Abstract
BACKGROUND
Cerebrospinal fluid (CSF) leaks increase postoperative risk for complication, likelihood of reoperation, and costs.
OBJECTIVE
To investigate a novel, self-adhering polyethylene glycol-coated collagen pad (PCC) as a dural substitute relative to Duragen XS (DGX; Integra LifeSciences Corporation, Plainsboro, New Jersey) and as a dural sealant relative to Tachosil (Takeda Austria GmbH, Linz, Austria), a fibrinogen and thrombin-coated collagen pad (FTC).
METHODS
A canine supratentorial durotomy surgical model was used to investigate the safety and efficacy of PCC. For safety, 4 animals were bilaterally treated with DGX or PCC and recovered for 1, 8, or 16 wk; total 24 animals. Each animal underwent physical and neurological examinations weekly and 16-wk animals underwent a magnetic resonance imaging (MRI) examination at each time point. For efficacy, 9 animals were unilaterally treated with FTC or PCC and underwent a burst pressure test intraoperatively or 14 d postoperatively; total 36 animals.
RESULTS
In the safety study, no abnormal clinical signs or changes were noted on physical and neurological examinations, or in clinical pathology, CSF analysis or histopathology of DGX or PCC-treated animals. No consistent signs of cerebral compression, CSF leak, hemorrhage, or hydrocephalus were noted on MRI. In the efficacy study, no significant difference was found between FTC and PCC at each time point or overall (13.9 vs 12.3 mm Hg, n = 18 per group, P = .46).
CONCLUSION
PCC is safe for use as a dural substitute and effective as a dural sealant. The novel, self-adhering combination of a polyethylene glycol-based sealant and a collagen pad may offer unique benefits to the advancement of duraplasty.
Keywords: Cerebrospinal fluid leak, Dura mater, Collagen, Polyethylene glycol, Hemopatch, Tachosil, Duragen
ABBREVIATIONS
- ANOVA
analysis of variance
- CSF
cerebrospinal fluid
- DGX
DuraGen XS
- FTC
fibrinogen and thrombin-coated collagen pad
- IV
intravenous
- MRI
magnetic resonance imaging
- PCC
PEG-coated collagen pad
- PEG
polyethylene glycol
- SC
subcutaneous
- SEM
scanning electron microscopy
Cerebrospinal fluid (CSF) leaks occur within up to 10.7% of patients undergoing a cranial durotomy.1 Patients with a CSF leak have increased postoperative risk for complications, likelihood of reoperations, and costs.1-3 In preventing CSF leaks, neurosurgeons have widely used synthetic and biological sealants to reinforce suture lines.1,4,5 However, new investigations explore the use of dural patches that both seal suture lines and bridge dural gaps to act as a dural sealant and substitute.6
A novel, reactive polyethylene glycol (PEG)-coated collagen pad (PCC) has been demonstrated to be an effective sealing hemostat in animal models and clinical investigations,7 and demonstrated to provide clinically relevant adherence for use as a dural sealant in an in vitro model.8 Reactive PEGs are monomers that rapidly form hydrogels and crosslink with proteins on tissue within seconds without being exothermic. The formed hydrogel is highly biocompatible and has adequate strength to prevent postoperative CSF leaks.9 In addition, collagen is known to have a biodegradable profile suitable for use as a dural substitute.10-14
Therefore, the objective of this study is to investigate the safety and efficacy of a novel, sealing dural substitute PCC.
METHODS
Dural Substitutes and Sealants
PEG-Coated Collagen Pad
Hemopatch (Sealing Hemostat; Baxter AG, Vienna, Austria) is a sealing hemostat being investigated as a dural substitute and sealant. It is an absorbable collagen pad derived from bovine epidermis, types I and III collagen, and coated with N-hydroxysuccinimide functionalized PEG. The uncoated, nonactive surface is marked with blue squares. The blue squares are a low concentration of Brilliant Blue (FD&C Blue No 1), a clinically established and well-tolerated dye used as a colorant in drug formulations and dural sealants.7 PCC is applied dry with digital pressure for 2 min using dry gauze. Currently, PCC is not cleared by FDA.
DuraGen XS Dural Substitute
DuraGen XS (DGX; Dural Regeneration Matrix; Integra LifeSciences Corporation, Plainsboro, New Jersey) is an absorbable collagen pad derived from bovine Achille's tendon and composed of type I collagen. DGX is cleared by FDA as a dural substitute for the repair of dura mater for which clinical acceptability and biocompatibility characteristics have been established.14,15 DGX was applied dry then moistened with saline followed by digital pressure for 2 min with dry gauze.
TachoSil (Fibrinogen and Thrombin-Coated Collagen Pad) Dural Sealant
TachoSil (Absorbable Fibrin Sealant Patch; Takeda Austria GmbH, Linz, Austria) is a fibrinogen and thrombin-coated collagen pad (FTC). Tachosil is not FDA approved for dural sealing; however, its use as a dural sealant is well accepted in the scientific and clinical literature.16-21 It is an appropriate comparator due to the similar self-adhering properties. A liquid-based dural sealant is not an appropriate comparator to seal a dural gap. FTC was applied dry with digital pressure for 3 min using moistened gauze.
Stereomicrograph and Scanning Electron Micrograph Images
Dry, naïve pieces of DGX, FTC, and PCC were characterized using stereomicrography and scanning electron microscopy (SEM). Each was removed from their packaging, cut into smaller specimens, coated with metal to enhance conductance, and examined in a JSM 7600F Thermal Field Emission SEM (JEOL, Peabody, Massachusetts).
Animal Welfare Statement
Animal activities were performed according to the Guide for the Care and Use of Laboratory Animals and the United States Animal Welfare Act in an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International following Institutional Animal Care and Use Committee Approval. The canine durotomy model is the accepted animal model to investigate dural sealants and substitutes.9,22-25 When compared to clinical data, the model is predictive of clinical performance.4
Canine Supratentorial Durotomy Model
Animals received acepromazine (0.1 mg/kg, subcutaneous [SC]) and atropine (0.05 mg/kg, SC) for sedation and Propofol (6 mg/kg, intravenous [IV]) for induction then intubated and maintained on isoflurane. The skin overlying the cranium was then prepared with aseptic solutions and infiltrated with bupivacaine (up to 2 mg/kg, SC). Buprenorphine (0.02 mg/kg, IV) and buprenorphine SR (0.06 mg/kg, SC) were administered for perioperative analgesia. Cefazolin (25 mg/kg, IV) and cefovecin (8 mg/kg, SC) were administered prophylactically as antibiotics. Lactated Ringer's solution (10-11 mL/kg/h, IV) was administered with anesthesia.
