Abstract
Background
In the current study, we determined the effects of interleukin (IL)-21 on human natural killer (NK) cells and monocyte responses during Mycobacterium tuberculosis (Mtb) infection.
Methods
We found that Mtb stimulated CD4+ and NK T cells from healthy individuals with latent tuberculosis infection (LTBI+) are major sources of IL-21. CD4+ cells from tuberculosis patients secreted less IL-21 than did CD4+ cells from healthy LTBI+ individuals. Interleukin-21 had no direct effect on Mtb-stimulated monocytes.
Results
Interleukin-21-activated NK cells produced interferon (IFN)-γ, perforin, granzyme B, and granulysin; lysed Mtb-infected monocytes; and reduced Mtb growth. Interleukin-21-activated NK cells also enhanced IL-1β, IL-18, and CCL4/macrophage-inflammatory protein (MIP)-1β production and reduced IL-10 production by Mtb-stimulated monocytes. Recombinant IL-21 (1) inhibited Mtb growth, (2) enhanced IFN-γ, IL-1β, IL-18, and MIP-1β, and (3) reduced IL-10 expression in the lungs of Mtb-infected Rag2 knockout mice.
Conclusions
These findings suggest that activated T cells enhance NK cell responses to lyse Mtb-infected human monocytes and restrict Mtb growth in monocytes through IL-21 production. Interleukin-21-activated NK cells also enhance the immune response by augmenting IL-1β, IL-18, and MIP-1β production and reducing IL-10 production by monocytes in response to an intracellular pathogen.
Keywords: cytokine, human, IL-21, NK cells, tuberculosis
Interleukin-21 produced by Mtb-activated CD4+ cells activate NK cells to produce IFN-γ and antimicrobial peptides to lyse Mtb-infected monocytes and to inhibit Mtb growth. Interleukin-21-activated NK cells enhanced IL-1β, IL-18, and MIP-1β production and reduced IL-10 production by Mtb-stimulated monocytes.
Natural killer (NK) cells play a central role in innate immunity to microbial pathogens [1]. They mediate protection against viruses, bacteria, and parasites by killing infected cells and secreting cytokines that shape the adaptive immune response [2]. Human NK cells lyse Mycobacterium tuberculosis (Mtb)-infected monocytes and alveolar macrophages and upregulate CD8+ T-cell responses [3, 4]. Natural killer cells produce interleukin (IL)-22, which inhibits intracellular growth of Mtb. Furthermore, NK cells lyse Mtb-expanded CD4+ regulatory T cells (Tregs) [5]. Blocking NK cells at the time of Bacillus Calmette-Guérin (BCG) vaccination enhances expansion of Tregs [6].
Natural killer cells express receptors for soluble factors including cytokines, which modulate NK cell function [7, 8]. It is well known that IL-2 produced by T cells is essential for optimal NK cell responses [9]. There is limited information available about the effect of other T-cell cytokines on NK cell responses. Recent studies have demonstrated that IL-21 produced by T cells enhances NK cell responses [10].
Interleukin-21 is a pleotropic cytokine that belongs to the class 1 family of cytokines [11]. The biological effects of IL-21 are mediated through IL-21R, which uses the common gamma chain (γc), as do other members of this family, including IL-2, IL-4, IL-7, IL-9, and IL-15 [12]. Activated CD4+ and NK T cells are major sources of IL-21 and affect the proliferation of T, B, and NK cells [12, 13]. Interleukin-21 has antitumor effects and is being tested in phase 2 clinical trials for treatment of patients with metastatic melanoma [14]. In viral infections, IL-21 contributes to the control of the persistent lymphocytic choriomeningitis virus [15] and improves T and NK cell function in individuals infected with human immunodeficiency virus (HIV) [16, 17]. In Mtb infection, memory-like NK cells contribute to vaccine-induced protective immune responses against Mtb infection, and IL-21 has been shown to mediate the development and expansion of memory-like NK cells in a murine model [18]. Interleukin-21 produced by CD4+ T cells promotes CD8+ T cell expansion and effector functions and is essential for the optimal control of Mtb infection in mice [19, 20]. However, the effect of IL-21 on the activation of human NK cells during Mtb and other bacterial infections has not been studied.
In the current study, using blood samples from individuals with latent tuberculosis infection (LTBI), patients with active tuberculosis (TB), and Rag2 knockout (KO) mice infected with Mtb, we determined the contribution of IL-21 towards NK cell-mediated host defenses against Mtb infection.
METHODS
Patient Population
Blood was obtained from 30 healthy LTBI individuals, 15 tuberculin-negative donors, and 10 HIV-seronegative patients with culture-proven pulmonary TB who had received anti-TB therapy for <4 weeks. Acid-fast stains of sputum were positive for 8 patients. All studies were approved by the Institutional Review Board of the University of Texas Health Science Center (Tyler, TX) and the Institutional Review Board of Blue Peter Public Health Research Centre (Hyderabad, India), and written informed consent was obtained from all participants.
Animals
All animal studies were performed on specific-pathogen-free 8-week-old female C57BL/6 (Jackson Laboratory, Bar Harbor, ME) and Rag2 KO mice (Taconic Biosciences, Rensselaer, NY). The Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler approved the studies. Animal procedures involving the care and use of mice were in accordance with the guidelines of National Institutes of Health/Office of Laboratory Animal Welfare.
Antibodies and Other Reagents
For flow cytometry, we used fluorescein isothiocyanate (FITC) anti-CD14, phycoerythrin (PE)-CY7 anti-CD3, FITC anti-CD4, APC anti-CD8, FITC anti-CD56 (all from BioLegend), and PE anti-IL-21 (eBioscience). For confocal microscopy, we used anti-granulysin, anti-perforin (Thermo Fisher Scientific), and anti-granzyme B (R&D Systems) as primary antibodies, and the secondary antibodies were goat anti-rabbit IgG (H+L) - Alexa Fluor 488 and goat anti-mouse IgG (H+L) - Alexa Fluor 647, obtained from Life Technologies; fluoroshield mounting medium with 4’,6-diamidino-2-phenylindole (DAPI) from Abcam (ab104139) was also used.
