Abstract
Translation of mRNA into protein has a fundamental role in neurodevelopment, plasticity, and memory formation; however, its contribution in the pathophysiology of depressive disorders is not fully understood. We investigated the involvement of MNK1/2 (MAPK-interacting serine/threonine-protein kinase 1 and 2) and their target, eIF4E (eukaryotic initiation factor 4E), in depression-like behavior in mice. Mice carrying a mutation in eIF4E for the MNK1/2 phosphorylation site (Ser209Ala, Eif4e ki/ki), the Mnk1/2 double knockout mice (Mnk1/2−/−), or mice treated with the MNK1/2 inhibitor, cercosporamide, displayed anxiety- and depression-like behaviors, impaired serotonin-induced excitatory synaptic activity in the prefrontal cortex, and diminished firing of the dorsal raphe neurons. In Eif4e ki/ki mice, brain IκBα, was decreased, while the NF-κB target, TNFα was elevated. TNFα inhibition in Eif4e ki/ki mice rescued, whereas TNFα administration to wild-type mice mimicked the depression-like behaviors and 5-HT synaptic deficits. We conclude that eIF4E phosphorylation modulates depression-like behavior through regulation of inflammatory responses.
Translation of mRNA contributes to neuronal function and complex behaviours, and inflammation is thought to contribute to depression. Here the authors show that mice lacking phosphorylation sites in eIF4E (eukaryotic initiation factor 4E) display anxiety- and depression-like behaviour and decreased IkBα expression; furthermore TNFα delivery to the medial prefrontal cortex induces depression-like behaviour and deficits in serotonergic transmission.
Introduction
Major depressive disorder (MDD) is one of the leading causes of disability worldwide1, with a lifetime prevalence of 16.6 % in the United States2. Current treatments are ineffective in about one third of patients3, indicating the urgent need to better understand MDD pathophysiology and identify novel therapeutic targets. The MAPK/ERK pathway is impaired in the brains of MDD patients4,5, however a causal role for downstream mRNA translation in depression has not been studied. mRNA translation (protein synthesis) plays a major role in the control of gene expression, allowing for rapid and spatially restricted changes in protein levels6. A rate-limiting step in the initiation of mRNA translation is the recruitment of the eukaryotic initiation factor 4E (eIF4E) to the mRNA 5′cap structure (m7GpppN, where N is any nucleotide). eIF4E binds to the scaffolding protein eIF4G, which also binds to the RNA helicase eIF4A, to form the eIF4F complex, which mediates the recruitment of preinitiation complex6. eIF4E is a target of the mammalian target of rapamycin complex 1 (mTORC1) and MAPK/ERK pathways;7 the activation of which stimulates protein synthesis.
The MAP kinases ERK1/2 and p38 control mRNA translation through the MAPK-interacting serine/threonine-protein kinase 1 and 2 (MNK1/2). MNKs phosphorylate eIF4E8 on Ser2099–11. Phosphorylation of eIF4E engenders increased translation of a subset of mRNAs12, some of which play a role in memory formation13 and regulation of circadian rhythms14.
In MDD-suicide subjects, the activity, protein, and mRNA levels of ERK1/2 are decreased in post-mortem prefrontal and hippocampal areas4,5. The consequence of reduced activation of ERK1/2 in the pathophysiology of depression and on the anti-depressant effect of selective serotonin reuptake inhibitors (SSRIs)15,16 and mood stabilizers17 is well understood. In this regard, MAPK kinase (MEK) inhibition induces depression-like behaviors and blocks the antidepressant action of SSRIs and tricyclic compounds15. Furthermore, reduction in P38 MAPK phosphorylation has been observed in an animal model of depression and aggressive behavior, induced by dietary deprivation of n-3 polyunsaturated fatty acids18. Also, P38 MAPK activation contributes to the induction of depression-like behavior by chronic stress and immune mediators19,20.
Here we demonstrate that genetic and pharmacological inhibition of eIF4E phosphorylation in mice resulted in a depression-like state, impaired serotonin excitatory activity in the prefrontal cortex, and diminished firing of raphe serotonergic neurons. Inhibition of eIF4E phosphorylation caused decreased translation of IκBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha) mRNA in the brain. Decreased IκBα, a repressor of the transcription factor NF-κB, led to increased expression of the cytokine TNFα and exacerbated microglial responsiveness to inflammatory stimuli with lipopolysaccharide (LPS). Increased inflammation in the brain is a hallmark of several psychiatric disorders, including MDD19,21, in which patients have elevated cytokine levels22,23, and microgliosis24. Increased peripheral and central inflammatory markers in MDD are thought to contribute to the pathophysiology and treatment responsiveness19,21. Importantly, inhibition of TNFα ameliorated the depression-like behavior, as well as serotonergic synaptic and cell firing alterations induced by the lack of eIF4E phosphorylation.
Results
The MNK1/2-eIF4E pathway controls depression-like behaviors
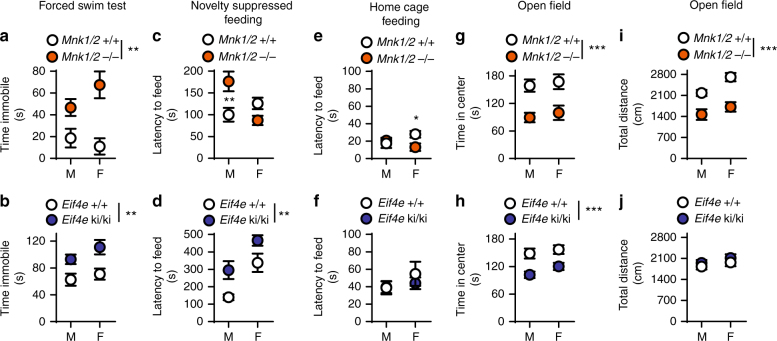
To study the role of eIF4E phosphorylation in depression pathophysiology, we examined the Mnk1 and Mnk2 double knock-out (Mnk1/2−/−)11, and the Eif4e Ser209Ala knock-in (Eif4e ki/ki)12 mice in several validated paradigms for depression-like behavior. In the forced swim test (FST), immobility time was increased in male (M) and female (F) Mnk1/2−/− (by 28.1 ± 13.6 s in M and 56.5 ± 18.7 s in F mice) and Eif4e ki/ki mice (by 30.4 ± 10.9 s in M and 40.0 ± 17.3 s in F) (Fig. 1a, b). Male Mnk1/2−/− and Eif4e ki/ki mice were more immobile than wild-type in the tail suspension test (by 70.2 ± 27.5 s and 35.8 ± 12.0 s, respectively) (Supplementary Fig. 1a, b). This test could not be accurately assessed in females, since a large proportion climbed on their own tails while suspended, as previously reported25.
Fig. 1.
Genetic inhibition of eIF4E phosphorylation induced anxiety- and depression-like behaviors. a Male (M) and female (F) mice mutant for the genes encoding MNK1 and MNK2 (Mnk1/2−/− mice) and their wild-type littermates (Mnk1/2+/+) were assessed for despair-like behavior in the forced swim test (FST) (M, n = 8 Mnk1/2+/+, n = 9 Mnk1/2−/−; F, n = 3 Mnk1/2+/+, n = 9 Mnk1/2−/−). b Male and female mice bearing a mutation in the Eif4e gene (Ser209 was substituted to Ala, Eif4e ki/ki) were also tested in the FST (M, n = 19 Eif4e+/+, n = 19 Eif4e ki/ki; F, n = 7 Eif4e+/+, n = 8 Eif4e ki/ki). c Novelty suppressed feeding (NSF) was assessed in Mnk1/2−/− (M, n = 9; F, n = 10) and wild-type mice (M, n = 8; F, n = 10). d NSF was also assayed in Eif4e+/+ and ki/ki mice (M, n = 9 Eif4e+/+, n = 9 Eif4e ki/ki; F, n = 8 Eif4e+/+, n = 9 Eif4e ki/ki). e Latency to feed in the home cage (home cage feeding, HCF) was determined in the Mnk1/2−/− and f Eif4e ki/ki mice and their littermates. g Time spent in the center of an open field (OF) arena was measured in Mnk1/2−/− (M, n = 12 Mnk1/2+/+, n = 8 Mnk1/2−/−; F, n = 7 Mnk1/2+/+, n = 13 Mnk1/2−/−) and h Eif4e ki/ki mice and their littermates (M, n = 17 Eif4e+/+, n = 18 Eif4e ki/ki; F, n = 11 Eif4e+/+, n = 19 Eif4e ki/ki). i Locomotion was also measured in Mnk1/2−/− and j Eif4e ki/ki mice. ** p < 0.01, *** p < 0.001 vs. wild-type littermates (see Supplementary Table 2 for detailed results of statistical tests)
In the novelty-suppressed feeding (NSF) test, a measure of hyponeophagia that is responsive to chronic treatment with antidepressants26,27, the latency to feed in a novel context was increased in male Mnk1/2−/− (by 73.3 ± 23.4 s) and Eif4e ki/ki (by 156.1 ± 56.7 s), as well as female Eif4e ki/ki mice as compared to control littermates (by 128.5 ± 57.4 s) (Fig. 1c, d). The mutant mice did not differ in the latency to feed in their home cage as compared to wild-type (HCF, Fig. 1e, f). In contrast, the female Mnk1/2−/− were not affected in the latency to feed in the novel context (Fig. 1c) but exhibited a decreased latency to feed in the home cage of 14.8 ± 5.7 s (Fig. 1e). This suggests a female-specific role in feeding behavior for MNK1/2, independent of eIF4E phosphorylation.