A craniotomy, measuring 1.5 × 1.5 cm for the safety study and 2 × 2 cm for the efficacy study, was created using a 3-mm diameter Carbide round cutting bur (MicroAire, Charlottesville, Virginia) on a MiniMag Micro drill (DePuy Synthes Power Tools, Palm Beach Gardens, Florida). The bone flap was then removed and a 5-mm diameter supratentorial durotomy was performed and treated (Figure 1).
FIGURE 1.

Treatment of a canine supratentorial durotomy with Hemopatch, a polyethylene glycol-coated collagen pad. A 5-mm diameter supratentorial durotomy is performed through a 1.5 × 1.5 cm craniotomy (A), which is then treated with Hemopatch (B). Hemopatch is a 2.0-mm thick collagen pad composed of types I and III collagen that is coated with N-hydroxysuccinimide functionalized polyethylene glycol to be a self-adherent dural sealant and substitute.
The bone flaps of animals being recovered were reattached using 2-0 polypropylene suture. The overlying muscle was sutured together and the SC tissues were closed with absorbable suture. The skin was approximated with subcuticular suture and skin glue. Animals were then recovered and received meloxicam (0.2 mg/kg, SC q 24 h) for 3 d.
Safety Study
Twelve male and 12 female (7.8±1.1 kg) naïve beagle dogs underwent a bilateral craniotomy and durotomy, and were bilaterally treated with either DGX or PCC (1.5 × 1.5 cm), so that 2 animals per sex and per group were maintained for a 1, 8, or 16-wk recovery period. Each dura substitute was placed in direct contact with the parenchymal surface of the brain in an onlay fashion without suturing.
Physical and neurological examinations (Table 1) were performed by a veterinarian prior to and weekly following surgery. Prior to surgery and at euthanasia, blood was collected to evaluate hematology, coagulation parameters, and blood chemistry. Concurrently, CSF was collected from the cisterna magna.
TABLE 1.
Physical and Neurological Examination
| Physical examination | |
| General appearance: body weight and condition, mentation, posture and gait, and hydration status | |
| Vital signs: temperature, heart rate and rhythm, peripheral pulse strength, respiratory rate, rhythm and effort, and mucous membrane color | |
| Body systems: Eyes, nose and nares, oral cavity, lymph nodes, limbs and joints, feet and nails, skin and hair coat, and abdominal palpation | |
| Neurological examination: cranial nerves | |
| Pupil light reflexes (left and right, direct and consensual) | |
| Palpebral response (left and right) | |
| Eye position (left and right) | |
| Gag reflex | |
| Tongue movement | |
| Neurological examination: central and peripheral nerves | |
| Conscious proprioception (left and right, fore and hind limbs) | |
| Hopping reflex (left and right, fore and hind limbs) | |
| Righting response (left, right) | |
| Withdrawal reflex (left and right, fore and hind limbs) | |
| Panniculus | |
| Patellar reflex (left and right hind limbs) | |
| Anal reflex | |
| Superficial pain | |
Each dog was evaluated prior to study and weekly while on study for abnormal physical and neurological signs.
Animals were euthanized with sodium pentobarbital solution and exsanguinated via perfusion fixation with 10% neutral buffered formalin. The implant sites were collected en bloc with the underlying brain tissue intact and stored in 10% neutral buffered formalin. Tissue samples were trimmed, processed, embedded in paraffin, sectioned, and stained with hematoxylin and eosin and Masson's trichrome. Histological slides were evaluated using a semiquantitative scale by a board-certified veterinary pathologist (SDR) for implant degradation and cellular response.
Sixteen-week animals had MRI scans performed prior to and following surgery, and at 1, 8, and 16 wk to detect signs of cerebral compression, CSF leakage, hydrocephalus, infection, or hemorrhage. T1- and T2-weighted images without contrast were generated using a Philips 1.5 T Intera MRI System (Philips Healthcare, Amsterdam, the Netherlands).
Efficacy Study
Thirty-six (7.5±1.2 kg) naïve beagle dogs underwent a unilateral craniotomy and durotomy, and were treated with either FTC or PCC (1.5 × 1.5 cm); 9 animals (5 males and 4 females) per group underwent a dural burst pressure test at the time of surgery and following a 14-d recovery.
A 3F Millar catheter was placed in the subarachnoid space of the contralateral hemisphere to obtain pressure measurements. After a baseline pressure was obtained (P0, mm Hg), saline containing 0.01 mg/mL methylene blue was administered into the cisterna magna at a rate of 0.45 mL/min to increase subarachnoid pressure. Once a CSF leak was observed visually, the burst strength (Pmax, mm Hg) and failure mode (cohesive, adhesive, and/or substrate) were recorded.26
A rate of 0.45 mL/min is the rate of CSF production in humans.27 Therefore, pressurization was at 2 times the normal rate of CSF production (ie, 0.9 mL/min, where the animal produced 0.45 mL/min and the syringe delivered 0.45 mL/min). Animals spontaneously breathed during the pressure test to avoid ventilator-induced pressure increases.28
Statistical Methods
For continuous endpoints, descriptive statistics consisted of means, standard deviations, and group size. For categorical endpoints, descriptive statistics consisted of incident counts.
In the safety comparison, sexes were pooled and a Levene's test was performed. If not significant, a mean square error was computed for a 1-way analysis of variance (ANOVA) and used by a Dunnett's comparison.
In the efficacy comparison, a 3-way ANOVA was used to compare Pmax for the effects of treatment, time, and sex. Results of pairwise comparisons are reported at the .05 and .01 significance levels. All tests were 2-tailed tests.
