Abstract
The following is a summary of the recommendations and good practice points for the BTS Guideline for the initial outpatient management of pulmonary embolism. Please refer to the full guideline for full information about each section.
Keywords: pulmonary embolism
Introduction
The full guideline for the initial outpatient management of pulmonary embolism (PE) is published in Thorax.1 The key features of the guideline are highlighted in a short article published to accompany the full guideline.2 The following is a summary of the recommendations and good practice points (GPPs). The sections referred to in the summary refer to the full guideline.
Background
The British Thoracic Society (BTS) Guideline for the initial outpatient management of PE provides guidance on how to risk-stratify patients with suspected and confirmed PE and subsequently manage them in an outpatient or ambulatory care setting.
Over the last 10 years there has been an increasing drive to manage many conditions traditionally treated during an inpatient admission as outpatients. This has become widespread practice in managing deep vein thrombosis, and data are increasing to support this strategy in PE. With the licensing of the direct oral anticoagulants (DOACs) for the treatment of acute PE which do not require a low-molecular-weight heparin (LMWH) run-in or International Normalised Ratio (INR) monitoring, outpatient management of suspected PE has become more straightforward. This is clearly an opportunity to improve patient experience and reduce hospital length of stay, but it also presents a risk if the wrong patients are identified for outpatient management.
Many acute hospital trusts have begun outpatient management of both suspected and confirmed PE, as evidenced by the large number of abstracts presented at conference proceedings, in addition to peer-reviewed publications. However, it is readily apparent from these reports that practice varies, and it is of concern that in some units no validated risk assessment is being undertaken. Given that PE can be fatal, this raises concerns over the safety of some local protocols. This safety concern is further highlighted by the fact that a study of outpatient management of PE was terminated early by the Drug and Safety Monitoring Board after two deaths in the outpatient arm (one right ventricular thrombus and one fatal bleed).3
The National Institute for Health and Care Excellence guidelines on the management of thromboembolic diseases published in 2012 and updated in 2015 provided no recommendations on how to risk-stratify for outpatient management.4 While both the European Society of Cardiology and the American College of Chest Physicians have also published guidance on the management of acute PE which both support outpatient management, neither provides detailed practical recommendations on risk stratification.5–7
Finally, the advent of the DOACs facilitates early discharge from hospital since patients no longer need to be kept in hospital until they have reached a therapeutic INR. This raises the question of how to identify moderate-risk patients appropriate for early discharge.
In conclusion, there is a need for a standardised approach to identify low-risk patients for outpatient management and also to aid clinicians in deciding when moderate-risk patients can be discharged early. The aim of this guideline is to standardise the approach to the initial outpatient management of PE (up to 3 weeks post diagnosis) and to reduce the risk to patients and hospital trusts, while ensuring best value to the National Health Service.
Target audience for the guideline
This guideline is aimed at clinicians, in particular physicians, clinical nurse specialists and advanced nurse practitioners, at all levels of seniority delivering emergency, acute, ambulatory and inpatient care who are involved in the management of acute PE. The main specialties referring to the guidance are likely to be acute, emergency, general (internal) and respiratory medicine. It is also designed to inform general practitioners involved in the delivery of ambulatory care or thrombosis pathways. This document may also be used by healthcare commissioners and hospital management to ensure appropriate staffing and resourcing of ambulatory care facilities to integrate PE management into ambulatory care pathways.
Scope of the guideline
The guideline covers
adults (≥16 years) with suspected and confirmed acute PE;
haemodynamically stable PE;
use of DOACs in relation to suspected PE and outpatient management;
risk stratification for identifying patients suitable for outpatient management or early discharge;
special subgroups of patients (pregnant patients, those with cancer and intravenous drug abusers).
The guideline does not cover
the diagnostic algorithm for PE;
evidence for cancer screening;
detailed comparisons of anticoagulant drugs;
who should be treated and not treated, specifically how to manage subsegmental PE;
duration of anticoagulation and thrombophilia investigations.
