Abstract
In order to explore the defense mechanism by which retrotransposons are repressed, we assessed the ability of methyl-CpG-binding protein 2, MeCP2, to influence LINE-1 (L1) and Alu transcription and, furthermore, L1 retrotransposition. In transient transfection assays, targeting of the transcriptional-repression domain (TRD) of MeCP2 (via a linked Gal4 DNA-binding domain) to the transcriptional start site of L1 promoter-driven reporter constructs efficiently repressed transcription. The Gal4-linked TRD of the related methyl-CpG-binding protein MBD1 also repressed transcription but not that of MBD2. Furthermore, full-length MeCP2 effectively repressed transcription of a HpaII-methylated L1 reporter. Secondly, we used a genetic assay employing a full-length neo-marked L1 reporter construct to study L1 retrotransposition. We found the Gal4-linked TRD of MeCP2 to repress effectively L1 retrotransposition when targeted to the retrotransposition reporter. Retrotransposition was also reduced in response to in vitro HpaII methylation of the reporter and was further decreased by co-expressed full-length MeCP2. In striking contrast expression of the Gal4-linked TRD of MeCP2 had no inhibiting effect on transcription of an AluSx reporter tagged with a 7S-upstream sequence. Furthermore, full-length MeCP2 abrogated the methylation-induced repression of this reporter. Our results indicate that MeCP2 serves a role in repression of L1 expression and retrotransposition but has no inhibiting effect on Alu transcription.
INTRODUCTION
DNA methylation is important for developmental regulation of gene expression, imprinting, X-chromosome inactivation and repression of transposable elements, and plays a significant role in cancer. More than 90% of the methylated cytosines in the human genome occur in retrotransposons, a few percent in satellite sequences, and only a minority in exons and regulatory sequences. LINE-1 (L1) elements, the major class of non-LTR retrotransposons, are interspersed repeated elements at 516 000 copies per human genome (accounting for 16.9% of its mass), containing an internal promoter and two long open reading frames. The overwhelming majority of L1s are retrotransposition defective, because they are 5′ truncated, internally rearranged, or mutated (1). Only a subset of ∼60 full-length L1s remained retrotransposition competent (2,3). Evidence for this subset is documented by 14 known cases of human diseases that arose from new L1 transpositions. Retrotransposition involves intermediate formation of full-length transcripts, reverse transcription and insertion into new genomic loci (4). The promoter, located at the 5′ end of the element, is unusual in that it contains a CpG island that is very heavily methylated (5). Methylation includes symmetric and asymmetric methylation at CpG dinucleotides as well as methylation at non-CpG sites. Alu repeats, the major class of SINEs in the human genome, are also barely transcribed despite the transcriptional potential of 1.09 million templates accounting for 10.6% of the mass of the genome. Alu elements are ∼300 bp in length, have internal RNA polymerase III promoter elements, and transcription produces a non-coding, poly(A) tail-ended RNA. Alu mobilization likely occurs through L1-encoded proteins, because Alu elements are flanked by target site duplications that bear close resemblance to the target site duplications of L1 elements (4,6). It is believed that hypermethylation of L1 as well as Alu repeats is a major defense mechanism to repress these genetic elements that could be otherwise very damaging if actively transcribed (5,7).
Repression by DNA methylation is thought to be established through binding of members of the MBD (methyl-CpG-binding domain) protein family, recruitment of histone deacetylases and generation of a transcriptionally inactive chromatin structure (8,9). The founder member of this family is MeCP2 (methyl-CpG-binding protein 2), which is characterized by a typical modular organization (10,11). Methyl-DNA binding resides in an 85-amino acid domain that folds into a β-sheet–α-helix–hairpin loop sandwich structure (12). The transcriptional-repressor domain (TRD) of MeCP2 residing between amino acids 207 and 310 mediates repression through binding to mSin3A within the mSin3A-HDAC1 and 2 co-repressor complex (13–15). Further results show that MeCP2 can also repress transcription through a histone deacetylase-independent mechanism, in part through direct and indirect interactions with the general transcription factor TFIIB (13,16,17). In mouse chromosomes, MeCP2 localizes with great abundance to the highly methylated major satellite DNA in the centromeric region (10). In human interphase nuclei, EGFP–MeCP2 fusion protein experiments suggested that MeCP2 is uniformly spread (18).
MBD1, the largest member of the MBD family, is expressed either as a 605-amino acid full-length isoform (MBD1v1) or as one of several shorter isoforms, resulting from alternative splicing events (PCM1, MBD1v2, MBD1v3 and MBD1v4) (8,19). They share an MBD at the N-terminus and a TRD, consisting of a short hydrophobic sequence, close to the C-terminus, but differ in the number of centrally located CxxC motifs and/or absence of short connecting regions (19,20). The physiological relevance of these variants is unknown. MBD1 has been localized in human chromosomes to the heavily methylated classical satellites 2 and 3 found in the pericentromeric heterochromatin of chromosomes 1, 9, 15 and 16, as well as to euchromatic regions (20). The third MBD family member, which can act as a transcriptional repressor (MBD2) is an integral component of an oligomeric complex (MeCP1), which additionally contains mSin3A, HDAC1 and 2, and RbAp46 and 48 (21). In full-length MBD2 (also named MBD2a), the MBD occupies a central position of the protein and overlaps with the TRD (22). A shorter isoform, named MBD2b, starts at the second methionine, thus lacking the sequence N-terminal of the MBD.
Here we explore the possible capability of MeCP2 to silence L1 and Alu transcription and, furthermore, L1 retrotransposition. To this end, we used two assays. First, using transient transfection assays, we found that the TRD of MeCP2 represses transcription from L1 promoter-driven luciferase constructs and that full-length MeCP2 represses transcription from a methylated version of the construct. Secondly, in a genetic assay employing a full-length neo-marked L1 reporter construct, the TRD of MeCP2 effectively reduced L1 retrotransposition. Furthermore, the methylation-induced repression of L1 retrotransposition was increased by full-length MeCP2. In sharp contrast, neither the TRD of MeCP2 nor the full-length protein exhibited any repressive effect on Alu transcription. Our results suggest that MeCP2 is involved in the defense response to the potentially damaging action of L1 elements.