Next, we tested exploratory behavior in the open field. Both male and female Mnk1/2−/− and Eif4e ki/ki mice showed a decrease in the time spent in the center of the arena (Mnk1/2−/−: by 69.6 ± 21.4 s in M and 67.7 ± 22.0 s in F; Eif4e ki/ki: by 46.4 ± 12.1 s in M and 36.6 ± 13.6 s in F) (Fig. 1g, h). While no changes in locomotion were observed in Eif4e ki/ki mice compared to their control littermates (Fig. 1j), the Mnk1/2 mice showed decreased locomotion (by 717.8 ± 214 s in M and 990 ± 219.8 s in F; Fig. 1i). A second exposure to the open field abrogated the difference in locomotion between Mnk1/2−/− and wild-type mice (Supplementary Fig. 1c–e). However, the difference in time spent in the center of the field persisted (Supplementary Fig. 1d–f), suggesting that novelty-related anxiety, rather than hypo-activity, caused decreased locomotion during the 1st exposure to the open field in the Mnk1/2−/− mice. Taken together, these results demonstrate that reduction of eIF4E phosphorylation, either by knocking out the Mnk1 and Mnk2 genes or by mutating the phosphorylation site in Eif4e, elicits depression- and anxiety-like behaviors.
eIF4E phosphorylation controls 5-HT neurotransmission
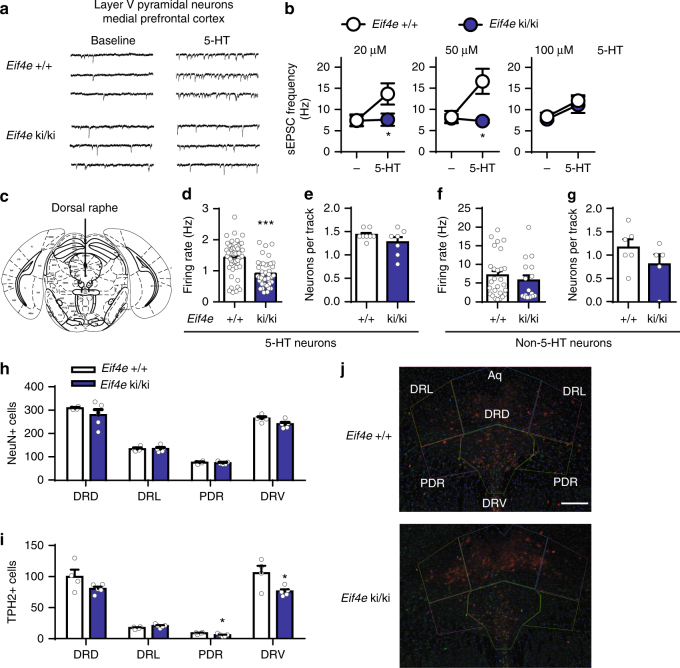
To understand the synaptic mechanisms underlying the depression-like state caused by inhibition of eIF4E phosphorylation, we first focused on serotonin (5-HT) neurotransmission in the medial prefrontal cortex (mPFC), a brain region central in depression pathophysiology28–30. Using whole-cell recording from layer V pyramidal neurons, we found no difference in the frequency of spontaneous excitatory postsynaptic current (sEPSCs) between wild-type and Eif4e ki/ki mice in baseline conditions (Fig. 2a,b). 5-HT stimulates sEPSCs in layer V pyramidal neurons31, mediated by excitatory 5-HT2A postsynaptic receptors32–34. 5-HT (20 μM) augmented the frequency of sEPSCs in wild type by 84.55 ± 24.09 %, but not in Eif4e ki/ki mice (Fig. 2a, b). The NMDA-blocker and rapidly-acting antidepressant, ketamine, enhances 5-HT-induced sEPSCs35. We therefore tested whether the enhancement occurs in Eif4e ki/ki mice. After a single injection of ketamine (IP, 10 mg/kg) 24 h prior to recording, 5-HT increased the frequency of sEPSCs by 336.80 ± 64.88% over baseline in wild-type but not in Eif4e ki/ki mice (Supplementary Fig. 4a, b). In either condition, there were no changes in sEPSC rise and decay time (Supplementary Fig. 2b and 4d, e), and amplitude (Supplementary Fig. 2a and 4c), except for an increase in sEPSC decay time by 5-HT in saline-treated Eif4e wild-type and ki/ki mice (Supplementary Fig. 2c). To determine whether a greater dose of 5-HT would stimulate sEPSC in Eif4e wild type and ki/ki mice, we treated layer V mPFC slices with 50 and 100 μM of 5-HT. While both doses were excitatory in control mice, only the 100 μM dose induced an increase in sEPSC frequency as compared to that of control mice (Fig. 2b and Supplementary Fig. 2d, e). These results indicate that the depression-like phenotypes in Eif4e ki/ki mice are associated with decreased sensitivity to 5-HT excitatory neurotransmission in layer V mPFC.
Fig. 2.
Serotonergic dysfunction in Eif4e ki/ki mice. a Representative whole cell recordings of spontaneous excitatory post-synaptic currents (sEPSCs) from layer V pyramidal neurons of the medial prefrontal cortex (mPFC) of wild-type and Eif4e ki/ki mice. Traces represent consecutive recordings of synaptic currents before (baseline) and after 5-HT (20 μM) treatment from single pyramidal cells in each group. b Frequency of sEPSCs from pyramidal neurons of wild-type and Eif4e ki/ki mice before (−) and after 5-HT at 20 μM (n = 7 cells from 5 Eif4e+/+; n = 8 cells from 7 Eif4e ki/ki), 50 μM (n = 4 cells from 3 Eif4e+/+; n = 5 cells from 3 Eif4e ki/ki) or 100 μM (n = 5 cells from 3 Eif4e+/+; n = 6 cells from 3 Eif4e ki/ki). c In vivo single-unit extracellular recordings of dorsal raphe neurons were performed in anesthetized Eif4e+/+and ki/ki mice. d Firing rate of dorsal raphe 5-HT neurons, measured in Eif4e+/+and ki/ki mice (n = 56 single unit recordings from 8 Eif4e+/+mice; n = 43 single unit recordings from 7 Eif4e ki/ki mice). e 5-HT neurons recorded per descent (track) in the dorsal raphe of wild-type and Eif4e ki/ki mice (n = 8 Eif4e+/+mice; n = 7 Eif4e ki/ki mice). f Firing rate of non-5-HT neurons in the DR (n = 27 single unit recordings from 6 Eif4e+/+mice; n = 16 single unit recordings from 5 Eif4e ki/ki mice). g Number of non-5-HT neurons per descent in the DR (n = 6 Eif4e+/+mice; n = 5 Eif4e ki/ki mice). h Number of NeuN + cells in the DR nuclei, which was subdivided into DR dorsal part (DRD), DR lateral part (DRL), posterodorsal raphe nucleus (PRN), and DR ventral part (DRV). Data are means of 4 slides per animal (n = 4 Eif4e+/+mice; n = 5 Eif4e ki/ki mice). i Number of tryptophan hydroxylase 2 (TPH2) positive cells in the DRN. j Representative images of the DR IHC; blue: nuclear DAPI staining; red: TPH2; green: NeuN. Scale bar is 100 μM. * p < 0.05, *** p < 0.001 vs. wild-type littermates (see Supplementary Table 2 for detailed results of statistical tests)
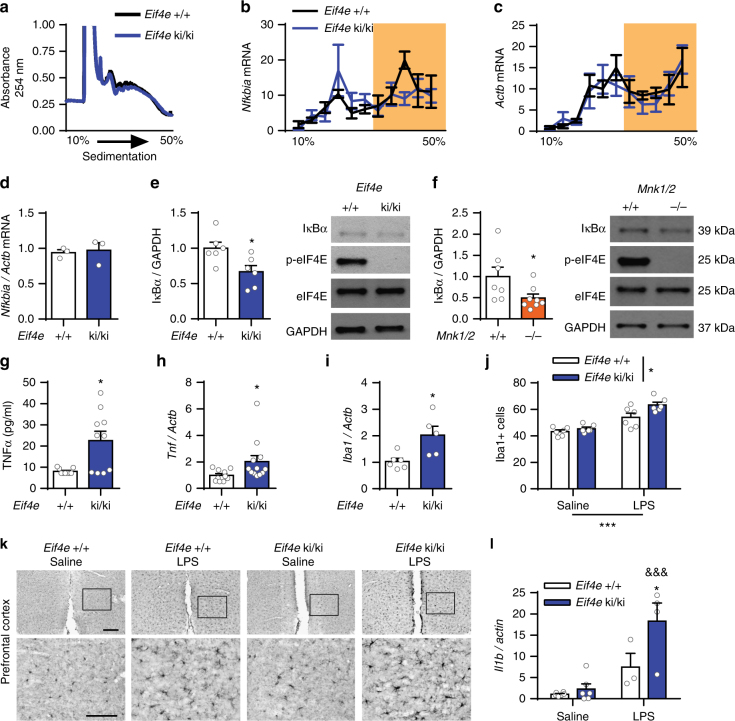
Fig. 5.
Translation of Nfkbia mRNA is reduced in Eif4e ki/ki mice. a Representative polysome profile of cortex-hippocampus from Eif4e+/+and ki/ki mice (1 mouse per genotype). b Distribution of the Nfkbia mRNA in the polysome fractions of wild-type and Eif4e ki/ki mice (n = 4 Eif4e+/+; n = 5 Eif4e ki/ki). c, Actb mRNA was measured in both mouse strains as a control. d Total mRNA levels of Nfkbia in the cortex-hippocampus of Eif4e+/+and ki/ki mice. e Average protein levels and representative western blot images of the Nfkbia mRNA product, IκBα, in the mPFC of Eif4e wild-type and ki/ki mice (n = 6/group). f Average protein levels and representative western blot images IκBα, in the mPFC of Mnk1/2+/+ and −/− mice (n = 7 Mnk1/2+/+; n = 8 Mnk1/2−/−). g Levels of the cytokine tumor necrosis factor α (TNFα) in the mPFC in wild-type and Eif4e ki/ki mice (n = 9 Eif4e+/+; n = 10 Eif4e ki/ki). h Total levels of Tnf mRNA in the mPFC in wild-type and Eif4e mutant mice (n = 10 Eif4e+/+; n = 12 Eif4e ki/ki). i mRNA levels of the microglial marker Iba1 (Aif1) in the mPFC in wild-type and Eif4e mutant mice (n = 6 Eif4e+/+; n = 5 Eif4e ki/ki). j Number of Iba1 positive cells (Iba1+) per 0.01 mm2 of mPFC from wild-type and Eif4e ki/ki mice 48 h after saline or LPS (IP, 2.5 mg kg−1) treatment (n = 3/group). k Representative images of the mPFC of wild-type or Eif4e ki/ki mice 48 h after saline or LPS treatment. l mRNA levels of Il1b in the mPFC of wild-type or Eif4e ki/ki mice 48 h after saline or LPS treatment (n = 6 Eif4e+/+Saline, n = 6 Eif4e ki/ki Saline, n = 3 Eif4e+/+LPS; n = 4 Eif4e ki/ki LPS). * p < 0.05, *** p < 0.001 vs. wild-type; &&& p < 0.001 vs. saline-treated Eif4e ki/ki (see Supplementary Table 2 for detailed results of statistical tests)
Serotonergic neurons that innervate the mPFC reside in the dorsal raphe (DR) nuclei36. We thus determined whether the activity of DR neurons is affected in Eif4e ki/ki mice. To this end, we performed in vivo single-unit extracellular recordings of DR neurons37 (Fig. 2c). Male and female Eif4e ki/ki had a 36.19 ± 6.54 and 40.87 ± 16.27% decrease, respectively, in the firing rate of electrophysiologically defined 5-HT neurons compared to control littermates, respectively (Fig. 2d and Supplementary Fig. 3c), in the absence of changes in the number of spontaneously active neurons (neurons per descent; Fig. 2e and Supplementary Fig. 3d). Furthermore, the firing rate and number of non-5-HT neurons in DR was the same between genotypes (Fig. 2f, g). To determine whether the decreased firing rate of DR neurons was due to increased activity of the 5-HT1A inhibitory autoreceptor38, DR neurons were recorded at baseline and following the administration of the 5-HT1A antagonist, WAY-100635 (IP, 0.1-0.3 mg/kg). The latter induced a similar dose-dependent increase in DR neuron firing rate in wild-type and Eif4e ki/ki mice (Supplementary Fig. 3a, b), suggesting that the 5-HT1A autoreceptors do not contribute to the diminished spontaneous activity of DR neurons in Eif4e ki/ki mice. These results indicate that reduction of eIF4E phosphorylation impaired serotonergic DR neuron firing and 5-HT post-synaptic excitation in the mPFC, in a manner similar to depression-inducing chronic stress31,39.
