Abstract
Purpose
This phase III trial evaluated the efficacy and safety of pazopanib versus placebo in patients with locally advanced renal cell carcinoma (RCC) at high risk for relapse after nephrectomy.
Patients and Methods
A total of 1,538 patients with resected pT2 (high grade) or ≥ pT3, including N1, clear cell RCC were randomly assigned to pazopanib or placebo for 1 year; 403 patients received a starting dose of 800 mg or placebo. To address toxicity attrition, the 800-mg starting dose was lowered to 600 mg, and the primary end point analysis was changed to disease-free survival (DFS) for pazopanib 600 mg versus placebo (n = 1,135). Primary analysis was performed after 350 DFS events in the intent-to-treat (ITT) pazopanib 600 mg group (ITT600mg), and DFS follow-up analysis was performed 12 months later. Secondary end point analyses included DFS with ITT pazopanib 800 mg (ITT800mg) and safety.
Results
The primary analysis results of DFS ITT600mg favored pazopanib but did not show a significant improvement over placebo (hazard ratio [HR], 0.86; 95% CI, 0.70 to 1.06; P = .165). The secondary analysis of DFS in ITT800mg (n = 403) yielded an HR of 0.69 (95% CI, 0.51 to 0.94). Follow-up analysis in ITT600mg yielded an HR of 0.94 (95% CI, 0.77 to 1.14). Increased ALT and AST were common adverse events leading to treatment discontinuation in the pazopanib 600 mg (ALT, 16%; AST, 5%) and 800 mg (ALT, 18%; AST, 7%) groups.
Conclusion
The results of the primary DFS analysis of pazopanib 600 mg showed no benefit over placebo in the adjuvant setting.
INTRODUCTION
Approximately 75% of patients diagnosed with renal cell carcinoma (RCC) have localized disease where surgical resection of the primary tumor is the current standard of care.1,2 Using the stage, size, grade, and necrosis (SSIGN) score, the 5-year relapse rate for intermediate- and high-risk RCC after nephrectomy is predicted to be 30% to 40%.2 The identification of adjuvant therapy is an unmet need for locally advanced RCC. Cytokines and girentuximab showed no improvement in disease-free survival (DFS).3-5 However, these agents demonstrated low antitumor activity in advanced RCC. Antiangiogenic agents including pazopanib and sunitinib demonstrated clinical efficacy for patients with advanced RCC and are the mainstays of first-line therapy.6 The study of antiangiogenic drugs in the adjuvant setting was warranted with the intent of preventing disease recurrence and improving DFS. One phase III study (ASSURE [Adjuvant Sorafenib or Sunitinib for Unfavorable Renal Carcinoma]) conducted in patients with RCC at intermediate or high risk of relapse failed to demonstrate an improvement in DFS with adjuvant sunitinib or sorafenib.7 Recently, a significant improvement in DFS was reported from the phase III S-TRAC (Sunitinib Treatment of Renal Adjuvant Cancer) study, which evaluated the efficacy of adjuvant sunitinib in high-risk RCC after nephrectomy.8
Pazopanib demonstrated a positive benefit-risk ratio in patients with advanced RCC and has received regulatory approval as first-line treatment.9-11 PROTECT (Pazopanib As Adjuvant Therapy in Localized/Locally Advanced RCC After Nephrectomy [VEG113387]) evaluated the efficacy and safety of pazopanib as an adjuvant therapy for patients with locally advanced RCC at high risk of relapse after nephrectomy. The trial was initiated using 800 mg of pazopanib versus placebo. However, to address intolerance and toxicity attrition, the 800-mg starting dose was lowered to 600 mg, and the primary end point analysis changed to DFS for the 600-mg cohort.
PATIENTS AND METHODS
Patients
Eligible patients were age ≥ 18 years and had resected nonmetastatic (M0) clear-cell or predominant clear-cell RCC histology, fulfilling any of the following combinations of pathologic staging based on TNM classification per the American Joint Committee on Cancer (2010 version) and Fuhrman nuclear grades: pT2G3-4N0, pT3-T4 GanyN0, or pTanyGanyN1. Baseline imaging assessment by independent radiologist review excluded metastasis. Patients had Karnofsky performance score of ≥ 80 and adequate organ function. Exclusion criteria are detailed in the Appendix (online only).
Study Design
Patients were stratified by: partial versus radical nephrectomy and TNM staging per the American Joint Committee on Cancer (2010 version) and Fuhrman nuclear grades (pT2G3-G4N0, pT3GanyN0, pT4GanyN0, or pTanyGanyN1). Patients were randomly assigned at a one-to-one ratio to receive pazopanib or matching placebo for 1 year. The study was designed with pazopanib 800 mg once daily as the starting dose; however, the initial dose was subsequently reduced to 600 mg once daily because the treatment discontinuation rate was higher than expected based on blinded aggregate safety review. The decision to change the primary end point analysis of this study was taken jointly by the sponsor and the study steering committee. After the first 8 to 12 weeks of treatment, the dose could be maintained or escalated to 800 mg daily, based on a patient’s safety and tolerability. Assessments for recurrence and safety were performed at regular intervals (Appendix). Dose adjustments and treatment discontinuation based on adverse events (AEs) occurred according to protocol guidelines (Appendix).
Study Oversight
The study was approved by local institutional review boards and conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent. The study was a collaboration of the authors and the sponsor (Novartis/GlaxoSmithKline).
End Points and Assessments
The primary end point was investigator-assessed DFS (independent review was used for eligibility but not for assessment of primary end point), defined as time from random assignment to development of local disease recurrence and/or metastasis or death resulting from any cause. The primary objective was to evaluate DFS with pazopanib 600 mg. Secondary objectives were to evaluate overall survival (OS), defined as time from random assignment to death resulting from any cause; DFS rates at yearly time points; DFS and OS with pazopanib 800 mg daily as initial dose compared with placebo; safety; and measures of patient-reported health outcomes and quality of life. Details of the quality-of-life health outcome assessments are provided in the Appendix.
