Abstract
Objectives:
To explore the possible association of childhood residence, education levels, and occupation with declining incidence rates of dementia in 2 cohorts of elderly African Americans.
Methods
African Americans residing in Indianapolis without dementia were enrolled in 1992 and 2001 and evaluated every 2–3 years. The cohorts consist of 1,440 participants in 1992 and 1,835 participants in 2001 aged 70 years and older. Cox proportional hazard regression models were used to compare cohort differences in dementia and Alzheimer’s disease (AD) risk.
Results
The 2001 cohort had significantly decreased risk of both incident dementia and AD (hazard ratio [HR]: 0.62/0.57 for dementia/AD). Years of education was associated with decreased risk of dementia (HR = 0.93; p = .0011). A significant interaction (p = .0477) between education and childhood rural residence was found for the risk of AD that higher education level is significantly associated with reduced AD risk (HR = 0.87) in participants with childhood rural residence, but no association in those with urban upbringing. The cohort difference for dementia rates were attenuated by adjusting for the 3 risk factors but remained significant (HR = 0.75; p = .04).
Discussion
These results emphasize the importance of early life factors including rural residence and education for the risk for dementia later in life.
Keywords: Alzheimer’s disease, Education, Employment, Rural–Urban
There are now several studies primarily from countries in the developed world that have reported decreasing prevalence and incidence rates of dementia (Doblhammer, Fink, & Fritze, 2015; Grasset et al., 2016; Langa et al., 2008; Langa et al., 2017; Manton, Gu, & Ukraintseva, 2005; Matthews et al., 2016; Satizabal et al., 2016; Schrijvers et al., 2012) although it should be noted that not all studies have reported this decline (Chan et al., 2013; Hall et al., 2009; Ohara et al., 2017; Rocca et al., 2011). No single protective factor has been identified but improvement in living conditions often associated with economic development, occupation, urbanization, and higher levels of education have been proposed (Wu et al., 2017). Several studies have suggested that these factors are particularly influential in early life (Contador, Bermejo-Pareja, Puertas-Martin, & Benito-Leon, 2015; Moceri, Kukull, Emanuel, van Belle, & Larson, 2000).
The Indianapolis-Ibadan project (IIDP) is a longitudinal study comparing dementia and Alzheimer’s disease (AD) prevalence, incidence, and risk factors in two elderly community-based cohorts of African Americans and Yoruba. Recruitment to the study was conducted at two time points, 1992 and 2001. In a recent article, it was reported that the incidence rates for dementia and AD were declining for African Americans but not for Yoruba (Gao et al., 2016). In an earlier study involving the 1992 cohort, it was reported that the relationship between education levels and dementia/AD in the African American community was complex involving an interaction between years of schooling and childhood rural residence. It was concluded that it was possible that low education may be a marker for other accompanying deleterious socioeconomic or environmental influences in childhood. In a separate analysis also involving the 1992 cohort education, childhood rural residence, and occupation were highly intercorrelated but only education and childhood rural residence were independently associated with increased risk for both cognitive impairment and dementia/AD, while occupation status was associated only with risk for cognitive impairment (Callahan et al., 1996; Hall, Gao, Unverzagt, & Hendrie, 2000).
The purpose of this article is to explore the possible association of childhood residence, education levels, and occupation with the declining rates of dementia and AD in African Americans by extending the analysis to include both the 1992 and 2001 cohorts.
Methods
Study Participants
Participants were from the IIDP, a longitudinal study comparing dementia and AD prevalence, incidence, and other health outcomes in two community-based cohorts. Recruitment to the study was conducted at two time points. In the first recruitment in 1992, cohorts of African Americans aged 65 or older living in Indianapolis and Yoruba aged 65 or older living in Ibadan, Nigeria, were enrolled in the study. For this study, only the Indianapolis cohorts were used. In Indianapolis, interviewers went door-to-door to randomly sampled addresses to invite African Americans (self-identified) aged 65 years and older to participate. In 1992, 2,212 African Americans were enrolled, whereas 249 (9.6%) refused, and 121 (4.7%) were too sick to participate.
In 2001, the IIDP conducted another wave of enrollment in both populations. In Indianapolis, community-dwelling subjects were randomly selected from Medicare records, who identified themselves as African Americans and were at least 70 years old. The age cutoff for the 2001 cohort was chosen to maintain comparability with the survivors in the 1992 cohort because the youngest participants in the 1992 cohort had since turned 70. Of 7,583 eligible individuals, interviewers were able to contact 4,433 by telephone or home visit. Of those contacted, 100 were deceased, 54 had moved to nursing homes, and 14 were not African American. Of the remaining 4,265 eligible individuals, 1,892 (44%) were enrolled, and 2,373 (56%) refused or were too ill. All participants agreed to undergo regular follow-up cognitive assessment and clinical evaluations. The study was approved by the Institutional Review Boards of Indiana University-Purdue University of Indianapolis and University of Ibadan. All enrolled participants provided informed consent.
