Abstract
The neurological disorder cerebral palsy (CP) is caused by unprogressive lesions of the immature brain and affects movement, posture, and the musculoskeletal system. Vibration therapy (VT) is increasingly used to reduce the signs and symptoms associated with this developmental disability. The purpose of this narrative review was systematically to appraise published research regarding acute and long-term effects of VT on functional, neuromuscular, and structural parameters. Systematic searches of three electronic databases identified 28 studies that fulfilled the inclusion criteria. Studies were analyzed to determine participant characteristics, VT-treatment protocols, effect on gross motor function (GMF), strength, gait, posture, mobility, spasticity, reflex excitability, muscle tone, mass, and bone strength within this population, and outcome measures used to evaluate effects. The results revealed that one acute session of VT reduces reflex excitability, spasticity, and coordination deficits. Subsequently, VT has a positive effect on the ability to move, manifested for GMF, strength, gait, and mobility in patients with CP. Effects persist up to 30 minutes after VT. Long-term effects of VT manifest as reduced muscle tone and spasticity occurring concomitantly with improved movement ability in regard to GMF, strength, gait, and mobility, as well as increased muscle mass and bone-mineral density. Posture control remained unaffected by VT. In conclusion, the acute and chronic application of VT as a nonpharmacological approach has the potential to ameliorate CP symptoms, achieving functional and structural adaptations associated with significant improvements in daily living. Even though further studies including adult populations validating the neuromuscular mechanisms underlying the aforementioned adaptations should be fostered, growing scientific evidence supports the effectiveness of VT in regard to supplementing conventional treatments (physiotherapy and drugs). Therefore, VT could reduce CP-associated physical disability and sensorimotor handicaps. Goals for patients and their caregivers referring to greater independence and improved safety may be achieved more easily and time efficiently.
Keywords: spasticity, muscle, gait, posture, reflex, neuromuscular
Introduction
Cerebral palsy (CP) is an umbrella term used to classify individuals with unprogressive lesions of the immature brain.1 CP is a disease with a prevalence of two cases per 1,000 live-born neonates, characterized by motor and mental dysfunction.2 Individuals with CP experience a variety of concomitant health problems as a result of their diagnosis, including movement disorders, difficulty with motor planning and control, and cognitive impairments.3 The level of severity is categorized by the Gross Motor Function Classification System (GMFCS), with values of I–V. Major motor impairments comprise increased cocontraction and joint stiffness,4 muscle weakness,5,6 decreased strength and power,4 restricted single- and multijoint range of motion (ROM),7 and gait and balance deteriorations.8–10 In spastic CP, as an upper-motor-neuron lesion, hypertonicity can be observed, being composed of neural and secondary nonneural components, including muscle structure or connective tissues.11,12 The pathophysiology of sensorimotor dysfunction due to lesions in the immature brain of humans with CP is manifested as insufficient control of afferents,13,14 spinal hyperexcitability,3,15 increased stretch reflexes, and increased cocontraction of antagonists8,16 concomitantly with diminished voluntary control of body movement.6,16
Interventions to counteract motor impairments in patients with CP range from drug administration and surgery to therapeutic exercise.17,18 Nonpharmacological nonoperative approaches comprise training modalities in the settings of neurorehabilitation conceptualized to stimulate the impaired sensorimotor system of patients with CP that may – due to plasticity of the brain and spinal cord – profit and ameliorate motor syndromes. Exercise interventions aim to improve development and function by capitalizing on the innate capacity of the neural system to change and adapt throughout the life span.18 Moderate–intense exercises include resistance training and stretching, physiotherapy, and systematic locomotor or postural exercises.19 Therefore, the severity of motor and mental dysfunction in CP patients significantly narrows the applicability of the aforementioned training modalities, which often require voluntary motor skills and solid cognitive understanding to follow instructions and perform the exercises. In particular, limited options exist for CP patients with GMFCS scores of IV and V. As a consequence, vibration therapy (VT) is an alternative easy-to-apply and time-efficient intervention that has recently moved into focus.20
VT is a training modality that uses mechanical oscillations as an indirect stimulus to act on human neuromuscular structures.21–23 Vibration determinants refer to the frequency (number of complete cycles per second, 5–200 Hz), amplitude (vertical displacement 0.5–10 mm), and type of VT (sinusoidal vertical and side-alternating). Two distinct categories can be identified: focal vibration, which acts with regional emphasis directly on the muscle belly or a tendon,21 and whole-body vibration (WBV), during which mechanical oscillations are transmitted indirectly through the entire body.24 Disregarding the topographic distinctions and the stimulus specified for the aforementioned VT modalities, the feasibility and efficacy of both focal and WBV therapy are independent of subjects’ movement ability, health, and mental status. As a consequence, the clinical application of vibration in neurorehabilitation has emerged as a particularly valuable tool, as there are no decisive motor prerequisites or particular cognitive requirements.25,26
Therefore, in the last few decades, research to investigate the effect of VT on patients with the central nervous system disorder CP has increased considerably. Scientific objectives include acute and long-term effects of VT on gross motor function, strength, and gait and posture control, as well as the effects on bone and muscle, which are of high relevance for everyday life. Additionally, numerous experiments have been executed with emphasis placed on the sensorimotor system to evaluate mechanisms underlying the aforementioned functional adaptations to the vibratory stimulus: methodologies including electromyography coupled with electrophysiology have allowed assessment of the excitability of spinal and corticospinal pathways, as well as afferent reflex loops related to CP-induced spasticity. It is thus important to conduct a review to examine systematic evidence guiding clinical decision making.
The objective of this narrative review is to provide a systematic overview of the literature related to the immediate and chronic effects of VT in CP and outline the sensorimotor mechanisms and adaptations, their functional consequences in terms of movement control, and resulting structural changes for muscle and bone. In accordance with the close relationship between successful treatment of CP symptoms and clear comprehension of the underlying pathophysiology, natural history, and impact on patient performances, we further address the neuromuscular mechanisms underlying the effect of VT evaluated in the context of CP-deconditioning prevention or rehabilitation.
Methods
This study was a systematic review of the available literature reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and guidelines.27
Search strategy
We performed an electronic database search on Medline/PubMed, Google Scholar, and Web of Knowledge. The keywords “cerebral palsy” AND “vibration” were included in our final Boolean search strategy. Results were limited to articles in English and German languages and studies published in the period from January 1900 to December 2017. Broad search terms were used to ensure that all articles with potential for inclusion in this review were evaluated. Furthermore, we scanned each article’s reference list in an effort to identify additional suitable studies for inclusion in the database.
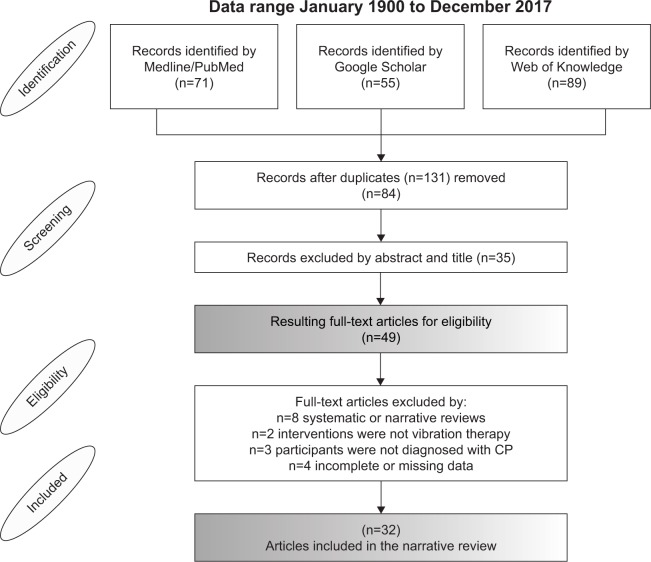
Available data identified by the electronic search were extracted independently by two well-trained researchers to determine whether the article met our inclusion criteria. In cases of disagreement, a third author was consulted. If the title or abstract did not provide sufficient information to determine eligibility, the article was obtained to determine whether it met the criteria. The number of articles that were included or excluded and the reasons for exclusion are detailed in Figure 1.
Figure 1.
PRISMA flow diagram of findings.
Note: Step-by-step approach for study identification, screening, and eligibility procedures, and final number of articles included.
Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; CP, cerebral palsy.
Eligible studies
The eligibility criteria for selection were human participants with a diagnosis of CP, intervention of vibration treatment, full-text original articles, studies with an experimental design, randomized controlled trials, controlled trials, cohort studies, case–control studies, pre–post studies, or case reports, no inconsistencies in “Methods” and “Results” sections, and complete data.
Data synthesis and analysis
Three reviewers extracted the data from each of the studies independently and cross-checked the results to eliminate any errors. Key demographic characteristics of the participants, vibration-intervention protocols, and outcomes of each study were systematically collected. Articles were divided into categories based on the type of study design and vibration intervention the participants received, in order to compare the effectiveness of vibration in patients with CP. The categories longitudinal and cross-section study designs and focal and WBV with side-alternating, vertical vibration or “other” were used.
Quality of evidence
We used the PEDro scale to estimate the quality of evidence. This scale assesses the methodological quality of a study based on such criteria as concealed allocation, intention-to-treat analysis, and adequacy of follow-up. These characteristics make the PEDro scale a useful tool for assessing the quality of therapy and rehabilitation trials.28 Methodological quality was independently assessed by two researchers. Studies were scored on the PEDro scale based on a Delphi list29 that consisted of eleven items. One item on the PEDro scale (eligibility criteria) is related to external validity and is generally not used to calculate the method score, leaving a score range of 0–10.
Results
The search strategy identified 215 articles (71 PubMed, 55 Google Scholar, and 89 Web of Knowledge), including 131 duplicate publications. After screening both abstracts and titles, 49 full-text articles were retrieved. After reviewing the articles to determine whether they met all the inclusion criteria, 28 studies remained and were included in the review. Figure 1 shows details of the flow of studies throughout the review.
Study characteristics
The 28 studies were divided into two intervention categories: longitudinal studies (18), documenting training effects, and cross-sectional studies (10), documenting the acute effects of vibration. Defined subcategories contain training attributes referring to focal and WBV. A total of 21 studies had been executed with subjects under the age of 18 years, only seven studies experimented with adult volunteers. A general description of the 28 studies is presented in Tables 2 and 4, including information about the study design, participant demographics, intervention, dosing parameters, outcome measures, and results.
Table 2.