Methodology
This guideline is based on the best available evidence. The methodology used to write the guideline adheres strictly to the criteria as set by the AGREE II collaboration, which is available online at http://www.agreetrust.org/resource-centre/agree-ii/. The BTS Standards of Care Committee (SOCC) guideline production manual is available at https://www.brit-thoracic.org.uk/standards-of-care/guidelines/.
Clinical questions and literature search
Clinical questions were structured in the Population, Intervention, Control, Outcome format (see online supplementary web appendix 1) to define the scope of the guideline and inform the literature search.
Systematic electronic database searches were conducted in order to identify potentially relevant studies for inclusion in the guideline. For each topic area the following databases were searched: Ovid MEDLINE (including MEDLINE In Process), Ovid EMBASE and the Cochrane Library (including the Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effects) from 1980.
The searches were first run in July 2014 and updated in October 2015 (see online supplementary web appendix 1 for search strategy). Searches included a combination of indexed terms and free-text terms and were limited to English-language publications only. The initial search identified 2385 potential abstracts and the second search 432 other abstracts.
Appraisal of literature
Appraisal was performed to be compliant with the AGREE II instrument. Two individuals (LH, RP) read the title and abstract of each article retrieved by the literature searches and decided whether the paper was definitely relevant, possibly relevant or not relevant to the project. Criteria formulated for categorising the abstracts into these three groups were
whether the study addressed the clinical question;
whether the appropriate study type was used to produce the best evidence to answer the clinical question;
review articles were excluded;
abstract was in English.
Abstracts were not rejected on the basis of the journal of publication, the country in which the research was performed or published or the date of publication.
The screened abstracts were allocated to the relevant section(s) of the guideline and two group members allocated to each of those sections. The full paper was obtained for all relevant or possibly relevant abstracts.
The first screening process identified 153 of the initial 2385 reference abstracts to be definitely or possibly relevant to the guideline. Two guideline reviewers per section independently reviewed the abstracts to identify papers to be appraised for the guideline. The two reviewers for each section then independently appraised each paper assigned to them using the Scottish Intercollegiate Guidelines Network (SIGN) critical appraisal checklists. The reliability of the evidence in each individual study was graded using the SIGN critical appraisal check lists and is shown in the evidence tables (++, + or –). The body of evidence for each recommendation was summarised into evidence statements and graded using the SIGN grading system (see table 1).
Table 1.
Key to evidence statements
| Grade | Evidence |
| 1++ | High-quality meta-analyses, systematic reviews of RCTs or RCTs with a very low risk of bias |
| 1+ | Well-conducted meta-analyses, systematic reviews of RCTs or RCTs with a low risk of bias |
| 1− | Meta-analyses, systematic reviews of RCTs or RCTs with a high risk of bias |
| 2++ | High-quality systematic reviews of case–control or cohort studies or high-quality case–control or cohort studies with a very low risk of confounding, bias or chance and a high probability that the relationship is causal |
| 2+ | Well-conducted case–control or cohort studies with a low risk of confounding, bias or chance and a moderate probability that the relationship is causal |
| 2− | Case–control or cohort studies with a high risk of confounding, bias or chance and a significant risk that the relationship is not causal |
| 3 | Non-analytic studies, eg, case reports, case series |
| 4 | Expert opinion |
RCT, randomised controlled trial.
Disagreements were resolved by discussion with the section partner. The second literature search in October 2015 yielded 432 abstracts. Of these, 13 were identified as definitely or possibly relevant to the guideline. However, all of the pertinent ones from this search had been identified by the Guideline Development Group (GDG) in the meantime and already incorporated.
Considered judgement and grading of evidence
The GDG used the evidence tables to judge the body of evidence and grade recommendations for this guideline. The evidence tables are available in online supplementary web appendix 2. Where evidence was lacking to answer the formulated clinical questions, expert opinions were obtained through consensus. The following were considered in the grading of the recommendations:
the available volume of the body of evidence;
how applicable the obtained evidence was in making recommendations for the defined target audience of this guideline;
whether the evidence was generalisable to the target population for the guideline;
whether there was a clear consistency in the evidence obtained to support recommendations;
what the implications of recommendations would be on clinical practice in terms of resources and skilled expertise.
