Abstract
Nogo-A has been well described as a myelin-associated inhibitor of neurite outgrowth and functional neuroregeneration after central nervous system (CNS) injury. Recently, a new role of Nogo-A has been identified as a negative regulator of synaptic plasticity in the uninjured adult CNS. Nogo-A is present in neurons and oligodendrocytes. However, it is yet unclear which of these two pools regulate synaptic plasticity. To address this question we used newly generated mouse lines in which Nogo-A is specifically knocked out in (1) oligodendrocytes (oligoNogo-A KO) or (2) neurons (neuroNogo-A KO). We show that both oligodendrocyte- and neuron-specific Nogo-A KO mice have enhanced dendritic branching and spine densities in layer 2/3 cortical pyramidal neurons. These effects are compartmentalized: neuronal Nogo-A affects proximal dendrites whereas oligodendrocytic Nogo-A affects distal regions. Finally, we used two-photon laser scanning microscopy to measure the spine turnover rate of adult mouse motor cortex layer 5 cells and find that both Nogo-A KO mouse lines show enhanced spine remodeling after 4 days. Our results suggest relevant control functions of glial as well as neuronal Nogo-A for synaptic plasticity and open new possibilities for more selective and targeted plasticity enhancing strategies.
Keywords: dendritic spines, myelin-derived Nogo-A, neuronal Nogo-A, synaptic plasticity, two-photon microscopy
Introduction
To accomplish its functions and adapt to new environments, the mature central nervous system (CNS) relies on a fine-tuned balance between dynamic remodeling and stability. Dendritic architecture as well as number and shape of dendritic spines influence neuronal function and have been correlated with activity-dependent processes such as synaptic plasticity (Xu et al. 2009; Fu et al. 2012; Zemmar et al. 2014). Whereas molecules that promote plasticity are well studied, little is known about factors that govern stability, for example, the consolidation of the newly formed architecture to secure storage of novel information of the network. Recent research has begun to elucidate the physiological functions and importance of these stabilizing molecules suggesting that they act as gatekeepers to protect the CNS from excess plasticity and hyper-reactivity with consequential clinical disorders such as neuropsychiatric diseases or epilepsy (Mironova and Giger 2013; Baldwin and Giger 2015). Accumulating evidence shows that the membrane protein Nogo-A is an important candidate among network-stabilizing molecules. Since Nogo-A is currently involved in clinical trials for spinal cord injury, stroke, multiple sclerosis, and amyotrophic lateral sclerosis, it is of vital importance to further understand the physiological functions of this protein in order to minimize side effects and maximize its beneficial neuroregenerative potential (Schwab and Strittmatter 2014).
Nogo-A was originally found in oligodendrocytes and thus described as a myelin-derived inhibitor of axonal outgrowth and neuroregeneration in the lesioned mature CNS (Caroni and Schwab 1988; Schnell and Schwab 1990; Chen et al. 2000; Fournier et al. 2001; GrandPre et al. 2002). For a long time, oligodendrocytes were thought to be the exclusive source of Nogo-A until neuronal expression of the protein was identified (Josephson et al. 2001; Huber et al. 2002; Wang et al. 2002; Jin et al. 2003; Liu et al. 2003). Further research revealed that Nogo-A and its receptors restrict structural and functional synaptic plasticity in several brain regions of the uninjured adult CNS (McGee et al. 2005; Syken et al. 2006; Raiker et al. 2010; Zagrebelsky et al. 2010; Delekate et al. 2011; Wills et al. 2012; Akbik et al. 2013; Jitsuki et al. 2016; Karlsson et al. 2016) including the motor cortex (Zemmar et al. 2014). It has been hypothesized that neuronal Nogo-A may act as a direct local restrictor of synaptic and dendritic plasticity while oligodendrocytic Nogo-A acts as an inhibitor of axonal outgrowth (Schwab 2010; Mironova and Giger 2013). However, no experimental study has yet investigated the specific functions of neuronal and oligodendrocytic Nogo-A in the uninjured adult CNS.
We used newly generated mouse lines in which Nogo-A was specifically knocked out in either oligodendrocytes (oligoNogo-A KO; Cnp-Cre+/-xRtn4flox/flox) or neurons (neuroNogo-A KO; Thy1-Cretg+xRtn4flox/flox) to analyze the contribution of these 2 Nogo-A synthesizing cell types toward dendritic morphology and synapse development in the uninjured adult mouse motor cortex. In motor cortex layer 2/3 cells, we find an increase in dendritic branching complexity and spine density in both Nogo-A KO mouse lines with spatial compartmentalization and a stronger effect in oligoNogo-A KO mice. Analysis of dynamic formation and elimination of layer 5 dendritic spines reveals a higher spine turnover rate in both Nogo-A KO mouse lines compared with control animals.
Materials and Methods
Animals
Control Rtn4flox/flox (FL/FL control), neuron-specific Thy1-Cretg+ xRtn4flox/flox (neuroNogo-A KO) and oligodendrocyte-specific Cnp-Cre+/-xRtn4flox/flox (oligoNogo-A KO) Nogo-A KO mouse lines were generated as described previously (Vajda et al. 2015). Animal experiments were performed in accordance with the guidelines and under the permits of the Veterinary Office of the Canton of Zurich as well as the University of California Santa Cruz.
Tissue Processing
Animals were deeply anesthetized with pentobarbital (Nembutal, 40 mg/kg body weight, intraperitoneally; Abbott Laboratories) and transcardially perfused with PBS (pH 7.4, room temperature) followed by fixative (4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4) (Zemmar et al. 2014). Brains were dissected immediately after perfusion, postfixed in the same fixative for 24 h at 4 °C, cryoprotected in 30% sucrose, sectioned at 40 μm in a cryostat, collected in PBS and transferred into antifreeze solution (15% ethylene glycol and 30% sucrose in 50 mM PB, pH 7.4) and stored at −20 °C until used.