Detailed Methods for the Following Sections Were Provided in Supplementary Methods
Isolation of monocytes and CD3-CD56+ cells, culture of human peripheral blood mononuclear cells (PBMCs), culture of human CD3-CD56+ cells, and monocytes, flow cytometry, determination of Mtb H37Rv growth in human monocytes, aerosol infection of mice with Mtb H37Rv, quantitative real-time polymerase chain reaction (qRTPCR) for quantification, measurement of cytokine production, cytotoxicity assay, and confocal microscopy ([18, 21] and Supplementary Methods) were provided in the Supplementary methods.
Statistical Analysis
Prism 7 software (GraphPad Software, San Diego, CA) was used for statistical analyses. P values less than .05 were considered to be statistically significant. The results are expressed as the mean ± standard error. For data that were normally distributed, comparisons between groups were assessed by a paired or unpaired t test and one-way analysis of variance, as appropriate. For data that were not normally distributed, the Wilcoxon rank-sum test was used.
RESULTS
Interleukin-21 Production by Mycobacterium tuberculosis-Stimulated Peripheral Blood Mononuclear Cells
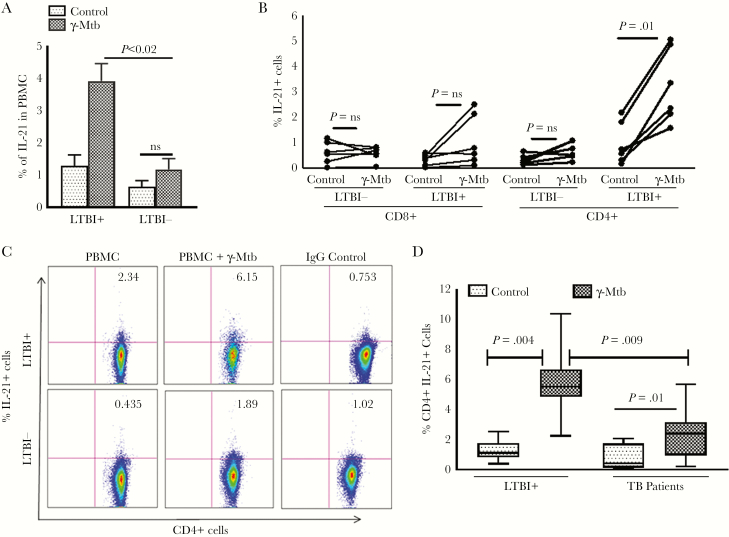
To determine whether IL-21 production depends on prior antigen exposure, we isolated PBMCs of 8 LTBI+ individuals, and we cultured them in the presence or absence of γ-Mtb. After 24 to 120 hours, IL-21 messenger ribonucleic acid (mRNA) was quantified by real-time PCR, normalized to glyceraldehyde 3-phosphate dehydrogenase mRNA to control for the efficiency of RNA extraction, and reverse transcribed in different samples. In the first 48 hours, a small increase was observed in the IL-21 mRNA levels by γ-Mtb-stimulated PBMCs of LTBI+ individuals. γ-Mtb induced a 5-fold, 11-fold, and 20-fold increase in IL-21 expression after 72 hours, 96 hours, and 120 hours, respectively (P = .001; Supplementary Figure 1A). γ-Mtb increased the percentage of IL-21+ cells (gated on total cells) in PBMCs of 6 LTBI+ individuals (1.0% ± 0.01% to 3.85% ± 1.23%, P < .02; Figure 1A), as determined by intracellular staining. By contrast, γ-Mtb did not significantly increase CD4+IL-21+ cells (gated on total PBMC) in PBMCs of 6 LTBI− individuals (Figure 1A). Our results suggest that IL-21 production depends on prior antigen exposure. We also found CD4+ and NK T cells are the major source for IL-21 in LTBI+ individuals (Figure 1B, C, Supplementary Figure 1B and Supplementary Results). Furthermore, our results demonstrate that IL-21 production by CD4+ cells is defective in TB patients (Figure 1D and Supplementary Results).
Figure 1.
Interleukin (IL)-21 production by Mycobacterium tuberculosis (Mtb)-stimulated peripheral blood mononuclear cells (PBMCs). Freshly isolated PBMCs from 6 healthy latent tuberculosis infection (LTBI)+ individuals were cultured with or without γ-irradiated M tuberculosis H37Rv (γ-Mtb, 10 μg/mL) for 120 hours. (A) The percentage of IL-21+ cells in total PBMCs (gated on all cells) was determined by flow cytometry. (B) The percentages of CD8+ cells and CD4+ cells that are IL-21+ were determined by flow cytometry. (C) A representative flow cytometry image (gated on CD4+IL-21+ cells) of 6 independent experiments is shown. (D) Freshly isolated PBMCs from healthy LTBI+ individuals and tuberculosis patients with active disease (10 donors in each group) were cultured with or without γ-irradiated Mtb H37Rv (γ-Mtb, 10 μg/mL). After 120 hours, the percentage of CD4+IL-21+ cells was determined by flow cytometry. The mean values, standard errors, and P values are shown.
