ABSTRACT
Currently, diagnosis of Pneumocystis jirovecii pneumonia (PJP) relies on analysis of lower respiratory specimens, either by microscopy or quantitative real-time PCR (qPCR). Thus, bronchoscopy is required, which is associated with increased risk of respiratory failure. We assessed the value of noninvasive serologic β-d-glucan (BDG) testing for laboratory diagnosis of PJP using a newly available turbidimetric assay. We identified 73 cases of PJP with positive qPCR results from lower respiratory specimens for Pneumocystis and serology samples dating from 1 week before to 4 weeks after qPCR. In addition, 25 sera from controls with suspected PJP but specimens negative for Pneumocystis by qPCR were identified. Sera were tested with a turbidimetric BDG assay (Fujifilm Wako Chemicals Europe GmbH, Neuss, Germany), using an 11-pg/ml cutoff. Sensitivity and specificity were calculated based on qPCR test results as a reference. The turbidimetric BDG assay identified 63/73 patients with positive or slightly positive qPCR tests for an overall sensitivity of 86%; after exclusion of cases with only slightly positive qPCR results, sensitivity was 91%. No correlation between serum BDG levels and respiratory specimen DNA levels was found. Serologic BDG testing was negative in 25/25 controls with negative qPCR for a specificity of 100% using the predefined cutoff. In 22/25 samples (88%), no BDG was detected. Serologic BDG testing using the turbidimetric assay showed high sensitivity and specificity compared to qPCR of lower respiratory specimens for the diagnosis of PJP. Both turnover time and test performance will allow clinicians to delay or in some cases forego bronchoscopy.
KEYWORDS: β-d-glucan, BDG, Fungitell, PJP, Pneumocystis, Wako, human immunodeficiency virus
INTRODUCTION
Pneumocystis jirovecii is an opportunistic fungal pathogen that can cause life-threatening pneumonia associated with high morbidity and mortality. Individuals with impaired immunity are at increased risk for P. jirovecii pneumonia (PJP). Patients suffering from PJP are in most cases HIV-infected individuals with low CD4+ T-lymphocyte count (1). According to the CDC classification system, HIV-positive patients with the diagnosis of PJP are suffering from AIDS. However, PJP is being diagnosed increasingly among patients on immunosuppressive regimens— e.g., in the context of hematological malignancies or autoimmune disorders (2, 3).
Initial clinical signs of PJP such as fever and weight loss are often nonspecific. Later on, the symptoms can culminate in life-threatening respiratory failure necessitating life support in intensive care units (3, 4). Early diagnosis is an essential requirement for the targeted therapy of PJP and has a severe impact on the clinical outcome (4). Diagnosis of PJP is complicated by nonspecific radiological and clinical findings. Hence, definitive diagnosis relies on microscopic examination or quantitative real-time PCR (qPCR) of bronchoalveolar lavage (BAL) fluid (5, 6). However, BAL is an invasive and resource-intensive procedure. It is associated with physical stress for the patient and increases the risk of respiratory failure.
A contributive laboratory tool for the diagnosis of PJP is the detection of β-d-glucan (BDG), which is a major constituent of fungal cell walls (5, 6). This antigen can be found in the blood of patients suffering from PJP but also other invasive fungal infections, such as candidiasis or aspergillosis. Several tests for the detection of BDG in human blood are commercially available (7, 8). Of these, only the FDA-approved (2004) and CE-marked (2008) Fungitell BDG assay (Associates of Cape Cod, East Falmouth, MA) is commonly used in Europe and America. This assay is challenging for microbiological laboratories due to methodological and economic reasons (9). For example, the test is designed to analyze only 21 samples in duplicate in order to reduce the high risk of contamination. Each assay run requires 12 standards and controls, which is basically prohibitive for testing single samples. Therefore, BDG testing still is rarely provided in emergency settings such as acute PJP. This fact and the low incidence of PJP result in a very limited number of larger-scale studies evaluating the usefulness of BDG testing in the setting of PJP (10, 11). In this study, which is based on 73 clinical cases that were tested positive for P. jirovecii DNA in respiratory specimens, we evaluated the sensitivity and specificity of a recently CE-marked turbidimetric BDG assay (Fujifilm Wako Chemicals Europe GmbH, Neuss, Germany) newly available in Europe as a diagnostic test to detect PJP. The assay is approved for the diagnosis of invasive fungal infections and was originally developed for the analysis of plasma specimens. We show that serum is an appropriate specimen type and demonstrate that serologic BDG testing using this assay results in a high sensitivity and specificity for laboratory diagnosis of PJP.