Statistical Analysis
PROTECT was originally designed to evaluate pazopanib at a starting dose of 800 mg daily. The sample size was estimated to allow 85% power to detect a 23% reduction in disease-recurrence risk (hazard ratio [HR], 0.77). The overall 1-, 3-, and 5-year DFS rates for patients receiving placebo were assumed to be 70%, 54%, and 49%, respectively. Later, the pazopanib dose was amended to a lower starting dose (600 mg daily). The choice of 600 mg was based on the dose used for first dose reduction from 800 mg in a large phase III study in advanced RCC.12 The study sample size was re-estimated to allow 85% power to detect a 30% reduction in disease-recurrence risk with pazopanib 600 mg daily as initial dose compared with placebo (HR, 0.70). The assumptions of the overall 1-, 3-, and 5-year DFS rates for patients receiving placebo were changed to 77%, 63%, and 54%, respectively. A total of 319 DFS events were needed, which required 1,100 patients (550 per treatment arm) to be enrolled. The total study enrollment was 1,538 patients; 403 patients were randomly assigned to the 800 mg daily starting dose (pazopanib, n = 198; placebo, n = 205), and 1,135 patients were randomly assigned to the 600 mg daily starting dose (pazopanib, n = 571; placebo, n = 564). The primary analysis was conducted when 350 events had occurred (data cutoff, October 15, 2015), and the DFS follow-up analysis was conducted with a data cutoff of October 15, 2016.
The primary analysis group for efficacy included all randomly assigned patients who received a starting dose of 600 mg daily or placebo, designated as the intent-to-treat pazopanib 600 mg group (ITT600mg). Two secondary groups were analyzed for efficacy: ITT800mg included all randomly assigned patients who received a starting dose of 800 mg daily or placebo, and ITTAll included all randomly assigned patients, regardless of starting dosage. The Pike estimator with 95% CI of the treatment HR based on the stratified log-rank test was provided.13 The safety group included all randomly assigned patients who received at least one dose of study treatment (ie, safety600mg, safety800mg, and safetyAll).
DFS was summarized using Kaplan-Meier survival curves. Patients who received subsequent anticancer therapy before documented recurrence or death and those who did not have a documented date of recurrence or death were censored at date of last adequate assessment (before initiation of new therapy). DFS rates and 95% CIs were summarized at yearly time points. The OS analysis of the ITT600mg group used the Haybittle-Peto method,14,15 which assessed statistical significance, and a stratified log-rank test to compare treatment arms. A two-sided significance level of .001 was set for the latest interim OS analysis (cutoff date, October 15, 2016). Data cutoff for the final OS analysis is April 15, 2019.
RESULTS
Patients
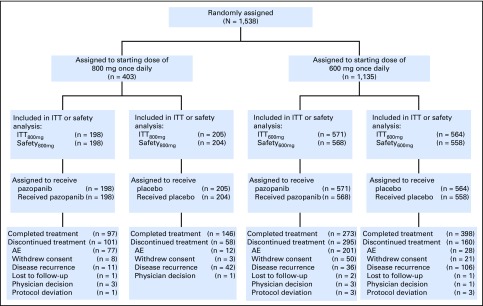
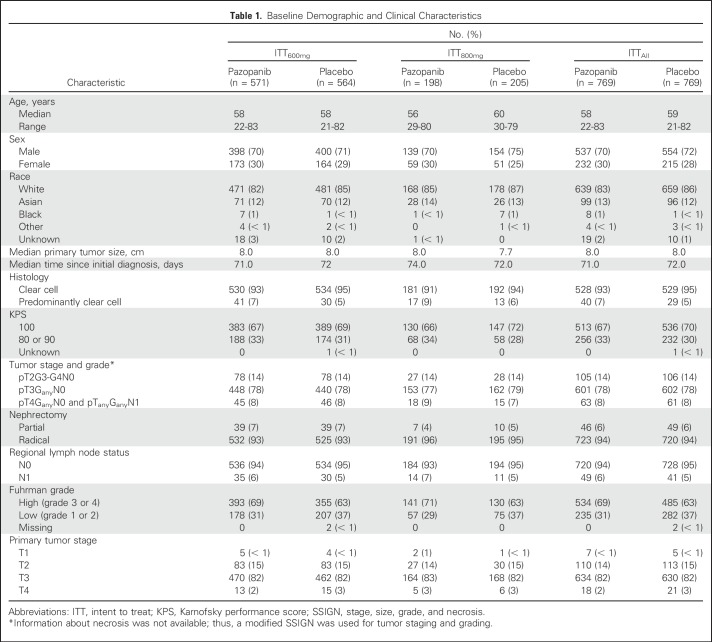
From December 9, 2010, through September 10, 2013, 1,538 eligible patients were enrolled at 263 centers in 26 countries globally. There were 571 patients receiving pazopanib and 564 receiving placebo in the primary efficacy group (ITT600mg) and 198 patients receiving pazopanib and 205 receiving placebo in the ITT800mg group (Fig 1). Ten patients were randomly assigned but never treated and excluded from the safety group, including three patients in the ITT800mg pazopanib group, one in the ITT800mg placebo group, and six in the ITT600mg placebo group. Demographic and clinical characteristics at baseline were similar in the ITT600mg, ITT800mg, and ITTAll groups and were balanced between treatment arms (Table 1).
Fig 1.
CONSORT diagram. AE, adverse event; ITT, intent to treat.
Table 1.