Interleukin-21 Enhances Proinflammatory Cytokine and Chemokine Production by Peripheral Blood Mononuclear Cells of Latent Tuberculosis Infection+ Individuals
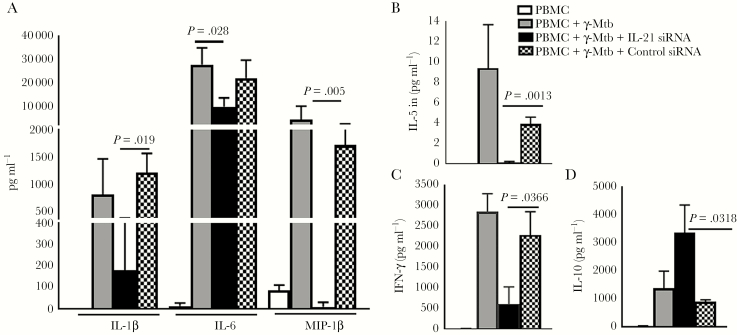
Previous studies have demonstrated that IL-21 regulates immune responses during infections and cancers [22, 23]. Because cytokines and chemokines play an important role during Mtb infection, we determined whether IL-21 has any effect on cytokine and chemokine production by PBMC of LTBI+ individuals. Peripheral blood mononuclear cells from 6 LTBI+ individuals were isolated, and cells were transfected with control or IL-21 small interfering RNA (siRNA). Control and IL-21 siRNA-transfected PBMCs were cultured with or without γ-Mtb. Interleukin-21 siRNA significantly inhibited IL-21 expression as determined by real-time PCR (Supplementary Figure 2A). After 120 hours, culture supernatants were collected and levels of 17 cytokines and chemokines were measured by Bio-Plex enzyme-linked immunosorbent assay. Interleukin-21 siRNA inhibited γ-Mtb-induced interferon (IFN)-γ, IL-1β, IL-6, MIP-1β, and IL-5 production (Figure 2A–C). By contrast, IL-21 siRNA enhanced γ-Mtb-induced IL-10 production by PBMCs (Figure 2D). Our results suggest that IL-21 produced by CD4+ cells and NK T cells during Mtb infection enhances IFN-γ, IL-1β, IL-6, MIP-1β, and IL-5 and inhibits IL-10 production.
Figure 2.
Effect of interleukin (IL)-21 small interfering ribonucleic acid (siRNA) on cytokine and chemokine production by peripheral blood mononuclear cells (PBMCs) of latent tuberculosis infection (LTBI)+ individuals. The PBMCs from 6 LTBI+ individuals were isolated, and cells were transfected with control or IL-21 siRNA as mentioned under Methods. Control PBMCs and siRNA-transfected PBMCs were cultured with or without γ-Mycobacterium tuberculosis (Mtb). After 120 hours, culture supernatants were collected and levels of various chemokines and cytokines were measured by multiplex enzyme-linked immunosorbent assay. The mean values, standard errors, and P values are shown.
Interleukin-21 Had No Direct Effect on Cytokine and Chemokine Production by Mycobacterium tuberculosis Stimulated Monocytes
In the above-mentioned experiment, we observed that IL-21 significantly enhances the production of cytokines predominantly produced by antigen presenting cells. We next determined the direct effect of IL-21 on cytokine production in Mtb-stimulated monocytes. We used recombinant IFN-γ, which is known to enhance cytokine production in Mtb-stimulated monocytes, as a positive control. Recombinant IFN-γ significantly enhanced IL-1β and MIP-1β and inhibited IL-10. By contrast, recombinant IL-21 had no direct effect on γ-Mtb-induced cytokine production by monocytes (Supplementary Figure 3). These results suggest that IL-21 has no direct effect on and acts through another immune cell population for enhancing cytokine production by Mtb-stimulated monocytes.
Interleukin-21-Activated Natural Killer Cells Enhance Proinflammatory Cytokine Production by Mycobacterium tuberculosis-Stimulated Monocytes
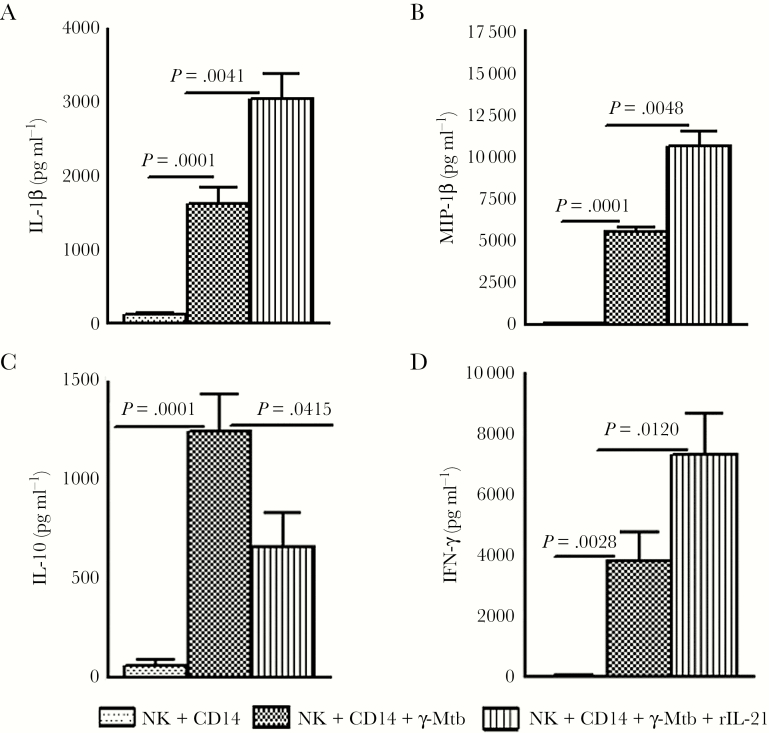
In a previous study, we demonstrated that IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mtb [18]. We found that γ-Mtb-cultured NK cells expressed significantly higher levels of IL-21R compared with monocytes (Supplementary Figure 2B). Here, we investigated whether culturing NK cells and monocytes in the presence of recombinant IL-21 had any effect on cytokine production by monocytes. Freshly isolated NK cells and monocytes were cultured in the presence or absence of recombinant IL-21 and γ-Mtb for 48 hours, as mentioned under Methods. Interleukin-1β and MIP-1β levels were significantly higher and IL-10 levels were lower in the culture supernatants in the presence of recombinant IL-21 (Figure 3). We also measured IFN-γ levels in these culture supernatants. γ-Mtb increased IFN-γ production from 30.00 ± 15.44 pg/mL to 3829 ± 944.5 pg/mL (P = .0028; Figure 3). Addition of IL-21 increased IFN-γ production further to 7307.4 ± 1337.8 (P = .012; Figure 3D). As previously observed, IL-21 has no effect on NK cells in the absence of antigen-primed antigen presenting cells [18]. Our findings demonstrate that IL-21-activated NK cells enhance monocyte responses against Mtb.