Study quality on the PEDro scale: long-term adaptations to vibration
| PEDro scale items | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahlborg et al49 | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Camerota et al64 | ✓ | CR | ||||||||||
| el-Shamy51 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Gusso et al48 | ✓ | ✓ | 1 | |||||||||
| Ibrahim et al59 | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Ko et al58 | ✓ | ✓ | ✓ | ✓ | 4 | |||||||
| Lee and Chon50 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Reyes et al57 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Ruck et al52 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Semler et al53 | ✓ | CR | ||||||||||
| Stark et al55 | ✓ | ✓ | ✓ | ✓ | 3 | |||||||
| Stark et al56 | ✓ | ✓ | ✓ | ✓ | 3 | |||||||
| Stark et al54 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 | ||
| Tupimai et al36 | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Unger et al46 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Myśliwiec et al47 | ✓ | ✓ | ✓ | ✓ | 3 | |||||||
| Wren et al60 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 7 | |||
| Yabumoto et al63 | CR | |||||||||||
| Score per item/average score | 14 (15) | 11 (15) | 5 (15) | 10 (15) | 1 (15) | 0 (15) | 7 (10) | 11 (15) | 6 (15) | 10 (15) | 14 (15) | 4 |
Notes: Longitudinal experiments investigating the chronic effect of vibration therapy on neuromuscular and functional parameters, as well as muscle and bone tissue. The bottom line illustrates the relative number per PEDro item and the overall average score. Note that CRs were excluded from evaluation, as scaling and items are not applicable for these study designs. 1, Eligibility criteria and source of participants; 2, random allocation; 3, concealed allocation; 4, baseline comparability; 5, blinded participants; 6, blinded therapists; 7, blind assessors; 8, adequate follow-up; 9, intention-to-treat analysis; 10, between-group comparisons; 11, point estimates and variability. Item 1 does not contribute to the total score.
Abbreviation: CR, case report.
Table 4.
Long-term adaptations to vibration
| Study | Intervention (duration, training protocol, exercise, control group) | Vibration characteristics (frequency, amplitude, duration) | Subjects (n), age, classification | Outcome measures | Results: significant long-term effects |
|---|---|---|---|---|---|
| Ahlborg et al49 | 8 weeks, three times a week Ex: 5-minute warm-up, static standing with 50° in hips and knees on platform, progressive training program (11 levels of intensity), finished with short-muscle stretching Con: resistance training on leg press, same warm-up and stretching, three sets of 10–15 reps with 2-minute rest (progressive training program) | vWBV, 25–40 Hz, 4 mm, 6 minutes (rest included) | n=14 (Con 7), 21–41 years, diplegic, GMFCS: NA | Spasticity (MAS), isokinetic muscle strength, 6MWT, TUG, GMFM88 | ↓Spasticity in knee extensors of stronger leg, ↑concentric and eccentric work and peak torque in the weak leg at angle speed 90°/second. Both groups improved at 30°/second, no difference between groups, no change, no change, ↑dimensions D (standing) and E (walking) |
| Camerota et al64 | 3 consecutive days, one session/day, postmeasurement 1 month later | Sinusoidal rMV on triceps surae, 100 Hz, 0.3–0.5 mm, 3×10 minutes, 1-minute break | n=1, 5 years, tetraplegic, GMFCS: II | Spatiotemporal gait parameters, kinematic gait parameters | ↑Step length, ↓stance duration relative to length of gait cycle, ↑gait velocity, ↑gait symmetry, ↓pelvic tilt anteversion and hip flexion |
| el-Shamy51 | 3 months, five times a week Ex: static/dynamic (squats, lateral shifting, tilting) Con: proprioceptive training | sWBV, 12–18 Hz, 2–6 mm, 3×3 minutes | n=30 (Con 15), 8–12 years, diplegic, GMFCS I–III | Isokinetic muscle strength, balance (Biodex) | ↑Knee-extension peak torque, ↑postural stability |
| Gusso et al48 | 20 weeks, four times a week Ex: standing knees slightly flexed Con: no | sWBV, 12–20 Hz, amplitude NA, 1×1–3×3 minutes | n=40, 11.3–20.8 years, GMFCS II–III | 6MWT, pQCT BMD of total body, DXA BMC of total body, DXA lean (muscle) mass, chair-rise test, force plate | ↑6MWT, ↑BMD, ↑BMC, ↑muscle mass, ↓chair-rising time |
| Ibrahim et al59 | 12 weeks Ex: standing knees slightly flexed Con: 1-hour conventional PT | sWBV, 12–18 Hz, 2–6 mm, 3×3 minutes, 3-minute break (add-on to 1-hour conventional PT) | n=30 (Con 15), 8–12 years, diplegic, GMFCS: NA | Isometric strength, spasticity (MAS), 6MWT, balance, GMFM88 | ↑Isometric muscle strength of knee extensors, ↓spasticity of knee extensors in stronger leg, ↑6MWT, no change between groups in walking balance, ↑dimensions D (standing) and E (walking) |
| Ko et al58 | 3 weeks, twice a week Ex: standing knees flexed 30° Con: conventional PT | sWBV, 20–24 Hz, 1–2 mm, 3×3 minutes, 3-minute break (add-on to 30-minute conventional PT twice a week) | n=24 (Con 12), 7–12 years, diplegia, hemiplegia, GMFCS I–III | JPS (joint-position sense), balance, gait (2-D) | ↑Ankle JPS, no difference between groups, ↑gait speed and step width |
| Lee and Chon50 | 8 weeks, five times a week Ex: static upright with handle bars Con: stretching, massage, sensorimotor training | sWBV, 5–25 Hz, 1–9 mm, 6×3 minutes | n=30 (Con 15), 10±2.26 years, spastic diplegia or quadriplegia, GMFCS NA | Gait test (3-D), ultrasonography | ↑Gait speed, stride length, cycle time, ↑ankle dorsiflexion and plantar flexion, ↑muscle thickness musculus tibialis anterior and soleus, no change in gastrocnemii |
| Reyes et al57 | 6 months, 7 days/week Con: placebo, 60 Hz and 90 Hz (three groups) | FV on radius and femur, 60 or 90 Hz, 0.3 g, 5 minutes a day | n=61 (placebo 21, 60 Hz 22, 90 Hz 18), 6–9 years, first neuron and some second neuron and other diseases | DXA BMD, DXA BMC, dynamometric muscle-strength measure, GMFM, MFM (gross motor), Quality-of-life questionnaire (PedsQL) | ↑BMD at ultradistal radius for 60 Hz group, ↑BMC at ultradistal radius for 60 Hz and 90 Hz groups, ↑muscle-force upper limb for 60 Hz group, no change in lower limb, no change, ↑in daily activity item, no change for other items |
| Ruck et al52 | 6 months, five times a week Ex: static/tilt angle (35° vertical) Con: school PT program | sWBV, 13–18 Hz, 2–6 mm, 3×3 minutes | n=20 (Con 10), 6.2–12.3 years, GMFCS II–IV | 10 m gait test, GMFM88 dimensions D (standing) and E (walking), DXA BMC lumbar spine and distal femur | ↑Walking speed, no significant difference, no positive treatment effect on bone |
| Semler et al53 | 6 months, five times a week at home Ex: static/tilt angle 40° | sWBV, 13–18 Hz, 0–6 mm, 4×3 minutes | n=1, 5 years, GMFCS NA | Spasticity assessment, gait tests | ↓Spasticity, ↑walking ability, ↑walking distance, ↑ability to perform unassisted steps |
| Stark et al55 | 6 months, ten times a week at home Ex: individualized according to goal-setting combined with multimodal treatment concept | sWBV (with and without tilt table), 5–25 Hz, amplitude NA, 3×3 minutes | n=78, 9.76 years, spastic diplegia, GMFCS I–V | DXA BMC of whole body without head, DXA lean (muscle) mass, modified GMFM, muscle force and angle of verticalism | ↑Percentage changes for BMC, ↑percentage changes for muscle mass, ↑modified GMFM, ↑percentage changes for muscle force and angle of verticalism |
| Stark et al56 | 6 months, ten times a week at home Ex: individualized according to goal setting combined with a multimodal treatment concept | sWBV (with and without tilt table), 5–25 Hz, amplitude NA, 3×3 minutes | n=356, 8.6±4.2 years | GMFM66 and GMFM88 | ↑GMFM66 total score, ↑All GMFM-88 dimensions |
| Stark et al54 | 14 weeks, ten times a week at home Ex: standing (if possible in squatting), sitting and four-point position with hands on platform Con: treatment as usual | sWBV (with and without tilt table), 12 and 22 Hz, maximum 2.5 mm, 3×3 minutes | 24 (Con 12), 12–24 months, GMFCS II–IV | GMFM66, PEDI (motor function) | Training was safe, developmental motor changes were similar in both groups |
| Tupimai et al36 | 6 weeks, five times a week Ex: standing with handle bar if necessary Con: prolonged passive-muscle stretching (PMS) on a tilt table for 40 minutes, PMS + WBV vs PMS alone |
Oscillating WBV (Aiko), 20 Hz, 2 mm, 1×5 minutes, 1-minute break | n=12, 6–18 years, spastic, GMFCS I–III | Spasticity (MAS), FTSST, balance (PBS) | ↓Spasticity, ↓time in FTSST, ↑balance on the PBS |
| Unger et al46 | 4 weeks, twice a week in week 1, three times a week in week 2, four to five times a week in weeks 3 and 4 Ex: trunk-oriented (sit-up variation, hip and lumbar extensions, plank) Con: inactive – groups switched after 4 weeks | vWBV, 35–40 Hz, 2–4 mm, 8×30 seconds (progression 45 seconds and 60 seconds) | n=27, 6–13 years, spastic diplegic, GMFCS I–III | 1MWT, posture (2-D photographic), ultrasound resting abdominal thickness, number of sit-ups (strength) | ↑Distance walked, ↑upright posture, ↑resting thickness of all four abdominal muscles (transversus abdominis, obliquus internus, obliquus externus, rectus abdominis), ↑sit-ups executed in 1 minute |
| Myśliwiec et al47 | 4 weeks, three times a week | vWBV, 20 Hz, 2 mm, 2×1 minute, 10-minute break | n=3, 22–30 years, spastic quadriplegic, GMFCS NA | ROM | ↑ROM in knee joint, no change in hip joint |
| Wren et al60 | 6 months, daily at home Ex: standing not specified Con: standing without WBV, groups switched after 6 months | Micro-impact (Juvent), 30 Hz 0.3 g, 10 minutes | n=31, 6–12 years, spastic hemi-, di-, tri-, quadriplegia, GMFCS I–IV | CT vertebral CBD, cross-sectional area, CBD tibia and geometry, plantar flexor strength | ↑Cortical bone area and moments of inertia, no difference in CBD or muscle, no correlation between compliance and outcome, similar in all GMFCS levels, no differences between vibration and standing for any muscle or strength variables |
| Yabumot et al63 | 5 weeks, PT twice a week + WBV for standing Ex: standing | vWBV, 30 Hz, 1–3 mm | n=1, 8 years, spastic diplegic, GMFCS III | 5 m walk test, spasticity (MAS), GMFM88, ROM with MTS (Tardieu) | ↑Number of steps, no change in spasticity, ↑GMFM88 dimension C (sitting) and D (standing), trend for increased ROM after intervention |
Note: Chronic effects in response to VT.