MATERIALS AND METHODS
Plasmid constructs
To construct reporter plasmids L1.3-Luc and L1RP-Luc the promoter region of L1.3 (nucleotides 1–909) and L1RP (nucleotides 1–905), respectively, (gifts of J. V. Moran, University of Michigan, Ann Arbor, USA and H. H. Kazazian, University of Pennsylvania, Philadelphia, USA; 23,24) were amplified by PCR and inserted into the HindIII site upstream of the luciferase cDNA in the pGL3-Basic vector (Promega). To generate the derivatives G5L1.3-Luc and G5L1RP-Luc a blunted fragment containing five Gal4 (G5) recognition motifs (from pFR-Luc; Stratagene) was inserted into the SmaI sites of the parent plasmids. The reporter plasmid 7SLAluSxTMBC1 contains the 7SL-upstream activating sequence, the consensus sequence for the PS (Sx) Alu subfamily and a diagnostic 20 bp sequence derived from the BC1 gene (gift of P. L. Deiniger, Tulane University Medical Center, New Orleans, USA; 25). The G5 derivative of 7SLAluSxTMBC1 was obtained by insertion of the G5 fragment into the unique EcoRI site. The retrotransposition reporter JM101/L1.2ΔCMV was a generous gift of J.V. Moran (26). Insertion of fragment G5 into the unique NotI site created G5JM101/L1.2ΔCMV. Methylated plasmids were obtained by incubation with M.HpaII or SssI methylase and controlled by digestion with HpaII and the methylation-insensitive isoschizomer MspI. Expression plasmid Gal4-TRDMeCP2 containing the C-terminal half of MeCP2 (amino acids 196–486) and a plasmid expressing full-length human MeCP2 have been described previously (16). Human MBD1v3 and MBD2b were cloned by RT–PCR using HeLa cell RNA and specific pairs of primers. The cDNAs were FLAG-tagged at their N-termini by further PCRs using 5′ primers encoding the FLAG epitope, and finally inserted into vectors pcDNA1.1/Amp and pcDNA3 (Invitrogen). Insertion of a PCR product encoding amino acids 383–605 of MBD1 between the KpnI and BamHI sites of pcDNA3-Gal4BD (16) generated Gal4-TRDMBD1. Insertion of the sequence encoding amino acids 45–262 of MBD2b into the BamHI site of pcDNA3-Gal4BD created Gal4-TRDMBD2.
Transfections and northern dot blotting
Transient transfections into human HEK293 and HeLa cells with the indicated plasmids and the Renilla luciferase reference plasmid pRL-TK (Promega), as well as luciferase activity assays, were performed as described (16). For northern dot blot analysis total RNA was isolated from transfected cells using the High Pure RNA Isolation Kit (Roche), and equal amounts were dotted onto a nitrocellulose membrane and hybridized overnight at 42°C in 6× SSC, 10× Denhardt’s and 150 µg/ml of salmon sperm DNA utilizing a labeled oligonucleotide (5′-TGTGTGTGCCAGTTACCTTG-3′) complementary to the unique BC1 region (25). The oligo was end-labeled with [γ-32P]ATP using T4 polynucleotide kinase. Blots were washed at 42°C successively with 6×, 5× and 4× SSC and subjected to autoradiography or quantified using a phosphoimager.
L1 retrotransposition assay
Retrotransposition frequency was determined using the rapid and quantitative transient L1 retrotransposition assay described previously (27). Briefly, for each transfection reaction, HeLa cells were seeded in 6-well dishes at 2 × 105 cells/well. The following day, triplicate dishes were co-transfected with 1 µg of reporter plasmid and 0.1 µg of effector plasmid using 3 µl of FuGene 6 transfection reagent (Roche) according to the manufacturer’s protocol. Antibiotic selection with G418 (400 µg/ml) was started 72 h post transfection. After 12 days, G418 foci were fixed and stained as described previously (26). In order to control for transfection, HeLa cells seeded in parallel were co-transfected with 0.5 µg of plasmid pHHR-GFP-BGH (gift of H.-G. Kräusslich, University of Heidelberg, Heidelberg, Germany) expressing the green fluorescent protein and 1 µg of reporter plasmid. Two days later transfection rates of 60–70% were determined by flow cytometry.
Immunoblot analysis
Equal amounts of cells were mixed with Laemmli buffer, boiled, and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and proteins were transferred by electroblotting to a nitrocellulose membrane. The membrane was blocked for 1 h at room temperature with Tris-buffered saline (TBS) containing 5% non-fat milk and incubated with anti-Gal4BD monoclonal antibody (Clontech) or anti-FLAG monoclonal antibody M2 (Sigma) in TBS. After two washes in TBS, the membrane was incubated with anti-mouse IgG horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). After the membrane had been washed six times with TBS/0.5% Tween-20, once with TBS/3% Tween-20 and twice with TBS, visualization was performed using an enhanced chemiluminescence detection system (Amersham).
RESULTS
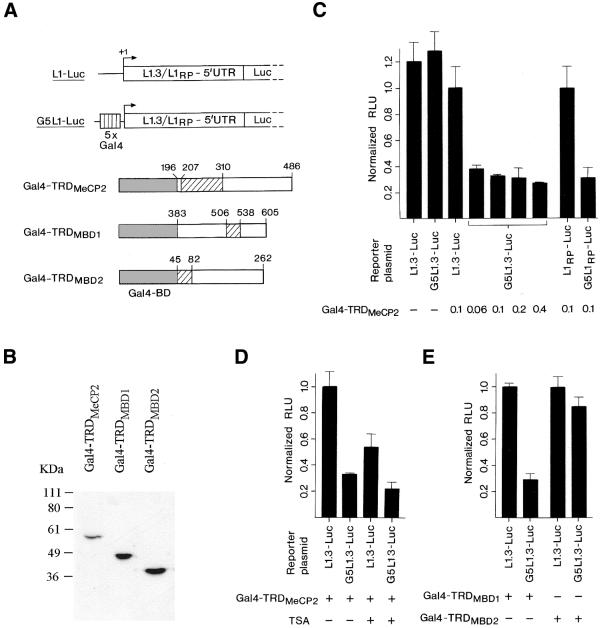
To monitor the effects of MeCP2 on L1 transcription we used transient co-transfection experiments in HEK293 cells employing a luciferase reporter plasmid driven by the internal L1.3 promoter (nucleotides 1–909) and an effector plasmid (Gal4-TRDMeCP2) encoding a fusion protein that links the transcriptional-repression domain of human MeCP2 (amino acids 196–486) to the heterologous Gal4 DNA-binding domain (Gal4) (23,16) (Fig. 1A). Immunoblot analysis using an anti-Gal4BD monoclonal antibody documented efficient expression of the fusion protein (Fig. 1B). Transcription from the L1.3 promoter was about 1.5-fold more effective than from the SV40 minimal promoter (data not shown). In control experiments, neither insertion of five copies of the Gal4 DNA-binding sequence (G5) upstream of the L1.3 promoter nor co-transfection of the effector plasmid Gal4-TRDMeCP2 appreciably affected expression of the L1.3-Luc reporter (Fig. 1A and C). However, co-transfection of Gal4-TRDMeCP2 and the G5L1.3-luciferase reporter targeted the TRD of MeCP2 to the L1.3 promoter and repressed expression by 63–72%, depending on the dose of effector plasmid (Fig. 1C). Identical results were obtained in HeLa cells (data not shown). A 24 h treatment with trichostatin A (TSA), a specific inhibitor of all known histone deacetylases, generally decreased the metabolic activity of the transfected cells (Fig. 1D). Comparison of the expression levels of both reporters in the absence (L1.3:G5L1.3 = 1:0.33) and presence (L1.3:G5L1.3 = 1:0.41) of TSA indicated that TSA only slightly relieved TRDMeCP2 mediated transcriptional repression, suggesting a minor contribution of histone deacetylases to the repression. We also tested the promoter region of another retrotransposition-competent L1 element, L1RP, which was isolated from a patient suffering from X-linked retinitis pigmentosa 2 and is thought to have undergone retrotransposition very recently (24,28). The L1RP promoter (nucleotides 1–905) turned out to be 2.1-fold more active than the SV40 minimal promoter (data not shown) and was repressed by the Gal4-linked TRD of MeCP2 by 69%, i.e. as efficiently as the L1.3 promoter (Fig. 1C). Identical results were obtained in HEK293 and HeLa cells.
