Abstract
Background
Long-term data on the influence of smoking on risk of microscopic colitis are limited. We therefore sought to examine and characterize the association between smoking and risk of incident microscopic colitis in two large prospective cohorts of women.
Methods
We conducted a prospective study of 231015 women enrolled in the Nurses’ Health Study [NHS] and NHSII. Information regarding smoking, other lifestyle factors and medications were collected biennially from 1976 to 2012 in NHS and from 1989 to 2013 in NHSII. Incident cases of microscopic colitis were confirmed through physician medical record review. We used Cox proportional hazards modelling to examine the association between smoking and risk of microscopic colitis.
Results
We documented 166 incident cases of microscopic colitis over 6122779 person-years of follow up. Compared to non-smokers, the multivariable-adjusted hazard ratio [HR] for microscopic colitis was 2.52 (95% confidence interval [CI] 1.59–4.00) amongst current smokers and 1.54 [95% CI 1.09–2.17] amongst past smokers. The risk increased with higher pack-years of smoking [p trend = 0.001] and diminished following smoking cessation [p trend = 0.017]. Current smoking appeared to be more strongly associated with risk of collagenous colitis [HR 3.68; 95% CI 1.94–6.97] than lymphocytic colitis [HR 1.71; 95% CI 0.83–3.53].
Conclusion
In two large prospective cohort studies, we observed an association between current smoking and risk of microscopic colitis. Risk of microscopic colitis appeared to increase with higher pack-years and diminish following smoking cessation. Future studies focused on characterizing the biological mechanisms underlying these associations are warranted.
Keywords: Smoking, microscopic colitis, collagenous colitis, lymphocytic colitis, Nurses’ Health Study, tobacco
1. Introduction
Microscopic colitis is a debilitating and increasingly recognized inflammatory disorder of the large intestine, characterized by an increased number of intraepithelial lymphocytes in the colon with or without expansion of collagen fibres in the lamina propria.1 In contrast to inflammatory bowel disease [IBD], mucosal abnormalities are rarely observed in microscopic colitis.2 Nevertheless, the incidence and prevalence of the disease have been rising over the past three decades.1,3 Despite the significant morbidity associated with the disease, data on potential aetiological factors are sparse.
Smoking, through its effect on the gut microbiota, mucosal barrier functions and microvasculature, is widely thought to be associated with an increased risk of Crohn’s disease while exerting a protective effect on the risk of ulcerative colitis.4–6 However, there are limited data on the link between smoking and microscopic colitis. Studies that have examined the association between smoking and risk of microscopic colitis are few, and are limited by their retrospective or case control design,7–9 limited ascertainment of exposures,7,8,10–13 and use of historical data or hospital-based cohorts to define smoking prevalence amongst controls,8,10 therefore increasing the risk of exposure misclassification and recall bias. Furthermore, no prior study has prospectively examined the long-term impact of intensity of smoking or smoking cessation on microscopic colitis. We therefore propose to investigate these associations in two large prospective cohorts of US women.
2. Methods
2.1. Study population
The Nurses’ Health Study [NHS] is a prospective cohort study established in 1976 when 121700 female nurses aged 30–55 years residing in 11 US states returned mailed health questionnaires. The NHSII is a similar prospective cohort study established in 1989 with a mailed questionnaire to 116686 female nurses aged 25–42 years. For both cohorts, follow-up questionnaires regarding medical and lifestyle information have been mailed every 2 years with a greater than 90% response rate. For this study, follow up was through the 2012 questionnaire for NHS and 2013 questionnaire for NHSII. At baseline, we excluded participants who reported a history of IBD or cancer [except for non-melanoma skin cancer], or did not provide information on smoking at baseline. This study was approved by the Partners HealthCare Institutional Review Board.
2.2. Assessment of smoking
We have previously detailed our assessment of smoking in the NHS and NHSII cohorts.14,15 Briefly, in baseline questionnaires, 1976 for NHS and 1989 for NHSII, participants were asked to provide information about their smoking status [current or past], the number of cigarettes smoked per day [either current or at the time they last smoked if previous smokers] and the age at which they started smoking. Follow-up biennial questionnaires asked participants to update their smoking status and the number of cigarettes smoked per day [either current or at the time they last smoked]. Number of cigarettes per day was also queried in categories [‘1 to 4’, ‘5 to 14’, ‘15 to 24’, ‘25 to 34’, ‘35 to 44’ or ‘45 or more’ cigarettes per day], and was converted to pack-years by converting cigarettes per day to packs [one pack = 20 cigarettes] and multiplying by cumulative exposure in years. For past smokers, months since quitting were derived from an open-ended response for age at last cigarette on the baseline questionnaire. This number was updated with each biennial questionnaire based on report of smoking status. Rates of smoking in NHS have been shown to reflect US national smoking rates over time.15
2.3. Assessment of covariates
Height, weight, menopausal status, oral contraceptive use [never, past, current], menopausal hormone therapy [MHT] [never, past, current] and diabetes were queried in the 1976 questionnaire for NHS and the 1989 questionnaire for NHSII. These variables were updated biennially over the follow-up period. Body mass index [BMI] was calculated using height and weight. Self-reported weight was validated in 1990 through measurements of a subset of 140 NHS participants, with high correlation [r = 0.97].16 Oral contraceptive use was validated by telephone interview of structured life events in a 1997 study of 215 randomly selected NHSII participants, with 99% agreement between the responses from the interview and the general questionnaires.17 Menopausal status was also validated in a subset of NHS participants, with reproducibility of menopause status of 98.8% between the 1978 and 1980 questionnaires.18
Race and ethnicity were queried in the 1992 and 2004 questionnaires for NHS and the 1989 and 2005 questionnaires for NHSII. These categories included South European/Mediterranean ancestry, Scandinavian ancestry, other Caucasian ancestry, African-American ancestry, Hispanic ancestry, Asian ancestry, Native American ancestry and other.
