Abstract
The newly emerging, highly pathogenic avian influenza (HPAI) H5N8 virus of clade 2.3.4.4 was recently detected in wild birds and domestic poultry in Egypt in the 2016/2017 winter season. Vaccination based on commercial H5 vaccines is used as an essential control strategy in Egyptian poultry. Here, we studied the efficacy of the eight most common commercial H5 poultry vaccines in the Egyptian market and compared them with an experimental vaccine based on the Egyptian LPAI H5N8 virus that was prepared by using reverse genetics. The experimental vaccine and Re-5 commercial vaccine were able to completely protect chickens and significantly reduce virus shedding. Our results indicate that most of the commercial poultry H5 vaccines used in the present study were ineffective because the seed viruses in these vaccines are genetically distinct from the H5N8 viruses currently circulating in Egypt. Although some of the commercial vaccines protected chickens from mortality, they failed to prevent chickens from shedding the virus. Accordingly, we recommend updating and reinforcing the H5N8 prevention and control strategies in Egypt. The vaccination strategy should be reconsidered based on currently circulating viruses.
Introduction
Since 2006, clade 2.2.1 of highly pathogenic avian influenza (HPAI) H5N1 viruses has been widely circulating in Egypt, causing massive economic losses in the Egyptian poultry industry1. The Egyptian veterinary authorities tried to apply a comprehensive response plan to control the spread of the virus but had limited success due to the presence of gaps in implementation. The control plan included establishing biosafety and biosecurity measures in poultry farms, increasing public awareness through the media, culling infected poultry, restricting poultry movement between governorates, and implementing emergency vaccination2,3. Despite these efforts, the H5N1 virus became enzootic, and the virus evolved into 3 antigenically distinct subclades (2.2.1.1, 2.2.1.1a, and 2.2.1.2)4.
At least 24 commercial avian influenza H5 vaccines have been licensed for use for H5N1 prophylaxis in Egyptian poultry3. The vaccine seed strains of these commercial products were based either on classic low pathogenicity H5NX viruses or on H5N1 reassortants harboring two surface glycoprotein (HA and NA) genes of H5N1 (clade 0, 1, 2.2.1, 2.3.2, and 2.3.4) viruses in the genetic background of the A/Puerto Rico/8/1934 (H1N1) strain3. Interestingly, some of the tested vaccines were immunogenic and protected chickens challenged with the Egyptian highly pathogenic H5N1 viruses under laboratory conditions5–7. However, the H5N1 and H5N2 experimentally prepared vaccines fared poorly when tested in the field in Egypt8. Previous studies on the cross-reactivity of commercial H5 poultry vaccines against H5N1 Egyptian isolates in the field in Egypt showed that only one vaccine based on an Egyptian H5N1 virus induced high cross-reactive antibody titers9.
The emerging clade 2.3.4.4 HPAI H5N8 virus was first detected in a live bird market in China in 201010. By 2014, H5N8 viruses had caused different outbreaks in domestic poultry and wild birds in South Korea. Several outbreaks were subsequently recorded either in wild or domesticated birds in many Eurasian and North American countries between 2014 and 2017. The HPAI H5N8 virus has been recently detected in wild birds and domestic poultry in Egypt11,12.
However, little is known about the efficacy and cross reactivity of commercial vaccines against the HPAI H5N8 virus. Herein, we assessed the efficacy of eight commercial vaccines based on different lineages of AI/H5 viruses against the newly emerging HPAI H5N8 virus in Egypt.
Results
Eight of the most common commercial AIV vaccines and an experimental inactivated vaccine based on the Egyptian LPAI H5N8 virus were used to vaccinate 9 chicken groups. All the collected swabs from the chickens up to 6 weeks of age were tested negative by RT-PCR, suggesting that the chickens were not exposed to natural AIV infection during the immune response follow up period. The maternal antibodies at one week of age cross-reacted with H5N1, H5N8, and H9N2 viruses, with log2 mean titers of 4.5, 1.7, and 3, respectively. At 2 weeks of age (day of vaccination), the reactivities against H5N1, H5N8, and H9N2 viruses were 4, 3, and 3 log2 mean titer, respectively. Serological responses to different types of vaccines were assessed weekly until 4 weeks post vaccination (wpv).
Chickens vaccinated with the experimental vaccine showed significantly higher antibody titers against the homologous virus at two wpv with a mean HI titer of 5.8 log2 significantly higher than the titer of the control group (p < 0.01, Fig. 1). The mean HI titer of chickens vaccinated with the experimental vaccine increased notably to 9.1 log2 at 3 wpv and continued increasing to reach 9.5 log2 at 4 wpv (p < 0.001 compared to the control group).