Study Design
A prospective cohort design was used with a baseline evaluation followed by regular evaluations scheduled 2–3 years apart in both populations using identical assessment instruments. Participants in the 1992 cohorts were evaluated for up to seven times, in 1992, 1995, 1998, 2001, 2004, 2007, and 2009. Participants in the 2001 cohort were evaluated for up to four times, in 2001, 2004, 2007, and 2009.
A two-stage design was used at each evaluation with in-home cognitive and functional evaluations for all participants followed by a full diagnostic workup of selected participants based on the performance of stage one cognitive tests. After each stage one evaluation, study participants were divided into three performance groups (good, intermediate, and poor) based on their cognitive and functional scores obtained during the in-home assessment and changes in scores from previous evaluations (Hendrie et al., 2001). Percentages sampled from each performance category were chosen to ensure that participants with the highest probability of dementia would be clinically assessed. All participants in the poor performance group were invited to be clinically assessed. Participants were randomly sampled from the intermediate performance group until 50% had clinical assessments and from the good performance group until 5% had clinical assessments.
Each clinically assessed participant was evaluated for the diagnosis of dementia or normal cognition, with further subtypes for those diagnosed with dementia (Clinical Evaluation section). All individuals diagnosed with dementia were no longer followed for in-person evaluations.
Cognitive Instruments
The community screening interview for dementia (CSID) was used during the first stage in-home assessment with a cognitive assessment of the study participant and an interview with a close relative evaluating the daily functioning of the participant. The CSID was developed by a group specifically for use in comparative epidemiologic studies of dementia in culturally disparate populations (Hall et al., 1996, 2000). The cognitive assessment in CSID evaluates multiple cognitive domains (language, attention and calculation, memory, orientation, praxis, and comprehension and motor response), and details of its content and development are described elsewhere (Morris et al., 1989; Hall, Gao, Emsley, et al., 2000; Hendrie et al., 2006).
Clinical Evaluation
Clinical evaluations included (a) a neuropsychological battery adapted from the Consortium to Establish a Registry of Alzheimer’s Disease (Morris et al., 1989); (b) a standardized neurologic and physical examination and functional status review (the clinician home-based interview to assess function; Hendrie et al., 2006); and (c) a structured interview with an informant familiar with the participant (most often a close relative) adapted from the Cambridge Examination for Mental Disorders of the Elderly informant interview (Hendrie et al., 1988; Roth et al., 1986). Diagnosis was made in a consensus diagnostic conference of clinicians reviewing the neuropsychological test battery, the physician’s assessment, the informant interview, and available medical records. Dementia was diagnosed with both the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition (American Psychiatric Association, 1987) and International Classification of Diseases, 10th Revision (American Psychiatric Association Press, 1992) criteria. AD was diagnosed using criteria proposed by the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS)/Alzheimer’s Disease and Related Disorder Association (ADRDA) (McKhann et al., 1984).
Other Information
Demographic information including age, sex, and education were available on all study participants. Information was also collected on whether the participant ever consumed alcohol or smoked regularly. In addition, medical history of coronary heart disease, cancer, diabetes, heart attack, hypertension, Parkinson’s disease, stroke, and depression was collected from self or informant reports as affirmative answers to whether the participants had ever been diagnosed or treated for these conditions. In addition, medication use was also ascertained at each evaluation starting in 2001.
Childhood residence was divided into rural (population < 2,500) and urban (population > 2,500) as reported in the U.S. Census (US Department of Commerce, 1904, 1975). The classification of rural or urban was by self-report. If respondents were not sure about the classification, they discussed it with the interviewer. The name of the location was reported, and we independently verified the classification using historical U.S. Census data (US Department of Commerce, 1922).
For occupation, participants’ primary occupation was recorded together with the number of years involved. This information was recoded into 12 mutually exclusive categories using the Standard Occupation Classification Manual (Standardized Occupational Classification Manual, 1980). White collar occupation involved sales, professional, and clerical/administrative categories.
Statistical Analyses
Participants with prevalent dementia (62 cases in the 1992 cohort and 57 cases in the 2001 cohort) were excluded from this analysis. Descriptive statistics including means and standard deviations for continuous variables and frequency counts and percentages for categorical variables were calculated for each of the two cohorts. t-Tests and Fisher’s exact tests were used to compare the two cohorts. For participants with incident dementia/AD, age at dementia/AD diagnosis was the survival outcome. Age at dementia/AD was censored at the time of last evaluation for participants who did not receive a diagnosis of dementia/AD during the study. Cox proportional hazards regression models were used to compare the differences on age to dementia or AD between the 1992 and 2001 cohorts adjusting for baseline age and gender. We further examined cohort differences after adjusting for childhood rural residence, years of education, and occupation. For the models on time to AD, those with non-AD dementia were excluded. Plots using the Kaplan–Meier technique with log-rank tests were used to compare the cohorts on age at dementia or AD.
