Abstract
MicroRNAs (miRNAs) are known to be crucial players in governing the differentiation of human induced pluripotent stem cells (hiPSCs). Despite their utter importance, identifying key lineage specifiers among the myriads of expressed miRNAs remains challenging. We believe that the current practice in mining miRNA specifiers via delineating dynamic fold-changes only is inadequate. Our study, therefore, provides evidence to pronounce “lineage specificity” as another important attribute to qualify for these lineage specifiers. Adopted hiPSCs were differentiated into representative lineages (hepatic, nephric and neuronal) over all three germ layers whilst the depicted miRNA expression changes compiled into an integrated atlas. We demonstrated inter-lineage analysis shall aid in the identification of key miRNAs with lineage-specificity, while these shortlisted candidates were collectively known as “lineage-specific miRNAs”. Subsequently, we followed through the fold-changes along differentiation via computational analysis to identify miR-192 and miR-372-3p, respectively, as representative candidate key miRNAs for the hepatic and nephric lineages. Indeed, functional characterization validated that miR-192 and miR-372-3p regulate lineage differentiation via modulation of the expressions of lineage-specific genes. In summary, our presented miRNA atlas is a resourceful ore for the mining of key miRNAs responsible for lineage specification.
Introduction
Extensive evidence has shown that human pluripotent stem cells (hPSCs) can give rise to all somatic cell types in an adult human1–4. Understanding the mechanisms by which hPSCs differentiate during early embryogenesis is crucial to understanding human development. Moreover, a comprehensive understanding of the mechanisms underlying hPSC differentiation is required for their application in disease studies, where intentionally differentiated functional cells derived from hPSCs have been widely used for disease modeling and replacement therapy5–7.
A previous in-depth analysis of the transcriptome reported a temporal change in microRNA (miRNA) expression levels during hPSC differentiation8, suggesting that the expression dynamics of miRNAs are associated with hPSC differentiation9. In addition, the identification of miRNAs regulating lineage specification such as miR-375, which regulates pancreatic islet formation, further confirms that miRNAs can serve as cell fate determinants8,10–15. However, identification of miRNAs regulating lineage specification, hereafter referred to as key miRNAs in this manuscript, remains challenging since there are massive numbers of miRNAs potentially regulating lineage formation16. In previous studies, key miRNAs were mainly revealed by the analysis of expression changes during differentiation of a single lineage (intra-lineage analysis)9,17,18. Following such analysis, only those key miRNAs with the greatest fold-changes were selected for further study19–21, while miRNAs that changed less dramatically were avoided.
Another criterion, lineage specificity, has also been used to identify key miRNAs, which alleviates the drawbacks of solely considering fold-change as the filtration criterion. Lineage-specific miRNAs have been reported previously22,23; several of them have been confirmed to determine cell identity10. For instance, miR-124 and miR-9, which are neuron-specific miRNAs, have been reported to direct neuronal differentiation and regulate brain development24. Many lineage-specific miRNAs have been revealed by the comparison of the miRNA transcriptome (miRNAome) between terminally differentiated tissues23,25,26. However, few studies have examined different lineages at the earliest stages of development. Therefore, profiling of the miRNAome of the three germ layers and further lineages induced from hPSCs would allow the identification of lineage-specific miRNAs that affect the earliest cell fate decisions.
Human embryonic stem cells (hESCs) are the most frequently used hPSCs in in vitro studies of human development27, although the full-scale application of hESCs in regenerative medicine and disease modeling is hindered by ethical and technical issues28. Human induced PSCs (hiPSCs), which resemble hESCs, have become an alternative cell type for disease modeling and drug selection7. hiPSCs induced from somatic cells of individual patients enable the establishment of hiPSC banks corresponding to all HLA haplotypes. Considering the necessity of integrating information from different patients, generation of a reference dataset of miRNAome based on hiPSCs of healthy individuals that could be used for comparison with any patient-specific hiPSCs is urgently required29.
In this study, we performed comparisons among three lineages (inter-lineage analysis) to reveal lineage-specific miRNAs at the early stages of development. From these miRNAs, we further identified novel key miRNAs and validated their key regulatory roles in early lineage specification. To perform the inter-lineage analysis, we induced hiPSCs into three representative lineages for the three germ layers and profiled the expression changes of miRNAs during differentiation. By integrating all profiling results, we built a miRNA atlas that allows comparisons of miRNA expression across three lineages. Based on this atlas, those miRNAs with lineage-specific expressions, such as miR-192 and miR-372-3p, were easily identified. After selecting key miRNA candidates by inter-lineage analysis, we conducted extensive experiments to determine whether they were true key regulators. Targets of miR-192 and miR-372-3p in lineage specification were predicted computationally. Their repression on predicted targets and their regulatory effects on lineage differentiation were validated experimentally. These results strongly supported the hypothesis that new key miRNAs could be precisely identified from our atlas using inter-lineage analysis.
Taken together, we have developed the inter-lineage analysis methodology to identify key miRNAs of early hPSCs differentiation by considering lineage specificity. The inter-lineage analysis is complementary to intra-lineage analysis and allows key miRNAs with less dramatic fold-changes to be identified. Our study differs from previous studies primarily in terms of: (1) determining which miRNAs affect the very earliest stages of hPSCs differentiation (key miRNAs); (2) the development of a computational method to identify key miRNAs from lineage-specific miRNAs; and (3) the generation of an integrated miRNA expression atlas for all three germ layers and their derived lineages.
Results
Step-wise in vitro differentiation of hiPSCs into hepatocytes, nephron progenitors, and neural progenitors
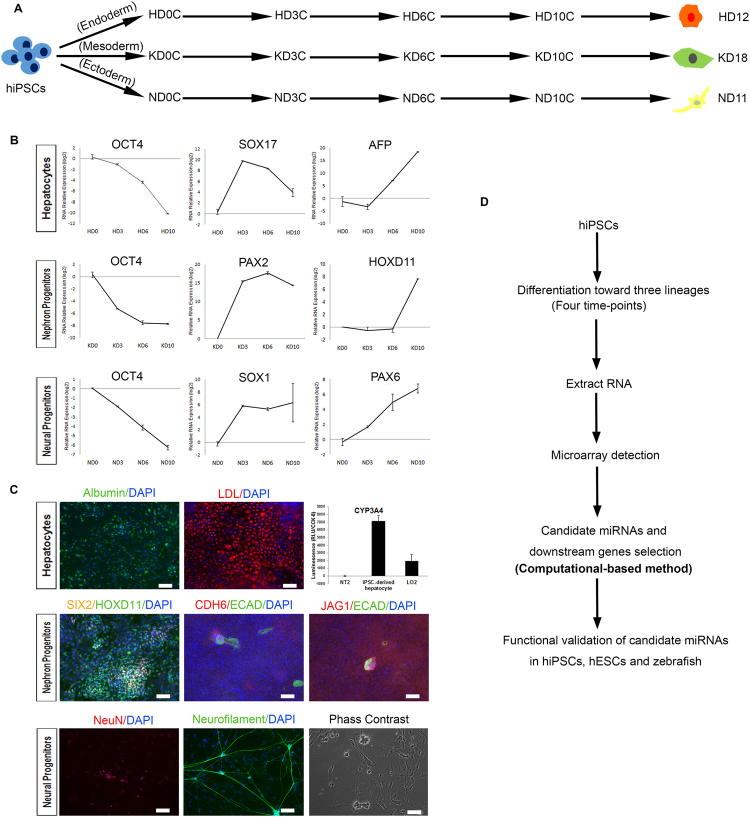
To clarify the miRNA dynamics involved in hiPSCs differentiation, we established a multi-lineage differentiation system following published protocols that show high differentiation efficiency30–32. We differentiated hiPSCs into representative lineages of the three germ layers: hepatocytes for the endoderm (12 days), nephron progenitors for the mesoderm (18 days), and neural progenitors for the ectoderm (11 days), respectively (Fig. 1A and Supplementary Fig. S1A). The hiPSC line used in this study (iBC 1.2) meets all established criteria for hiPSCs, as reported in our previous studies33,34. The expression of representative markers for each lineage was detected by quantitative real-time PCR (qPCR) to confirm the cell identity at each time-point (Fig. 1B and Supplementary Fig. S1B–D). The expression of the pluripotent marker OCT4 declined during differentiation of all three lineages, indicating a loss of pluripotency. Meanwhile, germ layer markers and lineage-specific markers appeared in a sequential order, as expected. Markers for hepatocytes, metanephric mesenchyme (MM), and neural progenitors were confirmed using immunofluorescent staining (IFC) analysis and flow-cytometric analysis, indicating that the purities of the three lineages were all around 50% (Supplementary Fig. S1E–K). Hepatocytes and neurons were additionally confirmed by functional assays and IFC, respectively (Fig. 1C).
Figure 1.