Figure 1.
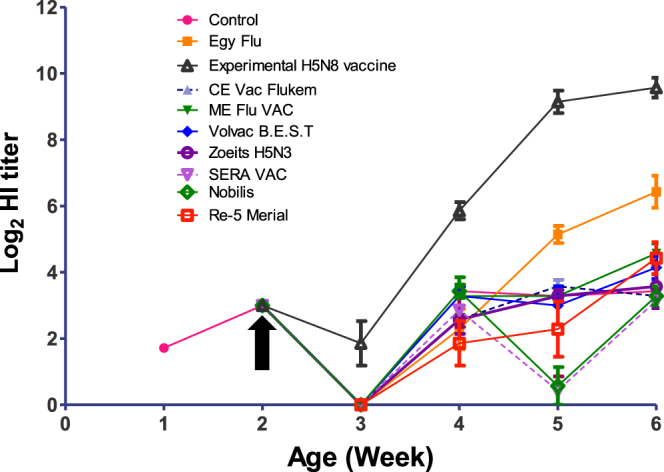
Profile of weekly log2 HI antibody responses of chickens vaccinated with eight commercial H5 vaccines and the experimental RG H5N8 vaccine against the Egyptian H5N8 virus. Chickens were vaccinated at 2 weeks of age (indicated by an arrow). Data are presented as the mean ± standard deviation.
No tested commercial vaccines induced any significant HI titers (p > 0.05) against the heterologous H5N8 virus until 3 wpv. At 4 wpv, only Egyflu had a significant cross-reactive log2 HI titer of 6.4 (p ≤ 0.001), whereas the other vaccines had no significant HI titer (p > 0.05, Fig. 1).
The HPAI A/duck/Egypt/F13666A/2017(H5N8) virus isolated from domestic poultry was lethal to unvaccinated chickens. It showed 100% mortality at four days post infection, with clinical signs of AIV infection including cyanotic combs and wattle, hemorrhagic legs, lethargy, anorexia, and diarrhea.
All chickens vaccinated with the Re-5 Merial, Zoetis H5N3, and experimental H5N8 vaccines survived until 10 days post infection with no clinical signs of AIV infection (Fig. 2). In contrast, chickens vaccinated with the remaining commercial vaccines succumbed to the viral infection, with mortality rates ranging from 60% for the Nobilis, ME Flu VAC and SER-VAC vaccines to 80% for the CEVac Flukem, Egyflu and Volvac (B.E.S.T.) vaccines, with milder clinical signs of AIV infection than those in unvaccinated chickens. Nevertheless, these groups showed significantly higher survival rates compared with the survival rate of the unvaccinated chicken group (p < 0.01).
Figure 2.
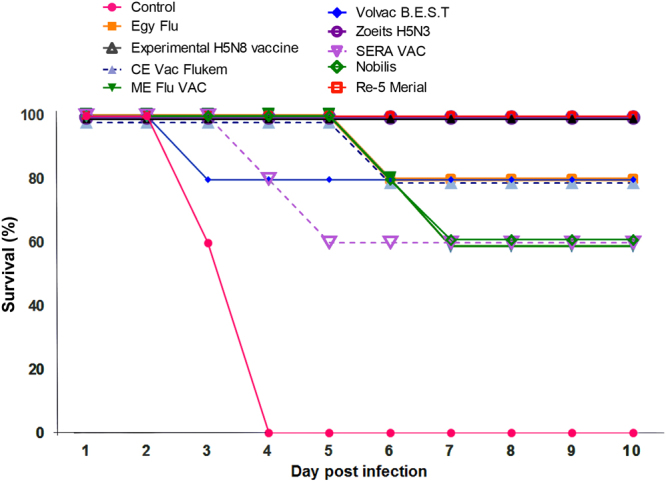
Survival curves of unimmunized and immunized poultry with tested vaccines after challenge with 0.5 mL of 107.5 EID50 of the HP H5N8 virus.
