB Gold Plus
INTRODUCTION
Infection with Mycobacterium tuberculosis can take the form of either active disease or latency, an asymptomatic disease state. While multiple diagnostic approaches are available to identify active tuberculosis (TB), including molecular assays able to be performed directly from patient specimens, the detection of latent TB infection (LTBI) remains challenging, with limited diagnostic assays available. Accurate identification of patients with LTBI is important from a disease prevention perspective, as approximately 5% to 10% of untreated otherwise-healthy adults will progress to active TB in their lifetime (1). This percentage increases substantially among immunocompromised patients with LTBI, including those who are receiving immunosuppressive therapy or are immunosuppressed as a result of certain underlying comorbidities or diseases, including uncontrolled human immunodeficiency virus (HIV) infection (1, 2). The detection of LTBI in these patients and appropriate treatment significantly diminish the risk of developing active TB, and, therefore, accurate diagnostic tests for LTBI are needed.
Currently, diagnostic testing for LTBI is performed either in vivo using the tuberculin skin test (TST), which involves intradermal injection of purified protein derivative from an M. tuberculosis strain, or ex vivo using an interferon gamma release assay (IGRA). Both methods rely on the cellular immune response to M. tuberculosis antigens in patients previously exposed to the organism. The TST, which has been in clinical use for over a century, has certain limitations, including a required repeat visit 48 to 72 h after placement for result interpretation, reader variability, the possibility of false-positive results in patients vaccinated with the bacillus Calmette-Guérin (BCG) Mycobacterium bovis strain, and false-negative results as a result of either immunosuppression or anergy in cases of active disease (3). In an effort to improve upon the TST, IGRAs were developed to measure released interferon gamma (IFN-γ) from T cells stimulated in vitro using antigens largely specific to the M. tuberculosis complex. There are currently three FDA-cleared IGRAs, including the T-SPOT.TB test (Oxford Diagnostic Laboratories, Memphis, TN), the QuantiFERON-TB Gold In-Tube (QFT-GIT; Qiagen, Germantown, MD) test, and most recently, the QuantiFERON-TB Gold Plus (QFT-Plus; Qiagen) assay, which received Food and Drug Administration (FDA) clearance in June 2017.
The QFT-GIT assay is designed to stimulate IFN-γ release from CD4+ T cells in a single TB antigen tube using long peptides from three M. tuberculosis antigens, including the early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10), both encoded within the M. tuberculosis region of difference 1 (RD1) locus, and the TB7.7 antigen. In contrast, the new QFT-Plus IGRA contains peptides from only the ESAT-6 and CFP-10 antigens and has two TB antigen tubes (TB1 and TB2). While the QFT-Plus TB1 tube is identical to the QFT-GIT, with the exception of TB7.7, and stimulates CD4+ T cells, the QFT-Plus TB2 tube has a cocktail of both long and short ESAT-6 and CFP-10 peptides to elicit IFN-γ release from both CD4+ and CD8+ T cells. CD8+ cytotoxic T cells have emerged as an important component of host immunity to and control of M. tuberculosis, with studies showing significantly higher CD8+ T-cell responses in patients with smear-positive or active pulmonary TB than in smear-negative or LTBI patients (4, 5). A strong CD8+ T-cell response has also been identified among recently exposed contacts of patients with active tuberculosis infection (6). Finally, patients coinfected with M. tuberculosis and HIV have been shown to maintain CD8+ T-cell antigen responses to M. tuberculosis in the setting of low CD4+ T-cell counts (7). Based on these findings, the inclusion of peptides for the stimulation of CD8+ T cells has been proposed to improve upon the sensitivity of the QFT-GIT assay for detecting TB infection (both latent and active), including infection as a result of recent exposure and, possibly, in patients with depleted CD4+ T-cell counts. Initial studies evaluating the QFT-Plus IGRA have shown equivalent sensitivity and high overall agreement with the QFT-GIT assay in patients with suspected active TB, among recent contacts of patients with active TB, and in health care workers (HCWs) in low-TB-incidence settings (8–13). However, there are limited comparative data evaluating these assays across the spectrum of TB infection, ranging from low-risk HCWs to previously treated HCWs, and among immigrants from regions endemic for TB undergoing evaluation for LTBI or active M. tuberculosis infection in the United States.
In this prospective study, the performance of the QFT-Plus IGRA was evaluated among adult patients presenting to the Olmsted County Public Health Services (OCPHS) TB clinic (Rochester, MN) for evaluation of or follow-up care for LTBI or active TB infection. Additionally, the QFT-Plus assay was simultaneously compared with the QFT-GIT assay in both TST-reactive and -negative HCWs.
MATERIALS AND METHODS
Study design.
This study was divided into two arms. First, adult patients, including those with refugee or immigrant status from countries endemic for TB, who presented to the OCPHS TB clinic for initial evaluation of or follow-up care for suspected LTBI or active TB, prospectively consented and were enrolled from January to April 2017. Patient charts were reviewed, and risk factors for active TB infection or LTBI were recorded, including demographic information, country of birth, travel to countries endemic for TB, prior BCG vaccination status, prior TST or IGRA results, known exposure to individuals with active TB infection, employment or time spent in high-risk facilities, prior treatment for active TB infection or LTBI, imaging studies, symptoms, HIV status, underlying chronic disease, and immunosuppressive therapy. Diagnosis of active TB infection or LTBI was based on criteria established by the recently updated TB diagnostic guidelines (14). Briefly, a microbiologic diagnosis of active TB was defined as either isolation of M. tuberculosis in culture or positivity by an M. tuberculosis molecular assay on any specimen source. In the absence of microbiologic detection of M. tuberculosis, a clinical diagnosis of active TB infection was established in patients suspected of having TB who responded favorably to combination anti-TB drug therapy either clinically and/or radiologically, and following the exclusion of an alternative diagnosis. LTBI was diagnosed in asymptomatic patients with both risk factors for TB infection (travel to or origin from a country endemic for TB, known TB exposure, etc), and a positive TST (induration, ≥10 mm) and/or QFT-GIT result, in whom active TB infection was excluded by history, physical examination, and radiographic studies. All other patients were categorized as having no evidence of active TB infection or LTBI.
