Abstract
Background
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations are recommended for glomerular filtration rate (GFR) estimation in the general population. They have not been evaluated in community-based populations, including Blacks at higher levels of GFR, but are commonly applied in such populations.
Methods
In an ancillary study of Multi-Ethnic Study of Atherosclerosis conducted at one site, we evaluated the performance of the CKD-EPI equations for creatinine (eGFRcr), cystatin C (eGFRcys) or the combination (eGFRcr–cys) compared with GFR measured as plasma clearance of iohexol.
Results
Among 294 participants, the mean age was 71 (SD 9) years, 47% were Black, 48% were women and the mean measured GFR (mGFR) was 72.6 (SD 18.8) mL/min/1.73 m2. The CKD-EPI equations overestimated mGFR with a larger median bias for eGFRcr and eGFRcr–cys than eGFRcys [−8.3 (95% confidence interval −9.7, −6.5), −7.8 (−9.2, −6.2) and −3.7 (−5.0, −1.8) mL/min/1.73 m2, respectively], with smaller bias for those with lower compared with higher eGFR and by race compared with sex.
Conclusion
The small differential bias of the CKD-EPI equation between races suggests that they can be used in Blacks as well as Whites in older community-based adults. The large differential bias in women versus men in all equations is in contrast to other studies and is unexplained. Further studies are required in multiracial and multiethnic community-based cohorts, taking into account differences in GFR measurement methods.
Keywords: creatinine, cystatin C, glomerular filtration rate, multiethnic study of atherosclerosis
INTRODUCTION
Assessment of kidney function is critical to care for all patients. Kidney Disease: Improving Global Outcomes guidelines recommend GFR estimating equations using creatinine and cystatin C derived by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) unless more accurate equations are available [1]. The CKD-EPI GFR estimating equations were developed from studies in diverse populations, including studies in populations selected because they were known either to have or not to have CKD, but did not include studies in community-based populations, and thus did not include many older adults or Blacks at higher levels of GFR [2, 3]. The CKD-EPI equations are commonly applied in such populations and therefore it is important to evaluate their performance in these settings. Since then, several studies have shown that the CKD-EPI equations perform well in community-based populations of Whites, but these studies did not include Blacks [4–8]. We measured GFR in an ancillary study within the Multi-Ethnic Study of Atherosclerosis (MESA), a community-based cohort of older Black and White individuals (MESA-Kidney).
We previously reported differences in measured GFR (mGFR) between Blacks and Whites and between men and women in MESA-Kidney [9]. Our goal in this article is to evaluate the performance of the CKD-EPI creatinine and cystatin C equations compared with mGFR in this study population, focusing on performance in subgroups defined by race and sex. We also evaluated performance by height and weight, because prior studies have shown differential accuracy of these equations by body size [10]. The CKD-EPI equations were developed using urinary clearance of 125I-iothalamate to measure GFR. It is difficult to perform urinary clearance measurements and to use radioisotopes in community-based populations, so recent studies have used plasma clearance of iohexol to measure GFR. Other equations have been developed using plasma clearance of iohexol, and a systematic review of GFR measurement methods has drawn attention to differences in mGFR using these two methods [8, 11, 12]. In a sensitivity analysis, we also explored whether the method used to measure GFR is a possible cause for the inaccuracies that we observed.
MATERIALS AND METHODS
Study population
MESA was designed to compare the prevalence of multiple measures of subclinical cardiovascular disease (CVD), risk factors for subclinical CVD and rates of progression to clinical CVD in individuals from various races and ethnicities who were free of clinical CVD at the baseline examination [13, 14]. MESA participants underwent their fifth visit between April 2010 and December 2011. As we have described previously, Black and White participants at the Johns Hopkins University MESA field center who completed the fifth visit were eligible for inclusion in MESA-Kidney, as were participants who completed the third or fourth visit but not the fifth visit [9]. MESA-Kidney participants were recruited between May 2012 and April 2014.
Laboratory methods
We measured GFR using plasma clearance of iohexol. Details of the GFR measurement procedure were reported previously [9, 15]. In brief, 5 mL of iohexol [Omnipaque 300 (300 mg/mL of organic iodine)] were administered intravenously over a period of 30 s followed by a 10 mL normal saline flush. Blood samples for plasma clearance measurements were taken from a second catheter at approximately 10, 30, 120, 240 and 300 min, with the exact times recorded [16]. Iohexol was assayed using high-performance liquid chromatography. Full details of the protocol have been previously described [9]. Iohexol values were reviewed to ensure they were consistent with linear decline over time on the log scale. We calculated GFR from the plasma clearance of iohexol using all time points, using a two-compartment model if both early and late time points were available. A total of six participants (2%) did not have early time points, in whom GFR was calculated using the Bröchner–Mortensen equation [17]. We calculated body surface area using the DuBois and DuBois formula and expressed GFR indexed per 1.73 m2 and not indexed for body surface area [18].
Serum creatinine was measured using the Roche/Hitachi Modular P instrument with Roche enzymatic Creatinine Plus reagent and calibrators (Coefficient of Variability was 2.3% for creatinine assay), which yields creatinine results that are traceable to National Institute of Standards and Technology isotope dilution mass spectrometry reference materials [19]. Comparability between the instruments was tested. Serum cystatin C was measured on the Roche COBAS 6000 using Gentian assays as described by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Working Group for the Standardization of Serum Cystatin C and the Institute for Reference Materials and Measurements (ERM-DA471/IFCC) [20–22].