A Cox proportional hazards model was used to compare mortality risk between the two cohorts. Two additional Cox proportional hazards models were conducted to ensure that our results are robust to the differential length of follow-up in the two cohorts. One model was run using only follow-up data to 2001 in the 1992 cohort so that the two cohorts would have comparable length of follow-up (approximately 9 years). Another model was used using data collected from the 2001 wave forward for both cohorts.
Results
For comparison with the 2001 cohort, this analysis excluded participants in the 1992 cohorts who were younger than 70 at enrollment. The 1992 African American cohort included 1,440 participants, 191 (13.3%) of whom developed incident dementia, 161 of whom had AD (11.2%). The 2001 African American cohort included 1,836 African Americans without dementia at 2001; of these, 94 (5.1%) were diagnosed with dementia during follow-up and 72 with AD (3.9%). The age range is 70–102 for the 1992 cohort and 70–98 for the 2001 cohort, and the birth years are 1891–1923 for the 1992 cohort and 1903–1931 in the 2001 cohort.
The 1992 cohort were significantly older, had significantly less years of education, were significantly more likely to report a childhood rural residence, less likely to have a white collar occupation, more likely to report a history of smoking, and had significantly less reported rates of hypertension, depression, stroke, diabetes, and cancer than participants in the 2001 cohort (Table 1). The 2001 cohort reported significantly greater use of statins (p =.0001), antihypertensive (p = .0091), and antidiabetic medication (p = .0163) than did the 1992 cohort.
Table 1.
Comparison of Baseline Demographic Variables and Medical Conditions Between the 1992 and 2001 Cohorts
| Variable name | 1992 Cohorta | 2001 Cohortb | p-Value |
|---|---|---|---|
| Age, mean (SD) | 77.7 (5.9) | 77.2 (5.5) | .0133 |
| Female, % | 65.5 | 65.5 | 1.0000 |
| Years of education, mean (SD) | 9.3 (3.1) | 11.4 (2.7) | <.0001 |
| Rural [to age 19], % | 33.7 | 24.6 | <.0001 |
| White collar job, % | 22.8 | 44.7 | <.0001 |
| Has ApoE4 allele, % | 37.9 | 34.5 | .2183 |
| History of smoking, % | 62.4 | 56.0 | .0003 |
| History of alcohol, % | 37.6 | 38.0 | .8243 |
| Hypertension, % | 65.1 | 75.5 | <.0001 |
| Depression, % | 6.4 | 10.9 | <.0001 |
| Heart attack, % | 15.7 | 14.0 | .1798 |
| Stroke, % | 12.2 | 15.2 | .0145 |
| CHD, % | 28.1 | 30.7 | .0973 |
| Diabetes, % | 24.7 | 29.2 | .0049 |
| Cancer, % | 12.1 | 16.8 | .0002 |
| Parkinson’s disease, % | 1.1 | 0.9 | .6026 |
| Statin usec | 12.0 | 24.5 | <.0001 |
| Antihypertensive medication usec | 70.6 | 77.0 | .0091 |
| Antidiabetic medication usec | 16.4 | 21.9 | .0163 |
Note. SD = standard deviation; CHD = coronary heart disease.
a N = 1,440.
b N = 1,836.
cMedication use taken from first assessment wave.
The three variables are highly correlated. Participants with childhood rural residence were more likely to have fewer years of education than those from urban residence (mean years of education 9.1 vs 11.0, p < .001). Those with childhood rural residence were also less likely to hold white-collar jobs (rural residence 21%, urban residence 40.5%, p < .001; see also Supplementary Table 3s).
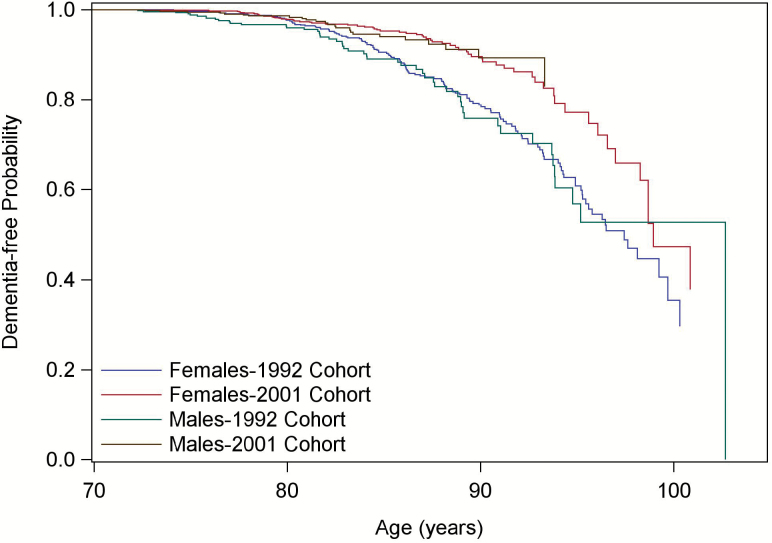
A Kaplan–Meier plot illustrates the significant difference in time to dementia between the 1992 and 2001 cohorts (p < .0001, Figure 1). The plot for time to AD was similar and is not shown.