Starting in 1990 and every 2 years since then, the NHS questionnaire was expanded to include information on use of non-steroidal anti-inflammatory drugs [NSAIDs].19–21 Similarly, in NHSII, information on regular use of NSAIDs was collected at baseline [1989 questionnaire] and updated biennially through follow up. Consistent with previous analyses, we defined regular use of NSAIDs as intake of two or more tablets per week.19–21
Information on proton pump inhibitors [PPIs], selective serotonin reuptake inhibitors [SSRIs], angiotensin-converting enzyme inhibitors [ACEIs], beta blockers, statins and diuretics has been collected and consistently updated in NHS and NHSII as they became available in the US market. Consistent with their prescription patterns in the US, starting in 2000 in NHS and 2001 in NHSII, information on use of all of these medications was available and updated over follow-up time.
2.4. Ascertainment of cases
In both cohorts, a diagnosis of microscopic colitis was obtained through self-report on the general questionnaire and validated through physician medical record review. In brief, since 1976 in NHS and 1989 in NHSII, participants have reported diagnoses of IBD, including Crohn’s disease, ulcerative colitis and microscopic colitis, through open-ended response on biennial surveys. In addition, a diagnosis of IBD has been specifically queried since 1982 in NHS and 1991 in NHSII. When a diagnosis was reported on any biennial questionnaire, a supplementary questionnaire was sent asking participants specifically for diagnoses of Crohn’s disease, ulcerative colitis or microscopic colitis. Related medical records were requested and reviewed by two gastroenterologists blinded to exposure information.
We excluded participants who subsequently denied the diagnosis of microscopic colitis on the supplementary questionnaire or declined permission to review their records. Data were extracted on laboratory findings, microbiology studies [e.g. stool studies], endoscopic findings and histopathology. Confirmation of all cases was based on two-physician review of histopathology records confirming a pathologist diagnosis of microscopic colitis. Information on type of microscopic colitis, namely lymphocytic colitis and collagenous colitis, were also obtained when available.
Of 241 reports of microscopic colitis, we confirmed 166 [69%] incident cases through follow up after medical record review. Of these, 154 cases had pathology reports that contained enough histological detail to discriminate between subtypes of collagenous colitis [n = 78] and lymphocytic colitis [n = 76]. The remaining 12 histopathology records stated a diagnosis of ‘microscopic colitis’ without further detail.
2.5. Statistical analysis
Participants began to accrue person-time of follow-up beginning at the date of return of their baseline questionnaire through to the date of diagnosis of microscopic colitis, death, last returned questionnaire or end of follow-up, whichever came first. We observed no heterogeneity in the association of smoking and risk of microscopic colitis in separate analyses of NHS and NHSII [p for heterogeneity > 0.50]. Thus, we pooled individual-level data from NHS and NHSII and adjusted for cohort in all analyses.
For our primary analysis, we used age-adjusted and multivariable-adjusted Cox proportional hazards modelling with time-varying exposure and covariate data to determine the hazard ratio [HR] and 95% confidence interval [CI] for microscopic colitis according to smoking status. We included age [months], cohort, BMI, oral contraceptive use, menopausal hormone therapy use and diabetes as covariates in the multivariable model. As NSAID use was not queried until 1990 in NHS, we performed a sensitivity analysis limiting our follow up to after 1990 in NHS and 1989 in NHSII when NSAID information was available. We also performed a sensitivity analysis limiting our follow up to after 2000, when other medications, including PPIs, SSRIs, beta blockers, ACEIs, statins and diuretics, were consistently available.
For secondary analyses, we performed age-adjusted and multivariable-adjusted Cox proportional hazards analysis to evaluate the relationship between pack-years [cumulative exposure] of smoking and time since cessation and risk of microscopic colitis. Missing exposure values for pack-years and time since smoking cessation were present in ≤ 1% of ever smokers and were set to the median values for ever smokers. We used the median value for each category of pack-years and ordinal categories of years since smoking cessation to evaluate for significance of linear trends. We additionally evaluated the association between smoking and microscopic colitis according to strata defined by cohort, BMI and NSAID use, and evaluated for potential interactions using cross-classified categories of these risk factors and smoking. We tested the significance of interactions by using the log-likelihood ratio test comparing the model with cross-classified categories with a model that included these factors as independent variables. We tested for the proportional hazards assumption by examining the interaction between age in categories and smoking variables and observed no violations [all p > 0.65]. A two-sided p-value < 0.05 was considered statistically significant. All analyses were done using SAS 9.4.
3. Results
Amongst 231015 women, we documented 166 incident cases of microscopic colitis over 6122779 person-years of follow up. The baseline characteristics of women according to smoking status are presented in Table 1. At baseline, 54.5% of participants were non-smokers, 22.3% were former smokers and 23.2% were current smokers. Compared to never users, current smokers were more likely to be post-menopausal and regular users of NSAIDs, and less likely to be taking oral contraceptives. Baseline characteristics of participants according to age, BMI and menopausal hormone therapy were similar according to smoking status.
Table 1.
Baseline characteristics of participants in the Nurses’ Health Study [NHS] and NHSII according to smoking status1.
| Smoking status | |||
|---|---|---|---|
| Never [n = 125857] | Past [n = 51547] | Current [n = 53582] | |
| NHS, n | 50 851 | 26942 | 38149 |
| NHSII, n | 75006 | 24605 | 15433 |
| Age in years, mean [SD] | 37.8 [7.2] | 39.5 [7.0] | 40.6 [7.3] |
| Body mass index, mean [SD] | 24.1 [4.8] | 24.1 [4.6] | 23.5 [4.2] |
| White race, % | 95.3 | 97.7 | 97.2 |
| Oral contraceptive use, % | |||
| Never | 33.7 | 31.9 | 39.4 |
| Ever | 66.3 | 68.1 | 60.6 |
| Post-menopausal, % | 15.1 | 18.2 | 25.2 |
| Menopausal hormone therapy use2, % | |||
| Past | 15.8 | 17.4 | 18.7 |
| Current | 28.5 | 30.0 | 26.3 |
| Regular NSAID use3, % | 18.1 | 20.8 | 24.1 |
| Diabetes4, % | 1.2 | 1.3 | 1.4 |
1Baseline data derived from 1976 questionnaire for NHS and 1989 questionnaire for NHSII for all data except for race. Race derived from 1992 and 2004 questionnaires for NHS, and 1989 and 2005 questionnaires for NHSII.