To determine whether the number of DR 5-HT neurons was altered in Eif4e ki/ki mice, we used tryptophan hydroxylase 2 (TPH2) as a histological marker. The number of TPH2 + cells was decreased in two subregions of the DR, the ventral part (DRV) and the posterodorsal raphe nucleus (PDR, Fig. 2h, j), while the total number of neurons (NeuN + cells) was the same between genotypes (Fig. 2i, j). When expressed as percent of the total neuronal population, the difference was only evident in the PDR (10.71 ± 1.039 vs 6.637 ± 1.179 % of NeuN + cells in PDR), suggesting a restricted reduction in 5-HT neurons in Eif4e ki/ki mice. These results are in agreement with the unchanged number of spontaneously active 5-HT neurons observed in in vivo electrophysiology (Fig. 2e and Supplementary Fig. 3d).
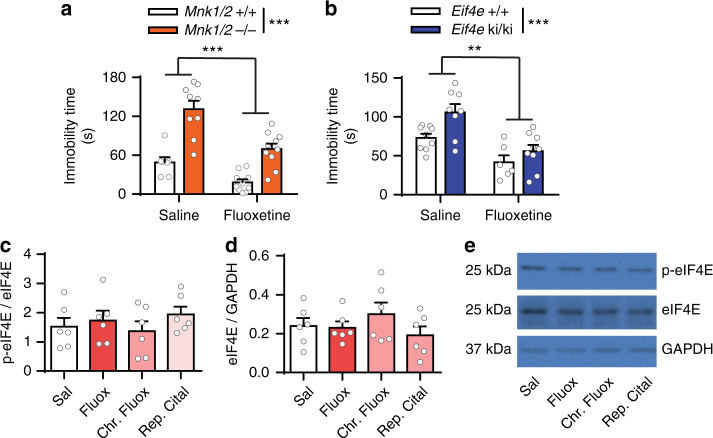
The finding of impaired serotonergic neurotransmission prompted us to investigate the behavioral response of Mnk1/2−/− and Eif4e ki/ki mice to the SSRI, fluoxetine. Acute treatment with fluoxetine (IP, 3 mg kg−1, 30 min) induced a significant decrease in immobility in the FST in both Mnk1/2−/− and their wild-type littermates by 46.88 ± 9.52 % and 62.83 ± 27.23 %, respectively (Fig. 3a). Fluoxetine also reduced immobility in Eif4e+/+and ki/ki mice by 43.00 ± 16.85 % and 47.03 ± 10.75 %, respectively (Fig. 3b). Importantly, neither acute (IP, 3 mg kg−1, 0.5 h) nor chronic (IP, 14 × 10 mg kg−1) fluoxetine, or repeated (IP, 3 × , 10 mg kg−1) citalopram treatment changed the phosphorylation of eIF4E in the brain of wild-type mice (Fig. 3c, e). Similarly, ketamine treatment (IP, 10 mg kg−1, 1 h) did not change eIF4E phosphorylation in wild-type mice (Supplementary Fig. 4g–i), although it reduced immobility in the FST in both Eif4e ki/ki and their wild-type littermates (Supplementary Fig. 4f). These results indicate that these treatments do not engage the MNK1/2-eIF4E signaling pathway to induce anti-depressant-like effects.
Fig. 3.
Normal response to Fluoxetine in Eif4e ki/ki mice. a Male wild-type and Mnk1/2−/− mice were treated with saline or fluoxetine (IP, 3 mg kg−1) and tested in the FST after 30 min (n = 7 Mnk1/2+/+Saline, n = 9 Mnk1/2−/− Saline; n = 10 Mnk1/2+/+Fluoxetine, n = 10 Mnk1/2−/− Fluoxetine). b Male Eif4e+/+ and ki/ki were also treated with saline or fluoxetine (IP, 3 mg kg−1) and performed the FST after 30 min (n = 9 Eif4e+/+Saline, n = 8 Eif4e ki/ki Saline; n = 6 Eif4e+/+Fluoxetine, n = 9 Eif4e ki/ki Fluoxetine). c eIF4E phosphorylation in the mPFC/HPC of male wild-type mice treated with saline, 1 dose of fluoxetine (0.5 h, IP, 3 mg kg−1), chronic fluoxetine (14 × , IP, 10 mg kg−1) or repeated citalopram (3 × , IP, 10 mg kg−1) (n = 6/group). d Total eIF4E levels in the same samples as in (d). e Representative western blots for mice treated with vehicle or SSRIs. ** p < 0.01, *** p < 0.001 vs wild-type (see Supplementary Table 2 for detailed results of statistical tests)
We also determined that adult hippocampal progenitor proliferation (Ki67 + cells, Supplementary Fig. 5a, b), survival (BrdU + cells, 28 d after treatment, Supplementary Fig. 5c, d) and differentiation (DCX + cells, Supplementary Fig. 5e, f) was not changed in male and female Eif4e ki/ki mice.
Inhibition of MNK1/2 induces depression-like phenotypes
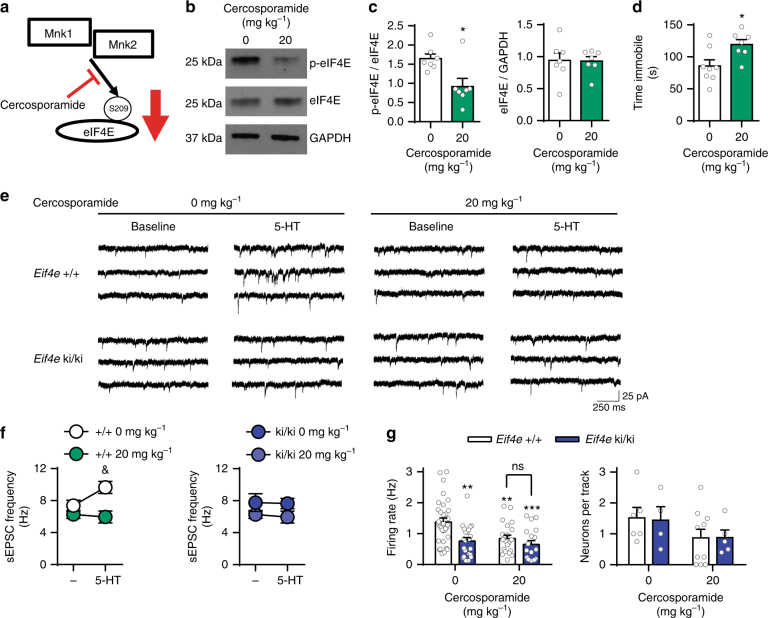
To rule out developmental/compensatory mechanisms in the depression- and anxiety-like behaviors in Mnk1/2−/− and Eif4e ki/ki mice, we examined whether the depression-like behavior could be induced by pharmacological inhibition of MNK1 and 2 in adult wild-type mice using cercosporamide, an inhibitor of MNK1 and MNK240 (Fig. 4a). Systemic administration of cercosporamide (IP, 5 × 20 mg kg−141) decreased eIF4E phosphorylation in the brain by 44.18 ± 14.76 % (Fig. 4b, c), and enhanced immobility in the FST by 33.49 ± 12.66 s (Fig. 4d).
Fig. 4.
Pharmacological inhibition of MNK1/2 induces despair-like behavior and 5-HT synaptic impairments. a Cercosporamide, an MNK1 and MNK2 inhibitor, was administered for 5 consecutive days to wild-type mice (IP, 20 mg kg−1). b Representative western blot images for phosphorylated eIF4E (p-eIF4E), total eIF4E and GAPDH in the cortex-hippocampus of mice treated with vehicle or cercosporamide. c Average levels of p-eIF4E in vehicle and cercosporamide-treated wild-type mice (vehicle (0 mg kg−1), n = 7; cercosporamide, n = 7). d Cercosporamide administration induced increased immobility in the Forced Swim Test (FST) in wild-type mice (vehicle (0 mg kg−1), n = 8; cercosporamide, n = 7). e Representative sEPSC traces from recordings in layer V mPFC from wild-type or Eif4e ki/ki mice treated with vehicle or cercosporamide. f Layer V mPFC sEPSC frequency of wild-type (left panel, vehicle n = 5 neurons from three mice, cercosporamide n = 6 neurons from two mice) or Eif4e ki/ki mice (right panel, vehicle n = 5 neurons from 2 mice, cercosporamide n = 7 neurons from 3 mice) treated with vehicle or cercosporamide. g Firing rate of 5-HT DR neurons in wild-type and Eif4e ki/ki mice treated with vehicle or cercosporamide (Eif4e+/+: Vehicle 33 neurons from 6 mice, cercosporamide 22 neurons from 10 mice; Eif4e ki/ki: vehicle 22 neurons from 4 mice, cercosporamide 18 neurons from 4 mice). * p < 0.05, ** p < 0.01, ***p < 0.001 vs vehicle-treated wild-type mice; & p < 0.05 vs. saline-treated Eif4e+/+ (see Supplementary Table 2 for detailed results of statistical tests)
Furthermore, in wild-type mice cercosporamide also inhibited the excitatory response of 5-HT in layer V mPFC pyramidal neurons (Fig. 4e, f) and decreased the firing rate of 5-HT DR neurons (Fig. 4g). Importantly, cercosporamide failed to change any of these parameters in Eif4e ki/ki mice (Fig. 4e, g), indicating an effect through eIF4E phosphorylation and not via unknown MNK1/2 targets. These results strongly support a direct role of eIF4E phosphorylation in the pathophysiology of depression.
Dysregulated TNFα synthesis in the brain of Eif4e ki/ki mice
To gain insight into the molecular mechanism by which the lack of eIF4E phosphorylation results in depression-like behavior and altered serotonergic neurotransmission, we inspected the list of mRNAs whose translation is significantly reduced in the absence of eIF4E phosphorylation12. Among these, the Nfkbia mRNA, encoding for the negative regulator of NF-κB, IκBα, is of particular interest12,42 because of the prominent role of inflammatory mechanisms in the pathophysiology of MDD and impaired serotonergic neurotransmission19,21. Translation initiation of the Nfkbia mRNA was determined based on its association with polysomes using sucrose density gradients (Fig. 5a). In Eif4e ki/ki mice, the association of Nfkbia mRNA with the heavy polysome fractions is decreased in cortico-hippocampal tissue (including mPFC), consistent with a reduction in the translation of this mRNA in the brain (Fig. 5b). As a control, the distribution of the Actb mRNA was not changed (Fig. 5c), and total levels of Nfkbia mRNA were the same in control versus Eif4e ki/ki mice (Fig. 5d). To demonstrate that the altered polysome profile of the Nfkbia mRNA resulted in reduced amounts of IκBα protein, we used Western blotting to measure its levels in the mPFC. In Eif4e ki/ki and Mnk1/2−/− mice, IκBα levels were decreased (by 50.88 ± 23.11 % and 33.33 ± 12.44 %, respectively) as compared to their corresponding littermate controls (Fig. 5e, f). Taken together, these results demonstrate dysregulated mRNA translation of IκBα in the mPFC upon inhibition of eIF4E phosphorylation.