Statistical analysis
Approach
The aim of this study was to evaluate the performance of the CKD-EPI equations within MESA-Kidney. In this study we observed relatively large errors as well as large differential bias between men and women, and therefore explored possible causes of these errors as related to the following two factors. First, since both creatinine and cystatin C are affected by body composition independent of GFR, we hypothesized the source of error may be related to body size, which on average was larger in MESA-Kidney than in prior studies [2, 3, 15] and differed between men and women. Second, since recent data suggest that plasma clearance of iohexol may be lower than urinary clearance of iothalamate, we hypothesized that the source of error may be related to a systematic difference in GFR measurement methods used in the development of CKD-EPI equations and in MESA-Kidney [12, 23].
Equation performance
Estimated GFR (eGFR) was first computed from equations developed by the CKD-EPI using creatinine, cystatin C and creatinine–cystatin C (see Supplementary data, Table S1). For comparisons with mGFR not indexed for body surface area, we converted bovine serum albumin (BSA)-indexed eGFR to nonindexed eGFR using the following equation: nonindexed eGFR (mL/min) = BSA-indexed eGFR (mL/min/1.73 m2) * BSA (m2)/1.73. The performance of these equations compared with mGFR was evaluated using metrics for bias, precision and accuracy, similar to previous reports [24]. Bias was assessed as the median difference between mGFR and eGFR (mGFR − eGFR). Precision was assessed as the interquartile range (IQR) of the differences between mGFR and eGFR. Accuracy was assessed as the percentage of eGFR within 30% of mGFR (P30) as a measure of large errors, and the root mean squared error (RMSE) was calculated as the square root of the squared differences between mGFR and eGFR. The 95% confidence intervals (CIs) around the median difference, IQR of the difference, P30 and RMSE were calculated using the bootstrap method (1000 bootstraps). For all four metrics, differences between equations were determined by nonoverlapping 95% CIs, allowing use of a consistent approach for comparison across all metrics, whereas P-values could only be determined for differences in bias and P30.
Errors related to body size of the study population
Within race and sex groups, we determined whether bias differed by height and weight by fitting linear regression models for the characteristic of interest on the difference between mGFR and eGFR. We explored whether there were any interactions between race and sex groups and the characteristic.
Errors related to differences in GFR measurement methods
We performed a sensitivity analysis of the performance of the CKD-EPI equations after increasing mGFR values in MESA-Kidney (plasma clearance of iohexol) to account for possible differences from the GFR measurement method used to develop the CKD-EPI equation (urinary clearance of iothalamate) [12, 23]. We selected adjustment factors of 5 and 10% as comparisons of the urinary clearance of iothalamate and urinary clearance of iohexol to estimate the difference at 15%, but plasma clearance of iohexol is generally lower than the urinary clearance of iothalamate by <10% [25]. We next compared the performance of the CKD-EPI equations to GFR estimating equations that were developed predominantly using plasma clearance of iohexol [8, 11, 26] (Supplementary data, Table S1 shows the equations).
RESULTS
Demographic and clinical characteristics of the study population
A total of 294 participants with complete data were included in MESA-Kidney, as has been previously described. In MESA-Kidney, the mean age was 71 (SD 9) years, 47% were Black, 48% were women and the mean mGFR was 72.6 (SD 18.8) mL/min/1.73 m2. Table 1 compares the clinical characteristics across race and sex groups. Some but not all measures of body size were greater in Blacks versus Whites and in men versus women, with larger differences between men and women than between Blacks and Whites. Black women from the fifth visit who were included were younger and had higher eGFR for creatinine (eGFRcr) than those who did not participate, but otherwise there were no significant differences between those included and excluded (Supplementary data, Tables S2 and S3). Supplementary data, Table S4 shows the eGFR using the various equations across the four groups.
