Abstract
Background
A mouse model of subarachnoid hemorrhage (SAH) investigated the effects of melatonin treatment on the generation of reactive oxygen species (ROS) and the activation of the SIRT3 gene in early brain injury (EBI).
Material/Methods
Male C57BL/6J mice were assigned to three groups: the SAH group; the sham group; and the SAH + melatonin-treated group (intraperitoneal dose, 150 mg/kg). TUNEL was used to study apoptosis of neuronal cells, Western-blot and immunohistochemistry detected expression of Sirt3, Bcl-2, superoxide dismutase 2 (SOD2), Bax, and cleaved caspase-3. Real-time polymerase chain reaction (PCR) and a luciferase reporter assay evaluated the effects of melatonin on SIRT3 gene expression. Malondialdehyde (MDA) and the reactive oxygen species (ROS) scavenger, reduced glutathione (GSH), and its ratio with oxidized glutathione (GSSG) was measured.
Results
The increase in neurological score and increase in cerebral edema following SAH were reduced in the SAH + melatonin-treated group. Neuronal apoptosis following SAH was reduced in the SAH + melatonin-treated group. Increased levels of SOD2, Bax, and cleaved caspase-3 following SAH were reduced in the SAH + melatonin-treated group; reduced levels of Sirt3 and Bcl-2 following SAH were increased in the SAH + melatonin-treated group. The GSH: GSSG ratio was increased, and the MDA level was decreased when melatonin treatment was used following SAH. Melatonin upregulated SIRT3 expression by increasing the transcription efficiency of the SIRT3 promoter in human glioma cell lines U87 and U251.
Conclusions
Melatonin provided protection from the effects of EBI following SAH by regulating the expression of murine SIRT3.
MeSH Keywords: Brain Injuries, Melatonin, Reactive Oxygen Species, Sirtuin 3, Subarachnoid Hemorrhage
Background
Subarachnoid hemorrhage (SAH) is a serious clinical condition that is usually due to rupture of an intracerebral aneurysm, resulting in arterial blood in the subarachnoid space. SAH is unpredictable and affects mainly younger individuals. In western countries, the annual incidence of SAH is 6–7 per 100,000, half of affected individuals are <55 years-of-age, and the mortality rate following SAH is up to 50% [1]. Early brain injury (EBI) is the term used for an acute injury in the brain that usually occurs in the first 72 hours following SAH, and has been identified as a critical factor in determining the overall mortality of SAH [2,3]. Apoptosis of neurons is thought to be the cellular basis of EBI [2]. Also, studies have shown that the apoptosis of neuronal cells may represent a novel target for the treatment or control of EBI that occurs following SAH [2].
The prevention or limitation of EBI and its consequences remain a challenge in the goal of reducing morbidity and mortality for patients with SAH [3]. Previous studies have shown that the pathogenesis of EBI is associated with oxidative stress [4]. In the early stage immediately following SAH, excessive amounts of reactive nitrogen species (RNS) and reactive oxygen species (ROS), including as nitric oxide, superoxide anion, hydroxyl radicals, peroxynitrite, and hydrogen peroxide, have been identified, which are known to attack non-enzymatic and enzymatic antioxidant defense systems [5]. Sirtuins, which are a class of highly conserved NAD-dependent enzymes are involved in the regulation of mitochondrial functions and transcriptional silencing, including in neuronal tissues [6]. In particular, Sirt3 protein (SIRT3 gene) is one of the members of the sirtuin family. Sirt3 is primarily localized in mitochondria, where, under pathophysiological conditions such as oxidative stress and metabolic disorders, it may have a protective role by scavenging oxygen free radicals [7]. Also, expression of SIRT3 has been reported to be involved in regulation of mitochondrial biogenesis, fusion, mitophagy, and fission, as well as the deacetylation of antioxidant enzymes, such as superoxide dismutase 2 (SOD2), which might be on the mechanism underlying its ability to protect the cells against apoptosis [8].
During EBI following SAH, the expression of Sirt3 was shown to be reduced in cortical neurons in a time-dependent manner, and a positive correlation between SOD2 and Sirt3 expression has been demonstrated, indicating that Sirt3 might be regarded as a key antioxidant factor during post-SAH EBI [9]. Also, the generation of ROS is considered to be a by-product of oxidative phosphorylation in the mitochondria, which is a major target of ROS-induced damage. However, melatonin can reduce the levels of ROS, thus reducing the extent of lipid peroxidation and preventing the deterioration of cellular membranes [10]. For example, it has been shown that melatonin treatment is associated with the reduction in increased levels of ROS in the oocytes of mice fed a high-fat diet, thus reducing the rate of meiotic abnormalities [11].
Melatonin has been reported to protect the brain against tissue damage following SAH [12]; melatonin has also recently been shown to protect against diabetic cardiomyopathy via Mst1/Sirt3 signaling pathways [13]. In 2016, Huang et al. reported that decreased expression of SIRT3 was associated with ROS generation in rat cortical neurons during EBI following experimental SAH [14].
In this study, an established mouse model of SAH was used with the aim of evaluating the effects of melatonin treatment on the generation of reactive oxygen species (ROS) and the activation of the SIRT3 gene in early brain injury (EBI).
Material and Methods
Animals
Healthy adult male C57BL/6J mice, weighing between 22–25 gm, were used in all of the experiments. The mice were purchased from the Experimental Animal Center of the Secondary Military Medical University. The study was approved by the local Ethics Committee of our institute. All animal experiments were conducted in accordance with National Institutes of Health (NIH) Guide to Laboratory Animal Care and Use Guide.