The second arm of the study was performed by consenting and enrolling TST-negative and TST-reactive (TST induration, ≥10 mm) HCWs at Mayo Clinic (Rochester, MN), between June 2016 and January 2017. Mayo Clinic, a large tertiary-care medical center, is classified as medium risk for exposure to TB according to current Centers for Disease Control and Prevention (CDC) criteria, although a recent study suggests that the facility has a low incidence of TB (15, 16). Annual TB screening at Mayo Clinic is performed by TST placement for previously TST-negative individuals or by a standard TB symptom questionnaire for previously TST-reactive HCWs. Medical records were only available for review of TST-reactive HCWs, and identical information was extracted for these individuals (if available), as described for the OCPHS patients. Both arms of this study were approved by the Mayo Clinic institutional review board.
Specimen collection and QFT-GIT/QFT-Plus testing.
Forty-two adult patients presenting to the OCPHS TB Clinic and meeting enrollment criteria were consented. A whole-blood (WB; 7 ml/patient) sample was collected into a single lithium heparin tube and transported to the laboratory at ambient temperature, and 0.8 to 1 ml of WB was aliquoted into the three QFT-GIT and four QFT-Plus tubes (tube order, QFT-GIT nil, QFT-Plus nil, QFT-GIT TB, QFT-Plus TB1, QFT-Plus TB2, QFT-GIT mitogen, and QFT-Plus mitogen) using new filtered tips between tubes, within 8 h of collection. WB from 119 HCWs were collected directly into the three QFT-GIT and four QFT-Plus tubes and transported to the laboratory within 8 h of collection.
The QFT-GIT and QFT-Plus assays were performed as per the manufacturer's instructions. Briefly, following aliquoting or receipt in the laboratory, all QFT tubes were inverted 10 times each to coat the sides of the tubes and placed into a 37°C incubator for 16 to 24 h. The tubes were subsequently centrifuged to separate the plasma and tested by the IFN-γ enzyme-linked immunosorbent assay (ELISA) immediately or stored at 4°C for up to 48 h prior to testing. The QFT-GIT and QFT-Plus IFN-γ ELISAs were both performed on the Agility (Dynex Technologies, Chantilly, VA) automated ELISA processor, which also performed all calculations on board to determine the nil, mitogen minus nil, and TB antigen minus nil IFN-γ values for the QFT-GIT assay. Data reduction and interpretation for the QFT-Plus assay were performed using the Qiagen Analysis Software (version 2.71). An eight-point standard curve was used for the QFT-GIT assay, and a four-point standard curve was used for the QFT-Plus IFN-γ ELISA. Results for the QFT-GIT were considered positive if the nil value was ≤8.0 IU/ml, and the TB antigen minus nil IFN-γ value was ≥0.35 IU/ml and at least 25% of the nil value, irrespective of the mitogen minus nil value. As per the manufacturer, the same criteria apply to the QFT-Plus assay; however, results are considered positive if either one or both of the TB antigen tubes are positive using the criteria described above.
Statistics.
The GraphPad software was used to calculate positive, negative, and overall percent agreement and 95% confidence intervals (CIs) (GraphPad, La Jolla, CA, USA; http://graphpad.com/quickcalcs). This software was also used to calculate Cohen's kappa values; values of <0.40, 0.40 to 0.75, and >0.75 were interpreted to indicate poor, fair, and excellent agreement, respectively (17). Microsoft Excel 2010 was used to create correlation plots and to determine Pearson's coefficients (R).
RESULTS
Subject characteristics.
A total of 167 individuals were enrolled, 6 of whom were excluded from the study due to improper specimen collection and/or transport. Among the remaining 161 individuals, 42 were patients presenting to the OCPHS TB Clinic, and 119 patients were Mayo Clinic HCWs (Table 1). The median age for patients enrolled at OCPHS was 36 years, and nearly half of the patients (48%) were female. The majority of patients were foreign born, with 23 patients (54.8%) originating from African or Middle Eastern countries, and 13 patients (31%) had a documented history of BCG vaccination. At the time of sample collection, three patients (7.1%) were on treatment for active pulmonary, extrapulmonary, or disseminated TB infection, 28 patients (66.7%) had LTBI with either incomplete or no prior LTBI treatment, and one patient (2.4%) had completed LTBI therapy in 2013. Following clinical and laboratory evaluation, 10 patients (23.8%) lacked evidence of either LTBI or active TB infection.
TABLE 1.
Characteristics of enrolled patients presenting to the OCPHS TB Clinic and Mayo Clinic health care workers
| Characteristic by patient groupa | Data |
|---|---|
| Enrolled subjects with correctly processed WB samples | |
| Total no. | 161 |
| Age (median [range]) (yr) | 41 (18–79) |
| No. (%) of females | 89 (55) |
| Patients presenting to OCPHS TB Clinic | |
| No. enrolled | 42 |
| Age (median [range]) (yr) | 36 (18–79) |
| No. (%) of females | 20 (48) |
| Region of birth (no. [%]) | |
| Asia | 10 (23.8) |
| Africa and Middle East | 23 (54.8) |
| South America | 3 (7.1) |
| North America | 6 (14.3) |
| No. (%) BCG vaccinated | 13 (31) |
| TB status | |
| Active TB Dx | 3 (7.1) |
| LTBI, partial, or no Tx | 28 (66.7) |
| LTBI, Tx completed | 1 (2.4) |
| No LTBI | 10 (23.8) |
| Health care workers | |
| No. enrolled | 119 |
| Age (median [range]) (yr) | 41 (25–62) |
| No. (%) of females | 69 (58) |
| No. of HCWs with reactive TST ≥10 mm (% of total HCWs) | 19 (16) |
| BCG vaccinatedb | 5 (26.3) |
| Documented Tx Hx for LTBIb | 7 (36.8) |
| Unknown Tx Hx for LTBIb | 3 (15.8) |
| No LTBIb,c | 9 (47.4) |
WB, whole blood; TB, tuberculosis; BCG, bacille Calmette-Guerin; Dx, diagnosis; Tx, treatment; Hx, history; LTBI, latent tuberculosis infection.