Table 1.
Clinical characteristics by race and sex
| Total | Black | White | P-value | Men | Women | P-value | |
|---|---|---|---|---|---|---|---|
| (n = 294) | (n = 139) | (n = 155) | (n = 154) | (n = 140) | |||
| Age (years) | 70.7 ± 8.6 | 69.5 ± 8.6 | 71.8 ± 8.5 | 0.02 | 71.2 ± 8.5 | 70.2 ± 8.7 | 0.3 |
| Smoking, n (%) | 0.1 | <0.001 | |||||
| Current | 26 (9) | 17 (12) | 9 (6) | 15 (10) | 11 (8) | ||
| Former | 131 (45) | 56 (40) | 75 (48) | 86 (56) | 45 (32) | ||
| Never | 137 (47) | 66 (47) | 71 (46) | 53 (34) | 84 (60) | ||
| Hypertension, n (%) | 188 (64) | 99 (71) | 89 (57) | 0.01 | 91 (59) | 97 (69) | 0.07 |
| Systolic blood pressure (mmHg) | 128.1 ± 17.4 | 130.0 ± 17.3 | 126.4 ± 17.3 | 0.08 | 127.8 ± 16.6 | 128.3 ± 18.2 | 0.8 |
| Diastolic blood pressure (mmHg) | 71.1 ± 9.6 | 72.2 ± 9.1 | 70.2 ± 9.9 | 0.07 | 72.4 ± 9.4 | 69.7 ± 9.7 | 0.02 |
| Diabetes, n (%) | 73 (25) | 47 (34) | 26 (17%) | 0.001 | 43 (28) | 30 (21) | 0.2 |
| LDL cholesterol (mg/dL) | 104.9 ± 36.4 | 110.2 ± 38.4 | 100.2 ± 34.0 | 0.02 | 101.8 ± 35.9 | 108.4 ± 36.8 | 0.1 |
| HDL cholesterol (mg/dL) | 55.4 ± 18.1 | 55.3 ± 20.1 | 55.4 ± 16.2 | 0.9 | 49.1 ± 12.5 | 62.3 ± 20.6 | <0.001 |
| Taking lipid lowering medications, n (%) | 126 (43) | 4086 (55) | 8640 (29) | <0.001 | 70 (45) | 56 (40) | 0.3 |
| CVD (at exam 5), n (%) | 14 (5) | 7 (5) | 7 (5) | 0.8 | 11 (7) | 3 (2) | 0.04 |
| Weight (kg) | 84.6 ± 17.1 | 87.1 ± 16.8 | 82.3 ± 17.1 | 0.02 | 91.1 ± 15.0 | 77.4 ± 16.3 | <0.001 |
| Height (cm) | 168.5 ± 9.7 | 168.7 ± 9.5 | 168.4 ± 10.0 | 0.8 | 175.5 ± 7.0 | 160.9 ± 5.7 | <0.001 |
| Body surface area (m2) | 1.94 ± 0.22 | 1.97 ± 0.20 | 1.92 ± 0.23 | 0.06 | 2.07 ± 0.17 | 1.81 ± 0.18 | <0.001 |
| Body mass index (kg/m2) | 29.7 ± 5.4 | 30.6 ± 5.8 | 28.9 ± 4.9 | 0.005 | 29.6 ± 4.7 | 29.9 ± 6.0 | 0.6 |
| Extracellular volume (L) | 16.6 ± 4.2 | 17.2 ± 4.3 | 16.1 ± 4.1 | 0.02 | 18.4 ± 3.9 | 14.6 ± 3.5 | <0.001 |
| Extracellular volume (L/kg) | 0.20 ± 0.04 | 0.20 ± 0.04 | 0.20 ± 0.04 | 0.5 | 0.20 ± 0.04 | 0.19 ± 0.04 | 0.003 |
| Measured GFR (mL/min/1.73 m2) | 72.6 ± 18.8 | 74.1 ± 19.7 | 71.2 ± 17.9 | 0.2 | 77.0 ± 19.6 | 67.7 ± 16.6 | <0.001 |
| Measured GFR (mL/min) | 82.1 ± 25.6 | 84.8 ± 26.0 | 79.7 ± 25.1 | 0.09 | 92.3 ± 26.1 | 70.9 ± 19.8 | <0.001 |
| Urine ACR (mg/g), median (IQR) | 10.0 (5.8–20.9) | 8.6 (5.0–20.0) | 11.1 (6.6–20.9) | 0.5 | 9.4 (5.0–26.3) | 10.5 (6.6–19.0) | 0.4 |
| Urine albumin (mg/L), median (IQR) | 8.0 (4.0–20.0) | 9.0 (4.0–19.0) | 8.0 (3.0–20.0) | 0.4 | 8.0 (4.0–27.5) | 8.0 (3.0–15.0) | 0.6 |
| Urine creatinine (mg/dL), median (IQR) | 90 (48–138) | 107 (59–157) | 70 (42–125) | <0.001 | 97 (57–152) | 74 (38–127) | 0.02 |
Values are presented as mean ± SD, unless stated otherwise. LDL, low-density lipoprotein; HDL, high-density lipoprotein; CVD, cardiovascular disease; GFR, glomerular filtration rate; ACR, albumin-to-creatinine ratio; IQR, interquartile range.
Equation performance overall and by level of eGFR in race and sex subgroups
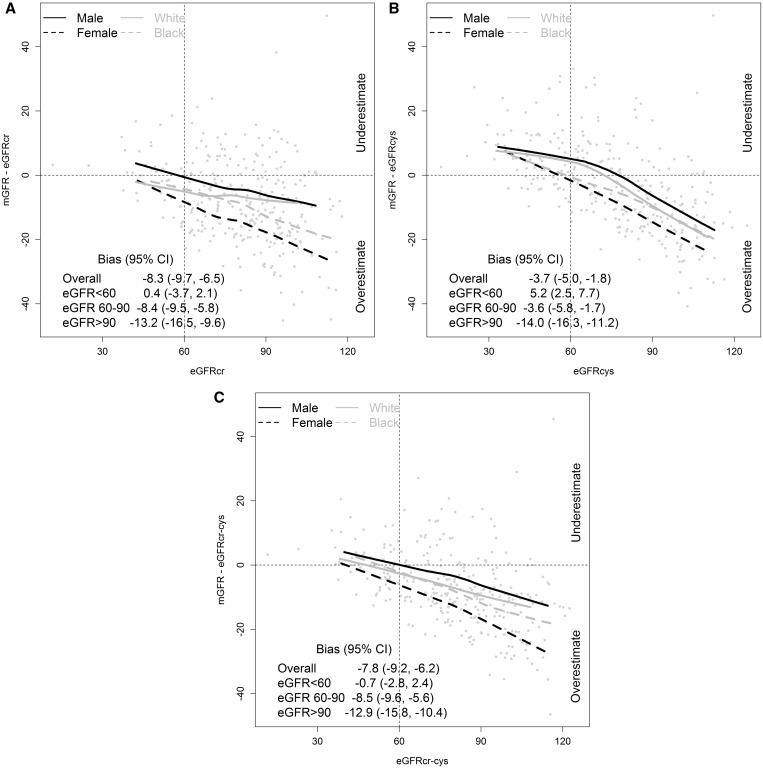
Table 2 and Figure 1 show the performance of the CKD-EPI creatinine (eGFRcr), cystatin C (eGFRcys) and creatinine–cystatin C equations (eGFRcr–cys) overall and by eGFR across race and sex groups. Table 3 shows the performance by sex and race subgroups. All three equations overestimated mGFR in the overall population with a larger median bias for eGFRcr and eGFRcr–cys than eGFRcys [−8.3 (95% CI −9.7, −6.5), −7.8 (−9.2, −6.2) and −3.7 (−5.0, −1.8) mL/min/1.73 m2, respectively] (Table 2). For all equations, differences were observed across the range of GFRs, with small to negligible bias at lower levels of GFR and larger bias at higher eGFRs (Figure 1). For all equations, consistent differences were observed across race and sex groups; median bias was smallest in men and largest in women and intermediate in Whites and Blacks (Table 3). Evaluation in race–sex subgroups showed median bias was smallest for White men, largest for Black women and intermediate for Black men and White women (Table 3). Precision was better for eGFRcr–cys than for eGFRcr and eGFRcys, and was similar among sex and race groups. In the overall population, accuracy was best for eGFRcr–cys, but differences among sex and race groups were similar to those for bias. Results were similar using mGFR and eGFR not indexed for body surface area.