Figure 1.
The TRDs of MeCP2 and MBD1 repress L1 transcription. (A) Schematic maps of the reporters L1.3/L1RP-Luc (L1-Luc) and G5L1.3/L1RP-Luc (G5L1-Luc) and the expression constructs Gal4-TRDMeCP2, Gal4-TRDMBD1 and Gal4-TRDMBD2. Numbers indicate relevant amino acids of the MBD protein portion in each fusion, and the respective TRDs are shown as hatched boxes. (B) Immunoblot analysis of HEK293 cells transfected with expression constructs Gal4-TRDMeCP2, Gal4-TRDMBD1 or Gal4-TRDMBD2 (0.1 µg each) using an anti-Gal4BD monoclonal antibody. The fusion proteins exhibit apparent molecular masses of 57, 46 and 39 kDa, respectively. (C) HEK293 cells were transfected with reporter constructs L1.3-Luc or L1RP-Luc that did or did not contain upstream Gal4 DNA-binding sites (G5) and with or without the indicated amounts (0.06–0.4 µg) of expression construct Gal4-TRDMeCP2. Relative luciferase activity of reporter constructs lacking Gal4 DNA-binding sites co-transfected with 0.1 µg of plasmid Gal4-TRDMeCP2 was set as 1.0. Columns represent mean luciferase activities (± standard deviations) of three to five independent experiments. (D) HEK293 cells were co-transfected with reporter plasmids L1.3-Luc or G5L1.3-Luc and expression construct Gal4-TRDMeCP2 (0.1 µg). After 24 h, transfected cells were treated with TSA (100 ng/ml) and incubated for another 24 h. (E) HEK293 cells were co-transfected with reporter constructs L1.3-Luc or G5L1.3-Luc and expression constructs Gal4-TRDMBD1 or Gal4-TRDMBD2 (0.1 µg each).
MeCP2 shares with MBD family members MBD1 and MBD2 the ability to repress transcription, though their TRDs bear no obvious similarities (19,20,22). We therefore analyzed whether the TRDs of MBD1 and MBD2 would also affect L1 expression. A fusion protein that tethers the Gal4 DNA-binding domain to the TRD of human MBD1 (amino acids 383–605 of isoform v1; Gal4-TRDMBD1) repressed transcription of the L1.3 promoter by 70% (Fig. 1A and E). In contrast, a Gal4 fusion protein containing the TRD of MBD2 (amino acids 45–262 of isoform b; Gal4-TRDMBD2) failed to repress transcription of the L1.3 promoter appreciably (Fig. 1A and E). These data strongly suggest that the TRDs of MeCP2 and MBD1, but not that of MBD2, can specifically inhibit L1 promoter activity.
Several lines of evidence indicate that the heavy methylation density of L1 elements plays a key role in their silencing (5,29). Therefore we analyzed the effects of co-expressed full-length FLAG-tagged MeCP2 on transcription of the L1.3 reporter construct in response to its methylation by HpaII methylase in transient transfection assays. We chose this enzyme, which methylates the L1.3 promoter at four nucleotide positions (36, 101, 304 and 481) for two reasons (Fig. 2A). First, MeCP2 can bind to a single symmetrically methylated CpG (30) and, secondly, methylation at nucleotide positions 36 and 304 has been shown previously to correlate inversely with L1 expression in cultured cell lines (29). Methylation of the L1.3 reporter in the absence of ectopic MeCP2 led to a weak decrease in transcription, probably due to binding of abundant endogenous MeCP2 (Fig. 2B). Furthermore, overexpression of FLAG-tagged MeCP2 (Fig. 2C) slightly reduced transcription from the unmethylated L1.3 promoter, possibly due to a weak affinity of MeCP2 to the unmethylated template (11). However, the transcription rate of the methylated promoter was strongly reduced by overexpressed MeCP2. We infer that MeCP2 can bind to methylated L1 in vivo and controls its transcription.
Figure 2.