MATERIALS AND METHODS
Study population and data collection.
Between January 2011 and August 2017, 104 patients at the 2,000-bed University of Munich medical center tested positive for P. jirovecii DNA by qPCR from respiratory tract specimens. Corresponding serum samples from 73 cases were accessible, which dated from 1 week before to 4 weeks after diagnosis. The vast majority of sera (95%) were obtained within 7 days before or after qPCR-based testing. Twenty-six (36%) of the patients were positive for HIV, 21 (29%) were suffering from hematological malignancy, and 14 (19%) received immunosuppressive therapy (for more details, see Table 1). Two patients were suffering from end-stage solid organ malignancies and one individual from innate immunodeficiency. Information about the underlying diseases and conditions of the remaining cases was not available due to data protection requirements.
TABLE 1.
Demographic characteristics and underlying disease of included patients
| Parameter | qPCR result |
|
|---|---|---|
| Positive | Negative | |
| Patients, no. | 73 | 25 |
| Age (mean), yr | 51 | 42 |
| Female, no. (%) | 23 (32) | 8 (32) |
| Underlying disease, no. (%) | ||
| HIV | 26 (36) | 9 (36) |
| Hematological malignancy | 21 (29) | 16 (64) |
| Immunosuppressive therapy | 14 (19) | 0 |
| Others/not defined | 12 (16) | 0 |
Samples of 25 randomly selected patients who had clinical signs of PJP but tested negative for P. jirovecii DNA from BAL fluids by qPCR during the same period were used as a control group. This group comprised 16 patients suffering from hematological malignancies and 9 patients infected with HIV. Demographic and clinical characteristics of the control group are also presented in Table 1.
This retrospective study was reviewed and approved by the ethics committee of our university hospital (Ethikkommission der Medizinischen Fakultät der LMU München); a waiver of informed consent was granted. The following basic clinical information was retrospectively analyzed using the test request forms: age, sex, and underlying disease. For this study, sample processing and data analysis were performed anonymously.
P. jirovecii qPCR.
PJP testing was performed on all samples using an in-house qPCR assay targeting the gene coding for P. jirovecii β-tubulin. The specimens analyzed were either BAL fluid (95%) or in fewer cases tracheal suction fluid. Detection of >1,000 genome equivalents (geq)/μl was classified as “positive,” 100 to 1,000 geq/μl as “slightly positive,” and <100 geq/μl (representing the limit of detection) as “negative.” The categorization was based on a previous in-house validation, where specimens found to be microscopically positive for P. jirovecii cysts in Grocott's methenamine silver stain were defined as “positive” results in qPCR testing and specimens that were microscopically negative but yielded positive qPCR results were defined as “slightly positive” qPCR results. This is in agreement with current guidelines (5).
BDG testing.
BDG in serum samples was measured using a turbidimetric BDG assay (Wako BDG assay) following the manufacturer's instructions. Clinical information and reference standard results were not available to the personnel performing and reading the test. According to the test protocol, BDG levels of ≥11 pg/ml were classified positive, indicating an invasive fungal infection. The limit of detection of the test is 6 pg/ml BDG according to specifications provided by the manufacturer. Samples without BDG are reported as <2.26 pg/ml for technical reasons.
RESULTS
In qPCR-positive patients, the sensitivity of BDG testing is >90%.