Table 2.
Performance of CKD-EPI GFR estimating equations overall and by race and sex subgroups
| Equation | Subgroup | Bias | Precision | Accuracy (error rate) |
|
|---|---|---|---|---|---|
| Median difference mGFR − eGFR (95% CI) | IQR difference mGFR − eGFR (95% CI) | 1-P30% eGFR > 30% of mGFR (95% CI) | Root mean square error (95% CI) | ||
| CKD-EPI creatinine | Overall | −8.3 (−9.7, −6.5) | 17.6 (15.2, 19.5) | 19.0 (14.6, 23.5) | 0.203 (0.186, 0.218) |
| Women | −14.2 (−16.5, −10.9) | 15.0 (11.6, 17.9) | 32.1 (24.3, 40.0) | 0.242 (0.219, 0.264) | |
| Men | −3.4 (−6.3, 0.0) | 15.7 (12.2, 18.2) | 7.1 (3.2, 11.7) | 0.160 (0.141, 0.178) | |
| Black | −9.0 (−12.5, −7.5) | 16.7 (13.8, 20.8) | 20.9 (15.1, 28.1) | 0.217 (0.192, 0.245) | |
| White | −6.5 (−9.3, −3.5) | 18.1 (14.8, 20.6) | 17.4 (11.6, 23.9) | 0.189 (0.170, 0.208) | |
| CKD-EPI cystatin C | Overall | −3.7 (−5.0, −1.8) | 18.0 (16.1, 20.9) | 11.2 (7.8, 15.0) | 0.190 (0.175, 0.207) |
| Women | −5.0 (−9.3, −3.3) | 18.5 (14.6, 21.2) | 13.6 (8.6, 19.6) | 0.191 (0.173, 0.210) | |
| Men | −1.5 (−3.9, 1.6) | 18.6 (15.2, 21.4) | 9.1 (4.5, 14.0) | 0.190 (0.164, 0.216) | |
| Black | −4.7 (−6.8, −2.5) | 16.8 (12.4, 18.8) | 10.8 (5.8, 15.8) | 0.183 (0.162, 0.205) | |
| White | −2.8 (−4.2, 0.9) | 21.2 (17.7, 24.3) | 11.6 (7.1, 16.8) | 0.197 (0.174, 0.221) | |
| CKD-EPI creatinine– cystatin C | Overall | −7.8 (−9.2, −6.2) | 14.0 (12.1, 16.2) | 12.6 (8.8, 16.3) | 0.176 (0.163, 0.188) |
| Women | −10.7 (−13.1, −9.0) | 14.2 (11.9, 17.9) | 22.1 (15.7, 29.3) | 0.203 (0.185, 0.222) | |
| Men | −4.3 (−7.0, −2.3) | 13.5 (11.2, 15.7) | 3.9 (1.3, 7.1) | 0.146 (0.130, 0.162) | |
| Black | −9.6 (−11.1, −7.8) | 15.2 (11.1, 19.3) | 17.3 (10.8, 23.7) | 0.194 (0.175, 0.214) | |
| White | −4.9 (−8.6, −3.4) | 13.0 (11.0, 16.0) | 8.4 (4.5, 12.9) | 0.157 (0.142, 0.172) | |
FIGURE 1.
Bias versus eGFR by race and sex subgroups. (A) CKD-EPI creatinine; (B) CKD-EPI cystatin C and (C) CKD-EPI creatinine–cystatin C. Solid lines indicate the median difference (median bias). Units of difference, ml/min/1.73m2
Table 3.