Cost-effectiveness was not reviewed in detail as in-depth economic analysis of recommendations falls beyond the scope of this guideline.
Recommendations were graded from A to D, as indicated by the strength of the evidence, as in table 2. In line with SIGN guidance, evidence appraised as ‘minus’ was considered in context but in the absence of other supporting evidence appraised as ‘plus’, it was discussed among the GDG regarding that point and any recommendation hence made was grade D. Important practical points currently lacking any research evidence and assessed as unlikely to have research evidence in the future were highlighted as GPPs.
Table 2.
Grades of recommendations
| Grade | Type of evidence |
| A | At least one meta-analysis, systematic review or RCT rated as 1++ and directly applicable to the target population or |
| A systematic review of RCTs or a body of evidence consisting principally of studies rated as 1+ directly applicable to the target population and demonstrating overall consistency of results. | |
| B | A body of evidence including studies rated as 2++ directly applicable to the target population and demonstrating overall consistency of results or |
| Extrapolated evidence from studies rated as 1++ or 1+. | |
| C | A body of evidence including studies rated as 2+ directly applicable to the target population and demonstrating overall consistency of results or |
| Extrapolated evidence from studies rated as 2++. | |
| D | Evidence level 3 or 4 or. |
| Extrapolated evidence from studies rated as 2+. | |
| ✔ | Important practical points for which there is no research evidence, nor is there likely to be any research evidence. The guideline committee wishes to emphasise these as good practice points. |
RCT, randomised controlled trial.
Drafting the guideline
The GDG corresponded regularly by email, and meetings of the full group were held in April and October 2014, March, May and October 2015, and January 2016 as well as a number of teleconferences. The BTS SOCC reviewed the draft guideline in March 2016. The draft guideline was made available online in January/February 2017 for public consultation and circulated to all the relevant stakeholders. The BTS SOCC re-reviewed the revised draft guideline in June 2017, and final SOCC approval was granted in December 2017.
This BTS Guideline will be reviewed within 5 years of publication.
Declarations of interest
All members of the Guideline Group made declarations of interest in line with BTS Policy, and further details can be obtained on request from BTS. GDG members are listed in Appendix 1 to the full guideline.
Representation and stakeholder organisations
Dr Vincent Connolly represented the Society for Acute Medicine; Dr Chris Davies represented the Royal College of Physicians, London; Dr Daniel Horner and Dr Laura Hunter represented the Royal College of Emergency Medicine; Ms Wendy Preston represented the Association of Respiratory Nurse Specialists; Dr Campbell Tait represented the British Committee for Standards in Haematology.
Summary of recommendations
Outcomes of outpatient care for low-risk PE
Recommendations
Patients with PE should be assessed for suitability for management as outpatients. Grade B
Patients assessed as low risk and suitable for outpatient management should be offered treatment in an outpatient setting where a robust pathway exists for follow-up and monitoring. Grade B
Research recommendation
Research is required to enhance the evidence base regarding patient experience and cost effectiveness.