2Amongst post-menopausal women.
3NSAID = non-steroidal anti-inflammatory drug; amongst patients in the NHSII 1989 questionnaire. NSAID data not available at baseline [1976 questionnaire] for patients in NHS. Regular use is defined as ≥2 tablets per week.
4Type I or type II.
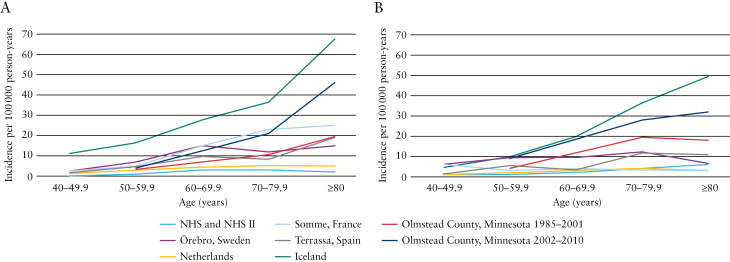
Figure 1 demonstrates the age-related incidence rate of collagenous colitis and lymphocytic colitis in the NHS and NHSII, as compared to cohorts from Örebro, Sweden;22 Somme, France;23 the Netherlands;24 Iceland;25 Terrassa, Spain;26 and Olmstead County, Minnesota.3,27 The incidence rate of microscopic colitis rose steadily from 1/100000 person-years in women aged 40–49.9 years to 9/100000 person-years in women aged ≥ 80 years, similar to that described in many European cohorts,22–24,28 but lower than that described in some North American cohorts.3,27,29
Figure 1.
Comparison of age-specific incidence rates of collagenous colitis [A] and lymphocytic colitis [B] in the Nurses’ Health Study and Nurses’ Health Study II and cohorts in France, Sweden, the Netherlands, Spain, Iceland and Minnesota, USA.
The mean age of microscopic colitis in this cohort was 64.7 years [SD 11.1]. There was no significant difference in mean age of onset of microscopic colitis between ever smokers [65.1 years; SD 9.6] and never smokers [64.0 years; SD 13.3; p = 0.56]. Compared to never smokers, the age-adjusted HR of microscopic colitis was 1.56 [95% CI 1.11–2.19] amongst past smokers and 2.59 [95% CI 1.64–4.10] amongst current smokers. These estimates were not significantly altered when adjusted for age, BMI, oral contraceptive use, MHT use, diabetes and cohort. The multivariable-adjusted HR of microscopic colitis was 1.54 [95% CI 1.09–2.17] amongst past smokers and 2.52 [95% CI 1.59–4.00] amongst current smokers, compared to never smokers [p trend < 0.0001; Table 2].
Table 2.
Smoking status, pack-years and years since smoking discontinuation, and risk of microscopic colitis in the Nurses’ Health Study [NHS] and NHSII.
| No. of cases | No. of person-years | Age-adjusted hazard ratio [95% CI] | Multivariable-adjusted hazard ratio [95% CI]1 | |
|---|---|---|---|---|
| Smoking status | ||||
| Never | 61 | 3285487 | [reference] | [reference] |
| Past | 78 | 1966863 | 1.56 [1.11, 2.19] | 1.54 [1.09, 2.17] |
| Current | 27 | 870429 | 2.59 [1.64, 4.10] | 2.52 [1.59, 4.00] |
| p trend | <0.0001 | <0.0001 | ||
| Pack-years of smoking | ||||
| Never | 61 | 3285487 | [reference] | [reference] |
| ≤ 10 years | 35 | 1102419 | 1.56 [1.03, 2.37] | 1.53 [1.00, 2.32] |
| 10.1–20 years | 17 | 687487 | 1.28 [0.75, 2.20] | 1.27 [0.74, 2.18] |
| 20.1–30 years | 19 | 410589 | 2.28 [1.36, 3.84] | 2.23 [1.32, 3.75] |
| 30.1–50 years | 24 | 444067 | 2.32 [1.43, 3.75] | 2.31 [1.42, 3.75] |
| > 50 years | 10 | 192729 | 1.79 [0.90, 3.55] | 1.79 [0.90, 3.56] |
| p trend2 | 0.0042 | 0.0013 | ||
| Years since smoking discontinuation | ||||
| Current smokers | 27 | 870429 | [reference] | [reference] |
| 0–5 years | 11 | 310251 | 0.94 [0.47–1.90] | 0.97 [0.47–1.95] |
| >5 years | 67 | 1656601 | 0.57 [0.36–0.89] | 0.57 [0.36–0.91] |
| Never smokers | 61 | 3285498 | 0.39 [0.24–0.61] | 0.40 [0.24–0.63] |
| p trend3 | 0.012 | 0.017 | ||
1Adjusted for age [months], cohort [NHS, NHS2], body mass index [<20, 20–24.9, 25–29.9, ≥ 30], oral contraceptive use [never, past, current], menopausal hormone therapy [MHT] use [never-MHT use, past MHT use, current MHT use], diabetes.
2Linear test for trend using ordinal variables based on median pack-years for each category.
3 p trend was estimated by entering years since smoking discontinuation in the model as a continuous variable excluding the ‘never smokers’ category.