Baseline Demographic and Clinical Characteristics
Efficacy
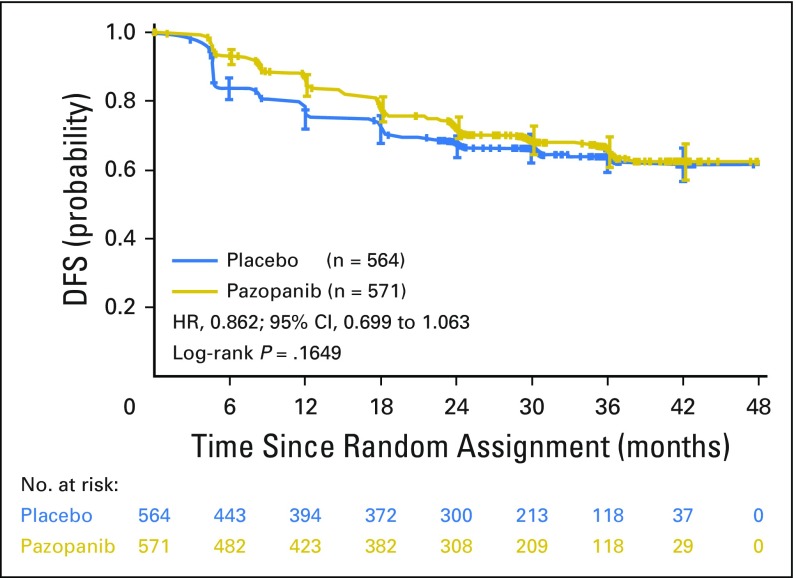
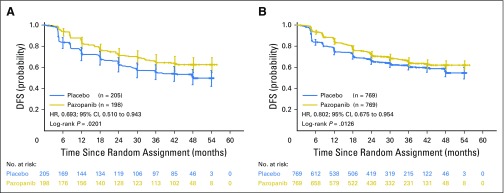
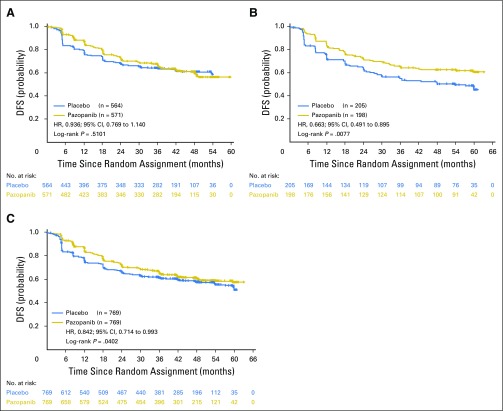
The study did not meet the primary DFS end point in the ITT600mg group (HR, 0.86; 95% CI, 0.70 to 1.06; P = .16; Fig 2). The median duration of follow-up in the ITT600mg group was 30.4 and 30.7 months for the pazopanib and placebo arms, respectively. The primary DFS analysis in the ITT600mg group was not statistically significant. The results of the secondary DFS analysis demonstrated a benefit for the ITT800mg (HR, 0.69; 95% CI, 0.51 to 0.94; nominal P = .02) and ITTAll (HR, 0.80; 95% CI, 0.68 to 0.95; nominal P = .01) groups, respectively (Figs 3A and 3B). The median duration of follow-up for both treatment arms in the ITT800mg group was 47.9 months.
Fig 2.
Disease-free survival (DFS) in the intent-to-treat pazopanib 600 mg (ITT600mg) group. HR, hazard ratio.
Fig 3.
Disease-free survival (DFS) in the secondary cohorts of the (A) intent-to-treat pazopanib 800 mg (ITT800mg) and (B) ITTAll groups. HR, hazard ratio.
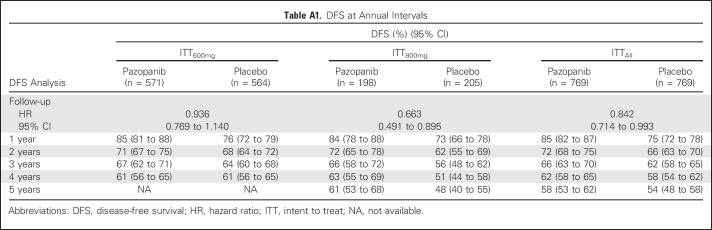
After 1 additional year of follow-up, the updated DFS analysis in the ITT600mg group yielded an HR of 0.94 (95% CI, 0.77 to 1.14) and two-sided nominal P value of .51 (stratified log-rank test; Appendix Fig A1A, online only).The median DFS was not attained in either treatment arm. The DFS follow-up analysis in the ITT800mg group yielded a 33.7% decrease in the relative risk of recurrence or death (HR, 0.66; 95% CI, 0.49 to 0.90; two-sided nominal P = .008 [stratified log-rank test]; Appendix Fig A1B). The median DFS was 54.0 months in the placebo arm and not attained in the pazopanib 800 mg arm. The proportion of patients alive and disease free decreased over time (Appendix Table A1, online only). For example, the DFS rate at 1 year was 85% (95% CI, 81% to 88%) and 76% (95% CI, 72% to 79%) for pazopanib and placebo, respectively, in the ITT600mg group and 84% (95% CI, 78% to 88%) and 73% (95% CI, 66% to 78%) for pazopanib and placebo, respectively, in the ITT800mg group. At 3 years, the DFS rate was 67% (95% CI, 62% to 71%) and 64% (95% CI, 60% to 68%) for pazopanib and placebo, respectively, in the ITT600mg group and 66% (95% CI, 58% to 72%) and 56% (95% CI, 48% to 62%) for pazopanib and placebo, respectively, in the ITT800mg group.
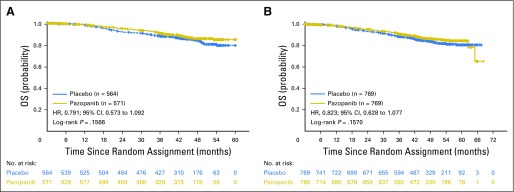
At the time of the DFS follow-up analysis (October 2016), the OS evaluation in the ITT600mg group (based on 148 events) yielded an HR of 0.79 (95% CI, 0.57 to 1.09) and two-sided, stratified, log-rank test P value of .16. Survival evaluation in the ITT800mg and ITTAll groups yielded HRs of 0.89 (95% CI, 0.54 to 1.46; P = .65) and 0.82 (95% CI, 0.62 to 1.07; P = .15), respectively (Fig 4).
Fig 4.
Interim overall survival (OS) in the (A) intent-to-treat pazopanib 600 mg (ITT600mg) and (B) ITTAll groups. HR, hazard ratio.
Treatment Administration and Safety
The median duration of exposure was similar in the two pazopanib dose groups: 10.6 months (range, 0 to 13 months) in the safety600mg and 10.2 months (range, 0 to 12 months) in the safety800mg groups. The percentage of patients completing 12 months of treatment was 49% in both pazopanib dose groups and 72% and 75% in the placebo safety600mg and safety800mg groups, respectively. The median daily dose in the pazopanib arms was 593.7 mg (range, 129 to 800 mg) in the safety600mg group and 648.4 mg (range, 178 to 800 mg) in safety800mg group, whereas in the placebo arms, it was 749.2 mg (range, 200 to 800 mg) in the safety600mg group and 800 mg (range, 360 to 800 mg) in the safety800mg group. Twenty-one percent of patients receiving pazopanib in the safety600mg group had a protocol-defined dose escalation by week 12 according to predefined safety criteria.