Percentage calculated using the number of patients with reactive TST results as the denominator (n = 19).
A reactive TST result was considered false positive.
The median age for the 119 enrolled HCWs was 41 years, and 58% of the HCWs were female (Table 1). Nineteen (16%) HCWs had a prior reactive TST result, and five (26.3%) of these individuals had received the BCG vaccine (based on patient history). Nineteen enrolled HCWs had reactive TST results; however, upon follow-up evaluation and further testing (including QFT-GIT) as part of routine clinical care, nine (47.4%) HCWs did not meet LTBI criteria and were felt to have falsely reactive TST results, as documented by their occupational medicine provider. Among the remaining 10 HCWs, seven (36.8%) HCWs had received prior LTBI therapy, and three (15.8%) HCWs lacked documented evidence of an LTBI treatment regimen.
Comparison of the QFT-Plus and QFT-GIT IGRAs.
The QFT-Plus assay showed agreement with the QFT-GIT in 31/34 (91.2%) positive tests and 124/126 (98.4%) negative tests, and had an overall agreement of 156/161 (96.6%) among all subjects, with a Cohen's kappa value of 0.91 (excellent interrater agreement) (Table 2). The five discordant subjects included one HCW and four OCPHS patients (Table 3). The IFN-γ levels for these discordant patients ranged from 0.19 IU/ml to 0.66 IU/ml between the QFT-GIT and QFT-Plus TB antigen tubes.
TABLE 2.
Qualitative comparison of QFT-Plus and QFT-GIT assays in HCWs and patients presenting to the OCPHS TB Clinica
| QFT-Plus result | QFT-GIT result |
% agreement (95% CI) |
Kappa value | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Indet. | Positive | Negative | Overall | ||
| Positive | 31 | 2 | 0 | 91.2 | 98.4 | 96.9 | 0.91 |
| Negative | 3 | 124 | 0 | (76.3–97.7) | (94–99.9) | (92.7–98.9) | (0.83–0.98) |
| Indet. | 0 | 0 | 1 | ||||
Total n = 161. Indet., indeterminate; CI, confidence interval.
TABLE 3.
Summary of clinical information for patients and HCWs with discordant QFT-GIT and QFT-Plus assay resultsa
| Subject no.b | Age (yr)/sexc | BCG vaccine | TST induration (yr) | Diagnosis of LTBI | QFT-GIT result | QFT-GIT TB Ag − nil (IU/ml) | QFT-Plus result | QFT-Plus TB1 Ag − nil (IU/ml) | QFT-Plus TB2 Ag − nil (IU/ml) | Additional patient and diagnostic informationd |
|---|---|---|---|---|---|---|---|---|---|---|
| HCW-1 | 49/F | Unknown | Negative | Unknown | Negative | 0.24 | Positive | 0.52 | 0.19 | NA |
| OCPHS-1 | 46/F | No | NA | Yes | Positive | 0.36 | Negative | 0.24 | 0.21 | Positive QFT-GIT result (TB tube value, 0.56 IU/ml) performed 1 yr prior to enrollment, employed in nursing facility, normal chest X ray, not treated |
| OCPHS-2 | 34/F | Yes | 15 mm (2012) | Yes | Positive | 0.45 | Negative | 0.32 | 0.32 | Positive QFT-GIT result (TB tube value, 1.16 IU/ml) performed 6 mo prior to enrollment during pregnancy, immigrant from South Korea, not treated |
| OCPHS-3 | 61/M | No | NA | Yes | Positive | 0.66 | Negative | 0.23 | 0.20 | Positive QFT-GIT result (TB tube value, 3.65 IU/ml) performed 10 mo prior to enrollment, 8 yr in refugee camp in Ethiopia, completed 5/6 mo of INH at time of enrollment |
| OCPHS-4 | 79/F | Yes | Negative | Yes | Negative | 0.25 | Positive | 0.27 | 0.50 | Positive QFT-GIT result (TB tube value, 7.55 IU/ml) 2 yr prior, Cambodian immigrant, calcified granuloma in left lung, INH and rifampin not tolerated |
HCW, health care worker; NA, not applicable; Ag, antigen.
OCPHS, Olmsted County Public Health Services.
F, female; M, male.
INH, isoniazid.
Among the 100 HCWs with prior negative TST results, all enrollees were negative by both the QFT-GIT and QFT-Plus IGRAs, except for three subjects, two of whom were positive by both the QFT-GIT (TB tube values, 0.81 IU/ml and 0.83 IU/ml) and QFT-Plus (TB1 tube values, 0.44 IU/ml and 0.86 IU/ml; TB2 tube values, 0.27 IU/ml and 1.04 IU/ml, respectively) assays, and one of whom was positive by the QFT-Plus IGRA only (TB1 tube value, 0.52 IU/ml; TB2 tube value, 0.19 IU/ml; HCW-1 in Table 3). Follow-up clinical and testing information on these individuals were not available to the study personnel.
Nineteen enrolled HCWs had a prior reactive TST result, among whom five HCWs were positive by both the QFT-GIT and QFT-Plus assays, with all three TB antigen tubes having IFN-γ concentrations of ≥0.35 IU/ml. Three of these five QFT-GIT/QFT-Plus positive HCWs had documentation of a completed LTBI treatment regimen in the past (1980 to 2012). Both IGRA versions were negative in the remaining 14 TST-reactive HCWs, which included four individuals who had completed LTBI therapy previously (1993 to 2012), one enrollee with LTBI but with an undocumented treatment history, and in all nine HCWs for whom the reactive TST was deemed a false-positive result by their treating physician (data not shown).