Immunostaining
Free-floating sections from oligoNogo-A KO and neuroNogo-A KO mice were processed under identical conditions to minimize staining variability. Sections were incubated free-floating overnight at 4 °C with primary antibodies (rabbit anti-Nogo-A serum, “Laura,” Rb173A, diluted 1:250) (Oertle et al. 2003; Dodd et al. 2005); mouse anti-NeuN (1:250, Millipore); mouse anti-APC (1:250, Calbiochem); diluted in PBS containing 4% normal goat serum and 0.05% Triton X-100. The sections were then rinsed with buffer, incubated with fluorescence-conjugated secondary antibodies raised in donkey coupled to indocarbocyanine Cy3 (antimouse) or to Alexa Fluor 488 (antirabbit). After final washing steps in PB, sections were mounted on slides in 50 mM Tris pH8.0 and cover-slipped with Mowiol (Calbiochem).
Golgi Impregnation and Identification of Pyramidal Neurons in Layer 2/3 in the Primary Motor Cortex (M1)
Brains were processed with a Golgi protocol as previously described (Chen et al. 2015). In brief, mice were sacrificed with an euthanizing agent (Euthusol, Virbac). Their brains were harvested, rinsed with double distilled water, and transferred to a Golgi–Cox solution composed of potassium dichromate, mercuric chloride, and potassium chromate (Rapid GolgiStain™ Kit,FD Neurotechnologies, Inc.) and stored at room temperature for 12–14 days. Brains were then transferred to a cryoprotectant solution and stored at 4 °C for another 4–7 days in the dark. Processed brains were sectioned at 200 μm in the coronal plane in a cryostat, and sections transferred onto triple-dipped gelatin (0.5% concentration) slides and allowed to air-dry at room temperature in the dark. Sections were then rehydrated with double distilled water, reacted in a developing solution (FD Neurotechnologies, Rapid Golgi Stain Kit), and dehydrated 4 min each in 50%, 75%, 95%, and 100% ethanol. Finally, sections were defatted in xylene-substitute and cover-slipped using Permount (ThermoFisher Scientific). Golgi staining was chosen as it is a standard method to investigate the qualitative and morphometric properties of neurons in the mouse cerebral cortex (Chen et al. 2015).
Neuron Selection, Reconstruction, and Dendritic Spine Analysis
To localize the motor cortex, we used parameters of the Paxinos Brain Atlas. Visualization of pyramidal neurons with their somata located in layers 2/3 and their respective dendrites and spines was accomplished with the Zeiss Axioscope system (Carl Zeiss, Inc.). Neuronal reconstruction followed the protocol previously described (Chen et al. 2012). Apical dendrites were defined as thickest dendrite with part of cytoplasm oriented toward the pia mater. Neurons were reconstructed and dendritic spines were counted under a 63x (oil immersion, NA 1.4) objective with the Neurolucida System (version 11, MicroBrightField Bioscience). Spines were counted on dendritic segments greater than 100 um (from soma to dendritic tip), typically on the longest dendritic segment that could be found for a particular neuron. The microscope was equipped with a digital camera (AxioCam MRc), a mechanical stage (Ludl), and x–y–z axis encoder connected to a computer. The experimenter was blind to the respective group (neuronal KO, oligodendrocyte-specific KO, control). Neuronal morphological variables (e.g., dendritic length, number of dendritic intersections) were derived from traced files and quantified using NeuroExplorer software (MicroBrightField Bioscience). The dendritic length and intersection analysis at different distances (away from the soma) was followed as previously described, using a Sholl analysis approach (Chen et al. 2015). All statistical analyses (Analysis of Variance followed by Tukey pair-wise posthocs, unless otherwise indicated) were performed using Statistica 12.0 Software (Dell) on a PC computer.
Image Analysis
Microscopic analyses were conducted under strictly blind conditions. Images were acquired using a Leica SP2 confocal microscope equipped with a 40X oil immersion objective. For each animal, densitometric measurements were carried out per frame or per region of interest (e.g., single cells) by using the software ImageJ (NIH). The background-corrected optical densities were averaged per brain region and animal. The mean relative optical densities of the control Rtn4flox/flox animals were set as 1.0 for each marker and brain region.
In Vivo 2Photon Imaging Procedure and Data Quantification
The surgical procedure of transcranial two-photon imaging and data quantification have been previously described (Yu et al. 2013). In brief, the skulls of H-line YFP+ anaesthetized mice were surgically thinned using a rotary drill to the point of complete transparency (~20 μm thickness), which allows clear visualization of spines located at apical dendrites under high magnification (60x, NA 1.1 objectives). Images were acquired at a resolution of 512 × 512 pixels at 3X zoom, encompassing ~50 um length at X/Y plane. The brain of the mouse was then imaged through the thinned skull using a Prairie Ultima IV multiphoton microscope with a TiSapphire laser tuned to the excitation wavelength for YFP (920 nm), in which image stacks (step size 0.7 um) were collected. The patterns of blood vessels and neuronal processes were also imaged for relocation of the same dendrites at subsequent imaging sessions. Following imaging, mice were sutured and allowed to recover until the reimaging session. Changes in the total spine number over 4 days interval were relative to the first imaging session and calculated as percentage of formation and elimination of dendritic spines per animal. Image presentations were followed as previously described (Yu et al. 2013) sparsely labeled dendrites with minimal crossings were chosen for representation. Two-dimensional maximum intensity projections of the three-dimensional image stacks containing in-focus dendritic segments of interest were used for all figures. Each image in a Z-stack plane was aligned using recursive alignment of stacks of images using Stackreg (ImageJ). The images were then thresholded, rotated to an appropriate angle, and smoothed linearly using Gaussian filter for presentation. All statistical analyses (Analysis of Variance followed by pair-wise posthoc tests) were performed using Statistica 12.0 Software (Dell) on a PC computer.