Inclusion and exclusion criteria for outpatient management or early discharge
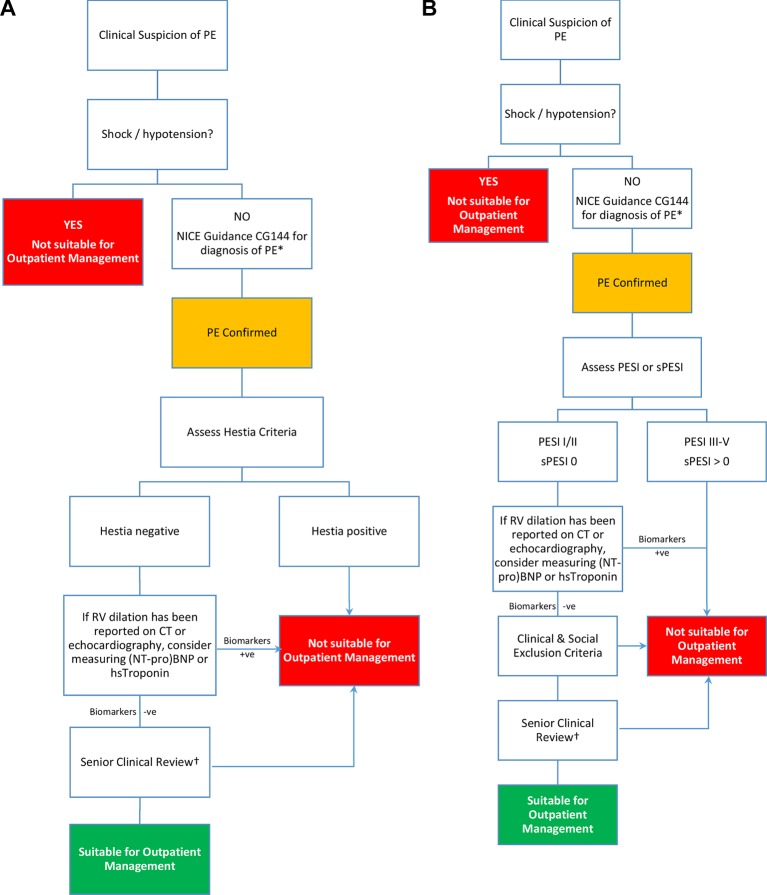
See figure 1a and 1b and tables 3, 4, 5, 6 and box 1.
Figure 1.
Algorithm for the outpatient management of pulmonary embolism (PE).*If imaging cannot be undertaken same day, then patients may be considered for empirical treatment with either low-molecular-weight heparin or apixaban or rivaroxaban and asked to return within 24 hours for definitive diagnosis, providing they fulfil the remainder of the criteria for outpatient management. †Patients with cancer or those who are pregnant or within 6 weeks post partum may be considered for outpatient management. BNP, B-type natriuretic peptide; NICE, National Institute for Health and Care Excellence; NT-proBNP, N-terminal B-type natriuretic peptide; PESI, Pulmonary Embolism Severity Index; sPESI, simplified PESI; RV, right ventricular.
Table 3.
Pulmonary Embolism Severity Index
| Parameter | Score | Risk class | Total points |
| Demographic features | I: very low II: low III: intermediate IV: high V: very high |
≤65 66–85 86–105 106–125 ≥126 |
|
| Age | Age in years | ||
| Male sex | +10 | ||
| Comorbid conditions | |||
| Cancer | +30 | ||
| Heart failure | +10 | ||
| Chronic lung disease | +10 | ||
| Clinical findings | |||
| Pulse≥110 bpm | +20 | ||
| SBP<100 mm Hg | +30 | ||
| RR≥30/min | +20 | ||
| Temp<36°C | +20 | ||
| Altered mental status* | +60 | ||
| Arterial blood oxygen saturation<90%† | +20 |
*Defined as disorientation, lethargy, stupor or coma.
†With or without the administration of supplemental oxygen.
RR, respiratory rate; SBP, systolic blood pressure.
Table 4.
Simplified Pulmonary Embolism Severity Index
| Parameter | Score | Risk class | Total points |
| Age>80 years | 1 | Low High |
0 ≥1 |
| Cancer* | 1 | ||
| Chronic cardiopulmonary disease | 1 | ||
| Pulse≥110 bpm | 1 | ||
| SBP<100 mm Hg | 1 | ||
| Arterial blood oxygen saturation<90%† | 1 |
*Defined as active cancer (diagnosed within last 12 months or undergoing treatment, personal communication from Prof David Jimenez).
†With or without the administration of supplemental oxygen.
SBP, systolic blood pressure.
Table 5.
Geneva score
| Parameter | Score | Risk class | Total points |
| Cancer | 2 | Low | ≤2 |
| Heart failure | 1 | High | >2 |
| Previous DVT | 1 | ||
| SBP<100 mm Hg | 2 | ||
| PaO2<8 kPa (60 mm Hg) | 1 | ||
| DVT confirmed on USS | 1 |
DVT, deep vein thrombosis; SBP, systolic blood pressure; USS, ultrasound.
