This editorial refers to ‘Electrical coupling between ventricular myocytes and myofibroblasts in the infarcted mouse heart’ by M. Rubart et al., pp. 389–400.
Cardiac arrhythmias are a very important clinical problem. Ventricular tachyarrhythmias (VTs) are a leading cause of death in the developed world,1 and atrial fibrillation (AF) is a major cause of morbidity, mortality and preventable stroke.2 The underlying mechanisms are under intense investigation, in order to better understand the underlying pathophysiology and design improved mechanism-based therapies.3
1. Role and mechanisms of arrhythmia promotion by cardiac fibrosis
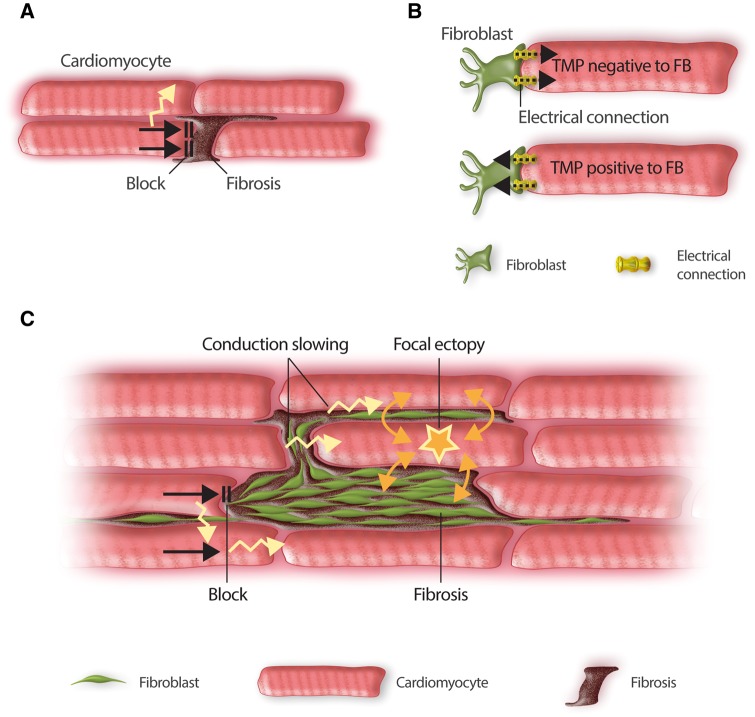
Cardiac fibrosis has been shown to play an important role in the occurrence of both VTs4 and AF.5 Fibrosis is known to interfere with cardiac bioelectricity in a number of potential ways (Figure 1). Replacement (or reparative) fibrosis occurs in response to cardiomyocyte cell death and replaces dead cardiomyocytes by scar tissue. This occurs in settings of significant cardiomyocyte loss, including after a myocardial infarction,4 in the ventricles of patients with long-standing heart failure,4 and in the atria in association with a wide variety of cardiac diseases and risk factors.2,5 The resulting collagen scar physically separates cardiomyocytes in muscle bundles,6 producing conduction blocks and slowing (Figure 1A) that enhance the likelihood of re-entrant excitation. In addition, profibrotic conditions cause fibroblast proliferation and differentiation to larger and more structurally complex myofibroblasts.7 There is evidence that fibroblasts, and more so myofibroblasts, can form electrical connections with cardiomyocytes.5,7 Fibroblasts possess ion-channels and have a typical resting potential of about −30 mV, although they do not have an active Phase-0 current or generate action potentials (APs).5 When coupled to cardiomyocytes, fibroblasts interact electrically (Figure 1B), depolarizing cardiomyocytes when the cardiomyocyte transmembrane potential (TMP) is negative to the fibroblast resting potential (e.g. during Phase 4) and repolarizing cardiomyocytes when the cardiomyocyte TMP is positive to the fibroblast resting potential (e.g. during the AP peak and plateau).
Figure 1.