RESULTS
Stereomicrograph and Scanning Electron Micrograph Images
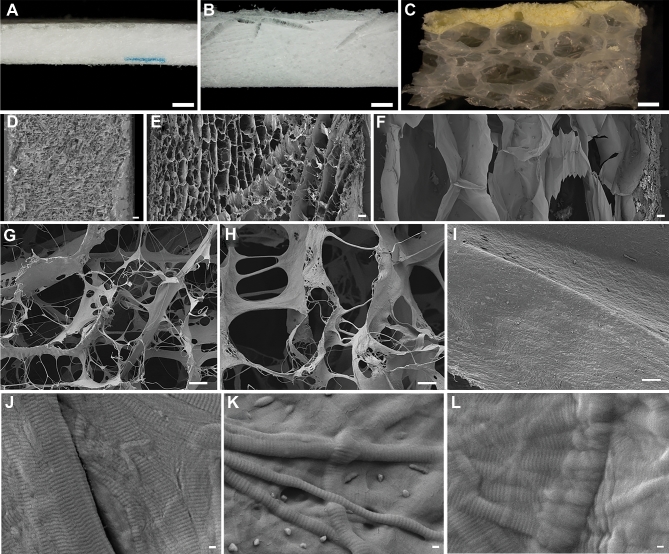
DGX and PCC have similar gross appearances with DGX being approximately 2 times thicker than PCC, whereas FTC has a dissimilar gross appearance and is thicker than DGX (Figures 2A-2C). FTC has the least dense cross-sectional structure and PCC has the most dense (Figures 2D-2F). The uncoated surfaces of DGX and PCC appear more open than that of FTC (Figures 2G-2I). The collagen fiber morphology is similar among the 3 collagen pads (Figures 2J-2L).
FIGURE 2.
Stereomicrograph and scanning electron micrograph images of Hemopatch, Duragen XS and Tachosil (left to right). Hemopatch is a sealing hemostat being investigated as a self-adherent dural sealant and substitute. Duragen XS is a dural substitute composed of type I collagen. Tachosil is a dural sealant composed of a fibrinogen and thrombin-coated collagen pad. Stereomicrographs (A-C) with active surfaces of Hemopatch and Tachosil upward (scale bar is 1 mm). Scanning electron micrographs (D-F) of cross-sections with active surface to the right (scale bar, 100 μm), (G-I) face of uncoated surfaces (scale bar, 10 μm), and (J-L) collagen structure (scale bar, 100 nm).
Safety Study
In-life Physical and Neurological Examination Findings
All animals survived the surgical procedure without complication. Localized SC seromas were noted on the rostral aspect of the frontal bones in some animals, which were transient and considered secondary to the surgical procedure. Prior to and weekly following the surgical procedure, no abnormal clinical signs were observed on physical and neurological examinations. No abnormal changes or significant differences were noted in hematology, coagulation, or clinical chemistry between groups. No signs indicative of a generalized inflammatory response or bacterial infection were noted in the CSF analyses (Table 2). All animals gained weight normally.
TABLE 2.
CSF Analysis of Animals Treated With DuraGen XS, a Collagen Dural Substitute, or Hemopatch, a Polyethylene Glycol-Coated Collagen Dural Sealant and Substitute, Prior to Surgery and 1, 8, and 16 wk Following Surgery
| DuraGen XS | Hemopatch | ||||
|---|---|---|---|---|---|
| Parameter | Study interval | n | Mean (SD) | n | Mean (SD) |
| Total red blood cell count (cells/μL) | Presurgery | 12 | 2508 (6492) | 12 | 2264 (6937) |
| 1 wk | 4 | 3.0 (3.5) | 4 | 6.5 (7.6) | |
| 8 wk | 4 | 14.5 (28.3) | 4 | 0.8 (1.0) | |
| 16 wk | 4 | 37.8 (74.8) | 4 | 32.0 (37.2) | |
| Total white blood cell count (cells/μL) | Presurgery | 12 | 2.5 (6.0) | 12 | 1.7 (3.0) |
| 1 wk | 4 | 1.3 (2.5) | 4 | 1.5 (1.7) | |
| 8 wk | 4 | 1.3 (1.5) | 4 | 0.0 (0.0) | |
| 16 wk | 4 | 0.0 (0.0) | 4 | 1.0 (2.0) | |
| Protein (mg/dL) | Presurgery | 12 | 17.5 (4.4) | 12 | 18.8 (5.9) |
| 1 wk | 4 | 19.0 (2.2) | 4 | 17.5 (2.4) | |
| 8 wk | 4 | 19.3 (2.6) | 4 | 18.8 (1.5) | |
| 16 wk | 4 | 17.0 (1.4) | 4 | 19.5 (3.0) | |
Samples were taken percutaneously from the cisterna magna. There are no remarkable differences between groups over time or indication of infection. The high red blood cell counts are due to sample contamination during collection. Data presented as mean (standard deviation).
MRI Images
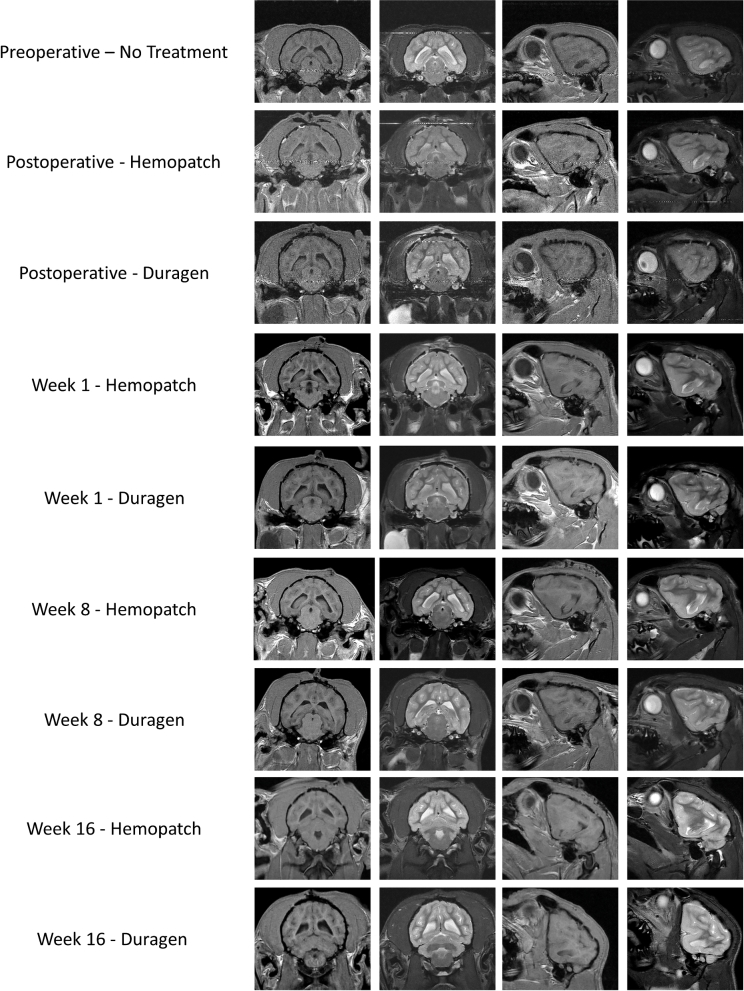
All 16-wk group animals had no persistent signs of cerebral compression, CSF leakage, hydrocephalus, infection, or hemorrhage (Figure 3). Following surgery, signs suggestive of slight cerebral compression were present at the surgical site in most animals of both groups, which resolved by week 1 and considered a result of the surgical procedure. One animal in the DGX group had signs suggestive of CSF leak or hemorrhage, which were not present on subsequent MRI examinations. One animal in the PCC group had signs suggestive of a cerebral compression and CSF leak or hemorrhage, which were also not present on subsequent MRI examinations.