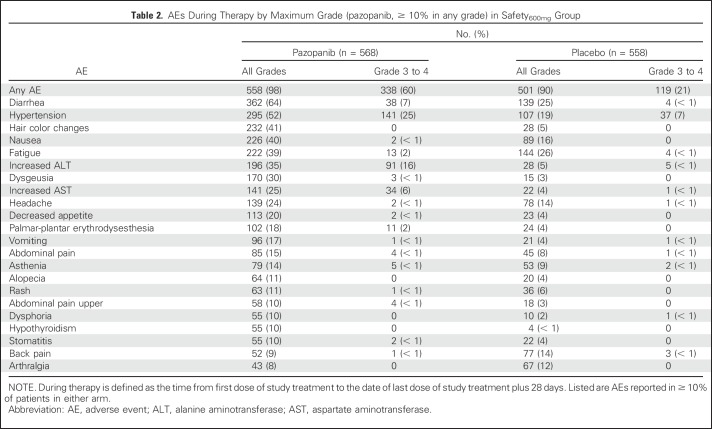
Dose reductions in the pazopanib arm were performed in 289 patients (51%) in the safety600mg group and 119 (60%) in the safety800mg group. Discontinuations because of AEs were similar in the pazopanib safety600mg and safety800mg groups (35% and 39%, respectively) and in the two placebo arms (5% and 6% for safety600mg and safety800mg, respectively). The most common AEs leading to pazopanib discontinuation were elevations of ALT (safety600mg, 16%; safety800mg, 18%) and AST (safety600mg, 5%; safety800mg, 7%).
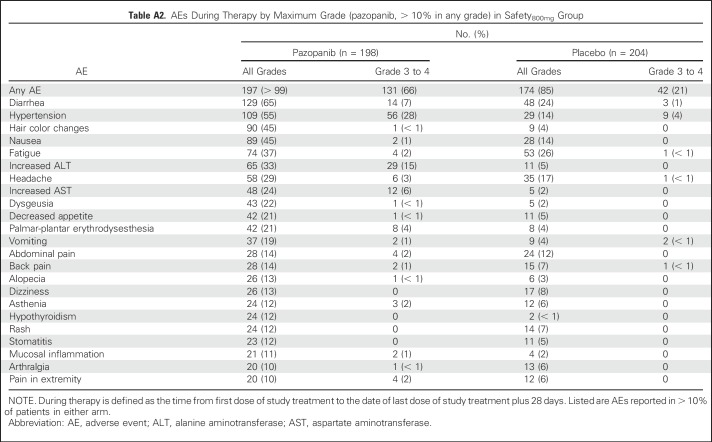
Overall, 98% of pazopanib-treated and 90% of placebo-treated patients in the safety600mg group experienced at least one AE during therapy (Table 2). Similar AE findings during treatment were reported for patients in the safety800mg group (Appendix Table A2, online only).
Table 2.
AEs During Therapy by Maximum Grade (pazopanib, ≥ 10% in any grade) in Safety600mg Group
Two grade 5 AEs occurred during the follow-up period with placebo and four with pazopanib; three of the pazopanib deaths occurred in the safety600mg group and were considered unrelated to treatment (CNS hemorrhage, cerebral hypoxia, and renal failure), and one occurred in the safety800mg group and was considered related to treatment (cardiomyopathy).
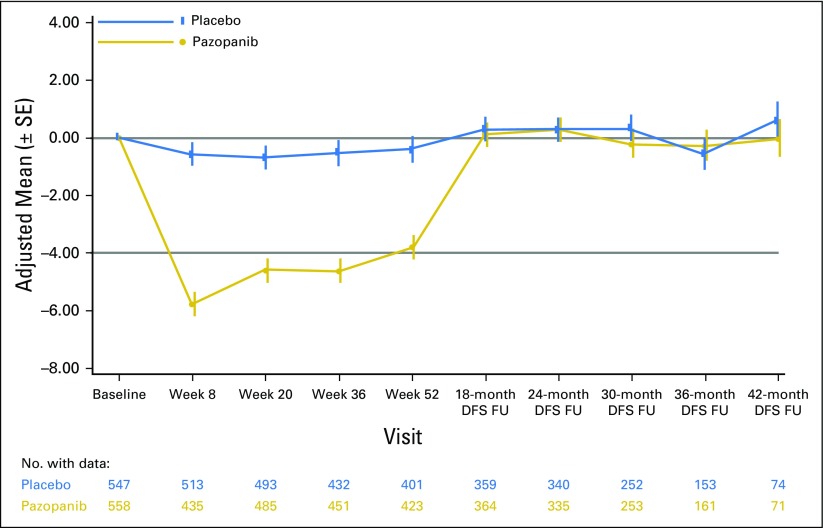
The Functional Assessment of Cancer Therapy-Kidney Symptom Index 19 (FKSI-19) questionnaire was used to gauge quality of life in the ITT600mg group, and scores were similarly high in the ITT800mg and ITTAll groups (data not shown). Pazopanib-treated patients had greater reductions from baseline in FKSI-19 total score compared with patients receiving placebo at all assessment points throughout the 12 months of treatment in the ITT600mg (Appendix Fig A2, online only) and ITT800mg and ITTAll groups (data not shown). The 1-year adjusted mean change from baseline in total FKSI-19 score with pazopanib was −4.49 for the ITT600mg group compared with −0.47 with placebo (P < .001). Although significant, these changes in FKSI-19 total score did not reach the prespecified minimally important difference of 5 to be deemed clinically relevant,16 with the exception of week 8, when the mean change from baseline was −5.77 for the ITT600mg group. The FKSI-19 total scores returned to baseline after treatment cessation in the pazopanib group, with no apparent difference between treatment arms during the follow-up period.
DISCUSSION
The PROTECT study did not meet the primary end point of DFS in the ITT600mg group. The secondary DFS analysis in the ITT800mg group showed a decrease in the relative risk of recurrence or death. However, the ITT800mg group represented approximately one third of the overall study group, and DFS in this group was a secondary end point. Results of the PROTECT study showed disparate outcomes observed in the ITT600mg and ITT800mg groups. Considering the DFS follow-up analysis results, the difference in treatment effect between the ITT600mg and ITT800mg groups could be related to the different starting dose, but it is not likely to be a result of a difference in duration of follow-up. The difference in treatment effect between the two groups might be explained by the better performance of the placebo arm in the ITT600mg group compared with that in the ITT800mg group, although this observation is not based on a randomized comparison. After 1 year of follow-up, the DFS rate in the placebo arm of the ITT600mg group was 76% compared with 73% in the placebo arm of the ITT800mg group. After 3 years of follow-up, the DFS rates in the placebo arms were 64% and 56% in the ITT600mg and ITT800mg groups, respectively. One factor that could explain differences in the outcomes of placebo groups includes unidentified patient demographic characteristics.