Clinical diagnoses and treatment statuses were available for the 42 prospectively enrolled OCPHS patients (Table 4). Following chart review, 10 of these individuals lacked evidence of LTBI or active TB infection; all 10 individuals were negative by both QFT IGRAs. The QFT-GIT and QFT-Plus results were both qualitative positive (IFN-γ values >10 IU/ml in all three TB antigen tubes) for the single patient who had completed LTBI therapy in 2013. Among the 28 patients diagnosed with LTBI at the time of enrollment, the QFT-GIT and QFT-Plus assays agreed in 24 (85.7%) patients; 22 patients were positive and two patients were negative by both assays (Table 4). For the two LTBI patients negative by both IGRAs, LTBI diagnoses were based on a reactive TST result (20 mm) for one patient who recently immigrated from Ethiopia (BCG vaccination status unknown), and due to a prior positive QFT-GIT result (TB antigen value, 0.64 IU/ml) in a pregnant woman immigrating from Somalia. The QFT-GIT and QFT-Plus results were discordant in four of the 28 LTBI patients (OCPHS 1 to 4, Table 3). In all four cases, positivity for the QFT-GIT or QFT-Plus IGRA was due to low-level IFN-γ reactivity, ranging from 0.36 IU/ml to 0.66 IU/ml.
TABLE 4.
Performance of QFT-Plus and QFT-GIT in clinically characterized patientsa
| Result by assay | Active TB (n = 3) | LTBI (n = 28) | No. completed LTBI treatment (n = 1) | No LTBI (n = 10) |
|---|---|---|---|---|
| QFT-GIT | ||||
| Positive | 1 | 25 | 1 | 0 |
| Negative | 1 | 3 | 0 | 10 |
| Indeterminate | 1 | 0 | 0 | 0 |
| QFT-Plus | ||||
| Positive | 1 | 23 | 1 | 0 |
| Negative | 1 | 5 | 0 | 10 |
| Indeterminate | 1 | 0 | 0 | 0 |
Total n = 42.
Finally, three patients presented with active tuberculosis at the time of enrollment, and in all three cases, the QFT-GIT and QFT-Plus results were concordant (Table 5). Notably, among these three patients, the QFT-GIT and QFT-Plus IGRAs were both indeterminate due to a low mitogen value in a patient with newly diagnosed HIV (41 cells/mm3 CD4+ T cells) and a positive M. tuberculosis sputum culture. Another patient with culture-negative M. tuberculosis lymphadenitis was negative by both the QFT-GIT and QFT-Plus assays. M. tuberculosis lymphadenitis in this patient was diagnosed based on prior M. tuberculosis exposure, positive acid-fast bacillus (AFB) staining from a lymph node biopsy specimen, and response to treatment.
TABLE 5.
Summary of clinical information for patients with active tuberculosis
| Subject no. | Age (yr)/sex | BCG vaccinated | TST induration (yr)a | Symptom(s) at presentation | Imaging studies | HIV status (CD4 cells/mm3) | M. tuberculosis studyb | QFT-Gold result (TB Ag − nil)c | QFT-Plus result (TB1 − nil/TB2 − nil) | Additional patient and diagnostic informationd |
|---|---|---|---|---|---|---|---|---|---|---|
| OCPHS-5 | 47/M | No | 20 mm (2017) | Multifocal lymphadenopathy | Normal | Negative | LN biopsied showed AFB, culture negative | Negative (0.26 IU/ml) | Negative (0.27/0.27 IU/ml) | DM, lives in United Arab Emirates, known exposure to active TB patient, Dx of extrapulmonary TB |
| OCPHS-6 | 52/M | No | NA | Transaminitis, anorexia, wt loss, hand lesion | Right lung cavitary lesion | Positive (41) | Positive sputum culture | Indeterminate due to low mitogen (0.01 IU/ml) | Indeterminate due to low mitogen (0/0 IU/ml) | DM, Somali immigrant, sputum culture positive for M. tuberculosis, Dx of disseminated TB |
| OCPHS-7 | 27/M | No | Reactivee | Asymptomatic | Worsening pulmonary nodular opacities | Unknown | Negative sputum culture | Positive (1.46 IU/ml) | Positive (1.34/2.01 IU/ml) | Recent completion of prison sentence and travel to Mexico, Dx of asymptomatic pulmonary TB |
NA, not applicable.
LN, lymph node; AFB, acid-fast bacilli.
Ag, antigen.
Dx, diagnosis; DM, diabetes mellitus.
TST induration not documented.
Assessment of QFT-GIT and QFT-Plus IFN-γ levels.
Among the 161 paired samples, the difference in absolute quantitative IFN-γ levels between the QFT-GIT TB antigen tube and the QFT-Plus TB1 and TB2 antigen tubes ranged from 0 IU/ml to 7.8 IU/ml (median difference, 0.03 IU/ml) and 0 IU/ml to 7.39 IU/ml (median difference, 0.03 IU/ml), respectively. The difference in absolute quantitative IFN-γ levels between the QFT-Plus TB1 and TB2 antigen tubes ranged from 0 IU/ml to 4.15 IU/ml (median difference, 0.02 IU/ml). For enrollees with LTBI, the median IFN-γ values in the QFT-GIT TB and QFT-Plus TB1 and TB2 tubes were 3.04 IU/ml (interquartile range [IQR], 0.26 to 3.32), 2.22 IU/ml (IQR, 0.31 to 6.20), and 2.44 IU/ml (IQR, 0.21 to 6.31), respectively. Overall, a high degree of correlation was observed between IFN-γ levels in the QFT-GIT TB antigen tube and each of the QFT-Plus TB antigen tubes, as well as between the QFT-Plus TB1 and TB2 tubes (Pearson's correlation coefficients [R] > 0.95) (Fig. 1). The correlation between IFN-γ values was also calculated using only samples with a positive result by either one or both of the QFT IGRAs (n = 36). While slightly lower, the correlation between the QFT-GIT and QFT-Plus IFN-γ levels remained high, with Pearson's coefficients of 0.91 for QFT-GIT versus QFT-Plus TB1, 0.93 for QFT-GIT versus QFT-Plus TB2, and 0.97 for QFT-Plus TB1 versus TB2 tubes (data not shown). Finally, the results for the QFT-Plus TB1 and TB2 antigen tubes matched qualitatively for all but three patients, with IFN-γ levels ranging from 0.19 IU/ml to 0.52 IU/ml between the discordant TB1 and TB2 tubes on those three cases.