Performance of CKD-EPI GFR estimating equations overall and by race and sex subgroups
| Equation | Subgroup | Bias | Precision | Accuracy (error rate) |
|
|---|---|---|---|---|---|
| Median difference mGFR − eGFR (95% CI) | IQR difference mGFR − eGFR (95% CI) | 1 P30% eGFR > 30% of mGFR (95% CI) | Root mean square error (95% CI) | ||
| CKD-EPI creatinine | Overall | −8.3 (−9.7, −6.5) | 17.6 (15.2, 19.5) | 19.0 (14.6, 23.5) | 0.203 (0.186, 0.218) |
| White female | −12.3 (−15.8, −8.3) | 15.7 (11.2, 18.5) | 30.0 (18.6, 41.4) | 0.220 (0.188, 0.251) | |
| Back female | −16.4 (−18.9, −12.4) | 16.0 (11.6, 21.4) | 34.3 (24.3, 45.7) | 0.261 (0.226, 0.296) | |
| White male | −1.6 (−6.2, 0.8) | 17.6 (12.6, 20.4) | 7.1 (2.4, 12.9) | 0.159 (0.136, 0.182) | |
| Black male | −5.8 (−7.4, −2.4) | 13.3 (9.6, 16.9) | 7.2 (1.4, 13.0) | 0.160 (0.132, 0.186) | |
| CKD-EPI cystatin C | Overall | −3.7 (−5.0, −1.8) | 18.0 (16.1, 20.9) | 11.2 (7.8, 15.0) | 0.190 (0.175, 0.207) |
| White female | −5.0 (−11.4, −2.8) | 18.7 (14.4, 23.5) | 15.7 (7.1, 24.3) | 0.199 (0.176, 0.223) | |
| Back female | −5.0 (−9.5, −2.5) | 18.0 (12.3, 22.0) | 11.4 (4.3, 18.6) | 0.183 (0.156, 0.209) | |
| White male | 1.8 (−3.3, 5.4) | 18.1 (14.2, 23.3) | 8.2 (3.5, 14.1) | 0.194 (0.156, 0.236) | |
| Black male | −4.3 (−8.1, 0.1) | 15.4 (10.6, 20.7) | 10.1 (4.3, 17.4) | 0.184 (0.150, 0.217) | |
| CKD-EPI creatinine– cystatin C | Overall | −7.8 (−9.2, −6.2) | 14.0 (12.1, 16.2) | 12.6 (8.8, 16.3) | 0.176 (0.163, 0.188) |
| White female | −9.8 (−12.2, −6.7) | 14.2 (9.8, 16.7) | 17.1 (8.6, 26.4) | 0.185 (0.162, 0.206) | |
| Back female | −13.5 (−16.9, −9.5) | 15.6 (11.7, 19.0) | 27.1 (17.1, 37.1) | 0.220 (0.194, 0.246) | |
| White male | −2.7 (−4.6, 0.3) | 13.4 (10.6, 17.4) | 1.2 (0.0, 3.5) | 0.130 (0.111, 0.150) | |
| Black male | −7.3 (−9.6, −4.3) | 12.8 (8.4, 15.7) | 7.2 (1.4, 13.0) | 0.164 (0.138, 0.188) | |
Associations of equation performance with body size
Supplementary data, Figure S1 shows the associations of height and weight with bias for the three equations. For all three equations, there was less variation in bias by height than by weight. There was an overestimate of mGFR at lower weight and an underestimate of mGFR at higher weight. Similar patterns were observed across sex and race subgroups for all three equations, except the comparison of difference of bias by weight, where the underestimate at higher weight was greater for Whites than Blacks for eGFRcys and eGFRcr–cys (P-value for the interaction of 0.004 and 0.0001, respectively).
Sensitivity analysis accounting for GFR measurement methods
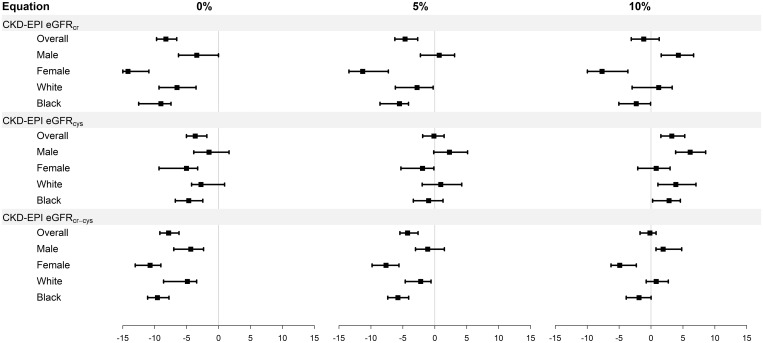
Figure 2 and Supplementary data, Table S5 compare bias assuming mGFR using plasma clearance of iohexol is 0, 5 or 10% lower than urinary clearance of iothalamate. As expected, the median bias (overestimate) of eGFRcr, eGFRcys and eGFRcr-cys was smaller after correction, and accuracy improved, but the pattern of larger differences in median bias between men and women than between Whites and Blacks persisted.
FIGURE 2.
Bias by race and sex subgroups assuming 0, 5 and 10% lower mGFR using plasma clearance of iohexol than urinary clearance of iothalamate. CKD-EPI, Chronic Kidney Disease Epidemiology Consortium; cr, creatinine; cys, cystatin C; cr-cys, creatinine-cystatin C. Units of difference, ml/min/1.73m2
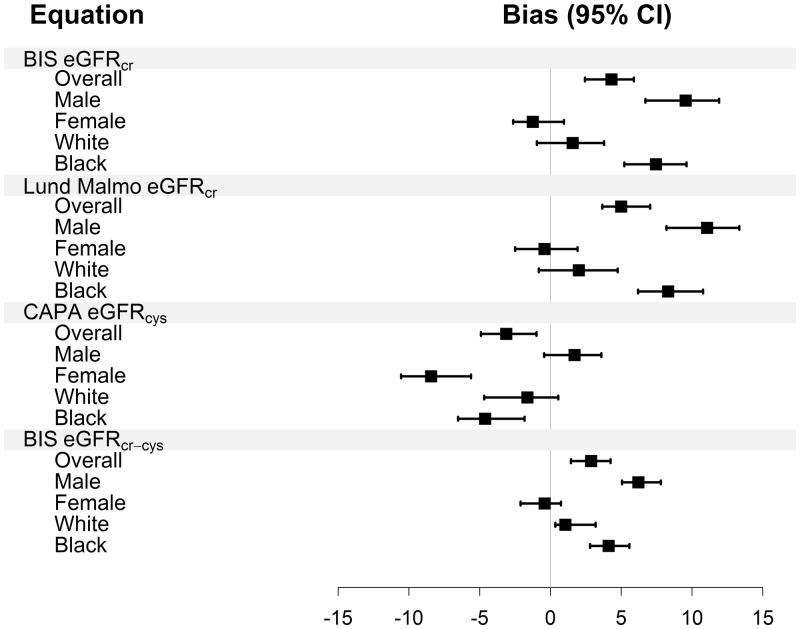
Figure 3 and Supplementary data, Table S6 shows the performance in equations that were developed predominantly using plasma clearance of iohexol. The BIScr (Berlin Initiative Study) and revised Lund–Malmö equations underestimated mGFR and the CAPA (Caucasian, Asian, pediatric and adult) equation overestimated mGFR, whereas the BIScr–cys equation was unbiased. There was large variation across race and sex subgroups for all equations except for the BIScr–cys equation. For all equations, the pattern of larger difference in median bias between men and women than between Whites and Blacks was observed.