We examined the risk of microscopic colitis according to intensity of smoking defined by pack-years of use and observed a statistically significant trend [p trend = 0.001; Table 2]. As compared to never smokers, the multivariable-adjusted HRs were 1.27 [95% CI 0.74–2.18] for 10.1–20 pack-years of use, 2.23 [95% CI 1.32–3.75] for 20.1–30 pack-years of use, 2.31 [95% CI 1.42–3.75] for 30.1–50 pack-years of use and 1.79 [95% CI 0.90–3.56] for > 50 pack-years of use.
We also examined the relationship between smoking cessation and risk of microscopic colitis among ever smokers and observed that the risk declined significantly over time [p trend = 0.017; Table 2]. As compared to current smokers, past smokers within 5 years since quitting had a multivariable-adjusted HR of 0.97 [95% CI 0.47–1.95], while those greater than 5 years since quitting had a multivariable-adjusted HR of 0.57 [95% CI 0.36–0.91].
We explored the association between smoking and risk of microscopic colitis according to different subtypes of microscopic colitis, collagenous colitis and lymphocytic colitis. While current smoking was associated with an increased risk of collagenous colitis, we did not observe a statistically significant association between smoking and risk of lymphocytic colitis [Table 3]. As compared to never smokers, the multivariable-adjusted HR of collagenous colitis was 1.82 [95% CI 1.09–3.06] in past smokers and 3.68 [95% CI 1.94–6.97] in current smokers [p trend ≤ 0.0001]. In contrast, the multivariable-adjusted HRs of lymphocytic colitis in past smokers [HR 1.18; 95% CI 0.72–1.94] and current smokers [HR 1.71; 95% CI 0.83–3.53] were not statistically significantly increased as compared to never smokers [p heterogeneity for microscopic colitis subtype = 0.24].
Table 3.
Smoking and risk of microscopic colitis according to disease subtype1.
| Never | Past | Current | p heterogeneity | |
|---|---|---|---|---|
| Collagenous colitis | 0.24 | |||
| No. of cases | 25 | 37 | 16 | |
| Person-years | 3285521 | 1966903 | 870436 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.83 [1.09, 3.07] | 3.79 [2.01, 7.15] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 1.82 [1.09, 3.06] | 3.68 [1.94, 6.97] | |
| Lymphocytic colitis | ||||
| No. of cases | 33 | 33 | 10 | |
| Person-years | 3285513 | 1966902 | 870442 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.22 [0.75, 1.99] | 1.80 [0.88, 3.68] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 1.18 [0.72, 1.94] | 1.71 [0.83, 3.53] | |
1Amongst patients with microscopic colitis with confirmed histological subtype.
2Adjusted for age [months], cohort [NHSI, NHSII], body mass index [< 20, 20–24.9, 25–29.9, ≥ 30], oral contraceptive use [never, past, current], menopausal hormone therapy [MHT] use [never-MHT use, past MHT use, current MHT use], diabetes.
As NSAID use has been widely implicated as a risk factor for microscopic colitis, we first performed a sensitivity analysis restricting our follow up to after 1990 in NHS and 1989 in NHSII when information on NSAID intake was also available. Compared to never smokers, the multivariable-adjusted HR for microscopic colitis was 1.50 [95% CI 1.06–2.12] among past smokers and 2.45 [95% CI 1.52–3.95] among current smokers [p trend = 0.0002], adjusting for NSAID use, age, cohort, BMI, oral contraceptive use, menopausal hormone therapy use and diabetes. On multivariable analysis, regular NSAID use was independently associated with incident microscopic colitis [HR 1.75; 95% CI 1.27–2.40].
We also considered the possibility that other medications previously shown to be associated with risk of microscopic colitis may at least in part explain our observed association and therefore performed a sensitivity analysis restricting our follow up to after 2000 in NHS and 2001 in NHSII, when these medications were both available in the US and were consistently ascertained in our cohorts. Compared to never smokers, the multivariable-adjusted HRs for microscopic colitis were 1.49 [95% CI 1.01–2.18] amongst past smokers and 2.83 [95% CI 1.57–5.11] amongst current smokers after further adjusting our models for use of NSAIDs, PPIs, SSRIs, ACEIs, beta blockers, statins and diuretics. Notably, use of diuretics [HR 1.71; 95% CI 1.16–2.53], PPIs [HR 1.59; 95% CI 1.07–2.36] and SSRIs [HR 1.61; 95% CI 1.01–2.57] were also independently associated with risk of microscopic colitis.
We considered the possibility that our observed associations may be due to differential health-seeking behaviours amongst women according to smoking status. Therefore, we examined the association between smoking and microscopic colitis amongst women who reported an annual physical examination in their last follow-up questionnaire and observed similar findings. Compared to never smokers, the multivariable-adjusted HRs of microscopic colitis were 1.55 [95% CI 1.06–2.26] amongst past smokers and 3.10 [95% CI 1.87–5.14] amongst current smokers. Similarly, amongst women who reported having undergone colonoscopy or sigmoidoscopy over follow-up, compared to never smokers, the multivariable-adjusted HRs of microscopic colitis were 1.48 [95% CI 0.85–2.56] amongst past smokers and 2.41 [95% CI 1.02–5.69] amongst current smokers.
Finally, we considered the possibility that age, BMI or NSAID use may modify the association between smoking and risk of microscopic colitis. We therefore examined the association between smoking and risk of microscopic colitis according to strata defined by age, age of diagnosis, cohort, BMI and NSAID use. Within each subgroup, we observed generally consistent associations [all p interaction ≥ 0.41, Table 4].
Table 4.