FIGURE 3.
MRI of dogs treated with DuraGen XS, a collagen dural substitute, or Hemopatch, a polyethylene glycol-coated collagen dural sealant and substitute. Serial coronal and sagittal T1- and T2-weighted images were obtained to investigate adverse effects on the brain. Images were obtained preoperatively and postoperatively, and at weeks 1, 8, and 16. Neither collagen duraplasty material is present on MRI. Cerebral compression secondary to the surgical procedure is seen on postoperative images in both groups, which resolves in subsequent MRI. Neither duraplasty material was associated with persistent signs of CSF leak, hydrocephalus, infection, or hemorrhage up to 16 wk after application to the durotomy.
Macroscopic Tissue Evaluation
There were no abnormal macroscopic findings associated with either dural substitute at any time point. Week 1 animals did not have full fusion of the craniotomy site, so one bone flap was removed while the contralateral bone flap was left in place to allow for undisturbed tissue analysis. Neither dural substitute showed signs of migration, stretch/shrinkage, or thickening/thinning nor were signs suggestive of CSF leakage, hydrocephalus, or hemorrhage observed.
Microscopic Tissue Evaluation
Both dural substitutes had excellent local biocompatibility as evidence by low-severity subacute inflammation that resolved by week 8 (Table 3). DGX was not detectable at week 16, while PCC was variably present at week 16. Vacuolated macrophages were present in only the PCC-treated animals, which indicate phagocytic bioresorption. The underlying brain was normal in both groups, except for very minimal, occasional, and model-related cortical hemorrhage beneath the durotomy. Neither dural substitute appeared to impede healing at the surgical site in any significant way.
TABLE 3.
Histological Evaluation of Brain and Dura Treated With DuraGen XS, a Collagen Dural Substitute, or Hemopatch, a Polyethylene Glycol-Coated Collagen Dural Sealant and Substitute, 1, 8, and 16 wk After Implantation to Treat a 0.5-mm Diameter Durotomy (n = 8 per Time Point per Group)
| DuraGen XS | Hemopatch | |||||
|---|---|---|---|---|---|---|
| Histopathology evaluation | 1 wk | 8 wk | 16 wk | 1 wk | 8 wk | 16 wk |
| Implant | ||||||
| Implant resorptiona | 0.0 (0.0) | 3.1 (0.4) | 4.0 (0.0) | 0.0 (0.0) | 3.0 (0.8) | 2.9 (0.8) |
| Response to implantb,c | ||||||
| Overall inflammation | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Polymorphonuclear cells | 0.3 (0.5) | 0.0 (0.0) | 0.0 (0.0) | 0.8 (0.5) | 0.0 (0.0) | 0.0 (0.0) |
| Macrophages | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Lymphocytes | 0.1 (0.4) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Plasma cells | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Giant cells | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Vacuolated macrophages | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.4 (0.5) | 1.6 (0.5) | 1.3 (0.5) |
| Fibrosis | 1.0 (0.0) | 1.6 (0.7) | 1.6 (0.5) | 0.3 (0.5) | 1.8 (0.5) | 1.9 (0.6) |
| Granulation tissue | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 1.4 (0.5) | 0.0 (0.0) | 0.0 (0.0) |
| Necrosis | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Vascularization | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Foreign body response | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.4) | 1.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Edema/hemorrhage | 1.5 (0.5) | 0.0 (0.0) | 0.0 (0.0) | 1.4 (0.5) | 0.0 (0.0) | 0.0 (0.0) |
| Bacteria presence | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Brain responsec | ||||||
| Gliosis | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.4) | 0.0 (0.0) |
| Neurodegeneration | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
| Adhesion: dura to pia/arachnoid | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.4) | 0.6 (0.5) | 0.3 (0.5) |
| Inflammation: pia (durotomy), subacute | 0.3 (0.5) | 0.0 (0.0) | 0.0 (0.0) | 0.9 (0.4) | 0.1 (0.4) | 0.0 (0.0) |
| Hemorrhage | 0.3 (0.5) | 0.0 (0.0) | 0.0 (0.0) | 0.1 (0.4) | 0.0 (0.0) | 0.0 (0.0) |
| Necrosis | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) |
aImplant resorption scale grade 0: no implant resorption; grade 1: minimal resorption (<25%); grade 2: <50% resorption; grade 3: >50% resorption; grade 4: fully resorbed.
bInflammatory cell scale grade 0: not present, none; grade 1: rare, estimated 1 to 5 cells per high-power field (phf), grade 2: mild, estimated 5 to 10 cells phf; grade 3: moderate, heavy infiltrate; grade 4: severe, packed cells.
cAll other parameters. Grade 0: absent/none; grade 1: minimal, present but minimal feature; grade 2: mild, notable feature; grade 3: moderate; prominent feature that does not disrupt tissue architecture and is not overwhelming; grade 4: severe, overwhelming feature or feature that effaces or disrupts tissue architecture.
DuraGen XS degraded faster than Hemopatch. Tissues treated with Hemopatch had a greater number of vacuolated macrophage than DuraGen XS. Data presented as mean (standard deviation).