Outline of the experimental design and summary of the multi-lineage induction. (A) Schematic overview of hiPSC differentiation into hepatocytes, nephron progenitors, and neural progenitors. (B) qPCR results showing the expression tendencies of pluripotency marker (OCT4), markers for endoderm (SOX17), mesoderm (PAX2), ectoderm (SOX1), and representative markers for hepatocytes (AFP), metanephric mesenchyme (HOXD11), and neural progenitors (PAX6) at four time-points. Values represent means ± SD (n = 2 independent cultures for each time-point). (C) Functional characterization of terminal cells, including hepatocytes (12 days), nephron progenitors (18 days) and neurons (40 days). For hepatocyte differentiation, Albumin (green fluorescence) indicates hepatocytes; LDL uptake assay indicates the LDL receptor activity in hepatocytes; and CYP450 assays show the cytochrome P450 activity of hepatocytes. For kidney differentiation, early metanephric mesenchyme marked by SIX2 (yellow fluorescence) and HOXD11 (green fluorescence), and nephron vesicles marked by CDH6 (green fluorescence), ECAD (red fluorescence) and JAG1 (red fluorescence) were induced successfully. For neuron differentiation, the nucleus and the axons of neurons were marked by NeuN (red fluorescence) and Neurofilament (green fluorescence), respectively. Phase contrast imaging showing the morphology of an induced neuron. Scale bars represent 100 μm. (D) Schematic overview of the experimental design. HD: hepatocyte differentiation; KD: nephron progenitor differentiation; ND: neural progenitor differentiation.
Intra-lineage analysis of differentially expressed miRNAs confirms previously reported key miRNAs
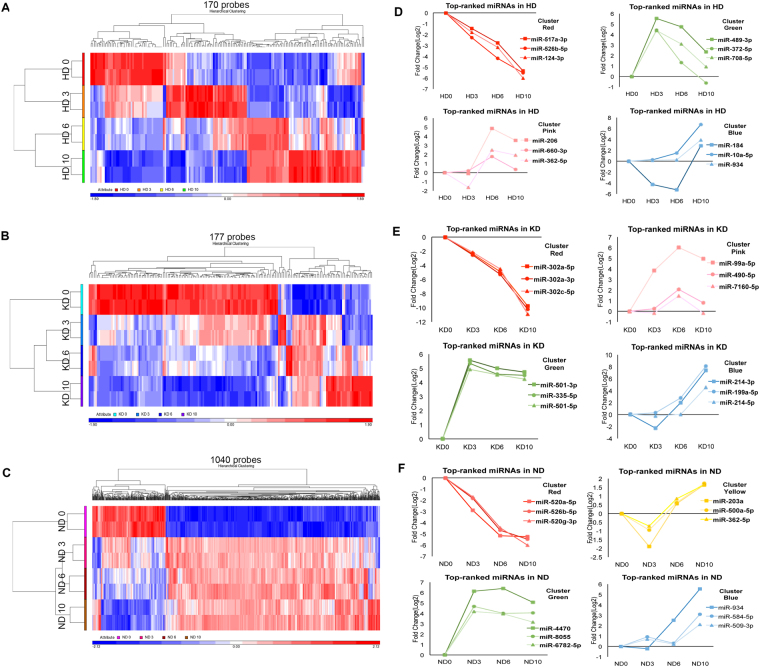
We first asked how miRNAs are differentially expressed when hiPSCs are differentiated into individual lineages (intra-lineage analysis). RNA was collected at days 0, 3, 6, and 10 (n = 2) of each lineage differentiation, as illustrated in Fig. 1D. The miRNA transcriptome (miRNAome) was examined by small RNA microarray analysis. We processed the microarray results with Partek Genomic Suite software following a standard workflow for miRNA expression. The differentially expressed small RNAs within each lineage are summarized in Supplementary Table S1. Specifically, a one-way ANOVA analysis was used to determine which small RNAs had differences in expression between time-points. Subsequent pairwise comparison between successive time-points was used to identify when small RNAs demonstrated significant differential expression (post-hoc testing, fold-change ≥2 or ≤−2, false discovery rate <0.05). We used the false discovery rate (FDR) instead of the P value to decrease false positives, as performed in previous studies8,33. These analyses identified 170, 177, and 1040 differentially expressed small RNAs in hepatocyte differentiation (HD), nephron progenitor differentiation (KD), and neural progenitor differentiation (ND), respectively (Fig. 2A–C).
Figure 2.
Intra-lineage analysis of differentially expressed miRNAs. (A–C) Hierarchical clustering of 170 differentially expressed small RNAs in the first ten days of HD, 177 differentially expressed small RNAs of KD, and 1,040 differentially expressed small RNAs of ND, respectively. The values of two biological replicates (indicated in color) for each time-point are shown. (D–F) The miRNAs with differential dynamics changes were plotted in different colors: small RNAs that decreased with differentiation (red); small RNAs enriched in stage 1 and 4 but downregulated at stage 2 (yellow); small RNAs increased at stage 2 but decreased at later stages (green); small RNAs enriched in stage 3 (pink); and small RNAs only increased at the latest stage (blue).
Bi-directional hierarchical clustering indicated distinct expression dynamics of small RNAs when different lineages were established (Fig. 2A–C). In HD, stage 1 and 2 samples clustered together, while stage 3 and 4 samples shared a similar pattern (Fig. 2A). In contrast, in KD and ND, stage 2, 3 and 4 samples clustered together, separate from stage 1 samples (Fig. 2B,C). Within each lineage, small RNAs were separated into different groups according to their differential dynamics changes. To clearly reveal these dynamics, miRNAs with the greatest fold-changes in each group were plotted in different colors (Fig. 2D–F). To ascertain the dynamic range of the microarray platform is appropriate for reliable detection, the expression of the five most-upregulated miRNAs in each lineage were further validated by qPCR (Supplementary Fig. S2).
Using the intra-lineage analysis, we were able to recapitulate previously reported key miRNAs. Specifically, we ranked the absolute fold-changes of miRNAs during lineage differentiation. Functions of miRNAs with the largest fold-change (top 10 ranking) were identified from previously reported studies and are summarized in Table 1. As expected, most of the miRNAs with large fold-changes (9 of 10) in both HD and KD were associated with corresponding lineage establishment. For example, miR-122, which changes the most from HD 0 to HD 6 (positive fold-change of 1,022), has been reported to be a liver-specific miRNA that functions in the maintenance of liver phenotype and metabolism of cholesterol35,36.
Table 1.
Top 10 miRNAs with previously reported development-associated functions.
| miRNAs | Expression pattern | Reported functions in lineages |
|---|---|---|
| miR-10a-5p | HD 10 (up) | Liver fibrosis62 |
| miR-122-5p | HD 6-10 (up) | Expressed in developing liver tissue63/Liver-specific miRNA and maintenance of adult-liver phenotype35,36/Cholesterol and lipid metabolism36,64 |
| miR-124-3p | HD 3–10 (down) | Cholangiocyte proliferation65/Brain-specific miRNA65,66 |
| miR-146b-5p | HD 10 (up) | Intestinal epithelial cell differentiation67/Visceral preadipocyte proliferation and differentiation68/Regulator of inflammation in epithelial cells, lung fibroblasts and others69 |
| miR-184 | HD 10 (up) | — |
| miR-192-5p/194-3p | HD 6–10 (up) | Liver-abundant miRNAs70/Intestinal epithelial cell differentiation71 |
| miR-489-3p | HD 3 (enriched) | Increased in all germ layers, highest in the endoderm11 |
| miR-517a-3p/-526b-5p | HD 3–10 (down) | Members of C19MC cluster, which is involved in pluripotent stem cell status72,73 |
| miR-10a-5p | KD 6 (enriched) | Biomarkers of kidney injury74 |
| miR-10b-5p | KD 6 (enriched) | — |
| miR-181a-5p | KD 10 (up) | Expressed in kidney tissue75/Hematopoietic lineage differentiation75 |
| miR-214–3p | KD 10 (up) | Differentiation of ESCs into endothelial cells76/Osteogenic differentiation77 |
| miR-302a-5p/3p/-302c-5p/-302d-5p | KD 10 (down) | hPSC-specific miRNAs22/Definitive endoderm formation12 |
| miR-371a-3p | KD 10 (down) | hPSC-specific miRNAs22/Mesendoderm specification78 |
| let-7e-5p | KD 10 (up) | Early nephrogenic differentiation38 |
| miR-10a-5p | ND 10 (up) | Neuron differentiation79 |
| miR-181a-2-3p | ND 3 (down) | Regulation of synaptic functions80 |
| miR-1208 | ND 6 (enriched) | — |
| miR-375 | ND 6–10 (up) | Inhibition of neurite differentiation81/Spinal motor neuron development82 |
| miR-4470 | ND 3–6 (up) | — |
| miR-520a-5p/-520g-3p/-526b-5p | ND 3–10 (down) | Members of C19MC cluster, which is involved in pluripotent stem cell status72,73 |
| miR-664b-5p | ND 3–10 (up) | — |
| miR-934 | ND 6–10 (up) | — |
In summary, the success in recapitulating many previously reported key miRNAs by intra-lineage analysis effectively supports the use of our profiling data as a reliable source for identifying key miRNAs.