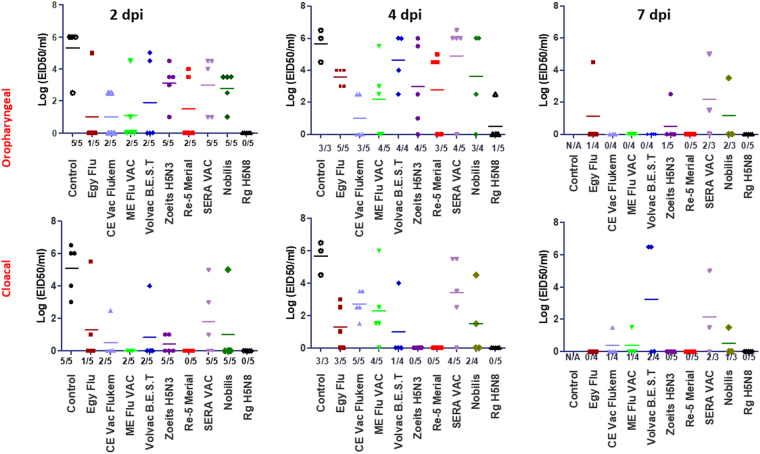
Virus shedding was monitored by the quantification of viral titers in oropharyngeal and cloacal swabs collected at 2, 4, and 7 days post infection. The virus titers in the oropharyngeal swabs were higher than those in the cloacal swabs. Virus shedding peaked at the fourth day post infection. In the unvaccinated chickens, the virus was detected in both oropharyngeal and cloacal swabs at day 2, with mean titers of 5.3 and 5 log10 EID50/mL, respectively. The virus was isolated from the three surviving chickens of the control group at day 4 post infection with the same virus titer of 5.66 log10 EID50/mL in the oral and cloacal swabs. All commercial vaccines reduced viral shedding in challenged vaccinated chickens. No virus was recovered from the oral and cloacal swabs collected from chickens vaccinated with the experimental vaccine at days 2 and 7 post infection. The virus was recovered from only one oral swab at day 4 post infection with a titer of 2.5 log10 EID50/mL (Fig. 3).
Figure 3.
Titration of viral shedding from collected oropharyngeal and cloacal swabs after challenge of unimmunized and immunized chickens with the Egyptian HPAIV H5N8 isolate at 2, 4 and 7 dpi. Five chickens from each group were individually infected with the HP H5N8 virus. Virus shedding was monitored by titration of log10 EID50/mL for each collected sample from live animals. Each dot represents the viral titer of each chicken. The bar for each vaccine is the mean of viral shedding in the group. The detection limit was <1 log10 EID50/0.1 mL.
No virus was detected in the cloacal swabs of chickens vaccinated with Re-5 Merial. However, it was detected in the oropharyngeal swabs at days 2 and 4 with titers of 1.5 and 2.8 log10 EID50/mL, respectively. Another commercial vaccine, Zoetis H5N3, provided protection against morbidity/mortality to vaccinated chickens, but virus shedding was observed in oropharyngeal swabs at days 2, 4, and 7 post infection with virus titers of 3.1, 3, and 0.5 log10EID50/mL, respectively.
Oropharyngeal shedding at 4 dpi was detected in chickens vaccinated with Egyflu (5/5 chickens), CEvac Flukem (3/5 chickens), ME Flu VAC (4/5 chickens), Volvac (B.E.S.T.) (4/4 chickens), Nobilis (3/4 chickens), and SERA-VAC (4/5 chickens) with virus titers of 3.6, 1, 2.2, 4.6, 4.9, and 3.6 log10 EID50/mL, respectively. The virus was detected in the cloacal swabs of vaccinated chickens at 4 dpi with Egyflu (3/5 chickens), CEVac Flukem (3/5 chickens), ME Flu VAC (4/5 chickens), Volvac (B.E.S.T.) (4/4 chickens), Nobilis (3/4 chickens), and SERA-VAC (4/5 chickens) with titers of 1.3, 2.7, 2.3, 1, 3.6 and 4.9 log10 EID50/mL, respectively.
Discussion
Multiple factors influence poultry vaccine efficacy. One of these critical factors is the genetic and antigenic matching between the circulating viruses and commercial vaccine strains13. According to the manual for vaccine evaluation by the World Organization for Animal Health (OIE), an effective poultry vaccine should protect at least 80% of vaccinated chickens from death and should reduce viral shedding after a challenge infection.