Abbreviations: Ex, exercise; vWBV, vertical whole-body vibration; GMFCS, Gross Motor Function Classification System; 1MWT, 1-minute walking test; MAS, modified Ashworth Scale; 6MWT, 6-minute walking test; TUG, timed up and go; GMFM, Gross Motor Function Measure; rMV, repeated muscle vibration; sWBV, side-alternating whole-body vibration; Con, control; pQCT, peripheral quantitative computed tomography; BMD, bone-mineral density; DXA, dual-energy X-ray absorptiometry; BMC, bone-mineral content; PT, physiotherapy; FV, focal vibration; MFM, motor-function measure; PEDI, Pediatric Evaluation of Disability Inventory; WBV, whole-body vibration; FTSST, five-time sit-to-stand test; PBS, pediatric balance scale; 2-D, two dimensional; ROM, range of motion; CT, computed tomography; CBD, cancellous bone density; MTS, modified Tardieu Scale.
Methodological quality of included trials
The methodological quality of included trials assessed with the PEDro scale ranged from high to poor for the cross-sectional (total scores 3–6, average 5) and longitudinal studies (total scores 1–8, average 4). Detail for each item is provided in Tables 1 and 2. No article scored more than 8 (out of 10) on this scale. Not all the criteria on the PEDro scale were able to be satisfied in the selected studies. Items with considerably weak scores were identified as items associated with “subject allocation” and “blinding of participants and operators” (2, 3, 6, and 7). Most of the studies fulfilled criteria 4, 8, 10, and 11, indicating baseline comparability, and most subjects undertook the designated training program and outcome measures were reported.
Table 1.
Study quality on the PEDro scale: acute adaptations to vibration
| PEDro scale items | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannon et al30 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Cheng et al38 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6 | ||||
| Dickin et al34 | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Eklund and Steen31 | ✓ | ✓ | ✓ | 3 | ||||||||
| Krause et al32 | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Leonard et al15 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Park et al35 | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Singh et al33 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Tardieu et al37 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 5 | |||||
| Tupimai et al36 | ✓ | ✓ | ✓ | ✓ | ✓ | 4 | ||||||
| Score per item/average score | 9 (10) | 2 (10) | 0 (10) | 10 (10) | 3 (10) | 0 (10) | 1 (10) | 7 (10) | 10 (10) | 5 (10) | 9 (10) | 5 |
Notes: Ten cross-section studies investigating the acute effect of vibration therapy on neuromuscular and functional parameters. The bottom line illustrates the relative number per PEDro item and the overall average score. 1, Eligibility criteria and source of participants; 2, random allocation; 3, concealed allocation; 4, baseline comparability; 5, blinded participants; 6, blinded therapists; 7, blind assessors; 8, adequate follow-up; 9, intention-to-treat analysis; 10, between-group comparisons; 11, point estimates and variability. Item 1 does not contribute to the total score.
Acute effects
Acute modulation refers to immediate changes manifested after acute vibration exposure. The definition of vibration exposure varies between single30–33 and repetitive bouts of vibration.15,34–37 The duration of the applied vibration varies: 3,15 10,37 and 30 seconds31,33 and 1,32,34,36 3,30 and 10 minutes.35 Details of vibration application can be found in Table 3. The results are summarized and clustered by functional category (Table 5).
Table 3.
Acute adaptations to vibration
| Study | Vibration characteristics (frequency, amplitude, duration) | Subjects (n), age, classification | Outcome measures | Results: significant acute effects |
|---|---|---|---|---|
| Cannon et al30 | FV 119 Hz, 0.5 mm, 3 minutes | n=3 (one with CP), 1–3 years, grading of GMF | Head-erect behavior (observations), paraspinal muscle activity (TVR), frequency of seizures | ↑Duration of head erect behavior (during vibration), ↑paraspinal muscle activation (during vibration), no effects on number of seizures |
| Cheng et al38 | vWBV 20 Hz, 2 mm, 1 minute (5×) | n=16, 9.8±2.3 years, GMFCS I–III | Spasticity MAS, active and passive ROM (AJ and KJ), relaxation index (Wartenberg pendulum index), gait 6MWT, and functional mobility TUG | ↓Spasticity score, knee extensors (MAS), ↑active ROM, no effects on passive ROM, ↑relaxation index, ↑distance for 6MWT, ↓time required for TUG |
| Dickin et al34 | vWBV 30–50 Hz, 2 mm, 1 minute (5×) | n=8, 20–51 years, CP 5–8 | Passive and dynamic ROM (AJ and KJ), gait parameters (speed, cadence, step, stride length, step and stride time, AJ and KJ angles) | ↑Dynamic ROM (ankle), ↑walking speed, ↑stride length |
| Eklund and Steen31 | FV 100–200 Hz, 1.5 mm, 30 seconds (maximum 1–2 minutes) | n>200, <18 years | Hyper- and hypotonia, acts of voluntary motor control (observations) | ↑Voluntary power (agonist), ↓hypertonicity (antagonist), ↑normal patterns of movement (dystonic syndrome), ↑body image function, kinesthesia, ↑spontaneous movement of weak muscles, ↑voluntary motor control, eg, memory of motor acts, pronunciation, feeding pattern |
| Krause et al32 | sWBV 16–24 Hz, 1.5–3 mm, 1 minute | n=44, 4–22 years, GMFCS II–IV | Stretch reflex, voluntary muscle activation, muscle coordination, aROM (AJ and KJ) | ↓Muscle-spindle reflex amplitude, ↑voluntary muscle activation, ↑muscle coordination, aROM (KJ) |
| Leonard et al15 | FV 200 Hz, amplitude NA, 3 seconds (5–10×) | n=6 (six Con), 12–16 years | Spinal excitability (H-reflex), shank-muscle activity during dorsiflexion, plantar flexion, and vibration | ↑Reflex amplitude during dorsiflexion (antagonist), ↑reflex amplitude during plantar flexion (agonist), but less compared to Con, ↓H-reflex amplitude during vibration (agonist), but less inhibition compared to Con |
| Park et al35 | sWBV 20 Hz, 2 mm, 10 minutes (2×) | n=17, 3–14 years, GMFCS I–IV | Spasticity (MAS, MTS) of plantar flexors (AJ), persistency of results | ↓Spasticity score (MAS and MTS), persistent results at 1 hour (MAS) and 2 hours (MTS) |
| Singh et al33 | Micro-impact (Juvent) 30–37 Hz, amplitude NA, 30 seconds | n=18 (10 Con), 4–12 years, GMFCS I–III | local-high intensity vibration (HLV) signal to tibia and femur, correlation of spasticity (MAS), and vibratory transmission | CP: ↑HLV in tibia, ↓HLV in femur, negative correlation with MAS Con: ↑HLV in tibia, ↑HLV in femur |
| Tardieu et al37 | FV 70 Hz, 0.5 mm, 10 seconds (3×) | n=22 (18 Con), 8–15 years, GMFCS | Vibration illusion | ↑Kinesthesia: vibration predicts reliably muscle contraction and joint movement |
| Tupimai et al36 | Oscillating WBV 20 Hz, 2 mm, 1 minute (5×) | n=12, 6–18 years, GMFCS I–III | Spasticity (MAS), muscle strength (FTSST), balance (PBS) | ↓Spasticity score (MAS), ↓time (FTSST), no effect on balance control |
Note: Illustration of chronic effects in response to VT.
Abbreviations: FV, focal vibration; CP, cerebral palsy; TVR, tonic vibration reflex; vWBV, vertical whole-body vibration; GMFCS, Gross Motor Function Classification System; MAS, modified Ashworth Scale; ROM, range of motion; 6MWT, 6-minute walking test; TUG, timed up and go; sWBV, side-alternating whole-body vibration; Con, control; aROM, active ROM; AJ, ankle joint; KJ, knee joint; NA, not available; MTS, modified Tardieu Scale; WBV, whole-body vibration; FTSST, five times sit-to-stand test; PBS, pediatric balance scale.
Table 5.
Conclusive effects of vibration therapy
| Clustering of parameters | Acute adaptations: effects of VT after a single bout | Chronic adaptations: effects of VT after weeks and months of application |
|---|---|---|
| Neuromuscular parameters | ||
| Spasticity, reflex activation, muscle tone, cocontraction of antagonists | Reduced spasticity in dorsal extensors, plantar flexors,35 knee flexors,31,36 knee extensors,36,38 and hip adductors36 assessed in static and dynamic measures, reduced H-reflex15 and muscle-spindle stretch reflex amplitude32 in muscles vibrated, reduced muscle hypertonicity of antagonists,31,38 reduced cocontraction of ankle and knee muscles32 | Reduced spasticity in dorsal extensors, plantar flexors,36,53 knee extensors,36,49,53,59 and hip adductors36 assessed in static and dynamic measures |
| Voluntary activation | Increased voluntary activation of ankle and knee muscles,32 spontaneous activation of weak muscles,31 and paraspinal muscle activation,30 improvements in voluntary motor control31,32 and coordination32 approaching normative values of healthy subjects31 | |
| Kinesthesia | Improved joint-position sense,31,37 awareness of level of muscle contraction,31,37 body image,31 and awareness of body segments31 | Improved segmental position sense in the ankle joint58 |
| Functional parameters | ||
| GMFM, total score, particular dimensions, particular tasks | Increased movement speed,31 edurance,30 precision,31 and spontaneous movement of weak musculature,31 improved recognition of body parts,31 pronunciation,31 and feeding patterns,31 increased memory of motor acts31 and repeatability after breaks31 of VT | Increased GMFM66 total score,55,56,58 improvement in all GMFM88 dimensions:56 C (sitting),63 D (standing),49,59,63 and E (walking)49,59 |
| Strength, torque, work, force, power, particular tasks | Increase in voluntary muscle power in upper and lower extremities,31 increased duration for head-erect behavior,30 reduced time to accomplish a sit-to-stand test36 | Increased concentric peak torque,49,51 eccentric peak torque,49 concentric49 and eccentric work,49 and increased muscle force55,57,59 (in upper and lower limb musculature), chair-rising: reduced time to accomplish the required number of repetitions48 |
| Gait, spatiotemporal characteristics, joint kinematics, complex locomotor movements | Increased gait speed34 and walking distance,38 augmented stride length,34 increased angular excursion in the limb joints,34 reduced time to accomplish TUG38 | Increased gait speed48,50,52,54,58 walking distance,46,48,53,59 walking abilty,53 walking agility,53 and number of steps,63 augmented stride length,50 increased step length54 and width,58 reduced relative stance time,64 cycle time,50 and gait symmetry,54 increased dorsiflexion and plantar flexion,50 reduced hip flexion,64 and pelvic tilt anteversion64 |
| Posture control and balance | No effects of VT36 | No effects of VT,36,58 improved postural stability,51 increased verticality in upright stance55 |
| Mobility | Increased active and passive range of motion in the ankle34,38 and knee joints32,38 | Increased active and passive range of motion in the ankle,50 knee,49 and hip joints64 |
| Quality of life | Increased well-being31 | Increased daily activity57 |
| Structural components | ||
| Muscle thickness, lean body mass | Increased muscle thickness of shank thigh muscles48,50 and lean mass of limbs,48 trunk,48 and whole body without head48,55 | |
| BMD, BMC | Increased BMD in vertebral bodies,48,60 limbs,57,48 and total body48 and BMC in lumbar spine,48 limb,48,57 and whole body without head48,55 |
Note: Note that table sums up positive effects of VT only, without considering insignificant study results manifesting no effects.