Week 1
The healing response was similar all in groups and showed slight inflammatory response along the periphery, mainly macrophages with a few neutrophils and rare giant cells. There was an increase in low-severity subacute inflammation in the pia beneath PCC compared to DGX. The subjacent cerebral cortex showed no significant changes. There was no evidence of neurodegeneration or gliosis in the cortex of any of the animals.
Week 8
The inflammatory response in both groups was completely resolved and replaced by dense and mature fibrous connective tissue. Low-severity adhesions were present between the pia and healed dura in the PCC-treated group, while not seen in the DGX-treated group. Both dural substitutes underwent intermediate to advanced resorption.
Week 16
The dense and mature fibrous connective tissue did not progress, while vacuolated macrophages slightly decreased. The presence of pia-to-dura adhesions was reduced in the PCC-treated group.
Efficacy Study
There was no significant difference between FTC and PCC-treated durotomy sites when pressurized intraoperatively, on day 14 or overall (Table 4). The common failure mode for FTC was adhesive, while for PCC was cohesive (Table 5).
TABLE 4.
Subarachnoid Pressure Test
| Baseline (mm Hg) | Pmax (mm Hg) | |||||
|---|---|---|---|---|---|---|
| Time point | n | Tachosil | Hemopatch | Tachosil | Hemopatch | P Value |
| Overall | 18 | 3.23 (1.50) | 4.21 (3.06) | 13.9 (6.74) | 12.3 (3.47) | .46 |
| Day 0 | 9 | 2.73 (1.23) | 3.75 (2.19) | 13.8 (8.52) | 12.9 (4.41) | .85 |
| Day 14 | 9 | 3.74 (1.64) | 4.67 (3.81) | 14.0 (4.89) | 11.7 (2.32) | .39 |
TachoSil, a fibrinogen and thrombin-coated collagen pad, and Hemopatch, a polyethylene glycol-coated collagen dural sealant and substitute, withstood similar subarachnoid pressure prior to failure. Data presented as mean (standard deviation).
TABLE 5.
Subarachnoid Pressure Test Failure Mode
| Tachosil | Hemopatch | |||||
|---|---|---|---|---|---|---|
| Failure mode | Overall | Day 0 | Day 14 | Overall | Day 0 | Day 14 |
| Cohesive | 2 | 0 | 2 | 14 | 8 | 6a |
| Adhesive | 16 | 9 | 7 | 5 | 1 | 4a |
| Substrate | 0 | 0 | 0 | 0 | 0 | 0 |
aBoth failure modes were noted simultaneously in one animal.
Tachosil failed adhesively, while Hemopatch failed cohesively when the subarachnoid pressure was increased. Data presented in frequencies.
DISCUSSION
This is the first investigation of the safety and efficacy of PCC as a dural substitute and sealant. When used as a dural substitute and sealant in a canine durotomy model, PCC was not associated with signs of neurological deficits, central nervous system inflammation, delayed wound healing, or CSF leakage. PCC was biocompatible as compared to DGX for use as a dural substitute and was effective as FTC as a dural sealant.
Safety of PCC
Consistent with clinical use, DGX and PCC were well tolerated and had an acceptable biodegradation profile when used in dogs. Both collagen pads primarily contain type I collagen, which is the same collagen composing human dura.29 In contrast to DGX, PCC was associated with an increase presence of vacuolated macrophage and a slower degradation. The increased presence of vacuolated macrophage is consistent with the breakdown of PEG-based hydrogels.9,22 The low-severity adhesions in PCC-treated animals are also consistent with other PEG-based dural sealants in this model.24 The formation of such adhesions should be considered if a reoperation is anticipated. The slower degradation is likely due to the collagen pad density. Consistent with other investigations, PCC has a lower porosity and greater density of collagen fibers than DGX as seen on SEM.7,25 The lower porosity and greater density may reduce the rate of cellular ingress leading to a slower degradation. In contrast, DGX is reported to degrade quickly leaving the brain exposed to the calvarium.30 Though both materials are thicker than normal human dura, 0.4 to 0.6 mm25, neither was associated with consistent signs suggestive of cerebral compression on MRI.
PEG hydrogels are hydrophilic and are known to swell 50% to 300% in volume.31,32 As seen in stereomicrograph images, the dry, nonactivated thickness of PCC is less than DGX. In addition, PCC has a noncompressed thickness of 2.0 mm and a maximum thickness when submerged in citrated human plasma for 24 h of 2.8 mm.8 The extensive swelling of other PEG-based dural sealants is associated with postoperative complications due to “mass effect.”32-37 By coating a collagen pad with only one type of reactive PEG, the amount of swelling is limited thereby reducing the risk of “mass effect.”
Based on a cellular analysis of the CSF, no animals treated with PCC or DGX suggested an inflammatory response or infection. This study could have been strengthened by including CSF ELISAs to assess interleukin-6 for inflammation, neuron-specific enolase for neuronal injury, and S-100B protein for brain injury.38 Similarly, a test for β2-transferrin could rule out postoperative seromas from being a postoperative pseudomeningocele. These sensitive assays, however, were not available for this study. Additionally, the study could have been strengthened by including a no treatment control to confirm and characterize the clinical presence of postoperative CSF leaks on MRI in this model.