Basic mechanisms through which fibrosis can affect cardiac electrical function. (A) Fibrotic extracellular matrix (primarily cross-linked collagen) acting as a physical conduction barrier between cardiomyocytes, causing conduction block and forcing conduction to take circuitous, slower routes. (B) Electrical connections between fibroblasts (FBs) and cardiomyocytes (CMs), e.g. mediated by connexin channels or nanotubes, allow current to pass whenever their TMP differs. Positive ions travel from FBs to cardiomyocytes whenever CM TMP is negative to that of FBs (e.g. depolarizing CMs during phase 4) and from CMs to FBs when CM TMP is positive to that of FBs (e.g. reducing the AP overshoot and abbreviating the early AP-plateau). (C) The arrhythmic consequences of fibrosis. Fibrotic tissue that acts as a barrier to conduction promotes re-entry by causing unidirectional conduction block and forcing conduction to travel transversely through more circuitous routes. FB–CM coupling can favour re-entry by acting as an electrotonic load that slows conduction and by abbreviating AP-duration. In addition, FBs can produce spontaneous arrhythmic activity in coupled CMs via depolarization-induced automaticity.
The consequences of these changes are summarized in Figure 1C. Replacement fibrosis causes conduction blocks that can initiate local re-entry and force the impulse into slow and circuitous conduction pathways that favour the maintenance of re-entry.6,8 When a sufficiently large number of myofibroblasts are coupled to cardiomyocytes, several arrhythmogenic mechanisms can result. Depolarization induced by current drawn from cardiomyocytes during Phase-4 causes spontaneous activity,9 while conduction-slowing and repolarization-abbreviation promote re-entry.10
2. Requirements for arrhythmia-promotion by fibroblast-cardiomyocyte coupling and experimental challenges
The ability of replacement fibrosis to promote arrhythmogenesis by acting as a physical barrier to conduction is well-documented.4,6,8 The most convincing evidence of arrhythmogenesis caused by myofibroblast–cardiomyocyte coupling has been in heterocellular fibroblast/cardiomyocyte co-cultures.9,10 The biological requirements for such interactions have been well-established in biophysical models.11 Critical determinants include the number of fibroblasts coupled to each cardiomyocyte and the extent of cardiomyocyte-fibroblast coupling.11 Both fibroblasts and myofibroblasts are small cells with sparse cytoplasm, making precise visualization of their structures and interactions with cardiomyocytes difficult. Histological studies have had great difficulty identifying structures, like connexins and nanotubes, that could mediate functional communication between fibroblasts/myofibroblasts and cardiomyocytes.12
3. Novel technical approaches provide powerful new insights
Advances in cell-type specific transgenic (Tg) expression of reporter-genes have opened up new avenues for the assessment of cardiomyocyte-fibroblast electrical connection. Quinn et al.13 expressed a voltage-sensitive dye (VSPF2.3) in Tg mice under the control of cardiomyocyte- (myosin heavy-chain, MHC) or non-cardiomyocyte- (Wilm’s tumour suppressor-1) specific promoters to record APs from either cell-type. With this technology, they were able to demonstrate cardiomyocyte-like APs from non-cardiomyocyte cells at the border of healed cryoinjury-lesions, providing strong evidence for heterocellular coupling. Furthermore, they identified tunnelling nanotubes as a potential structural basis.