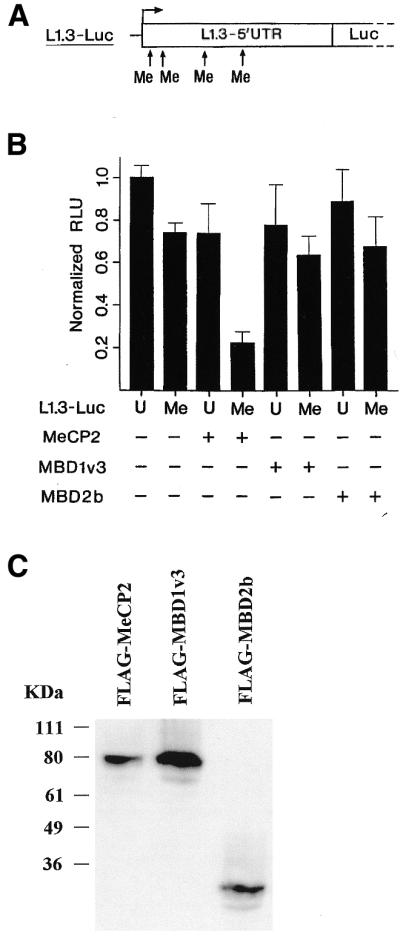
MeCP2 represses transcription from a methylated L1 promoter. (A) Schematic representation of the sites (nucleotides 36, 101, 304 and 481) in the L1.3–5′UTR methylated by M.HpaII methylase. (B) HEK293 cells were co-transfected with unmethylated (U) or HpaII-methylated (Me) reporter L1.3-Luc and expression constructs encoding full-length FLAG-tagged MeCP2, MBD1v3 or MBD2b. Relative luciferase activity of the unmethylated reporter in the absence of coexpressed genes was set as 1.0. Columns represent mean luciferase activities (± standard deviations) of three to five independent experiments. (C) Expression of FLAG-tagged MeCP2, MBD1v3 and MBD2b was controlled for by immunoblot analysis with an anti-FLAG monoclonal antibody. The tagged proteins exhibit apparent molecular masses of 81, 81 and 29 kDa, respectively.
Like MeCP2, MBD1 and MBD2 can bind to a fragment with widely separated methylated CpGs and even to a single methylated CpG (8). To study their effects on transcription from a HpaII-methylated L1.3 promoter, we selected the MBD1 splice variant v3 (MBD1v3) and the short initiation isoform MBD2b. MBD1v3 lacks the third CxxC motif and, thereby, the complicating ability to bind to unmethylated promoters (19). Neither expression of FLAG-tagged MBD1v3 nor that of FLAG-tagged MBD2b significantly inhibited transcription from the unmethylated L1.3 promoter (Fig. 2B). Furthermore methylation with HpaII did not affect a significant change in the expression of the reporter. Immunoblot analysis in Figure 2C shows that, in comparison to the expression of FLAG-MeCP2, FLAG-MBD1v3 as well as FLAG-MBD2b are expressed in larger molar amounts. Thus, insufficient amounts of protein cannot be used as an explanation for the lack of repression. Summarizing, full-length MBD1v3 failed to decrease L1 expression in response to HpaII methylation, although its TRD is able to repress L1 transcription when targeted to the L1 promoter (see Fig. 1E). MBD2b, which has the ability to bind to a single methylated CpG (8), did not repress L1 transcription either in the targeting experiment (see Fig. 1E) or in response to reporter methylation. We conclude that solely full-length MeCP2 can repress transcription from an HpaII-methylated L1 promoter.
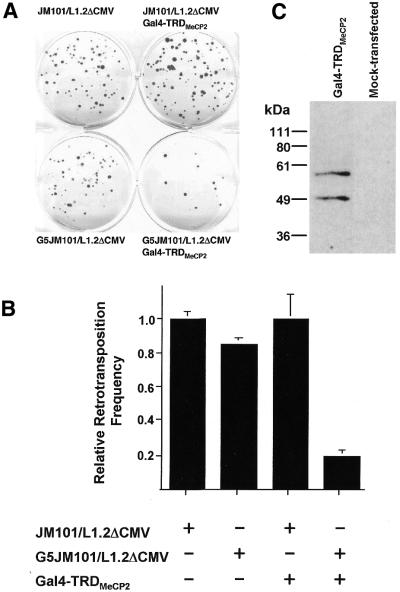
We next investigated the effects of MeCP2 on L1 retrotransposition, employing a recently developed genetic assay (26). The assay is based on an L1.2 reporter containing an antisense copy of the neo indicator gene disrupted by intron 2 of the γ-globin gene in sense orientation. Splicing and reverse transcription of RNAs from the marked L1.2 reporter allows expression of the neo gene and results in G418 resistance after integration of the cDNA into chromosomal DNA. Using reporter constructs JM101/L1.2ΔCMV and G5JM101/L1.2ΔCMV we measured average retrotransposition frequencies of 357 and 303 in 106 transfected cells, respectively (Fig. 3A; Table 1A). This is consistent with the previously reported transposition efficiency of L1.2 (26). Retrotransposition was previously demonstrated to start 48 h post-transfection and to proceed at a continuous high rate for at least 16 days (31). To verify the presence of Gal4-TRDMeCP2 while transcription and retrotransposition of the marked L1 was taking place, we first demonstrated its efficient expression 72 h after co-transfection with the L1 reporter, i.e. 1 day after the onset of transposition, by immunoblot analysis (Fig. 3C). While retrotransposition of JM101/L1.2ΔCMV was not affected by Gal4-TRDMeCP2, targeting of the repressor to the reporter through insertion of Gal4 DNA-binding sites drastically reduced retrotransposition by 82% (Fig. 3B). Since G418-resistant foci only arise if the reporter is transcribed and the RNA is spliced (26), we infer that downregulation of retrotransposition by the TRD of MeCP2 operates through its demonstrated ability to repress transcription (see Fig. 1C).
Figure 3.
The TRD of MeCP2 represses L1 retrotransposition. (A) Reporter construct JM101/L1.2ΔCMV or G5JM101/L1.2ΔCMV was co-transfected with the empty vector pcDNA1.1 or with expression construct Gal4-TRDMeCP2 into HeLa cells. Selection with G418 began 3 days after transfection and, after 12 days, G418 resistant foci were fixed and stained with Giemsa for visualization. Results of a representative transposition assay are shown. (B) Effect of Gal4-TRDMeCP2 on relative transposition frequencies of the neo-marked L1.2 reporters. The bar chart represents the data in Table 1A. Relative retrotransposition frequencies refer to the mean retrotransposition rate of construct JM101/L1.2ΔCMV (357 ± 17 × 106), which is set as 1.0. (C) Expression of Gal4-TRDMeCP2 72 h post-transfection was controlled for by immunoblot analysis with anti-Gal4BD antibody. The upper 57-kDa band represents the full-length Gal4-TRDMeCP2. The lower band very likely results from cellular protease activity, since the C-terminal half of MeCP2 exhibits prominent sensitivity to proteolysis (10).