The mice were assigned randomly into three groups: the subarachnoid hemorrhage (SAH) mouse model group; the sham-operated group; and the SAH mouse model group treated with intraperitoneal injection of melatonin (dose, 150 mg/kg).
Mouse model of subarachnoid hemorrhage (SAH)
The endovascular perforation method was used to generate the SAH mouse model. Briefly, 50 mg/kg of pentobarbital sodium was injected into the mice to induce anesthesia. A heat blanket was used to maintain the rectal temperature at 37±0.5°C. The internal carotid artery, external carotid artery, and left common carotid artery were exposed through a midline incision in the neck. Then, the left external carotid artery was ligated and cut to leave a 3 mm arterial stump. A nylon suture was inserted into the left internal carotid artery through the middle cerebral artery branch, and the suture was further advanced by approximately 3–5 mm to perforate the artery and create SAH with a duration of 10 seconds.
Except for the perforation of the middle cerebral artery, the sham-operated mice were treated using identical pre-operative and operative procedures.
Group assignment and melatonin treatment
The mice were assigned into the following groups: the SAH group; the sham group; and the SAH + melatonin-treated group. In the SAH + melatonin-treated group, dosing of melatonin was based on previously published studies. Melatonin was dissolved in 1 ml of sterile saline containing 1% ethanol, and injected intraperitoneally at a dose of 150 mg/kg, at two hours and 12 hours following SAH. Also, 1 mg/kg/day of luzindole was administered intraperitoneally for six consecutive days prior to induction of SAH.
Neurological deficit scores
Neurological deficits were assessed using an 18-point system including six tests conducted at 24 hours following induction of SAH in the mice. These six tests were: forelimb outstretching (score, 0–3), spontaneous activity (score, 0–3), climbing (score, 1–3), symmetry in movements of all limbs (score, 0–3), response to vibrissal touch (score, 1–3), and body proprioception (score, 1–3). A higher neurological score indicated decreased neurological function.
Brain water content (edema)
The standard wet-dry method was used to measure the brain water content (edema). Briefly, mice were sacrificed 24 hours following induction of SAH and their brains were immediately removed to isolate the right and left cerebellar hemispheres, the right and left cerebral hemispheres, and the brainstem. The separate parts of the brain were weighed obtain their initial wet weight. The brain slices were then desiccated at 105°C for 24 hours and weighed again to measure the dry weight. Finally, the percentage of brain water content was calculated as (wet weight–dry weight)/wet weight×100%
Cell culture and treatment
The human glioma cell lines, U87 and U251, were obtained from the Cell Bank at the Chinese Academy of Sciences and were maintained in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. The cells were cultured at 37°C and 5% CO2 in a humidified incubator. For the melatonin treatment experiments, the cells were cultured in 96-well plates and were treated with melatonin at a final concentration of 1 μM. When U87 and U251 cells were 70–80% confluence, they were transfected with Lipofectamine 2000 (Invitrogen, CA, USA) and corresponding constructs. All experiments were carried out in triplicates.
Cell proliferation assay
The human glioma cell lines, U87 and U251, were cultured in 96-well plates and treated with melatonin at a final concentration of 1 μM. Then, 10 μl of 5 mg/ml MTT were added to each well, and the plates were incubated for 4 hours at 37°C. After the removal of MTT solution, 100 μl of dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) were added into each well and incubated at 37°C for 10 min to dissolve the crystals. A spectrophotometric analysis (BioTek, Grand Island, NY, USA) was performed to detect cell viability at an absorbance of 490 nm. Each test was performed in triplicate.
RNA isolation and real-time polymerase chain reaction (PCR)
A microRNA (miRNA) isolation kit (Exiqon, Woburn, MA, USA) was used to extract total RNA from U87 and U251 cells in accordance with the manufacturer’s instructions. A NanoDrop spectrophotometer (Agilent Bioanalyzer, CA, USA) was used to measure the quantity and quality of isolated RNA. A High Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad, CA, USA) was used to synthesize SIRT3 cDNA, which was then loaded into an ABI PRISM 7900 SDS 2.1 machine (Life Technologies, Inc., Grand Island, NY, USA) to perform real-time quantitative PCR and to detect the expression of SIRT3. GADPH was used as the internal control for PCR. The 2-ΔΔCt method was used to calculate the relative expression of SIRT3 mRNA. All experiments were performed in triplicate.
Luciferase assay
For the promoter assays, the full sequence of the SIRT3 promoter was amplified using PCR. The PCR product was then cloned into pcDNA3.1 (+) at a position upstream of the firefly luciferase reporter gene. For transfection assays, the U87 and U251 cells were cultured in 96-well plates and transfected with constructs containing SIRT3. Also, the U87 and U251 cells were treated with melatonin at final concentrations of 100 nM, 1 μM, and 10 μM, respectively, and the luciferase activity in these cells was determined using a dual luciferase reporter assay system (Promega, Madison, WI, USA) following the manufacturer’s instructions. Each assay was performed in triplicate.
Determination of malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px)
The levels of SOD and MDA and the activity of GSH-Px levels was measured In brain tissues and cultured cells, using commercially available kits. A SpectraMax M5 microplate reader (Molecular Devices, CA, USA) was used for the measurements.