Inactivated AI/H5 vaccines based on phylogenetically distinct seed strains have been used in Egypt since 2006. Several experimental studies were conducted in chickens to evaluate the efficacy of different types of commercial vaccines against challenges with different Egyptian H5N1 viruses of clades 2.2.1, 2.2.1.1, 2.2.1.1a, and 2.2.1.2. The serological results showed that different types of commercial AI vaccines provided variable reactivity against the previously described antigens of Egyptian H5N1 viruses (isolates from 2006 to 2009, clade 2.2.1), but that reactivity declined with recent circulating viruses of clade 2.2.1.23. Except for Egyptian H5N1-based vaccines, the different types of commercial vaccines used in Egypt did not confer full protection against different clades of Egyptian H5N1 viruses7,9,14,15.
The HPAI H5N8 virus was recently detected in wild birds and domestic poultry in Egypt11,12. The most dominant control strategy for H5N1 in Egypt is vaccination using commercial AI/H5 vaccines. However, the detection of clade 2.3.4.4 H5N8 viruses in poultry in 2017 revealed the need to readdress the ability of the commercial H5 vaccine used in Egypt to protect poultry against the newly emerging H5N8 virus. We tested the efficacy of the eight most common commercial vaccines against a challenge with the HPAI A/duck/Egypt/F13666A/2017 (H5N8) virus (clade 2.3.4.4) in white leghorn chickens. Maternal antibodies were previously investigated as one of the possible factors affecting vaccine failure in chickens6,9,16. To exclude the interference of maternal antibodies with the different tested vaccines, chickens were vaccinated at 2 weeks of age. The experimental homologous H5N8 vaccine provided the best protection against a challenge with the clade 2.3.4.4 virus. The sera of chickens vaccinated with the Re-5 Merial, Zoetis, EgyFlu, CEVac Flukem and Volvac (B.E.S.T.) vaccines showed reduced cross-reactivity against the Egyptian H5N8 virus and provided ≥80% protection, while the Nobilis, ME Flu VAC, and SERA-VAC vaccines did not reach the protection limit recommended by the OIE. The Re-5 Merial (based on a clade 2.3.4 H5N1 virus) vaccine protected the chickens from mortality and reduced virus shedding. Most of the commercial vaccines protected chickens from mortality but did not reduce or prevent virus shedding. This suggests that the circulating H5N8 viruses may evade vaccine protection. The genetic dissimilarity and poor reactivity between the H5 commercial vaccines used in Egypt and the currently circulating H5N8 viruses proves that the vaccines might not be effective in the field or may introduce only partial protection and thus could lead to vaccine-induced escape mutant strains.
Materials and Methods
Vaccines and Viruses
Eight of the most common commercially available vaccines during 2017 were purchased from poultry vaccine distributors in Egypt (Table 1). The vaccines were based on several strains licensed for the control of the HPAI H5N1 virus in poultry. The Volvac® B.E.S.T. (Baculovirus Expression System Technology) is the commercial bivalent vaccine for AI/H5 and Newcastle disease viruses and is based on a recombinant baculovirus propagated in insect cells. The HA of the Volvac® B.E.S.T. vaccine originated from the A/duck/China/E319-2/03 (H5N1, clade 2.3.2) virus. Seven commercial oil-adjuvant inactivated vaccines were based on either reverse genetically engineered reassortant (RG) AI/H5 viruses (clades 1, 2.2.1.1, 2.2.1.2, and 2.3.4) or classic H5N2 vaccine seed strains.
Table 1.
List of H5 commercial vaccines used for the immunization of poultry against H5N8.