Smoking and risk of microscopic colitis according to selected strata.
| Never | Past | Current | p interaction | |
|---|---|---|---|---|
| Age | 0.65 | |||
| Age < 55 years | ||||
| No. of cases | 19 | 13 | 7 | |
| Person-years | 2124675 | 959899 | 594949 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.51 [0.74, 3.07] | 2.25 [0.94, 5.41] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 1.47 [0.72, 3.00] | 2.17 [0.90, 5.23] | |
| Age ≥ 55 years | ||||
| No. of cases | 42 | 65 | 20 | |
| Person-years | 1160812 | 1006965 | 275480 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.58 [1.07, 2.34] | 2.74 [1.60, 4.70] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 1.56 [1.05, 2.30] | 2.78 [1.62, 4.79] | |
| Cohort | 0.89 | |||
| NHS Cohort | ||||
| No. of cases | 39 | 62 | 17 | |
| Person-years | 1636244 | 1332836 | 630554 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.54 [1.03, 2.30] | 2.35 [1.32, 4.18] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 1.51 [1.01, 2.25] | 2.36 [1.32, 4.22] | |
| NHSII cohort | ||||
| No. of cases | 22 | 16 | 10 | |
| Person-years | 1649243 | 634027 | 239875 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.61 [0.84, 3.08] | 3.15 [1.48, 6.71] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 1.60 [0.83, 3.06] | 3.09 [1.45, 6.60] | |
| BMI2 | 0.63 | |||
| BMI < 25 kg/m2 | ||||
| No. of cases | 31 | 38 | 13 | |
| Person-years | 1712642 | 967000 | 537667 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.50 [0.93, 2.44] | 2.04 [1.06, 3.94] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 1.48 [0.91, 2.41] | 2.08 [1.08, 4.02] | |
| BMI ≥ 25 kg/m2 | ||||
| No. of cases | 30 | 40 | 14 | |
| Person-years | 1572845 | 999863 | 332761 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.57 [0.97, 2.55] | 3.23 [1.70, 6.15] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 1.50 [0.92, 2.44] | 3.20 [1.67, 6.10] | |
| NSAID use | 0.41 | |||
| No NSAID use3 | ||||
| No. of cases | 34 | 34 | 12 | |
| Person-years | 1473110 | 865113 | 256310 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.15 [0.71, 1.86] | 2.21 [1.14, 4.32] | |
| Multivariable-adjusted HR [95% CI]1 | [reference] | 1.12 [0.69, 1.82] | 2.23 [1.14, 4.36] | |
| Regular NSAID use3 | ||||
| No. of cases | 25 | 42 | 13 | |
| Person-years | 856610 | 539516 | 156981 | |
| Age-adjusted hazard ratio [95% CI] | [reference] | 1.99 [1.20, 3.31] | 2.94 [1.49, 5.82] | |
| Multivariable-adjusted hazard ratio [95% CI]1 | [reference] | 2.01 [1.21, 3.34] | 2.91 [1.46, 5.77] | |
1Adjusted for age [months], cohort [NHSI, NHSII], body mass index [<25, ≥25], oral contraceptive use [never, past, current], menopausal hormone therapy [MHT] use [never-MHT use, past MHT use, current MHT use], diabetes. Models adjusted for these covariates minus the strata variables [cohort, BMI, NSAID use].
2Body mass index.
3Amongst patients with reported NSAID data.
4. Discussion
In two large prospective cohorts of US women, we demonstrated a positive association between smoking and risk of microscopic colitis. The risk of microscopic colitis appeared to increase with higher pack-years of smoking. Although risk persisted for women who stopped smoking within 5 years, women who quit smoking for more than 5 years had an attenuated risk. In addition, our data suggest a stronger association between smoking and the collagenous colitis subtype of microscopic colitis as compared to lymphocytic colitis.
Our findings regarding the impact of smoking on the rate of incident microscopic colitis are consistent with prior studies. Vigren and colleagues demonstrated higher rates of smoking amongst collagenous colitis cases compared to the general population (37% vs 17%, odds ratio [OR] = 2.95).10 Other case-control studies have shown an increased risk of both collagenous and lymphocytic colitis with active smoking.8,9 Interestingly, Yen et al.8 demonstrated that the risk was stronger for collagenous colitis [OR = 5.36, 95% CI 2.67–10.75] compared to lymphocytic colitis [OR = 3.81, 95% CI 2.00–7.27]. Finally, Baert and colleagues demonstrated a higher rate of smoking amongst patients with collagenous colitis compared to lymphocytic colitis.7 Nevertheless, many of these studies were limited by a lack of detailed information on many potential risk factors for microscopic colitis, including BMI and NSAID or other medication use.8,10,11 Through rigorous sensitivity analyses accounting for other medications associated with microscopic colitis, we demonstrate that the association between smoking and microscopic colitis is independent of that explained by use of PPIs, SSRIs, ACEIs, diuretics, beta-blockers or NSAIDs. Additionally, in our cohort, consistent with prior studies, PPIs, diuretics and SSRIs are independently associated with risk of microscopic colitis.2,9,30–42 Furthermore, our long-term prospective cohort study adds novel data regarding the impact of cumulative tobacco exposure and smoking cessation on risk of microscopic colitis, demonstrating that while increased total dose of smoking is associated with an increased risk of microscopic colitis, this risk may be mitigated over time with smoking cessation. Therefore, our study, in addition to better characterizing the association between smoking and microscopic colitis, greatly expands upon prior studies examining this relationship.
The exact mechanism underlying the association between smoking and microscopic colitis remains largely unknown. Nevertheless, several plausible mechanisms regarding the role of smoking exist, including modification of human gut microbiota, regulation of immune function and modification of epithelial barrier function. Similar mechanisms have been proposed for the previously observed positive association between smoking and increased risk of incident Crohn’s disease, another chronic inflammatory disorder of the gastrointestinal tract.5,6,43–47 Further studies may provide novel connections in the pathogenesis of these two inflammatory bowel diseases.