Results
Specific Reduction of Neuronal Nogo-A and Oligodendrocytic Nogo-A in the Motor Cortex
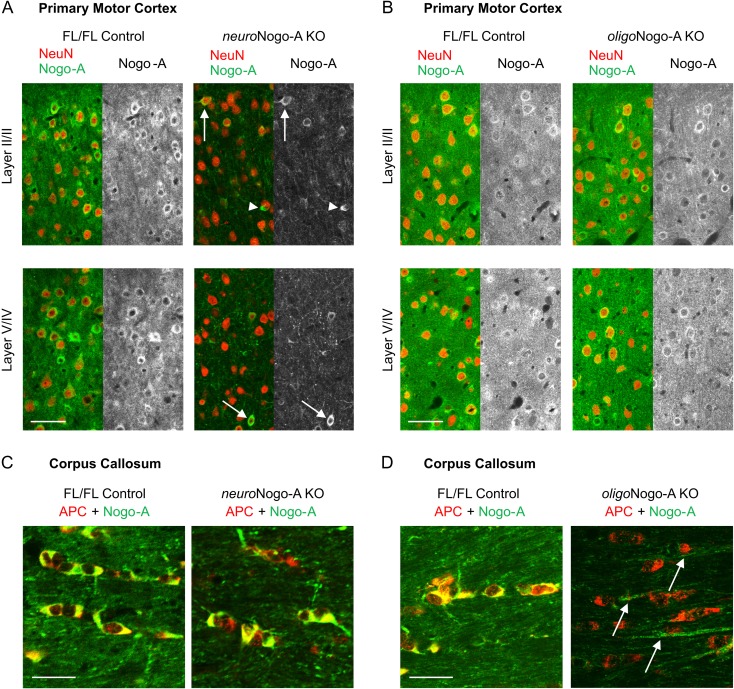
We characterized the previously described neuroNogo-A (Thy1-Cretg+xRtn4flox/flox) and oligoNogo-A (Cnp-Cre+/-xRtn4flox/flox) conditional knockout (KO) mouse lines (Vajda et al. 2015) in the motor cortex. Immunofluorescent stainings of NeuN positive neurons confirmed a significant reduction of Nogo-A expression in the primary motor cortex (Fig. 1A). In contrast, oligoNogo-A KO mice did not show decreased Nogo-A levels in the NeuN positive neurons within primary motor cortex (Fig. 1B). In the corpus callosum (CC), adenomatous polyposis coli (APC) positive oligodendrocytes expressed Nogo-A in control mice and neuroNogo-A KO mice (Fig. 1C), whereas Nogo-A was abolished in cell bodies and myelin of oligoNogo-A KO mice (Fig. 1D).
Figure 1.
Nogo-A expression in motor cortex (M1) and CC of neuroNogo-A KO and oligoNogo-A KO mice. (A) Absence of Nogo-A-immunostaining in the majority of neurons (due to thy-1-Cre expression) in neuroNogo-A KO mice. Nogo-A expression persists in the oligodendrocytes (arrowheads) and in a small population of NeuN positive neurons (arrows),probably due to their lack of thy-1-Cre expression. (B) Neuronal Nogo-A is preserved in M1 cells of oligoNogo-A KO mice. (C) In the CC, APC-positive oligodendrocytes express Nogo-A in control and neuroNogo-A KO mice. (D) APC-positive oligodendrocytes lack Nogo-A in the oligoNogo-A KO mice. The arrows indicate Nogo-A positive axons. Calibration bar: 50 μm in (A, B); 25 μm in (C, D).
In summary, downregulation of Nogo-A was observed mainly in pyramidal neurons in neuroNogo-A KO mice, and cell type-selective deletion of oligodendrocytic Nogo-A was seen in oligoNogo-A KO mice.
Dendritic Branching Complexity, Dendritic Length and Spine Density are Increased Upon Deletion of Neuronal and Oligodendrocytic Nogo-A
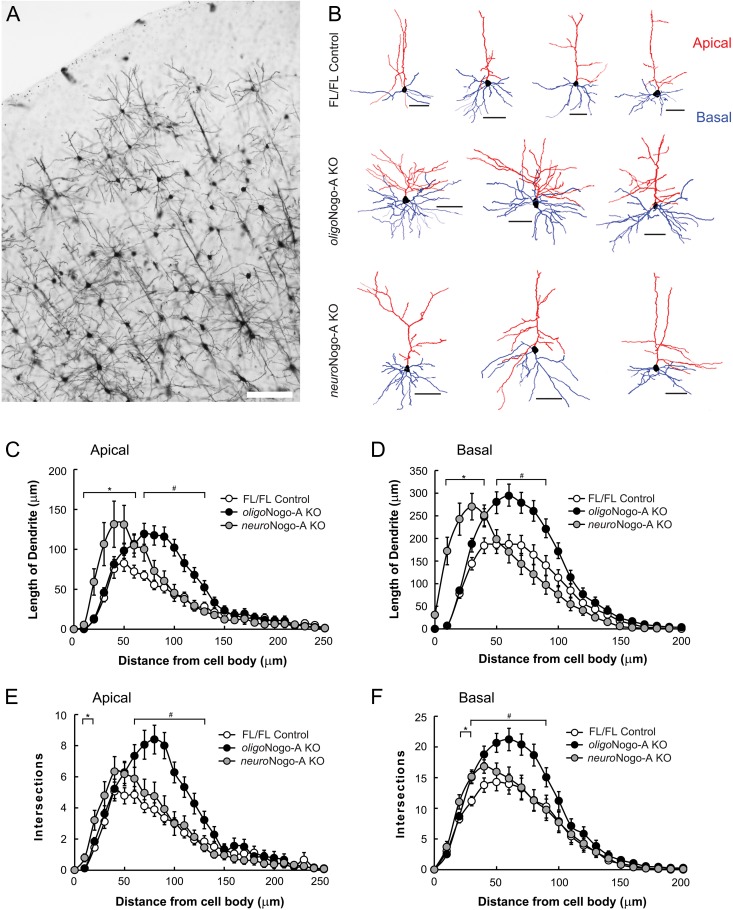
Antibody-mediated neutralization of Nogo-A or its receptor NgR1 were shown earlier to enhance synaptic plasticity in layer 2/3 neurons of the motor cortex in acute, adult cortical slices (Zemmar et al. 2014). To determine the effects of neuronal versus oligodendrocytic Nogo-A deletion on adult cortical dendritic morphology, we analyzed dendritic branching complexity and dendritic length of apical and basal dendrites of Golgi-impregnated layer 2/3 pyramidal neurons (n = 18 neurons for FL/FL control and oligoNogo-A KO, n = 24 neurons for neuroNogo-A KO from 4 animals per genotype). The length of apical and basal dendrites was increased in the distal regions of oligoNogo-A KO mice (Fig. 2B,C,D; P < 0.05), while neuroNogo-A KO mice displayed increased length in the proximal parts of apical and basal dendrites (Fig. 2B,C,D; P < 0.05). Sholl analysis revealed that oligoNogo-A KO mice displayed higher numbers of dendritic intersections, that is, higher branch complexity, compared with littermate controls in the distal regions of the apical and basal dendrites (Fig. 2E,F; P < 0.05). In contrast, neuroNogo-A KO mice showed slightly increased dendritic branching closer to the cell body in both apical and basal dendrites (Fig. 2E,F; P < 0.05), compared with control mice.