Determination of Sirt3 protein activity
A commercially available kit (Invitrogen, CA, USA) was used to measure the deacetylase activity of the Sirt3 protein. Briefly, 40 μg of purified Sirt3 were incubated with the substrates at 37°C for 45 min, followed by the addition of 25 μl of developer and 45 min of additional incubation. The SpectraMax M5 microplate reader (Molecular Devices, CA, USA) was used to determine the Sirt3 protein activity.
Western blot analysis
The U87 and U251 cells were washed twice using ice-cold phosphate-buffered saline (PBS) before being lysed in RIPA lysis buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.1% sodium dodecyl sulfate (SDS) and protease inhibitors (Roche, Diagnostics, Indianapolis, IN, USA). Then, the lysates were centrifuged for 15 min at 15,000 g at 4°C followed by boiling in 2-mercaptoethanol for 10 min. The samples were then separated using 8–12% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA), then incubated at 37 °C for 60 min in a Tris-buffered saline (TBS) containing 10% nonfat dried milk powder and 0.1% Tween20 to eliminate nonspecific antibody binding. The nitrocellulose membranes were incubated with the primary antibodies to Sirt3, Bax, Bcl-2, superoxide dismutase (SOD), and cleaved caspase-3 (1: 4,000 dilution) (Santa Cruz, Biotech) and a primary antibody against β-actin (1: 8,000 dilution, Santa Cruz, Biotech) for 12 hours at 4°C, followed by two hours of incubation at room temperature with the secondary antibodies (1: 5,000 dilution) (Sigma-Aldrich Co., USA). An Immobilon™ Western Chemiluminescent HRP Substrate (ECL) (Millipore, USA) was used to visualize the immunoreactive bands. All assays were performed in triplicate.
Immunohistochemistry
Tissue samples from the mouse cerebellum, cerebrum, and brainstem were collected and fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin wax and sectioned at 4 μm onto glass slides. Immunohistochemical staining of the tissue sections was performed using the rabbit anti-human monoclonal antibody against Sirt3 (1: 500 dilution, Santa Cruz, Biotech). The tissue sections were then incubated with peroxidase-conjugated anti-rabbit IgG antibodies (1: 1,000 dilution) for 60 min, followed by incubation with the brown chromogen, 3, 3-diaminobenzidine (DAB), in accordance with the manufacturer’s instructions. Finally, hematoxylin was used to counterstain the tissue sections, to allow for histological evaluation using light microscopy.
TUNEL assay for neuronal apoptosis
The apoptosis profile of the mouse brain tissue was evaluated using the terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay. Briefly, the tissue sections were treated with 50 μl of TUNEL reaction buffer and incubated at 37°C for 1 hour in a dark and humidified atmosphere. Then, the slides were incubated with 4′,6-diamidino-2-phenylindole (DAPI) at room temperature for 5 min to visualize the cell nuclei. The number of positive neurons was measured by flow cytometry. The ratio of positive neurons was calculated as the number of TUNEL positive neurons/the number of total neurons.
Statistical analysis
All numerical data values were shown as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS version 20.0 statistical software (SPSS Inc., Chicago, IL, USA). The chi-squared and Student’s t-test were used to compare the difference between two groups. Variance analysis with post hoc testing was used to compare the differences between more than two groups. P<0.05 was considered statistically significant.
Results
This study established a mouse model of subarachnoid hemorrhage (SAH) and early brain injury (EBI). Three groups of mice were studied: the SAH group; the sham group; and the SAH + melatonin-treated group (intraperitoneal dose, 150 mg/kg).
Melatonin treatment reduced the severity of brain edema and neurological deficit following subarachnoid hemorrhage (SAH) in the mouse model
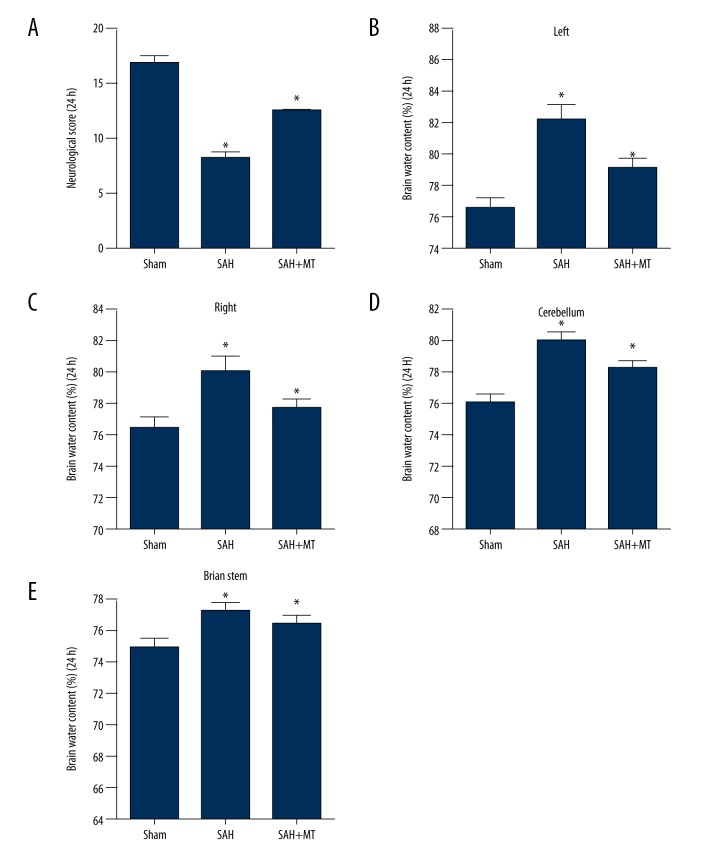
The brain water content (edema) and neurological deficit scores were measured within 24 hours following SAH. A significant reduction in the neurological score was found when compared with the sham control group (P<0.05) (Figure 1A). A significant increase in the water content (edema) of the brain tissue of the SAH + melatonin-treated group was found when compared with the sham control group (P<0.05), as well as an increase in the water content of different anatomical brain structures, including the left cerebrum (Figure 1B), right cerebrum (Figure 1C), cerebellum (Figure 1D) and brainstem (Figure 1E). A significant improvement in the neurological deficit was found in the SAH + melatonin-treated group when compared with the sham control group (P<0.05).