FIG 1.
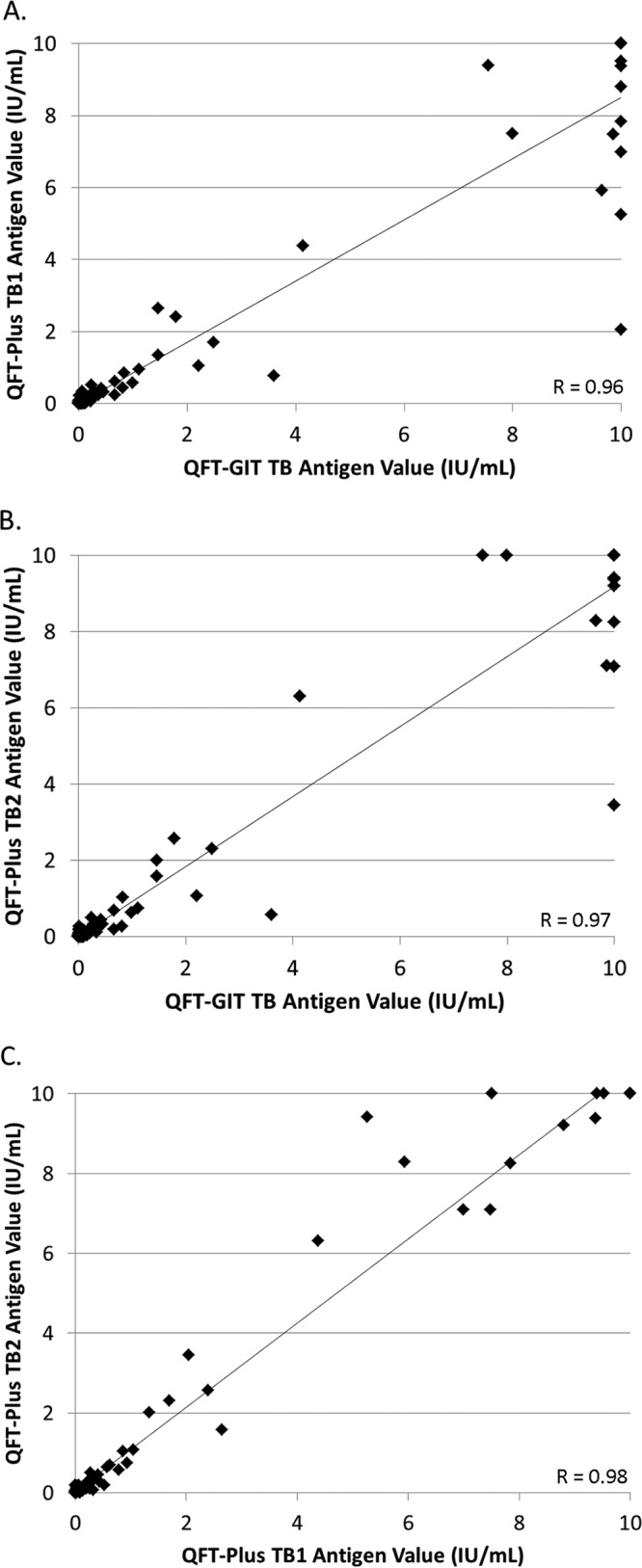
Correlation of QFT-GIT and QFT-Plus quantitative IFN-γ values. Graphs show correlations between quantitative results for the QFT-GIT TB antigen tube and QFT-Plus TB1 tube (A), the QFT-GIT TB antigen tube and QFT-Plus TB2 tube (B), and the QFT-Plus TB1 tube and QFT-Plus TB2 tube (C). R, Pearson's correlation coefficient.
DISCUSSION
This study compared the performance of the new FDA-cleared QFT-Plus IGRA to that of the QFT-GIT version of this assay using specimens collected from prospectively enrolled patients presenting to a county TB clinic and in both TST-reactive and TST-negative HCWs at Mayo Clinic, in Rochester, MN. We show high overall agreement (>95%) between the two QuantiFERON-TB IGRA versions in populations at either low or high risk for M. tuberculosis infection, including not only low-risk HCWs but also immigrants from areas endemic for TB and three patients with active TB disease. Studies evaluating the new QFT-Plus assay are continuing to emerge and have primarily been performed in patients outside North America with active TB infection or as part of TB contact investigations (9, 11, 12, 18, 19). Fewer reports have focused on the performance of this new IGRA in patients with LTBI (13, 20, 21). To date, a single manuscript evaluating this assay has been published from the United States, which compared the performance of the QFT-Plus to that of the QFT-GIT for TB screening of HCWs practicing in a low-TB-incidence setting (8). Our study adds to the growing body of evidence suggesting that the QFT-Plus IGRA performs equivalently to the QFT-GIT predicate for the assessment of patients at risk for LTBI and in HCWs.