Figure 1.
Kaplan–Meier plot of time to dementia by sex for the two cohorts.
We used a series of Cox proportional hazards models to determine whether the 2001 cohort differed from the 1992 cohort in dementia/AD risk and whether differences in childhood rural residence, education levels, and white-collar occupations accounted for the difference between the two cohorts (Table 2). Adjusting for baseline age and gender, Model 1 indicates that the 2001 cohort had significantly decreased risk of both dementia and AD compared to those in the 1992 cohort (hazard ratio [HR] = 0.62 for dementia and HR = 0.57 for AD). Models 2a, 2b, and 2c showed the separate association of childhood rural residence, lower education and nonwhite collar occupation with increased dementia/AD risk while differences between the two cohorts attenuated but remained significant. Model 3 in Table 2 included all three risk factors in the Cox proportional hazards model. We then investigated additional models including potential interactions among childhood rural residence, education, occupation, and cohort and found only one significant interaction between childhood rural residence and education for AD risk. Model 4 indicates that the effect of education on dementia/AD risk depends on childhood rural residence. For participants with urban upbringing, higher education level is not significantly associated with dementia/AD risk; But for participants with rural childhood residence, higher education level is significantly associated with reduced AD risk (HR = 0.87, p = .0477). The difference in dementia/AD risk between the two cohorts were attenuated mostly with the adjustment of educational level, but participants in the 2001 cohort remained at significantly lower risk for dementia and AD than those in the two cohorts.
Table 2.
Cohort Effect: Results From Cox Proportional Hazards Models Adjusting for Sex and Age
| Model number | Variables | Dementia | Alzheimer’s disease | ||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-Value | Hazard ratio (95% CI) | p-Value | ||
| 1 | 2001 cohort vs 1992 cohort | 0.62 (0.48–0.81) | .0004 | 0.57 (0.43–0.77) | .0002 |
| 2a | 2001 cohort vs 1992 cohort | 0.64 (0.49–0.84) | .0009 | 0.60 (0.44–0.80) | .0006 |
| Rural residence vs urban residence | 1.41 (1.11–1.80) | .0054 | 1.55 (1.19–2.02) | .0011 | |
| 2b | 2001 cohort vs 1992 cohort | 0.74 (0.57–0.98) | .0356 | 0.72 (0.53–0.98) | .0346 |
| Years of education | 0.92 (0.88–0.95) | <.0001 | 0.90 (0.86–0.94) | <.0001 | |
| 2c | 2001 cohort vs 1992 cohort | 0.66 (0.51–0.87) | .0026 | 0.62 (0.46–0.83) | .0017 |
| White collar vs. blue collar | 0.70 (0.54–0.91) | .0080 | 0.66 (0.49–0.89) | .0064 | |
| 3 | 2001 cohort vs 1992 cohort | 0.75 (0.57–0.99) | .0436 | 0.72 (0.53–0.99) | .0407 |
| Years of education | 0.93 (0.89–0.97) | .0011 | 0.91 (0.87–0.96) | .0002 | |
| Rural vs urban residence | 1.26 (0.98–1.62) | .0746 | 1.36 (1.03–1.79) | .0297 | |
| White collar vs blue collar | 0.90 (0.67–1.20) | .4651 | 0.91 (0.66–1.27) | .5858 | |
| 4 | 2001 cohort vs 1992 cohort | 0.75 (0.57–0.99) | .0393 | 0.72 (0.53–0.98) | .0363 |
| Rural vs urban residence | 2.34 (1.10–4.98) | .0264 | 2.97 (1.30–6.75) | .0095 | |
| White collar job vs nonwhite collar | 0.86 (0.64–1.16) | .3284 | 0.86 (0.62–1.21) | .3965 | |
| Years of education for urban residence | 0.96 (0.91–1.02) | .1656 | 0.95 (0.89–1.02) | .1371 | |
| Years of education for rural residence | 0.90 (0.85–0.95) | .0852* | 0.87 (0.82–0.93) | .0477* | |
*p-Values for testing the differences in hazard ratios for years of education between participants with urban and rural residence.