Figure 3.
Effect of interleukin (IL)-21 on cytokine and chemokine production by Mycobacterium tuberculosis (Mtb)-stimulated monocytes in the presence of natural killer (NK) cells. Freshly isolated monocytes from 6 latent tuberculosis infection (LTBI)+ individuals were cultured with CD3-CD56+ cells in the presence or absence of γ-Mtb as mentioned under Methods. Few cells were cultured with recombinant IL-21. After 48 hours, culture supernatants were collected, and (A) IL-1β, (B) macrophage-inflammatory protein (MIP)-1β, (C) IL-10, and (D) interfereon (IFN)- γ production were measured by enzyme-linked immunosorbent assay. The mean values, standard errors, and P values are shown.
Interleukin-21 Enhances Human Natural Killer Cell-Mediated Lysis of Mycobacterium tuberculosis-Infected Autologous Monocytes
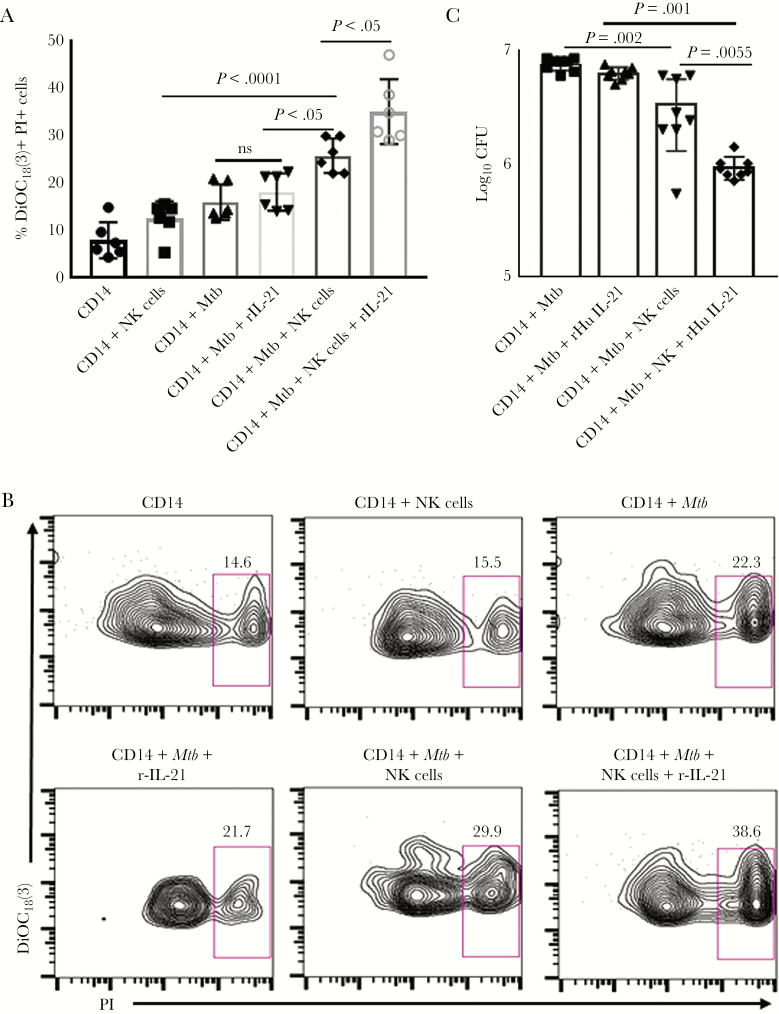
Cytokine and chemokine production by monocytes occurs during very early stages of Mtb infection [24]. In a previous study, we demonstrated that NK cells enhance cytokine production by Mtb-stimulated macrophages and lyse Mtb-infected macrophages [3]. We determined whether IL-21 has any effect on NK-cell mediated lysis of Mtb-infected monocytes as mentioned in Supplementary Methods. Natural killer cells lysed a higher percentage of H37Rv-infected monocytes than uninfected monocytes (25.77% ± 3.7% vs 12.41% ± 3.7%, respectively; P < .001), and this was further enhanced by the addition of recombinant IL-21 (35.03% ± 2.8%, P < .05; Figure 4A and B). Our results demonstrates that apart from enhancing proinflammatory cytokine production by Mtb-cultured monocytes, IL-21-activated NK cells can lyse Mtb-infected monocytes more efficiently than NK cells alone.
Figure 4.
Effect of interleukin (IL)-21 on natural killer (NK) cell responses against autologous Mycobacterium tuberculosis (Mtb)-infected monocytes. (A) Cytotoxicity. Freshly isolated monocytes were labeled with 3, 3ʹ-dioctadecyloxacarbocyanine (DiOC18(3)) and infected with Mtb H37Rv at a multiplicity of infection (MOI) of 1:2.5 (2.5 Mtb to 1 monocyte). Two hours after infection, cells were washed to remove extracellular bacteria, and 0.5 × 106 cells/well were mixed with 2.5 × 106 effector cells (NK cells) at an E:T cell ratio of 5:1 in 300 μL Roswell Park Memorial Institute (RPMI) medium 1640 with 10% heat-inactivated human serum in the presence or absence of recombinant IL-21. After 24 hours, 130 μL propidium iodide (PI) was added to each well, and nonviable cells were determined by flow cytometry. The mean values and standard errors (SEs) for the percentage of cytotoxicity are shown. (B) A representative flow cytometry image is shown. (C) Mycobacterium tuberculosis growth in monocytes. Monocytes were infected with H37Rv at an MOI of 2.5:1 as mentioned under Methods. Freshly isolated NK cells were added to a few wells, at a ratio of 5 NK cells:1 monocyte. Some infected monocytes and NK cells were cultured with recombinant IL-21 (10 ng/mL) for 2 days. The supernatant was aspirated, and monocytes were lysed. The supernatant was centrifuged to pellet the bacteria, and the pellets were added to the cell lysates. Bacterial suspensions were ultrasonically dispersed, serially diluted, and plated in triplicate on 7H10 agar. The number of colonies was counted after 3 weeks. The mean values and SEs are shown for the number of colony-forming units (CFUs) per well.