Table 6.
Clinical exclusion criteria for outpatient pulmonary embolism (PE) management
| Hestia criteria (Zondag et al)8 | Davies et al 9 | ||
| Is the patient haemodynamically unstable?* | Yes/no | Need for hospitalisation for another medical reason | Yes/no |
| Is thrombolysis or embolectomy necessary? | Yes/no | Additional monitoring required (ECG, oxygen, parenteral analgesia) | Yes/no |
| Active bleeding or high risk of bleeding?† | Yes/no | Likelihood of poor compliance/difficulty with follow-up | Yes/no |
| More than 24 hours of oxygen supply to maintain oxygen saturation>90%? | Yes/no | Previous PE/early recurrence of PE | Yes/no |
| Is PE diagnosed during anticoagulant treatment? | Yes/no | Coexisting major DVT | Yes/no |
| Severe pain needing intravenous pain medication for>24 hours? | Yes/no | Bleeding disorders, active bleeding | Yes/no |
| Medical or social reason for treatment in hospital>24 hours? | Yes/no | Pregnancy | Yes/no |
| Does the patient have a creatinine clearance<30 mL/min? | Yes/no | Patient preference for hospital stay | Yes/no |
| Does the patient have severe liver impairment? (discretion of clinician) | Yes/no | ||
| Is the patient pregnant? | Yes/no | ||
| Does the patient have a documented history of heparin-induced thrombocytopenia? | Yes/no | ||
| Eligible for outpatient treatment: no risk factors Ineligible for outpatient treatment: at least one risk factor present | |||
*SBP <100 mm Hg with HR >100 bpm; condition requiring admission to intensive care unit.
†Gastrointestinal bleeding in the preceding 14 days, recent stroke (<4 weeks ago), recent operation (<2 weeks ago), bleeding disorder, thrombocytopenia (platelet count <75×109/L), uncontrolled hypertension (SBP >180 mm Hg or DBP >110 mm Hg).
DVT, deep vein thrombosis; SBP, systolic blood pressure.
Box 1. Clinical exclusion criteria (Aujesky et al (2011))10 .
Exclusion criteria
Oxygen saturation<90%
Systolic blood pressure <100 mm Hg
Chest pain needing opiates
Active bleeding
High risk of bleeding (stroke within the preceding 10 days, gastrointestinal bleed within the last 14 days or platelet count<75 000/mm3)
Obesity (weight>150 kg)
Heparin-induced thrombocytopenia
Severe renal failure (creatinine clearance<30 mL/min)
Therapeutic anticoagulation (International Normalised Ratio ≥2.0)
Barriers to treatment adherence or follow-up
Recommendations
Patients with confirmed PE should be risk-stratified using a validated clinical risk score. Grade B
Patients in Pulmonary Embolism Severity Index (PESI) Class I/II, simplified PESI (sPESI) 0 or meeting the Hestia criteria should be considered for outpatient management of PE. Grade B.
- Where PESI or sPESI is used and indicates a low risk, a set of exclusion criteria should be applied to patients being considered for outpatient management of confirmed PE. Grade B
- Exclusion criteria include
- Haemodynamic instability (HR >110; systolic blood pressure <100 mm Hg; requirement for inotropes and critical care; requirement for thrombolysis or embolectomy).
- Oxygen saturations <90% on air.
- Active bleeding or risk of major bleeding (eg, recent gastrointestinal bleed or surgery, previous intracranial bleeding, uncontrolled hypertension).
- On full-dose anticoagulation at the time of the PE.
- Severe pain (eg, requiring opiates).
- Other medical comorbidities requiring hospital admission.
- Chronic kidney disease stages 4 or 5 (estimated glomerular filtration rate <30 mL/min) or severe liver disease.
- Heparin-induced thrombocytopenia within the last year and where there is no alternative to repeating heparin treatment.