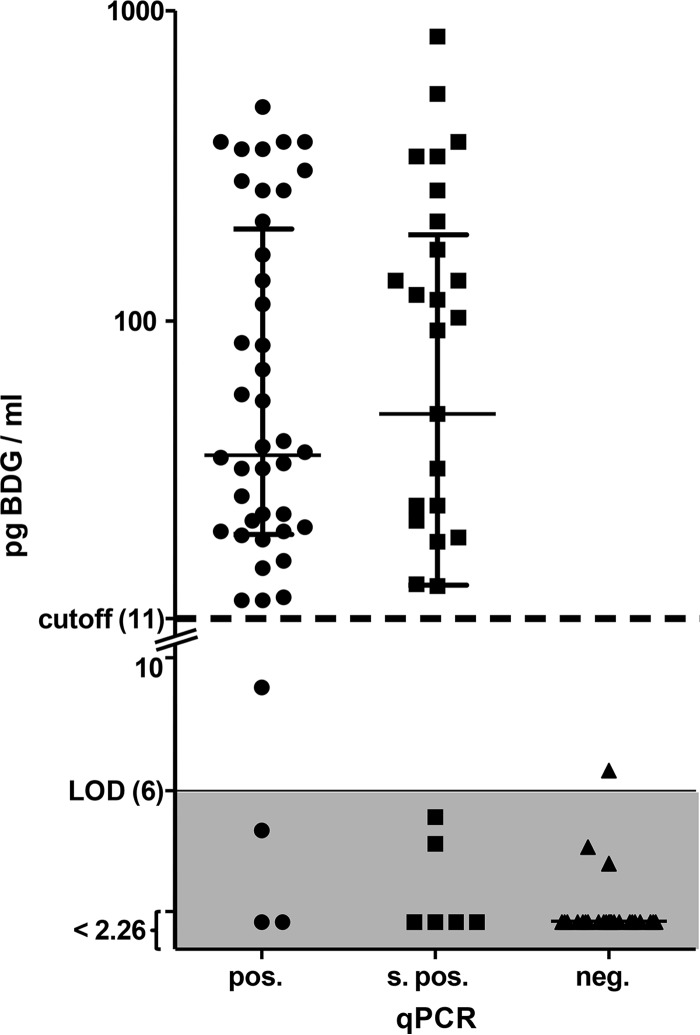
The sera of 73 patients with evidence of P. jirovecii DNA in lower respiratory samples were tested for BDG according to the manufacturer's instructions. The test yielded positive results for 63 of 73 patients (86%). When patients with slightly positive qPCR results were excluded, the sensitivity increased to 91%. The BDG assay was positive in 24 out of 26 cases, with HIV infection resulting in a sensitivity of 92% for this patient group. In one HIV-infected individual with positive qPCR, serum BDG content was determined to be 9.1 pg/ml and thereby missed the cutoff of 11 pg/ml by a narrow margin. The concentration of BDG in positive samples (>11 pg/ml) ranged from 12.6 up to 827 pg/ml (Fig. 1). This broad distribution of measurement results was found in all subgroups of underlying disease, age, or sex (data not shown). Interestingly, no correlation between BDG levels in serum and DNA levels in respiratory tract samples was found.
FIG 1.
Box plot (with median and interquartile ranges) of the BDG content (picograms per milliliter) in serum of patients with positive (pos.), slightly positive (s. pos.), and negative (neg.) qPCR results. The y axis is logarithmic for values of >10 pg/ml and linear for values of <10 pg/ml, as indicated by the break in the axis. Dotted and solid lines indicate the cutoff (11 pg/ml) and the limit of detection (LOD; 6 pg/ml) as specified by the manufacturer. Results beneath the LOD are plotted in the shaded area. BDG not detected is reported as <2.26 pg/ml for technical reasons.
BDG testing is highly specific in patients with suspected PJP.
We performed BDG testing in serum samples from 25 patients with clinical signs compatible with PJP but negative qPCR results for P. jirovecii from BAL fluids. All serum samples yielded BDG concentrations clearly below the cutoff. Twenty-two of 25 samples did not contain any detectable BDG. This indicates a high specificity of BDG testing in patients with suspected PJP.