Interleukin-21 Enhances Human Natural Killer Cell-Mediated Inhibition of Mycobacterium tuberculosis Growth in Autologous Monocytes
Based on the above-mentioned findings, we determined the effect of IL-21 on NK cell-mediated inhibition of intracellular mycobacterial growth in monocytes. Freshly isolated monocytes from 8 LTBI+ healthy individuals were infected with Mtb H37Rv at a multiplicity of infection of 1:2.5 and cultured with autologous NK cells. After 48 hours, 7.5 × 106 colony-forming units (CFU) per well of cultured monocytes alone were present. The addition of NK cells reduced the CFU 3.4 × 106 (P = .002; Figure 4C). Addition of recombinant IL-21 to these cells further reduced the CFU to 0.93 × 106 (P = .005; Figure 4C). Recombinant IL-21 had no effect on Mtb growth in monocytes in the absence of NK cells (Figure 4C). Our results demonstrate that IL-21-activated human NK cells inhibit Mtb growth in autologous monocytes more efficiently than NK cells alone.
Interleukin-21 Enhances Antimicrobial Peptide Expression by Human Natural Killer Cells
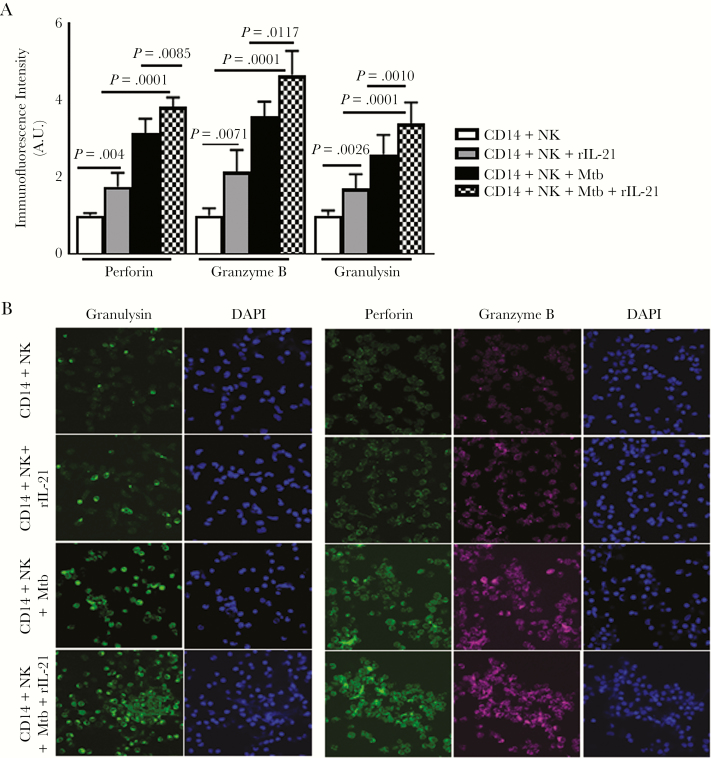
To determine the mechanism(s) by which IL-21 enhances NK cell-mediated inhibition of Mtb growth in human monocytes, we measured antimicrobial peptide expression by confocal microscopy. The addition of freshly isolated NK cells to Mtb-infected monocytes enhanced perforin, granzyme B, and granulysin expression (Figure 5A and B). These findings suggest that IL-21 enhances antimicrobial production by NK cells in response to Mtb-infected monocytes.
Figure 5.
Effect of interleukin (IL)-21 on antimicrobial peptide production by natural killer (NK) cells. (A) Monocytes were infected with H37Rv at a multiplicity of infection of 2.5:1, as mentioned under Method. Freshly isolated NK cells were added to few wells, at a ratio of 5 NK cells:1 monocyte. Some infected monocytes and NK cells were cultured with recombinant IL-21 (10 ng/mL) for 2 days. Monocytes and NK cells cultured with control or Mycobacterium tuberculosis (Mtb)-infected monocytes in the presence or absence of recombinant IL-21 were examined for antimicrobial peptides granulysin, perforin, and granzyme B production by confocal microscopy. The mean values, P values, and standard errors are shown. (B) A representative image of 6 independent experiments are shown.
Interleukin-21 Inhibits Mycobacterium tuberculosis Growth in Infected Rag2 Knockout Mice
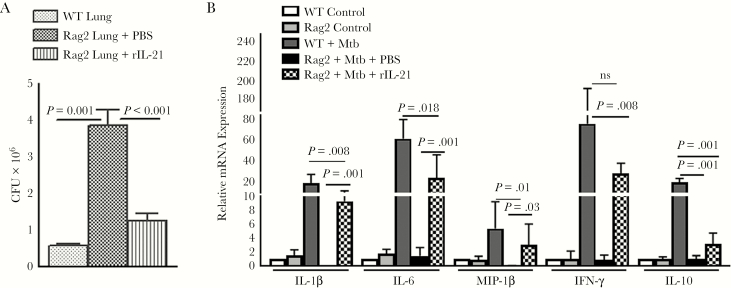
Rag2 KO mice have a germline mutation and fails to produce mature B and T lymphocytes [25]; however, their NK cell function is normal. To determine the in vivo relevance of the above-mentioned human studies, we evaluated the response of Rag2 KO mice treated with recombinant IL-21 and infected with Mtb as mentioned under Methods. Thirty days after infection, in Mtb-infected wild-type mice the CFU per lung was 0.57 ± 0.05 × 106, and this was increased to 3.8 ± 0.44 × 106 (P = .001; Figure 6A) in Rag2 KO mice. Recombinant IL-21 treatment reduced the bacterial burden in Rag2 KO mice to 1.25 ± 0.2 × 106 (P < .001; Figure 6A). We observed significant increases in IFN-γ, IL-1β, MIP-1β, and IL-6 and reduced IL-10 expressions in the lungs of Mtb-infected mice that received recombinant IL-21 (Figure 6B). These findings suggest that IL-21 enhances NK cell responses to limit bacterial replication in the lungs of Mtb-infected mice.