Figure 1.
Melatonin treatment reduced brain water content (edema) and neurological deficit following subarachnoid hemorrhage (SAH) in a mouse model. Three mouse groups were studied: the subarachnoid hemorrhage (SAH) group; the sham group; and the SAH + melatonin-treated group (intraperitoneal dose, 150 mg/kg). (A) The neurological score in the SAH group was significantly lower compared with the SAH + melatonin-treatment group, and both were significantly lower compared with the sham group. (B) Brain water content (edema) including left, right, cerebellum and brain stem in the SAH group was significantly greater compared with the SAH + melatonin-treatment group, and both were significantly lower compared with the sham group.
Melatonin suppressed SAH-induced apoptosis of neuronal cells in the brain cortex
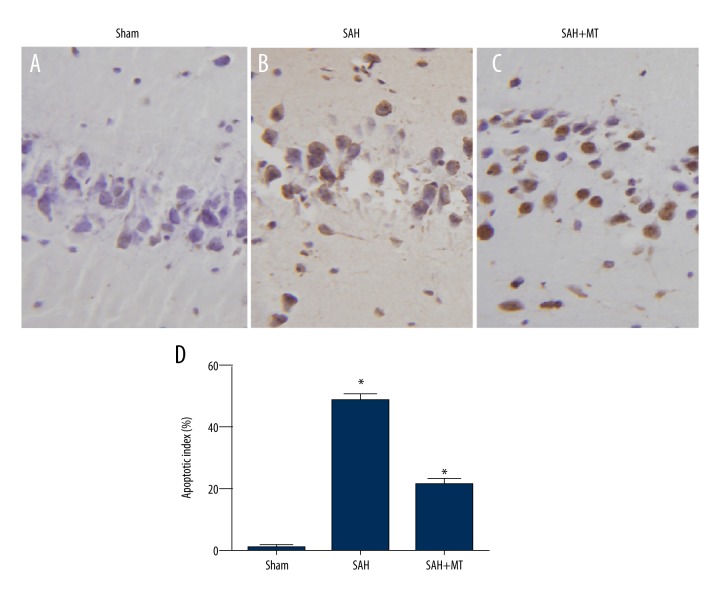
The TUNEL assay was utilized to determine the apoptotic profile of the neurons. As shown in Figure 2, a significant increase in the apoptotic index was observed in the SAH group when compared with the sham group (P<0.05). Also, treatment with melatonin significantly inhibited the apoptotic index of neurons in the brain at 24 hours following SAH (P<0.05).
Figure 2.
(A–D) Melatonin inhibited neuronal apoptosis in the brain cortex induced by subarachnoid hemorrhage (SAH).
Melatonin treatment of SAH-induced early brain injury (EBI) regulated the levels of redox and apoptotic proteins
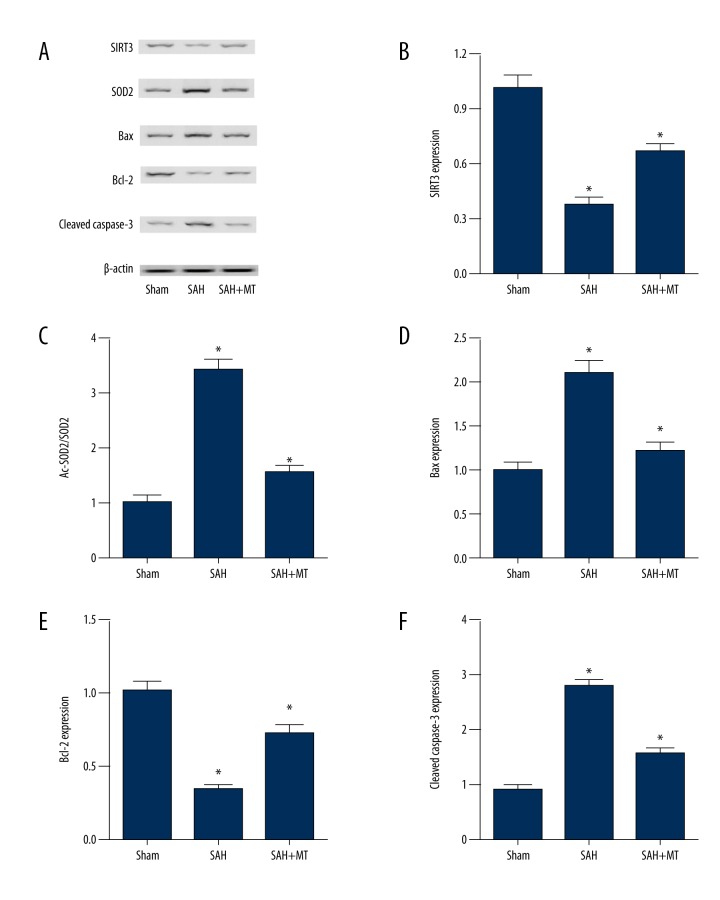
The effect of melatonin on the Sirt3/SOD2 and Bax/Bcl-2/cleaved caspase-3 apoptotic signaling pathways was studied by measuring the expression of redox and apoptotic protein expression levels in the sham, SAH, and SAH + melatonin-treated mouse groups at 24 hours following SAH. The results showed that SAH significantly downregulated the protein expression levels of apoptosis inhibiting genes when compared with the sham controls (P<0.05), including SIRT3 (Figure 3A, 3B) and BCL-2 (Figure 3A, 3E). However, mice with SAH treated with melatonin showed significant upregulation of the protein expression levels of apoptosis inhibiting genes when compared with the untreated SAH group (P<0.05), including BCL-2 and SIRT3.