Inter-lineage analysis reveals lineage-specific miRNAs
Identification of key miRNAs by intra-lineage analysis has traditionally relied on fold-change, leading to the identification of only key miRNAs with large fold-changes. Here, we applied another criterion to identify key miRNAs, one that focuses on their differential expression across lineages in addition to considering fold-change23,25,26.
According to previous studies, miRNAs with expression restricted to one lineage contribute to cell identity10, suggesting that the expression changes of miRNAs (upregulation or downregulation) have a significant potential to specify lineages. Therefore, we hypothesized that miRNAs that specifically change at one lineage are potential candidates for key miRNAs. For convenience, we termed these miRNAs “lineage-specific miRNAs”. Here, we describe how we identify them by comparing the expression of miRNAs across lineages (inter-lineage analysis).
First, we subjected all small RNA transcriptome data (24 samples in total for all three lineages) to principal component analysis (PCA). The 24 samples automatically segregated into four groups, including undifferentiated hiPSCs with different pre-induction methods (HD 0, ND 0, KD 0, yellow sphere), differentiating hepatocytes (HD 3–10, pink sphere), differentiating nephron progenitors (KD 3–10, green sphere), and differentiating neural progenitors (ND 3–10, blue sphere) (Fig. 3A).
Figure 3.
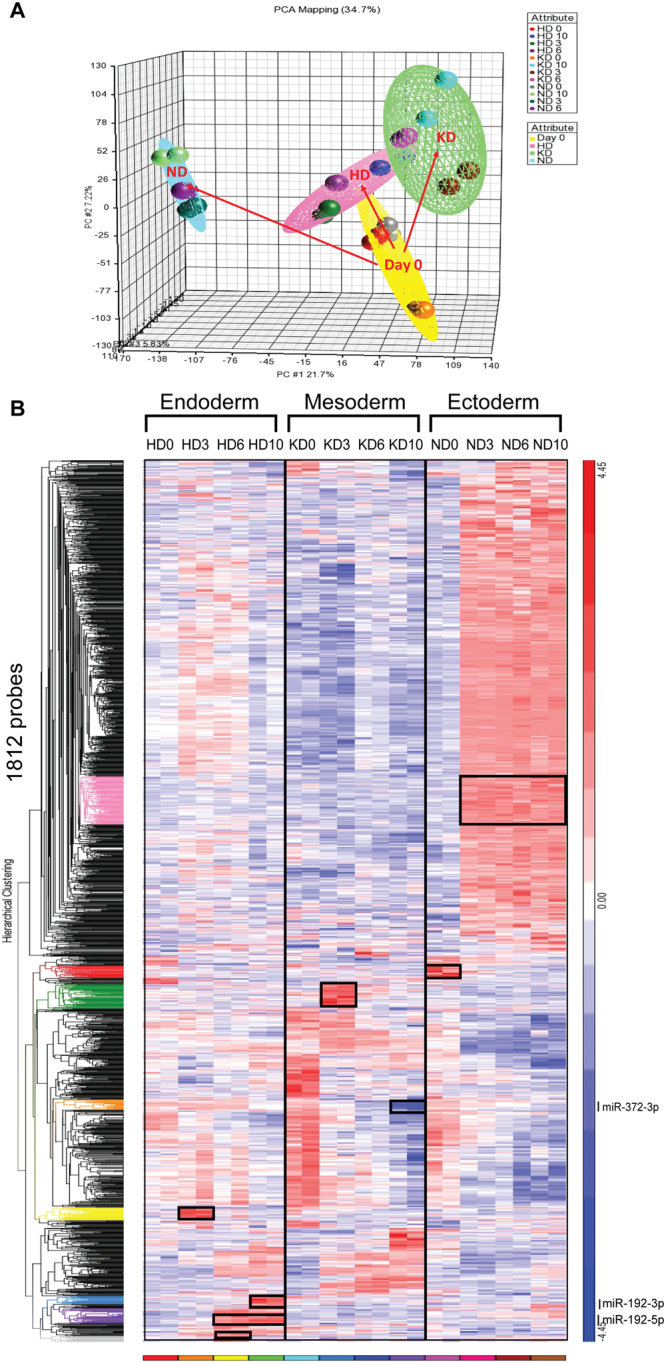
Inter-lineage analysis reveals lineage-specific miRNAs. (A) Principal component analysis (PCA) showing the unsupervised division of samples into a day 0 group (yellow sphere), an HD group (pink sphere), a KD group (green sphere) and a ND group (blue sphere). (B) Comprehensive heat map showing distinct dynamics of small RNA expression patterns during lineage specification. Colored dendrograms and black boxes indicate small RNAs that were only upregulated or downregulated in one lineage. Particularly, gray, purple, blue and yellow dendrograms indicate small RNAs that were only changed in HD. Orange and green dendrograms indicate small RNAs that were only changed in KD, Red and pink dendrograms indicate small RNAs that were only changed in ND. The newly identified key miRNAs (miR-192-3p/5p and miR-372-3p) are indicated in the dendrograms that they are included.
Next, to further show the expression patterns of small RNAs in the establishment of different lineages, we performed hierarchical clustering of all identified differentially expressed small RNAs (1,812 small RNAs) in three lineages (Fig. 3B). The bi-direction hierarchical clustering automatically resulted in several subclusters with distinct expression patterns (Fig. 3B)37. Within these subclusters of small RNAs, we focused primarily on miRNAs and denoted them as lineage-specific miRNAs. To clearly show these lineage-specific miRNAs in the atlas, we delineated them with black boxes in the heat-map and colored them in the dendrogram (Fig. 3B). For instance, the subcluster of miRNAs colored green in the dendrogram was specifically elevated in KD 3 but not in HD or ND (Fig. 3B). All lineage-specific miRNAs with normalized expression values are summarized in Supplementary Table S2. As expected, several of them have been previously reported to specify lineage, such as miR-124 in ND and let-7e in KD38,39, supporting lineage specificity as an effective criterion to identify key miRNAs.
Identification of novel key miRNAs from lineage-specific miRNAs
Lineage-specific miRNAs form a candidate set of key miRNAs (273 lineage-specific miRNAs). We next sought to select the most likely potential candidates for validation. However, since the cardinality of the candidate set (Supplementary Table S2) was large, it was difficult to evaluate every candidate and identify key miRNAs. Therefore, we focused on the HD 6–10 subcluster (purple and blue subcluster) and the KD 10 subcluster (orange subcluster). At these time-points, early lineages have formed, considering that hepatoblasts and metanephric mesenchyme are progenitors of hepatocytes and nephrons, respectively.
One straightforward method to identify key miRNAs from lineage-specific miRNAs is based on fold-change. By ranking the fold-change of lineage-specific miRNAs, we found that both miR-192-5p and miR-192-3p ranked the highest among the HD 6–10 subcluster (Fig. 3B and Supplementary Table S2), increasing our suspicion that they were candidate key miRNAs. However, selection by fold-change can only identify key miRNAs that change substantially, while it ignores those changing less dramatically. We therefore developed a computational method to expand the search range (Supplementary Fig. S3). Briefly, given that key miRNAs should regulate critical genes in specific lineages, we first searched miRNAs targeting critical genes in KD, and then intersected these identified miRNAs with lineage-specific miRNAs to obtain final key miRNA candidates (Supplementary Fig. S3A). We focused on PKD1 and PKD2, which are key regulatory genes that encode Polycystin 1 (PC1) and Polycystin 2 (PC2), respectively. They are upregulated synergistically during the early stages of embryonic renal development and are important in the developing kidney for maintaining the differentiated phenotype of tubular epithelium40. We then asked whether there were any miRNAs contributing to kidney development via regulation of PKD1 or PKD2. TargetScan generated a list of potential miRNAs for targeting PKD1/PKD2. Because PKD1/PKD2 were upregulated in KD (Supplementary Fig. S3B), we searched the specifically downregulated miRNAs in KD and compared the results with the TargetScan analysis. Using this approach, we recapitulated the miR-106b~25 cluster and miR-17~92 cluster (Supplementary Fig. S3C), which showed an increased expression tendency in a polycystic kidney disease (PKD) mouse model41. More importantly, the overexpression of the miR-17~92 cluster was sufficient to produce cyst-like structures in the mouse model41. In addition to previously reported miRNAs, we identified miR-372-3p, which has well-established enrichment in hESCs but has never been reported in kidney development42 (Supplementary Fig. S3D). Our array results indicated that miR-372-3p was specifically downregulated from KD 6 to 10 (Fig. 3B). TargetScan analysis showed that both PKD1 and PKD2 are targeted by miR-372-3p (Supplementary Fig. S3E).
Effects of novel key miRNAs on lineage specification
To experimentally validate that miR-192 and miR-372-3p indeed regulate lineage specification, we performed functional characterization to determine their effects. The experimental validations were performed in both hESCs (H1) and hiPSCs (iBC 1.2) to rule out the possibility of hiPSC-specific “artifacts”.