Our observation that smoking is particularly associated with an increased risk of collagenous colitis is biologically plausible. The pathogenesis of collagenous colitis appears to be related to myofibroblast dysfunction48 and fibrolysis imbalance,49 either as a primary process or as a response to microbiome changes or underlying lymphocytic infiltration.50–52 Indeed, there is increased expression of transforming growth factor-beta [TGF-β] and tissue inhibitor of metalloproteinase-1 [TIMP-1], important regulators of extracellular matrix breakdown and fibrosis, in the colonic mucosa of patients with collagenous colitis as compared to healthy controls.49,53 The hypothesis that fibrosis may occur as a reaction to underlying inflammatory or microbiome changes is supported by rat models of chronic colitis, in which colonic inoculation of anaerobic bacterial strains led to upregulation of TGF-β and an increase in collagen deposition.52 Smoking has been associated with dysbiosis in Crohn’s disease,5,47,54 and thus may also contribute to pathogenesis of collagenous colitis through its effect on the microbiome. Interestingly, smoking has also been shown to directly upregulate TGF-β and TIMP-1 in chronic obstructive pulmonary disease and pulmonary fibrosis, which in turn lead to fibrosis and airway remodelling.55–59 Smoking may alternatively have a similar effect in the large intestine and therefore play a key role in the pathogenesis of collagenous colitis. By either reactive upregulation of TGF-β or direct myofibroblast dysfunction, an increase in TGF-β and fibrinogenesis promoted by smoking may be responsible for the disproportionate association of smoking with collagenous colitis as compared to lymphocytic colitis.
Our study has several strengths. First, we utilized two large cohorts with prospective collection of detailed smoking exposure data for over 35 years to assess the relationship between smoking and microscopic colitis. Second, detailed and updated smoking exposure data allowed us to assess intensity of smoking and influence of smoking cessation to add novel, temporal characterization of these exposures with microscopic colitis. Third, outcomes were confirmed by medical record review by two physicians, an advantage over studies that used diagnostic coding to ascertain outcomes. Furthermore, > 90% survey response rates in this cohort60 and ability to adjust for numerous confounders through prospectively collected, detailed, time-varying covariate data minimized the biases that are typically observed in case control and retrospective studies.
Our study has several limitations that are worth noting. Although this was a large, nationwide prospective cohort study, the majority of participants in our cohort were white women, possibly limiting the generalizability of our findings. However, previous studies have demonstrated that the risk of microscopic colitis is highest amongst women,1,61,62 making our cohorts ideal populations to study risk factors for microscopic colitis. Although it is one of the largest longitudinal studies of microscopic colitis conducted, we did rely on self-report to initially ascertain outcomes, with confirmation by physician medical record review. As such, some cases of microscopic colitis may have been missed, either due to under-reporting or absence of colonoscopy performed for symptoms. However, all participants in this study were health professionals, increasing their likelihood of accurately reporting a medical diagnosis. In addition, such outcome misclassification would probably have biased our effect estimates toward the null. Lastly, our results were consistent across numerous sensitivity analyses.
In sum, this is the first prospective cohort study to confirm the previously reported association between smoking and risk of incident microscopic colitis and further characterize the relationship by examining the impact of intensity, duration and cessation of smoking on the risk. Further studies are required to understand the mechanistic basis for the association of smoking and microscopic colitis.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health [T32 DK007191 to KEB; K23 DK099681 to HK; K24 DK098311 to ATC; and P30 DK043351 to the Center for the Study of Inflammatory Bowel Disease at Massachusetts General Hospital]. It was also supported by a career development award from the American Gastroenterological Association [AGA] to HK, an MGH Research Scholars Award to ATC, and a career development award from the Crohn’s and Colitis Foundation to PL. The Nurses’ Health Study and Nurses’ Health Study II are supported by the National Cancer Institute at the National Institutes of Health [UM1 CA186107 and UM1 CA176726, respectively].
Conflict of Interest
HK receives consulting fees from Abbvie, Takeda and Samsung Bioepis. HK also receives grant support from Takeda. ANA serves on the scientific advisory board of Abbvie, Takeda and Merck. ATC receives consulting fees from Janssen, Pfizer Inc. and Bayer Healthcare. The remaining authors have no conflicts to disclose.
Acknowledgments
KEB: study concept and design, data acquisition, analysis and interpretation of data, drafting manuscript, critically revising manuscript; ANA: data acquisition, critically revising manuscript; PL: data acquisition, critically revising manuscript; OO: study concept and design, critically revising manuscript; JFL: study concept and design, critically revising manuscript; JMR: study concept and design, data acquisition, critically revising manuscript ATC: data acquisition, analysis and interpretation of data, critically revising manuscript; HK: study concept and design, data acquisition, analysis and interpretation of data, drafting manuscript, critically revising manuscript. All authors have approved the final version of the manuscript, including the authorship list.