Figure 3.
Melatonin was associated with early brain injury (EBI) after subarachnoid hemorrhage (SAH) by affecting redox and apoptotic genes. Three mouse groups were studied: the subarachnoid hemorrhage (SAH) group; the sham group; and the SAH + melatonin-treated group (intraperitoneal dose, 150 mg/kg). (A) Melatonin treatment increased Bcl-2 and Sirt3 protein levels, and decreased SOD2, Bax, and cleaved caspase-3 levels following SAH. (B) Sirt3 protein levels in the SAH group and SAH + melatonin-treatment group were much lower than in the sham group. (C) SOD2 levels in the SAH + melatonin-treatment group were much greater than in the sham group, and the SAH group. (D) Bax levels in the SAH group were much greater than in the SAH + melatonin-treatment group, and both of them were much greater than in the sham group. (E) Bcl-2 levels in the SAH and SAH + melatonin-treated groups were significantly lower than in the sham group. (F) Cleaved caspase-3 levels in the SAH + melatonin-treatment group were significantly greater than in the sham group, and were significantly greater than in the SAH group.
In contrast, the protein expression levels of Bax in the neuronal mitochondria (Figure 3A, 3D) were significantly elevated in mice following SAH when compared with the sham controls (P<0.05), but levels of Bax in the neuronal mitochondria were significantly reduced in the SAH + melatonin-treated group compared with the SAH group without melatonin treatment (P<0.05). When compared with the sham group, SAH significantly upregulated the protein levels of the proapoptotic factor, Bax, as well as the protein level of SOD2 (P<0.05) (Figure 3A, 3C) and cleaved caspase-3 (Figure 3A, 3F). When compared with the untreated SAH group, the SAH group treated with melatonin showed significant downregulation of the protein levels of Bax, SOD2 and cleaved caspase-3 (P<0.05)
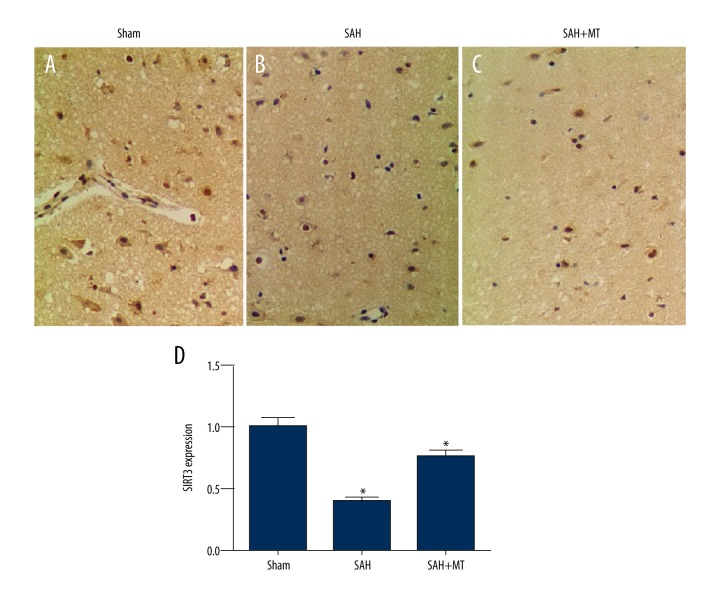
Levels of protein expression of Sirt3 in the mouse groups
Immunohistochemistry for the detection of protein levels of Sirt3 in the brain tissues of the sham group, the SAH group, and the SAH + melatonin-treated group showed that the protein expression of Sirt3 in the SAH group was significantly lower compared with that in the SAH + melatonin-treated group (P<0.05); the protein expression levels of Sirt3 in both SAH groups was significantly lower than that in the sham group (P<0.05) (Figure 4A–4D). These findings supported the protective role of melatonin in EBI by upregulating the expression of SIRT3.
Figure 4.
(A–D) Sirt3 protein levels in the subarachnoid hemorrhage (SAH) group were significantly lower than in the SAH + melatonin-treated group, and both were significantly lower than in the sham group. Three mouse groups were studied: the subarachnoid hemorrhage (SAH) group; the sham group; and the SAH + melatonin-treated group (intraperitoneal dose, 150 mg/kg).