To investigate the pathophysiological consequence of the decrease in IκBα, we used a multiplex immunoassay to determine the baseline levels of 23 cytokines, which are known to be regulated by IκBα/NF-κB, from the PFC of Mnk1/2−/− and Eif4e ki/ki mice (Supplementary Table 1). Only eotaxin and tumor necrosis factor α (TNFα) appeared increased in the PFC of both, Mnk1/2−/− and Eif4e ki/ki mice. We could not detect a difference in eotaxin levels from independent PFC samples using ELISA (Supplementary Fig. 6a), although it was reduced in plasma samples of Mnk1/2−/− and Eif4e ki/ki mice (Supplementary Fig. 6b). In contrast, TNFα levels were increased in the PFC of Eif4e ki/ki and Mnk1/2−/− mice by 101.9 ± 42.75% and 178.34 ± 55.33 % over controls, respectively (Fig. 5g and Supplementary Fig. 6c). Consistent with transcriptional regulation of Tnf, its mRNA levels were increased in the PFC of Eif4e ki/ki by 104.3 ± 46.83 % over control levels (Fig. 5h). In the circulation, TNFα levels were elevated only in Mnk1/2−/− (by 203.8 ± 91.62 %), but not in Eif4e ki/ki mice (Supplementary Fig. 6d, e). Furthermore, levels of the soluble TNF receptor 1 (sTNFR1) were induced in the serum of Eif4e ki/ki mice (Supplementary Fig. 6f). sTNFR1 stabilizes TNFα and its biological activity, thus promoting inflammation43.
Next, we investigated whether increased TNFα led to neuroinflammation. To this end, we measured the levels of Iba1 (Aif1) mRNA, a marker of microglial activation44. We observed increased levels of Iba1 in the PFC (99.02 ± 33.31 % over control) (Fig. 5i), and in the hippocampus (100.28 ± 34.73 % over control) of Eif4e ki/ki mice (Supplementary Fig. 6g). Next, we challenged Eif4e+/+and ki/ki mice with the immunogen, lipopolysaccharide (LPS, IP, 2.5 mg kg−1,), and analyzed the brains of the mice 48 h later; a timeline whereby acute inflammatory responses have receded, but depression-like behaviors persist45. Eif4e ki/ki mice had more Iba1 + cells in the prefrontal cortex (Fig. 5j, k), and the hippocampus (Supplementary Fig. 6h, i) following both saline and LPS treatments. Furthermore, Il1b mRNA was induced to greater levels in the Eif4e ki/ki mice compared to wild-type counterparts 48 h after LPS stimulation (145.42 ± 47.84% over Eif4e+/+treated with LPS) (Fig. 5l). Taken together, these results demonstrate that inhibition of eIF4E phosphorylation causes reduced translation of the Nfkbia mRNA, leading to increased levels of TNFα expression and an inflammatory state in the brain.
TNFα mediates depression-like phenotypes in Eif4e ki/ki mice
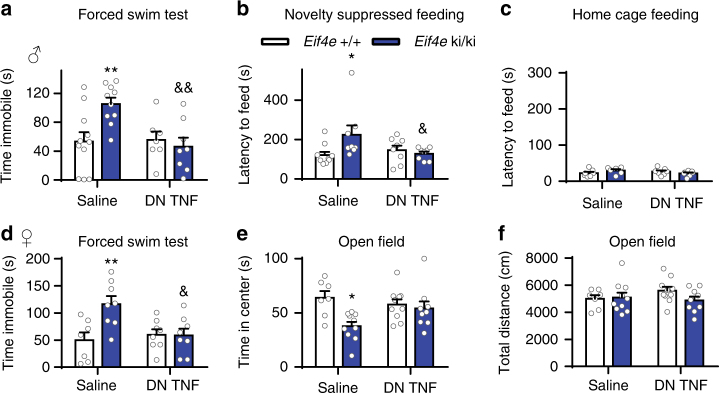
To investigate the contribution of the elevated brain levels of TNFα in Eif4e ki/ki and Mnk1/2−/− mice towards the behavioral and serotonergic alterations, we treated mice with a dominant negative mutant of TNFα (DN TNF, XPro159546). Central administration of DN TNF (ICV, 12 days, 10 mg ml−1 in 100 μl, 0.25 μl h−1) effectively reduced the increased immobility in the FST for the Mnk1/2−/− (Supplementary Fig. 7a) and Eif4e ki/ki mice (Fig. 6a) by 48.78 ± 13.59 and 59.20 ± 16.69 s, respectively, to levels indistinguishable from saline-treated control mice. DN TNF also reduced immobility in female Eif4e ki/ki by 57.59 ± 17.98 s (Fig. 6d). Importantly, the treatment did not affect FST immobility in wild-type littermates (Supplementary Fig. 7a and Fig. 6a, d). Furthermore, centrally administered DN TNF decreased the incremented latency to feed in the NSF test in Mnk1/2−/− (Supplementary Fig. 7b) and Eif4e ki/ki mice (Fig. 6b), by 268.0 ± 75.31 and 95.50 ± 39.72 s, respectively, to levels similar to wild-type controls. The latency to feed in the home cage was not affected by the DN TNF treatment in neither wild-type nor mutant mice (Supplementary Fig. 7c and Fig. 6c). Decreased exploration of the center of the open field was increased in female Eif4e ki/ki mice (Fig. 6e), but not general locomotion (Fig. 6f).
Fig. 6.
TNFα causes behavioral impairments in Eif4e ki/ki mice. a wild-type and Eif4e ki/ki mice were administered (ICV) a dominant negative mutant version of TNFα (DN TNF) or vehicle for 12 days (via an osmotic pump connected to a guide cannula). Male mice were then evaluated in the FST (n = 11 Eif4e+/+ Saline, n = 7 Eif4e+/+ DN TNF, n = 10 Eif4e ki/ki Saline, n = 8 Eif4e ki/ki DN TNF). b Mice were also tested in the novelty suppressed feeding task (n = 10 Eif4e+/+ Saline, n = 8 Eif4e+/+ DN TNF, n = 8 Eif4e ki/ki Saline, n = 8 Eif4e ki/ki DN TNF). c Latency to feed in their home cage was also measured. d Female mice also administered with vehicle or DN TNF (ICV) were evaluated in the FST (n = 7 Eif4e+/+ Saline, n = 8 Eif4e+/+ DN TNF, n = 8 Eif4e ki/ki Saline, n = 8 Eif4e ki/ki DN TNF). e Female mice exploratory behavior was also measured in the center of an open field (OF) (n = 7 Eif4e+/+ Saline, n = 10 Eif4e+/+ DN TNF, n = 10 Eif4e ki/ki Saline, n = 9 Eif4e ki/ki DN TNF). f Their locomotion in the OF was also measured. * p < 0.05, ** p < 0.01, *** p < 0.001 vs. wild-type saline; &, p < 0.05, && p < 0.01, &&& p < 0.001 vs. saline-treated Eif4e+/+or ki/ki (see Supplementary Table 2 for detailed results of statistical tests)
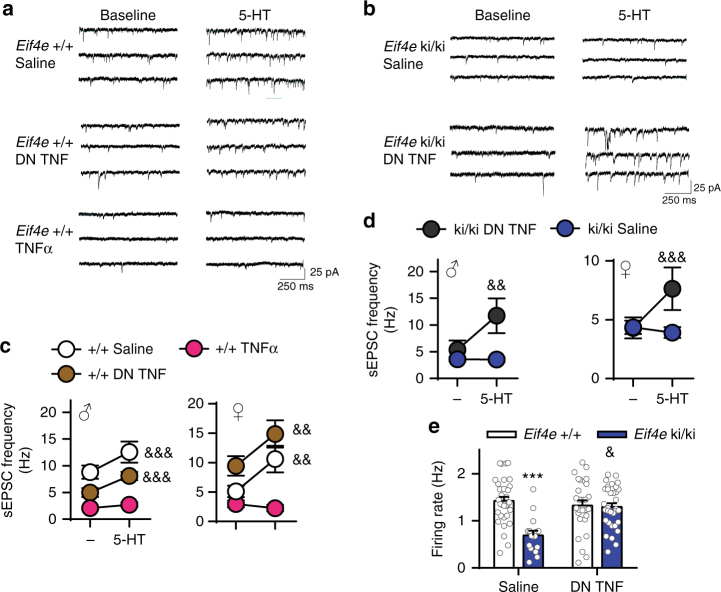
Moreover, 5-HT-induced excitatory synaptic activity in layer V pyramidal neurons was restored in male and female Eif4e ki/ki mice following direct treatment with DN TNF (200 ng ml−1, 1 h incubation) of the mPFC slices (Fig. 7a–c). The same treatment did not change the 5-HT-induced increase in sEPSC frequency (Fig. 7a, c) or amplitude (Supplementary Fig. 2g) in wild-type mice. Finally, in vivo treatment with DN TNF restored the firing rate of the DR neurons in Eif4e ki/ki mice to control levels in vivo (Fig. 7e).
Fig. 7.
TNFα causes serotonergic impairments in Eif4e ki/ki mice. a Representative sEPSC traces from mPFC pyramidal neurons in brain slices of wild-type mice treated with vehicle (saline), DN TNF (200 ng ml−1) or mouse recombinant TNFα (10 ng ml−1). Synaptic currents were recorded before (baseline) and after 5-HT (20 μM) administration. b Representative sEPSC traces from Eif4e ki/ki mice treated with either vehicle or DN TNF (200 ng ml−1), measured before and after 5-HT (20 μM) administration. c Frequency of sEPSCs in mPFC slices of male and female wild-type mice. Slices were directly treated with saline (M: n = 10 cells from 6 mice; F: n = 9 cells from 6 mice), DN TNF (200 ng ml−1; M: n = 8 cells from 5 mice; F: n = 7 cells from 4 mice) or mouse recombinant TNFα (10 ng ml−1; M: n = 9 cells from 6 mice; F: n = 6 cells from 5 mice), pre- and post-5-HT (20 μM) application. d Average sEPSC frequency in Eif4e ki/ki mice before and after 5-HT (20 μM) application; slices were treated with either saline (M: n = 11 cells from 6 mice; F: n = 9 cells from 7 mice) or DN TNF (200 ng ml−1; M: n = 8 cells from 6 mice; F: n = 9 cells from 7 mice). e, Firing rate of DR neurons in wild-type and Eif4e ki/ki mice chronically treated with DN TNF or saline (ICV, 12 days; n = 33 recordings from 6 Eif4e+/+ Saline mice; n = 31 recordings from 5 Eif4e+/+ DN TNF mice; n = 16 recordings from Eif4e ki/ki Saline mice; n = 33 recordings from 6 Eif4e ki/ki DN TNF mice). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. wild-type saline; & p < 0.05, && p < 0.01, &&& p < 0.001 vs. saline-treated Eif4e+/+or ki/ki (see Supplementary Table 2 for detailed results of statistical tests)
To demonstrate that TNFα causes deficits in 5-HT neurotransmission, as it does for behavior47, we determined the 5-HT response of layer V PFC neurons in wild-type mice following direct TNFα treatment to the slices (10 ng ml−1, 1 h). TNFα treatment ameliorated the 5-HT-induced increase in sEPSC frequency in male and female mice (Fig. 7a, c) without affecting their amplitude (Supplementary Fig. 2g). In vivo, an acute treatment with TNFα (ICV, 0.02 fg µl−1) also elicited a rapid decrease in the frequency of spontaneous firing rate of DR neurons in wild-type mice (Fig. 8a–c). These results support the conclusion that over-expression of TNFα is necessary and sufficient to induce depression-like behavior and impaired serotonergic neurotransmission as a consequence of decreased eIF4E phosphorylation.