DISCUSSION
Only a limited number of larger-scale studies focusing on BDG testing for diagnosis of PJP have been published (10, 11). To our knowledge, at least three BDG tests are commercially available: the Fungitell assay (Associates of Cape Cod), the Wako BDG assay (Fujifilm Wako Pure Chemical Corporation, Ltd., Osaka, Japan), and the Fungitec-G tests (Seikagaku, Kogyo, Tokyo, Japan). Of those, only the Fungitell assay has been FDA approved (in 2004) and CE marked (in 2008). The two other tests have been used in routine diagnostics in Japan for 20 years, but have not been FDA-approved and CE-marked, thereby limiting their application in routine diagnostics in Europe and the United States. Recently, the Wako BDG assay obtained CE marking in Europe. The test principles of all three tests rely on the same enzymatic cascades extracted from a Limulus (horseshoe crab) species. The Fungitell and Fungitec-G tests are colorimetric assays, while the Wako assay is turbidimetric.
While the Fungitell assay is designed for the analysis of serum samples, plasma was recommended for the Wako BDG assay in Japan. However, clinicians in Europe are used to collecting serum samples for infectious disease serology. Because of this, one aim of the present study was to evaluate the suitability of serum for the detection of BDG using the Wako assay. Since pairwise plasma and serum samples were not available for this study, a direct comparison of the different specimens was not possible. Another limitation is the fact that not all sera were sampled simultaneously with the corresponding BAL fluid or tracheal suction sample. This could potentially affect the direct comparability of the qPCR and BDG test results and the conclusions drawn from it. Nevertheless, our results indicate that antigenemia can be expected days before and after positive qPCR results, and the Wako test can detect BDG in serum of PJP patients with high sensitivity.
The sensitivities were 86% in the pooled cohort, 83% in the non-HIV group, and 92% in the HIV-infected subgroup (Table 2). This is in good agreement with a recent meta-analysis by Li et al. (sensitivities of 85 and 92%, respectively) (12) and suggests that BDG-testing for laboratory diagnosis of PJP could be best utilized in the high-risk group of HIV-infected patients. In our study, we identified PJP cases via qPCR, while diagnosis in previous studies typically relied on microscopy (12). Molecular detection of P. jirovecii results in higher sensitivity compared to staining (13). If the cases with slightly positive qPCR results, which are expected to be negative in microscopy, are excluded from the analysis, the sensitivity of BDG testing rises to 91% in the non-HIV-infected subgroup. Until now, a commonly accepted case definition for PJP has not been established. Therefore, qPCR was defined as the reference test method for this study. Hence, the study design puts serology at a disadvantage compared to PCR-based diagnostics and probably underestimates the performance of BDG testing.
TABLE 2.
Sensitivity and specificity of BDG testing for different subgroups
| Patient group | Sensitivity (%) | Specificity (%) |
|---|---|---|
| All patients | 86 | 100 |
| qPCR positive | 91 | |
| qPCR slightly positive | 79 | |
| HIV positive | 92 | 100 |
| qPCR positive | 90 | |
| qPCR slightly positive | 100 | |
| HIV negative | 83 | 100 |
| qPCR positive | 91 | |
| qPCR slightly positive | 75 |
We expected that the fungal load would correlate with the respective genome equivalents determined by qPCR from respiratory specimens and the BDG levels detected in serum. Surprisingly, there was no correlation. This suggests that serum BDG levels do not necessarily reflect the fungal load present in respiratory specimens. Besides this, slightly positive qPCR results were more frequently obtained in non-HIV patients (24 of 29 cases [83%]), which could match the lower fungal burden previously found in non-HIV-infected individuals with PJP (14). Of these 24 cases, 18 (75%) were found to be positive in BDG testing. Taken together, it can be speculated whether BDG testing is a marker for the invasiveness of the disease and could be a helpful tool to distinguish between colonization and infection, which remains challenging for PCR-based diagnostics (15, 16).
We found the specificity of the Wako BDG test to be 100% in a control group of 25 individuals with comparable underlying diseases and clinical suspicion of PJP. In contrast, in larger-scale studies using colorimetric tests (e.g., Fungitell or Fungitec G [Nissui Pharmaceutical]), specificities ranged from 65 to 92% (10, 11, 17). High specificity is a well-known feature of turbidimetric BDG testing reported in studies focusing on invasive candidiasis and invasive aspergillosis (8). It has to be kept in mind that BDG is a panfungal marker that is not specific for Pneumocystis (18). However, clinical symptoms and distinct radiological findings typically allow for discrimination between PJP and other causative agents (19, 20).