Figure 2.
Dendritic length in motor cortex layer 2/3 pyramidal cells of neuroNogo-A KO and oligoNogo-A KO mice. (A) Microphotograph of Golgi staining labeled neurons in primary motor cortex from a control animal. Image was taken with 10x objective (NA = 0.3). (B) Representative examples of layer 2/3 pyramidal cells of FL/FL control, oligoNogo-A KO and neuroNogo-A KO mice. Apical and basal dendrites are shown in red and blue tracings, respectively. (C, D) Compared with control animals (n = 18 neurons from 4 animals), dendritic length is enhanced in neuroNogo-A KO mice (n = 24 neurons from 4 animals) proximal and in oligoNogo-A KO mice (n = 18 neurons from 4 animals) distal to the cell body of apical (C) and basal dendrites (D). (E, F) Sholl analysis (10 μm spacing of concentric spheres) reveals that dendritic complexities (indicated by number of intersections) of apical (E) and basal dendrites (F) are significantly increased in the more distal parts upon deletion of oligodendrocytic Nogo-A, while neuroNogo-A KO mice showed enhanced dendritic complexity in the proximal regions (mean ± SEM. #P < 0.05 oligoNogo-A KO vs. FL/FL; *P < 0.05 neuroNogo-A KO vs. FL/FL). Calibration bar: 100 μm in (A), 50 μm in (B). Data are presented as mean ± SEM.
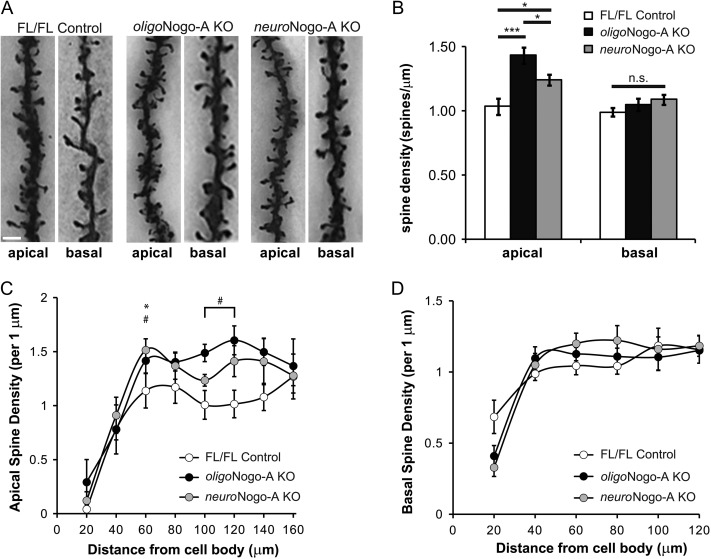
Spine density reflects the number of excitatory synapses in the postsynaptic neuron and thereby serves as an indicator of excitatory synaptic plasticity (Holtmaat and Svoboda 2009). We found that spine density of layer 2/3 pyramidal neurons of the motor cortex was significantly higher at more distal regions (Fig. 3C; P < 0.05) of apical but not basal dendrites (Fig. 3D) of both oligoNogo-A KO and neuroNogo-A KO mice compared with FL/FL control animals, with larger effects observed in oligoNogo-A KO mice (Fig. 3A,B; P < 0.001).
Figure 3.
Spine density in motor cortex layer 2/3 pyramidal cells of neuroNogo-A KO and oligoNogo-A KO mice. (A) Representative examples of Golgi-impregnated apical and basal dendrites from FL/FL control, oligoNogo-A KO mice and neuroNogo-A KO mice. (B) Spine density is significantly increased in apical but not basal dendrites in both KO lines with a stronger effect in oligoNogo-A KO mice (n = 15 cells for FL/FL control, n = 15 cells for oligoNogo-A KO, n = 16 cells for neuroNogo-A KO from 4 animals per condition). (C, D) Higher dendritic spine density is seen in the more distal parts of apical (C) but not basal (D) dendrites in both KO lines with a stronger effect in oligoNogo-A KO mice. Calibration bar: 2 μm in (A). Data are presented as mean ± SEM. #P < 0.05 oligoNogo-A KO versus FL/FL, *P < 0.05 neuroNogo-A KO versus FL/FL.
These results show that oligoNogo-A KO and neuroNogo-A both influence dendritic length and spine density in motor cortex layer 2/3 pyramidal cells with a compartmental effect.