Figure 6.
Interleukin (IL)-21 enhances immune responses and inhibits Mycobacterium tuberculosis (Mtb) growth in infected Rag2 mice. Wild-type (WT) and Rag2 knockout (KO) mice (both C57BL/6) were infected with 50–100 colony-forming units (CFU) of H37Rv by aerosol. Few Rag2 KO mice received recombinant IL-21 or phosphate-buffered saline (PBS) through tail vein injection. One month after infection (A), the bacterial burden in the lungs was measured. Data are representative of 2 independent experiments. Five mice per group were used for each independent experiment. (B) Lung cells were prepared as mentioned under Methods. Expression of various cytokines and chemokines in the lung messenger ribonucleic acid was determined by quantitative real-time polymerase chain reaction. The mean values, P values, and standard errors are shown.
DISCUSSION
Recent studies in animal models have demonstrated that IL-21 signaling is essential for optimal host resistance against Mtb infection and for the expansion of memory-like NK cells to provide protection against Mtb [18–20]. There is limited information available about production of IL-21 and its role in the human response to Mtb infection. In the current study, we observed the following. (1) Interleukin-21 is produced by human CD4+ and NK T cells. CD4+ cells of TB patients produce less IL-21 than that produced by CD4+ cells from LTBI+ individuals. (2) Interleukin-21 had no direct effect on cytokine production by Mtb-stimulated human monocytes. (3) Interleukin-21 enhances human NK cell ability to produce IFN-γ and antimicrobial peptides, lyse Mtb-infected monocytes, and restrict Mtb growth in human monocytes. (4) Interleukin-21-activated human NK cells enhance IL-1β, IL-18, and MIP-1β production and reduce IL-10 production by Mtb-stimulated monocytes. (5) Interleukin-21 inhibits Mtb growth in Rag2 KO mice and enhances NK cell responses.
Interleukin-21 is a pleiotropic cytokine produced primarily by CD4+ T-cell populations and NK T cells, and it acts on a broad range of target cells including B cells, T cells, NK cells, dendritic cells, macrophages, and epithelial cells [26]. Interleukin-21 has several biological functions and induces the activation, proliferation, and differentiation of T cells, NK cells, and NK T cells [27]. Interleukin-21 causes autoimmune disease [28], and IL-21 signaling is critical for the development of type 1 diabetes [29]; however, it may also contribute to resistance against intracellular pathogens, including viruses and mycobacteria [19, 20]. In human Mtb infection, NK T cells produce IL-21 at the site of disease [30]. Decreased levels of IL-21 were detected in pulmonary TB patients compared with those in LTBI+ individuals, and IL-21 levels were significantly restored after successful completion of the anti-TB treatment [31, 32]. In a murine model of Mtb infection, CD4+ cells have been demonstrated to be the major source of IL-21 [19]. We confirmed these findings and further demonstrated that antigen-experienced human CD4+ cells are also the major sources for IL-21 in human Mtb infection. In addition, our study also demonstrates that IL-21 has no direct effect on Mtb-infected monocytes but enhances monocyte responses against Mtb infection by enhancing NK cell responses.
T cells play a crucial role in protective immunity against Mtb and other intracellular pathogens [33], in part through production of IFN-γ and TNF-α, that are required for resistance to infection [34, 35]. There is limited information about the role of IL-21 (T-cell cytokine) in protective immune responses against Mtb infection. Memory-like NK cells contribute to vaccine-induced protective immune responses against Mtb infection, and IL-21 mediates the development and expansion of memory-like NK cells, as has been demonstrated in a murine model of Mtb infection [18]. Upon BCG immunization in mice and humans, NK T cells produce higher levels of IL-21 [30]. In Mtb-infected mice, IL-21 signaling is essential for optimal CD8+ T-cell priming, accumulation of T cells in the lungs, and enhancing T-cell cytokine production [19]. These results suggest that IL-21 signaling has an intrinsic role in promoting the protective capacity of T cells during Mtb infection. Members of the γc family, including cytokines such as IL-2 and IL-15, are essential for the activation of NK cells [36]. Interleukin-21 is a member of the γc family, and limited information is available regarding the role in the activation of human NK cells. During viral infections, IL-21 is primarily produced by CD4+ cells and enhances T and NK cell functions [37]. In the current study, we found that IL-21 enhances NK cell production of IFN-γ and antimicrobial peptides. Interleukin-21-activated NK cells enhanced cytokine and chemokine production by Mtb-stimulated monocytes, lysed Mtb-infected monocytes, and restricted Mtb growth in monocytes more efficiently than did control NK cells. Our current human study demonstrates that IL-21 is essential for optimal NK cell-mediated immune responses against Mtb infection.
CD4+ cells from TB patients produced less IL-21 in response to Mtb than did CD4+ cells from LTBI+ individuals. Reduced Mtb-induced IL-21 secretion by peripheral blood CD4+ cells in TB patients may be due to defective T-cell responses. In a previous study, we demonstrated that NK cells from TB patients produce less IFN-γ compared with that produced NK cells from LTBI+ individuals [3]. In the current study, we observed that IL-21 enhances IFN-γ production by human NK cells, suggesting that NK cells of TB patients are defective in IFN-γ production due to abnormalities in IL-21 production by T cells.
Natural killer cells are prominent components of the innate immune response; however, limited information is available on the role of NK cells in mycobacterial infection. In previous studies, we demonstrated that human NK cells lyse Mtb-infected monocyte-derived and alveolar macrophages, through the NKp46 receptor and NKG2D [3, 38], and NK cell-derived IL-22 inhibits intracellular growth of Mtb by inducing calgranulin A expression and enhancing phagolysosomal fusion [8, 39]. In the current study, we observed that IL-21 produced by antigen experienced CD4+ cells, and NK T cells enhances NK cell lysis of Mtb-infected monocytes and restricts Mtb growth in monocytes. Our results suggest a novel role for IL-21 in restricting Mtb growth in human Mtb infection.