Effects of miR-192 on hepatocyte differentiation
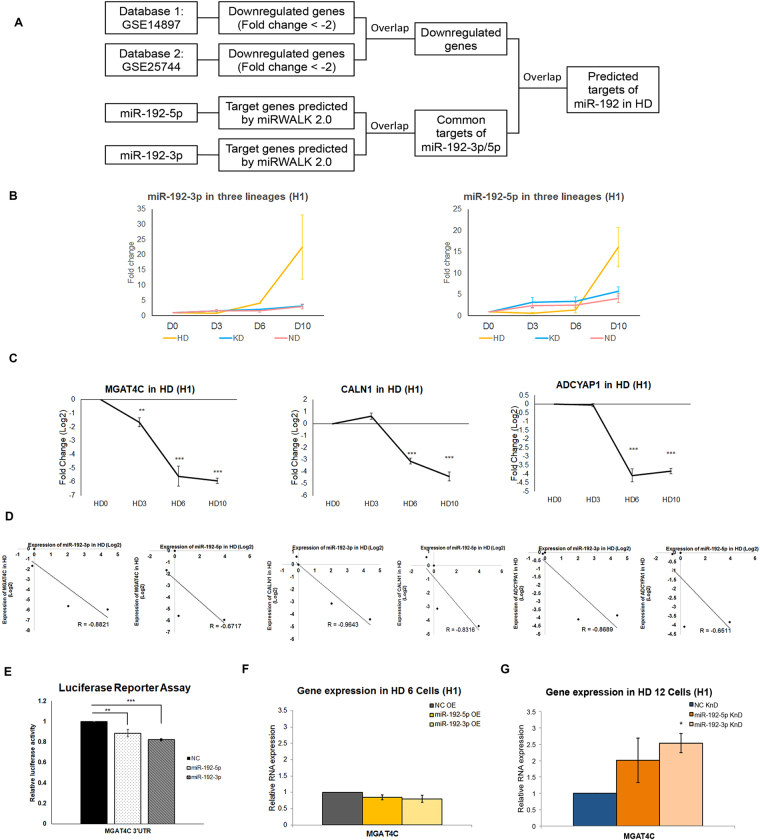
We first sought to determine what the targets of miR-192 in HD. To predict targets of miR-192 in a more deterministic manner, we applied bioinformatics analysis by combining published transcriptional datasets and the in silico target prediction (Fig. 4A). The qPCR analysis confirmed that miR-192-5p/3p were only upregulated during HD (Fig. 4B and Supplementary Fig. S4A). Due to inhibitory effects of miRNAs on target mRNAs, only downregulated genes (fold-change ≤−2) from two independent profiling studies were identified (Supplementary Fig. S4B and Table S3)43,44. With further filtration by miRWalk, in which the interactions between miRNAs and mRNAs could be predicted by different online tools, common targets of miR-192-3p and miR-192-5p were identified (Supplementary Fig. S4C and Table S3). By overlapping the list of downregulated genes and targets of miR-192, we identified 123 potential targets of miR-192 in HD (Supplementary Fig. S4D and Table S3).
Figure 4.
Identification of target genes of key miRNAs during hepatocyte differentiation. (A) Strategy for predicting common targets of miR-192-3p and miR-192-5p during HD. (B) TaqMan qPCR analysis confirming that miR-192-3p/5p were specifically upregulated during HD (n = 3 independent cultures for each time-point). (C) qPCR results showing the expression tendencies of common targets of miR-192-3p/5p during HD (n = 3 independent cultures for each time-point). (D) Correlation plot revealing reverse-correlations between miR-192-3p/5p and MGAT4C/CALN1/ADCYAP1, respectively. (E) Luciferase reporter assay confirming that miR-192-3p/5p could inhibit the 3’UTR of MGAT4C (n = 3 independent cultures for each group). (F) qPCR results showing the expression of ectodermal marker MGAT4C in HD 6 cells upon transfection of miR-192-3p/5p mimics (n = 3 independent cultures for each group). (G) qPCR results showing the expression of MGATC4C in HD 12 cells upon transfection of miR-192-3p/5p inhibitors (n = 3 independent cultures for each group). In (C), data are presented as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 for statistical comparisons between day 0 and other time-points (ANOVA plus Bonferroni’s post hoc test). In (E–G), data are presented as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 for statistical comparisons between control groups and experimental groups (ANOVA plus Bonferroni’s post hoc test). OE: overexpression; KnD: Knockdown; KD: nephron progenitor differentiation; NC: non-targeting control.
As the fold-changes of genes detected by the two datasets were modulated, we randomly selected several genes for validation in our HD samples. The qPCR analysis showed that Calneuron 1 (CALN1), MGAT4 family member C (MGAT4C) and adenylate cyclase activating polypeptide 1 (pituitary) receptor type I (ADCYAP1R1) were downregulated (Fig. 4C and Supplementary Fig. S4E). A negative correlation between miR-192-5p/3p and CALN1/MGAT4C/ADCYAP1R1 was observed (Fig. 4D and Supplementary Fig. S4F). Since CALN1 is a human brain-specific gene, whereas MGAT4C is normally expressed in mesodermal tissues like the kidney and ADCYAP1R1 encodes the receptor for peptide signals in response to stress in the brain45–47, we reasoned that miR-192 may promote HD via repressing genes normally expressed in the other two germ layer-derived lineages.
Given that both microarray and qPCR analysis detected a significant downregulation of MGAT4C, we further examined the molecular effects of miR-192 on this gene. Luciferase reporter assays showed that miR-192-5p and miR-192-3p mimics could suppress the MGAT4C 3′UTR (Fig. 4E). In addition to this, we also examined the expression change of MGAT4C upon disturbance of miR-192 expression. When H1 cells were transfected with miR-192-5p and miR-192-3p mimics from HD 0–6, MGAT4C transcript levels were moderately decreased (Fig. 4F). However, when miR-192-5p and miR-192-3p inhibitors were transfected from HD 6–12, we observed an upregulation of the MGAT4C transcript levels at HD 12 (Fig. 4G). Similar results were obtained in iBC 1.2 cells (Supplementary Fig. S4G,H).
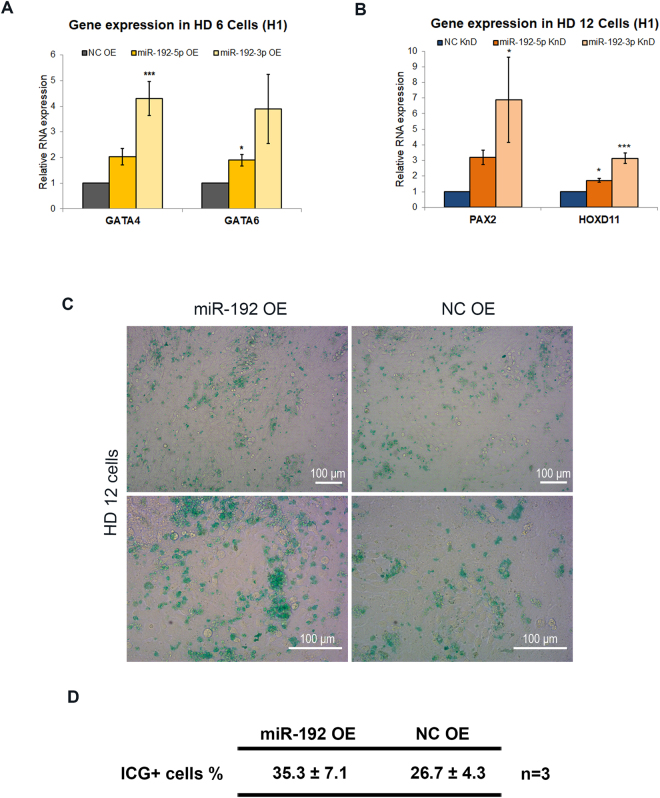
Next, we determined the regulatory effects of miR-192 on lineage specification. We examined the expression changes of lineage-specific genes while modulating miR-192 expression during HD. When H1 cells were transfected with miR-192-5p/3p mimics from HD 0–6, GATA4 and GATA6—which are expressed during definitive endoderm differentiation48—were elevated with the overexpression of miR-192-5p/3p (Fig. 5A). Conversely, the intermediate mesoderm marker PAX2 and the MM marker HOXD11 were significantly increased upon inhibition of miR-192-5p/3p from HD 6–12 (Fig. 5B and Supplementary Fig. S5), supporting that miR-192-5p/3p repress mesodermal differentiation.
Figure 5.