Melatonin treatment reduced SAH-induced oxidative stress in the brain
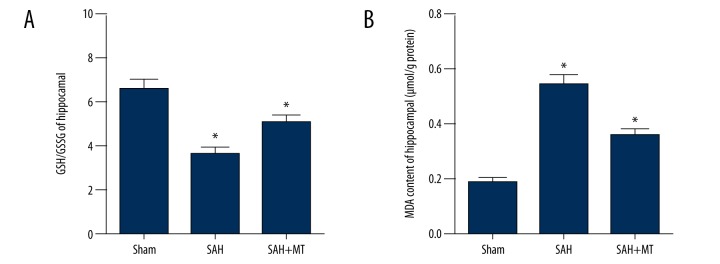
As shown in Figure 5A, reduced glutathione (GSH) and its ratio with oxidized glutathione (GSSG) (the GSH: GSSG ratio) was significantly reduced in the SAH group (Figure 5A) when compared with the sham group (P<0.05). Treatment of the SAH mice with melatonin partially increased the ratio GSH: GSSG compared with the untreated SAH mice (P<0.05).
Figure 5.
Melatonin treatment upregulated SIRT3 expression caused by oxidative stress in the brain following subarachnoid hemorrhage (SAH). Three mouse groups were studied: the subarachnoid hemorrhage (SAH) group; the sham group; and the SAH + melatonin-treated group (intraperitoneal dose, 150 mg/kg). (A) Reduced glutathione (GSH), and its ratio with oxidized glutathione (GSSG) (the GSH: GSSG ratio) was inhibited in the SAH group, and melatonin treatment partially reversed the change in GSH: GSSG ratio induced by SAH. (B) Malonaldehyde (MDA) was significantly increased in the SAH group; MDA was significantly suppressed following treatment with melatonin.
Malondialdehyde (MDA) a marker of lipid peroxidation, was significantly upregulated in the SAH group (Figure 5B) when compared with the sham group (P<0.05). MDA was significantly reduced in the SAH + melatonin treatment group compared with the untreated SAH mice (P<0.05). These findings supported the view that treatment with melatonin affected the redox homeostasis in the mouse brain, and that SAH -induced EBI could be reduced melatonin treatment.
The effects of melatonin on the expression of SIRT3 and the transcription efficiency of the SIRT3 promoter
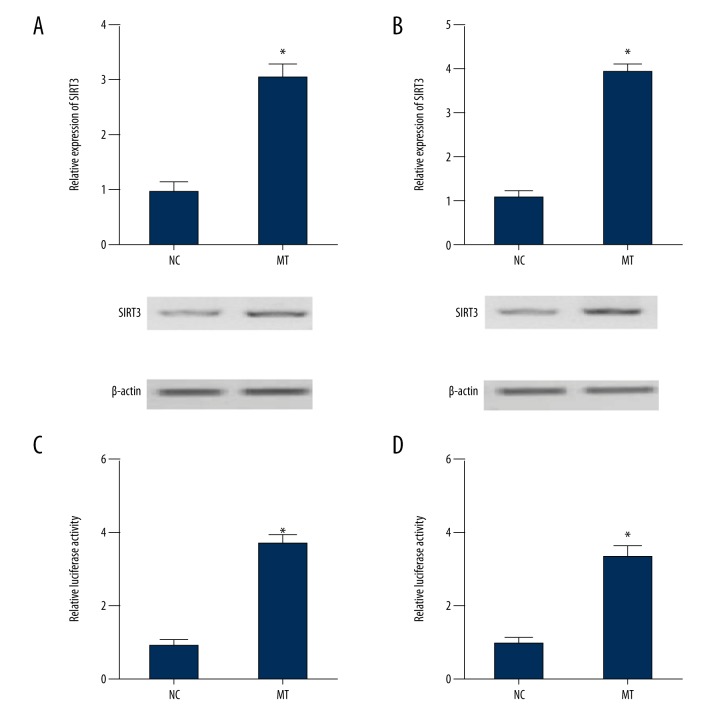
Real-time polymerase chain reaction (PCR), Western blot analysis, and the luciferase assay were conducted to investigate how melatonin affected the expression of SIRT3. Increasing concentrations of melatonin (100 nM, 1 mM, 10 mM) were used to treat cells in the human glioma cell lines, U87 and U251. As shown in Figure 6, the expression of SIRT3 in U87 cells (Figure 6A) and U251 cells (Figure 6B) were significantly increased following melatonin treatment when compared with the normal control (NC) (P<0.05). The luciferase activity of SIRT3 constructs in U87 cells (Figure 6C) and U251 cells (Figure 6D) was significantly upregulated following melatonin treatment compared with the normal control (NC) (P<0.05). These findings supported the view that melatonin enhanced SIRT3 expression by increasing the transcription efficiency of the SIRT3 promoter.
Figure 6.
Melatonin treatment upregulated SIRT3 expression by increasing SIRT3 promoter transcription efficiency. (A) Melatonin treatment upregulated SIRT3 expression in cells in the U87 cell line compared with the normal control (NC) group. (B) SIRT3 expression in cells in the U251 cell line was upregulated following treatment with melatonin when compared with NC group. (C) Treatment with melatonin increased luciferase activity driven by the SIRT3 promoter in cells in the U87 cell line. (D) The SIRT3 promoter was upregulated in cells in the U87 cell line following treatment with melatonin.
Discussion
Previous studies in humans and animals have shown that reactive oxygen species (ROS) are produced in the early stages following subarachnoid hemorrhage (SAH) and early brain injury (EBI) and that ROS can compromise the non-enzymatic and enzymatic antioxidant defense systems [15]. The mechanisms underlying ROS-induced brain injuries include disruption of the blood-brain barrier, production of strong vascular spasmogens, activation of pro-apoptosis enzymes, and damage to vascular endothelial cells and smooth muscle [15,16]. However, melatonin is known to have a variety of pharmacological activities, including anti-inflammation, anti-oxidation, and anti-apoptotic effects [17,18]. Also, melatonin has a protective effect on hemorrhagic tissue injury in the lung and brain [19,20].