Interleukin-1β is a proinflammatory cytokine that plays an important role in host defense against Mtb infection [41]. Beta chemokines, such as MIP-1β, are produced during Mtb infection and inhibit Mtb growth [42]. Interleukin-18 plays an important role in IFN-γ production during human TB infection [24, 43]. The anti-inflammatory cytokine, IL-10, is pathogenic during Mtb infection [44]. We observed that IL-21 had no direct effect on cytokine production by Mtb-stimulated human monocytes (Supplementary Figure 3); however, it activated human NK cells to enhance IL-1β, IL-18, and MIP-1β and reduce IL-10 production by Mtb-stimulated monocytes (Figure 3). Our findings demonstrate a novel role for IL-21 to enhance protective immune responses during Mtb infection. Interleukin-10 and IL-21 belong to the same family, and, in the current study, we observed that IL-21 inhibits IL-10 production by Mtb-stimulated monocytes by enhancing NK cell activity. Interleukin-21-activated human NK cells produced IFN-γ (Figure 3D), which is known to enhance monocyte effector functions [4]. Natural killer cells stimulate Mtb-exposed macrophages to produce IL-15 and IL-18, which in turn enhances the capacity of CD8+ T cells to produce IFN-γ and to lyse Mtb-infected monocytes and increase IFN-γ production by CD4+ cells [4, 45]. Our results suggest that IL-21-activated NK cells contribute to immune defenses by favoring the development of a protective type 1 response. As reported in our previous studies [43], during the initial stages of infection with intracellular pathogens, cells of the innate immune system, such as monocytes and macrophages, produce soluble mediators that shape the nature of the subsequent adaptive immune response by T cells, which in turn produce cytokines that activate macrophages and stimulate them to produce specific patterns of monokines [46]. Because IFN-γ is central to the protection against many organisms, several positive feedback loops have been described that favor production of this cytokine [47]. Our findings demonstrate the presence of an additional positive feedback loop involving IL-21. We suggest that when healthy LTBI+ individuals are exposed to Mtb, NK T or CD4+ T cells may quickly produce IL-21, leading to greater IFN-γ production by NK cells and therefore rapid elimination of mycobacteria.
Rag2 KO mice do not have functional B and T lymphocytes; however, their NK cell function is normal [25]. Corresponding with our human studies, recombinant IL-21 significantly enhanced IFN-γ, IL-1β, and MIP-1β expression and reduced IL-10 expression as well as inhibited Mtb growth in the lungs of Rag2 KO mice, thereby demonstrating the in vivo relevance to our human studies. We observed that CD4+ cells are a major source of IL-21 during Mtb infection. Moreover, a progressive defect in CD4+ cell function is observed in HIV infection, which leads to defective IL-21 production in HIV-infected individuals [48]. Natural killer cells are not the primary cellular targets of HIV, and NK cell activation should not enhance HIV replication. Our findings also lay the groundwork for development of novel IL-21-dependent methods to stimulate NK cell-mediated immunity against TB in HIV+ individuals and may contribute to the development of an effective vaccine to prevent TB and development of novel immunotherapies to treat active TB in HIV+ individuals. Our study also serves to improve the understanding of the mechanisms that mediate susceptibility to TB in HIV+LTBI+ individuals and facilitate the development of interventions to prevent progression of LTBI to active TB.
CONCLUSIONS
In summary, we observed that IL-21 produced by Mtb-activated CD4+ cells activated NK cells to produce IFN-γ and antimicrobial peptides to lyse Mtb-infected monocytes and to inhibit Mtb growth. Interleukin-21-activated NK cells also enhanced IL-1β, IL-18 and MIP-1β production and reduced IL-10 production by Mtb-stimulated monocytes. This suggests a novel role for IL-21 in protection against intracellular bacteria, such as Mtb. Further identification of IL-21-dependent and IL-21 receptor-dependent mechanisms that regulate NK cell responses will reveal regulatory pathways that may yield new targets for enhancing NK cell-mediated immunity during TB infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the National Institutes of Health, CRDF Global, The Cain Foundation for Infectious Disease Research, The Department of Pulmonary Immunology, and The Department of Biotechnology, New Delhi, India for providing financial support.