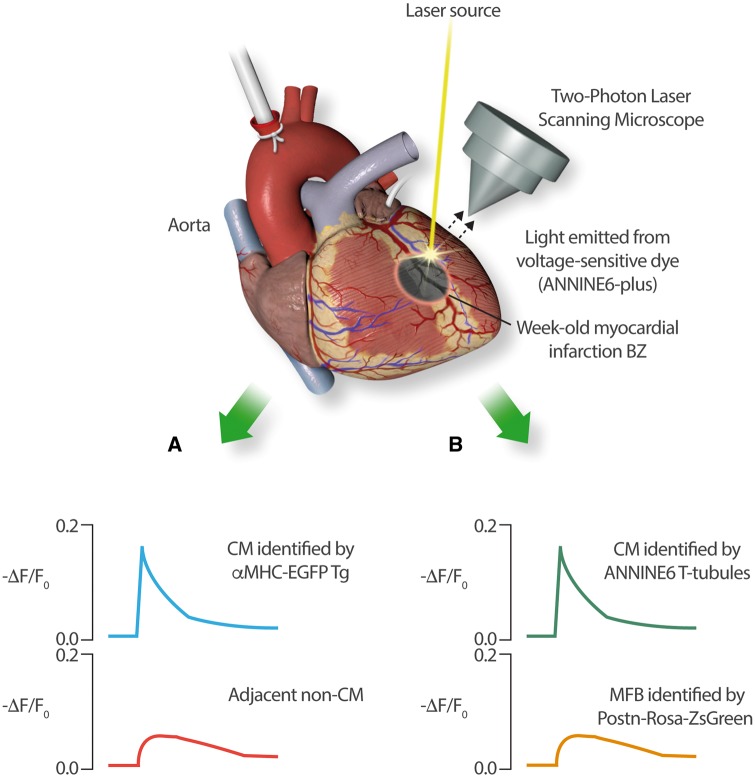
In the present issue of Cardiovascular Research, Rubart et al. take this technology further and provide compelling direct evidence for electrical interactions between cardiomyocytes and myofibroblasts in the peri-infarction border zone.14 Their experimental approach is illustrated in Figure 2. They created 2 cell-type specific Tg mouse-lines, one with enhanced green-fluorescent protein (EGFP) under the control of the cardiomyocyte-specific α-MHC promoter and the other expressing the Rosa-ZsGreen reporter under the control of the myofibroblast-specific periostin (Postn) promoter. They then induced anterior-wall myocardial infarctions by coronary–artery occlusion in each line and recorded cellular electrical activity in Langendorff-perfused hearts with the voltage-sensitive dye ANINE6 7–10 days post-infarction. Two-photon laser-scanning microscopy was used to simultaneously record electrical activity from cardiomyocyte and non-cardiomyocyte cells in the infarct border-zone. In the αMHC–EGFP mice, they recorded typical brisk APs from cardiomyocytes, as well as clear simultaneous activity, slower in upstroke and smaller in amplitude, from non-cardiomyocytes (Figure 2A). In Postn-Rosa-ZSGreen hearts, they recorded virtually identical slow/small-amplitude activity from myofibroblasts simultaneous to brisk APs in cardiomyocytes identified by ANINE6-staining of transverse-tubules (Figure 2B). In addition, they analyse connexin43 interconnections at cardiomyocyte-myofibroblast connections histochemically, estimating them to be expressed at ≈5% of the level in cardiomyocyte- intercalated-disc regions.
Figure 2.
A schematic drawing of the Rubart experimental paradigm (as described in reference14). Two-photon scanning confocal laser microscopy was used to record optical APs emitted by the voltage-dependent dye ANINE6-plus from adjacent cells. Reporter dyes expressed in Tg mice under the control of cell-type specific promoters were used to allow the identification of adjacent CM and non-CM cells in the border zone of 7- to 10-day-old myocardial infarctions. Two sets of Tg mice were studied: (A) α-MHC/ EGFP Tg-mice, in which CMs were identified by green fluorescence and (B) periostin (Postn)/Rosa-ZsGreen expressing mice, in which myofibroblasts (MFBs) fluoresced and adjacent CMs could be identified by ANINE6-plus staining of transverse (T)-tubules. In both sets of experiments, CMs recorded typical brisk APs, whereas MFBs showed simultaneous voltage-deflections (indicated by changes in fluorescence, F) that were slower in kinetics and much smaller in amplitude.
These results establish unequivocally for the first time that border-zone fibroblasts show coupled electrical activity with cardiomyocytes. Second, they show this in a clinically-relevant myocardial infarction model. Thirdly, they provide new insights into the kinetics and amplitudes of these responses. Finally, they provide a putative structural basis in terms of cardiomyocyte–fibroblast connexin43 junctions.