Previous studies have shown that melatonin can affect the generation of ROS, inflammatory responses and oxidative stress in the brain following SAH [19]. Treatment with melatonin has previously been suggested to play a neuroprotective role in SAH-induced EBI, generated through inhibition of NLRP3 inflammasome-dependent apoptosis [21]. A recent study has shown that antioxidants reduced the production of ROS reduced oxidative damage resulting from ischemia-reperfusion injury [22]. As an antioxidant, melatonin is also considered to act as a powerful scavenger of free radicals and a cellular protector against oxidative stress [23–25]. It has also been demonstrated in animal studies that melatonin could reduce the level of ROS and thus decrease the cellular damage caused by ischemia-reperfusion injury [26].
In the present study, a mouse model of SAH and EBI was established. Treatment with melatonin significantly inhibited apoptosis of neurons in the brain at 24 hours following SAH. In this study, the expression of Sirt3 protein levels and the SIRT3 gene were significantly downregulated in the SAH group, whereas the treatment with melatonin significantly increased the expression of Sirt3 protein levels and the SIRT3 gene. In support of the findings of this study, melatonin has previously been shown to activate the SIRT3 signaling pathway in a receptor-dependent manner and myocardial reperfusion and ischemic injury [27]. Also, in the hippocampus of sleep-deprived rats, melatonin has been shown to maintain the expression of SIRT1 [28]. Therefore, it is likely that the stimulatory action of melatonin may be associated with the expression of sirtuins.
In this study, when compared with the sham group, SAH upregulated the protein expression and levels of Bax, a proapoptotic factor, as well as the protein level of SOD2 (Figure 3A, 3C) and cleaved caspase-3 (Figure 3A, 3F); melatonin treatment of mice with SAH significantly downregulated the protein expression and levels of Bax, SOD2, and cleaved caspase-3. When compared with the sham group, the level of malondialdehyde (MDA) was upregulated, but the ratio of glutathione (GSH), and its ratio with oxidized glutathione (GSSG) (the GSH: GSSG ratio) was significantly reduced in the SAH group; melatonin treatment corrected this alteration in MDA and GSH: GSSG ratio.
In 2012, Giralt and Villarroya showed that Sirt3 is a NAD-dependent protein deacetylase and a member of the silent information regulator 2 (Sir2) family [29]. The SIRT3 gene plays a regulatory role in a diverse range of key biological activities, including neuroprotection, aging, cardiovascular disease, cancer, regulation of nuclear gene expression, and metabolic control [30–33]. In 2007, Lombard et al. showed that the Sirt3 protein is localized in mitochondria and regulates several mitochondrial processes, including protein de-acetylation [34]. In another previous study on calorie restriction and oxidative stress, the mechanism was shown to be mediated by SOD2 activation by the SIRT3 that induced an adaptive change to oxidative stress, maintaining ROS homeostasis and providing cellular protection against oxidative damages [35]. A further in vitro study showed that SIRT3 expression reduced the level of ROS and maintained ATP production, thus protecting PC12 cells against hypoxic damage via the SOD2 (MnSOD) and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) pathways [36]. The findings of this present study in a mouse model are supported by the findings in a rat model of SAH and EBI where the expression profile of SIRT3 and the cellular distribution of Sirt3 in the cerebral cortex have been characterized, also indicating the neuroprotective role during the early brain injury following SAH [14]. Genes that when upregulated offer protection from oxidative tissue damage have been proposed as targets for treatment or prevention of tissue damage and the findings from the present study support the possibility that SIRT3 and its anti-oxidative effects may become a potential therapeutic target for the treatment of EBI [37].