Table 1. Effects of Gal4-TRDMeCP2- (A) and MeCP2-expression (B) on L1 retrotransposition frequencies.
| Transfected constructs | N | Retrotransposition frequency | Activity | |
| |
|
|
(×10–6) mean ± SEM |
|
| A | JM101/L1.2ΔCMV | 6 | 357 ± 17 | 1.00 |
| G5JM101/L1.2ΔCMV | 6 | 303 ± 15 | 0.85 | |
| JM101/L1.2ΔCMV + Gal4- TRDMeCP2 | 6 | 359 ± 53 | 1.00 | |
| G5JM101/L1.2ΔCMV + Gal4-TRDMeCP2 | 6 | 65 ± 13 | 0.18 | |
| B | JM101/L1.2ΔCMV (U) | 3 | 612 ± 40 | 1.00 |
| JM101/L1.2ΔCMV (Me) | 3 | 259 ± 27 | 0.42 | |
| JM101/L1.2ΔCMV (U) + MeCP2 | 3 | 485 ± 3 | 0.79 | |
| JM101/L1.2ΔCMV (Me) + MeCP2 | 3 | 182 ± 24 | 0.30 |
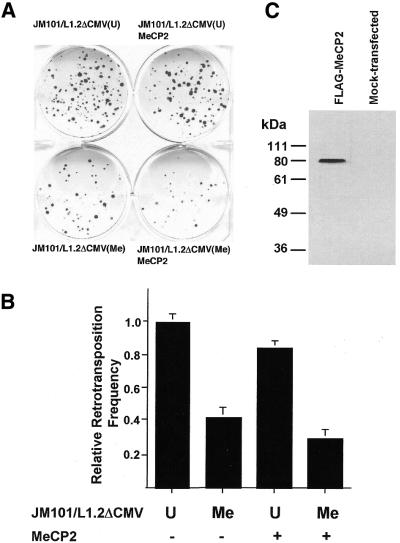
Next we tested the effects of methylation and co-expression of full-length MeCP2 on L1 retrotransposition (Fig. 4A; Table 1B). HpaII methylation of reporter JM101/L1.2ΔCMV significantly reduced its ability to retrotranspose (Fig. 4B). This is likely due to binding of methyl-CpG-binding proteins including endogenous MeCP2. Due to conflicting reports on the presence of MeCP2 in HeLa cells (21,32), we performed a comparative immunoblot analysis on several human and murine cell lines by use of an anti-MeCP2 antibody (Upstate) showing that HeLa cells contain as much MeCP2 as, for example, NIH3T3 cells (data not shown). Overexpression of FLAG-tagged MeCP2, as controlled by immunoblot analysis 72 h post-transfection (Fig. 4C), affected a further, although weak, reduction of the retrotransposition frequency (Fig. 4B). Methylation-induced repression in the presence and absence of over-expressed MeCP2 did not differ significantly. Summarizing our results from the luciferase activity assays and the retrotransposition assays, our data support the conclusion that MeCP2 can repress L1 retrotransposition.
Figure 4.
Methylated and overexpressed full-length MeCP2 diminishes L1 retrotransposition frequency. (A) Unmethylated (U) or HpaII-methylated (Me) reporter JM101/L1.2ΔCMV was co-transfected with empty vector pcDNA1.1 or with the expression construct for FLAG-tagged MeCP2. Results of a representative transposition assay are shown. (B) Effect of MeCP2 on relative retrotransposition frequencies of the methylated versus unmethylated neo-marked L1.2 reporter. The bar chart is based on the data in Table 1B. Relative retrotransposition frequencies refer to the mean retrotransposition rate of the unmethylated reporter JM101/L1.2ΔCMV (612 ± 40 × 106), which is set as 1.0. (C) Expression of FLAG-tagged MeCP2 (81 kDa) 72 h post-transfection was controlled for by immunoblot analysis with anti-FLAG antibody.
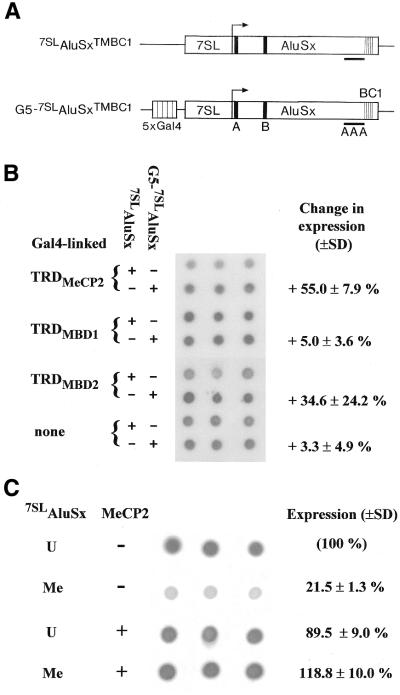
Since Alu elements are likely mobilized by the trans action of L1 proteins and share with L1 repeats a heavy methylation density, we also explored whether MeCP2 would affect Alu expression. To this aim, we employed a reporter plasmid, 7SLAluSxTMBC1, whose expression is greatly enhanced by a 7SL-upstream activating sequence (Fig. 5A) (25). The reporter contains a diagnostic unique 20 bp sequence derived from the BC1 gene to enable northern dot blot analysis of its activity. When targeted to the reporter by Gal4 DNA-binding sites the Gal4-linked TRD of MeCP2 affected some increase in the transcriptional activity of the reporter, while the TRDs of MBD1 and MBD2 had no statistically significant effect on its expression (Fig. 5B). Methylation of the 7SLAluSxTMBC1 reporter by HpaII methyltransferase did not affect its expression (data not shown), yet complete methylation using SssI methyltransferase drastically reduced its expression (Fig. 5C). Surprisingly, co-expression of full-length MeCP2 fully abrogated this repressive effect (Fig. 5C). Thus, in contrast to the repressive action of MeCP2 on L1 transcription, it influences Alu transcription positively. The molecular basis of this positive effect is unclear and various possibilities of direct and indirect interactions of MeCP2 with regulatory components of Alu transcription have to be considered (see Discussion).
Figure 5.
Effects of MeCP2 on Alu transcription. (A) Schematic diagram of the reporters 7SLAluSxTMBC1 and G5-7SLAluSxTMBC1. The location of promoter elements box A and box B, the adenine-rich region and the diagnostic unique BC1 region are indicated. (B) Effects of different TRDs on 7SLAluSx transcription. HEK293 cells were transfected with reporter construct 7SLAluSxTMBC1 or G5-7SLAluSxTMBC1 and expression constructs Gal4-TRDMeCP2, Gal4-TRDMBD1 or Gal4-TRDMBD2 or no expression construct. (C) Effects of methylation and full-length MeCP2 on 7SLAluSx transcription. HEK293 cells were transfected with unmethylated or SssI methylated 7SLAluSxTMBC1 and with or without an expression construct encoding full-length FLAG-tagged MeCP2. An autoradiogram of a northern dot blot analyzing three independent transfections is shown. In (B), expression levels using the G5 reporter are expressed as the change (% ± standard deviation) relative to the expression levels using the reporter without Gal4 DNA-binding sites.