Abbreviations: VT, vibration therapy; GMFM, Gross Motor Function Measure; TUG, timed up and go; BMD, bone-mineral density; BMC, bone-mineral content.
Functional modulation
Gross motor function
The effect of VT on gross motor function was assessed in three studies. Motor control was reported to be improved during vibration.30,31,37 Cannon et al used focal vibration in three children with CP and documented a longer duration of head-erect behavior.30 Eklund and Steen used focal vibration in a population of >200 patients with CP and noted in a descriptive approach increased movement speed, precision, and spontaneous movement of weak musculature concomitantly with improved awareness of the recognition of body parts and urge to move.31 These adaptations were accompanied by improved pronunciation and feeding patterns, as well increased memory of motor acts and repeatability after breaks in VT. Improved kinesthesia31 occurred concomitantly with reduced stretch sensation of the vibrated muscle,37 indicating vibration-induced modulations in the excitably of afferent pathways coupled with supraspinal changes involving brain structures.
Strength
Only minor evidence exists for acute effects of vibration on muscle strength, including differing paradigms and methodological approaches. Tupimai et al assessed the acute effect of oscillating WBV on time to complete “five-time sit to stand” in 12 children with spastic CP (GMFCS I–III), and found a trend toward a reduced duration to complete the task.36 Eklund and Steen used a descriptive approach and reported increased voluntary muscle power of the vibrated muscle.31 With emphasis on the upper back, neck, and head, Cannon et al documented longer duration of head-erect behavior, which may point toward increased strength.30
Gait
In two studies, the effect of VT on gait was assessed. Using vertical WBV, Dickin et al found increased walking speed and elevated stride length accompanied by increased angular excursion in eight diplegic and hemiplegic patients with CP. No effects were shown for cadence, step length, or step and stride time.34 Cheng et al found significantly increased distance covered in a predefined time-frame 6-minute walking test (6MWT) and reduced duration in a predefined distance (timed up and go) immediately after vertical WBV in 16 children with spastic diplegia or spastic quadriplegia.38
Posture control and balance
Only limited results have been registered in regard to postural control. Tupimai et al assessed the acute effect of oscillating WBV on the control of upright posture in 12 children with spastic CP (GMFCS I–III) by means of the pediatric balance scale, and found no significant effects.36
Mobility
Improved mobility after VT has been demonstrated by increased angular ROM in lower limb joints. Improvements were observed at ankle34,38 and knee joints.32,38 Krause et al assessed the acute effects of side-alternating WBV on active ROM, and found immediately increased ROM in the knee joint, whereas the ankle joint remained unaffected in 44 children with CP.32 Using vertical WBV, Dickin et al found increased active ROM during walking in the ankle joints of eight diplegic and hemiplegic patients.34 Likewise, Cheng et al found increased active ROM concomitant with increased Wartenberg relaxation-index scores after exposure to vertical WBV in the ankle and knee joint in 16 children with spastic CP.38 Passive ROM remained unchanged. Evidently, this increase was manifested only in dynamic measurements, such as during walking34 or for active ROM,39 but not in passive ROM.34,38 A possible explanation for these varying results might be found in the underlying neuromuscular modulation associated with the stretch-reflex excitability and hypertonicity.
Neuromuscular modulation
Independently of the classification of VT – including WBV and focal vibration – mechanical sinusoidal stimulation elicits a frequency- and amplitude-dependent succession of stretch reflexes.21,24 The responsiveness of secondary muscle-spindle endings to focal vibration has been manifested in animal models40 and human subjects,41–44 and the expression “tonic vibration reflex” is commonly used to describe this neuromechanical coupling. Whereas focal vibration elicits stretch-reflex responses via afferent reflex circuitry, mainly in the vibrated muscle,21,42 WBV acts throughout the whole organism and encompasses the muscles of all body segments.24,45 Although less pronounced, this phenomenon has also been described to be valid for patients with CP.37
Reflex excitability
Three studies assessed the effect of VT on reflex excitability and hypertonicity and consistently demonstrated vibration-induced inhibition. Leonard et al assessed spinal excitability, ie, afferent transmission in the α-motor-neuron pool, in six children with spastic CP during focal vibration to the Achilles tendon and found inhibition of H-reflex responses.15 This inhibition occurred approximately 300 milliseconds after the onset of vibration15 and was maintained after vibration. Although the vibration-induced effects were of statistical significance, percentage changes were smaller compared to reference values obtained in a sample of healthy controls. Krause et al investigated the effect of side-alternating WBV on the stretch-reflex response and found decreased stretch-reflex amplitude and slightly delayed latency immediately after treatment.32 Furthermore, Eklund and Steen used a descriptive approach including >200 subjects with CP and reported a reduction in hypertonicity in the vibrated muscles.31 Taken together, the outcomes indicate that VT inhibits reflex activity addressing afferent pathways.
Spasticity
After an acute bout of vibration, spasticity has been shown to be reduced in studies using clinical assessments,35,36,38 measurements of muscle tone and hypertonicity,31,38 and stretch-reflex activity.39 Clinical measures comprised the modified Ashworth Scale (MAS)35,36 and modified Tardieu Scale (MTS).35 With the exception of Eklund and Steen, who found a decline in spasticity in all body segments, spasticity reduction was recorded in the lower extremities only.31,35,36,39 Using side alternating WBV, Park et al found improved MAS and MTS in 17 children with GMFCS levels IV–V.35 Accordingly, Krause et al showed an immediate reduction in musculus soleus stretch-reflex response after VT in 44 children with CP.32 Furthermore, Tupimai et al found a reduction in spasticity in the MAS of the hip adductor, quadriceps, hamstrings, and soleus muscle of the stronger leg as well as the soleus of the weaker leg after passive muscle stretching (PMS) combined with the application of oscillating WBV in 12 children with spastic CP (GMFCS levels I–III) in comparison to PMS alone.36 Accordingly, Cheng et al found reduced MAS scores in knee extensors concomitant with increased Wartenberg pendulum-test relaxation-index scores after exposure to vertical WBV in 16 children with CP.38
At the same time, such symptoms as seizures have not been shown to be worsened during the application of vibration.30 Interestingly, the effects of reduced hypertonicity in response to VT were most prominent in subjects suffering from hypertonicity because of spasticity.31 However, it has to be taken into account that vibration not only affects symptoms of spasticity, but – vice versa – also effects vibration transmission: in patients with spastic CP, higher MAS scores were correlated with reduced vibratory signal transmission to the femur. Therefore, vibration may be dampened at the distal femur in spasticity.33
Neuromuscular coordination
In patients with CP, agonist-reflex muscle activation and antagonist-reflex muscle inhibition are reduced during voluntary movement compared to healthy controls.15 As an underlying mechanism, the authors discussed pathological impairments in reciprocal inhibition during voluntary movements around the ankle.15 In three studies, the effect of VT on neuromuscular coordination was assessed using electromyography. In addition to stretch-reflex inhibition, Krause et al demonstrated that the activation ratio of agonist:antagonist muscle is increased during maximal isometric voluntary contraction after side-alternating WBV.32 Likewise, focal vibration applied to the anterior tibial tendon causes a reflex inhibition in the antagonistic triceps surae.15 Eklund and Steen used a descriptive approach and reported increased spontaneous movement of weak muscles, as well as augmented movement speed and precision.31 Taken together, the results indicate that vibration modulates not only agonist- but also antagonist-muscle activity.15,31,32 This involves improvements in muscle coordination immediately after vibration.32
Duration of effects
The acute effects of VT on muscle activation and functional performance have been demonstrated during and also following vibration. Time windows varied between investigations, showing sustained effects immediately,39 as well as 1 (MAS) and 2 hours (MTS)35 following the application of vibration. As reported by Eklund and Steen, VT causes increased memory of motor acts and improved repeatability after breaks.31 Therefore, the period after VT is sufficiently long for therapists to teach the patient skilled movement after VT and benefit from reduced spasticity and hypertonicity in the skeletal muscle.31
Long-term effects
Long-term adaptations refer to chronic changes manifested after repeated sessions of VT executed over weeks or months. The duration of applied vibration ranges from 30 seconds46 to 1,47–49 3,48,50–56 5,57 9,58,59 and 10 minutes,36,60 with the number of sets ranging from one to six. A summary of methodological quality can be found in Table 2. Details of vibration application can be found in Table 4. In the following section, results are summarized and clustered by functional category (Table 5).
Functional adaptations
Gross motor function
The most frequently used assessment for gross motor function in CP is the Gross Motor Function Measure (GMFM). The GMFM is a semiquantitative, standardized, and validated assessment for motor function primarily developed for children with CP.61,62 The test consists of 88 items scored 0–3 points divided into five dimensions: A (laying and rolling), B (sitting), C (crawling), D (standing), and E (walking). The GMFM66 is the interval-scaled total score of the GMFM.