4. Potential significance, limitations and unanswered questions
Understanding the mechanisms by which fibrosis affects cardiac electrical function is very important. Fibrosis is an important contributor to arrhythmogenesis and new mechanistic insights are needed to devise innovative and more effective therapeutic approaches. If myofibroblast–cardiomyocyte electrical coupling is important in arrhythmogenesis, then targeting of fibroblast electrical properties (such as fibroblast ion-channels and fibroblast-cardiomyocyte connections) may be a viable antiarrhythmic strategy. Fibroblast ion-channel expression is altered by cardiac disease, and these alterations may contribute to arrhythmogenesis if there is significant fibroblast-to-cardiomyocyte coupling.15
Some limitations of the Rubart study need to be appreciated. First and foremost, both the Rubart study14 and that by Quinn et al.13 suggest that fibroblasts adjacent to a myocardial scar are coupled to cardiomyocytes, but neither indicates precisely how much or how they are coupled. Significant effects on cardiac arrhythmogenesis require substantial numbers of fibroblasts connected to cardiomyocytes with an important degree of connectivity.7,11 Whether or not biological coupling is sufficient to contribute to arrhythmogenesis remains unresolved. In addition, significant cell-to-cell communication is not the only way for adjacent cells to affect each other’s bioelectricity. If a non-excitable cell (like a fibroblast) cell-membrane is in close physical contact with the membrane of an excitable cell (like a cardiomyocyte) generating relatively large currents, the non-excitable cell-membrane may show measurable passive voltage-changes in the absence of direct electrical connection. This type of ephaptic response is compatible with the slow and small myofibroblast voltage-changes seen in in the Rubart study, which could simply represent charging of their membrane-capacitance. It would be of great interest to be able to selectively uncouple myofibroblasts from cardiomyocytes in an in situ model, and to determine the changes in cardiomyocyte APs and arrhythmogenesis that result. Second, the Rubart study (like the Quinn investigation) examines what may be the ‘best case’ scenario for myofibroblast–cardiomyocyte coupling, in the border region of a myocardial scar. How these observations apply to other fibrotic conditions, like the ventricular and atrial substrate in the presence of heart failure or other profibrotic conditions; remains to be determined. Nevertheless, these studies are an important step forward in the understanding of cardiomyocyte-fibroblast interaction, and the authors are to be congratulated on successfully completing elegant, innovative and challenging experimental series of great importance to the field.
Funding
The Canadian Institutes of Health Research (Foundation Grant 148401) and the Heart and Stroke Foundation of Canada (G-16-00012708).
Conflict of interest: none declared.
References
- 1. Steinberg C, Laksman ZW, Krahn AD.. Sudden cardiac death: a reappraisal. Trends Cardiovasc Med 2016;26:709–719. [DOI] [PubMed] [Google Scholar]
- 2. Andrade J, Khairy P, Dobrev D, Nattel S.. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 3. Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XH, Dobrev D, Nattel S.. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc Res 2016;109:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Disertori M, Rigoni M, Pace N, Casolo G, Masè M, Gonzini L, Lucci D, Nollo G, Ravelli F.. Myocardial Fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc Imaging 2016; 9:1046–1055. [DOI] [PubMed] [Google Scholar]
- 5. Yue L, Xie J, Nattel S.. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res 2011;89:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, Nattel S.. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res 2009;105:1213–1222. [DOI] [PubMed] [Google Scholar]
- 7. Rohr S. Arrhythmogenic implications of fibroblast-myocyte interactions. Circ Arrhythm Electrophysiol 2012;5:442–452. [DOI] [PubMed] [Google Scholar]
- 8. Tschabrunn CM, Roujol S, Nezafat R, Faulkner-Jones B, Buxton AE, Josephson ME, Anter E.. A swine model of infarct-related reentrant ventricular tachycardia: electroanatomic, magnetic resonance, and histopathological characterization. Heart Rhythm 2016;13:262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miragoli M, Salvarani N, Rohr S.. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res 2007;101:755–758. [DOI] [PubMed] [Google Scholar]
- 10. Zlochiver S, Muñoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J.. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys J 2008; 95:4469–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacCannell KA, Bazzazi H, Chilton L, Shibukawa Y, Clark RB, Giles WR.. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J 2007;92:4121–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kohl P, Gourdie RG.. Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue? J Mol Cell Cardiol 2014;70:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quinn TA, Camelliti P, Rog-Zielinska EA, Siedlecka U, Poggioli T, O’Toole ET, Knöpfel T, Kohl P.. Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics. Proc Natl Acad Sci USA 2016;113:14852–14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rubart M, Tao W, Lu XL, Conway SJ, Reuter SP, Lin SF, Soorpas MH, Electrical coupling between ventricular myocytes and myofibroblasts in the infarcted mouse heart. Cardiovasc Res 2018;114:389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aguilar M, Qi XY, Huang H, Comtois P, Nattel S.. Fibroblast electrical remodeling in heart failure and potential effects on atrial fibrillation. Biophys J 2014;107:2444–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]