DISCUSSION
Two lines of evidence indicate that MeCP2 efficiently represses L1 transcription. First, targeting the Gal4-linked TRD of MeCP2 to Gal4 DNA-binding sequences inserted upstream of L1 luciferase reporter constructs downregulated expression from two active L1 promoters, L1.3 and L1RP, by 70%. In a second approach, full-length MeCP2 efficiently repressed a HpaII-methylated L1.3 reporter by 77%. This is consistent with a previous report indicating an inverse correlation between L1 expression in cultured cell lines and methylation of two HpaII sites at nucleotide positions 36 and 304 (29). In vivo, L1 elements are heavily methylated over the entire CpG-rich promoter, which provides a reasonable explanation why repression of L1 elements in somatic tissues approaches 100% (5). Cre-mediated deletion of the maintenance methyltransferase gene Dnmt1 caused demethylation and an increase in the expression of L1 and retroviral elements in cultured murine fibroblasts (33). Thus, our results and their combination with reported findings suggest that MeCP2 also mediates repression of endogenous L1 elements. In contrast to two known positive regulatory factors of L1 transcription, the ubiquitous factor YY1 and a neuronally expressed SRY family transcription factor (SOX11), MeCP2 causes repression of L1 transcription (34,35). It remains to be elucidated whether MeCP2 interacts with YY1 or SOX11, or competes with these factors for binding to the promoter region. Interestingly, repression of endogenous L1 elements by MeCP2 may be relevant for our understanding of the molecular events leading to Rett syndrome, a neurological disorder that is caused by mutations in the gene encoding MeCP2 (36). While the detailed consequences of a non-functional MeCP2 on neuronal gene expression are currently unknown, two mutually non-exclusive hypotheses have been put forward (37). Rett syndrome may be caused by misregulation of genes crucial for neuronal physiology or by an excessive transcriptional ‘noise’. Through the recent availability of Mecp2-null mice it is now possible to analyze the effects of missing MeCP2 on endogenous L1 expression and their possible impact on Rett syndrome (38,39).
Furthermore, we used a genetic assay to show that the TRD of MeCP2 strongly represses retrotransposition of a full-length L1 reporter construct by 82%. Retrotransposition was also reduced after in vitro methylation of the reporter and was further decreased by co-expressed full-length MeCP2. Interestingly, L1 transposition and L1 transcription were repressed to the same degree in the two different assays. This may suggest that L1 transcription is the rate limiting step of retrotransposition. The evolutionary genetics of L1 require its expression and transposition in the germ line. In fact, L1 is expressed at various points of the murine gamete life cycle (40,41). The notion that endogenous L1 elements are repressed by MeCP2 would require an expression pattern of MeCP2 inverse to that of L1. In support of this notion, several studies at the mRNA as well as the protein level showed that MeCP2 is widely expressed in mammalian tissues, but a recent RT–PCR analysis indicated that MeCP2 mRNA is almost undetectable in murine male germ cells (32,42). Thus, MeCP2 is likely to be involved in repression of L1 expression and transposition in most somatic tissues of the mouse, but is not present in male germ cells where retrotransposition is taking place. Besides MeCP2, germ line-specific factors may participate in determining the restricted expression pattern of L1 (34). The L1 retrotransposition machinery is thought to be additionally used by Alu elements, since these have no coding capacity (4). A transcriptional control of L1 expression by MeCP2 therefore postulates a further indirect role of the repressor in the genomic spreading of Alu elements.
In striking contrast to the repressive effects of MeCP2 on L1 expression, the TRD of MeCP2 failed to decrease transcription of an AluSx reporter, but rather increased its transcription. Furthermore, MeCP2 completely abrogated the methylation-induced decrease in reporter transcription. Relief of repression could occur at several levels. One possible effect could be competition with binding of one or more proteins, which repress Alu transcription. Alternatively, MeCP2 could repress expression of such proteins at the transcriptional level. Previous in vitro transcription studies have indicated that methylation of two CpGs in the A box control region of Alu repeats is sufficient to inhibit transcription (43). Since this inhibition is relieved by competition with methylated plasmid DNA, it has been concluded that repression is caused by a methyl-CpG-binding protein present in limiting amounts. Based on our transient transfection results, MeCP2, MBD1 and MBD2 are not likely candidates for this protein.
MeCP2 has been shown previously to repress a number of cellular as well as viral gene promoters (13–17 and references therein). Notably, reports on promoters that are refractory to the repressive effect of MeCP2 were lacking so far, giving MeCP2 the image of a general repressor. We here report on the first example (to our knowledge) of a transcription unit that is not repressed by MeCP2. Thus, although Alu elements are likely mobilized by the trans action of L1 proteins, the role of MeCP2 in transcriptional regulation of Alu and L1 elements is completely different. Conceivably MeCP2 can only repress genes transcribed by RNA polymerase II, possibly through inhibition of factors solely engaged in polymerase II transcription. Interestingly, Alu and L1 repeats also differ in another fundamental chromosomal feature and its impact on gene expression. Compared to bulk chromatin, chromatin encompassing Alus is enriched in histone H4 acetylated at lysines 5, 8, 12 and 16 (44). Contrary to this, chromatin containing L1 repeats is depleted in H4 acetylated at any of the four acetylatable lysines. In the case of L1 elements, the correlation of a presumed involvement of MeCP2 in repression of endogenous L1 elements and the low level of histone H4 acetylation would support the assumption that recruitment of the mSin3A-histone deacetylase co-repressor complex through MeCP2 is partially responsible for the underacetylation of L1 associated chromatin (14,15).
Since Rett syndrome indicates that MeCP2 cannot be (fully) substituted by any other methyl-CpG-binding protein, we searched for potential differences in the L1-repressive effect among other MBD family members. In the Gal4 fusion protein experiment, the TRD of MBD1 repressed transcription from the L1 reporter. Thus, the failure of full-length MBD1v3 to repress an HpaII-methylated L1 reporter may well be explained by an insufficient affinity of MBD1v3 to bind to the few HpaII-methylated sites in the L1 promoter (19). In this context it is relevant to note that methylation at solely HpaII recognition sites does not reflect the in vivo situation with many more CpG sequences methylated (5). Unfortunately the in vivo situation is difficult to imitate, since most CpGs are hemimethylated and, furthermore, a few methylated cytosines are located outside of CpG sequences. In the Gal4 fusion protein experiment the TRD of MBD2 lacked L1-repressing activity. MBD2 is a component of the MeCP1 histone deacetylase complex (21). Recent purification of MeCP1 to homogeneity has revealed that the compositions of MeCP1 and the NuRD complex are identical except for the additional presence of MBD2 in MeCP1 (45). Both contain nine components, including Mi2 responsible for the chromatin remodeling activity of the complex. This suggests that MBD2 functions by recruiting the NuRD complex to methylated promoters.