Effects of side-alternating WBV on gross motor function have been assessed in five longitudinal studies. Ibrahim et al found improvements in GMFM88 dimensions D (standing) and E (walking, running, and jumping) after 12 weeks of side-alternating WBV application in 15 children (30 total) with diplegic CP in comparison to 1-hour traditional physiotherapy.59 Likewise, Stark et al found improvements in a modified version of the GMFM after 6 months of home-based side-alternating WBV application in addition to intensive, functional blocks of interval rehabilitation in 78 children with spastic diplegic or spastic quadriplegic CP.55 In 2013, Stark et al found improved GMFM66 total scores and improvements in all GMFM88 dimensions (A–E) after the same intervention in 356 children with CP (GMFCS levels I–V).56 However, other studies demonstrated no significant effects of WBV. Ruck et al found no difference in the GMFM88 dimensions D (standing) or E (walking, running, jumping) after 6 months of additional side-alternating WBV application to the conventional school physiotherapy program in ten children (20 total) with CP (GMFCS levels II–IV) in comparison to conventional physiotherapy alone.52 Likewise, Stark et al found no additional effects for GMFM66 and Pediatric Evaluation of Disability Inventory scores after 14 weeks of home-based side-alternating WBV application beyond to the standard therapeutic protocol in 12 (total 24) young (12–24 months) children with CP (GMFCS levels II–IV) in comparison to standard of care only.54
Two studies using vertical WBV documented positive effects on gross motor function. Ahlborg et al found improvement in GMFM88 dimensions D (standing) and E (walking, running, jumping) after eight weeks of vertical WBV application in seven children (14 total) with diplegic CP in comparison to resistance training,49 while Yabumoto et al, in their case report of an 8-year-old boy with CP (GMFCS level III), found that GMFM88 dimensions C (sitting) and D (standing) improved after 5 weeks of vertical WBV application complementing conventional physiotherapy.63 After 6 months of focal vibration, however, Reyes et al found no changes in GMFM scores among three groups – placebo vs 60 Hz vs 90 Hz vibration – in 20 children (total 61) with a first-neuron diagnosis (mixed with some second-neuron and other diseases).57
Strength
The effects of side-alternating WBV on strength have been assessed in three longitudinal studies. el Shamy found increased knee-extension peak torque at an angle speed of 90°/second and 60°/second in isokinetic muscle testing after 3 months of side-alternating WBV application in 15 children (30 total) with diplegic CP (GMFCS levels I–II) in comparison to traditional physiotherapy (muscle stretching, strengthening, balance, and proprioceptive training).51 Ibrahim et al found increased isometric knee-extensor muscle strength after 12 weeks of side-alternating WBV application in 15 children (30 total) with diplegic CP in comparison to 1 hour’s conventional physiotherapy.59 Stark et al found improved muscle force and angle of verticality after 6 months of home-based side-alternating WBV application with intensive, functional blocks of interval rehabilitation in 78 children with spastic diplegic or spastic quadriplegic CP.55
Three studies using vertical WBV documented different effects on strength in various paradigms. Ahlborg et al found increased concentric and eccentric work and peak torque in the weak leg at an angle speed of 90°/second in isokinetic muscle testing; both groups improved by 30°/second after 8 weeks of vertical WBV application in seven children (14 total) with diplegic CP in comparison to resistance training.49 There were no differences between groups. Unger et al found an increased number of sit-ups executed in 1 minute after 4 weeks of vertical WBV application in addition to school physiotherapy in 27 children with CP (GMFCS levels I–III) in comparison to school physiotherapy alone.46 Wren et al found no differences between vibration and standing for any of the muscle or strength variables after 6 months of microimpact vertical WBV application, with considerably smaller displacement amplitudes compared to the sham intervention (standing only) in 31 children (crossover design) with CP (all types, GMFCS levels I–V).60 They found no correlation between compliance and outcome and similar results for all GMFCS levels. After 6 months of focal WBV, Reyes et al found an increase in muscle force for the muscle group vibrated with 60 Hz of focal vibration on the radius and femur (high frequency, low magnitude) compared to placebo and 90 Hz vibration in 20 children (total 61) with a first-neuron diagnosis (mixed with some second neuron and other diseases).57 No changes were found for the lower limb.
Gait
For side-alternating WBV, Gusso et al found improved 6MWT after 20 weeks of side-alternating WBV application in 40 children with mild–moderate CP (GMFCS levels II–III).48 Ibrahim et al also found improved 6MWT after 12 weeks of side-alternating WBV application in 15 children (30 total) with diplegic CP in comparison to 1-hour conventional physiotherapy.59 Ko et al found improved gait speed and step width after 3 weeks of additional side-alternating WBV application to their conventional physiotherapy in 12 children (24 total) with diplegic or hemiplegic CP in comparison to physiotherapy alone.58 Lee and Chon also found improvements in gait speed, stride length, cycle time, and increase in ankle dorsiflexion and plantar-flexion excursions after 8 weeks of additional side-alternating WBV application to conventional physiotherapy in 15 children (30 total) with spastic diplegic or quadriplegic CP in comparison to conventional physiotherapy alone.50 Ruck et al found increased walking speed in the 10 m walking test after six months of additional side-alternating WBV application to the conventional school physiotherapy program in ten children (20 total) with CP (GMFCS levels II–IV) in comparison to conventional school physiotherapy alone.52 Semler et al reported improved walking ability, prolonged walking distance, and improved agility to perform unassisted steps after 6 months of side-alternating WBV application in one child with CP.53
The results for vertical WBV were comparable to those of side-alternating WBV. Unger et al found improved 1-minute walking test (1MWT) scores after 4 weeks of vertical WBV application in addition to school physiotherapy in 27 children with CP (GMFCS levels I–III) in comparison to physiotherapy alone.46 Yabumoto et al found in their case report of an 8-year-old boy with CP (GMFCS level III) that the number of steps for the 5 m walking test decreased after 5 weeks of vertical WBV application complementing conventional physiotherapy.63 Ahlborg et al found no difference in 6MWT and timed up-and-go test results after 8 weeks of vertical WBV application in seven children (14 total) with diplegic CP in comparison to resistance training.49 In a case report, Camerota et al reported higher gait velocity and symmetry concomitant with shorter stance time and step length after focal vibration applied to the calf muscle in a triplegic boy with GMFCS level II.64 Improvements in gait rhythm were accompanied by kinematic adaptations, characterized as increased functional ROM in joints of the lower extremities.
Posture control and balance
Using side-alternating WBV, el Shamy found increased anteroposterior and mediolateral postural stability after 3 months of application in 15 children (30 total) with diplegic CP (GMFCS levels I–II) in comparison to traditional physiotherapy (muscle stretching, strengthening, balance, and proprioceptive training).51 Tupimai et al also found improved balance on the pediatric balance scale after 6 weeks of prolonged PMS combined with oscillating WBV application in 12 children (crossover) with spastic CP (GMFCS levels I–III) in comparison to PMS alone.36 However, Ko et al found no difference in balance following 3 weeks of additional side-alternating WBV to their conventional physiotherapy in 12 children (24 total) with diplegic or hemiplegic CP in comparison to conventional physiotherapy alone.58 Using vertical WBV, Unger et al found more upright posture after 4 weeks of vertical WBV application additionally to school physiotherapy in 27 children with CP (GMFCS levels I–III) in comparison to school physiotherapy alone.46 In contrast, Ibrahim et al found no difference for walking balance after 12 weeks of vertical WBV application in 15 children (30 total) with diplegic CP in comparison to 1-hour conventional physiotherapy.59
Mobility
Myśliwiec et al found increased ROM in the knee joint but no change in the hip joint after 4 weeks of vertical WBV application in three female adults with spastic CP.47 Yabumoto et al found a trend in their case report of an 8-year-old boy with CP (GMFCS level III) for improved ROM after a 5-week intervention of vertical WBV application complementing conventional physiotherapy.63 Furthermore, Camerota et al observed increased passive ROM in the ankle joint and increased functional mobility in joints of the lower extremities during gait in a 1-month follow-up after 3-day focal vibration applied to the muscle belly of the triceps surae in a child with triplegic CP (GMFCS level II).64
Neuromuscular adaptations
Spasticity
The effects of side-alternating WBV on spasticity have been assessed in two longitudinal studies. Ibrahim et al found reduced spasticity of the knee extensor of the stronger leg on the MAS after 12 weeks of side-alternating WBV application in 15 children (30 total) with diplegic CP in comparison to 1-hour conventional physiotherapy.59 Semler et al reported reduced spasticity after 6 months of side-alternating WBV application in one child with CP.53 For vertical WBV, results were similar. Ahlborg et al found decreased spasticity in the stronger leg measured by the MAS in the knee extensors after 8 weeks of vertical WBV application in seven children (14 total) with diplegic CP in comparison to resistance training.49 In their case report of an 8-year-old boy with CP (GMFCS level III), Yabumoto et al found that MAS did not change after 5 weeks of vertical WBV application complementing conventional physiotherapy.63 Additionally, Tupimai et al found reduced spasticity on the MAS of the hip adductor, quadriceps, hamstrings, and soleus muscles of stronger and weaker legs after 6 weeks of PMS combined with oscillating WBV application in 12 children (crossover) with spastic CP (GMFCS levels I–III) in comparison to PMS alone.36
Structural adaptations
Muscle
The effects of side-alternating WBV on muscle structure have been assessed in three longitudinal studies. Gusso et al found increased lean body (muscle) mass measured by dual X-ray absorptiometry (DXA) after 20 weeks of side-alternating WBV application in 40 children with mild–moderate CP (GMFCS levels II–III).48 They also found improved muscle function on the chair-rise test (Leonardo mechanography). Stark et al also found increased lean body (muscle) mass measured by DXA after 6 months of home-based side-alternating WBV application in addition to intensive, functional blocks of interval rehabilitation in 78 children with spastic diplegic or spastic quadriplegic CP.55 Lee and Chon found increased muscle thicknesses of the tibialis anterior and soleus in ultrasound measurements and no change in the gastrocnemii after 8 weeks of side-alternating WBV application in addition to conventional physiotherapy in 15 children (30 total) with spastic diplegic or quadriplegic CP in comparison to conventional physiotherapy alone.50 Using vertical WBV, Unger et al found an increase in resting thickness of all four abdominal muscles (transversus abdominis, obliquus inter-nus, obliquus externus, rectus abdominis) after 4 weeks of VT in addition to school physiotherapy in 27 children with CP (GMFCS levels I–III) in comparison to school physiotherapy alone.46
Bone
The effects of side-alternating WBV on bone structure have been assessed in three longitudinal studies. Gusso et al found increased bone-mineral content (BMC) measured by DXA after 20 weeks of side-alternating WBV application in 40 children with mild–moderate CP (GMFCS levels II–III).48 They also found increased bone-mineral density (BMD) on peripheral quantitative computed tomography measurement. Stark et al found improved BMC measured by DXA after six months of home-based side-alternating WBV application in addition to intensive, functional blocks of interval-rehabilitation in 78 children with spastic diplegic or spastic quadriplegic CP.55 However, Ruck et al did not detect a positive treatment effect on bone (measured by DXA) after 6 months of additional side-alternating WBV application to a conventional school physiotherapy program in ten children (20 total) with CP (GMFCS levels II–IV) in comparison to conventional school physiotherapy alone.52
Using vertical WBV, Wren et al found an increase in cortical bone area and moments of inertia measured by computed tomography, but no difference in vertebral cancellous bone density after 6 months of microimpact WBV application compared to a sham intervention (standing only) in 31 children with CP (all types, GMFCS levels I–V).60 They also found no correlation between compliance and outcome and similar results for all GMFCS levels. After applying focal vibration on the radius and femur (high frequency, low magnitude), Reyes et al found an increase in BMD at the ultradistal radius for the group vibrated with 60 Hz after 6 months of VT compared to placebo and 90 Hz vibration in 20 children (total 61) with a first-neuron diagnosis (mixed with some second-neuron and other diseases).57 They also found an increase in BMC at the ultradistal radius for the group vibrated with 60 Hz (20 children) and the group with 90 Hz (16 children).