FIGURE 3.
Bias by race and sex subgroups for equations developed using GFR measured using plasma clearance of iohexol. CKD-EPI, Chronic Kidney Disease Epidemiology Consortium; CAPA, Caucasian, Asian, pediatric and adult; BIS, Berlin Initiative Study; cr, creatinine; cys, cystatin C; cr-cys, creatinine-cystatin C. Units of difference, ml/min/1.73m2
Differences in bias between race and sex groups
Table 4 shows differences in bias between Blacks and Whites and men and women for all equations. There were large differences in bias between men and women across all filtration markers and equations, with the exception of eGFRcys for the CKD-EPI equation. In contrast, there was variation in the difference in bias between Blacks and Whites, which differed among equations even for the same filtration marker. For eGFRcr, the difference in bias between Blacks and Whites was not significant for the CKD-EPI equation, but was significant for the BIS and Lund–Malmö equations. For eGFRcys, the difference in bias between Blacks and Whites was not significant for either the CKD-EPI or CAPA equations. For eGFRcr–cys, there was a significant difference in bias between Blacks and Whites for both CKD-EPI and BIS, but in the opposite direction.
Table 4.
Difference in bias between Blacks and Whites and between men and women for all equations
| Female versus male (95% CI) | Blacks versus White (95% CI) | |
|---|---|---|
| Creatinine | ||
| CKD-EPI | −10.7 (−14.4, −7) | −2.5 (−6.2, 1.1) |
| BIS | −10.8 (−13.9, −7.7) | 5.9 (2.6, 9.1) |
| Lund–Malmö | −11.4 (−14.8, −8.1) | 6.3 (3, 9.6) |
| Cystatin C | ||
| CKD-EPI | −3.7 (−7.9, 0.5) | −1.9 (−5, 1.2) |
| CAPA | −10.2 (−13.1, −7.2) | −3 (−6.1, 0.2) |
| Creatinine–cystatin C | ||
| CKD-EPI | −6.3 (−9.1, −3.6) | −4.7 (−7.5, −1.9) |
| BIS | −6.8 (−8.7, −4.8) | 3.1 (1.1, 5.0) |
BIS, Berlin Initiative Study. CAPA, Caucasian, Asian, pediatric and adult.
CONCLUSION
MESA-Kidney is the first evaluation of GFR estimating equations in a community-based population sample that includes both Blacks and Whites. In general, the CKD-EPI equations overestimated mGFR at higher eGFRs, while other equations underestimated mGFR, possibly reflecting differences in the exogenous filtration marker used to measure GFR in the development of the equations. All equations showed variation in the performance by race and sex, with larger and more consistent differences by sex than race. The small variation in the CKD-EPI equations by race supports their use in Blacks as well as Whites in the general population. The large and consistent variation in all equations by sex raises questions about possible differences in the study population in this study compared with prior evaluations.
Use of plasma clearance of iohexol in MESA-Kidney to measure GFR, rather than urinary clearance of iothalamate, as was used to measure GFR in the development of the CKD-EPI equations, could be a source of the observed bias. Seegmiller et al. [23] showed that urinary clearance of iohexol (thought to be ∼5–10% lower than clearance of iohexol) was approximately 15% lower than urinary clearance of iothalamate. A systematic review by Soveri et al. [12] found that some but not all prior studies demonstrated urinary clearances of iothalamate to overestimate and iohexol to underestimate the urinary insulin clearance, with smaller differences for the plasma clearance of iohexol. Prior studies comparing the performance of CKD-EPI equations have not demonstrated systematic differences based on exogenous filtration markers used to measure the GFR, but this may be due to differences among assays or small sample sizes [27]. A recent study in an elderly Icelandic population using plasma clearance of iohexol did not demonstrate a large bias of the CKD-EPI equation [4]. In a sensitivity analysis, we demonstrated that systematically higher mGFR (as may have been seen if urinary clearance of iothalamate had been used) in MESA-Kidney would lead to lesser bias of the CKD-EPI equations and attenuate the difference in performance between eGFRcr and eGFRcys, but would not attenuate the observed differences between men and women.
Prior studies could not assess the race coefficient for Blacks versus Whites at high levels of GFR [2, 3]. The CKD-EPI equations were developed in a diverse population including both Blacks and White, but did not include a large number of Blacks with higher GFR. They include a race coefficient in equations using creatinine as the filtration marker, but not in the equation using cystatin C as the sole filtration marker. The other equations evaluated were developed in predominantly European Caucasian populations and do not include a race coefficient. In MESA-Kidney, the similar performance of the CKD-EPI creatinine equation in Blacks and Whites, and the differential performance of the BIScr and revised Lund–Malmö equations in Blacks and Whites, support the requirement for a race coefficient in equations using creatinine as the sole filtration marker in populations with higher levels of GFR. This is consistent with the evaluation of the CKD-EPI equation in European Africans with CKD, which confirmed the need for a Black coefficient [28]. The similar performance of CKD-EPIcys and the CAPA equation in Blacks and Whites supports the absence of such a requirement in cystatin C equations.