The role of DNA methylation in tissue-specific gene expression during development and in the adult organism has been subject to much debate. A correlation between methylation and gene expression has been observed for several genes (33), but no correlation has been found for other genes (32). This might suggest that methylation is not necessary for transcriptional repression of all genes. Furthermore, it has been postulated that DNA methylation primarily evolved as a defense mechanism against transposable elements (7). Although our studies using transient transfections of cultured cells cannot resolve this controversy, they document that recruitment of MeCP2 by methylated L1 sequences has the potential to repress L1 expression in somatic tissues.
Acknowledgments
ACKNOWLEDGEMENTS
We thank J. V. Moran, H. H. Kazazian, P. L. Deininger and H.-G. Kräusslich for generous gifts of plasmid DNA. This article is based on the doctoral theses of F.Y. and N.Z. in the Faculties of Biology and Chemistry, respectively, at the University of Hamburg. This research was supported by grant SFB545/B2 (W.H.S.) and grants Schu1014/2-1 and Schu1014/2-2 (G.S.) of the Deutsche Forschungsgemeinschaft and grant AZ.10.01.1.104 (G.S.) of the Fritz-Thyssen Stiftung.
References
- 1.Kazazian H.H. and Moran,J.V. (1998) The impact of L1 retrotransposition on the human genome. Nature Genet., 19, 19–24. [DOI] [PubMed] [Google Scholar]
- 2.Sassaman D.M., Dombroski,B.A., Moran,J.V., Kimberland,M.L., Nass,T.P., DeBerardinis,R.J., Gabriel,A., Swergold,G.D. and Kazazian,H.H. (1997) Many human L1 elements are capable of retrotransposition. Nature Genet., 16, 37–43. [DOI] [PubMed] [Google Scholar]
- 3.International Human Genome Sequencing Consortium (2001) Initial sequencing and analysis of the human genome. Nature, 409, 860–921. [DOI] [PubMed] [Google Scholar]
- 4.Boeke J.D. (1997) LINEs and Alus – the polyA connection. Nature Genet., 16, 6–7. [DOI] [PubMed] [Google Scholar]
- 5.Woodcock D.M., Lawler,C.B., Linsenmeyer,M.E., Doherty,J.P. and Warren,W.D. (1997) Asymmetric methylation in the hypermethylated CpG promoter region of the human L1 retrotransposon. J. Biol. Chem., 272, 7810–7816. [DOI] [PubMed] [Google Scholar]
- 6.Jurka J. (1997) Sequence patterns indicate an enzymatic involvement in integration of mammalian retrotransposons. Proc. Natl Acad. Sci. USA, 94, 1872–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoder J.A., Walsh,C.P. and Bestor,T.H. (1997) Cytosine methylation and the ecology of intragenomic parasites. Trends Genet., 13, 335–339. [DOI] [PubMed] [Google Scholar]
- 8.Hendrich B. and Bird,A. (1998) Identification and characterization of a family of mammalian methyl-CpG binding proteins. Mol. Cell. Biol., 18, 6538–6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bird A.P. and Wolffe,A.P. (1999) Methylation-induced repression–belts, braces, and chromatin. Cell, 99, 451–454. [DOI] [PubMed] [Google Scholar]
- 10.Lewis J.D., Meehan,R.R., Henzel,W.J., Maurer-Fogy,I., Jeppesen,P., Klein,F. and Bird,A. (1992) Purification, sequence, and cellular localization of a novel chromosomal protein that binds to methylated DNA. Cell, 69, 905–914. [DOI] [PubMed] [Google Scholar]
- 11.Weitzel J.M., Buhrmester,H. and Strätling,W.H. (1997) Chicken MAR-binding protein ARBP is homologous to rat methyl-CpG-binding protein MeCP2. Mol. Cell. Biol., 17, 5656–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakefield R.I.D., Smith,B.O., Nan,X., Free,A., Soteriou,A., Uhrin,D., Bird,A.P. and Barlow,P.N. (1999) The solution structure of the domain from MeCP2 that binds to methylated DNA. J. Mol. Biol., 291, 1055–1065. [DOI] [PubMed] [Google Scholar]
- 13.Nan X., Campoy,F.J. and Bird,A. (1997) MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell, 88, 471–481. [DOI] [PubMed] [Google Scholar]
- 14.Nan X., Ng,H.-H., Johnson,C.A., Laherty,C.D., Turner,B.M., Eisenman,R.N. and Bird,A. (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature, 393, 386–389. [DOI] [PubMed] [Google Scholar]
- 15.Jones P.L., Veenstra,G.J.C., Wade,P.A., Vermaak,D., Kass,S.U., Landsberger,N., Strouboulis,J. and Wolffe,A.P. (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genet., 19, 187–191. [DOI] [PubMed] [Google Scholar]
- 16.Yu F., Thiesen,J. and Strätling,W.H. (2000) Histone deacetylase-independent transcriptional repression by methyl-CpG-binding protein 2. Nucleic Acids Res., 28, 2201–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaludov N.K. and Wolffe,A.P. (2000) MeCP2 driven transcriptional repression in vitro: selectivity for methylated DNA, action at a distance and contacts with the basal transcription machinery. Nucleic Acids Res., 28, 1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita N., Takebayashi,S.I., Okumura,K., Kudo,S., Chiba,T., Saya,H. and Nakao,M. (1999) Methylation-mediated transcriptional silencing in euchromatin by methyl-CpG binding protein MBD1 isoforms. Mol. Cell. Biol., 19, 6415–6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujita N., Shimotake,N., Ohki,I., Chiba,T., Saya,H., Shirakawa,M. and Nakao,M. (2000) Mechanism of transcriptional regulation by methyl-CpG binding protein MBD1. Mol. Cell. Biol., 20, 5107–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng H.-H., Jeppesen,P. and Bird,A. (2000) Active repression of methylated genes by the chromosomal protein MBD1. Mol. Cell. Biol., 20, 1394–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng H.-H., Zhang,Y., Hendrich,B., Johnson,C.A., Turner,B.M., Erdjument-Bromage,H., Tempst,P., Reinberg,D. and Bird,A. (1999) MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nature Genet., 23, 58–61. [DOI] [PubMed] [Google Scholar]