A Cox proportional hazards model using age at death as the outcome did not reveal a significant difference in mortality risk between the two cohorts (HR = 1.06, 95% CI: 0.96–1.17, p = .2784). The Cox proportional hazards model using only data collected in the 9-year follow-up period in the 1992 cohort also indicated a significantly lower dementia or AD risk in the 2001 cohort (HR = 0.65, p = .0015 for dementia, and HR = 0.65, p = .0057 for AD). The second sensitivity analysis using only data collected from 2001 onward in both cohorts also showed significantly lower dementia or AD risk in the 2001 cohort (HR = 0.58, p = .0105 for dementia, HR = 0.46, p = .0005 for AD).
This analysis focused primarily on the Indianapolis cohort. In contrast, there were no significant differences in rates for dementia and AD between the 1992 and 2001 cohorts for the Ibadan participants. The difference between the cohorts remained nonsignificant after adding age, sex, childhood residence, and education to the model (Supplementary Tables 1s and 2s).
Discussion
In this analysis, education levels, childhood rural residence, and white collar occupation were significantly associated with the rates of dementia/AD in both the 1992 and 2001 cohort in univariate models. However, when all three variables are included in the same model only education levels and to a lesser extent childhood rural residence are significantly associated with the dementia rates. When interactions between the three variables are included, the interaction between rural residence and levels of education is significantly associated with the cohort differences in AD rates and marginally in dementia (AD: p = .0477; dementia: p = .0852) but rural residence in childhood also remains significant for both dementia and AD. It should be noted that while the cohort difference for dementia rates were attenuated by adjusting for the three risk factors, most of the effect due to adjusting for education, it remained significant.
These results are similar to a previous report that rural childhood residence significantly increased the risk for dementia. Most of the elderly African Americans reporting childhood rural residence in this study came from the South including Kentucky and Tennessee. Approximately half may have been part of the “great migration” of southern African Americans to Northern Cities which occurred in the early part of the 20th century. The migration was stimulated by the increasingly drastic socioeconomic circumstances in the south, where many were sharecroppers, still under Jim Crow laws and now facing a catastrophic decline in the cotton industry due to extreme weather conditions and the invasion of the boll weevil (Marks, 1989). Participants with low education were likely subjected to particularly severe economic circumstances with families hovering on the brink of starvation as well as dealing with barriers to employment, health and social services as a result of institutionalized racism (Dollard, 1957). There is now considerable evidence that certain geographically defined communities, particularly in the south are at greater risk for chronic illnesses including cancer, vascular diseases, stroke, increased all-cause mortality rates, reduced life expectancy, and most recently, for greater recording of dementia/AD in death certificates (Dwyer-Lindgren et al., 2016; Karp et al., 2016; Labarthe et al., 2016; Rosenberg et al., 2016; Taylor, Greenlund, McGuire, Lu, & Croft, 2017). The explanations given for these geographically defined areas of increased risk include socioeconomic disparities, access to health care, lifestyle factors such as physical inactivity and smoking, obesity-increased rates of vascular risk factors, obesity, hypertension, and diabetes (Karp et al., 2016; Labarthe et al., 2016; Rosenberg et al., 2016). A disturbing finding from this study is that the increased risk for dementia and AD for residents of southern rural communities persists even after they have migrated to an urban environment. Early life risk factors have been associated with an increasing risk for AD in at least one other study, the risk being attributed to failure to reach complete levels of brain maturation (Moceri et al., 2000).
It is noteworthy that when all three variables, education, childhood rural residence, and occupational status are included in the dementia decline model, occupational status loses its significance (dementia: HR = 0.90, p = .4651; AD: HR = 0.91, p = .5858) reinforcing the concept that the major sociodemographic factors influencing dementia and AD rates may occur early in life. At least one other study involving rural and urban elderly populations has arrived at a similar conclusion, that occupation is not an independent protective factor against cognitive impairment, but is closely related to educational levels (Lorenzo-López et al., 2017). It is possible however that the occupational classification used may not identify elements of work that might have a more significant effect on dementia risk.
Higher education levels were significantly associated with the decline in dementia and AD rates as reported in other studies (Meng & D’Arcy, 2012; Satizabal et al., 2016; Langa et al., 2017). But when the interaction between residence and education levels are included in the models, higher levels of education only have a significant effect on AD rates and a trend for dementia rates for those participants from rural residence in childhood (AD: p = .0477, dementia: p = .0852). It has been suggested by Manly, Jacobs, Touradji, Small, and Stern (2002) that where quality of education is low, educational achievement rather than years of education may be a better predictor of cognitive outcomes than grade level (Manly et al., 2002; Sisco et al., 2015). There is considerable evidence that the quality of education in the rural South in 20s and 30s particularly for African Americans was low, considerably lower than those found in urban communities. Few schools were available in the rural South and those available taught only to the level of the eighth grade. These schools had high student to teacher ratios and in many of the schools attendance was often sporadic (Bauman, Bondi, Layman, McConnell, & Tompkins, 2001). Children from families wishing to obtain higher education had to leave home to attend centrally located boarding schools. It is possible therefore that the greater association for education in rural children reflected the need for more years of education to overcome the poor quality of education of the earlier childhood years. It is also possible that residence in boarding schools may have attenuated the risks of rural living. Most of the urban childhood residence group in this study lived in Indianapolis. Even though life was still hard in the segregated city, there were more job and educational opportunities in Indianapolis than in the rural south (Hall, Gao, Unverzagt et al., 2000). Compulsory Public Education and Mandatory School Attendance was in effect (Brady, 1996).