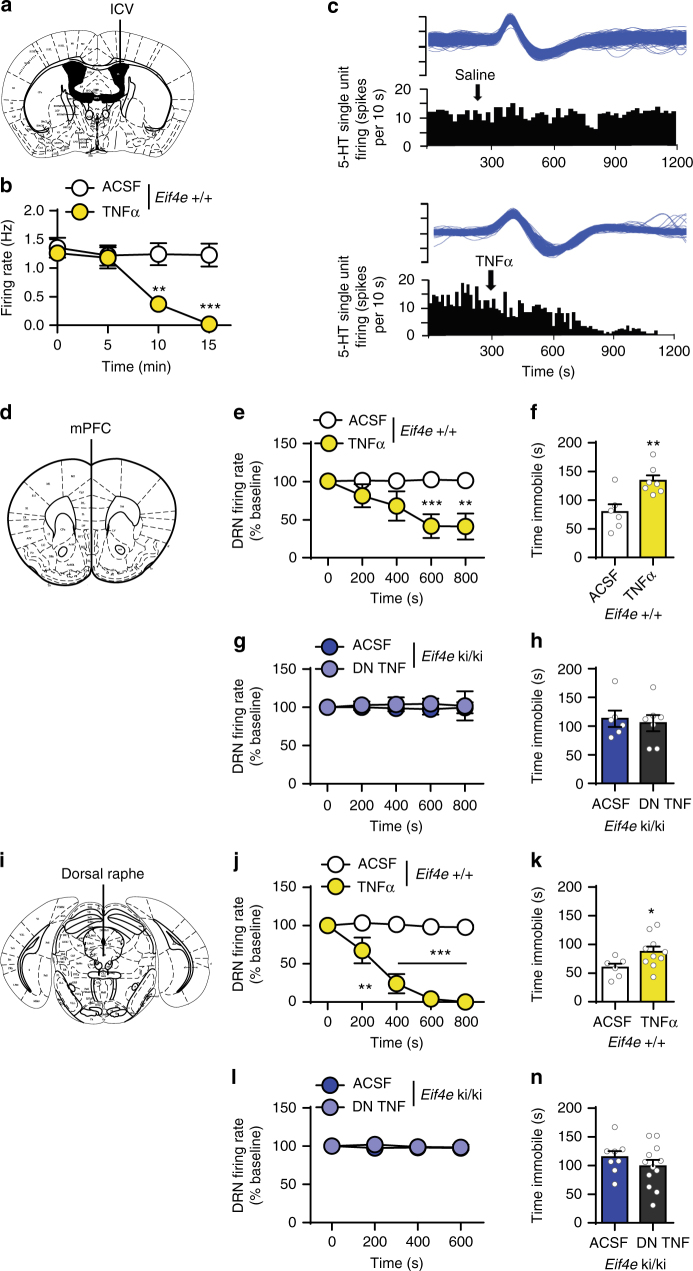
Fig. 8.
Simultaneous inhibition of mPFC and DR by TNFα underlies despair-like behavior. a Acute recombinant mouse TNFα (ICV, 0.02 pg ml−1) into wild-type mice. b Firing rate of DR neurons in wild-type mice treated with either artificial cerebrospinal fluid (ACSF) (n = 4) or mouse recombinant TNFα (n = 6) averaged in 5 minute bins. c Representative integrated firing rate histograms (spikes per 10 s) showing traces of DR neurons in wild-type mice treated with either saline or mouse recombinant TNFα. d Acute treatment of TNFα (0.1 fg in 0.5 µl ACSF) into the mPFC of wild-type mice or DN TNF (100 pg in 0.5 μl ACSF) to Eif4e ki/ki mice. d Firing rate of DRN 5-HT neurons of wild-type mice treated with ACSF (n = 6) or TNFα (n = 6) into the mPFC. e Immobility time in the FST, 20 min after ACSF (n = 6) or TNFα (n = 7) into the mPFC of wild-type mice. f, Firing rate of DRN 5-HT neurons of Eif4e ki/ki mice treated with ACSF (n = 5) or DN TNF (n = 5) into the mPFC. g FST immobility time 20 min after ACSF (n = 6) or DN TNF (n = 7) into the mPFC of Eif4e ki/ki mice. h Similar treatments were performed into the DRN. i 5-HT DR neuron firing rate after ACSF (n = 7) or TNFα (n = 6) in wild-type mice. j Despair-like behavior in wild-type mice treated with ACSF (n = 6) or TNFα (n = 10). k DRN 5-HT neurons in Eif4e ki/ki treated with ACSF (n = 5) or DN TNF (n = 4). l FST immobility in Eif4e ki/ki treated with ACSF (n = 8) or DN TNF (n = 12). * p < 0.05, ** p < 0.01, *** p < 0.001 vs. wild-type ACSF (see Supplementary Table 2 for detailed results of statistical tests)
To better understand the link between TNFα-induced deficits in 5-HT neurotransmission in DR and mPFC, we determined the effects of acute TNFα or DN TNF treatments in the mPFC (Fig. 8d) on despair-like behavior and DR firing. Acute infusion of TNFα in the mPFC of wild-type mice (0.1 fg in 0.5 µl of ACSF) rapidly induced immobility in the FST (Fig. 8f) and a decreased firing of DR 5-HT neurons (Fig. 8e). However, acute DN TNF administration to the mPFC of Eif4e ki/ki mice (100 pg in 0.5 μl of ACSF), which restores the 5-HT-induced sEPSC excitation in the mPFC (Fig. 7b, d), failed to rescue the immobility in the FST (Fig. 8h) and did not influence the DR 5-HT firing (Fig. 8g). Finally, acute TNFα infusion in the DR (Fig. 8i) of wild-type mice, also induced immobility in the FST (Fig. 8k) and decreased DR 5-HT neuronal firing (Fig. 8j). Like treatment to the mPFC, acute DN TNF microinjection to the DR did not rescue the increased FST (Fig. 8n) nor did it diminish the firing rate of DR 5-HT neurons in Eif4e ki/ki mice (Fig. 8l). These results show that in the Eif4e ki/ki mice, TNFα simultaneously targets the mPFC and DR to engender the decrease in function of the bidirectional mPFC-DR circuit48, leading to despair-like behavior.
Discussion
We demonstrated that in mice, a deficit in eIF4E phosphorylation triggers depression and anxiety phenotypes (Figs. 1, 4 and Supplementary Fig. 1), as well as reduced activity of DR neurons and impaired serotonin neurotransmission in the PFC (Figs. 2, 4 and Supplementary Fig. 3 and 4). These effects coincided with a reduction in brain levels of the NF-κB inhibitor, IκBα, leading to over production of TNFα, and hypersensitivity to the sequelae of inflammatory stimuli (Fig. 5 and Supplementary Fig. 6). Brain-wide blocking of TNFα diminished depressive behaviors and rescued the impairment in serotonergic neurotransmission (Figs. 6, 7 and Supplementary Fig. 7). Based on these findings, we offer a model (Supplementary Fig. 8) whereby reduction of eIF4E phosphorylation contributes to the development of depressive symptoms and 5-HT dysfunction by modulating the inflammatory response through the translational control of IκBα expression.
Clinical and preclinical data support a strong link between inflammation and mood disorders. First, the levels of cytokines, including TNFα and IL-6, are increased in MDD patients22,23, as well as in individuals that have suffered childhood trauma and abuse49, a well-documented risk factor for MDD and other mental disorders50. Second, these observations have been replicated in rodent models of depression induced by chronic stress51,52. Third, infection-triggered sickness behaviors, which are similar to symptoms of depression, can be treated with classical antidepressants45,53. Fourth, clinical trials suggest that non-steroidal anti-inflammatory drugs (NSAIDs) are beneficial for depression54, as well as anti-cytokine therapies, including those using blocking antibodies to target TNFα55.
Our findings support the important role that the NF-κB pathway plays in the pathophysiology of depressive phenotypes through the control of serotonergic neurotransmission19. In this regard, chronic stress can also increase the sensitivity of NF-κB activation to inflammatory stimuli in the PFC and hippocampus. This occurs via the glucocorticoid receptor, and can be reversed by MK-80156, an NMDA receptor antagonist with anti-depressant activity like ketamine57. Furthermore, the IκB Kinase (IKK) in the nucleus accumbens (NAc) plays an important role in controlling mood58 and mediating the effects of chronic social stress in synaptic morphology and in the behavioral expression of social defeat59. IKKs lie directly upstream of IκBα and are responsible for its phosphorylation and degradation, leading to NF-κB activation and nuclear translocation60. Activation of NF-κB by chronic stress is also responsible for the stress-induced decrease in neurogenesis in the hippocampus and anhedonia61. Importantly, in humans, it was reported that social stress increases NF-κB signaling in peripheral blood mononuclear cells of healthy subjects62 and this response is exaggerated in depressed patients63.
Targeting the NF-κB signaling pathway for the treatment of depression has the potential drawback of affecting a myriad of immune functions60, as well as impairing normal neuronal physiology and cognition64,65. In contrast, targeting TNFα would be more appealing in light of its role in the pathophysiology of depression, particularly deficits in serotonin neurotransmission21. A meta-analysis of clinical data suggests that blocking antibodies against TNFα are consistently effective in improving mood in patients with chronic inflammatory diseases independently from the outcome of their accompanying physical illness55.
While the current study was being considered for publication, a different research group reported depression-like behaviors and increased inflammatory responses in the Eif4e ki/ki mice66. Behavioral phenotypes in our Eif4e ki/ki mouse testing are consistent with those reported by reference66. In addition, our study demonstrated the involvement of MNK1 and MNK2 as the upstream kinases of eIF4E, and demonstrate the involvement of TNFα as a mediator of serotonergic neurotransmission impairment in the PFC and the DR, as a consequence of decreased eIF4E phosphorylation and attenuated translation of the mRNA encoding IκBα.
In summary, we demonstrated that decreased mRNA translation via inhibition of eIF4E phosphorylation regulates inflammatory responses via IκBα translation and induction of TNFα in mice, eliciting an inflammatory state, deficits in 5-HT neurotransmission and a depressive state that is clinically relevant to MDD.
Methods
Mice
Adult male and female mice (P60-180) were used in these studies. Mnk1/2 double knockout and Eif4e ki/ki mice have been reported11,12. Mice were housed in groups of 2–5 per cage with ad libitum access to food and water (except in experiments involving food deprivation), in temperature (21 °C) and humidity (~55%) controlled rooms, and a 12, h light/dark cycle. All procedures followed the Canadian Council on Animal Care guidelines and were approved by McGill University and Université de Montréal Animal Care Committees.