Exposure-efficacy analysis was performed among patients treated in this study.17 A correlation was observed between higher trough plasma concentration and longer DFS. A similar relationship has been reported in the metastatic setting.18 These results suggest that higher serum concentration may be associated with more clinical benefit.
With regard to OS, the results are inconclusive, because the data are not yet mature. The final data cutoff for OS analysis is planned for April 15, 2019.
The safety profile of pazopanib is consistent with that previously reported in advanced RCC studies, and the safety profile was similar for patients receiving pazopanib in the safety600mg and safety800mg groups. Although the intent of modifying the protocol dose of pazopanib from 800 to 600 mg was to reduce the rate of discontinuation and improve the safety profile, the proportions of patients in both cohorts had similar discontinuation rates and safety. The rate of pazopanib discontinuation because of AEs in PROTECT (35% to 39%) was higher compared with that observed in two large phase III studies in advanced or metastatic RCC (14% and 24%, respectively).10,12
Approximately 21% of patients receiving pazopanib 800 mg discontinued from study drug because of transaminase elevations. In a large phase III randomized trial (COMPARZ [Comparing the Efficacy, Safety and Tolerability of Pazopanib Versus Sunitinib]), 6% of patients discontinued pazopanib for abnormalities in liver function tests.12 This difference may be explained by more stringent criteria defined for the management of liver enzyme elevation in PROTECT. Overall, ALT increases were reversible and manageable with pazopanib dose interruptions or reductions provided in the protocol guidelines. The evaluation of outcomes for patients with ALT > 3× the upper limit of normal showed that 99% of all those receiving pazopanib recovered to grade ≤ 1. Health-related quality of life deteriorated during treatment with pazopanib, showing a slight trend toward improvement over time; quality of life was promptly restored back to baseline levels after treatment.
The efficacy results of PROTECT follow conflicting results from the two other large adjuvant trials in RCC. The ASSURE trial (comparing adjuvant sorafenib and sunitinib v placebo) failed to meet its primary end point,7 whereas the S-TRAC trial (comparing adjuvant sunitinib v placebo) met its primary end point.8 These three trials enrolled generally similar patient populations with intermediate to high risk of relapse. However, the stratification of the risk of relapse was performed differently in these studies; moreover, some differences existed between the respective study populations as well as the drug administered. For example, in PROTECT, the population consisted of patients with intermediate or high risk defined by the SSIGN scoring algorithm,19 whereas the UISS (University of California-Los Angeles Integrated Staging System) risk stratification system20 was used in S-TRAC and ASSURE. All patients in S-TRAC presented with stage T3 or T4 disease, whereas in PROTECT, T3 or T4 represented 84% of patients. The ASSURE trial enrolled a broader patient population, including patients with stage T1b high-grade disease (15%), and the proportion of patients with stage T3 or T4 disease was approximately 65%. ASSURE was the only trial that enrolled patients with non–clear cell histology; this subgroup comprised 20% of the patient population. Although the patient populations of these studies differed somewhat, they all enrolled patients with a high risk of relapse.13
Findings from PROTECT demonstrated differences in outcome between the 600- and 800-mg starting dose groups; however, the study did not meet its primary DFS end point. The results of the primary analysis of DFS with pazopanib 600 mg showed no benefit over placebo in the adjuvant setting. The safety profile of pazopanib was consistent with prior experience.
ACKNOWLEDGMENT
We thank the patients and their families, the investigators and site staff, and the study teams who participated in the PROTECT study. Editorial assistance was provided by Chris Ontiveros (ApotheCom, New York, NY) and Julia Burke (ApotheCom, Auckland, New Zealand).
Appendix
Exclusion Criteria
Patients were excluded if they had locally recurrent or bilateral renal cell carcinoma (RCC) or history of another malignancy, unless they had been disease free for 5 years or had a history of completely resected nonmelanomatous skin carcinoma or successfully treated in situ carcinoma; had clinically significant GI abnormalities that may increase the risk for GI bleeding or affect absorption of investigational product; active diarrhea of any grade; had history of HIV infection, chronic active hepatitis, or uncontrolled infection; had history of cardiac angioplasty or stenting, myocardial infarction, unstable angina, coronary artery bypass graft surgery, or symptomatic peripheral arterial vascular disease within the past 6 months; had history of class III/IV congestive heart failure defined per the New York Heart Association classification; had history of cerebrovascular accident, including transient ischemic attack, pulmonary embolism, or untreated deep venous thrombosis within the past 6 months; had corrected QT interval > 480 msec; had poorly controlled hypertension (≥ 140 mm Hg systolic or ≥ 90 mm Hg diastolic blood pressure); had evidence of active bleeding or bleeding diathesis; had any serious and/or unstable preexisting medical, psychiatric, or other condition that could interfere with patient safety, provision of informed consent, or compliance with study procedures; were unable or unwilling to discontinue use of various prohibited medications for at least 14 days or five half-lives of a drug (whichever was longer) before the first dose of study treatment and for the duration of the study; were receiving concurrent therapy to treat cancer, including treatment with an investigational agent, or concurrently participating in another clinical trial involving an anticancer investigational drug; had received any investigational drug within 30 days or five half-lives (whichever was longer) before the first dose of study treatment; had a known immediate or delayed hypersensitivity reaction or idiosyncrasy to drugs chemically related to pazopanib or excipients that in the opinion of the investigator contradicted their participation; or had used or were using systemic antivascular endothelial growth factor inhibitors or cytokines (eg, interferon, interleukin-2).
Disease, Safety, and Quality-of-Life Assessments
Disease assessments were performed using computed tomography (CT) or magnetic resonance imaging (MRI) at baseline; weeks 20, 36, and 52 during year 1; every 6 months during years 2 to 5; and yearly thereafter. Baseline imaging was performed a minimum of 4 weeks after nephrectomy and at least 2 weeks before random assignment. Baseline disease-free status was assessed locally and confirmed by central review. Local RCC recurrence or distal metastasis was diagnosed only when the clinical, imaging, or pathologic findings met predefined criteria consistent with recurrence or metastasis. Safety was assessed during clinic visits and as clinically indicated. After established disease recurrence, patients were observed for survival every 6 months until death, study completion, or early study termination.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). RCC symptoms were assessed using the Functional Assessment of Cancer Therapy Kidney Symptom Index 19 (FKSI-19). Quality-of-life (QoL) changes from baseline were evaluated for pazopanib and placebo during the treatment period, disease-free survival (DFS) period, and study period after disease recurrence. Plasma samples for clinical pharmacology assessments were also collected.