Financial support. This work was supported by grants from the National Institutes of Health (AI123310, AI120257 and A1085135; to R. V.), CRDF Global, The Cain Foundation for Infectious Disease Research, The Department of Pulmonary Immunology and The Department of Biotechnology (BT/PR9622/MED/15/109/2013 to V. L. V.), New Delhi, India.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Lanier LL. NK cell recognition. Annu Rev Immunol 2005; 23:225–74. [DOI] [PubMed] [Google Scholar]
- 2. Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol 2008; 9:503–10. [DOI] [PubMed] [Google Scholar]
- 3. Vankayalapati R, Wizel B, Weis SE et al. The NKp46 receptor contributes to NK cell lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 2002; 168:3451–57. [DOI] [PubMed] [Google Scholar]
- 4. Vankayalapati R, Klucar P, Wizel B et al. NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J Immunol 2004; 172:130–7. [DOI] [PubMed] [Google Scholar]
- 5. Roy S, Barnes PF, Garg A et al. NK cells lyse T regulatory cells that expand in response to an intracellular pathogen. J Immunol 2008; 180:1729–36. [DOI] [PubMed] [Google Scholar]
- 6. Dhiman R, Periasamy S, Barnes PF et al. NK1.1+ cells and IL-22 regulate vaccine-induced protective immunity against challenge with Mycobacterium tuberculosis. J Immunol 2012; 189:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol 2004; 22:405–29. [DOI] [PubMed] [Google Scholar]
- 8. Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH. Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 2011; 89:216–24. [DOI] [PubMed] [Google Scholar]
- 9. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol 2001; 22:633–40. [DOI] [PubMed] [Google Scholar]
- 10. Parrish-Novak J, Foster DC, Holly RD, Clegg CH. Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J Leukoc Biol 2002; 72:856–63. [PubMed] [Google Scholar]
- 11. Spolski R, Gromer D, Leonard WJ. The γ c family of cytokines: fine-tuning signals from IL-2 and IL-21 in the regulation of the immune response. F1000Res 2017; 6:1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Habib T, Nelson A, Kaushansky K. IL-21: a novel IL-2-family lymphokine that modulates B, T, and natural killer cell responses. J Allergy Clin Immunol 2003; 112:1033–45. [DOI] [PubMed] [Google Scholar]
- 13. Coquet JM, Kyparissoudis K, Pellicci DG et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 2007; 178:2827–34. [DOI] [PubMed] [Google Scholar]
- 14. Davis ID, Brady B, Kefford RF et al. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: a phase IIa trial. Clin Cancer Res 2009; 15:2123–9. [DOI] [PubMed] [Google Scholar]
- 15. Johnson LD, Jameson SC. Immunology. A chronic need for IL-21. Science 2009; 324:1525–6. [DOI] [PubMed] [Google Scholar]
- 16. Strbo N, de Armas L, Liu H et al. IL-21 augments natural killer effector functions in chronically HIV-infected individuals. AIDS 2008; 22:1551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iannello A, Boulassel MR, Samarani S et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol 2010; 184:114–26. [DOI] [PubMed] [Google Scholar]
- 18. Venkatasubramanian S, Cheekatla S, Paidipally P et al. IL-21-dependent expansion of memory-like NK cells enhances protective immune responses against Mycobacterium tuberculosis. Mucosal Immunol 2017; 10:1031–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Booty MG, Barreira-Silva P, Carpenter SM et al. IL-21 signaling is essential for optimal host resistance against Mycobacterium tuberculosis infection. Sci Rep 2016; 6:36720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheekatla SS, Tripathi D, Venkatasubramanian S et al. IL-21 receptor signaling is essential for optimal CD4+ T cell function and control of Mycobacterium tuberculosis infection in mice. J Immunol 2017; 199:2815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Apostol BL, Kazantsev A, Raffioni S et al. A cell-based assay for aggregation inhibitors as therapeutics of polyglutamine-repeat disease and validation in Drosophila. Proc Natl Acad Sci U S A 2003; 100:5950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brandt K, Singh PB, Bulfone-Paus S, Rückert R. Interleukin-21: a new modulator of immunity, infection, and cancer. Cytokine Growth Factor Rev 2007; 18:223–32. [DOI] [PubMed] [Google Scholar]
- 23. Leonard WJ, Wan C-K. IL-21 signaling in immunity. F1000Res 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Domingo-Gonzalez R, Prince O, Cooper A, Khader S. Cytokines and chemokines in Mycobacterium tuberculosis infection. MicrobiolSpectr 2016; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shinkai Y, Rathbun G, Lam KP et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 1992; 68:855–67. [DOI] [PubMed] [Google Scholar]
- 26. Spolski R, Leonard WJ. Interleukin-21: a double-edged sword with therapeutic potential. Nat Rev Drug Discov 2014; 13:379–95. [DOI] [PubMed] [Google Scholar]
- 27. Skak K, Frederiksen KS, Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008; 123:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Langrish CL, Chen Y, Blumenschein WM et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005; 201:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A 2008; 105:14028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu C, Li Z, Fu X et al. Antigen-specific human NKT cells from tuberculosis patients produce IL-21 to help B cells for the production of immunoglobulins. Oncotarget 2015; 6:28633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar NP, Sridhar R, Hanna LE et al. Decreased frequencies of circulating CD4⁺ T follicular helper cells associated with diminished plasma IL-21 in active pulmonary tuberculosis. PLoS One 2014; 9:e111098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumar NP, Banurekha VV, Nair D, Babu S. Diminished plasma levels of common γ-chain cytokines in pulmonary tuberculosis and reversal following treatment. PLoS One 2017; 12:e0176495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol 2001; 19:93–129. [DOI] [PubMed] [Google Scholar]
- 34. Cooper AM, Dalton DK, Stewart TA et al. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 1993; 178:2243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flynn JL, Chan J, Triebold KJ et al. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med 1993; 178:2249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ferlazzo G, Pack M, Thomas D et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A 2004; 101:16606–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science 2009; 324:1569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vankayalapati R, Garg A, Porgador A et al. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. J Immunol 2005; 175:4611–17. [DOI] [PubMed] [Google Scholar]
- 39. Dhiman R, Indramohan M, Barnes PF et al. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 2009; 183:6639–45. [DOI] [PubMed] [Google Scholar]
- 40. Dhiman R, Venkatasubramanian S, Paidipally P et al. Interleukin 22 inhibits intracellular growth of Mycobacterium tuberculosis by enhancing calgranulin A expre ssion. J Infect Dis 2014; 209:578–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mayer-Barber KD, Andrade BB, Oland SD et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature 2014; 511:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saukkonen JJ, Bazydlo B, Thomas M et al. Beta-chemokines are induced by Mycobacterium tuberculosis and inhibit its growth. Infect Immun 2002; 70:1684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vankayalapati R, Wizel B, Weis SE et al. Production of interleukin-18 in human tuberculosis. J Infect Dis 2000; 182:234–9. [DOI] [PubMed] [Google Scholar]
- 44. Redford PS, Murray PJ, O’Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol 2011; 4:261–70. [DOI] [PubMed] [Google Scholar]
- 45. Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med 1993; 178:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vankayalapati R, Wizel B, Lakey DL et al. T cells enhance production of IL-18 by monocytes in response to an intracellular pathogen. J Immunol 2001; 166:6749–53. [DOI] [PubMed] [Google Scholar]
- 47. Ma X, Chow JM, Gri G et al. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med 1996; 183:147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chevalier MF, Jülg B, Pyo A et al. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. J Virol 2011; 85:733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.