Duration of effects
The duration of vibration-induced effects on a long-term scale has not been studied to date. Investigations including a follow-up after termination of VT are missing.
Use of vibration treatment in patients with CP
Among the different intervention protocols, the analysis revealed no apparent dosage-dependency of VT. The great variation in VT protocols makes it difficult to highlight a clear favorite setting with efficiency beyond the others. Vibration has been applied at different levels: 5–50 Hz for WBV and reaching peak frequencies for focal vibration at 60–200 Hz. Amplitude utilized in the studies was 1–6 mm for WBV and much smaller for focal vibration: 0.3–0.5 mm. Smaller amplitude with increasing frequency is usually chosen for VT (Tables 3 and 4). In contrast to studies executed in samples of healthy subjects,21–23 protocols for patients with CP were often composed individually using personalized, progressively augmented dosages, instead of universal intervention settings. For acute and longitudinal studies using a WBV protocol, one to six sets of VT were applied with durations between 30 seconds and 10 minutes in static and dynamic conditions: standing upright with stance support, knees extended or flexed at 10°, 30°, or 50°, sitting and four-point position on knees and hands, dynamic squats and semisquats, lateral shifting and tilting, hip and lumbar extensions, and sit-up variations. Also, tilt tables at angles of 10°–50° have been used to apply WBV when the subjects could not stand independently or even with support. Focal vibration was simply applied to the muscle belly of interest or to the tendon attached to it. With increasing VT-treatment analysis, it became apparent that dosage depended on the severity level of the motor dysfunction in patients with CP. Simple static exercises coupled with low-frequency and low-amplitude VT were utilized for patients with high GMFCS levels (IV–V, lower functioning), whereas training settings for low GMFCS levels (I–III, higher functioning) contained high-frequency and high-amplitude VT concomitant with dynamic exercises.
Effect of age differences between children and adults
The majority of the studies cited (21) were executed in subjects aged 3–17 years. Seven cross-sectional experiments15,30,31,33,35,37,38 and 14 longitudinal trials46,50–60,63,64 elaborated the efficiency of VT in children, whereas only three32,34,36 cross-sectional and four36,47–49 longitudinal studies included adult volunteers older than 17 years. In the last few decades, scientific debate in the field of neurorehabilitation has consistently concluded that training regimes commonly achieve higher efficiency in children compared to adolescent patient groups.65,66 The child’s immature brain and spinal cord have higher plasticity potential, learning curves for children are steeper, and functional recovery after brain injuries is faster.65–67 Although VT demonstrably has a positive and age-independent influence on neuromuscular, functional, and structural factors associated with the disease-related deficits in patients with CP, we expect that VT will be particularly efficient in children, due to their advantage of neuroplasticity, and that VT may initiate long-term developmental effects that may persist into adulthood.65,66
Feasibility and side effects
VT can be applied regardless of subjects’ dysfunctional movement ability, health, and mental status with the help of a second person, and is thus appreciated as a feasible exercise modality providing a wide range of dosages tailored to individual requirements (Tables 3 and 4). Therefore, its neurorehabilitative application has emerged as a valuable alternative to other interventions, as there are no decisive motor prerequisites or particular cognitive requirements.25,26 Nevertheless, a few studies have documented adverse effects, including fatigue,52 pain,52 panic, and torsion spasms due to suddenly applied vibration.31 These side effects should be considered in the context of clinical VT and investigated further.
Although no study has been executed to establish the safety and feasibility of VT, there are major factors mentioned in the aforementioned manuscripts to be considered. First, VT is a machine-based therapy and thus requires hardware, installation, and supervision.20,54 Second, a specialist should guide each training session, secure the patient, and adjust vibration determinants.24,54,80 In particular, patients with high GMFCS levels (IV–V, lower functioning) may need help to activate (WBV) or fix (focal vibration) the device and adjust individual settings using an appropriate amplitude and frequency.24,80 Coupled with the high mass of WBV devices and transfer of mechanical oscillations to surroundings (floor, wall), the material and additional manpower necessary may be a bias for the application of VT in the clinical and home-based setting.
Discussion
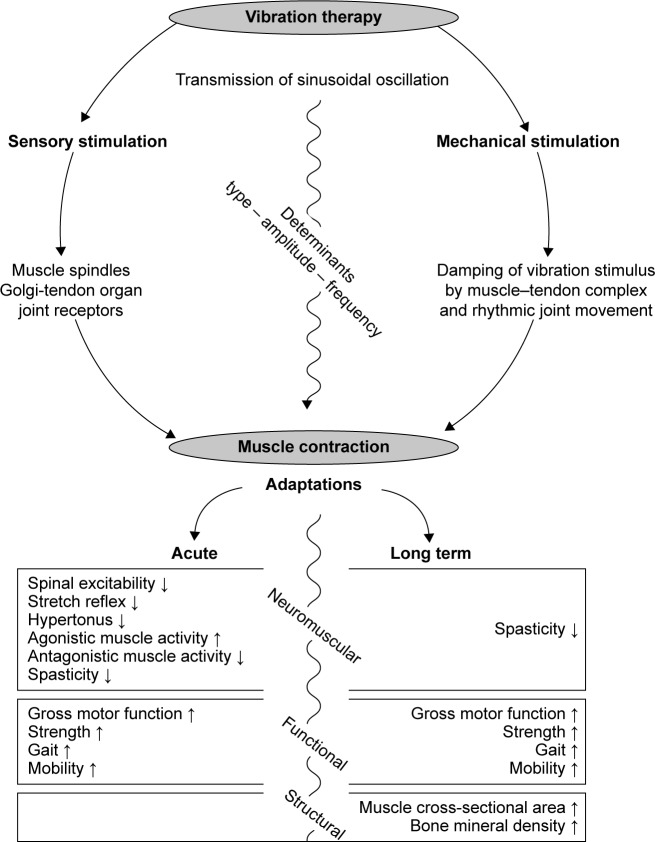
For decades, vibratory stimuli have been considered an auspicious exercise modality in neurorehabilitation. The goal of this evidence-based data search was to determine the beneficial outcomes of VT in the CP population. A thorough review of the currently available data demonstrates that VT elicits numerous desirable acute and long-term effects (Figure 2). Despite the bias of a limited article quantity of fair study quality, findings outlined that one session of VT reduced reflex excitability,15,32 hypertonicity,31 spasticity,35,36,38 and coordination deficits15,31,32 and subsequently had a positive influence on GMFM,30,31,37 strength,30,31,36 gait,34,38 and mobility32,34,38 in adults and children with CP (Figure 2). A condensed statement of the chronic effects of VT based on a considerable number of randomized controlled trials of fair study quality would include significant benefits in regard to spasticity,36,49,53 GMFM,49,55,56,59,63 muscle strength,46,49,51,55,59,60 gait,46,48–50,52,53,58,59,63,64 and mobility,63,64 as well as muscle mass48,50,55 and BMD.48,52,55,57,60 Acute and long-term effects of VT on posture control were inconsistent and remained insignificant.36,46,51,58,59
Figure 2.
Overview of results.
Note: Overview of mechanisms underlying vibration therapy and resulting short- and long-term effects.
The duration of the aforementioned effects varies greatly: acute effects were demonstrated during,30,31,37 immediately after,32 and up to 2 hours after VT.35 Using VT as an intervention, training periods varied from 3 weeks58 to 6 months.52,53,55–57,60 Therefore, VT is a training method that is on one hand used as a prerequisite of succeeding interventions (ie, strength training, stretching, and physiotherapy). Greater motor control might be enabled for the tasks selected (ie, squats, walking) being executed immediately following VT. On the other hand, VT may be used as a repetitive training method itself, enabling greater motor performance after a minimum intervention period of 3 weeks.
From neuromuscular effects to motor function and musculoskeletal tissue
Although minor evidence points toward causal relationships among the effects of VT on neuromuscular, functional, and structural components of the human body, which allow unambiguous chronological reasoning underlying the benefits of VT in CP populations, we would like to propose a conceptual model based on the pathophysiology and symptomatic of CP: while the region and magnitude of the brain injury differ greatly among patients with CP,3 they consistently show deficits in their neuromuscular control.6,16 Impaired muscle function6,16 is a common phenomenon, due to the cerebral lesion that subsequently hinders regular movement,68–71 resulting in deficient stimuli for muscle and bone growth.
We think that its specific inhibitory impact at the spinal level of the neuromuscular system might represent the origins of VT benefits. Outlined as gradually reduced spinal excitability after 300 milliseconds of VT15 and a persistent inhibition of reflex responses,32 VT might counteract disease-related spasticity35,36,38 and hypertonicity.31 As a consequence, factors interfering with precise movement execution are diminished for a certain period during and after VT, which may lead to greater synergistic30–32 and antagonistic muscle coordination32 associated with an increase in muscle strength,31,36 angular ROM,32 kinesthesia,31 gait,34,38 and mobility.32,34,38 Additionally, any kind of exercise executed in a sensible time frame during or after VT is carried out with greater precision,31 increased muscular activation intensities,30 and resulting forces,30,31,36 which CP patients could not reach without the support of VT.30,51,59 However, those mechanical forces acting on the human body are known to be essential for growth or at least the homeostasis of musculoskeletal tissues. Therefore, we expect significantly augmented myogenic and osteogenic stimuli in response to VT, causing muscle hypertrophy48,50,55 and increased BMC and BMD,48,52,55,57,60 which is important to improve the fragile and deficient health status of patients with CP.72,73 The logical effect chain outlined in this paragraph can help in understanding the prerequisites and broad range of VT efficiency associated with CP.
Using VT in clinical settings
In comparison to other exercise modalities, VT depicts a possible intervention that can be applied in multiple settings of neurorehabilitation. In particular, the following benefits emphasize its uniqueness among conventional intervention methods in CP. First, demands in regard to motor activation are low, while great acute and long-term performance enhancements can be achieved. Therefore, reflex-induced contraction and relaxation of muscles are realized passively, with the VT device being fixated to the body (focal vibration) or the subject sitting or standing on top of the vibration platform (WBV). Second, illustrated by numerous different devices, possible training applications are manifold, considering topographic preferences in regard to particular body segments. Third, the execution of training tasks does not require great cognitive ability of the subject (easy to learn) and can be easily integrated into conventional therapy sessions, because of its time-efficiency and task variability (easy to apply). After all, adding vibration to conventional therapy triggers neuromuscular modulation via spinal reflex circuitry, which offers great potential in settings of neurorehabilitation.