Efficacy of PCC
PCC was as effective as FTC, which is a clinically effective dural sealant.16-21 Furthermore, both sealants were effective over the range of normal human subarachnoid pressure, 7 to 15 mm Hg.39-41 In contrast, a collagen dural substitute applied in an onlay fashion to a 1.5-cm diameter durotomy provided a burst pressure of 7.2 ± 2.8 mmHg.9 Comparing data from different studies is, however, difficult, because test systems and methods vary (eg, durotomy size, anesthetics, pressure transducer placement, head position, etc.). In this study, the subarachnoid space was pressurized by increasing the CSF volume instead of reducing the subarachnoid space through a Valsalva maneuver. This method was selected to standardize the pressurization of the dura for impartial comparisons and to be consistent with previous studies.9,24
FTC failed adhesively with CSF separating the collagen pad from the dura. In contrast, PCC failed cohesively with CSF weeping through the collagen, which is the typical failure mode for collagen-based dural substitutes.9 The difference in failure mode is likely due to the different collagen architecture. As seen on SEM, FTC has a closed-cell structure while PCC has a porous structure. The porous structure of PCC is known to allow migration of fluid through the collagen pad, which reduces the fluid–tissue interface stress level and improves adherence.42,43 An adhesive failure leads to a continual CSF leak regardless of pressure, whereas a cohesive failure retains function following transient increases in CSF pressure (eg, coughing, sneezing). Recent studies investigating the complication rate of nonwater tight closures suggest that the complication rate is clinically acceptable and comparable to water tight closures,44-46 whereas true CSF leaks lead to worse surgical outcomes.1-3
Use of PCC as a Self-Adhering Duraplasty Material
PCC provides a novel combination of a PEG-based dural sealant and a collagen dural substitute, which is a self-adherent dural substitute that seals dural gaps. The active surface of the collagen pad is coated with PEG that effectively forms a hydrogel between the collagen pad and dura.7 In contrast, forming a hydrogel on top of a collagen pad and dura is reported with mixed results. In a preclinical investigation by Preul et al,9 this combination provided a CSF burst pressure of greater than 36 mmHg and prevented CSF leaks in 5 of 6 animals (83.3%) up to 8 wk after surgery.9 In a clinical investigation by Litvack et al,47 however, the combination was associated with a significantly increased risk of CSF leak compared to a collagen matrix alone. In other clinical investigations, the combination provided acceptable procedure-related complication rates for CSF leak48,49 and meningitis.49 Notably, synthetic dural sealants are not to be used with nonautologous duraplasty materials other than collagen.49,50
Use of a hydrogel sealant applied on top of a duraplasty material may introduce a thick layer between the brain and skull. The use of a collagen pad coated with a reactive PEG reduces the thickness and amount of material reducing the likelihood of causing a mass effect. The PCC may provide tensile strength when bridging dura across a cavity following removal of meningioma, metastatic brain tumor, or glioma, relative to nonself-adhering collagen pads.51
While collagen is a natural choice as the dural substitute, either a PEG based or fibrin sealant can be used to fix the collagen pad. PEG-based sealants are reported to have better clinical performance than fibrin-based sealants to seal dura,4,52 which favors the use of PCC. FTC is demonstrated to provide clinical benefit as a dural sealant.29 Some investigators have, however, identified complications related to application of thrombin into or on to the brain.37,53-56 In addition, the self-adherence of PCC removes the need for suturing which reduces surgical time.29
CONCLUSION
Based on this study, PCC is safe and effective in treating a supratentorial durotomy in dogs. In addition to clinical data, application to other cranial approaches and procedures is of future research interest (eg, posterior fossa, transsphenoidal, Chiari malformation corrections, etc.). The combination of a PEG-based sealant and a collagen pad may offer unique benefits for the advancement of duraplasty.
Disclosures
Funding for this study was provided by Baxter Healthcare Corporation. Each study was designed and performed using sound scientific methods for impartial data collection and comparison. Drs. Lewis and Baumgartner are employees of Baxter Healthcare Corporation. Dr. Gulle is an employee of Baxter Medical Products GmbH. The authors alone are responsible for the content and writing of the manuscript.
Acknowledgments
The authors thank their administrative and technical staff, and James P. DiOrio and Mary Anne Murphy for their scanning electron microscopy expertise.
COMMENT
The authors present a well-designed canine model for supratentorial durotomy to test the relative efficacy of a new combined collagen patch/polyethylene glycol sealant compared to DuraGen® (Integra LifeSciences Corporation, Plainsboro, New Jersey) and TachoSil® (Takeda Austria GmbH, Linz, Austria). The authors used a 1.5 × 1.5 cm craniotomy (2 cm for efficacy) but only a 5 mm durotomy. The study is well designed with thorough outcome measures including MIR, neurologic examinations, CSF sampling, and histological analyses. With such a small opening (5 mm), I question whether the sensitivity of the model is sufficient to detect any differences between the alternatives. Certainly, one may consider that a single piece of oxidized cellulose sponge (Gelfoam®; Pfizer, New York, New York) may be sufficient to cover this defect and prevent CSF leak. Overall, the authors found equivalence between the new substitute and the alternatives. However, the authors did find an increased rate of adhesions between the pia and the graft when compared to Duragen®. This might certainly be an important consideration in reoperation. While the current study demonstrates similar safety and efficacy to existing materials, it does not demonstrate a clear advantage. Further testing is necessary with more sensitive models (eg posterior fossa) to determine if there is an advantage to the new substitute and to determine its cost effectiveness.