Among the 161 subjects enrolled in our study, the QFT-GIT and QFT-Plus assays were qualitatively discordant in five (3.1%) individuals, and in all five cases, the QFT-GIT and QFT-Plus IFN-γ levels bordered the dichotomous 0.35 IU/ml cutoff for assay positivity, ranging from 0.19 IU/ml to 0.66 IU/ml. Prior reports assessing the reproducibility of the QFT-GIT IGRA have identified multiple factors (e.g., preanalytical, analytical, manufacturing, biologic, etc.), which can lead to assay variability as high as ±0.60 IU/ml around the cutoff, resulting in false-positive QFT results and conversion/reversion events (22–24). Given the borderline IFN-γ levels among the discordant samples, alongside our effort to minimize processing variables via side-by-side specimen collection, transport, preanalytical processing, and testing using an automated ELISA platform, the disparate results for these five patients may be a consequence of the intrinsic variability associated with the IFN-γ ELISA. Interestingly, two of these discordant patients (OCPHS-3 and OCPHS-4) had positive QFT-GIT results 2 to 8 years prior to enrollment into our study, with elevated IFN-γ levels (3.65 IU/ml to 7.55 IU/ml). These two patients received at least partial LTBI treatment prior to enrollment, which may explain the significantly decreased IFN-γ levels and provide an alternative cause for the discrepant results following retesting by the QFT-GIT and QFT-Plus assays. Of note, however, three other patients who were receiving LTBI treatment and four LTBI patients who had completed treatment at the time of enrollment were all positive by both QFT-IGRAs, supporting prior findings (specific to the QFT-GIT assay) that IFN-γ levels cannot be used as a measure of treatment response (25–29). Although our study has only a small number of LTBI patients on therapy or having completed a treatment regimen, our preliminary findings suggest that similar to the QFT-GIT, the qualitative QFT-Plus result cannot be relied upon as an indicator of treatment response for LTBI. Additional studies using a larger cohort of patients undergoing LTBI treatment are warranted, however, to assess the CD8+ T-cell response and its possible role in monitoring response to therapy.
Of the 100 HCWs with prior negative TST results, two were positive by both QFT IGRAs, and one was positive by only the QFT-Plus IGRA. The TB antigen IFN-γ levels all bordered the 0.35 IU/ml cutoff, except for one HCW who had a QFT-Plus TB2 antigen tube value of 1.04 IU/ml (TB1 value, 0.27 IU/ml). Mayo Clinic has a fairly low rate of TST skin conversions among HCWs, with only nine employee TST conversions occurring due to suspected workplace M. tuberculosis exposure over a nearly 17-year period (30). In a recently published study evaluating the QFT-GIT and QFT-Plus IGRAs for HCW TB screening, Moon and colleagues suggested a conservative approach for interpretation of the QFT-Plus qualitative result (8). Specifically, for HCWs with no known risk factors for TB working in a low-TB-incidence setting, they suggest that the QFT-Plus assay be considered positive only if both the TB1 and TB2 antigen tubes are greater than 0.35 IU/ml. Using these modified criteria, two of the three QFT-Plus-positive HCWs in our study would be considered QFT-Plus negative. While repeat testing and follow-up clinical findings were unavailable for these three HCWs, based on the known intrinsic variability of the IFN-γ ELISA and prior studies delineating the low positive predictive value of the QFT assays in low-risk HCWs, we speculate that the QFT-Plus and QFT-GIT assay results were falsely positive in these three employees (8, 31). As noted in numerous prior studies, the issues of variability and reproducibility around a single dichotomous cutoff can lead to challenges associated with result interpretation, particularly among low-risk patients or HCWs with positive IFN-γ values near the assay cutoff (22, 32). This limitation was acknowledged and addressed in the most recent American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention guidelines for the diagnosis of tuberculosis. The guidelines state that testing for LTBI should not be performed in individuals who are unlikely to be infected with M. tuberculosis; the guidelines also recognize that this practice occurs and recommend that low-risk individuals with an initially positive IGRA result be retested, with a negative result overriding the initial positive result (14). In theory, the modified QFT-Plus interpretive criteria, as defined by Moon and colleagues (8), would meet these recommendations (i.e., via individual assessment of the two TB antigen tubes) for low-risk individuals who require TB screening, although further validation of this alternative interpretive approach is needed.
Finally, we documented a high degree of correlation (R > 0.95) between the quantitative IFN-γ values from the QFT-GIT TB antigen tube and both the TB1 and TB2 QFT-Plus antigen tubes; this correlation is notably higher than those in previous studies performed in low-risk HCWs (R = 0.74 to 0.75) (8). A strong correlation was also observed between the QFT-Plus TB1 and TB2 antigen tubes, which may in part be explained by the higher level of precision reported for the QFT-Plus assay, an aspect particularly relevant for patients without a CD8+ T-cell response (33). Overall, our findings suggest that the absence of the TB7.7 antigen from the QFT-Plus IGRA does not significantly impact assay performance, consistent with conclusions from two prior studies (13, 18). These results notably differ, however, from those of a prior study, which documented higher IFN-γ levels by the QFT-GIT assay than by the QFT-Plus IGRA for patients with active TB infection or LTBI (9). While the patient populations evaluated by these studies differ, a definitive cause for these observed differences in IFN-γ levels is not immediately apparent. Additionally, among the 23 LTBI patients with positive QFT-Plus test results, we found similar levels of IFN-γ production in the TB1 and TB2 antigen tubes; only three enrolled subjects had qualitatively discordant results between the two TB antigen tubes, with IFN-γ values in these cases falling near the 0.35 IU/ml cutoff (0.19 to 0.52 IU/ml). Notably, TB2-specific CD8+ T-cell responses were more frequently observed in active TB disease cases versus patients with LTBI (44% versus 20%) in a small study from Italy; however, the only patient with active TB disease and a low CD4+ T-cell count in our study had indeterminate QFT-Plus and QFT-GIT results due to low mitogen values (34). While our data generally suggest equivalent performances between the two QFT-TB IGRAs, whether the addition of the TB2 antigen tube to the QFT-Plus IGRA provides a diagnostic advantage over the QFT-GIT version, particularly in patients with CD8+ T-cell-reliant disease states (e.g., HIV infection with low CD4 counts), remains to be defined.