Limitations
The research that is available on VT in patients with CP is limited and difficult to compare, due to the heterogeneous methodological approaches and multiple outcome measures. The study quality is satisfying, but not certified as good or excellent (Tables 1 and 2). Only a small number of studies considered adult subjects, and no study was executed to establish the safety and feasibility of VT. Furthermore, three other factors limit the scope of the results obtained in this systematic review. First, CP populations differ tremendously in their classification,3 affected brain areas, and handicapped body regions,74 as well as their responsiveness to nonpharmacological interventions.20,25,75 Subject variability is a major bias in clinical trials,57 and it is thus expected that the severity of motor and mental dysfunction significantly influences the feasibility and efficiency of exercises, including VT.20 Although scientific evidence clearly points out the benefits in regard to neural control of skeletal muscles, daily motion, and structural adaptations of muscle and bone tissue, conclusive statements are restricted to particular sub-populations within the CP community. Second, the health and performance status of patients with CP is influenced by clinical measures applied apart from VT. These entail drugs administered with differing dosages (ie, baclofen, diazepam, tizanidine),76 Botox injections,77 and surgery on connective tissue (ie, lengthening of the muscle–tendon complex, joint relocation)78 and nerves in the spinal cord (ie, rhizotomy).79 Although the benefits of clinical treatments are not included in this review, their impact on spasticity and movement biomechanics, as well as adverse effects entailing seizures and hypotension, most probably influence the effects of VT exercises.76–78 Therefore, the effectiveness of VT can hardly be interpreted independently of clinical treatments applied with considerable interindividual differences. Third, VT determinants varied among studies. While the sensorimotor stimulation is identical for all types of VT, the frequency and amplitude – outlined as the major determinants in regard to muscle activation and training intensity24,80 – have been used arbitrarily within a wide range from low to high. Therefore, while the success of VT has been outlined clearly in the aforementioned experiments, systematic judgment on the choice of VT parameters requires further investigation.
Prospects
This systematic review depicts an overview of the evidence regarding the potential and limitations of VT for humans with CP. For clinicians and therapists, the identification of specific fields of application while simultaneously considering VT-induced side effects might be useful for designing evidence-based VT interventions. Outstanding issues include the effect of VT on posture control and balance and evidence-based justification of the mechanisms. Even though further high-quality research is needed – especially in regard to the acute effects of VT and its efficiency in adults – the potential and possible benefits of VT in neurorehabilitation shall be pointed out. After all, enhancing quality of life for people with CP should be the main aim being implemented by an interdisciplinary approach to high-quality scientific research. Thereby, based on the manuscripts included in this data research, it became apparent that VT helps patients with CP to master daily life challenges by the facilitation of movement quality.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Krägeloh-Mann I, Cans C. Cerebral palsy update. Brain Dev. 2009;31(7):537–544. doi: 10.1016/j.braindev.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Yeargin-Allsopp M, Braun KN, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121(3):547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 3.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 4.Poon DM, Hui-Chan CW. Hyperactive stretch reflexes, co-contraction, and muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2009;51(2):128–135. doi: 10.1111/j.1469-8749.2008.03122.x. [DOI] [PubMed] [Google Scholar]
- 5.Elder GC, Kirk J, Stewart G, et al. Contributing factors to muscle weakness in children with cerebral palsy. Dev Med Child Neurol. 2003;45(8):542–550. doi: 10.1017/s0012162203000999. [DOI] [PubMed] [Google Scholar]
- 6.Stackhouse SK, Binder-Macleod SA, Lee SC. Voluntary muscle activation, contractile properties, and fatigability in children with and without cerebral palsy. Muscle Nerve. 2005;31(5):594–601. doi: 10.1002/mus.20302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Wang Y. Gait analysis of children with spastic hemiplegic cerebral palsy. Neural Regen Res. 2012;7(20):1578–1584. doi: 10.3969/j.issn.1673-5374.2012.20.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger W, Quintern J, Dietz V. Pathophysiology of gait in children with cerebral palsy. Electroencephalogr Clin Neurophysiol. 1982;53(5):538–548. doi: 10.1016/0013-4694(82)90066-9. [DOI] [PubMed] [Google Scholar]
- 9.Berger W. Characteristics of locomotor control in children with cerebral palsy. Neurosci Biobehav Rev. 1998;22(4):579–582. doi: 10.1016/s0149-7634(97)00047-x. [DOI] [PubMed] [Google Scholar]
- 10.Rose J, Wolff DR, Jones VK, Bloch DA, Oehlert JW, Gamble JG. Postural balance in children with cerebral palsy. Dev Med Child Neurol. 2002;44(1):58–63. doi: 10.1017/s0012162201001669. [DOI] [PubMed] [Google Scholar]
- 11.Bar-On L, Molenaers G, Aertbeliën E, et al. Spasticity and its contribution to hypertonia in cerebral palsy. Biomed Res Int. 2015;2015:317047. doi: 10.1155/2015/317047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheean G, McGuire JR. Spastic hypertonia and movement disorders: pathophysiology, clinical presentation, and quantification. PM R. 2009;1(9):827–833. doi: 10.1016/j.pmrj.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Brouwer B, Ashby P. Altered corticospinal projections to lower limb motoneurons in subjects with cerebral palsy. Brain. 1991;114(Pt 3):1395–1407. doi: 10.1093/brain/114.3.1395. [DOI] [PubMed] [Google Scholar]
- 14.Brouwer B, Smits E. Corticospinal input onto motor neurons projecting to ankle muscles in individuals with cerebral palsy. Dev Med Child Neurol. 1996;38(9):787–796. doi: 10.1111/j.1469-8749.1996.tb15113.x. [DOI] [PubMed] [Google Scholar]
- 15.Leonard CT, Moritani T, Hirschfeld H, Forssberg H. Deficits in reciprocal inhibition of children with cerebral palsy as revealed by H reflex testing. Dev Med Child Neurol. 1990;32(11):974–984. doi: 10.1111/j.1469-8749.1990.tb08120.x. [DOI] [PubMed] [Google Scholar]
- 16.Milner-Brown HS, Penn RD. Pathophysiological mechanisms in cerebral palsy. J Neurol Neurosurg Psychiatry. 1979;42(7):606–618. doi: 10.1136/jnnp.42.7.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krigger KW. Cerebral palsy: an overview. Am Fam Physician. 2006;73(1):91–100. [PubMed] [Google Scholar]
- 18.Aisen ML, Kerkovich D, Mast J, et al. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol. 2011;10(9):844–852. doi: 10.1016/S1474-4422(11)70176-4. [DOI] [PubMed] [Google Scholar]
- 19.Ryan JM, Cassidy EE, Noorduyn SG, O’Connell NE. Exercise interventions for cerebral palsy. Cochrane Database Syst Rev. 2017;6:CD011660. doi: 10.1002/14651858.CD011660.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verschuren O, Ketelaar M, Takken T, Helders PJ, Gorter JW. Exercise programs for children with cerebral palsy: a systematic review of the literature. Am J Phys Med Rehabil. 2008;87(5):404–417. doi: 10.1097/PHM.0b013e31815b2675. [DOI] [PubMed] [Google Scholar]
- 21.Souron R, Besson T, Millet GY, Lapole T. Acute and chronic neuromuscular adaptations to local vibration training. Eur J Appl Physiol. 2017;117(10):1939–1964. doi: 10.1007/s00421-017-3688-8. [DOI] [PubMed] [Google Scholar]
- 22.Cochrane DJ. Vibration exercise: the potential benefits. Int J Sports Med. 2011;32(2):75–99. doi: 10.1055/s-0030-1268010. [DOI] [PubMed] [Google Scholar]
- 23.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 24.Ritzmann R, Gollhofer A, Kramer A. The influence of vibration type, frequency, body position and additional load on the neuro-muscular activity during whole body vibration. Eur J Appl Physiol. 2013;113(1):1–11. doi: 10.1007/s00421-012-2402-0. [DOI] [PubMed] [Google Scholar]
- 25.Saquetto M, Carvalho V, Silva C, Conceição C, Gomes-Neto M. The effects of whole body vibration on mobility and balance in children with cerebral palsy: a systematic review with meta-analysis. J Musculoskelet Neuronal Interact. 2015;15(2):137–144. [PMC free article] [PubMed] [Google Scholar]
- 26.Sa-Caputo DC, Costa-Cavalcanti R, Carvalho-Lima RP, et al. Systematic review of whole body vibration exercises in the treatment of cerebral palsy: brief report. Dev Neurorehabil. 2016;19(5):327–333. doi: 10.3109/17518423.2014.994713. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 4.2.6. London: Cochrane Collaboration; 2006. [Google Scholar]
- 29.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–175. doi: 10.2522/ptj.20070147. [DOI] [PubMed] [Google Scholar]
- 30.Cannon SE, Rues JP, Melnick ME, Guess D. Head-erect behavior among three preschool-aged children with cerebral palsy. Phys Ther. 1987;67(8):1198–1204. doi: 10.1093/ptj/67.8.1198. [DOI] [PubMed] [Google Scholar]
- 31.Eklund G, Steen M. Muscle vibration therapy in children with cerebral palsy. Scand J Rehabil Med. 1969;1(1):35–37. [PubMed] [Google Scholar]
- 32.Krause A, Schönau E, Gollhofer A, et al. Alleviation of motor impairments in patients with cerebral palsy: acute effects of whole-body vibration on stretch reflex response, voluntary muscle activation and mobility. Front Neurol. 2017;8:416. doi: 10.3389/fneur.2017.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh H, Whitney DG, Knight CA, et al. Site-specific transmission of a floor-based, high-frequency, low-magnitude vibration stimulus in children with spastic cerebral palsy. Arch Phys Med Rehabil. 2016;97(2):218–223. doi: 10.1016/j.apmr.2015.08.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dickin DC, Faust KA, Wang H, Frame J. The acute effects of whole-body vibration on gait parameters in adults with cerebral palsy. J Musculoskelet Neuronal Interact. 2013;13(1):19–26. [PubMed] [Google Scholar]