References
- 1. Pardi DS, Kelly CP. Microscopic colitis. Gastroenterology 2011;140:1155–65. [DOI] [PubMed] [Google Scholar]
- 2. Kane JS, Rotimi O, Ford AC. Macroscopic findings, incidence and characteristics of microscopic colitis in a large cohort of patients from the United Kingdom. Scand J Gastroenterol 2017;52:988–94. [DOI] [PubMed] [Google Scholar]
- 3. Pardi DS, Loftus EV Jr, Smyrk TC et al. . The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut 2007;56:504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergeron V, Grondin V, Rajca S et al. . Current smoking differentially affects blood mononuclear cells from patients with Crohn’s disease and ulcerative colitis: relevance to its adverse role in the disease. Inflamm Bowel Dis 2012;18:1101–11. [DOI] [PubMed] [Google Scholar]
- 5. Benjamin JL, Hedin CR, Koutsoumpas A et al. . Smokers with active Crohn’s disease have a clinically relevant dysbiosis of the gastrointestinal microbiota. Inflamm Bowel Dis 2012;18:1092–100. [DOI] [PubMed] [Google Scholar]
- 6. Burke KE, Boumitri C, Ananthakrishnan AN. Modifiable environmental factors in inflammatory bowel disease. Curr Gastroenterol Rep 2017;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baert F, Wouters K, D’Haens G et al. . Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut 1999;45:375–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yen EF, Pokhrel B, Du H et al. . Current and past cigarette smoking significantly increase risk for microscopic colitis. Inflamm Bowel Dis 2012;18:1835–41. [DOI] [PubMed] [Google Scholar]
- 9. Fernández-Bañares F, de Sousa MR, Salas A et al. . RECOMINA Project, GETECCU Grupo Español de Enfermedades de Crohn y Colitis Ulcerosa. Epidemiological risk factors in microscopic colitis: a prospective case-control study. Inflamm Bowel Dis 2013;19:411–7. [DOI] [PubMed] [Google Scholar]
- 10. Vigren L, Sjöberg K, Benoni C et al. . Is smoking a risk factor for collagenous colitis?Scand J Gastroenterol 2011;46:1334–9. [DOI] [PubMed] [Google Scholar]
- 11. Roth B, Gustafsson RJ, Jeppsson B, Manjer J, Ohlsson B. Smoking- and alcohol habits in relation to the clinical picture of women with microscopic colitis compared to controls. BMC Womens Health 2014;14:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Verhaegh BPM, Pierik MJ, Goudkade D, Cuijpers YSMT, Masclee AAM, Jonkers DMAE. Early life exposure, lifestyle, and comorbidity as risk factors for microscopic colitis: a case-control study. Inflamm Bowel Dis 2017;23:1040–6. [DOI] [PubMed] [Google Scholar]
- 13. Wickbom A, Nyhlin N, Montgomery SM, Bohr J, Tysk C. Family history, comorbidity, smoking and other risk factors in microscopic colitis: a case-control study. Eur J Gastroenterol Hepatol 2017;29:587–94. [DOI] [PubMed] [Google Scholar]
- 14. Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol 2012;107:1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarna L, Bialous SA, Jun HJ, Wewers ME, Cooley ME, Feskanich D. Smoking trends in the Nurses’ Health Study (1976–2003). Nurs Res 2008;57:374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 17. Hunter DJ, Manson JE, Colditz GA et al. . Reproducibility of oral contraceptive histories and validity of hormone composition reported in a cohort of US women. Contraception 1997;56:373–8. [DOI] [PubMed] [Google Scholar]
- 18. Colditz GA, Stampfer MJ, Willett WC et al. . Reproducibility and validity of self-reported menopausal status in a prospective cohort study. Am J Epidemiol 1987;126:319–25. [DOI] [PubMed] [Google Scholar]
- 19. Ananthakrishnan AN, Higuchi LM, Huang ES et al. . Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann Intern Med 2012;156:350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan AT, Manson JE, Albert CM et al. . Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation 2006;113:1578–87. [DOI] [PubMed] [Google Scholar]
- 21. Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 2005;294:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olesen M, Eriksson S, Bohr J, Järnerot G, Tysk C. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993–1998. Gut 2004;53:346–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fumery M, Kohut M, Gower-Rousseau C et al. . On behalf on the Somme MC group; EPIMAD group. Incidence, clinical presentation, and associated factors of microscopic colitis in Northern France: a population-based study. Dig Dis Sci 2017;62:1571–9. [DOI] [PubMed] [Google Scholar]
- 24. Verhaegh BP, Jonkers DM, Driessen A et al. . Incidence of microscopic colitis in the Netherlands. A nationwide population-based study from 2000 to 2012. Dig Liver Dis 2015;47:30–6. [DOI] [PubMed] [Google Scholar]
- 25. Agnarsdottir M, Gunnlaugsson O, Orvar KB et al. . Collagenous and lymphocytic colitis in Iceland. Dig Dis Sci 2002;47:1122–8. [DOI] [PubMed] [Google Scholar]
- 26. Fernández-Bañares F, Salas A, Esteve M et al. . Evolution of the incidence of collagenous colitis and lymphocytic colitis in Terrassa, Spain: a population-based study. Inflamm Bowel Dis 2011;17:1015–20. [DOI] [PubMed] [Google Scholar]
- 27. Gentile NM, Khanna S, Loftus EV Jr et al. . The epidemiology of microscopic colitis in Olmsted County from 2002 to 2010: a population-based study. Clin Gastroenterol Hepatol 2014;12:838–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kane JS, Rotimi O, Ford AC. Macroscopic findings, incidence and characteristics of microscopic colitis in a large cohort of patients from the United Kingdom. Scand J Gastroenterol 2017;52:988–94. [DOI] [PubMed] [Google Scholar]
- 29. Williams JJ, Kaplan GG, Makhija S et al. . Microscopic colitis-defining incidence rates and risk factors: a population-based study. Clin Gastroenterol Hepatol 2008;6:35–40. [DOI] [PubMed] [Google Scholar]
- 30. Fernández-Bañares F, Esteve M, Espinós JC et al. . Drug consumption and the risk of microscopic colitis. Am J Gastroenterol 2007;102:324–30. [DOI] [PubMed] [Google Scholar]
- 31. Verhaegh BP, de Vries F, Masclee AA et al. . High risk of drug-induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther 2016;43:1004–13. [DOI] [PubMed] [Google Scholar]