Behavioral procedures
Mice were handled once 24 h before each experiment. All behavioral experiments were performed between 10:00 am and 3:00 pm. Behaviors (except for the open field) were recorded, stored, and analyzed as MPEG files using an automated behavioral tracking system (Videotrack, View Point Life Science, Montreal, Canada) equipped with infrared lighting-sensitive CCD cameras. The analog signals supplied by the camera were measurements of the luminosity of each point of the image scanned point-by-point and line-by-line at a rate of 25 images per second. The signals were transmitted to the Videotrack system and digitized on 8 bits by digital analog conversion. Before the experiments, animal/image background contrast detection thresholds were calibrated by visual inspection to distinguish different behavioral patterns.
For the forced swim test, mice were placed in a glass beaker (24 cm tall, 14 cm diameter) containing 3 L of water at 24 ± 2°C. Mice were allowed to swim for 6 minutes. Immobility was quantified in the last 4 minutes of the test using the Videotrack system.
For the tail suspension test, mice were suspended by their tail from a lever in a 30 × 30 × 30 cm white-painted enclosure. Movements were recorded and quantified for 5 min in a dimly lit environment.
For the novelty suppressed feeding, mice were food-deprived for 48 h, then each mouse was placed in a brightly illuminated (100 W, 350 lx) open arena (40 × 40 white-painted floor with white walls 30 cm in height) containing lab chow (3 pellets) placed at the center of the arena. The latency to initiate feeding (in seconds) was noted and used as an index of anxiety/depression-like behavior. The cut-off time was 600 s. Feeding latency was also observed in the home cage containing 3 pellets spread on the floor. The session was terminated immediately after mice had initiated feeding.
Locomotor activity and time spent in the center of an open field were quantified for 10 min with an infrared activity-monitoring acrylic apparatus with activity sensors located on the sides, front, and back of the boxes (VersaMax system, AccuScan Instruments, Columbus, OH, U.S.A.). Mnk1/2−/− and wild-type littermates were tested twice in the apparatus, with the second test occurring 1 week after the initial test.
Anti-depressant treatments
Fluoxetine hydrochloride (Tocris Bioscience, Oakville ON, Canada), escitalopram oxalate (Tocris Biosceicne) and ketamine (Narketan 10, Vetoquinol, Lavaltrie, QC, Canada) were diluted in 0.9% NaCl solution and administered IP as follows: acute fluoxetine, 1 dose of 3 mg kg‒1 (behavioral testing/tissue collection occurred 0.5 h after injection); chronic fluoxetine, 14 doses (daily) of 10 mg kg‒1 (tissue collection occurred 0.5 h after last injection); repeated citalopram 3 doses of 10 mg kg‒1 (24, 5 and 0.5 h before tissue collection); ketamine (10 mg kg‒1) 1 or 24 h before behavioral testing/tissue collection or mPFC whole-cell recordings.
Cercosporamide treatment
Intraperitoneal (i.p.) injections were carried out using an aqueous solution of 4% Tween 80 (Sigma-Aldrich) and 4% PEG-400 (Sigma-Aldrich). Cercosporamide was administered daily by i.p. injections for five consecutive days at 20 mg kg‒1 to 8 week-old C57BL/6 J male mice (Jackson Laboratory). Mice were tested in the FST 1 h after the last injection and immediately sacrificed for brain collection. Other cohorts of wild-type and Eif4e ki/ki mice were treated likewise and used for layer V mPFC whole-cell recordings or in vivo DR recordings, 1 h after the last injection.
Mouse serum and brain collection
Naive mice (Mnk1/2−/−, Eif4e ki/ki and their respective wild-type littermates) were briefly anesthetized using 5% isofluorane. Blood was collected by heart puncture and allowed to clot for 10 min at 4 °C. Serum was separated by centrifugation (10 min, 4 °C, 5000 × g) and stored at −80 °C. Brains were harvested immediately after heart puncture, prefrontal cortex and hippocampus dissected, flash frozen and kept at −80 °C until used.
In a separate experiment, Eif4e ki/ki and wild-type littermates were treated with saline (IP, 10 ml kg−1) or LPS (E. Coli 0111:B4, 2.5 mg kg−1 45, Sigma-Aldrich, St Louis MO, U.S.A.) and sacrificed 48 h later45. At that point, brains were harvested immediately, prefrontal cortex and hippocampus dissected, flash frozen and kept at −80 °C until used or processed for immunohistochemical analyses.
Polysome profiling
Polysome profiling was performed as previously reported67. Briefly, hippocampi and prefrontal cortex were dissected and snap frozen, then homogenized in 425 μl of hypotonic buffer [(5 mM Tris-HCl (pH 7.5), 2.5 mM MgCl2, 1.5 mM KCl and 1 × protease inhibitor cocktail (EDTA-free)], with 5 μl of 10 mg/ml cycloheximide, 1 μl of 1 M DTT, and 100 units of RNAse inhibitor. After homogenization, 25 μl of 10 % Triton X-100 (final concentration 0.5 %) and 25 μl of 10 % Sodium Deoxycholate (final concentration 0.5 %) were added. Following a 10 min incubation at 4 °C, samples were centrifuged (16,000 × g for 7 min at 4 °C). After adjusting for RNA concentration, the supernatant was applied to a 5–50% sucrose gradient and centrifuged at 222,228 × g (36,000 rpm), for 2 hr at 4 °C. Gradients were fractionated using an automated collector (Teledyne ISCO Foxy R1 Fraction Collector, Cole Palmer) connected to a syringe pump (Brandel) and a UV detector (Teledyne ISCO UA, 6 UV/VIS Detector). RNA was extracted from the collected fractions, along with an input sample, using TRIzol reagent (Ambion by Thermo Fisher Scientific). cDNA was then synthesized using Super Script III reverse transcriptase (Invitrogen by Thermo Scientific) using 250 ng of RNA as template and then Nfkiba and Actb mouse specific TaqMan assays (Applied Biosystems by Thermo Fisher Scientific). mRNA levels were estimated using the ΔΔCt method.
Bio-Plex Immunoassay
Flash-frozen prefrontal cortex from Eif4e ki/ki, Mnk1/2−/− and their respective wild-type littermate control mice were lysed using the Bio-Plex Cell Lysis Kit (Bio-Rad, Mississauga, ON, Canada) following the manufacturer’s instructions for tissue samples. Samples were assayed in the Bio-Plex Pro mouse cytokine 23-plex assay following the manufacturer’s instructions. The plate was read in the Bio-Plex 200 plate reader.
ELISA
Serum or prefrontal cortex lysate (using the Bio-Plex Cell Lysis Kit) were assayed for eotaxin or TNFα using Quantikine ELISAs for mouse eotaxine or TNFα (R&D Systems, Minneapolis, MN, U.S.A.) following the manufacturer’s instructions. Soluble TNF receptor 1 (sTNFRI) was also measured in mouse serum (Quantikine ELISA, R&D Systems, Minneapolis, MN, U.S.A.)
Real-time qPCR
Flash-frozen prefrontal cortex and hippocampus were processed for RNA extraction using TRIzol reagent (Ambion by Thermo Fisher Scientific). cDNA was then synthesized using Super Script III reverse transcriptase (Invitrogen by Thermo Scientific) using 500 ng of total RNA as template and then Tnf, Il1b, Iba1 (Aif1) and Actb mouse specific TaqMan assays (Applied Biosystems by Thermo Fisher Scientific). mRNA levels were estimated using the ΔΔCt method.
Western Blotting
Mouse tissues were dissociated using Bio-Plex Cell Lysis Kit (Bio-Rad, Mississauga, ON, Canada). Western blotting was performed as previously described68. Antibodies against indicated proteins were: phospho-eIF4E (Ser209, Abcam, 1:1000), eIF4E (BD Biosciences, 1:1000), IκBα (Cell Signaling Technology, 1:1000), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, coupled to Horseradish Peroxidase, Abcam, 1:5000) or β-actin (Sigma, 1:5000); secondary antibodies were anti-mouse and anti-rabbit (GE Healthcare). Quantification of immunoblots was performed using ImageJ (NIH), and expressed as a ratio (either p-eIF4E/eIF4E or eIF4E/GAPDH or eIF4E/β-actin, IκBα/GAPDH). Western blots experiments were replicated at least two times. Uncropped western blots are provided in Supplementary Figure 9.
Immunohistochemistry
For the analysis of neuroinflammation markers, coronal brain sections were thin cut (50 μm) using a Cryostat and placed in PBS containing 2 mM sodium azide and 3 mM NaF, pH 7.4. For the immunohistochemical staining, sections were first treated with 0.3% H2O2 and 20% methanol in PBS for 10 min to deactivate endogenous peroxidases and permeabilize the tissue, and then blocked for 1 h in 10% goat serum/PBS and incubated (overnight, 4 °C) in rabbit anti-IBA1 antibody (1:1000 final dilution). Next, tissue was incubated for 1.5 h at room temperature in biotinylated anti-rabbit IgG (1:200; Vector Laboratories, Burlingame, CA) and then placed in an avidin/biotin HRP complex for 1 h (prepared per instructions of the manufacturer; Vector Laboratories). Sections were washed in PBS (three times, 10 min/wash) between each labeling step. The signal was visualized using nickel-intensified DAB substrate (Vector Laboratories) and sections were mounted on gelatin-coated slides with Permount media (Fisher Scientific, Houston, TX).
Bright-field microscopy images were captured using a digital camera mounted on an inverted Zeiss microscope (Oberkochen, Germany). All data were quantified using Adobe Photoshop software (Adobe Systems Incorporated, San Jose, CA). For cell counting, an intensity threshold filter was initially applied to eliminate nonspecific background labeling, and then the number of detectable signals above the threshold (now defined as positive cells) were counted on the 100 × micrographs for the hippocampal CA1 area and the prefrontal cortex. A mean value for each animal was generated from 2 brain sections per animal; this value was then used to generate the group mean.
For the quantification of 5-HT and total number of DR neurons, coronal DR sections were fixed in 2% paraformaldehyde (10 min, RT) washed in PBS, and then incubated in 10% normal donkey serum (NDS) and 0.2% Triton X (1 h, RT). Then, primary antibodies were applied (in 10% NDS, 4 C for 48 h). Antibodies were: polyclonal rabbit anti-TPH2 (Novus Biologicals, 1:1000) and monoclonal mouse anti-NeuN (Millipore, 1:150). After washing, avidin/biotin blocking was performed (Avidin/Biotin blocking kit, Vector Laboratories), then slices were incubated with Cy3 donkey anti-rabbit (Linaris, 1:400) and biotinylated horse anti-mouse (Biozol, 1:2000, for 2 h, at RT. Following a washing step, FITC-avidin was applied (1:300, 1 h, RT), then DAPI staining performed (1:8000, 10 min, RT) and sections were cover slipped with Citifluor (Citifluor Ltd.). For image capture and analysis, a conventional light/fluorescent Olympus BX50 microscope (Olympus Optical, Hamburg, Germany) with a black and white Spot Insight camera (Diagnostic Instruments, Visitron systems, Puchheim, Germany) was used. By means of image editing software (MetaMorph 5.05) the individual images were combined into red/green/blue color figure plates and the counting tool was applied for the 4 areas as indicated; brightness and contrast were adjusted, and the images stored as TIFF files (Adobe Photoshop 5.05). All sections were processed the same way to enable comparison.