Even after all these variables are included in the models, cohort differences remain. It has been suggested that the declining rates of dementia can be attributed to better treatment of cardiovascular risk factors (Gao et al., 2016). In this study, the African Americans in the 2001 cohort actually had higher rates of medical conditions including diabetes, hypertension, and stroke, but they also had higher levels of medication use including the use of statins, antihypertensive and antidiabetic mediations. Intentions are to explore this further using a more comprehensive database.
Strengths
This study has the strength of long follow-ups in large dynamic cohorts of African Americans. Identical diagnostic criteria were used throughout the entire study period with the same basic group of clinicians conducting the clinical consensus process, thus ensuring diagnostic consistency. Detailed information was available for all three variables, childhood residence, occupation, and education levels.
Limitations
This is an observational study so the ability to make causal connections is limited. This is also a unique African American cohort, who lived through a time of extraordinary social and political upheaval. The generalizability of these findings to other populations including other African American populations may be limited. As the three variables are highly correlated, it is conceivable that differing sociodemographic circumstances could lead to different results. These models did not control for comorbidity rates and their treatment and a number of potentially important variables such as childhood family income, years of age at migration, and diet, were not available. The 2001 cohort had higher refusal rates than did the 1992 cohort raising the possibility of selection bias.
Conclusion
In conclusion, in this analysis rural residence in childhood and increased education levels particularly for the childhood rural residents were significantly associated with the rates of dementia and AD and accounted for some of the decline in rates between the 2001 and 1992 cohorts. Nevertheless, the cohort decline remained significant even after adjusting childhood residence and education. These results suggest the possible benefits of further exploration of the effects of both childhood residence and migration on health outcomes including dementia in elderly African Americans and the protective effects of higher levels of education for these residents, as well as a more intensive investigation into other possible protective factors such as better treatment of vascular risk factors.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (AG09956 and P30 AG012846) and the National Institutes of Health (R01 AG0145350).
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
Author Contributions: H. C. Hendrie planned the study including the instrumentation, interpreted data analysis, and wrote and revised the article. V. Smith-Gamble planned the study, assisted with the instrumentation, interpreted data analysis, and wrote and revised the article. K. A. Lane performed the statistical analyses and wrote and revised the article. C. Purnell wrote and revised the article. D. O. Clark interpreted the analysis and wrote and revised the article. S. Gao planned the study, supervised data analysis, and wrote and revised the article.
References
- American Psychiatric Association.(1987). Diagnostic and statistical manual of mental disorders (3rd Edition, revised). Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association Press (1992). ICD-10. The International Statistical Classification of Diseases and Related Health Problems: 1 and 2. Geneva: World Health Organization. [Google Scholar]
- Bauman J. S., Bondi V., Layman R., McConnell T., & Tompkins V (2001). The 1930s: Education: Overview. American Decades; Gale Virtual Reference Library Retrieved June 27, 2017, from go.galegroup.com/ps/i.do?p=GVRL&sw=w&u=iulib_iupui&v=2.1&id=GALE%7CCX3468301121&it=r&asid=72a0d33ee894f72accdb111c999b2664 [Google Scholar]
- Brady C. M. (1996). Indianapolis at the time of the Great Migration, 1900–1920. Black History News and Notes, 65. Retrieved from www.carolynbrady.com [Google Scholar]
- Callahan C. M., Hall K. S., Hui S. L., Musick B. S., Unverzagt F. W., & Hendrie H. C (1996). Relationship of age, education, and occupation with dementia among a community-based sample of African Americans. Archives of Neurology, 53, 134–140. doi:10.1001/archneur.1996.00550020038013 [DOI] [PubMed] [Google Scholar]
- Chan K. Y., Wang W., Wu J. J., Liu L., Theodoratou E., Car J., … Rudan I (2013). Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet, 381, 2016–2023. doi:10.1016/S0140-6736(13)60221–4 [DOI] [PubMed] [Google Scholar]
- Contador I., Bermejo-Pareja F., Puertas-Martin V., & Benito-Leon J (2015). Childhood and adulthood rural residence increases the risk of dementia: NEDICES study. Current Alzheimer Research, 12, 350–357. doi:10.2174/1567205012666150324181327 [DOI] [PubMed] [Google Scholar]