- 32. Masclee GM, Coloma PM, Kuipers EJ, Sturkenboom MC. Increased risk of microscopic colitis with use of proton pump inhibitors and non-steroidal anti-inflammatory drugs. Am J Gastroenterol 2015;110:749–59. [DOI] [PubMed] [Google Scholar]
- 33. Bonderup OK, Fenger-Grøn M, Wigh T, Pedersen L, Nielsen GL. Drug exposure and risk of microscopic colitis: a nationwide Danish case-control study with 5751 cases. Inflamm Bowel Dis 2014;20:1702–7. [DOI] [PubMed] [Google Scholar]
- 34. Fumery M, Kohut M, Gower-Rousseau C et al. . On behalf on the Somme MC group; EPIMAD group. Incidence, clinical presentation, and associated factors of microscopic colitis in Northern France: a population-based study. Dig Dis Sci 2017;62:1571–9. [DOI] [PubMed] [Google Scholar]
- 35. Thomson RD, Lestina LS, Bensen SP, Toor A, Maheshwari Y, Ratcliffe NR. Lansoprazole-associated microscopic colitis: a case series. Am J Gastroenterol 2002;97:2908–13. [DOI] [PubMed] [Google Scholar]
- 36. Wilcox GM, Mattia AR. Microscopic colitis associated with omeprazole and esomeprazole exposure. J Clin Gastroenterol 2009;43:551–3. [DOI] [PubMed] [Google Scholar]
- 37. Capurso G, Marignani M, Attilia F et al. . Lansoprazole-induced microscopic colitis: an increasing problem? Results of a prospecive case-series and systematic review of the literature. Dig Liver Dis 2011;43:380–5. [DOI] [PubMed] [Google Scholar]
- 38. O’Toole A, Coss A, Holleran G et al. . Microscopic colitis: clinical characteristics, treatment and outcomes in an Irish population. Int J Colorectal Dis 2014;29:799–803. [DOI] [PubMed] [Google Scholar]
- 39. Keszthelyi D, Jansen SV, Schouten GA et al. . Proton pump inhibitor use is associated with an increased risk for microscopic colitis: a case-control study. Aliment Pharmacol Ther 2010;32:1124–8. [DOI] [PubMed] [Google Scholar]
- 40. Law EH, Badowski M, Hung YT, Weems K, Sanchez A, Lee TA. Association between proton pump inhibitors and microscopic colitis. Ann Pharmacother 2017;51:253–63. [DOI] [PubMed] [Google Scholar]
- 41. Gentile NM, Yen EF. The incidence of microscopic colitis: microscopic no more. Dig Dis Sci 2017;62:1394–5. [DOI] [PubMed] [Google Scholar]
- 42. Tong J, Zheng Q, Zhang C, Lo R, Shen J, Ran Z. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:265–76; quiz 277. [DOI] [PubMed] [Google Scholar]
- 43. Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–17. [DOI] [PubMed] [Google Scholar]
- 44. Monick MM, Powers LS, Walters K et al. . Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol 2010;185:5425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sokol H, Pigneur B, Watterlot L et al. . Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 2008;105:16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Quévrain E, Maubert MA, Michon C et al. . Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut 2016;65:415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Allais L, Kerckhof FM, Verschuere S et al. . Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ Microbiol 2016;18:1352–63. [DOI] [PubMed] [Google Scholar]
- 48. Salas A, Fernández-Bañares F, Casalots J et al. . Subepithelial myofibroblasts and tenascin expression in microscopic colitis. Histopathology 2003;43:48–54. [DOI] [PubMed] [Google Scholar]
- 49. Günther U, Schuppan D, Bauer M et al. . Fibrogenesis and fibrolysis in collagenous colitis. Patterns of procollagen types I and IV, matrix-metalloproteinase-1 and -13, and TIMP-1 gene expression. Am J Pathol 1999;155:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Flejou JF, Grimaud JA, Molas G, Baviera E, Potet F. Collagenous colitis. Ultrastructural study and collagen immunotyping of four cases. Arch Pathol Lab Med 1984;108:977–82. [PubMed] [Google Scholar]
- 51. Mori S, Kadochi Y, Luo Y et al. . Proton pump inhibitor induced collagen expression in colonocytes is associated with collagenous colitis. World J Gastroenterol 2017;23:1586–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mourelle M, Salas A, Guarner F, Crespo E, García-Lafuente A, Malagelada JR. Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology 1998;114:519–26. [DOI] [PubMed] [Google Scholar]
- 53. Ståhle-Bäckdahl M, Maim J, Veress B, Benoni C, Bruce K, Egesten A. Increased presence of eosinophilic granulocytes expressing transforming growth factor-beta1 in collagenous colitis. Scand J Gastroenterol 2000;35:742–6. [DOI] [PubMed] [Google Scholar]
- 54. Opstelten JL, Plassais J, van Mil SW et al. . Gut microbial diversity is reduced in smokers with Crohn’s disease. Inflamm Bowel Dis 2016;22:2070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guan S, Liu Q, Han F et al. . Ginsenoside Rg1 ameliorates cigarette smoke-induced airway fibrosis by suppressing the TGF-β1/Smad pathway in vivo and in vitro. Biomed Res Int 2017;2017:6510198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Navratilova Z, Zatloukal J, Kriegova E, Kolek V, Petrek M. Simultaneous up-regulation of matrix metalloproteinases 1, 2, 3, 7, 8, 9 and tissue inhibitors of metalloproteinases 1, 4 in serum of patients with chronic obstructive pulmonary disease. Respirology 2012;17:1006–12. [DOI] [PubMed] [Google Scholar]
- 57. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J 2004;18:816–27. [DOI] [PubMed] [Google Scholar]
- 58. Higashimoto Y, Yamagata Y, Iwata T et al. . Increased serum concentrations of tissue inhibitor of metalloproteinase-1 in COPD patients. Eur Respir J 2005;25:885–90. [DOI] [PubMed] [Google Scholar]
- 59. Aschner Y, Downey GP. Transforming growth factor-β: master regulator of the respiratory system in health and disease. Am J Respir Cell Mol Biol 2016;54:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62. [DOI] [PubMed] [Google Scholar]
- 61. Larsson JK, Sonestedt E, Ohlsson B, Manjer J, Sjöberg K. The association between the intake of specific dietary components and lifestyle factors and microscopic colitis. Eur J Clin Nutr 2016;70:1309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Turner K, Genta RM, Sonnenberg A. Ethnic distribution of microscopic colitis in the United States. Inflamm Bowel Dis 2015;21:2634–9. [DOI] [PubMed] [Google Scholar]