For analysis of neurogenesis markers, immunohistochemistry was conducted on 50 µm thick free-floating sections cut on a freezing sliding microtome. Every 8th section was sampled for staining through the entire septo-temporal axis of the hippocampus.
The following pre-treatment were used prior to immunohistochemistry: for BrdU staining, sections were first incubated in 2 N HCl at 37 °C for 30 min to denature the DNA; for Ki67, antigen retrieval was performed with heated citrate buffer (pH = 6.0) in a steamer (95–100 °C) for 15 min, followed by a cool down at room temperature for 30 additional minutes; no pre-treatment was required for DCX staining.
For all markers, endogenous peroxidase was quenched in 3% H2O2 in PBS, followed by blocking (PBS/0.2% Triton x-100/2% normal serum from secondary antibodies host species) and overnight incubation in primary antibody solution diluted in blocking buffer at 4 °C (rat anti-BrdU 1:1000, Serotec; mouse anti-ki67 1:2000, Pharmingen; goat anti-DCX 1:1000, Santa Cruz Biotechnology). Sections were incubated for 1 hr at RT with biotinylated secondary antibodies at 1:200 (goat anti-rat, horse anti-mouse and horse anti-goat, respectively; Vector Laboratories), and the reaction was visualized with DAB after avidin-biotin peroxidase complex amplification (Vectastain, Vector Laboratories). Sections were then mounted on slides, left to dry overnight, dehydrated and coverslipped for stereological analysis.
Cell counting and density measurements were performed with an Olympus BX51 microscope under a ×40 objective lens and using Stereo Investigator software (MBF Bioscience). For each marker and in every 8th section, all positive cells within the mounted thickness of the granule cell layer of the dentate gyrus were counted blind to the experimental group. For each section, counts were divided by the area of the dentate gyrus to yield cellular density. Data presented correspond to the average of 9 sections per animal.
In vivo extracellular recording
In vivo single-unit extracellular recording electrophysiology experiments were conducted as previously37. Mice were anesthetized with chloral hydrate (400 mg kg−1 i.p., using a 2% solution) and placed in a stereotaxic frame (using the David Kopf mouse adaptor) with the skull positioned horizontally. To maintain a full anesthetic state in which there was no reaction to a tail or paw pinch, chloral hydrate supplements of 100 mg kg−1 were given as needed. Extracellular single-unit recordings were performed using single-barreled glass micropipettes pulled from 2 mm capillary glass (Stoelting, Wood Dale, IL, U.S.A.) on a PE-21 pipette puller (Narashige, Tokyo, Japan) and preloaded with fiberglass strands to promote capillary filling with 2 % Pontamine Sky Blue dye in 2 M NaCl for Dorsal Raphe Nuclei neuronal recordings. The micropipette tips were broken down to diameters of 1–3 μm to reach an electrode impedance of 2–6 MΩ.
For microinjection in the DRN, a cannula (33-gauge stainless steel tubing) was stereotaxically lowered using the following coordinates: AP: −3.42 mm from bregma; ML: ± 0.0 mm, DV: −1.97 mm from dura, 30° angle. For microinjections in the mPFC, a similar cannula was lowered at: AP: + 1.7 mm; ML: ± 2.0 mm; DV: −2.0 mm, 30° angle. Vehicle (ACSF: 2.5 mM KCl; 125 mM NaCl; 1.18 mM MgCl2; 1.26 mM CaCl2), TNFα (0.1 fg in 0.5 µl ACSF) or DN TNF (200 ng ml−1 in 0.5 ul) solutions were used to fill the cannula. The microinjection was made using a 5 μl Hamilton syringe that was connected to the injection cannula by a length of polyethylene (PE-10) tubing over a period of 60 s and the injection cannula was gently removed 2 min later.
The single-barreled glass micropipettes were positioned 0.5 to 1 mm posterior to the interaural line on the midline and lowered into the DRN, attained at a depth of between 2.5 and 3.5 mm from the brain surface. The DRN 5-HT neurons were then identified according to the following criteria: a slow (0.5–2.5 Hz) and regular firing rate with a long duration (0.8–1.2 ms) and positive action potentials. Single-unit activity was recorded as large-amplitude action potentials captured by a software window discriminator, amplified by an AC Differential MDA, 3 amplifier (BAK electronics, Umatilla, FL, U.S.A.), post-amplified and band-pass filtered by a Realistic 10 band frequency equalizer, digitized by a CED 1401 interface system (Cambridge Electronic Design, Cambridge, U.K.), processed online, and analyzed off-line using Spike2 software version 5.20 for Windows. The spontaneous single-spike activity of neurons was recorded for at least 2 min and 30 s immediately after detection to eliminate mechanical artifacts due to electrode displacement. The total number of spontaneously active 5-HT neurons and their firing rates were determined by monitoring their average discharge frequency for a minimum period of 1 min. The number of neurons recorded per electrode descent was also calculated, since the number of neurons recorded is a valid, indirect index of the percentage of neurons that are spontaneously discharging (active) during in vivo electrophysiological recordings69.
Intracerebroventricular (ICV) TNFα injection
For ICV implantation, mice were anesthetized with chloral hydrate and placed in a stereotaxic frame with the skull positioned horizontally. Burr holes were drilled in the skull on the side over one of the lateral ventricle and a guide stainless steel cannula (26 gauge) was stereotaxically lowered 2.5 mm using the following coordinates: AP: −0.2 mm from bregma, L: ± 0.9 from the midline and V: −2.5 mm from cortical surface. The cannula was fixed into the skull using dental screws and cement. A vehicle (ACSF: 2.5 mM KCl; 125 mM NaCl; 1.18 mM MgCl2; 1.26 mM CaCl2) or recombinant mouse TNFα (R&D System) (0.1 fg in 5 µl ACSF) solution prefilled inner cannula (33-gauge stainless steel tubing) was inserted and driven 0.5 mm below the tip of the guide. The microinjection was made using a 5 μl Hamilton syringe that was connected to the injection cannula by a length of polyethylene (PE-10) tubing over a period of 60 s and the injection cannula was gently removed 2 min later.
Whole cell recordings in PFC slices in vitro
Eif4e ki/ki and wild-type littermate mice (6–8-week-old) received intraperitoneal injections of ketamine (IP, 10 mg kg‒1) or saline 24 h before whole cell recording experiments. Serotonin creatine sulfate monohydrate (5-HT; 20, 50 or 100 µM; from Sigma) was freshly prepared in aCSF. Recombinant mouse TNF-α (R&D Systems) was dissolved in phosphate buffer containing 0.1% bovine serum albumin as stock solution (1000 × final concentration) and kept at ‒20 °C. The dominant-negative (DN) TNF, XPRO1595 (from Xencor), was freshly prepared in phosphate buffer. DN-TNF (200 ng ml‒1) and TNF-α (10 ng ml‒1) were freshly prepared in aCSF and slices were pre-incubated for 60 min before recording.
For brain slice preparation, Eif4e ki/ki and wild-type littermate mice (6 to 8-week-old) were anesthetized with isoflurane and the brain was removed and placed in ice-cold oxygenated (95% O2 / 5% CO2) cutting solution containing 87 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4, 7 mM MgSO4, 25 mM NaHCO3, 25 mM D-glucose, 75 mM sucrose, 1 mM ascorbic acid, 3 mM pyruvic acid and 0.5 mM CaCl2. Coronal slices (300 μm thickness) of medial prefrontal cortex were prepared using a Vibratome (Leica; VT1000S) and transferred to oxygenated artificial cerebrospinal fluid (aCSF) containing 124 mM NaCl, 5 mM KCl, 1.25 mM NaH2PO4, 2 mM MgSO4, 2 mM CaCl2, 26 mM NaHCO3 and 10 mM dextrose (pH = 7.3–7.4; 295-300 mOsmol) at room temperature. After a recovery period of at least 60 min, individual slices were transferred to a submersion recording chamber mounted on an upright microscope (Zeiss, Oberkochen, Germany) equipped with a long-range water immersion objective (40 ×) with Hoffmann optics (Modulation Optics, Greenvale, NY) and an infrared CCD camera, and perfused at 2.5 ml min‒1 with aCSF at 32 °C.
Recordings were obtained from layer V pyramidal cells under visual guidance using patch pipettes (3-5 MΩ) pulled from borosilicate glass capillaries (World Precision Instruments, Sarasota, USA) and filled with solution containing 120 mM CsMeSO3, 5 mM NaCl, 1 mM MgCl2, 10 mM di-Na-phosphocreatine, 10 mM HEPES, 2 mM ATP, 0.5 mM GTP, 2 mM QX314, 0.5 mM spermine and 0.3 % biocytin (pH 7.3; 285-295 mOsmol). Excitatory postsynaptic currents (EPSCs) were recorded in voltage-clamp mode using a Multiclamp 700 A amplifier (Molecular Devices) and digitized using Digidata 1440 A and pClamp10 software (Molecular Devices). Recordings were low-pass filtered at 2 kHz, digitized at 20 kHz and stored on a PC. Series resistance (15–25 MΩ) was regularly monitored during experiments and only cells with series resistance changes less than 20 % of initial value and stable holding current were included. Spontaneous EPSCs were recorded at −70 mV holding potential before, during, and after bath application of 5-HT (20 µM; 3 min application), and analyzed offline with Mini Analysis Version 6.0.3 software (Synaptosoft Inc., Decatur, GA, USA)70. The detection threshold of spontaneous EPSCs was set at twice baseline noise level and individual events were confirmed by visual inspection. Only cells that exhibited stable sEPSC frequency and amplitude over a 10 min control period were kept and only one cell was recorded per slice. After completion of recordings, slices were transferred to 4% paraformaldehyde (0.1 M phosphate buffer) and stored overnight at 4 °C. Slices were then processed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) and observed under a light microscope. Experimenter was blind to the mice genotype and codes were broken after data analysis.