We cannot fully explain the differential bias we observed between women and men for most of the equations evaluated here. All equations tested here except CAPA include a sex coefficient. Prior studies have not observed sex differences in performance for estimating equations [3, 4, 8, 11, 29]. There are several possible explanations for the discrepancy between these prior studies and our observations here. First, the plasma clearance of iohexol may have differential error in women versus men. Indeed, we also observed large differences in mGFR by sex in MESA-Kidney, with lower mGFR in women than men, which is consistent with the observed difference in bias of eGFR [9]. However, this method to measure GFR has been widely used, and prior studies have not suggested sex differences [8, 11, 26]. Second, the sex coefficients in the creatinine equations tested here may not be valid in mixed race (Black–White) populations with higher levels of GFR. Indeed, prior studies have shown lesser differences between men and women in their estimation of GFR from creatinine (i.e., an attenuated sex coefficient) in low-risk populations compared with CKD populations [30–33]. However, if sex coefficients for the CKD-EPI equations had been derived in MESA-Kidney, they would lead to lower estimates of GFR in women compared with the current coefficients. If such MESA-specific coefficients were applied to a population-based sample, they would lead to even lower mean eGFRs and higher prevalences of CKD in women compared with men; this would be even more discordant with the lower incidence of ESRD in women compared with men in the USA [34, 35]. Third, MESA-Kidney participants, particularly women, may differ in body composition or nutrition from participants included in prior studies. Body composition and nutrition can affect mGFR and eGFR [36]. We observed that bias varied with both height and weight, but not by sex. We also observed that the differential bias between men and women was larger for eGFRcr than eGFRcys. Indeed, this latter observation explains the better performance of eGFRcys versus eGFRcr in the overall dataset, as eGFRcr had larger bias than eGFRcys in women but not in men. Given all of these issues, we cannot conclude that the sex difference in GFR estimation between men and women observed here is representative of the larger population. We therefore think it would be premature to suggest revision of the sex coefficients in the CKD-EPI equations based on this study.
The strengths of this analysis are a well-characterized elderly cohort that included Blacks and Whites recruited from a community-based population, GFR was measured using a reference standard method and the use of creatinine and cystatin C assays traceable to international reference materials assayed in a laboratory that also developed the CKD-EPI equations. There are also several limitations. First, the small sample size, especially among subgroups, leads to wide CIs for many of the performance metrics and precludes strong conclusions. Second, the cohort is drawn from MESA participants at only one site and the results may not generalize to all of MESA or other populations. Third, there are differences among reference standards for GFR measurement, in particular between plasma clearance of iohexol and urinary clearance of iothalamate, and in some studies iohexol clearance is lower than iothalamate clearance [12]. However, we tried to account for this difference in the sensitivity analysis. Finally, the GFR estimating equations other than CKD-EPI were developed in predominantly European populations with differences in characteristics from MESA-Kidney, which may contribute to the poor performance of some of the equations.
In summary, we showed the CKD-EPI equations have differential performance across race, sex and the eGFR subgroups found in this community-based sample. Our interpretation is that the small differential bias between Blacks and Whites validates the use of these equations in Blacks as well as Whites in the general population. The large differential bias in women versus men in all equations is in contrast to other studies and is unexplained. This study should be repeated in other multiracial and multiethnic community-based cohorts. These findings support the need to better understand the non-GFR determinants of endogenous filtration markers and develop more accurate GFR estimating equations. Future development and validation of GFR estimation equations should take into account differences in GFR measurement methods.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
FUNDING
This research was supported by a grant from the National Institutes of Health (R01DK087961, contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169); the National Heart, Lung, and Blood Institute and National Center for Research Resources (UL1-TR-000040 and UL1-TR-001079).
CONFLICT OF INTEREST STATEMENT
L.A.I. reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health, National Kidney Foundation, Pharmalink, Gilead Sciences and Otsuka. A.S.L. reports funding to Tufts Medical Center for research and contracts with the National Institutes of Health, National Kidney Foundation, Amgen, Pharmalink, Gilead Sciences and Otsuka. J.C. reports funding to Johns Hopkins University for research and contracts with the National Institutes of Health and the National Kidney Foundation. J.H.E. is a consultant to Gentian, Moss, Norway. Siemens Healthcare Diagnostics, Tarrytown, NY, has provided free or steeply discounted reagents for studies performed in J.H.E.’s research laboratory. He also receives funding from the National Institutes of Health, the Centers for Disease Control and Prevention and several private research foundations. M.G.S. receives funding from the National Institutes of Health and serves as an advisor to Cricket Health and to Tai Diagnostics. T.S. and W.S.P. receive funding from the National Institutes of Health. H.T., C.J. and A.O. declare that they have no conflicts of interest.
L.A.I., A.S.L. and J.C. have a provisional patent [Coresh, Inker and Levey] filed 15 August 2014—‘Precise estimation of GFR from multiple biomarkers’ (PCT/US2015/044567). The technology is not licensed in whole or in part to any company. Tufts Medical Center, John Hopkins University and Metabolon have a collaboration agreement to develop a product to estimate GFR from a panel of markers.