Assessment of Recurrence, Safety, and Compliance
Assessment of the primary end point of DFS was based on evaluation of imaging scans. The preferred imaging assessment method for the chest, abdomen, and pelvis was CT with intravenous contrast. Clinical assessments included medical history and physical examination for possible palpable lesions (eg, lymph nodes) or visual lesions and for evaluation of other signs or symptoms that may be suggestive of local disease or metastatic lesions. Pathology assessments included cytology or histology by needle aspiration or biopsy.
Required baseline imaging consisted of CT/MRI of chest, abdomen, and pelvis. Baseline imaging was performed after a minimum of 4 weeks after nephrectomy. On the day of random assignment (day 1) before dosing, patients completed the QoL questionnaires and underwent a physical examination. New abnormal conditions or concurrent medications were to be documented, and blood samples for biomarker and pharmacogenetic assessments were drawn. CT or MRI of the chest, abdomen, and pelvis was performed at weeks 20, 36, and 52 (± 14 days) during the first year of treatment; every 6 months (± 21 days) from the start of year 2 through year 5; and every 12 months (± 21 days) from the start of year 6 onward. Brain and bone scans were to be performed as clinically indicated.
Once a patient presented with established disease recurrence, he or she was observed using the telephone every 6 months for survival until death, study completion, or early termination of study by the independent data monitoring committee.
Safety assessments included physical examination, clinical laboratory tests (including clinical chemistry, hematology, coagulation, thyroid function, and urinalysis), ECG, Karnofsky performance score, vital signs, evaluation of concomitant medications, and pregnancy test (if applicable). Screening safety assessments were not performed before recovery of patients from nephrectomy and/or its complications to grade ≤ 1 severity. Screening safety assessments were also used as baseline for patients who were eligible for the study, and the assessments were only valid if they were within 4 weeks (28 days) of random assignment. If treatment discontinuation occurred in between scheduled visits, safety assessments (with exception of ECG and thyroid function test) were performed if the previous assessments were reported > 4 weeks previously. ECG and thyroid function tests were performed if the previous assessments were > 12 weeks old.
The investigator or site staff was responsible for detecting, documenting, and reporting events that met the criteria for AEs. Information about the AEs volunteered by the patient, discovered by investigator questioning, or detected by other means was collected from the start of study treatment until follow-up. The following information on AEs was obtained: duration (start and stop dates), severity (mild, moderate, or severe), causality (reasonable possibility [yes or no]), and actions taken and outcome.
Abnormal laboratory test results (hematology, clinical chemistry, or urinalysis) or other safety assessments (eg, ECGs, radiologic scans, vital signs measurements), including those that worsened from baseline, and events felt to be clinically significant in the medical and scientific judgment of the investigator were recorded as AEs. Clinically significant safety assessments associated with the underlying disease, unless judged by the investigator to be more severe than expected for the patient’s condition, were not reported as AEs. All AEs were monitored until resolution or recovery to the baseline severity. Death resulting from disease during study was recorded.
Compliance with study treatment was confirmed by the sites through querying the patients during the site visits and documented. The number of study treatment tablets dispensed to and taken by each patient was recorded and reconciled with the study treatment and compliance records. Treatment start and stop dates, including dates for treatment delays and/or dose reductions, were recorded.
Source of Drug, Dose Adjustments, and Discontinuations Because of AEs
After completion of required screening/baseline assessments, eligible patients were registered in the GlaxoSmithKline Registration and Medication Ordering System (RAMOS) by the investigator or authorized site staff for stratification and random assignment. Patient identification and the following information for stratification were entered into the RAMOS system to obtain blinded study treatment assignment: nephrectomy procedure (partial v radical [including total nephrectomy]) and TNM stage and tumor grade groups, as described in Patients and Methods.
Patients were centrally assigned to the pazopanib or placebo arm at a one-to-one ratio. Treatment assignment remained blinded until a patient was confirmed to have disease recurrence or development of distal metastasis or until the study achieved the primary end point of DFS.
The maximum allowable pazopanib dose was 800 mg daily, and the minimum allowable dose was 400 mg daily. As a general rule, if dose reduction of study treatment was necessary, the dose was reduced stepwise by 200 mg at each step, and the patient was monitored for approximately 10 to 14 days at each dose level. If the investigator believed that further reduction to 200 mg daily was required, the investigator contacted the sponsor’s study physician to determine appropriateness.
After abatement of toxicity with modified dose and management of adverse effects, dose re-escalation back to the pre-event dose was attempted. For patients whose treatment was interrupted for liver events, study treatment was rechallenged at a dose of 400 mg daily. If a patient’s study treatment was interrupted for > 21 days, the investigator was contacted the study physician to review the patient’s condition to resume treatment.
Patients who were assigned to study treatment at the dose level of 600 mg daily did not receive a higher dose for the first 8 to 12 weeks of treatment. Starting at the week-8 visit, the investigator determined whether to escalate the dose to 800 mg daily or maintain the dose at 600 mg daily based on a patient’s safety and tolerability profile since the first dose and in accordance with the following dose-escalation criteria: no or only grade 1 treatment-related AEs, no ongoing grade 2 treatment-related AEs (could have had grade 2 treatment-related AEs that recovered to grade ≤ 1), no grade 3 or 4 treatment-related AEs, and ALT ≤ upper limit of normal.
AEs of hair color change and alopecia were excluded when applying these criteria for dose escalation. For patients with multiple ongoing treatment-related grade 1 AEs (eg, > three AEs), dose escalation to the 800-mg dose level was based on the investigator’s clinical judgment. If a patient could not be dose escalated at week 8 because of ongoing treatment-related grade 2 AEs, the patient was re-evaluated at week 12 for the possibility of dose escalation to the 800-mg dose level. If the dose could not be escalated to 800 mg daily between weeks 8 and 12, the patient maintained a maximum 600-mg dose for the rest of the treatment period unless subsequent dose reduction was necessary because of an AE.
If an AE was considered unlikely to be related to study treatment per the investigator’s clinical judgment, these dose modification rules did not apply.
Health Outcome Assessments
RCC symptoms affecting health-related QoL were assessed using the FKSI-19. This assessment was used to evaluate changes from baseline health-related QoL for pazopanib compared with placebo during the following study periods: 12 months of treatment, DFS, and after disease recurrence.