- Doblhammer G., Fink A., & Fritze T (2015). Short-term trends in dementia prevalence in Germany between the years 2007 and 2009. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 11, 291–299. doi:10.1016/j.jalz.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Dollard J. (1957). Caste and class in a southern town. Garden City, NJ: Anchor Books. [Google Scholar]
- Dwyer-Lindgren L., Bertozzi-Villa A., Stubbs R. W., Morozoff C., Kutz M. J., Huynh C., … Murray C. J (2016). US county-level trends in mortality rates for major causes of death, 1980–2014. JAMA, 316, 2385–2401. doi:10.1001/jama.2016.13645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S., Ogunniyi A., Hall K. S., Baiyewu O., Unverzagt F. W., Lane K. A., … Hendrie H. C (2016). Dementia incidence declined in African-Americans but not in Yoruba. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12, 244–251. doi:10.1016/j.jalz.2015.06.1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasset L., Brayne C., Joly P., Jacqmin-Gadda H., Peres K., Foubert-Samier A., … Helmer C (2016). Trends in dementia incidence: Evolution over a 10-year period in France. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 12, 272–280. doi:10.1016/j.jalz.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Hall K. S., Gao S., Baiyewu O., Lane K. A., Gureje O., Shen J., … Hendrie H. C (2009). Prevalence rates for dementia and Alzheimer’s disease in African Americans: 1992 versus 2001. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 5, 227–233. doi:10.1016/j.jalz.2009.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. S., Gao S., Emsley C. L., Ogunniyi A. O., Morgan O., & Hendrie H. C (2000). Community screening interview for dementia (CSI ‘D’); performance in five disparate study sites. International Journal of Geriatric Psychiatry, 15, 521–531. doi: 10.1002/1099–1166(200006)15:6<521::AID-GPS182>3.0.CO;2-F [DOI] [PubMed] [Google Scholar]
- Hall K. S., Gao S., Unverzagt F. W., & Hendrie H. C (2000). Low education and childhood rural residence: Risk for Alzheimer’s disease in African Americans. Neurology, 54, 95–99. doi:10.1212/WNL.54.1.95 [DOI] [PubMed] [Google Scholar]
- Hall K. S., Ogunniyi A. O., Hendrie H. C., Osuntokun B. O., Hui S. L., Musick B. S., … Baiyewu O. A (1996). A cross-cultural community based study of dementias: Methods and performance of the survey instrument: Indianapolis, U.S.A. and Ibadan, Nigeria. International Journal of Methods in Psychiatry Research, 6, 129–142. doi:10.1002/(SICI)1234-988X(199610)6:3%3C129::AID-MPR164%3E3.3.CO;2-A [Google Scholar]
- Hendrie H. C., Hall K. S., Brittain H. M., Austrom M. G., Farlow M., Parker J., & Kane M (1988). The CAMDEX: A standardized instrument for the diagnosis of mental disorder in the elderly: A replication with a US sample. Journal of the American Geriatrics Society, 36, 402–408. doi:10.1111/j.1532–5415.1988.tb02378.x [DOI] [PubMed] [Google Scholar]
- Hendrie H. C., Lane K. A., Ogunniyi A., Baiyewu O., Gureje O., Evans R., … Hall K. S (2006). The development of a semi-structured home interview (CHIF) to directly assess function in cognitively impaired elderly people in two cultures. International Psychogeriatrics, 18, 653–666. doi:10.1017/S104161020500308X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie H. C., Ogunniyi A., Hall K. S., Baiyewu O., Unverzagt F. W., Gureje O., … Hui S. L (2001). Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. JAMA, 285, 739–747. doi:10.1001/jama.285.6.739 [DOI] [PubMed] [Google Scholar]
- Karp D. N., Wolff C. S., Wiebe D. J., Branas C. C., Carr B. G., & Mullen M. T (2016). Reassessing the stroke belt: Using small area spatial statistics to identify clusters of high stroke mortality in the United States. Stroke, 47, 1939–1942. doi:10.1161/STROKEAHA.116.012997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarthe D. R., Howard G., Safford M. M., Howard V. J., Judd S. E., Cushman M., … Kissela B. M; Reasons for Geographic and Racial Differences in Stroke (REGARDS) Investigators (2016). Incidence and case fatality at the county level as contributors to geographic disparities in stroke mortality. Neuroepidemiology, 47, 96–102. doi:10.1159/000449102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa K. M., Larson E. B., Crimmins E. M., Faul J. D., Levine D. A., Kabeto M. U., & Weir D. R (2017). A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Internal Medicine, 177, 51–58. doi:10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa K. M., Larson E. B., Karlawish J. H., Cutler D. M., Kabeto M. U., Kim S. Y., & Rosen A. B (2008). Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity?Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 4, 134–144. doi:10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo-López L., Millán-Calenti J. C., López-López R., Diego-Diez C., Laffon B., Pásaro E., … Maseda A (2017). Effects of degree of urbanization and lifetime longest-held occupation on cognitive impairment prevalence in an older Spanish population. Frontiers in Psychology, 8, 162. doi:10.3389/fpsyg.2017.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly J. J., Jacobs D. M., Touradji P., Small S. A., & Stern Y (2002). Reading level attenuates differences in neuropsychological test performance between African American and White elders. Journal of the International Neuropsychological Society, 8, 341–348. doi:10.1017/S1355617702813157 [DOI] [PubMed] [Google Scholar]