To our knowledge, this is the first prospective study to compare QFT-GIT and QFT-Plus IGRAs across the spectrum of TB infection from low-risk or previously treated HCWs to immigrants from areas endemic for TB undergoing evaluation or follow-up for LTBI or active TB infection in a mid-to-low-TB-incidence setting in the United States. The limitations of our study include the lack of an LTBI diagnostic reference standard and use of the QFT-GIT IGRA to establish a clinical diagnosis of LTBI (thus introducing bias) and therefore an inability to resolve discordant results between the two IGRAs. Also, the majority of the enrolled subjects were immunocompetent, which precludes us from comparing the performances of these IGRAs in patient populations at high risk for LTBI and active TB disease. Last, while the QFT-Plus assay allows for WB collection into a lithium-heparin tube prior to aliquoting into the QFT-Plus tubes, this practice is not supported by the instructions in the QFT-GIT package insert. However, in an effort to alleviate the anxiety expressed by immigrants and refugees due to cultural beliefs and/or practices with respect to blood draws into more than one tube, WB from the lithium-heparin tube was also aliquoted into the QFT-GIT tubes. Notably, the high correlation between IFN-γ levels from the QFT-GIT and QFT-Plus levels suggests that deviation from the QFT-GIT specimen collection procedure still provided valid results.
In conclusion, we show high correlation and comparable results between the QFT-GIT and QFT-Plus assays in our study population composed of subjects across a spectrum of TB infection in a medium-to-low-TB-incidence area in the United States. Our findings suggest that the necessary transition to the QFT-Plus assay due to discontinuation of the QFT-GIT IGRA reagents will be associated with a minimal difference in assay performance characteristics.
ACKNOWLEDGMENTS
We thank all clinicians and language interpreters at the OCPHS TB Clinic for their assistance with patient enrollment and support of this study. We acknowledge Deke Haefner and Cassie DeMars from Mayo Validation Support Services and Shawn Einerson from the Mayo Clinic Department of Laboratory Medicine and Pathology Biospecimens Program for their invaluable assistance with this study. Additionally, we thank Robin Molella and Dawn Beck for their support of this project. Finally, we would like to acknowledge Qiagen for providing the QFT-Plus collection tubes and IFN-γ ELISAs. Qiagen had no role in the study design, data collection or interpretation.
REFERENCES
- 1.Horsburgh CR., Jr 2004. Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 350:2060–2067. doi: 10.1056/NEJMsa031667. [DOI] [PubMed] [Google Scholar]
- 2.Getahun H, Matteelli A, Chaisson RE, Raviglione M. 2015. Latent Mycobacterium tuberculosis infection. N Engl J Med 372:2127–2135. doi: 10.1056/NEJMra1405427. [DOI] [PubMed] [Google Scholar]
- 3.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. 2014. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozot V, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, Perreau M, Lazor-Blanchet C, Petruccioli E, Hanekom W, Goletti D, Bart PA, Nicod L, Pantaleo G, Harari A. 2013. Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur J Immunol 43:1568–1577. doi: 10.1002/eji.201243262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day CL, Abrahams DA, Lerumo L, Janse van Rensburg E, Stone L, O'Rie T, Pienaar B, de Kock M, Kaplan G, Mahomed H, Dheda K, Hanekom WA. 2011. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J Immunol 187:2222–2232. doi: 10.4049/jimmunol.1101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolova M, Markova R, Drenska R, Muhtarova M, Todorova Y, Dimitrov V, Taskov H, Saltini C, Amicosante M. 2013. Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn Microbiol Infect Dis 75:277–281. doi: 10.1016/j.diagmicrobio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland JS, Young JM, Peterson KL, Sanneh B, Whittle HC, Rowland-Jones SL, Adegbola RA, Jaye A, Ota MO. 2010. Polyfunctional CD4+ and CD8+ T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. J Immunol 184:6537–6544. doi: 10.4049/jimmunol.1000399. [DOI] [PubMed] [Google Scholar]
- 8.Moon HW, Gaur RL, Tien SS, Spangler M, Pai M, Banaei N. 2017. Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 55:1650–1657. doi: 10.1128/JCM.02498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann H, Avsar K, Gores R, Mavi SC, Hofmann-Thiel S. 2016. Equal sensitivity of the new generation QuantiFERON-TB Gold plus in direct comparison with the previous test version QuantiFERON-TB Gold IT. Clin Microbiol Infect 22:701–703. doi: 10.1016/j.cmi.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Barcellini L, Borroni E, Brown J, Brunetti E, Campisi D, Castellotti PF, Codecasa LR, Cugnata F, Di Serio C, Ferrarese M, Goletti D, Lipman M, Rancoita PM, Russo G, Tadolini M, Vanino E, Cirillo DM. 2016. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J 48:1411–1419. doi: 10.1183/13993003.00510-2016. [DOI] [PubMed] [Google Scholar]
- 11.Telisinghe L, Amofa-Sekyi M, Maluzi K, Kaluba-Milimo D, Cheeba-Lengwe M, Chiwele K, Kosloff B, Floyd S, Bailey SL, Ayles H. 2017. The sensitivity of the QuantiFERON-TB Gold Plus assay in Zambian adults with active tuberculosis. Int J Tuberc Lung Dis 21:690–696. doi: 10.5588/ijtld.16.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takasaki J, Manabe T, Morino E, Muto Y, Hashimoto M, Iikura M, Izumi S, Sugiyama H, Kudo K. 2018. Sensitivity and specificity of QuantiFERON-TB Gold Plus compared with QuantiFERON-TB Gold In-Tube and T-SPOT.TB on active tuberculosis in Japan. J Infect Chemother 24:188–192. doi: 10.1016/j.jiac.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Petruccioli E, Vanini V, Chiacchio T, Cuzzi G, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2017. Analytical evaluation of QuantiFERON-Plus and QuantiFERON-Gold In-Tube assays in subjects with or without tuberculosis. Tuberculosis (Edinb) 106:38–43. doi: 10.1016/j.tube.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, Keane J, Lewinsohn DA, Loeffler AM, Mazurek GH, O'Brien RJ, Pai M, Richeldi L, Salfinger M, Shinnick TM, Sterling TR, Warshauer DM, Woods GL. 2017. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 64:111–115. doi: 10.1093/cid/ciw778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen PA, Lambert LA, Iademarco MF, Ridzon R. 2005. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 54:1–141. [PubMed] [Google Scholar]