Regulation of lineage-specific gene expression by lineage-specific miRNAs during hepatocyte differentiation. (A) qPCR results showing the expression of definitive endoderm markers GATA4 and GATA6 in HD 6 cells with transfection of miR-192-3p/5p mimics (n = 3 independent cultures for each group). (B) qPCR results showing the expression of intermediate mesoderm marker PAX2 and metanephric mesenchyme marker HOXD11 in HD 12 cells upon transfection of miR-192-3p/5p inhibitors (n = 3 independent cultures for each group). (C) HD 12 cells were examined by indocyanine green staining (ICG). The cells transfected with miR-192 mimics (left column) and scramble controls (right column) were compared (Scale bars represent 100 μm). (D) Table showing the percentage of ICG+ cells at HD 12 with the transfection of miR-192 mimics and non-targeting controls (data are presented as the means ± SD. n = 3 independent cultures for each group). In (A,B), data are presented as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 for statistical comparisons between control groups and experimental groups (ANOVA plus Bonferroni’s post hoc test). OE: overexpression; KnD: Knockdown; NC: non-targeting control.
To demonstrate the effects of miR-192 on HD, we also determined the functional characteristics of HD 12 cells when modulating the expression of miR-192. Indocyanine green (ICG) is a non-toxic organic anion that can be eliminated exclusively by mature hepatocytes49. Therefore, uptake of ICG is used to measure the hepatic functions. When H1 cells were transfected with miR-192-5p mimics, ICG analysis showed that the percentage of ICG-positive cells at HD12 was increased substantially (Fig. 5C,D).
Taken together, we concluded that miR-192 is likely a key miRNA that promotes endodermal hepatic differentiation while inhibiting the formation of mesoderm.
Effects of miR-372-3p on the expression of KD-specific genes
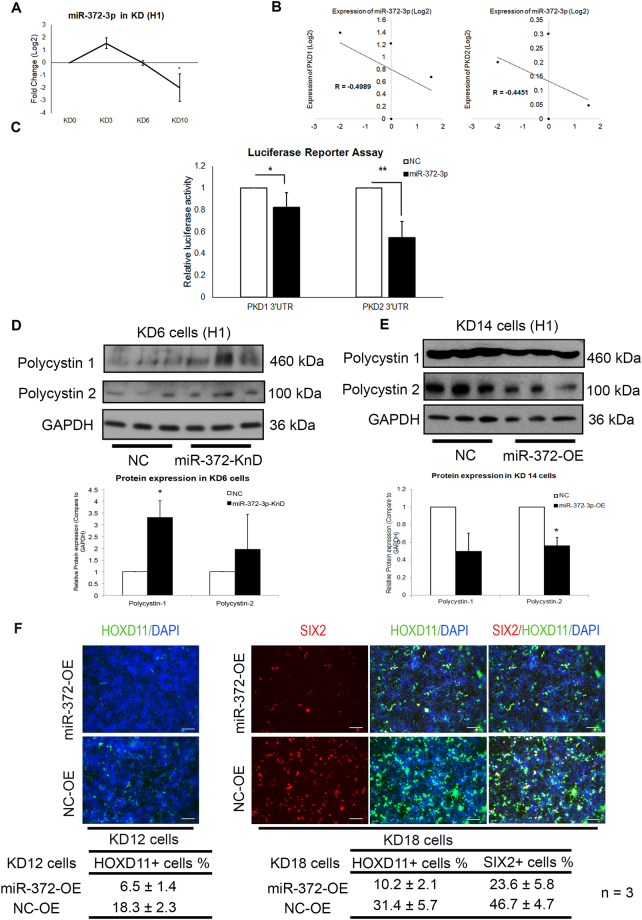
To assess the regulatory roles of miR-372-3p in KD, we first performed qPCR analysis to confirm the downregulation of miR-372-3p (Fig. 6A and Supplementary Fig. S6A). A negative correlation between miR-372-3p and PKD1/PKD2 was identified (Fig. 6B and Supplementary Fig. S6B). Next, we performed luciferase reporter assays and found that the 3′UTRs of PKD1/PKD2 were significantly inhibited by miR-372-3p (Fig. 6C). Since miRNAs can lead to mRNA decay or inhibit translation, we further measured the protein levels of PC1/PC2. Knockdown of miR-372-3p during early nephron differentiation (KD 0–6) resulted in increased PC1 and PC2 protein levels (Fig. 6D and Supplementary Fig. S6C). Conversely, overexpression of miR-372-3p at KD 8–14 resulted in decreased PC1 and PC2 protein levels (Fig. 6E and Supplementary Fig. S6D).
Figure 6.
Regulation of PKD1/PKD2 expression by lineage-specific miRNAs during nephron progenitor differentiation. (A) Taqman qPCR analysis showing the expression tendency of miR-372-3p during KD (n = 3 independent cultures for each time-point). (B) Correlation plot revealing reverse-correlations between miR-372-3p and PKD1/PKD2, respectively. (C) Luciferase reporter assay results confirming that miR-372-3p could inhibit the 3′UTR of both PKD1 and PKD2 (n = 4 independent cultures for each group). (D,E) Western blot results (upper panel) showing expression of Polycystin 1 and Polycystin 2 in KD 6 cells (D) with transfection of miR-372-3p mimics or non-targeting controls, and expression of Polycystin 1 and Polycystin 2 in KD 14 cells (E) upon transfection of miR-372-3p inhibitors or non-targeting controls. Normalized protein expressions of Polycystin 1 and Polycystin 2 are shown in lower panel (D,E). The expression of NC group of each experiment is set as 1. n = 3 independent cultures for each group. (F) KD 12 cells (left column) and KD 18 cells (right column) were examined by immunofluorescent staining (IFC) analysis. The cells transfected with miR-372-3p mimics (upper row) and scramble controls (lower row) were compared (Scale bars represent 200 μm). Table showing the percentage of HOXD11+ cells at KD 12, SIX2+ cells and HOXD11+ cells at KD 18, with the transfection of miR-372-3p mimics and non-targeting controls (data are presented as the means ± SD. n = 3 independent cultures for each group). In (A), data are presented as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 for statistical comparisons between day 0 and other time-points (Paired two-tailed t-test). In (C–E), data are presented as the means ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 for statistical comparisons between control groups and experimental groups (Paired two-tailed t-test). OE: overexpression; KnD: Knockdown; KD: nephron progenitor differentiation, NC: non-targeting control.
To demonstrate the effects of miR-372-3p on KD, we also determined the efficiency of KD when modulating the expression of miR-372-3p. Briefly, we measured the expression of cellular markers that are important for kidney development including SIX2 and HOXD11. IFC analysis showed that the percentage of cells expressed the early metanephric marker HOXD11 at KD 12 was decreased substantially when H1 cells were transfected with miR-372-3p mimics (Fig. 6F). Similarly, the percentage of cells that eventually expressed both HOXD11 and the nephric marker SIX2 at KD 18 was decreased upon the transfection (Fig. 6F), indicating that miR-372-3p inhibits the formation of nephron progenitors.
Taken together, these experiments validated that miR-372-3p is a key miRNA. According to intra-lineage analysis (Supplementary Table S1), miR-372-3p expression was not changed significantly; the change from KD 0 to KD 3 was ranked 99th among 177 small RNAs. As a result, it was difficult to identify miR-372-3p as a key miRNA by intra-lineage analysis. In contrast, inter-lineage analysis combined with bioinformatics analysis allowed us to identify miR-372-3p as a key miRNA, supporting the use of inter-lineage analysis to discover novel key miRNAs.
Discussion
MiRNAs have been found to act as critical regulators in lineage specification. Identification of key miRNAs regulating hPSCs differentiation has become a leading research topic. Intra-lineage analysis, which solely considers miRNAs with large fold-changes, has frequently been used to identify key miRNAs. More recently, inter-lineage analysis has been used to identify key miRNAs with lineage specificity. However, investigation of human-specific key miRNAs at the earliest stages of development has still been hindered by a lack of human samples; hPSC-derived lineages have been developed to overcome this challenge. Herein, we applied inter-lineage analysis to hPSC-derived lineages to identify key miRNAs with lineage specificity.
To identify key miRNAs by inter-lineage analysis, we built a miRNA atlas for human in vitro early cell lineage specification. This atlas depicts the dynamic expression of miRNAs during the three germ layer formation and lineage differentiation. With this atlas, we can easily identify lineage-specific miRNA in a readily-visualized manner. In this study, we identified miR-192 and miR-372-3p as key miRNAs regulating HD and KD, respectively. To further validate miR-192 and miR-372-3p as key miRNAs, we performed comprehensive experiments to validate their regulatory functions in HD and KD. First, we confirmed that miR-192 and miR-372-3p directly repressed their downstream targets. Next, we modulated their expression during lineage differentiation. The disruptions skewed lineage formation by affecting the expression of lineage markers. Particularly, our data showed that the overexpression of miR-192 increased the expressions of endodermal markers GATA4/6, while knockdown of miR-192 increased the expression of mesodermal marker PAX2. Given that endoderm and mesoderm share the same progenitors, perhaps the upregulation of miR-192 potentially affects the segregation of endodermal and mesodermal cell fate, leading to an increase in the formation of hepatic endoderm at the expense of mesodermal formation50. Finally, we performed functional characterization of miR-192 and miR-372-3p in lineage differentiation, establishing that miR-192 and miR-372-3p affect HD and KD, respectively. These validations prove that miR-192 and miR-372-3p are truly key miRNAs.