Conclusions
This study in a mouse model of subarachnoid hemorrhage (SAH) investigated the effects of melatonin treatment on the generation of reactive oxygen species (ROS) and the activation of the SIRT3 gene in early brain injury (EBI). Melatonin provided protection from the effects of EBI following SAH by regulating the expression of murine SIRT3.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Epidemiology of aneurysmal subarachnoid hemorrhage in Australia and New Zealand: incidence and case fatality from the Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Stroke. 2000;31:1843–50. doi: 10.1161/01.str.31.8.1843. [DOI] [PubMed] [Google Scholar]
- 2.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–53. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 3.Cahill J, Zhang JH. Subarachnoid hemorrhage: Is it time for a new direction? Stroke. 2009;40:S86–87. doi: 10.1161/STROKEAHA.108.533315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: Significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- 6.Villalba JM, Alcain FJ. Sirtuin activators and inhibitors. Biofactors. 2012;38:349–59. doi: 10.1002/biof.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn BH, Kim HS, Song S, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008;105:14447–52. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papa L, Germain D. SirT3 regulates the mitochondrial unfolded protein response. Mol Cell Biol. 2014;34:699–710. doi: 10.1128/MCB.01337-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Ratchford AM, Chi MM, et al. Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol Endocrinol. 2009;23:1603–12. doi: 10.1210/me.2009-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaefer M, Hardeland R. The melatonin metabolite N-acetyl-5-methoxykynuramine is a potent singlet oxygen scavenger. J Pineal Res. 2009;46:49–52. doi: 10.1111/j.1600-079X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 11.Han L, Wang H, Li L, et al. Melatonin protects against maternal obesity-associated oxidative stress and meiotic defects in oocytes via the SIRT3-SOD2-dependent pathway. J Pineal Res. 2017;63(3) doi: 10.1111/jpi.12431. [DOI] [PubMed] [Google Scholar]
- 12.Cao S, Shrestha S, Li J, et al. Melatonin-mediated mitophagy protects against early brain injury after subarachnoid hemorrhage through inhibition of NLRP3 inflammasome activation. Sci Rep. 2017;7:2417. doi: 10.1038/s41598-017-02679-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Lin J, Wang S, et al. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J Pineal Res. 2017;63(2) doi: 10.1111/jpi.12418. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, Huang Y, Huang RQ, et al. SIRT3 expression decreases with reactive oxygen species generation in rat cortical neurons during early brain injury induced by experimental subarachnoid hemorrhage. Biomed Res Int. 2016;2016 doi: 10.1155/2016/8263926. 8263926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Chen J, Mo H, et al. Minocycline protects against NLRP3 inflammasome-induced inflammation and P53-associated apoptosis in early brain injury after subarachnoid hemorrhage. Mol Neurobiol. 2016;53:2668–78. doi: 10.1007/s12035-015-9318-8. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Xia X, Li X. Plasmid pLXSN-mediated adrenomedullin gene therapy for cerebral vasospasm following subarachnoid hemorrhage in rats. Med Sci Monit. 2018;24:3293–302. doi: 10.12659/MSM.901914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HM, Zhang Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014;57:131–46. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 18.Agil A, Reiter RJ, Jimenez-Aranda A, et al. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J Pineal Res. 2013;54:381–88. doi: 10.1111/jpi.12012. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Wang L, Wu C, et al. Melatonin-enhanced autophagy protects against neural apoptosis via a mitochondrial pathway in early brain injury following a subarachnoid hemorrhage. J Pineal Res. 2014;56:12–19. doi: 10.1111/jpi.12086. [DOI] [PubMed] [Google Scholar]
- 20.Taslidere E, Esrefoglu M, Elbe H, et al. Protective effects of melatonin and quercetin on experimental lung injury induced by carbon tetrachloride in rats. Exp Lung Res. 2014;40:59–65. doi: 10.3109/01902148.2013.866181. [DOI] [PubMed] [Google Scholar]
- 21.Dong Y, Fan C, Hu W, et al. Melatonin attenuated early brain injury induced by subarachnoid hemorrhage via regulating NLRP3 inflammasome and apoptosis signaling. J Pineal Res. 2016;60:253–62. doi: 10.1111/jpi.12300. [DOI] [PubMed] [Google Scholar]
- 22.Cervantes MI, de Oca Balderas PM, de Jesus Gutierrez-Banos J, et al. Comparison of antioxidant activity of hydroethanolic fresh and aged garlic extracts and their effects on cerebral ischemia. Food Chem. 2013;140:343–52. doi: 10.1016/j.foodchem.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 23.Reiter RJ. Antioxidant actions of melatonin. Adv Pharmacol. 1997;38:103–17. doi: 10.1016/s1054-3589(08)60981-3. [DOI] [PubMed] [Google Scholar]
- 24.Tutunculer F, Eskiocak S, Basaran UN, et al. The protective role of melatonin in experimental hypoxic brain damage. Pediatr Int. 2005;47:434–39. doi: 10.1111/j.1442-200x.2005.02085.x. [DOI] [PubMed] [Google Scholar]
- 25.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–90. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupuis F, Regrigny O, Atkinson J, et al. Impact of treatment with melatonin on cerebral circulation in old rats. Br J Pharmacol. 2004;141:399–406. doi: 10.1038/sj.bjp.0705629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L, Sun Y, Cheng L, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: Role of SIRT1. J Pineal Res. 2014;57:228–38. doi: 10.1111/jpi.12161. [DOI] [PubMed] [Google Scholar]
- 28.Chang HM, Wu UI, Lan CT. Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res. 2009;47:211–20. doi: 10.1111/j.1600-079X.2009.00704.x. [DOI] [PubMed] [Google Scholar]
- 29.Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: Metabolism, cell death and aging. Biochem J. 2012;444:1–10. doi: 10.1042/BJ20120030. [DOI] [PubMed] [Google Scholar]
- 30.Kong X, Wang R, Xue Y, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellizzi D, Rose G, Cavalcante P, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is ssociated with survival at oldest ages. Genomics. 2005;85:258–63. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Alhazzazi TY, Kamarajan P, Verdin E, Kapila YL. SIRT3 and cancer: Tumor promoter or suppressor? Biochim Biophys Acta. 2011;1816:80–88. doi: 10.1016/j.bbcan.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–67. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 34.Lombard DB, Alt FW, Cheng HL, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–14. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu X, Brown K, Hirschey MD, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–67. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, Li L, Li CY, et al. SIRT3 protects cells from hypoxia via PGC-1alpha- and MnSOD-dependent pathways. Neuroscience. 2015;286:109–21. doi: 10.1016/j.neuroscience.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 37.Cui Y, Duan X, Li H, et al. Hydrogen sulfide ameliorates early brain injury following subarachnoid hemorrhage in rats. Mol Neurobiol. 2016;53:3646–57. doi: 10.1007/s12035-015-9304-1. [DOI] [PubMed] [Google Scholar]