Dendritic Spine Turnover is Enhanced Upon Deletion of Oligodendrocytic and Neuronal Nogo-A
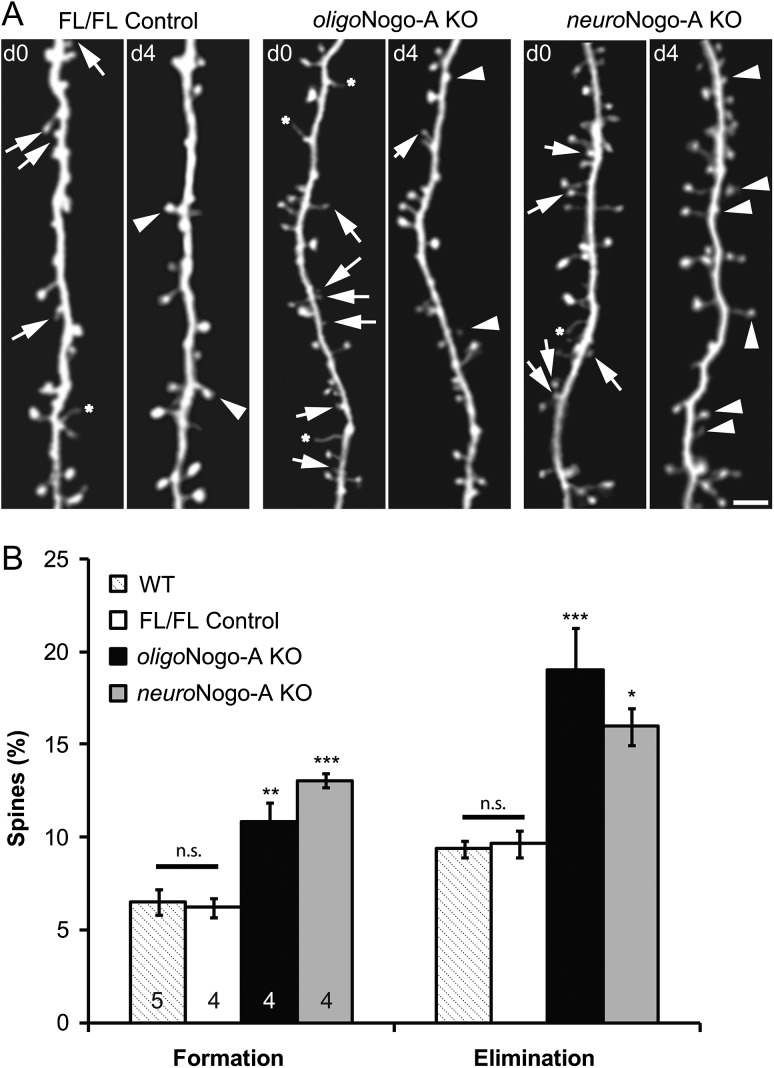
Next, we investigated the dynamics of dendritic spines by in vivo imaging in layer 5 pyramidal cells, the functionally crucial output neurons of the motor cortex. To visualize these cells, we crossed oligoNogo-A KO and neuroNogo-A KO mice with the YFP-H line, which expresses cytoplasmic yellow fluorescent protein (YFP) selectively in a population of layer 5 pyramidal cells (Feng et al. 2000). In 6- to 8-week-old adult mice, we repeatedly imaged motor cortex dendritic spines over a period of 4 days. We found that spine formation and elimination were significantly increased in both Nogo-A KO mouse lines (n = 4, respectively) compared with FL/FL control animals (n = 4) and WT mice (n = 5) (Fig. 4A–D; P < 0.001 for both formation and elimination). Between the 2 Nogo-A KO lines no significant difference was found for spine formation (P = 0.18) or spine elimination (P = 0.35).
Figure 4.
Dynamic spine turnover in neuroNogo-A and oligoNogo-A KO mice. (A–C) Repeated two-photon in vivo imaging of the same motor cortex layer 5 dendritic segments at day 0 (d0) and day 4 (d4) reveals formation of new spines (arrowheads), spine elimination (arrows), and filopodia (stars) in control (A), oligoNogo-A KO (B), and neuroNogo-A KO (C) adult mice (6–8 weeks of age). Scale bar, 2 um. (D) Percentage of dendritic spines that are newly formed or eliminated over 4 days (n = 5 animals for WT control, n = 5 animals for FL/FL control, n = 4 animals for oligoNogo-A KO, n = 4 for neuroNogo-A KO; mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001).
These data demonstrate that oligodendrocytic and neuronal Nogo-A both contribute to similar degrees to restrict synapse remodeling over the time period of 4 days.
Discussion
This study provides the first analysis of the separate effects of neuronal versus oligodendrocytic Nogo-A deletion on dendritic and synaptic plasticity in the mouse motor cortex. In layer 2/3 cells, we found increased dendritic complexity, dendritic length and spine density in both Nogo-A KO lines with a stronger effect in oligoNogo-A KO mice on apical spine density. The spine turnover rate of motor cortex layer 5 cells was enhanced upon deletion of both oligodendrocytic and neuronal Nogo-A.
Specific Functions of Neuronal and Oligodendrocytic Nogo-A
The discovery of neuronal Nogo-A expression (Josephson et al. 2001; Huber et al. 2002; Wang et al. 2002; Jin et al. 2003; Liu et al. 2003) and the functionally important influence of Nogo-A and its receptors NgR1, PirB and S1PR2 at synapses (McGee et al. 2005; Syken et al. 2006; Zagrebelsky et al. 2010; Delekate et al. 2011; Wills et al. 2012; Akbik et al. 2013; Bochner et al. 2014; Kempf et al. 2014; Zemmar et al. 2014; Jitsuki et al. 2016; Karlsson et al. 2016) raise the question of the specific roles of oligodendrocytic and neuronal Nogo-A.