Varun R. Kshettry
Cleveland, Ohio
REFERENCES
- 1. Grotenhuis JA. Costs of postoperative cerebrospinal fluid leakage: 1-year, retrospective analysis of 412 consecutive nontrauma cases. Surg Neurol. 2005;64(6):490-493. [DOI] [PubMed] [Google Scholar]
- 2. Piek J, Weber C, Kundt G et al. . Pharmacoeconomical consequences of postoperative CSF leaks after intracranial surgery—a prospective analysis. J Neurol Surg A Cent Eur Neurosurg. 2012;73(1):25-28. [DOI] [PubMed] [Google Scholar]
- 3. Weber C, Piek J, Gunawan D. Health care costs of incidental durotomies and postoperative cerebrospinal fluid leaks after elective spinal surgery. Eur Spine J. 2015;24(9):2065-2068. [DOI] [PubMed] [Google Scholar]
- 4. Osbun JW, Ellenbogen RG, Chesnut RM et al. . A multicenter, single-blind, prospective randomized trial to evaluate the safety of a polyethylene glycol hydrogel (Duraseal Dural Sealant System) as a dural sealant in cranial surgery. World Neurosurg. 2012;78(5):498-504. [DOI] [PubMed] [Google Scholar]
- 5. Kim KD, Wright NM. Polyethylene glycol hydrogel spinal sealant (DuraSeal Spinal Sealant) as an adjunct to sutured dural repair in the spine: results of a prospective, multicenter, randomized controlled study. Spine (Phila Pa 1976). 2011;36(23):1906-1912. [DOI] [PubMed] [Google Scholar]
- 6. Hutter G, von Felten S, Sailer MH, Schulz M, Mariani L. Risk factors for postoperative CSF leakage after elective craniotomy and the efficacy of fleece-bound tissue sealing against dural suturing alone: a randomized controlled trial. J Neurosurg. 2014;121(3):735-744. [DOI] [PubMed] [Google Scholar]
- 7. Lewis KM, Kuntze E, Gulle H. Control of bleeding in surgical procedures: Critical appraisal of HEMOPATCH [Sealing Hemostat]. Med Devices (Auckl). 2016;9:1-10. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4694675/. Accessed Aug 25, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lewis KM, Spazierer D, Slezak P, Baumgartner B, Regenbogen J, Gulle H. Swelling, sealing, and hemostatic ability of a novel biomaterial: A polyethylene glycol-coated collagen pad. J Biomater Appl. 2014;29(5):780-788. [DOI] [PubMed] [Google Scholar]
- 9. Preul MC, Campbell PK, Bichard WD, Spetzler RF. Application of a hydrogel sealant improves watertight closures of duraplasty onlay grafts in a canine craniotomy model. J Neurosurg. 2007;107(3):642-650. [DOI] [PubMed] [Google Scholar]
- 10. Esposito F, Grimod G, Cavallo LM, Lanterna L, Biroli F, Cappabianca P. Collagen-only biomatrix as dural substitute: What happened after a 5-year observational follow-up study. Clin Neurol Neurosurg. 2013;115(9):1735-1737. [DOI] [PubMed] [Google Scholar]
- 11. Narotam PK, van Dellen JR, Bhoola KD. A clinicopathological study of collagen sponge as a dural graft in neurosurgery. J Neurosurg. 1995;82(3):406-412. [DOI] [PubMed] [Google Scholar]
- 12. Barbolt TA, Odin M, Léger M, Kangas L. Pre-clinical subdural tissue reaction and absorption study of absorbable hemostatic devices. Neurol Res. 2001;23(5):537-542. [DOI] [PubMed] [Google Scholar]
- 13. Narotam PK, Van Dellen JR, Bhoola K, Raidoo D. Experimental evaluation of collagen sponge as a dural graft. Br J Neurosurg. 1993;7(6):635-641. [DOI] [PubMed] [Google Scholar]
- 14. Stendel R, Danne M, Fiss I et al. . Efficacy and safety of a collagen matrix for cranial and spinal dural reconstruction using different fixation techniques. J Neurosurg. 2008;109(2):215-221. [DOI] [PubMed] [Google Scholar]
- 15. Khorasani L, Kapur RP, Lee C, Avellino AM. Histological analysis of DuraGen in a human subject: case report. Clin Neuropathol. 2008;27(5):361-364. [DOI] [PubMed] [Google Scholar]
- 16. Chauvet D, Tran V, Mutlu G, George B, Allain JM. Study of dural suture watertightness: an in vitro comparison of different sealants. Acta Neurochir (Wien). 2011;153(12):2465-2472. [DOI] [PubMed] [Google Scholar]
- 17. Hutter G, von Felten S, Sailer MH, Schulz M, Mariani L. Risk factors for postoperative CSF leakage after elective craniotomy and the efficacy of fleece-bound tissue sealing against dural suturing alone: a randomized controlled trial. J Neurosurg. 2014;121(3):735-744. [DOI] [PubMed] [Google Scholar]
- 18. Nistor RF, Chiari FM, Maier H, Hehl K. The fixed combination of collagen with components of fibrin adhesive-a new hemostypic agent in skull base procedures. Skull Base Surg. 1997;7(1):23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biroli F, Esposito F, Fusco M et al. . Novel equine collagen-only dural substitute. Neurosurgery. 2008;62(3 suppl 1):273-274; discussion 274. [DOI] [PubMed] [Google Scholar]
- 20. Reddy M, Schöggl A, Reddy B, Saringer W, Weigel G, Matula C. A clinical study of a fibrinogen-based collagen fleece for dural repair in neurosurgery. Acta Neurochir (Wien). 2002;144(3):265-269; discussion 269. [DOI] [PubMed] [Google Scholar]
- 21. Ulivieri S, Peri G, Tiezzi G, Mileo E, Giorgio A, Oliveri G. From surgery to neurosurgery: our experience on the efficacy of fleece-bound sealing (TachoSil®) for dural repair. G Chir. 2014;35(7-8):195-198. [PMC free article] [PubMed] [Google Scholar]
- 22. Preul MC, Bichard WD, Spetzler RF. Toward optimal tissue sealants for neurosurgery: use of a novel hydrogel sealant in a canine durotomy repair model. Neurosurgery. 2003;53(5):1189-1198. [DOI] [PubMed] [Google Scholar]
- 23. Hadley MN, Martin NA, Spetzler RF, Sonntag VK, Johnson PC. Comparative transoral dural closure techniques: a canine model. Neurosurgery. 1988;22(2):392-397. [DOI] [PubMed] [Google Scholar]
- 24. Hutchinson RW, Mendenhall V, Abutin RM, Muench T, Hart J. Evaluation of fibrin sealants for central nervous system sealing in the mongrel dog durotomy model. Neurosurgery. 2011;69(4):921-928. [DOI] [PubMed] [Google Scholar]
- 25. Zerris VA, James KS, Roberts JB, Bell E, Heilman CB. Repair of the dura mater with processed collagen devices. J Biomed Mater Res B Appl Biomater. 2007;83(2):580-588. [DOI] [PubMed] [Google Scholar]
- 26. ASTM Standard F2392-04(2015) Standard Test Method for Burst Strength of Surgical Sealants. ASTM International. www.astm.org. Accessed Apr 25, 2017. [Google Scholar]