| Vaccine trade name | Virus used | Lineage | Manufacturer, Country | HA nucleotide sequence % similarity to Egyptian H5N8 |
|---|---|---|---|---|
| Nobilis Influenza H5N2 | A/duck/Potsdam/1402-6/1986(H5N2) | Classic | Intervet, The Netherlands | 87 |
| CEVac Flukem | A/chicken/Mexico/232/1994 (H5N2) | Classic | Ceva, Mexico | 80 |
| Zoetis H5N3 | A/chicken/Vietnam/C58/2004 (H5N3) | Clade 1 | Zoetis, USA | 91 |
| EgyFlu | RG A/chicken/Egypt/18-H/2009 (H5N1) | Clade 2.2.1.1 | Harbin Veterinary Research Institute, China | 89 |
| ME Flu VAC | RG A/duck/ Egypt /M2583D /2010 (H5N1) | Clade 2.2.1.2 | ME-VAC, Egypt | 90 |
| SERA-VAC | RG A/chicken/Egypt/M2583D/2010(H5N1) | Clade 2.2.1.2 | Veterinary Serum and Vaccine Institute, Egypt | 90 |
| Volvac (B.E.S.T.) | A/duck/China/E319-2/2003 (H5N1) + ND | Clade 2.3.2 | Boehringer Ingelheim, Mexico | 93 |
| Reassortant AIV (strain Re-5) Re-5, Merial | RG A/duck/Anhui/1/2006(H5N1) (Re-5) | Clade 2.3.4 | Merial, China | 94 |
The HPAI H5N8 virus, A/green winged teal/Egypt/871/2016 (clade 2.3.4.4), was used to prepare an experimental vaccine. A plasmid-based reverse genetics system was applied using six internal genes of the A/Puerto Rico/8/34(H1N1) virus, the LP form of HA (accession no. MF037851.1) and the NA (accession no. MF037848.1) genes of the H5N8 virus17. The generated RG vaccine strain was propagated in 10-day-old specific pathogen-free embryonated chicken eggs (SPF-ECE) (Koum Oshiem SPF Chicken Farm, Fayoum, Egypt) for 3 passages of 48 h each and did not result in embryo death. To create the experimental vaccine, allantoic fluid containing the RG H5N8 virus (titer = 7 log2 HA/50 µL) was inactivated by the addition of 0.1% formalin and mixed with Montanide ISA 70 VG (Seppic, France) in the ratio recommended by the manufacturer (30 antigen/70 adjuvant). The LPAI A/chicken/Egypt/S10489C/2015(H9N2) and A/chicken/Egypt/D10552B/2015 (H5N1) viruses were used as antigens to detect antibodies against H9N2 and H5N1 viruses, respectively18. The plaque-purified HPAI A/duck/Egypt/F13666A/2017(H5N8) virus was used for laboratory challenge experiments.
Immunization of chickens and serological assays
A total of 100 one-week-old Lohmann White chicks were divided into 10 groups (10 chicks/group). Serum samples collected from 10 randomly selected chicks were tested for H5N1, H5N8, and H9N2 antibodies resulting from maternally transmitted immunity at ages one and two weeks by hemagglutination inhibition (HI) assay using chicken RBCs18. Nine groups of chickens received 0.5 mL/chick of a vaccine by intramuscular injection into the thigh at 14 days old. One group was used as an unvaccinated control. Once a week for the first three weeks post vaccination (wpv), blood samples were withdrawn from 7 chickens in each group to evaluate antibody titers against the H5N8 virus by HI. Chickens were monitored daily for morbidity and mortality. Cloacal and oral swabs were obtained weekly from each group post vaccination to monitor influenza A virus infection.
Challenge infection and determination of virus shedding
From each group, five animals were randomly selected at four wpv. Each of the five was infected with a 0.5 mL total dose containing 107.5 EID50 HP H5N8 challenge virus via intranasal and intratracheal routes (0.25 mL/route). Birds were then monitored daily for morbidity and mortality until 10 days post infection. Cloacal and oral swabs were obtained from each bird at 2, 4, and 7 days post infection for virus shedding titration in each bird in different groups by calculating the EID50 per 1 mL of virus. All animal experiments were approved by the Ethics Committee of the National Research Center, Egypt. Experimental infection was performed at biosafety level 3 negative-pressure chicken isolators (PLAS LABS) and under controlled laboratory and biosafety conditions. Any chickens that showed a rapid onset of paralysis, disorientation, reluctance to feed, lethargy and loss of body weight was culled as the humane endpoint.
Statistical analyses
Statistical analyses were performed with GraphPad Prism V5 (GraphPad Inc., CA, USA). An ANOVA with Tukey’s post hoc test was used to compare antibody titers and viral shedding yields. Differences were considered statistically significant at p ≤ 0.05.
Ethical approval
All animal experiments were conducted in strict accordance with and adherence to the relevant policies regarding animal handling as mandated under international, national, and/or institutional guidelines for the care of animals and were approved by the Research Ethical Committee at the National Research Center, Cairo, Egypt. This article does not contain any studies with human participants performed by any of the authors.
Acknowledgements
This work was funded by the following: The National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under the contract number HHSN272201400006C; the Deanship of Scientific Research, King Abdulaziz University, Jeddah, Saudi Arabia, contract number I-005-436; and by the Science and Technology Development Fund (STDF) in Egypt, under contract number 5175. This work was also supported by the American Lebanese Syrian Associated Charities (ALSAC).