In addition to revealing lineage-specific miRNAs, our miRNA atlas can also be used as a roadmap for lineage distinction, as it reveals lineage-specific patterns of miRNA expression. In HD, for example (Fig. 2A), four clusters represented small RNAs highly expressed at HD 0, 3, 6 and 10, respectively. Another cluster remained high from HD 6 to 10. Similar to HD, KD was also characterized by stage-specific clusters (Fig. 2B). In ND, however, the pattern was remarkably different (Fig. 2C). We observed two major clusters, the expression levels of which were dramatically changed as early as ND 3. Meanwhile, this atlas clearly identified many small RNAs that changed specifically. For instance, the miRNAs that increased from ND 3 to ND 10 (purple dendrogram) had low expression in the other two lineages. These lineage-specific clusters can be used as markers to identify lineages. Notably, since the hiPSCs were induced to generate HD 0 cells, KD 0 cells and ND 0 cells with different pre-induction methods (Supplementary data), the expression patterns of miRNAs at day 0 were variable in different lineages (Fig. 3).
Table 1 shows that the top 10 miRNAs in ND were much less likely to have known functions in neural development compared to those in HD and KD. This might be due to the increasing complexity of development from small mammals to primates, especially in the neuronal system51. More miRNAs are involved in ND of primates during evolution, whereas the majority of developmental studies have been performed in small mammals52, leading to many neuron-specific primate miRNAs that have remained functionally undescribed, requiring specific studies with primate samples53.
In addition to human developmental processes, our miRNA atlas can be applied to the study of complex congenital disorders. Mutations in the PKD1/PKD2 genes or abnormal expression of PC1/PC2 are known to cause the formation of renal cysts54–56. We newly identified the regulation of miR-372-3p on PKD1/PKD2, indicating the possible association of miR-372-3p with polycystic kidney disease for the first time. Moreover, the key miRNAs identified from our atlas are also potential markers and therapeutic targets for various diseases, as treatments in which the miRNA activity is inhibited are in various stages of preclinical or clinical trials57,58. There was a differential change of miR-372-3p expression between hiPSC- and hESC-derived KD 3 cells that might be due to known differences in microRNA expression between these two hPSC types59. However, after KD 3, miR-372-3p expression decreased in both hPSC-derived cells. Together with its inhibitory effects on PC1/PC2 at KD 6, the substantial decline in miR-372-3p expression levels after KD 3 was sufficient to increase the expression levels of PC1/PC2, despite the differences at earlier time-points.
Importantly, we selected hiPSCs but not hESCs for the construction of the atlas. Considering the necessity of integrating information from different patients, we attempted to produce a reference dataset based on hiPSCs of healthy individuals that could be used for comparison with any patient-specific hiPSCs29. Given that hESCs are a widely used model for experimental subjects27, we used these for testing to provide fair comparisons between hiPSCs and hESCs and to confirm the reliability of our dataset. Compatible expression patterns of the most upregulated miRNAs in hiPSC- and hESC-derived cells (Supplementary Fig. S2) suggested that differential open chromatin states did not affect the expression patterns of important miRNAs. The hiPSCs were reprogrammed from male fibroblasts, which is the most common model despite the existence of male skin lineage memories. Given that the expression profile of miRNAs is different between women and men in many somatic tissues60, our miRNA atlas is more applicable for studies of male tissues.
To construct the atlas, we followed three well-established protocols to establish the multi-lineage differentiation systems. After successful inductions of HD, KD, and ND, we measured the differentiation efficiencies using IFC and flow-cytometric analysis to, by which the purities of desired somatic cell types were indicated up to 70%. These results suggested that the data outputted from these derived cells represents a mixture of miRNAs changed from wanted cells and unwanted cells. Therefore, to construct a more reliable atlas, an analysis of pure population will be important and necessary in the future.
A statistical test with non-adjusted P values has an increased chance of drawing false conclusions when multiple tests are performed61. As a result, a non-adjusted P < 0.05 is more likely to appear in our case when testing many outcomes from a single intervention. Herein, we adopted a more stringent criterion for multiple testing, the FDR-adjusted P value61. Compared to the non-adjusted P value, fewer but more reliable results have been generated by FDR. Using HD as an example, 826 and 170 differentially expressed miRNAs could be identified with a P < 0.05 and an FDR < 0.05, respectively. As a tradeoff, an FDR < 0.05 excluded several important candidates, such as miR-375; FDR was too conservative in this case. When we ran the analysis with a P < 0.05, miR-375 was the number 1 upregulated miRNA (fold-change = 104, P = 0.001291) from HD 0–3, which is consistent with its implicated functions in the endodermal formation8,11–15. Therefore, the statistical criterion using the P value can be applied to our dataset when more candidate miRNAs need to be identified.
In summary, our inter-lineage analysis generated a list of lineage-specific miRNAs during hiPSC differentiation. This list can serve as a source for the identification of key miRNAs and further investigation of the molecular mechanisms underlying hPSC differentiation. Moreover, the integrated miRNA expression atlas can further serve as a reference for studying human developmental processes and human congenital disease.
Materials and Methods
hiPSC culture and differentiation
iBC 1.2 is a hiPSC line that has been previously reported. iBC 1.2 was generated from a “normal” individual by a viral infection with genetic integration. H1 is a widely used hESC line. Both iBC 1.2 and H1 are male PSC lines. hiPSCs and hESCs were maintained with mTeSR medium (05870, StemCell Technologies, Vancouver, BC, Canada) on Matrigel matrix (354277, Corning, NY, USA)-coated plates and passaged with dispase (354235, Corning, NY, USA) every 4–6 days. They were induced according to previously established protocols for hepatocytes, nephron progenitors, and neural progenitors. The details of the three lineage induction procedures are described in the Supplemental Methods.
Immunofluorescence Microscopy
Cells were fixed in 4% formaldehyde for 20 minutes and permeabilized with 0.1% Triton X-100 for 45 min, after which the cells were blocked with 1% BSA and 4% goat serum in PBS for 45 min. The cells were then incubated with primary antibodies at 4 °C overnight and with secondary antibodies at room temperature for 1 hour. Finally, nuclei were stained with DAPI. The details regarding the antibodies and dilution ratio are listed in the Supplemental Information. Cells were imaged with a Nikon Ti-E Live-Cell Imaging System.
RNA extraction and real-time quantitative PCR
Total RNA, including miRNAs, were extracted from cells using the RecoverAll™ Total Nucleic Acid Isolation Kit for FFPE (AM1975, Thermo Fisher Scientific). RNA quantitation was performed using a NanoDrop 2000 Spectrophotometer (Thermo Scientific). RNA quality was examined using an Agilent 2100 Bioanalyzer. For cell samples prepared for microRNA microarray detection, an RNA integrity number larger than 9.0 was accepted. For gene expression analysis, cDNA was synthesized with PrimeScirpt™ Reverse Transcriptase (RR036A, Takara). SYBR Green master mix was used for PCR in a QuantStudio™ 7 Flex System (Life Technologies). GAPDH and 18 S RNA were used as internal controls. For microRNA expression analysis, RNA was reverse-transcribed with a TaqMan MicroRNA Reverse Transcription Kit, and amplification was performed using TaqMan probes with Taqman Universal Master Mix II, without UNG (Thermo Fisher Scientific). RNU6B was used as an endogenous control for normalization of miRNAs. The samples were plotted relative to control samples (HD0, KD0, and ND0), and the standard deviation of at least three measurements was calculated.
MicroRNA microarray analysis and data processing
MiRNA microarray expression data were acquired on an Affymetrix miRNA 4.0 platform (2,578 mature miRNAs, 2,025 stem-loop miRNAs, and 1,996 other small RNAs), using 500 ng total RNA per sample. Microarray data were deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession number GEO: GSE97952. miRNA expression data were then analyzed in the Partek® Genomics Suite® following the workflow for miRNA microarray analysis to detect differentially expressed miRNAs between samples. MicroRNAs of interest were filtered using a cut-off fold-change value of ≥2 or ≤−2 with an FDR <0.05. Both hierarchical clustering results and a heat map were generated with the Partek® Genomics Suite® platform. miRNA target prediction algorithms were performed with TargetScanHuman 6.2 (http://www.targetscan.org/vert_61/) and miRWALK (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/). The accession projects supporting the prediction of downstream targets of miR-192 are GSE14897 and GSE25744.
Luciferase reporter assay
50,000 HEK 293 cells were plated in wells of 24-well plates 24 h before transfection. Reporter plasmids with MGAT4C, PKD1, PKD2 3′UTR were purchased from GeneCopoeia. 10 pmol synthetic miRNA mimics of specific miRNAs, including miR-192-3p, miR-192-5p, and miR-372-3p or non-targeting control (GenePharma, Shanghai, China), and 200 ng reporter plasmids were co-transfected with 1 μl Lipofectamine 2000 according to the manufacturer’s instructions. The medium was changed to DMEM supplemented with 10% FBS for 18 h post-transfection. At 48–72 h post-transfection, media were collected for analysis. Luciferase activities were determined with a Secrete-Pair™ Dual Luminescence Assay Kit (SPDA-D010, GeneCopoeia, Rockville, MD, USA).