- 35.Park C, Park ES, Choi JY, Cho Y, Rha DW. Correction: immediate effect of a single session of whole body vibration on spasticity in children with cerebral palsy. Ann Rehabil Med. 2017;41(4):722–723. doi: 10.5535/arm.2017.41.4.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tupimai T, Peungsuwan P, Prasertnoo J, Yamauchi J. Effect of combining passive muscle stretching and whole body vibration on spasticity and physical performance of children and adolescents with cerebral palsy. J Phys Ther Sci. 2015;28(1):7–13. doi: 10.1589/jpts.28.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tardieu G, Tardieu C, Lespargot A, Roby A, Bret MD. Can vibration- induced illusions be used as a muscle perception test for normal and cerebral-palsied children? Dev Med Child Neurol. 1984;26(4):449–456. doi: 10.1111/j.1469-8749.1984.tb04470.x. [DOI] [PubMed] [Google Scholar]
- 38.Cheng HY, Ju YY, Chen CL, Chuang LL, Cheng CH. Effects of whole body vibration on spasticity and lower extremity function in children with cerebral palsy. Hum Mov Sci. 2015;39:65–72. doi: 10.1016/j.humov.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Krause A, Gollhofer A, Freyler K, Jablonka L, Ritzmann R. Acute corticospinal and spinal modulation after whole body vibration. J Mus-culoskelet Neuronal Interact. 2016;16(4):327–338. [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory JE, Proske U. The responses of muscle spindles in the kitten to stretch and vibration. Exp Brain Res. 1988;73(3):606–614. doi: 10.1007/BF00406620. [DOI] [PubMed] [Google Scholar]
- 41.Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindles and Golgi tendon organs. Muscle Nerve. 2007;36(1):21–29. doi: 10.1002/mus.20796. [DOI] [PubMed] [Google Scholar]
- 42.Burke D, Hagbarth KE, Löfstedt L, Wallin BG. The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol. 1976;261(3):673–693. doi: 10.1113/jphysiol.1976.sp011580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desmedt JE, Godaux E. Mechanism of the vibration paradox: excitatory and inhibitory effects of tendon vibration on single soleus muscle motor units in man. J Physiol. 1978;285:197–207. doi: 10.1113/jphysiol.1978.sp012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lance JW. The reflex effects of muscle vibration. Proc Aust Assoc Neurol. 1966;4:49–56. [PubMed] [Google Scholar]
- 45.Zaidell LN, Mileva KN, Sumners DP, Bowtell JL, Woloschak GE. Experimental evidence of the tonic vibration reflex during whole-body vibration of the loaded and unloaded leg. PLoS One. 2013;8(12):e85247. doi: 10.1371/journal.pone.0085247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unger M, Jelsma J, Stark C. Effect of a trunk-targeted intervention using vibration on posture and gait in children with spastic type cerebral palsy: a randomized control trial. Dev Neurorehabil. 2013;16(2):79–88. doi: 10.3109/17518423.2012.715313. [DOI] [PubMed] [Google Scholar]
- 47.Myśliwiec A, Kuszewski M, Saulicz E, et al. The influence of vibration on the quality of gait in women with cerebral palsy. Int J Disabil Hum Dev. 2015;14(2):125–129. [Google Scholar]
- 48.Gusso S, Munns CF, Colle P, et al. Effects of whole-body vibration training on physical function, bone and muscle mass in adolescents and young adults with cerebral palsy. Sci Rep. 2016;6:22518. doi: 10.1038/srep22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahlborg L, Andersson C, Julin P. Whole-body vibration training compared with resistance training: effect on spasticity, muscle strength and motor performance in adults with cerebral palsy. J Rehabil Med. 2006;38(5):302–308. doi: 10.1080/16501970600680262. [DOI] [PubMed] [Google Scholar]
- 50.Lee BK, Chon SC. Effect of whole body vibration training on mobility in children with cerebral palsy: a randomized controlled experimenter-blinded study. Clin Rehabil. 2013;27(7):599–607. doi: 10.1177/0269215512470673. [DOI] [PubMed] [Google Scholar]
- 51.el-Shamy SM. Effect of whole-body vibration on muscle strength and balance in diplegic cerebral palsy: a randomized controlled trial. Am J Phys Med Rehabil. 2014;93(2):114–121. doi: 10.1097/PHM.0b013e3182a541a4. [DOI] [PubMed] [Google Scholar]
- 52.Ruck J, Chabot G, Rauch F. Vibration treatment in cerebral palsy: a randomized controlled pilot study. J Musculoskelet Neuronal Interact. 2010;10(1):77–83. [PubMed] [Google Scholar]
- 53.Semler O, Fricke O, Vezyroglou K, Stark C, Schoenau E. Preliminary results on the mobility after whole body vibration in immobilized children and adolescents. J Musculoskelet Neuronal Interact. 2007;7(1):77–81. [PubMed] [Google Scholar]
- 54.Stark C, Herkenrath P, Hollmann H, et al. Early vibration assisted physiotherapy in toddlers with cerebral palsy: a randomized controlled pilot trial. J Musculoskelet Neuronal Interact. 2016;16(3):183–192. [PMC free article] [PubMed] [Google Scholar]
- 55.Stark C, Nikopoulou-Smyrni P, Stabrey A, Semler O, Schoenau E. Effect of a new physiotherapy concept on bone mineral density, muscle force and gross motor function in children with bilateral cerebral palsy. J Musculoskelet Neuronal Interact. 2010;10(2):151–158. [PubMed] [Google Scholar]
- 56.Stark C, Semler O, Duran I, et al. Intervallrehabilitation mit häuslichem Training bei Kindern mit Zerebralparese. Monatsschr Kinderheilkd. 2013;161(7):625–632. [Google Scholar]
- 57.Reyes ML, Hernandez M, Holmgren LJ, Sanhueza E, Escobar RG. High-frequency, low-intensity vibrations increase bone mass and muscle strength in upper limbs, improving autonomy in disabled children. J Bone Miner Res. 2011;26(8):1759–1766. doi: 10.1002/jbmr.402. [DOI] [PubMed] [Google Scholar]
- 58.Ko MS, Sim YJ, Kim DH, Jeon HS. Effects of three weeks of whole-body vibration training on joint-position sense, balance, and gait in children with cerebral palsy: a randomized controlled study. Physiother Can. 2016;68(2):99–105. doi: 10.3138/ptc.2014-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahim MM, Eid MA, Moawd SA. Effect of whole-body vibration on muscle strength, spasticity, and motor performance in spastic diplegic cerebral palsy children. Egypt J Med Hum Genet. 2014;15(2):173–179. [Google Scholar]
- 60.Wren TA, Lee DC, Hara R, et al. Effect of high-frequency, low-magnitude vibration on bone and muscle in children with cerebral palsy. J Pediatr Orthop. 2010;30(7):732–738. doi: 10.1097/BPO.0b013e3181efbabc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31(3):341–352. doi: 10.1111/j.1469-8749.1989.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 62.Michaelis U. Gross Motor Function Measure (GMFM66 and GMFM88) User’s Manual, 2nd edition. Dev Med Child Neurol. 2015;57(12):1188. [Google Scholar]
- 63.Yabumoto T, Shin S, Watanabe T, et al. Whole-body vibration training improves the walking ability of a moderately impaired child with cerebral palsy: a case study. J Phys Ther Sci. 2015;27(9):3023–3025. doi: 10.1589/jpts.27.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Camerota F, Galli M, Celletti C, et al. Quantitative effects of repeated muscle vibrations on gait pattern in a 5-year-old child with cerebral palsy. Case Rep Med. 2011;2011:359126. doi: 10.1155/2011/359126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Damiano DL. Rehabilitative therapies in cerebral palsy: the good, the not as good, and the possible. J Child Neurol. 2009;24(9):1200–1204. doi: 10.1177/0883073809337919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnston MV. Clinical disorders of brain plasticity. Brain Dev. 2004;26(2):73–80. doi: 10.1016/S0387-7604(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 67.Berger MS, Pitts LH, Lovely M, Edwards MS, Bartkowski HM. Outcome from severe head injury in children and adolescents. J Neurosurg. 1985;62(2):194–199. doi: 10.3171/jns.1985.62.2.0194. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Woollacott MH. Lower extremity kinetics for balance control in children with cerebral palsy. J Mot Behav. 2007;39(4):306–316. doi: 10.3200/JMBR.39.4.306-316. [DOI] [PubMed] [Google Scholar]
- 69.Donker SF, Ledebt A, Roerdink M, Savelsbergh GJ, Beek PJ. Children with cerebral palsy exhibit greater and more regular postural sway than typically developing children. Exp Brain Res. 2008;184(3):363–370. doi: 10.1007/s00221-007-1105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuzson AE, Granata KP, Abel MF. Spastic velocity threshold constrains functional performance in cerebral palsy. Arch Phys Med Rehabil. 2003;84(9):1363–1368. doi: 10.1016/s0003-9993(03)00199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woollacott MH, Shumway-Cook A. Postural dysfunction during standing and walking in children with cerebral palsy: what are the underlying problems and what new therapies might improve balance? Neural Plast. 2005;12(2–3):211–219. doi: 10.1155/NP.2005.211. discussion 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shortland A. Muscle deficits in cerebral palsy and early loss of mobility: can we learn something from our elders? Dev Med Child Neurol. 2009;51(Suppl 4):59–63. doi: 10.1111/j.1469-8749.2009.03434.x. [DOI] [PubMed] [Google Scholar]
- 73.Houlihan CM, Stevenson RD. Bone density in cerebral palsy. Phys Med Rehabil Clin N Am. 2009;20(3):493–508. doi: 10.1016/j.pmr.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kent RM. Cerebral palsy. In: Barnes MP, Good DC, editors. Handbook of Clinical Neurology: Neurological Rehabilitation. Vol. 110. Amsterdam: Elsevier; 2013. pp. 443–459. [DOI] [PubMed] [Google Scholar]
- 75.Naro A, Leo A, Russo M, et al. Breakthroughs in the spasticity management: are non-pharmacological treatments the future? J Clin Neurosci. 2017;39:16–27. doi: 10.1016/j.jocn.2017.02.044. [DOI] [PubMed] [Google Scholar]
- 76.Kita M, Goodkin DE. Drugs used to treat spasticity. Drugs. 2000;59(3):487–495. doi: 10.2165/00003495-200059030-00006. [DOI] [PubMed] [Google Scholar]
- 77.Graham HK, Aoki KR, Autti-Ramo I, et al. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture. 2000;11(1):67–79. doi: 10.1016/s0966-6362(99)00054-5. [DOI] [PubMed] [Google Scholar]
- 78.Abel MF, Damiano DL, Pannunzio M, Bush J. Muscle-tendon surgery in diplegic cerebral palsy: functional and mechanical changes. J Pediatr Orthop. 1999;19(3):366–375. [PubMed] [Google Scholar]
- 79.Fasano VA, Broggi G, Barolat-Romana G, Sguazzi A. Surgical treatment of spasticity in cerebral palsy. Childs Brain. 1978;4(5):289–305. doi: 10.1159/000119785. [DOI] [PubMed] [Google Scholar]
- 80.Abercromby AF, Amonette WE, Layne CS, McFarlin BK, Hinman MR, Paloski WH. Variation in neuromuscular responses during acute whole-body vibration exercise. Med Sci Sports Exerc. 2007;39(9):1642–1650. doi: 10.1249/mss.0b013e318093f551. [DOI] [PubMed] [Google Scholar]