- Social reasons which may include inability to return home, inadequate care at home, lack of telephone communication, concerns over compliance, etc.
No specific assessment of bleeding risk is required in patients who are deemed low risk by PESI/sPESI/ Hestia criteria. Grade B
Measurement of right ventricular:left ventricular ratio on CT or assessment of right ventricular function on echocardiography is not obligatory for the identification of low-risk patients for outpatient management. Grade C
Where right ventricular dilatation has been identified on CT scanning or echocardiography in patients who are suitable for outpatient management, consider measuring laboratory cardiac biomarkers (B-type natriuretic peptide (BNP), N-terminal B-type natriuretic peptide, high-sensitivity troponin I or high-sensitivity troponin T). Normal values may be used to identify low-risk patients; elevated biomarkers in this context should prompt inpatient admission for observation. Grade C
Good practice point
In the context of low-risk PE and an incidental finding of elevated troponin, senior review is required and alternative causes for troponin release should be considered.
Management of patients with suspected PE, where a diagnosis has yet to be confirmed
Recommendation
Patients with suspected PE should, where reasonably practical, undergo investigation on the same day of presentation to exclude a diagnosis of PE. An alternative strategy of anticoagulation followed by outpatient imaging within 24 hours may be considered in patients with suspected PE, who have been deemed low risk and eligible for outpatient care as per confirmed PE. Robust systems should be in place to facilitate next day investigation and review. Grade D
Treatment of suspected/confirmed PE in the outpatient setting
(See tables 7 and 8).
Table 7.
Key randomised trials of direct oral anticoagulants in the treatment of acute pulmonary embolism (PE)
| Study patients (n) | Treatment arm (vs heparin/warfarin) | Efficacy | Safety |
| (study drug vs warfarin) | |||
| RE-COVER (2009) n=2564 |
LMWH≥5 days followed by dabigatran 150 mg twice daily | Recurrent VTE or fatal PE: 2.4% vs 2.1% | Major bleeding: 1.6% vs 1.9% |
| RE-COVER II (2014) n=2589 |
LMWH≥5 days followed by dabigatran 150 mg twice daily | Recurrent VTE or fatal PE: 2.3% vs 2.2% | Major bleeding: 15 patients vs 22 patients |
| EINSTEIN PE (2012)* n=4833 |
Rivaroxaban 15 mg twice daily for 3 weeks followed by 20 mg once daily | Recurrent VTE or fatal PE: 2.1% vs 1.8% | Major or CRNM bleeding: 10.3% vs 11.4% |
| AMPLIFY study (2013) n=5400 |
Apixaban 10 mg twice daily for 7 days followed by 5 mg twice daily | Recurrent VTE or fatal PE: 2.3% vs 2.7% | Major bleeding: 0.6% vs 1.8% |
| HOKUSAI-VTE (2013) n=8292 |
LMWH≥5 days followed by edoxaban 60 mg once daily (30 mg once daily if creatinine clearance 30–50 mL/min or body weight<60 kg) | Recurrent VTE or fatal PE: 3.2% vs 3.5% | Major or CRNM bleeding: 8.5% vs 10.3% |
*Only EINSTEIN PE included exclusively patients with PE.
CRNM, clinically relevant non-major; LMWH, low-molecular-weight heparin.
Table 8.
Relative risk of major and non-major bleeding using direct oral anticoagulants compared with Vitamin K antagonist
| Study | Drug | Risk ratio of major and clinically relevant non-major bleeding events |
| RE-COVER (2009) | Dabigatran | 0.64 (0.48–0.85) |
| RE-COVER II (2014) | Dabigatran | 0.63 (0.47–0.86) |
| EINSTEIN-DVT (2010) | Rivaroxaban | 1.00 (0.80–1.25) |
| EINSTEIN PE (2012) | Rivaroxaban | 0.91 (0.77–1.07) |
| AMPLIFY study (2013) | Apixaban | 0.44 (0.36–0.55) |
| HOKUSAI-VTE (2013) | Edoxaban | 0.83 (0.72–0.95) |
DVT, deep vein thrombosis; VTE, venous thromboembolism.