The role of BDG testing in PJP is still a matter of debate in different guidelines (5, 6, 21). For patients with hematological malignancies and stem cell transplant recipients with suspicion of PJP, BDG testing is recommended in situations when BAL is not applicable (5). According to the guidelines of the European Conference on Infections in Leukemia (ECIL), negative results in the BDG assay can rule out the suspicion of PJP (5). Our data also confirm the value of a positive test result: There was not a single false-positive result in the control group, which consisted of patients with the suspicion of PJP and respective risk factors: i.e., HIV infection or hematological malignancy. Therefore, immediate BDG testing can be applied as an adjunctive tool to establish the indication for or against BAL. This could potentially prevent complications, such as the need for prolonged ventilation after BAL in unstable patients. Compared to the alternative colorimetric BDG tests, the Wako assay is probably more cost-efficient for single-sample testing and reports positive results typically within 45 min. In the clinical setting, a rapid turnover time of laboratory BDG-based testing for P. jirovecii will be critical for the diagnosis or exclusion of PJP, potentially allowing the physician to postpone or even skip BAL.
In summary, we were able to show that the newly available turbidimetric BDG assay is a valuable diagnostic tool in the setting of PJP. To our knowledge, this is the second largest study to evaluate the suitability of BDG testing for the diagnosis or exclusion of PJP and the largest study to evaluate the turbidimetric Wako BDG assay in this context. In comparison to the published data on the well-known Fungitell assay, this study demonstrates equivalent sensitivity and likely indicates superior specificity.
ACKNOWLEDGMENTS
This study was supported by Fujifilm Wako Chemicals Europe GmbH.
We thank Helen Müller for technical support.
REFERENCES
- 1.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, Holmberg S, Jones JL. 2000. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis 30(Suppl 1):S5–S14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 2.Avino LJ, Naylor SM, Roecker AM. 2016. Pneumocystis jirovecii pneumonia in the non-HIV-infected population. Ann Pharmacother 50:673–679. doi: 10.1177/1060028016650107. [DOI] [PubMed] [Google Scholar]
- 3.Cordonnier C, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Alanio A, Hauser PM, Lagrou K, Melchers WJ, Helweg-Larsen J, Matos O, Bretagne S, Maertens J. 2016. Pneumocystis jirovecii pneumonia: still a concern in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 71:2379–2385. doi: 10.1093/jac/dkw155. [DOI] [PubMed] [Google Scholar]
- 4.Limper AH, Adenis A, Le T, Harrison TS. 2017. Fungal infections in HIV/AIDS. Lancet Infect Dis 17:e334–e343. doi: 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- 5.Alanio A, Hauser PM, Lagrou K, Melchers WJG, Helweg-Larsen J, Matos O, Cesaro S, Maschmeyer G, Einsele H, Donnelly JP, Cordonnier C, Maertens J, Bretagne S. 2016. ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 71:2386–2396. doi: 10.1093/jac/dkw156. [DOI] [PubMed] [Google Scholar]
- 6.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. July 2017. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf.