It has been speculated that myelin-derived Nogo-A influences growth inhibition (McGee et al. 2005; Schwab 2010) and neuronal Nogo-A affects synaptic plasticity (Raiker et al. 2010; Wills et al. 2012; Akbik et al. 2013; Karlsson et al. 2016). Our data provide the first evidence that both Nogo-A sources surprisingly contribute to dendritic plasticity and synaptic development. We also show spatial compartmentalization of the effects of the two Nogo-A pools, which may be an important aspect of the specific functions of neuronal versus glial Nogo-A. Neuronal function is highly dependent on spatially and temporally precise signaling. Increasing evidence indicates that the complex morphology of neurons has created compartments that subdivide the neuron into spatially distinct signaling domains important for neuronal function (Llinas et al. 1995; Chen and Sabatini 2012). The compartmentalized effect observed in our study may indicate a direct local function for neuronal Nogo-A on dendrites located close to the soma allowing faster information exchange with the cell body. Possible direct protein synthesis of neuronal Nogo-A at the dendritic spine (Holt and Schuman 2013) could further facilitate this effect. Since neuronal Nogo-A modulates fast dendritic spine dynamics (Kellner et al. 2016), such a mechanism may rapidly provide readily available neuronal Nogo-A after depletion, enabling a fast turnover (minutes to hours). It may also allow fast interaction with plasticity promotors such as neurotrophic factors as suggested by the finding that NgR1 counteracts FGF2 action on LTP (Lee et al. 2008). On the other hand, oligodendrocytic Nogo-A could be located further away from the soma as suggested by its presence around axons (Dodd et al. 2005). Myelin-derived Nogo/Nogo receptor containing signalosomes may travel retrogradely in neurites and confer growth/plasticity inhibitory signals to the soma (Dodd et al. 2005; Joset et al. 2010). The oligodendrocytic pool may be relevant for long-term effects mediated by the Nogo/Nogo receptor complex such as memory formation (Karlen et al. 2009; Karlsson et al. 2016) and consolidation of neuronal circuits by myelination (McGee et al. 2005). In summary, oligodendrocytic Nogo-A may have a global plasticity-restricting, network-stabilizing function in the adult myelinated CNS originating from myelin-axon interactions while neuronal Nogo-A may act more locally and on a faster timescale in dendritic and synaptic domains.
Mechanism of Action and Synaptic Localization of the Nogo Complex
The processes of synaptic plasticity and neuronal outgrowth share similar underlying mechanisms (Llinas 1979; Bloom and Morgan 2011). Several growth inhibitors and repulsive guidance cues were found to also restrict synaptic plasticity whereas attractive guidance cues and promotors of neuronal outgrowth can facilitate synaptic plasticity (Shen and Cowan 2010). This opens the intriguing hypothesis that Nogo/NgR signaling may utilize the same underlying mechanism at the growth cone and at the synapse. One auspicious target is the actin cytoskeleton: it is well known that the Nogo/Nogo receptor signaling cascade mediates growth cone collapse through modulation of the Rho/ROCK pathway and its downstream effectors Lim kinase (LIMK-1) and cofilin (Montani et al. 2009; Nash et al. 2009). Actin and RhoA are also enriched in presynaptic endings and in dendritic spines (Santos Da Silva et al. 2004). Actin dynamics are involved in pre- and postsynaptic control of synaptic transmission (Dillon and Goda 2005) and change in response to Nogo-A manipulations (Kellner et al. 2016). Another mechanistically relevant aspect is the synaptic localization of Nogo-A and NgR1. In the motor cortex Nogo-A is located postsynaptically while NgR1 is at the presynaptic terminal (Zemmar et al. 2014). This opposing arrangement of receptor and ligand allows signaling via different mechanisms: Cis interaction of Nogo-A with postsynaptic glutamate receptors (Peng et al. 2011) or trans-synaptic interaction of Nogo-A with NgR1 may modulate synaptic alterations through reverse signaling as suggested for Ephrin/Eph interactions (Klein 2009).
Compensatory Upregulation of Other Plasticity-Inhibitors
Global knockout of Nogo-A in mice is accompanied by a compensatory upregulation of developmental axon guidance molecules (Kempf et al. 2013). Thus, it is possible that animals who develop without neuronal or glial Nogo-A compensate this loss by either increased developmental expression of other plasticity-restricting factors or decreased synthesis of plasticity promoting factors. An intriguing possibility to test the function of the two Nogo-A pools more specifically is the acute loss of neuronal or glial Nogo-A utilizing selective function-blocking antibodies against neuronal or oligodendrocytic Nogo-A in future studies.
Relevance for Targeted Neuroregeneration
Factors that influence rewiring of injured neurites and neurons by enhancing synaptic plasticity, axonal sprouting and growth represent a powerful target to improve neural repair and regeneration after CNS injury In case of Nogo-A, recent studies have shown specific effects for neuronal and glial Nogo-A after CNS injury: oligodendrocytic Nogo-A KO mice (in which neuronal Nogo-A is spared), showed significantly increased cell survival after optic nerve injury (Vajda et al. 2015) suggesting a cell autonomous role for neuronal Nogo-A in improving neuronal survival, for example, by protection against oxidative damage (Mi et al. 2012; Guo et al. 2013) or recruitment of cytoprotective proteins (Erb et al. 2003). Moreover, neuronal, but not oligodendrocytic Nogo-A enhanced regenerative axon growth after optic nerve injury (Pernet et al. 2012; Vajda et al. 2015). Another important aspect for effective pro-regenerative effects after CNS injury is the time-dependent neutralization of Nogo-A. Anti-Nogo-A antibody application in the early phase after large forebrain stroke in rats and subsequent intensive rehabilitative training resulted in full recovery of forelimb function whereas simultaneous application of anti-Nogo-A antibody treatment and intensive training led to impaired outcomes after stroke and spinal cord injury in adult rats (Maier et al. 2008; Wahl et al. 2014). Further investigations are required to determine whether neuronal and glial Nogo-A have separate effects in early and later phases of regeneration, rewiring and stabilization of neuronal connections after CNS injury.