The FKSI-19 is a disease-specific assessment that measures disease- and treatment-related symptoms specifically in patients with renal cancer (Rosenbloom SK, et al: J Clin Oncol 25, 2007 [suppl; abstr 6524]; Rosenbloom S, et al: Res Hum Cap Dev 16:53-66, 2008; Rao D, et al: J Pain Symptom Manage 38:291-298, 2009). It includes patient self-reports on experience of symptoms in the past 7 days (eg, lack of energy, pain, bone pain, shortness of breath, fatigue, blood in urine).
These questionnaires were administered following the same schedule as the CT scans (ie, at baseline [day 1 predose]; weeks 8, 20, 36, and 52; every 6 months during years 2 to 5; and once every year starting at year 6). In addition, QoL questionnaires were completed by patients via mail approximately 3 months after documented disease recurrence.
Table A1.
DFS at Annual Intervals
Table A2.
AEs During Therapy by Maximum Grade (pazopanib, > 10% in any grade) in Safety800mg Group
Fig A1.
Disease-free survival (DFS) in the (A) intent-to-treat pazopanib 600 mg (ITT600mg), (B) ITT800mg, and (C) ITTAll groups (follow-up analysis of DFS on October 15, 2016).
Fig A2.
Health-related quality-of-life outcomes in the intent-to-treat pazopanib 600 mg group assessed using the National Comprehensive Cancer Network/Functional Assessment of Cancer Therapy Kidney Symptom Index 19 questionnaire total score. DFS, disease-free survival; FU, follow-up.
Footnotes
Supported by Novartis, which also funded medical writing and editorial support (provided by ApotheCom) and previously supported by GlaxoSmithKline (pazopanib is an asset of Novartis AG as of March 2, 2015). Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by Memorial Sloan Kettering Cancer Center Support Grant/Core Grant No. P30 CA008748.
Clinical trial information: NCT01235962.
See accompanying Editorial on page 3895
AUTHOR CONTRIBUTIONS
Conception and design: Robert J. Motzer, Naomi B. Haas, Brian I. Rini, Ho Yeong Lim, Paul Russo, Cora N. Sternberg
Provision of study materials or patients: Robert J. Motzer, Naomi B. Haas, Frede Donskov, Marine Gross-Goupil, Jae Lyun Lee, Bohuslav Melichar, Brian I. Rini, Toni K. Choueiri, Lori A. Wood, Paul Russo
Collection and assembly of data: Robert J. Motzer, Frede Donskov, Marine Gross-Goupil, Sergei Varlamov, Evgeny Kopyltsov, Jae Lyun Lee, Bohuslav Melichar, Brian I. Rini, Toni K. Choueiri, Milada Zemanova, Lori A. Wood, M. Neil Reaume, Arnulf Stenzl, Simon Chowdhury, Ray McDermott, Agnieszka Michael, Marlene J. Carrasco-Alfonso, Paul Russo, Cora N. Sternberg
Data analysis and interpretation: Robert J. Motzer, Naomi B. Haas, Frede Donskov, Jae Lyun Lee, Bohuslav Melichar, Brian I. Rini, Toni K. Choueiri, Simon Chowdhury, Ray McDermott, Weichao Bao, Marlene J. Carrasco-Alfonso, Paola Aimone, Maurizio Voi, Christian Doehn, Paul Russo, Cora N. Sternberg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Robert J. Motzer
Consulting or Advisory Role: Pfizer, Novartis, Eisai, Exelixis
Research Funding: Pfizer (Inst), GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Genentech (Inst)
Naomi B. Haas
Consulting or Advisory Role: Cerulean Pharma, Exelixis, Pfizer, Novartis
Expert Testimony: Eli Lilly (I)
Stock or Other Ownership: Tetralogic
Frede Donskov
Research Funding: GlaxoSmithKline (Inst), Novartis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Exelixis
Marine Gross-Goupil
Honoraria: Pfizer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Ipsen
Consulting or Advisory Role: Pfizer, Novartis, GlaxoSmithKline, Bristol-Myers Squibb
Speakers' Bureau: Novartis
Research Funding: GlaxoSmithKline (Inst), Pfizer (Inst), Ipsen (Inst), Roche (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), Astellas (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Amgen, Merck Sharp & Dohme, Pfizer, Novartis, Roche, Bristol-Myers Squibb
Sergei Varlamov
No relationship to disclose
Evgeny Kopyltsov
No relationship to disclose
Jae Lyun Lee
Honoraria: Pfizer, Astellas Pharma, Novartis
Consulting or Advisory Role: Astellas Pharma, AstraZeneca, Eisai
Research Funding: Pfizer, Janssen, Novartis, Exelixis, Bayer
Bohuslav Melichar
Honoraria: Roche Pharma AG, Novartis, Ipsen, Bristol-Myers Squibb, Astellas Pharma, Pfizer, Eli Lilly, Janssen-Cilag, Merck, Merck Sharp & Dohme, Angelini Pharma, Bayer HealthCare Pharmaceuticals
Consulting or Advisory Role: Roche Pharma AG, Novartis, Bristol-Myers Squibb, Astellas Pharma, Bayer HealthCare Pharmaceuticals, Pfizer, Merck, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Novartis, Roche, Bristol-Myers Squibb, Merck
Brian I. Rini
Consulting or Advisory Role: Pfizer, Roche, Bristol-Myers Squibb, Acceleron, Novartis, GlaxoSmithKline
Research Funding: Pfizer (Inst), Genentech (Inst), Bristol-Myers Squibb (Inst), Acceleron (Inst), Immatics (Inst), Peloton (Inst)
Travel, Accommodations, Expenses: Pfizer
Toni K. Choueiri
Honoraria: National Comprehensive Cancer Network, UpToDate
Consulting or Advisory Role: Pfizer, Bayer HealthCare Pharmaceuticals, Novartis, Merck, Bristol-Myers Squibb, Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Peloton Therapeutics, Exelixis, Prometheus, Alligent, GlaxoSmithKline, Roche
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Genentech (Inst), Celldex (Inst), Agensys (Inst), Roche (Inst)
Milada Zemanova
Consulting or Advisory Role: Novartis
Honoraria: Eli Lilly
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Boehringer Ingelheim
Lori A. Wood
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Novartis (Inst), Pfizer (Inst), Roche (Inst), AstraZeneca (Inst),
M. Neil Reaume
Consulting or Advisory Role: Novartis, Sanofi, Pfizer, Janssen, Roche Canada
Arnulf Stenzl
Consulting or Advisory Role: Ipsen, Janssen, Amgen, Roche Pharma AG, Bayer HealthCare Pharmaceuticals, Takeda Pharmaceuticals, CureVac, Intuitive Surgical SARL, Bristol-Myers Squibb, Alere
Honoraria: Ipsen Pharma, Janssen, Alere
Speakers’ Bureau: Ipsen, Janssen, Alere, General Electric, Amgen, CureVac, Erbe, Novartis, Karl Storz
Research Funding: Novartis (Inst), Ipsen (Inst), Bayer AG (Inst), CureVac (Inst), Immatics Biotechnologies (Inst), Janssen (Inst), Convance (Inst), Johnson & Johnson, Amgen