- 22.Boeke J., Ammerpohl,O., Kegel,S., Moehren,U. and Renkawitz,R. (2000) The minimal repression domain of MBD2b overlaps with the methyl-CpG binding domain and binds directly to Sin3A. J. Biol. Chem., 275, 34963–34967. [DOI] [PubMed] [Google Scholar]
- 23.Moran J.V., DeBerardinis,R.J. and Kazazian,H.H. (1999) Exon shuffling by L1 retrotransposition. Science, 283, 1530–1534. [DOI] [PubMed] [Google Scholar]
- 24.Kimberland M.L., Divoky,V., Prchal,J., Schwahn,U., Berger,W. and Kazazian,H.H. (1999) Full-length human L1 insertions retain the capacity for high frequency retrotransposition in cultured cells. Hum. Mol. Genet., 8, 1557–1560. [DOI] [PubMed] [Google Scholar]
- 25.Roy A.M., West,N.C., Rao,A., Adhikari,P., Alemán,A., Barnes,A.P. and Deininger,P.L. (2000) Upstream flanking sequences and transcription of SINEs. J. Mol. Biol., 302, 17–25. [DOI] [PubMed] [Google Scholar]
- 26.Moran J.V., Holmes,S.E., Naas,T.P., DeBerardinis,R.J., Boeke,J.D. and Kazazian,H.H. (1996) High frequency retrotransposition in cultured mammalian cells. Cell, 87, 917–927. [DOI] [PubMed] [Google Scholar]
- 27.Wei W., Morrish,T.A., Alisch,R.S. and Moran,J.V. (2000) A transient assay reveals that cultured human cells can accommodate multiple LINE-1 retrotransposition events. Anal. Biochem., 284, 435–438. [DOI] [PubMed] [Google Scholar]
- 28.Schwahn U., Lenzner,S., Dong,J., Feil,S., Hinzmann,B., van Duijnhoven,G., Kirschner,R., Hemberger,M., Bergen,A.A., Rosenberg,T. et al. (1998) Positional cloning of the gene for X-linked retinitis pigmentosa 2. Nature Genet., 19, 327–332. [DOI] [PubMed] [Google Scholar]
- 29.Thayer R.E., Singer,M.F. and Fanning,T.G. (1993) Undermethylation of specific LINE-1 sequences in human cells producing a LINE-1-encoded protein. Gene, 133, 273–277. [DOI] [PubMed] [Google Scholar]
- 30.Nan X., Meehan,R.R. and Bird,A. (1993) Dissection of the methyl-CpG binding domain from the chromosomal protein MeCP2. Nucleic Acids Res., 21, 4886–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostertag E.M., Luning Prak,E.T., DeBerardinis,R.J., Moran,J.V. and Kazazian,H.H. (2000) Determination of L1 retrotransposition kinetics in cultured cells. Nucleic Acids Res., 28, 1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller C., Readhead,C., Diederichs,S., Idos,G., Yang,R., Tidow,N., Serve,H., Berdel,W.E. and Koeffler,H.P. (2000) Methylation of the cyclin A1 promoter correlates with gene silencing in somatic cell lines, while tissue-specific expression of cyclin A1 is methylation independent. Mol. Cell. Biol., 20, 3316–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson-Grusby L., Beard,C., Possemato,R., Tudor,M., Fambrough,D., Csankovszki,G., Dausman,J., Lee,P., Wilson,C., Lander,E. and Jaenisch,R. (2001) Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nature Genet., 27, 31–39. [DOI] [PubMed] [Google Scholar]
- 34.Tchénio T., Casella,J.-F. and Heidmann,T. (2000) Members of the SRY family regulate the human LINE retrotransposons. Nucleic Acids Res., 28, 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Becker K.G., Swergold,G.D., Ozato,K. and Thayer,R.E. (1993) Binding of the ubiquitous nuclear transcription factor YY1 to a cis regulatory sequence in the human LINE-1 transposable element. Hum. Mol. Genet., 2, 1697–1702. [DOI] [PubMed] [Google Scholar]
- 36.Amir R.E., Van den Veyver,I.B., Wan,M., Tran,C.Q., Francke,U. and Zoghbi,H.Y. (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet., 23, 185–188. [DOI] [PubMed] [Google Scholar]
- 37.Van den Veyver I.B. and Zoghbi,H.Y. (2000) Methyl-CpG-binding protein 2 mutations in Rett syndrome. Curr. Opin. Genet. Dev., 10, 275–279. [DOI] [PubMed] [Google Scholar]
- 38.Chen R.Z., Akbarian,S., Tudor,M. and Jaenisch,R. (2001) Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genet., 27, 327–331. [DOI] [PubMed] [Google Scholar]
- 39.Guy J., Hendrich,B., Holmes,M., Martin,J.E. and Bird,A. (2001) A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genet., 27, 322–326. [DOI] [PubMed] [Google Scholar]
- 40.Branciforte D. and Martin,S.L. (1994) Developmental and cell type specificity of LINE-1 expression in mouse testis: implications for transposition. Mol. Cell. Biol., 14, 2584–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trelogan S.A. and Martin,S.L. (1995) Tightly regulated, developmentally specific expression of the first open reading frame from LINE-1 during mouse embryogenesis. Proc. Natl Acad. Sci. USA, 92, 1520–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reichwald K., Thiesen,J., Wiehe,T., Weitzel,J., Strätling,W.H., Kioschis,P., Poustka,A., Rosenthal,A. and Platzer,M. (2000) Comparative sequence analysis of the MECP2-locus in human and mouse reveals new transcribed regions. Mamm. Genome, 11, 182–190. [DOI] [PubMed] [Google Scholar]
- 43.Liu W.-M. and Schmid,C.W. (1993) Proposed roles for DNA methylation in Alu transcriptional repression and mutational inactivation. Nucleic Acids Res., 6, 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson C.A., O’Neill,L.P., Michell,A. and Turner,B.M. (1998) Distinctive patterns of histone H4 acetylation are associated with defined sequence elements within both heterochromatic and euchromatic regions of the human genome. Nucleic Acids Res., 26, 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng Q. and Zhang,Y. (2001) The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes. Genes Dev., 15, 827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]