Recommendations
Patients with confirmed PE being treated in the outpatient setting should be offered treatment with either LMWH and dabigatran, LMWH and edoxaban or a single-drug regimen (apixaban or rivaroxaban). Grade A
Patients with suspected PE being treated in the outpatient setting may be treated with apixaban or rivaroxaban pending diagnosis as an alternative to LMWH. Grade D
Good practice point
Using a single DOAC in a pathway is preferred to minimise potential confusion over dosing and administration.
Assessing patients transitioning from inpatient care to early discharge/outpatient care?
Recommendation
Patients who have been admitted with an intermediate risk PE (PESI Class III) can be considered for early discharge when they meet the criteria for low risk (PESI class I/II or sPESI score 0). Grade C
Good practice points
Those with PESI-48 class III or sPESI-48 score of >0 are considered to be at higher risk of adverse outcome and senior review is necessary prior to discharge; PESI and sPESI may remain elevated due to non-reversible factors (eg, cancer, age) which should be taken into consideration when using clinical judgement.
Consideration should be given to repeating assessment of right ventricular function with echocardiography or biomarkers in those admitted with right ventricular dysfunction or biomarker elevation at baseline.
Level of seniority of review
Good practice points
Patients with confirmed or suspected PE should be reviewed by a consultant prior to discharge on an outpatient PE pathway. If no consultant is available, then patients may be reviewed by a senior trainee (ST3 or above; ST4 in the case of emergency medicine) by a staff grade or similar substantive career grade doctor, advanced nurse practitioner or clinical nurse specialist designated to undertake this role within the department with consultant advice available.
If patients are on an outpatient pathway for suspected PE and being considered for discharge and scanning the following day, a local protocol should be in place to guide a full cardiorespiratory assessment to exclude other causes for symptoms (including full history, examination, ECG and chest radiograph), including risk assessment.
Follow-up of patients specific to those managed in the outpatient setting
Recommendations
Patients with confirmed PE who are eligible for outpatient care should be provided with verbal and written information on the signs and symptoms of recurrence, major bleeding and additional complications. Individual centres should also provide an appropriate point of contact in the event of complications or concerns, both in and out of hours. Grade B
Patients should have a formal review (telephone/face to face) at least once during the first week after discharge to ensure therapeutic compliance along with the absence of complications. Grade B
Hospitals should have local protocols and pathways in place for follow-up of all PE patients, whether treated as an inpatient or outpatient. This should include assessment of ongoing symptoms (with further directed investigation as appropriate) and consideration of optimal duration/modality of anticoagulation. Grade D
Good practice points
Consider initial assessment of provoking risk factors for the index PE at an early stage, for example, immobility, surgery, cancer, intercurrent illness, etc, since this will determine duration of anticoagulation. Screening policies for malignancy are out of scope for this guideline, but when screening investigations are performed, a mechanism should be in place to review results within a prompt time frame.
Follow-up of PE should be performed by clinicians with a special interest in venous thromboembolism.
Research recommendation
Further studies evaluating the role of technology for remote monitoring, such as virtual consultations and data gathering, are needed.
Management of PE in the outpatient setting in specific circumstances
Pregnancy/puerperium
Good practice points
All pregnant and postpartum women presenting with suspected PE or confirmed PE should be reviewed by a consultant and discussed with maternity services prior to discharge.
Outpatient care pathways may be considered for suspected or confirmed PE in pregnancy and/or the postpartum period.
Clinical risk scores derived for non-pregnant patients, such as PESI/sPESI, should not be used in pregnant women.
DOACs or Vitamin K antagonists should not be used in pregnant patients with suspected or proven PE.
Research recommendation
Studies addressing the safety and efficacy of outpatient care pathways for pregnant and postpartum patients with suspected or confirmed PE are needed.