Notes
We thank Xinzhu Yu and Anthony Gilmore for technical comments and fruitful discussions. Conflict of Interest: None declared.
Funding
This work was funded by grants of the Swiss National Science Foundation (grant 31003A-149315-1 to M.E.S. and grant IZK0Z3-150809 to A.Z.), to A.Z. the Heidi Demetriades Foundation, to M.E.S. the European Research Council (“Nogorise”) and the Christopher and Dana Reeve Foundation (CDRF), to YZ the National Institute of Mental Health (R01MH104227) and National Institute of Neurological Disorders and Stroke (R01NS078791).
References
- Akbik FV, Bhagat SM, Patel PR, Cafferty WB, Strittmatter SM. 2013. Anatomical plasticity of adult brain is titrated by nogo receptor 1. Neuron. 77:859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin KT, Giger RJ. 2015. Insights into the physiological role of CNS regeneration inhibitors. Front Mol Neurosci. 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom OE, Morgan JR. 2011. Membrane trafficking events underlying axon repair, growth, and regeneration. Mol Cell Neurosci. 48:339–348. [DOI] [PubMed] [Google Scholar]
- Bochner DN, Sapp RW, Adelson JD, Zhang S, Lee H, Djurisic M, Syken J, Dan Y, Shatz CJ. 2014. Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia. Sci Trans Med. 6:258ra140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab ME. 1988. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1:85–96. [DOI] [PubMed] [Google Scholar]
- Chen CC, Bajnath A, Brumberg JC. 2015. The impact of development and sensory deprivation on dendritic protrusions in the mouse barrel cortex. Cereb Cortex. 25:1638–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Tam D, Brumberg JC. 2012. Sensory deprivation differentially impacts the dendritic development of pyramidal versus non-pyramidal neurons in layer 6 of mouse barrel cortex. Brain Struct Funct. 217:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. 2000. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 403:434–439. [DOI] [PubMed] [Google Scholar]
- Chen Y, Sabatini BL. 2012. Signaling in dendritic spines and spine microdomains. Curr Opin Neurobiol. 22:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delekate A, Zagrebelsky M, Kramer S, Schwab ME, Korte M. 2011. NogoA restricts synaptic plasticity in the adult hippocampus on a fast time scale. Proc Natl Acad Sci U S A. 108:2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon C, Goda Y. 2005. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 28:25–55. [DOI] [PubMed] [Google Scholar]
- Dodd DA, Niederoest B, Bloechlinger S, Dupuis L, Loeffler JP, Schwab ME. 2005. Nogo-A, -B, and -C are found on the cell surface and interact together in many different cell types. J Biol Chem. 280:12494–12502. [DOI] [PubMed] [Google Scholar]
- Erb M, Steck AJ, Nave KA, Schaeren-Wiemers N. 2003. Differential expression of L- and S-MAG upon cAMP stimulated differentiation in oligodendroglial cells. J Neurosci Res. 71:326–337. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. 2000. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 28:41–51. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. 2001. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 409:341–346. [DOI] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. 2012. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 483:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GrandPre T, Li S, Strittmatter SM. 2002. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 417:547–551. [DOI] [PubMed] [Google Scholar]
- Guo F, Jin WL, Li LY, Song WY, Wang HW, Gou XC, Mi YJ, Wang Q, Xiong L. 2013. M9, a novel region of amino-Nogo-A, attenuates cerebral ischemic injury by inhibiting NADPH oxidase-derived superoxide production in mice. CNS Neurosci Ther. 19:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Schuman EM. 2013. The central dogma decentralized: new perspectives on RNA function and local translation in neurons. Neuron. 80:648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. 2009. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 10:647–658. [DOI] [PubMed] [Google Scholar]
- Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. 2002. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci. 22:3553–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin WL, Liu YY, Liu HL, Yang H, Wang Y, Jiao XY, Ju G. 2003. Intraneuronal localization of Nogo-A in the rat. J Comp Neurol. 458:1–10. [DOI] [PubMed] [Google Scholar]
- Jitsuki S, Nakajima W, Takemoto K, Sano A, Tada H, Takahashi-Jitsuki A, Takahashi T. 2016. Nogo receptor signaling restricts adult neural plasticity by limiting synaptic AMPA receptor delivery. Cereb Cortex. 26:427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson A, Widenfalk J, Widmer HW, Olson L, Spenger C. 2001. NOGO mRNA expression in adult and fetal human and rat nervous tissue and in weight drop injury. Exp Neurol. 169:319–328. [DOI] [PubMed] [Google Scholar]
- Joset A, Dodd DA, Halegoua S, Schwab ME. 2010. Pincher-generated Nogo-A endosomes mediate growth cone collapse and retrograde signaling. J Cell Biol. 188:271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlen A, Karlsson TE, Mattsson A, Lundstromer K, Codeluppi S, Pham TM, Backman CM, Ogren SO, Aberg E, Hoffman AF, et al. . 2009. Nogo receptor 1 regulates formation of lasting memories. Proc Natl Acad Sci U S A. 106:20476–20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson TE, Smedfors G, Brodin AT, Aberg E, Mattsson A, Hogbeck I, Wellfelt K, Josephson A, Brene S, Olson L. 2016. NgR1: a tunable sensor regulating memory formation, synaptic, and dendritic plasticity. Cereb Cortex. 26:1804–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner Y, Fricke S, Kramer S, Iobbi C, Wierenga CJ, Schwab ME, Korte M, Zagrebelsky M. 2016. Nogo-A controls structural plasticity at dendritic spines by rapidly modulating actin dynamics. Hippocampus. 26:816–831. [DOI] [PubMed] [Google Scholar]
- Kempf A, Montani L, Petrinovic MM, Schroeter A, Weinmann O, Patrignani A, Schwab ME. 2013. Upregulation of axon guidance molecules in the adult central nervous system of Nogo-A knockout mice restricts neuronal growth and regeneration. Eur J Neurosci. 38:3567–3579. [DOI] [PubMed] [Google Scholar]