Knockdown and overexpression of miRNAs
Mimics of non-targeting control, miR-192-3p, miR-192-5p, and miR-372-3p miRNAs were purchased from GenePharma. Inhibitors of negative control, miR-192-3p, miR-192-5p, and miR-372-3p were purchased from Thermo Fisher Scientific. Cells cultured in 12-well plates were transfected with either mimics or inhibitors. First, 12.5 pmol mimics or inhibitors were diluted in 80 μl Opti-MEM. Second, 3.5 μl Lipofectamine RNAiMAX reagent was diluted in 80 μl Opti-MEM. Third, the Opti-MEM containing miRNA mimics and the Opti-MEM containing Lipofectamine® RNAiMAX Reagent were mixed at a ratio of 1:1. The mixture was incubated at room temperature for 20 minutes and added to the differentiated cells. The medium was changed the next day. Transfection was performed every two days three times. Then, the transfected cells were collected for further analysis.
Western blot analysis
Cells were lysed in RIPA buffer with 2% proteinase inhibitor. 30 μg of total proteins were resolved on 4–20% precast polyacrylamide gels (4561096, Bio-Rad, Hercules, CA, USA), and transferred to PVDF membranes (ISEQ. 00010, Merck Millipore, Darmstadt, Germany). After transfer, the membranes were blocked with 5% Blotting-grade Blocker (1706404, Bio-Rad, Hercules, CA, USA) in PBS-T for 1 hour, and incubated with specific primary antibodies in the blocking solutions overnight at 4 °C. The membranes were then washed with PBST three times and incubated with secondary antibodies for 1 hour at room temperature. Then, the antibodies were detected using an ECL HRP substrate system (K-12045-D20, Advansta, Menlo Park, CA, USA). The protein band intensities were quantified using Image Lab software. Information regarding the antibodies is shown in the Supplemental Information.
Statistical analysis
For microarray analysis, one-way ANOVA testing was used to determine which small RNAs had significant differences in expression between time-points. Subsequent pairwise comparisons between successive time-points were used to identify when small RNAs demonstrated significant differential expression (post-hoc testing, fold-change ≥2 or ≤−2, false discovery rate <0.05). For qPCR analysis, luciferase reporter assays and Western blotting analysis, statistical significance was determined using one-way ANOVA followed by Bonferroni’s post-hoc testing. A paired two-tailed Student’s t-test was performed when only two groups were compared. A single asterisk indicates P < 0.05, a double asterisk indicates P < 0.01, and a triple asterisk indicates P < 0.001. In all Figures, the mean ± SD is plotted.
Data availability statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Electronic supplementary material
Acknowledgements
We thank Ms. Tin Yan Ha for help with qPCR analysis; Dr. Yick Keung Suen, Mr. Wai Nok Law, Dr. Bi Feng Chen, Ms. Dan Dan Cao, and Dr. Jia Jie Tu for helpful guidance with respect to cell culture; Dr. Chun Shui Luk for helpful guidance with respect to bioinformatics analyses; and Dr. Jianzhen Li for helpful guidance with the zebrafish experiments. This work was supported in part by the CUHK VC One-Off Discretionary Fund (Project 4930732) and the One-Off Funding for Joint Lab/Research Collaboration (Project 3132966) provided to the CUHK-CAS GIBH Joint Laboratory on Stem Cell and Regenerative Medicine, the Lo Kwee-Seong Biomedical Research Fund, and Project Stem Cell Therapy of Liver Diseases: An Investigation (2015CB964700), 973 Scheme, Ministry of Science and Technology, China.
Author Contributions
L.L., K.K.M., S.G. and W.Y.C. conceived the project. L.L., K.K.M., S.G. and H.H.C. designed the experiments. L.L., K.K.M. and S.G. conducted the experiments. L.L. analyzed the data and wrote the manuscript. W.Y.C. and H.H.C. edited the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-27719-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hoi-Hung Cheung, Email: cheunghh@cuhk.edu.hk.
Wai-Yee Chan, Email: chanwy@cuhk.edu.hk.
References
- 1.Leonardo TR, Schultheisz HL, Loring JF, Laurent LC. The functions of microRNAs in pluripotency and reprogramming. Nature cell biology. 2012;14:1114–1121. doi: 10.1038/ncb2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boland MJ, Nazor KL, Loring JF. Epigenetic regulation of pluripotency and differentiation. Circulation research. 2014;115:311–324. doi: 10.1161/CIRCRESAHA.115.301517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiagarajan RD, Morey R, Laurent LC. The epigenome in pluripotency and differentiation. Epigenomics. 2014;6:121–137. doi: 10.2217/epi.13.80. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto T, Furusawa C, Kaneko K. Pluripotency, Differentiation, and Reprogramming: A Gene Expression Dynamics Model with Epigenetic Feedback Regulation. PLoS computational biology. 2015;11:e1004476. doi: 10.1371/journal.pcbi.1004476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung Y, Bauer G, Nolta JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30:42–47. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox IJ, et al. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science. 2014;345:1247391. doi: 10.1126/science.1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avior Y, Sagi I, Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nature reviews. Molecular cell biology. 2016;17:170–182. doi: 10.1038/nrm.2015.27. [DOI] [PubMed] [Google Scholar]
- 8.Liao X, et al. Matched miRNA and mRNA signatures from an hESC-based in vitro model of pancreatic differentiation reveal novel regulatory interactions. Journal of cell science. 2013;126:3848–3861. doi: 10.1242/jcs.123570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivey KN, Srivastava D. microRNAs as Developmental Regulators. Cold Spring Harbor perspectives in biology. 2015;7:a008144. doi: 10.1101/cshperspect.a008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinton A, et al. A distinct microRNA signature for definitive endoderm derived from human embryonic stem cells. Stem cells and development. 2010;19:797–807. doi: 10.1089/scd.2009.0224. [DOI] [PubMed] [Google Scholar]
- 12.Hinton A, et al. sRNA-seq analysis of human embryonic stem cells and definitive endoderm reveals differentially expressed microRNAs and novel IsomiRs with distinct targets. Stem Cells. 2014;32:2360–2372. doi: 10.1002/stem.1739. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa D, et al. miRNome Profiling of Purified Endoderm and Mesoderm Differentiated from hESCs Reveals Functions of miR-483-3p and miR-1263 for Cell-Fate Decisions. Stem cell reports. 2017;9:1588–1603. doi: 10.1016/j.stemcr.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joglekar MV, Joglekar VM, Hardikar AA. Expression of islet-specific microRNAs during human pancreatic development. Gene expression patterns: GEP. 2009;9:109–113. doi: 10.1016/j.gep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Fogel, G. B., Tallon, T., Wong, A. S., Lopez, A. D. & King, C. C. 93–102 (Springer International Publishing).