- 16.Dobler CC, Farah WH, Alsawas M, Mohammed K, Breeher LE, Murad MH, Molella RG. 2017. Tuberculin skin test conversions and occupational exposure risk in US healthcare workers. Clin Infect Dis 66:706–711. doi: 10.1093/cid/cix861. [DOI] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 18.Yi L, Sasaki Y, Nagai H, Ishikawa S, Takamori M, Sakashita K, Saito T, Fukushima K, Igarashi Y, Aono A, Chikamatsu K, Yamada H, Takaki A, Mori T, Mitarai S. 2016. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 6:30617. doi: 10.1038/srep30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, Dal Monte P, Di Serio C, Goletti D, Lombardi G, Lipman M, Rancoita PM, Tadolini M, Cirillo DM. 2016. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J 47:1587–1590. doi: 10.1183/13993003.02033-2015. [DOI] [PubMed] [Google Scholar]
- 20.Pieterman ED, Liqui Lung FG, Verbon A, Bax HI, Ang CW, Berkhout J, Blaauw G, Brandenburg A, van Burgel ND, Claessen A, van Dijk K, Heron M, Hooghiemstra M, Leussenkamp-Hummelink R, van Lochem E, van Loo IHM, Mulder B, Ott A, Pontesilli O, Reuwer A, Rombouts P, Saegeman V, Scholing M, Vainio S, de Steenwinkel JEM. 2018. A multicentre verification study of the QuantiFERON-TB Gold Plus assay. Tuberculosis (Edinb) 108:136–142. doi: 10.1016/j.tube.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Igari H, Ishikawa S, Nakazawa T, Oya Y, Futami H, Tsuyuzaki M, Suzuki K, Matsumura R. 2018. Lymphocyte subset analysis in QuantiFERON-TB Gold Plus and T-Spot.TB for latent tuberculosis infection in rheumatoid arthritis. J Infect Chemother 24:110–116. doi: 10.1016/j.jiac.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. 2013. Test variability of the QuantiFERON-TB Gold In-Tube assay in clinical practice. Am J Respir Crit Care Med 187:206–211. doi: 10.1164/rccm.201203-0430OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitworth WC, Hamilton LR, Goodwin DJ, Barrera C, West KB, Racster L, Daniels LJ, Chuke SO, Campbell BH, Bohanon J, Jaffar AT, Drane W, Maserang D, Mazurek GH. 2012. Within-subject interlaboratory variability of QuantiFERON-TB Gold In-Tube tests. PLoS One 7:e43790. doi: 10.1371/journal.pone.0043790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banaei N, Gaur RL, Pai M. 2016. Interferon gamma release assays for latent tuberculosis: what are the sources of variability? J Clin Microbiol 54:845–850. doi: 10.1128/JCM.02803-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiappini E, Fossi F, Bonsignori F, Sollai S, Galli L, de Martino M. 2012. Utility of interferon-gamma release assay results to monitor anti-tubercular treatment in adults and children. Clin Ther 34:1041–1048. doi: 10.1016/j.clinthera.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Adetifa IM, Ota MO, Jeffries DJ, Lugos MD, Hammond AS, Battersby NJ, Owiafe PK, Donkor SD, Antonio M, Ibanga HB, Brookes RH, Aka P, Walton R, Adegbola RA, Hill PC. 2013. Interferon-gamma ELISPOT as a biomarker of treatment efficacy in latent tuberculosis infection: a clinical trial. Am J Respir Crit Care Med 187:439–445. doi: 10.1164/rccm.201208-1352OC. [DOI] [PubMed] [Google Scholar]
- 27.Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. 2006. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am J Respir Crit Care Med 174:831–839. doi: 10.1164/rccm.200511-1783OC. [DOI] [PubMed] [Google Scholar]
- 28.Chee CB, KhinMar KW, Gan SH, Barkham TM, Pushparani M, Wang YT. 2007. Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. Am J Respir Crit Care Med 175:282–287. doi: 10.1164/rccm.200608-1109OC. [DOI] [PubMed] [Google Scholar]
- 29.Escalante P, Peikert T, Van Keulen VP, Erskine CL, Bornhorst CL, Andrist BR, McCoy K, Pease LR, Abraham RS, Knutson KL, Kita H, Schrum AG, Limper AH. 2015. Combinatorial immunoprofiling in latent tuberculosis infection. Toward better risk stratification. Am J Respir Crit Care Med 192:605–617. doi: 10.1164/rccm.201412-2141OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobler CC, Murad MH. 2017. Interpreting diagnostic tests with continuous results and no gold standard: a common scenario explained using the tuberculin skin test. Evid Based Med 22:199–201. doi: 10.1136/ebmed-2017-110825. [DOI] [PubMed] [Google Scholar]
- 31.Dorman SE, Belknap R, Graviss EA, Reves R, Schluger N, Weinfurter P, Wang Y, Cronin W, Hirsch-Moverman Y, Teeter LD, Parker M, Garrett DO, Daley CL, Tuberculosis Epidemiologic Studies Consortium. 2014. Interferon-gamma release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med 189:77–87. doi: 10.1164/rccm.201302-0365OC. [DOI] [PubMed] [Google Scholar]
- 32.Nemes E, Rozot V, Geldenhuys H, Bilek N, Mabwe S, Abrahams D, Makhethe L, Erasmus M, Keyser A, Toefy A, Cloete Y, Ratangee F, Blauenfeldt T, Ruhwald M, Walzl G, Smith B, Loxton AG, Hanekom WA, Andrews JR, Lempicki MD, Ellis R, Ginsberg AM, Hatherill M, Scriba TJ, C-040-404 Study Team, the Adolescent Cohort Study Team. 2017. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis infection. Am J Respir Crit Care Med 196:638–648. doi: 10.1164/rccm.201704-0817OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher D, Manissero D, Stocking C, Pyne C. 2016. Preliminary data on precision of QuantiFERON-TB Plus performance. Eur Respir J 48:953–954. doi: 10.1183/13993003.00596-2016. [DOI] [PubMed] [Google Scholar]
- 34.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2016. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect 73:588–597. doi: 10.1016/j.jinf.2016.09.008. [DOI] [PubMed] [Google Scholar]