- Manton K. C., Gu X. L., & Ukraintseva S. V (2005). Declining prevalence of dementia in the U.S. elderly population. Advances in Gerontology, 16, 30–37. [PubMed] [Google Scholar]
- Marks C. (1989). Farewell-We’re good and gone. Bloomington, IN: Indiana University Press. [Google Scholar]
- Matthews F. E., Stephan B. C., Robinson L., Jagger C., Barnes L. E., Arthur A., … Brayne C; Cognitive Function and Ageing Studies (CFAS) Collaboration (2016). A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nature Communications, 7, 11398. doi:10.1038/ncomms11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., & Stadlan E. M (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology, 34, 939–944. doi:10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- Meng X., & D’Arcy C (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One, 7, e38268. doi:10.1371/journal.pone.0038268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moceri V. M., Kukull W. A., Emanuel I., van Belle G., & Larson E. B (2000). Early-life risk factors and the development of Alzheimer’s disease. Neurology, 54, 415–420. doi:10.1212/WNL.54.2.415 [DOI] [PubMed] [Google Scholar]
- Morris J. C., Heyman A., Mohs R. C., Hughes J. P., van Belle G., Fillenbaum G., … Clark C (1989). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology, 39, 1159–1165. [DOI] [PubMed] [Google Scholar]
- Ohara T., Hata J., Yoshida D., Mukai N., Nagata M., Iwaki T., … Ninomiya T (2017). Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology, 88, 1925–1932. doi:10.1212/WNL.0000000000003932 [DOI] [PubMed] [Google Scholar]
- Rocca W. A., Petersen R. C., Knopman D. S., Hebert L. E., Evans D. A., Hall K. S., … White L. R (2011). Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 7, 80–93. doi:10.1016/j.jalz.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg B. L., Kellar J. A., Labno A., Matheson D. H., Ringel M., VonAchen P., … Moses H. III (2016). Quantifying geographic variation in health care outcomes in the United States before and after risk-adjustment. PLoS One, 11, e0166762. doi:10.1371/journal.pone.0166762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Tym E., Mountjoy C. Q., Huppert F. A., Hendrie H., Verma S., & Goddard R (1986). CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. The British Journal of Psychiatry: The Journal of Mental Science, 149, 698–709. doi:10.1192/bjp.149.6.698 [DOI] [PubMed] [Google Scholar]
- Satizabal C. L., Beiser A. S., Chouraki V., Chêne G., Dufouil C., & Seshadri S (2016). Incidence of dementia over three decades in the Framingham Heart Study. The New England Journal of Medicine, 374, 523–532. doi:10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers E. M., Verhaaren B. F., Koudstaal P. J., Hofman A., Ikram M. A., & Breteler M. M (2012). Is dementia incidence declining? Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology, 78, 1456–1463. doi:10.1212/WNL.0b013e3182553be6 [DOI] [PubMed] [Google Scholar]
- Sisco S., Gross A. L., Shih R. A., Sachs B. C., Glymour M. M., Bangen K. J., … Manly J. J (2015). The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 70, 557–567. doi:10.1093/geronb/gbt133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Office of Federal Statistical Policy and Standards. Standardized Occupational Classification Manual. (1980). Washington, DC: United States Department of Commerce, Office of Federal Statistical Policy and Standards. [Google Scholar]
- Taylor C. A., Greenlund S. F., McGuire L. C., Lu H., & Croft J. B (2017). Deaths from Alzheimer’s Disease—United States, 1999–2014. Morbidity and Mortality Weekly Report, 66, 521–526. doi:10.15585/mmwr.mm6620a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Commerce.(1904). Statistical abstract of the United States. Washington, DC: Government Printing Office. [Google Scholar]
- U.S. Department of Commerce.(1922). Fourteenth census of the United States taken in 1920. Washington, DC: Government Printing Office. [Google Scholar]
- U.S. Department of Commerce.(1975). US Bureau of the Census historical statistics of the United States, colonial times to 1970 (Bicentennial ed. Part I ed. Vol. A43). Washington, DC: Government Printing Office. [Google Scholar]
- Wu Y. T., Beiser A. S., Breteler M. M. B., Fratiglioni L., Helmer C., Hendrie H. C., … Brayne C (2017). The changing prevalence and incidence of dementia over time -—Current evidence. Nature Reviews. Neurology, 13, 327–339. doi:10.1038/nrneurol.2017.63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.