Cancer
Recommendation
The Hestia criteria may be used to evaluate patients with active cancer for suitability for outpatient management of PE. Grade D
Good practice points
Patients with active cancer should be reviewed by a consultant prior to discharge given the higher risk of 30-day mortality. Good practice point
Patients with incidental PE should be managed in the same way with respect to outpatient management as those with symptomatic PE.
Research recommendation
Further studies are needed to validate risk stratification tools specific to patients with cancer.
Intravenous drug use
Good practice point
Intravenous drug abusers with suspected PE should be admitted for further investigation and management.
Patient information and support needs
Recommendation
Written patient information and education should be integral to outpatient PE pathways. Grade D
Good practice point
Succinct written information should be provided to the patient on discharge, using non-technical language and including telephone numbers/email addresses for advice on dealing with any subsequent changes in the patient’s condition. An example, from Sheffield Teaching Hospitals, is included in online appendix 3. The information material produced by the thrombosis charity Thrombosis UK (http://www.thrombosisuk.org) may also prove helpful.
Healthcare providers need to use clinical judgement, knowledge and expertise when deciding whether it is appropriate to apply recommendations for the management of patients. The recommendations presented here are a guide and may not be appropriate for use in all situations. The guidance provided does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of each patient, in consultation with the patient and/or their guardian or carer.
Acknowledgments
The British Thoracic Society and the Guideline Group members are particularly grateful to Dr Rodney Hughes and Dr Matthew Fay who contributed to the early work of the guideline group; the BTS Standards of Care Committee.
Footnotes
Contributors: LSH chaired the guideline group, and as lead author led the drafting and revision of the document. All authors drafted sections of the guideline and undertook revisions of the paper. LSH had final responsibility for the guideline.
Competing interests: RC received funding from BTG, Actelion and MSD; BE received funding from Astra Zeneca and Diageo; DH received finding from Creavotech; LSH received funding from GSK, Endotronix, Actelion, Bayer, MSD, BTG and BMS; RP received funding from Bayer and Daiichi Sankyo; KS received funding from Actelion; CT received funding from Bayer, Daiichi Sankyo and Novo Nordisk; CNP received funding from UCB and Alliance; WP received funding from ARNS, AZ and BI.
Provenance and peer review: Commissioned; internally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Howard L S, et al. . BTS Guideline for Initial outpatient management of pulmonary embolism. Thorax 2018. 73 Suppl 2. [DOI] [PubMed] [Google Scholar]
- 2. Howard L S. BTS Guidelines for the initial outpatient management of pulmonary embolism: there’s no place like home. Thorax 2018. 73. [DOI] [PubMed] [Google Scholar]
- 3. Otero R, Uresandi F, Jiménez D, et al. . Home treatment in pulmonary embolism. Thromb Res 2010;126:e1–e5. doi:10.1016/j.thromres.2009.09.026 [DOI] [PubMed] [Google Scholar]
- 4. NICE. Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. 2012. CG144 https://www.nice.org.uk/guidance/cg144. [PubMed] [Google Scholar]
- 5. Kearon C, Akl EA, Comerota AJ, et al. . Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. 141 9th ed: Chest, 2012:e419S–96. doi:10.1378/chest.11-2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kearon C, Akl EA, Ornelas J, et al. . Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315–52. doi:10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 7. Konstantinides SV, Torbicki A, Agnelli G, et al. . ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014. 35:3033-69. [DOI] [PubMed] [Google Scholar]
- 8. Zondag W, Mos IC, Creemers-Schild D, et al. . Hestia Study Investigators. Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost 2011;9:1500–7. doi:10.1111/j.1538-7836.2011.04388.x [DOI] [PubMed] [Google Scholar]
- 9. Davies CW, Wimperis J, Green ES, et al. . Early discharge of patients with pulmonary embolism: a two-phase observational study. Eur Respir J 2007;30:708–14. doi:10.1183/09031936.00140506 [DOI] [PubMed] [Google Scholar]
- 10. Aujesky D, Roy PM, Verschuren F, et al. . Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 2011;378:41–8. doi:10.1016/S0140-6736(11)60824-6 [DOI] [PubMed] [Google Scholar]