- Kempf A, Tews B, Arzt ME, Weinmann O, Obermair FJ, Pernet V, Zagrebelsky M, Delekate A, Iobbi C, Zemmar A, et al. . 2014. The sphingolipid receptor S1PR2 is a receptor for Nogo-a repressing synaptic plasticity. PLoS Biol. 12:e1001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. 2009. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat Neurosci. 12:15–20. [DOI] [PubMed] [Google Scholar]
- Lee H, Raiker SJ, Venkatesh K, Geary R, Robak LA, Zhang Y, Yeh HH, Shrager P, Giger RJ. 2008. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J Neurosci. 28:2753–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YY, Jin WL, Liu HL, Ju G. 2003. Electron microscopic localization of Nogo-A at the postsynaptic active zone of the rat. Neurosci Lett. 346:153–156. [DOI] [PubMed] [Google Scholar]
- Llinas R. 1979. The role of calcium in neuronal function In: Schmitt FOWF, editor. The neurosciences fourth study program. Cambridge, MA: MIT Press; p. 555–571. [Google Scholar]
- Llinas R, Sugimori M, Silver RB. 1995. The concept of calcium concentration microdomains in synaptic transmission. Neuropharmacology. 34:1443–1451. [DOI] [PubMed] [Google Scholar]
- Maier IC, Baumann K, Thallmair M, Weinmann O, Scholl J, Schwab ME. 2008. Constraint-induced movement therapy in the adult rat after unilateral corticospinal tract injury. J Neurosci. 28:9386–9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. 2005. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 309:2222–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi YJ, Hou B, Liao QM, Ma Y, Luo Q, Dai YK, Ju G, Jin WL. 2012. Amino-Nogo-A antagonizes reactive oxygen species generation and protects immature primary cortical neurons from oxidative toxicity. Cell Death Differ. 19:1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironova YA, Giger RJ. 2013. Where no synapses go: gatekeepers of circuit remodeling and synaptic strength. Trends Neurosci. 36:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani L, Gerrits B, Gehrig P, Kempf A, Dimou L, Wollscheid B, Schwab ME. 2009. Neuronal Nogo-A modulates growth cone motility via Rho-GTP/LIMK1/cofilin in the unlesioned adult nervous system. J Biol Chem. 284:10793–10807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash M, Pribiag H, Fournier AE, Jacobson C. 2009. Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol. 40:224–235. [DOI] [PubMed] [Google Scholar]
- Oertle T, van der Haar ME, Bandtlow CE, Robeva A, Burfeind P, Buss A, Huber AB, Simonen M, Schnell L, Brosamle C, et al. . 2003. Nogo-A inhibits neurite outgrowth and cell spreading with three discrete regions. J Neurosci. 23:5393–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Kim J, Zhou Z, Fink DJ, Mata M. 2011. Neuronal Nogo-A regulates glutamate receptor subunit expression in hippocampal neurons. J Neurochem. 119:1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Joly S, Dalkara D, Schwarz O, Christ F, Schaffer D, Flannery JG, Schwab ME. 2012. Neuronal Nogo-A upregulation does not contribute to ER stress-associated apoptosis but participates in the regenerative response in the axotomized adult retina. Cell Death Differ. 19:1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiker SJ, Lee H, Baldwin KT, Duan Y, Shrager P, Giger RJ. 2010. Oligodendrocyte-myelin glycoprotein and Nogo negatively regulate activity-dependent synaptic plasticity. J Neurosci. 30:12432–12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Da Silva J, Schubert V, Dotti CG. 2004. RhoA, Rac1, and cdc42 intracellular distribution shift during hippocampal neuron development. Mol Cell Neurosci. 27:1–7. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. 1990. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature. 343:269–272. [DOI] [PubMed] [Google Scholar]
- Schwab ME. 2010. Functions of Nogo proteins and their receptors in the nervous system. Nat Rev Neurosci. 11:799–811. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Strittmatter SM. 2014. Nogo limits neural plasticity and recovery from injury. Curr Opin Neurobiol. 27:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Cowan CW. 2010. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2:a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. 2006. PirB restricts ocular-dominance plasticity in visual cortex. Science. 313:1795–1800. [DOI] [PubMed] [Google Scholar]
- Vajda F, Jordi N, Dalkara D, Joly S, Christ F, Tews B, Schwab ME, Pernet V. 2015. Cell type-specific Nogo-A gene ablation promotes axonal regeneration in the injured adult optic nerve. Cell Death Differ. 22:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl AS, Omlor W, Rubio JC, Chen JL, Zheng H, Schroter A, Gullo M, Weinmann O, Kobayashi K, Helmchen F, et al. . 2014. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 344:1250–1255. [DOI] [PubMed] [Google Scholar]
- Wang X, Chun SJ, Treloar H, Vartanian T, Greer CA, Strittmatter SM. 2002. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 22:5505–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills ZP, Mandel-Brehm C, Mardinly AR, McCord AE, Giger RJ, Greenberg ME. 2012. The nogo receptor family restricts synapse number in the developing hippocampus. Neuron. 73:466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. 2009. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 462:915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang G, Gilmore A, Yee AX, Li X, Xu T, Smith SJ, Chen L, Zuo Y. 2013. Accelerated experience-dependent pruning of cortical synapses in ephrin-A2 knockout mice. Neuron. 80:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrebelsky M, Schweigreiter R, Bandtlow CE, Schwab ME, Korte M. 2010. Nogo-A stabilizes the architecture of hippocampal neurons. J Neurosci. 30:13220–13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemmar A, Weinmann O, Kellner Y, Yu X, Vicente R, Gullo M, Kasper H, Lussi K, Ristic Z, Luft AR, et al. . 2014. Neutralization of Nogo-A enhances synaptic plasticity in the rodent motor cortex and improves motor learning in vivo. J Neurosci. 34:8685–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]