- 7.Yu J, Li R-Y, Gao L-J, Lu Q-Y, Wang X-H. 2010. Utility of galactomannan enzyme immunoassay and (1,3)beta-d-glucan assay in invasive fungal infection. Zhonghua Yi Xue Za Zhi 90:371–374. (In Chinese.) [PubMed] [Google Scholar]
- 8.Yoshida K, Shoji H, Takuma T, Niki Y. 2011. Clinical viability of Fungitell, a new (1→3)-β-D: -glucan measurement kit, for diagnosis of invasive fungal infection, and comparison with other kits available in Japan. J Infect Chemother 17:473–477. doi: 10.1007/s10156-010-0198-6. [DOI] [PubMed] [Google Scholar]
- 9.Tran T, Beal SG. 2016. Application of the 1,3-β-d-glucan (Fungitell) assay in the diagnosis of invasive fungal infections. Arch Pathol Lab Med 140:181–185. doi: 10.5858/arpa.2014-0230-RS. [DOI] [PubMed] [Google Scholar]
- 10.Sax PE, Komarow L, Finkelman MA, Grant PM, Andersen J, Scully E, Powderly WG, Zolopa AR. 2011. Blood (1->3)-beta-d-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis 53:197–202. doi: 10.1093/cid/cir335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salerno D, Mushatt D, Myers L, Zhuang Y, de la Rua N, Calderon EJ, Welsh DA. 2014. Serum and BAL beta-d-glucan for the diagnosis of Pneumocystis pneumonia in HIV positive patients. Respir Med 108:1688–1695. doi: 10.1016/j.rmed.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li W-J, Guo Y-L, Liu T-J, Wang K, Kong J-L. 2015. Diagnosis of pneumocystis pneumonia using serum (1-3)-β-d-glucan: a bivariate meta-analysis and systematic review. J Thorac Dis 7:2214–2225. doi: 10.3978/j.issn.2072-1439.2015.12.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakefield AE, Pixley FJ, Banerji S, Sinclair K, Miller RF, Moxon ER, Hopkin JM. 1990. Detection of Pneumocystis carinii with DNA amplification. Lancet 336:451–453. doi: 10.1016/0140-6736(90)92008-6. [DOI] [PubMed] [Google Scholar]
- 14.Alanio A, Desoubeaux G, Sarfati C, Hamane S, Bergeron A, Azoulay E, Molina JM, Derouin F, Menotti J. 2011. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect 17:1531–1537. doi: 10.1111/j.1469-0691.2010.03400.x. [DOI] [PubMed] [Google Scholar]
- 15.Hauser PM, Bille J, Lass-Flörl C, Geltner C, Feldmesser M, Levi M, Levi M, Patel H, Muggia V, Alexander B, Hughes M, Follett SA, Cui X, Leung F, Morgan G, Moody A, Perlin DS, Denning DW. 2011. Multicenter, prospective clinical evaluation of respiratory samples from subjects at risk for Pneumocystis jirovecii infection by use of a commercial real-time PCR assay. J Clin Microbiol 49:1872–1878. doi: 10.1128/JCM.02390-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mühlethaler K, Bögli-Stuber K, Wasmer S, von Garnier C, Dumont P, Rauch A, Mühlemann K, Garzoni C. 2012. Quantitative PCR to diagnose Pneumocystis pneumonia in immunocompromised non-HIV patients. Eur Respir J 39:971–978. doi: 10.1183/09031936.00095811. [DOI] [PubMed] [Google Scholar]
- 17.Esteves F, Lee C-H, de Sousa B, Badura R, Seringa M, Fernandes C, Gaspar JF, Antunes F, Matos O. 2014. (1-3)-Beta-d-glucan in association with lactate dehydrogenase as biomarkers of Pneumocystis pneumonia (PcP) in HIV-infected patients. Eur J Clin Microbiol Infect Dis 33:1173–1180. doi: 10.1007/s10096-014-2054-6. [DOI] [PubMed] [Google Scholar]
- 18.Obayashi T, Yoshida M, Mori T, Goto H, Yasuoka A, Iwasaki H, Teshima H, Kohno S, Horiuchi A, Ito A, Yamaguchi H. 1995. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17–20. doi: 10.1016/S0140-6736(95)91152-9. [DOI] [PubMed] [Google Scholar]
- 19.Greene RE, Schlamm HT, Oestmann J-W, Stark P, Durand C, Lortholary O, Wingard JR, Herbrecht R, Ribaud P, Patterson TF, Troke PF, Denning DW, Bennett JE, de Pauw BE, Rubin RH. 2007. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis 44:373–379. doi: 10.1086/509917. [DOI] [PubMed] [Google Scholar]
- 20.Kanne JP, Yandow DR, Meyer CA. 2012. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR Am J Roentgenol 198:W555–W561. doi: 10.2214/AJR.11.7329. [DOI] [PubMed] [Google Scholar]
- 21.Cooley L, Dendle C, Wolf J, Teh BW, Chen SC, Boutlis C, Thursky KA. 2014. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J 44:1350–1363. doi: 10.1111/imj.12599. [DOI] [PubMed] [Google Scholar]