Statistical analyses
Data were expressed as mean ± S.E.M. and analyzed using GraphPad Prism 6 (GraphPad Software Inc.). Statistical results are detailed in Supplementary Table 2. When comparing two data sets, (Figs. 2d, i, 4c, d, 5d, i, 8f, h, k, n, Supplementary Figs. 1a, b, 3c, d, 6a–g) unpaired Student’s t-test were performed; when variance was significantly different between groups, Welch’s correction was used. One-way ANOVA was used for data in Fig. 3c, d. Data sets depicted in Figs. 1a–j, 3a, b, 4g, 5j, l, 6a–f, 7e, Supplementary Figs. 3a, 4f–h, 5a, 5c, 5e, 6h, and 7a–c were analyzed using two-way ANOVA, with genotype and gender (Fig. 1a, j, Supplementary Figs. 5a, c, e) or treatment (Figs. 3a, b, 4g, 5j, l, 6a–f, 7e, Supplementary Figs. 4f–h, 6h, 7a–c) as independent factors; when interaction was significant, Bonferroni pair-wise comparisons were used. Data from Figs. 2b, 4f, 7c, d, 8b, 8e, g, j, l and Supplementary Figs. 1c–f, 2a–c, 2f, g, 3a, b and 4b–e were analyzed using mixed-design two-way ANOVA with genotype (Fig. 2b, Supplementary Figs. 1c–f, 2a–c, 3a, b and 4b–e) or treatment (Figs. 4f, 7c, d, 8b, e, g, j, l, and Supplementary Figs. 2f, g) as independent factor, and pre- post- 5-HT (Figs. 2b, 4f, I, j, Supplementary Figs. 2a–c, 2f, g and 4b–e), time after treatment (Figs. 8b, d, f, i, k), testing session (Supplementary Figs. 1c–f) or drug dose (Supplementary Fig. 3a, b) as repeated measure; Bonferroni was used for pair-wise comparisons when interaction was significant. Values of p < 0.05 were considered significant.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electronic supplementary material
Acknowledgements
We thank David E. Szymkowski (Xencor, Inc., Monrovia, CA) for the generous donation of XPro1595; Jeremy Graff (Eli Lilly, Indianapolis, IN) for the generous donation of cercosporamide, and Annie Sylvestre, Annik Lafrance, Isabelle Harvey, Isabel Laplante, Luca Posa, Ada Posner, Grace Ling, Joshua Byer, Doreen Marks and Hansen Tsui for technical assistance. We thank Prof. Joseph Rochford for his help in data analysis. A.A.-V. was recipient of FRQS (Fond Recherche Québec-Santé) and CIHR (Canadian Institutes for Health Research) postdoctoral fellowships. D.D.G. was a recipient of FQRNT (Fonds de Recherche du Québec – Nature et technologies) postdoctoral fellowship. N.N. was supported by the Quebec Network on Suicide, Mood Disorders and Related Disorders. J.-C.L. is the recipient of the Canada Research Chair in Cellular and Molecular Neurophysiology. This work was supported by a CIHR Foundation grant to N.S. (FND-148423), a CIHR operating grant to J.-C.L. (MOP-125985), and a Fonds de la Recherche du Québec - Santé (FRQS) Group grant (Groupe de Recherche sur le Système Nerveux Central [GRSNC]) to J.-C.L.
Author contributions
A.A-V. designed the study, performed experiments, analyzed data and wrote the manuscript. N.H. and M.J.E. performed whole-cell electrophysiology experiments, analyzed data, and contributed to writing the manucript. D.D.G. performed in vivo electrophysiology experiments, analyzed data, and contributed to writing the manucript. E.M-C. performed polysome experiments, and edited the manuscript. J.P. performed western blot experiments and analyzed data. V.S. perfromed mouse cannulations. R.C. performed immunohistochemical stainings and quantifcation, and contributed to writing the manuscript. C.R., A.T., and N.M. performed immunohistochemical analysis. S.W. performed and assisted in some behavioral and western blot experiments, and edited the manuscript. S.C. assisted in some behavioral experiments, and edited the manuscript. N.N., G.T. and R.N. contributed to data analysis. G.L. participated in LPS-treatment experiments. J-C.L., G.G., and N.S. supervised students, designed experiments and contributed to the writing of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors jointly supervised this work: Jean-Claude Lacaille, Gabriella Gobbi, Nahum Sonenberg.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-04883-5.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Global Burden of Disease Study, C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kessler RC, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Dwivedi Y, et al. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J. Neurochem. 2001;77:916–928. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Fuster MJ, et al. FADD adaptor and PEA-15/ERK1/2 partners in major depression and schizophrenia postmortem brains: basal contents and effects of psychotropic treatments. Neuroscience. 2014;277:541–551. doi: 10.1016/j.neuroscience.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Topisirovic I, Sonenberg N. Translational control by the eukaryotic ribosome. Cell. 2011;145:333–334. doi: 10.1016/j.cell.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 8.Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi B, et al. Phosphorylation of eukaryotic protein synthesis initiation factor 4E at Ser-209. J. Biol. Chem. 1995;270:14597–14603. doi: 10.1074/jbc.270.24.14597. [DOI] [PubMed] [Google Scholar]
- 10.Flynn A, Proud CG. Serine 209, not serine 53, is the major site of phosphorylation in initiation factor eIF-4E in serum-treated Chinese hamster ovary cells. J. Biol. Chem. 1995;270:21684–21688. doi: 10.1074/jbc.270.37.21684. [DOI] [PubMed] [Google Scholar]
- 11.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol. Cell. Biol. 2004;24:6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furic L, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc. Natl Acad. Sci. USA. 2010;107:14134–14139. doi: 10.1073/pnas.1005320107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bramham CR, Jensen KB, Proud CG. Tuning specific translation in cancer metastasis and synaptic memory: control at the MNK-eIF4E Axis. Trends Biochem Sci. 2016;41:847–858. doi: 10.1016/j.tibs.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Cao R, et al. Light-regulated translational control of circadian behavior by eIF4E phosphorylation. Nat. Neurosci. 2015;18:855–862. doi: 10.1038/nn.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol. Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Gourley SL, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol. Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Manji HK. The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers. Curr. Opin. Psychiatry. 2006;19:313–323. doi: 10.1097/01.yco.0000218604.63463.cd. [DOI] [PubMed] [Google Scholar]
- 18.Rao JS, et al. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol. Psychiatry. 2007;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 19.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruchas MR, et al. Selective p38alpha MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AH, Haroon E, Felger JC. Therapeutic implications of brain-immune interactions: treatment in translation. Neuropsychopharmacology. 2017;42:334–359. doi: 10.1038/npp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol. Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry. 2016;21:1696–1709. doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres-Platas SG, Cruceanu C, Chen GG, Turecki G, Mechawar N. Evidence for increased microglial priming and macrophage recruitment in the dorsal anterior cingulate white matter of depressed suicides. Brain Behav. Immun. 2014;42:50–59. doi: 10.1016/j.bbi.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Mayorga AJ, Lucki I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacol. (Berl.) 2001;155:110–112. doi: 10.1007/s002130100687. [DOI] [PubMed] [Google Scholar]
- 26.Samuels, B. A. & Hen, R. Mood and anxiety related phenotypes in mice: characterization using behavioral tests Vol. II (ed Todd D. Gould) 107–121 (Humana Press, New York, 2011).
- 27.Bodnoff SR, Suranyi-Cadotte B, Quirion R, Meaney MJ. A comparison of the effects of diazepam versus several typical and atypical anti-depressant drugs in an animal model of anxiety. Psychopharmacol. (Berl.) 1989;97:277–279. doi: 10.1007/BF00442264. [DOI] [PubMed] [Google Scholar]
- 28.Rajkowska G, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol. Psychiatry. 1999;45:1085–1098. doi: 10.1016/S0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 29.Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev. Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- 30.Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog. Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- 31.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc. Natl Acad. Sci. USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puig MV, Celada P, Diaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb. Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- 33.Beique JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc. Natl Acad. Sci. USA. 2007;104:9870–9875. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avesar D, Gulledge AT. Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits. 2012;6:12. doi: 10.3389/fncir.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parent A, Descarries L, Beaudet A. Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience. 1981;6:115–138. doi: 10.1016/0306-4522(81)90050-6. [DOI] [PubMed] [Google Scholar]
- 37.Gobbi G, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc. Natl Acad. Sci. USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall H, et al. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]way-100635. Brain Res. 1997;745:96–108. doi: 10.1016/S0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- 39.Bambico FR, Nguyen NT, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur. Neuropsychopharmacol. 2009;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Konicek BW, et al. Therapeutic inhibition of MAP kinase interacting kinase blocks eukaryotic initiation factor 4E phosphorylation and suppresses outgrowth of experimental lung metastases. Cancer Res. 2011;71:1849–1857. doi: 10.1158/0008-5472.CAN-10-3298. [DOI] [PubMed] [Google Scholar]
- 41.Gkogkas CG, et al. Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile X syndrome-like phenotypes. Cell Rep. 2014;9:1742–1755. doi: 10.1016/j.celrep.2014.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herdy B, et al. Translational control of the activation of transcription factor NF-kappaB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat. Immunol. 2012;13:543–550. doi: 10.1038/ni.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aderka D, Engelmann H, Maor Y, Brakebusch C, Wallach D. Stabilization of the bioactivity of tumor necrosis factor by its soluble receptors. J. Exp. Med. 1992;175:323–329. doi: 10.1084/jem.175.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korzhevskii DE, Kirik OVBrain. Microglia and microglial markers. Neurosci. Behav. Physiol. 2016;46:284–290. doi: 10.1007/s11055-016-0231-z. [DOI] [Google Scholar]
- 45.Aguilar-Valles A, Kim J, Jung S, Woodside B, Luheshi GN. Role of brain transmigrating neutrophils in depression-like behavior during systemic infection. Mol. Psychiatry. 2014;19:599–606. doi: 10.1038/mp.2013.137. [DOI] [PubMed] [Google Scholar]
- 46.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues AL. Depressive-like behavior induced by tumor necrosis factor-alpha in mice. Neuropharmacology. 2012;62:419–426. doi: 10.1016/j.neuropharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Warden MR, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol. Psychiatry. 2016;21:642–649. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am. J. Psychiatry. 2012;169:141–151. doi: 10.1176/appi.ajp.2011.11020335. [DOI] [PubMed] [Google Scholar]
- 51.Kreisel T, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 52.Hodes GE, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc. Natl Acad. Sci. USA. 2014;111:16136–16141. doi: 10.1073/pnas.1415191111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Connor JC, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohler O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 55.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry. 2016;23:335–343. doi: 10.1038/mp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Munhoz CD, et al. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J. Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christoffel DJ, et al. Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology. 2012;37:2615–2623. doi: 10.1038/npp.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christoffel DJ, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 61.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl Acad. Sci. USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bierhaus A, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl Acad. Sci. USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pace TW, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 64.Boersma MCH, et al. A requirement for NF-κB in developmental and plasticity-associated synaptogenesis. J. Neurosci. 2011;31:5414–5425. doi: 10.1523/JNEUROSCI.2456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 66.Amorim IS, et al. Loss of eIF4E phosphorylation engenders depression-like behaviors via selective mRNA translation. J. Neurosci. 2018;38:2118–2133. doi: 10.1523/JNEUROSCI.2673-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gandin, V. et al. Polysome fractionation and analysis of mammalian translatomes on a genome-wide scale. J. Vis. Exp.87, 51455 (2014). [DOI] [PMC free article] [PubMed]
- 68.Bidinosti M, et al. Postnatal deamidation of 4E-BP2 in brain enhances its association with raptor and alters kinetics of excitatory synaptic transmission. Mol. Cell. 2010;37:797–808. doi: 10.1016/j.molcel.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J. Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haji N, et al. TNF-alpha-mediated anxiety in a mouse model of multiple sclerosis. Exp. Neurol. 2012;237:296–303. doi: 10.1016/j.expneurol.2012.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.