Travel, Accommodations, Expenses: Alere, Amgen, CureVac, Ipsen, Novartis, Janssen, Erbe, Karl Storz, Astellas Pharma, Roche
Simon Chowdhury
Honoraria: GlaxoSmithKline, Novartis
Consulting or Advisory Role: GlaxoSmithKline, Novartis, Pfizer, Johnson & Johnson, Astellas, Bayer
Research Funding: Sanofi
Speakers’ Bureau: GlaxoSmithKline, Johnson & Johnson, Astellas, Bayer
Travel, Accommodations, Expenses: GlaxoSmithKline, Novartis, Johnson & Johnson, Astellas, Bayer
Ho Yeong Lim
No relationship to disclose
Ray McDermott
Consulting or Advisory Role: Clovis Oncology, Pfizer, Amgen, Bristol-Myers Squibb
Honoraria: Bristol-Myers Squibb, Sanofi, Janssen, Celgene, Pfizer, Novartis, Astellas, Bayer
Research Funding: Pfizer, Janssen, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Merck, Sharp & Dohme, Astellas, Sanofi
Travel, Accommodations, Expenses: Pfizer, Celgene
Agnieszka Michael
Honoraria: Pfizer, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Ipsen
Weichao Bao
Employment: Novartis
Stock or Other Ownership: Novartis
Marlene J. Carrasco-Alfonso
Employment: Novartis
Stock or Other Ownership: Novartis, Kite Pharma (I), Juno Therapeutics (I), Bluebird Bio (I), Bellicum Pharmaceuticals (I), Cellectis (I), Lion Biotechnologies (I), Adaptimmune (I), Intellia Therapeutics (I), Editas Medicine (I)
Paola Aimone
Employment: Novartis Pharma AG
Stock or Other Ownership: Novartis Pharma AG
Travel, Accommodations, Expenses: Novartis Pharma AG
Maurizio Voi
Employment: Novartis
Stock or Other Ownership: Novartis, Pfizer, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Bristol-Myers Squibb
Christian Doehn
Stock or Other Ownership: AstraZeneca, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Fresenius
Honoraria: Amgen, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, Roche
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, Novartis, Pfizer, Bayer HealthCare Pharmaceuticals, Eisai, GlaxoSmithKline, Ipsen, Roche
Travel, Accommodations, Expenses: Amgen, Bristol-Myers Squibb, Novartis, Pfizer, Roche
Paul Russo
No relationship to disclose
Cora N. Sternberg
Honoraria: Pfizer, Bristol-Myers Squibb, Novartis, Janssen, Bayer HealthCare Pharmaceuticals, Astellas Pharma, Sanofi, Eisai, Ipsen, GlaxoSmithKline, Merck Sharp & Dohme, Eli Lilly
Research Funding: Exelixis (Inst)
REFERENCES
- 1.Janzen NK, Kim HL, Figlin RA, et al. : Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 30:843-852, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Janowitz T, Welsh SJ, Zaki K, et al. : Adjuvant therapy in renal cell carcinoma-past, present, and future. Semin Oncol 40:482-491, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messing EM, Manola J, Wilding G, et al. : Phase III study of interferon alfa-NL as adjuvant treatment for resectable renal cell carcinoma: An Eastern Cooperative Oncology Group/Intergroup trial. J Clin Oncol 21:1214-1222, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Clark JI, Atkins MB, Urba WJ, et al. : Adjuvant high-dose bolus interleukin-2 for patients with high-risk renal cell carcinoma: A cytokine working group randomized trial. J Clin Oncol 21:3133-3140, 2003 [DOI] [PubMed] [Google Scholar]
- 5. doi: 10.1001/jamaoncol.2016.4419. Chamie K, Donin NM, Klöpfer P, et al: Adjuvant weekly girentuximab following nephrectomy for high-risk renal cell carcinoma: The ARISER randomized clinical trial. JAMA Oncol 3:913-920, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network: NCCN guidelines in oncology: Kidney cancer. https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf.
- 7.Haas NB, Manola J, Uzzo RG, et al. : Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 387:2008-2016, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravaud A, Motzer RJ, Pandha HS, et al. : Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 375:2246-2254, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Bukowski RM: Pazopanib: a multikinase inhibitor with activity in advanced renal cell carcinoma. Expert Rev Anticancer Ther 10:635-645, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Sternberg CN, Davis ID, Mardiak J, et al. : Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol 28:1061-1068, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Hutson TE, Davis ID, Machiels JP, et al. : Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. J Clin Oncol 28:475-480, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Cella D, et al. : Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722-731, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Berry G, Kitchin RM, Mock PA: A comparison of two simple hazard ratio estimators based on the logrank test. Stat Med 10:749-755, 1991 [DOI] [PubMed] [Google Scholar]
- 14.Haybittle JL: Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol 44:793-797, 1971 [DOI] [PubMed] [Google Scholar]
- 15.Peto R, Pike MC, Armitage P, et al. : Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer 35:1-39, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yost KJ, Sorensen MV, Hahn EA, et al. : Using multiple anchor- and distribution-based estimates to evaluate clinically meaningful change on the Functional Assessment of Cancer Therapy-Biologic Response Modifiers (FACT-BRM) instrument. Value Health 8:117-127, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Sternberg CN, Donskov F, Haas NB, et al. : Pazopanib exposure-response assessment as adjuvant therapy for patients with localized or locally advanced renal cell carcinoma (RCC) following nephrectomy. J Clin Oncol 35, 2017. (suppl; abstr 4564) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suttle AB, Ball HA, Molimard M, et al. : Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Br J Cancer 111:1909-1916, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leibovich BC, Blute ML, Cheville JC, et al. : Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 97:1663-1671, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lam JS, Shvarts O, Leppert JT, et al. : Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol 174:466-472, discussion 472, quiz 801, 2005 [DOI] [PubMed] [Google Scholar]