Author Contributions
A.K., G.K., A.A.K. and M.A.A. designed the experiments. A.K., A.A. and A.N. performed the experiments. A.K., A.N., G.K. and M.A.A. analyzed the data. G.K., J.S., M.S., E.M., M.A.A. and R.W. contributed reagents, materials, and analysis tools. A.K., J.S., G.K. and M.A.A. wrote the paper and revised the final manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Ahmed Kandeil and Jamal S. M. Sabir contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ghazi Kayali, Email: ghazi@human-link.org.
Mohamed A. Ali, Email: mohamedahmedali2004@yahoo.com
References
- 1.Aly MM, Arafa A, Hassan MK. Epidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 2006. Avian Dis. 2008;52:269–277. doi: 10.1637/8166-103007-Reg.1. [DOI] [PubMed] [Google Scholar]
- 2.Kayali G, et al. Active surveillance for avian influenza virus, Egypt, 2010-2012. Emerg. Infect. Dis. 2014;20:542–551. doi: 10.3201/eid2004.131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kayali G, et al. Avian Influenza A(H5N1) Virus in Egypt. Emerg. Infect. Dis. 2016;22:379–388. doi: 10.3201/eid2203.150593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelwhab EM, et al. Diversifying evolution of highly pathogenic H5N1 avian influenza virus in Egypt from 2006 to 2011. Virus Genes. 2012;45:14–23. doi: 10.1007/s11262-012-0758-1. [DOI] [PubMed] [Google Scholar]
- 5.Dmitriev RI, et al. [Tissue specificity of alternative splicing products of mouse mRNA encoding new protein hampin homologous to the Drosophila MSL-1 protein] Bioorg Khim. 2005;31:363–371. doi: 10.1007/s11171-005-0045-1. [DOI] [PubMed] [Google Scholar]
- 6.Kim JK, et al. Puzzling inefficiency of H5N1 influenza vaccines in Egyptian poultry. Proc. Natl. Acad. Sci. USA. 2010;107:11044–11049. doi: 10.1073/pnas.1006419107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grund C, et al. Highly pathogenic avian influenza virus H5N1 from Egypt escapes vaccine-induced immunity but confers clinical protection against a heterologous clade 2.2.1 Egyptian isolate. Vaccine. 2011;29:5567–5573. doi: 10.1016/j.vaccine.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Kandeil A, et al. Novel reassortant H9N2 viruses in pigeons and evidence for antigenic diversity of H9N2 viruses isolated from quails in Egypt. J. Gen. Virol. 2016;98:548–562. doi: 10.1099/jgv.0.000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayali G, et al. Do commercial avian influenza H5 vaccines induce cross-reactive antibodies against contemporary H5N1 viruses in Egypt? Poult. Sci. 2013;92:114–118. doi: 10.3382/ps.2012-02637. [DOI] [PubMed] [Google Scholar]
- 10.Lee YJ, et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg. Infect. Dis. 2014;20:1087–1089. doi: 10.3201/eid2006.140233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kandeil A, et al. Genetic characterization of highly pathogenic avian influenza A H5N8 viruses isolated from wild birds in Egypt. J. Gen. Virol. 2017;98:1573–1586. doi: 10.1099/jgv.0.000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.OIE. Update on avian influenza in animals. http://www.oie.int/wahis_2/public%5C..%5Ctemp%5Creports/en_fup_0000023232_20170314_163139.pdf (2017).
- 13.Wong SS, Webby RJ. Traditional and new influenza vaccines. Clin. Microbiol. Rev. 2013;26:476–492. doi: 10.1128/CMR.00097-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spackman E, et al. Variation in protection of four divergent avian influenza virus vaccine seed strains against eight clade 2.2.1 and 2.2.1.1. Egyptian H5N1 high pathogenicity variants in poultry. Influenza Other Respir. Viruses. 2014;8:654–662. doi: 10.1111/irv.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Zoghby EF, et al. Isolation of avian influenza H5N1 virus from vaccinated commercial layer flock in Egypt. Virol. J. 2012;9:294. doi: 10.1186/1743-422X-9-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelwhab EM, et al. Influence of maternal immunity on vaccine efficacy and susceptibility of one day old chicks against Egyptian highly pathogenic avian influenza H5N1. Vet. Microbiol. 2012;155:13–20. doi: 10.1016/j.vetmic.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Webby RJ, et al. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet. 2004;363:1099–1103. doi: 10.1016/S0140-6736(04)15892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. WHO Manual on Animal Influenza Diagnosis and Surveillance. World Health Organization. http://www.wpro.who.int/emerging_diseases/documents/docs/manualonanimalaidiagnosisandsurveillance.pdf?ua=1 (2002).