Supplementary Material
REFERENCES
- 1. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 2. Levey AS, Stevens LA, Schmid CH. et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Inker LA, Schmid CH, Tighiouart H. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan L, Levey AS, Gudnason V. et al. Comparing GFR estimating equations using cystatin C and creatinine in elderly individuals. J Am Soc Nephrol 2015; 26: 1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilbride HS, Stevens PE, Eaglestone G. et al. Accuracy of the MDRD (modification of diet in renal disease) study and CKD-EPI (CKD epidemiology collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis 2013; 61: 57–66 [DOI] [PubMed] [Google Scholar]
- 6. Alshaer IM, Kilbride HS, Stevens PE. et al. External validation of the Berlin equations for estimation of GFR in the elderly. Am J Kidney Dis 2014; 63: 862–865 [DOI] [PubMed] [Google Scholar]
- 7. Vidal-Petiot E, Haymann JP, Letavernier E. et al. External validation of the BIS (Berlin initiative study)-1 GFR estimating equation in the elderly. Am J Kidney Dis 2014; 63: 865–867 [DOI] [PubMed] [Google Scholar]
- 8. Schaeffner ES, Ebert N, Delanaye P. et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 2012; 157: 471–481 [DOI] [PubMed] [Google Scholar]
- 9. Inker LA, Shafi T, Okparavero A. et al. Effects of race and sex on measured GFR: the multi-ethnic study of atherosclerosis. Am J Kidney Dis 2016; 68: 743–751 [DOI] [PubMed] [Google Scholar]
- 10. Fan L, Inker LA, Rossert J. et al. Glomerular filtration rate estimation using cystatin C alone or combined with creatinine as a confirmatory test. Nephrol Dial Transplant 2014; 29: 1195–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bjork J, Grubb A, Sterner G. et al. Revised equations for estimating glomerular filtration rate based on the Lund–Malmo Study cohort. Scand J Clin Lab Invest 2011; 71: 232–239 [DOI] [PubMed] [Google Scholar]
- 12. Soveri I, Berg UB, Bjork J. et al. Measuring GFR: a systematic review. Am J Kidney Dis 2014; 64: 411–424 [DOI] [PubMed] [Google Scholar]
- 13. Kramer H, Jacobs DR Jr, Bild D. et al. Urine albumin excretion and subclinical cardiovascular disease. The multi-ethnic study of atherosclerosis. Hypertension 2005; 46: 38–43 [DOI] [PubMed] [Google Scholar]
- 14. Bild DE, Bluemke DA, Burke GL. et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002; 156: 871–881 [DOI] [PubMed] [Google Scholar]
- 15. Inker LA, Okparavero A, Tighiouart H. et al. Midlife blood pressure and late-life GFR and albuminuria: an elderly general population cohort. Am J Kidney Dis 2015; 66: 240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Omnipaque (Iohexol) Injection [Package Insert]. Princeton, NJ: Amersham Health Inc, 2004 [Google Scholar]
- 17. Brochner-Mortensen J. A simple method for the determination of glomerular filtration rate. Scand J Clin Lab Invest 1972; 30: 271–274 [DOI] [PubMed] [Google Scholar]
- 18. Du Bois D, Du Bois EF.. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5: 303–311; discussion 312–303 [PubMed] [Google Scholar]
- 19. Levey AS, Coresh J, Greene T. et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53: 766–772 [DOI] [PubMed] [Google Scholar]
- 20. Grubb A, Blirup-Jensen S, Lindstrom V. et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 2010; 48: 1619–1621 [DOI] [PubMed] [Google Scholar]
- 21. Blirup-Jensen S, Grubb A, Lindstrom V. et al. Standardization of Cystatin C: development of primary and secondary reference preparations. Scand J Clin Lab Invest Suppl 2008; 241: 67–70 [DOI] [PubMed] [Google Scholar]
- 22. Selvin E, Juraschek SP, Eckfeldt J. et al. Calibration of cystatin C in the National Health and Nutrition Examination Surveys (NHANES). Am J Kidney Dis 2013; 61: 353–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seegmiller JC, Burns BE, Schinstock CA. et al. Discordance between iothalamate and iohexol urinary clearances. Am J Kidney Dis 2016; 67: 49–55 [DOI] [PubMed] [Google Scholar]
- 24. Stevens LA, Zhang Y, Schmid CH.. Evaluating the performance of equations for estimating glomerular filtration rate. J Nephrol 2008; 21: 797–807 [PMC free article] [PubMed] [Google Scholar]
- 25. Seegmiller JC, Sviridov D, Larson TS. et al. Comparison of urinary albumin quantification by immunoturbidimetry, competitive immunoassay, and protein-cleavage liquid chromatography-tandem mass spectrometry. Clin Chem 2009; 55: 1991–1994 [DOI] [PubMed] [Google Scholar]
- 26. Grubb A, Horio M, Hansson LO. et al. Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 2014; 60: 974–986 [DOI] [PubMed] [Google Scholar]
- 27. Earley A, Miskulin D, Lamb EJ. et al. Estimating equations for glomerular filtration rate in the era of creatinine standardization: a systematic review. Ann Intern Med 2012; 156: 785–795 [DOI] [PubMed] [Google Scholar]
- 28. Flamant M, Vidal-Petiot E, Metzger M. et al. Performance of GFR estimating equations in African Europeans: basis for a lower race-ethnicity factor than in African Americans. Am J Kidney Dis 2013; 62: 182–184 [DOI] [PubMed] [Google Scholar]
- 29. Pottel H, Hoste L, Dubourg L. et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 2016; 31: 798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poggio ED, Wang X, Greene T. et al. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 2005; 16: 459–466 [DOI] [PubMed] [Google Scholar]
- 31. Rule AD, Torres VE, Chapman AB. et al. Comparison of methods for determining renal function decline in early autosomal dominant polycystic kidney disease: the consortium of radiologic imaging studies of polycystic kidney disease cohort. J Am Soc Nephrol 2006; 17: 854–862 [DOI] [PubMed] [Google Scholar]
- 32. Rule AD, Larson TS, Bergstralh EJ. et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004; 141: 929–937 [DOI] [PubMed] [Google Scholar]
- 33. Rule AD, Rodeheffer RJ, Larson TS. et al. Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc 2006; 81: 1427–1434 [DOI] [PubMed] [Google Scholar]
- 34. Coresh J, Turin TC, Matsushita K. et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 2014; 311: 2518–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. US Renal Data Systems. 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2015 [Google Scholar]
- 36. Levey AS, Inker LA, Coresh J.. GFR estimation: from physiology to public health. Am J Kidney Dis 2014; 63:820–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.