- 27. Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Re. 2008. doi:10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Apuzzo JL, Wiess MH, Petersons V, Small RB, Kurze T, Heiden JS. Effect of positive end expiratory pressure ventilation on intracranial pressure in man. J Neurosurg. 1977;46(2):227-232. [DOI] [PubMed] [Google Scholar]
- 29. Biroli F, Esposito F, Fusco M et al. . Novel equine collagen-only dural substitute. Neurosurgery. 2008;62(3 suppl 1):273-274; discussion 274. [DOI] [PubMed] [Google Scholar]
- 30. Sekhar LN, Mai JC. Dural repair after craniotomy and the use of dural substitutes and dural sealants. World Neurosurg. 2013;79(3-4):440-442. [DOI] [PubMed] [Google Scholar]
- 31. Wallace DG, Cruise GM, Rhee WM et al. . A tissue sealant based on reactive multifunctional polyethylene glycol. J Biomed Mater Res. 2001;58(5):545-555. [DOI] [PubMed] [Google Scholar]
- 32. Thavarajah D, De Lacy P, Hussain R, Redfern RM. Postoperative cervical cord compression induced by hydrogel (DuraSeal): a possible complication. Spine (Phila Pa 1976). 2010;35(1):E25-E26. [DOI] [PubMed] [Google Scholar]
- 33. Neuman BJ, Radcliff K, Rihn J. Cauda equina syndrome after a TLIF resulting from postoperative expansion of a hydrogel dural sealant. Clin Orthop Relat Res. 2012;470(6):1640-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blackburn SL, Smyth MD. Hydrogel-induced cervicomedullary compression after posterior fossa decompression for Chiari malformation. Case report. J Neurosurg. 2007;106(4 suppl):302-304. [DOI] [PubMed] [Google Scholar]
- 35. Mulder M, Crosier J, Dunn R. Cauda equina compression by hydrogel dural sealant after a laminotomy and discectomy: case report. Spine (Phila Pa 1976). 2009;34(4):E144-E148. [DOI] [PubMed] [Google Scholar]
- 36. Epstein NE. Dural repair with four spinal sealants: focused review of the manufacturers' inserts and the current literature. Spine J. 2010;10(12):1065-1068. [DOI] [PubMed] [Google Scholar]
- 37. Lee SH, Park CW, Lee SG, Kim WK. Postoperative cervical cord compression induced by hydrogel dural sealant (DuraSeal®). Korean J Spine. 2013;10(1):44-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kassam A, Nemoto E, Balzer J et al. . Effects of Tisseel fibrin glue on the central nervous system of nonhuman primates. Ear Nose Throat J. 2004;83(4):246-248, 250, 252 passim. [PubMed] [Google Scholar]
- 39. Liu D, Michon J. Measurement of the subarachnoid pressure of the optic nerve in human subjects. Am J Ophthalmol. 1995;119(1):81-85. [DOI] [PubMed] [Google Scholar]
- 40. Ghajar J. Traumatic brain injury. Lancet 2000;356(9233):923-929. [DOI] [PubMed] [Google Scholar]
- 41. Wise SK, Schlosser RJ. Evaluation of spontaneous nasal cerebrospinal fluid leaks. Curr Opin Otolaryngol Head Neck Surg. 2007;15(1):28-34. [DOI] [PubMed] [Google Scholar]
- 42. Baumgartner B, Draxler W, Lewis KM. Treatment of severe aortic bleeding using hemopatch in swine on dual antiplatelet therapy. J Invest Surg. 2016;22:1-9. Available at: http://www.tandfonline.com/doi/full/10.3109/08941939.2016.1154627. Accessed Aug 25, 2016. [DOI] [PubMed] [Google Scholar]
- 43. Matonick JP, Hammond J. Hemostatic efficacy of EvarrestTM, fibrin sealant patch vs. TachoSil® in a heparinized swine spleen incision model. J Invest Surg. 2014;27(6):360-365. [DOI] [PubMed] [Google Scholar]
- 44. Kshettry VR, Lobo B, Lim J, Sade B, Oya S, Lee JH. Evaluation of non-watertight dural reconstruction with collagen matrix onlay graft in posterior fossa surgery. J Korean Neurosurg Soc. 2016;59(1):52-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barth M, Tuettenberg J, Thomé C, Weiss C, Vajkoczy P, Schmiedek P. Watertight dural closure: is it necessary? A prospective randomized trial in patients with supratentorial craniotomies. Neurosurgery. 2008;63(4 suppl 2):352-358; discussion 358. [DOI] [PubMed] [Google Scholar]
- 46. Sade B, Oya S, Lee JH. Non-watertight dural reconstruction in meningioma surgery: results in 439 consecutive patients and a review of the literature. Clinical article. J Neurosurg. 2011;114(3):714-718. [DOI] [PubMed] [Google Scholar]
- 47. Litvack ZN, West GA, Delashaw JB, Burchiel KJ, Anderson VC. Dural augmentation: part I-evaluation of collagen matrix allografts for dural defect after craniotomy. Neurosurgery. 2009;65(5):890-897; discussion 897. [DOI] [PubMed] [Google Scholar]
- 48. Burkett CJ, Patel S, Tabor MH, Padhya T, Vale FL. Polyethylene glycol (PEG) hydrogel dural sealant and collagen dural graft matrix in transsphenoidal pituitary surgery for prevention of postoperative cerebrospinal fluid leaks. J Clin Neurosci. 2011;18(11):1513-1517. [DOI] [PubMed] [Google Scholar]
- 49. Weinstein JS, Liu KC, Delashaw JB Jr et al. . The safety and effectiveness of a dural sealant system for use with nonautologous duraplasty materials. J Neurosurg. 2010;112(2):428-433. [DOI] [PubMed] [Google Scholar]
- 50. Confluent Surgical, Inc. DuraSeal dural sealant system instructions for use Available at: http://www.covidien.com/imageServer.aspx/doc178555.pdf?contentID=13915&contenttype=application/pdf. Accessed Aug 25, 2016. [Google Scholar]
- 51. Balasubramanian C, Coley E, Whittle IR. Dural bridge sutures to prevent sinking of dural substitutes: technical note. Acta Neurochir (Wien). 2009;151(2):155-157. [DOI] [PubMed] [Google Scholar]
- 52. Than KD, Baird CJ, Olivi A. Polyethylene glycol hydrogel dural sealant may reduce incisional cerebrospinal fluid leak after posterior fossa surgery. Neurosurgery. 2008;63(1 suppl 1):ONS182-ONS186; discussion ONS186-187. [DOI] [PubMed] [Google Scholar]
- 53. Lee KR, Betz AL, Keep RF, Chenevert TL, Kim S, Hoff JT. Intracerebral infusion of thrombin as a cause of brain edema. J Neurosurg. 1995;83(6):1045-1050. [DOI] [PubMed] [Google Scholar]
- 54. Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996;84(1):91-96. [DOI] [PubMed] [Google Scholar]
- 55. Gong C, Boulis N, Qian J, Turner DE, Hoff JT, Keep RF. Intracerebral hemorrhage-induced neuronal death. Neurosurgery. 2001;48(4):875-882; discussion 882-883. [DOI] [PubMed] [Google Scholar]
- 56. Lee KR, Drury I, Vitarbo E, Hoff JT. Seizures induced by intracerebral injection of thrombin: a model of intracerebral hemorrhage. J Neurosurg. 1997;87(1):73-78. [DOI] [PubMed] [Google Scholar]