- 16.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic acids research. 2014;42:D68–73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letzen BS, et al. MicroRNA expression profiling of oligodendrocyte differentiation from human embryonic stem cells. PloS one. 2010;5:e10480. doi: 10.1371/journal.pone.0010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petriv OI, et al. Comprehensive microRNA expression profiling of the hematopoietic hierarchy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15443–15448. doi: 10.1073/pnas.1009320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia DM, et al. Weak seed-pairing stability and high target-site abundance decrease the proficiency of lsy-6 and other microRNAs. Nature structural & molecular biology. 2011;18:1139–1146. doi: 10.1038/nsmb.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu SD, et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic acids research. 2011;39:D163–169. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic acids research. 2015;43:D146–152. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suh MR, et al. Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 2004;270:488–498. doi: 10.1016/j.ydbio.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Londin E, et al. Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E1106–1115. doi: 10.1073/pnas.1420955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Jin P. Roles of small regulatory RNAs in determining neuronal identity. Nature reviews. Neuroscience. 2010;11:329–338. doi: 10.1038/nrn2739. [DOI] [PubMed] [Google Scholar]
- 25.McCall MN, et al. Toward the human cellular microRNAome. Genome research. 2017;27:1769–1781. doi: 10.1101/gr.222067.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juzenas S, et al. A comprehensive, cell specific microRNA catalogue of human peripheral blood. Nucleic acids research. 2017;45:9290–9301. doi: 10.1093/nar/gkx706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Z, Huangfu D. Human pluripotent stem cells: an emerging model in developmental biology. Development. 2013;140:705–717. doi: 10.1242/dev.086165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medvedev SP, Shevchenko AI, Zakian SM. Induced Pluripotent Stem Cells: Problems and Advantages when Applying them in Regenerative Medicine. Acta naturae. 2010;2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 29.Singh VK, Kalsan M, Kumar N, Saini A, Chandra R. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Frontiers in cell and developmental biology. 2015;3:2. doi: 10.3389/fcell.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YF, et al. Rapid generation of mature hepatocyte-like cells from human induced pluripotent stem cells by an efficient three-step protocol. Hepatology. 2012;55:1193–1203. doi: 10.1002/hep.24790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohamad O, et al. Vector-free and transgene-free human iPS cells differentiate into functional neurons and enhance functional recovery after ischemic stroke in mice. PloS one. 2013;8:e64160. doi: 10.1371/journal.pone.0064160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takasato M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nature cell biology. 2014;16:118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, et al. Idiopathic Autism: Cellular and Molecular Phenotypes in Pluripotent Stem Cell-Derived Neurons. Molecular neurobiology. 2017;54:4507–4523. doi: 10.1007/s12035-016-9961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung HH, et al. Telomerase protects werner syndrome lineage-specific stem cells from premature aging. Stem cell reports. 2014;2:534–546. doi: 10.1016/j.stemcr.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lagos-Quintana M, et al. Identification of tissue-specific microRNAs from mouse. Current biology: CB. 2002;12:735–739. doi: 10.1016/S0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 36.Girard M, Jacquemin E, Munnich A, Lyonnet S, Henrion-Caude A. miR-122, a paradigm for the role of microRNAs in the liver. Journal of hepatology. 2008;48:648–656. doi: 10.1016/j.jhep.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Yin H, et al. Phylogenetic tree-informed microRNAome analysis uncovers conserved and lineage-specific miRNAs in Camellia during floral organ development. Journal of experimental botany. 2016;67:2641–2653. doi: 10.1093/jxb/erw095. [DOI] [PubMed] [Google Scholar]
- 38.Vinas JL, et al. miRNA let-7e modulates the Wnt pathway and early nephrogenic markers in mouse embryonic stem cell differentiation. PLoS One. 2013;8:e60937. doi: 10.1371/journal.pone.0060937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Molecular cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gainullin VG, Hopp K, Ward CJ, Hommerding CJ, Harris PC. Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. The Journal of clinical investigation. 2015;125:607–620. doi: 10.1172/JCI76972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel V, et al. miR-17~92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10765–10770. doi: 10.1073/pnas.1301693110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakshmipathy U, et al. MicroRNA expression pattern of undifferentiated and differentiated human embryonic stem cells. Stem cells and development. 2007;16:1003–1016. doi: 10.1089/scd.2007.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan GJ, et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329–335. doi: 10.1002/hep.23335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jozefczuk J, Prigione A, Chavez L, Adjaye J. Comparative analysis of human embryonic stem cell and induced pluripotent stem cell-derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem cells and development. 2011;20:1259–1275. doi: 10.1089/scd.2010.0361. [DOI] [PubMed] [Google Scholar]
- 45.Wu YQ, Lin X, Liu CM, Jamrich M, Shaffer LG. Identification of a human brain-specific gene, calneuron 1, a new member of the calmodulin superfamily. Molecular genetics and metabolism. 2001;72:343–350. doi: 10.1006/mgme.2001.3160. [DOI] [PubMed] [Google Scholar]
- 46.Yang TL, et al. Runs of homozygosity identify a recessive locus 12q21.31 for human adult height. The Journal of clinical endocrinology and metabolism. 2010;95:3777–3782. doi: 10.1210/jc.2009-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ressler KJ, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiyaboonchai A, et al. GATA6 Plays an Important Role in the Induction of Human Definitive Endoderm, Development of the Pancreas, and Functionality of Pancreatic beta Cells. Stem cell reports. 2017;8:589–604. doi: 10.1016/j.stemcr.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berk PD, Stremmel W. Hepatocellular uptake of organic anions. Progress in liver diseases. 1986;8:125–144. [PubMed] [Google Scholar]
- 50.Li N, Wei C, Olena AF, Patton JG. Regulation of endoderm formation and left-right asymmetry by miR-92 during early zebrafish development. Development. 2011;138:1817–1826. doi: 10.1242/dev.056697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nature reviews. Neuroscience. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berezikov E, et al. Diversity of microRNAs in human and chimpanzee brain. Nature genetics. 2006;38:1375–1377. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- 53.Lopez JP, et al. miR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nature medicine. 2014;20:764–768. doi: 10.1038/nm.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris PC, Torres VE. Polycystic kidney disease. Annual review of medicine. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney international. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapin HC, Caplan MJ. The cell biology of polycystic kidney disease. The Journal of cell biology. 2010;191:701–710. doi: 10.1083/jcb.201006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel V, Noureddine L. MicroRNAs and fibrosis. Current opinion in nephrology and hypertension. 2012;21:410–416. doi: 10.1097/MNH.0b013e328354e559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noureddine L, Hajarnis S, Patel V. MicroRNAs and Polycystic Kidney Disease. Drug discovery today. Disease models. 2013;10:e137–e1743. doi: 10.1016/j.ddmod.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chin MH, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell stem cell. 2009;5:111–123. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai R, Ahmed SA. Sexual dimorphism of miRNA expression: a new perspective in understanding the sex bias of autoimmune diseases. Therapeutics and clinical risk management. 2014;10:151–163. doi: 10.2147/TCRM.S33517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verhoeven KJF, Simonsen KL, McIntyre LM. Implementing false discovery rate control: increasing your power. Oikos. 2005;108:643–647. doi: 10.1111/j.0030-1299.2005.13727.x. [DOI] [Google Scholar]
- 62.Van Keuren-Jensen KR, et al. microRNA changes in liver tissue associated with fibrosis progression in patients with hepatitis C. Liver international: official journal of the International Association for the Study of the Liver. 2016;36:334–343. doi: 10.1111/liv.12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang J, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 2004;1:106–113. doi: 10.4161/rna.1.2.1066. [DOI] [PubMed] [Google Scholar]
- 64.Esau C, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 65.Xiao Y, et al. Dysregulated miR-124 and miR-200 expression contribute to cholangiocyte proliferation in the cholestatic liver by targeting IL-6/STAT3 signalling. Journal of hepatology. 2015;62:889–896. doi: 10.1016/j.jhep.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 66.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nature neuroscience. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao Y, Zhang M, Lonnerdal B. Growth factor TGF-beta induces intestinal epithelial cell (IEC-6) differentiation: miR-146b as a regulatory component in the negative feedback loop. Growth factor TGF-beta induces intestinal epithelial cell (IEC-6) differentiation: miR-146b as a regulatory component in the negative feedback loop. 2013;8:69–78. doi: 10.1007/s12263-012-0297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen L, et al. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol. Cell. Endocrinol. 2014;393:65–74. doi: 10.1016/j.mce.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 69.Comer BS, et al. MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;307:L727–734. doi: 10.1152/ajplung.00174.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Starkey Lewis PJ, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54:1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 71.Hino K, et al. Inducible expression of microRNA-194 is regulated by HNF-1alpha during intestinal epithelial cell differentiation. RNA. 2008;14:1433–1442. doi: 10.1261/rna.810208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hromadnikova I, et al. Expression profile of C19MC microRNAs in placental tissue in pregnancy-related complications. DNA and cell biology. 2015;34:437–457. doi: 10.1089/dna.2014.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stadler B, et al. Characterization of microRNAs involved in embryonic stem cell states. Stem cells and development. 2010;19:935–950. doi: 10.1089/scd.2009.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang N, et al. Urinary microRNA-10a and microRNA-30d serve as novel, sensitive and specific biomarkers for kidney injury. PloS one. 2012;7:e51140. doi: 10.1371/journal.pone.0051140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 76.Yoder MC. Differentiation of pluripotent stem cells into endothelial cells. Curr. Opin. Hematol. 2015;22:252–257. doi: 10.1097/MOH.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shi K, et al. MicroRNA-214 suppresses osteogenic differentiation of C2C12 myoblast cells by targeting Osterix. Bone. 2013;55:487–494. doi: 10.1016/j.bone.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 78.Rosa A, Spagnoli FM, Brivanlou AH. The miR-430/427/302 family controls mesendodermal fate specification via species-specific target selection. Dev. Cell. 2009;16:517–527. doi: 10.1016/j.devcel.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 79.Beveridge NJ, Tooney PA, Carroll AP, Tran N, Cairns MJ. Down-regulation of miR-17 family expression in response to retinoic acid induced neuronal differentiation. Cellular signalling. 2009;21:1837–1845. doi: 10.1016/j.cellsig.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 80.Saba R, et al. Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons. Molecular and cellular biology. 2012;32:619–632. doi: 10.1128/MCB.05896-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdelmohsen K, et al. miR-375 inhibits differentiation of neurites by lowering HuD levels. Molecular and cellular biology. 2010;30:4197–4210. doi: 10.1128/MCB.00316-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhinge A, et al. MiR-375 is Essential for Human Spinal Motor Neuron Development and May Be Involved in Motor Neuron Degeneration. Stem Cells. 2016;34:124–134. doi: 10.1002/stem.2233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).