Abstract
Evidence shows that asthma originates in early life. Studies have found that phototherapy and/or neonatal jaundice may be associated with asthma. We investigated the association between neonatal bilirubin levels and childhood asthma without phototherapy intervention in the Collaborative Perinatal Project, a multicenter prospective cohort study conducted in the United States from 1959 to 1965. A total of 54,795 livebirths were included, and 40,063 children were followed up until 7 years of age or older. Total serum bilirubin (TSB) levels were examined at 48 hours postpartum in newborns with birthweights of 2,250 g or more. Information on asthma and other diseases through age 7 years was summarized and confirmed by a group of pediatricians and child neurologists. Among 28,807 term infants, the overall prevalence of asthma was 5.26%. Risks of asthma increased with both maximum TSB levels and TSB levels at 48 hours postpartum (P for trend < 0.01). Neonatal maximum TSB levels greater than 15 mg/dL were associated with a 61% increase in the risk of childhood asthma (odds ratio = 1.61, 95% confidence interval: 1.04, 2.08) after adjustment for confounders. In this prospective cohort study of infants born at a time when phototherapy was unavailable, neonatal hyperbilirubinemia was associated with an increased risk of childhood asthma.
Keywords: asthma, bilirubin, immaturity, perinatal exposure, phototherapy
Editor's note: An invited commentary on this article appears on page 1698.
Asthma is one of the most common chronic diseases, affecting nearly 25 million Americans. Children aged 0–17 years have a higher prevalence of asthma (9.5%) than do adults aged 18 years and older (7.7%) (1). There is widespread concern that the incidence of asthma is still rising in developed countries (2). However, the causes of asthma are not fully understood. More and more evidence shows that asthma has its origins in early life or even in utero (3, 4). Several perinatal factors have been consistently suggested to be associated with asthma, such as maternal smoking, young maternal age, preterm birth, and low birthweight (5).
More than half (50%–60%) of neonates develop visible jaundice due to elevated bilirubin concentration in the first week of life (6). Although most cases of neonatal jaundice are physiological, the long-term effects of neonatal bilirubin exposure on children and even adults remain controversial (7). Several studies found that phototherapy and/or neonatal jaundice was associated with asthma. The odds ratios ranged from 1.30 (95% confidence interval (CI): 1.16, 1.47) to 1.64 (95% CI: 1.36, 1.98) after adjustment for confounders (8, 9). In these studies, neonatal jaundice was defined in children who underwent phototherapy according to data in the national register system but for whom information on bilirubin levels was unavailable. Because phototherapy is a primary treatment for neonatal jaundice, it may be difficult to separate phototherapy from neonatal hyperbilirubinemia in current clinical practice. Thus, it remains unclear whether neonatal jaundice, phototherapy, or both are associated with an increased risk of asthma. We examined the association between neonatal bilirubin levels and childhood asthma without the complication of phototherapy in the Collaborative Perinatal Project (CPP), a large prospective study of births occurring in the United States in 1959–1965, when phototherapy had yet to be applied clinically (10).
MATERIALS AND METHODS
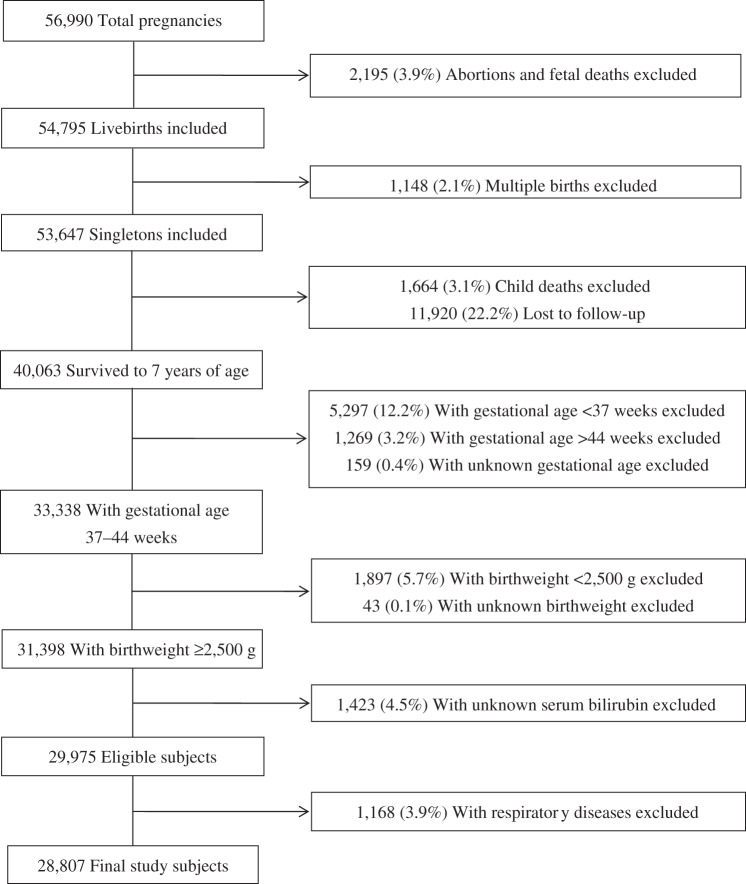
Study population
The CPP was a prospective study that recruited 46,021 women with 56,990 pregnancies at 12 centers in the United States from 1959 to 1965. During the 7-year study period, 18% of participants had more than 1 birth. Children were followed with detailed assessments of neurological, neurosensory, and cognitive development, as well as general health and physical growth at 8 months and 1, 3, 4, and 7 years of age. A detailed description of the study has been provided elsewhere (11). We included 54,795 livebirths. Among 53,647 singletons, 74.7% (n = 40,063) were followed until 7 years of age (Figure 1). Preterm (gestational age <37 weeks) and low-birthweight infants (birthweight <2,500 g) were excluded, as were children with unknown bilirubin levels (n = 1,423). We further excluded infants who had experienced neonatal respiratory diseases, because these children may have higher bilirubin levels and a potentially a higher risk of asthma (12). Finally, 28,807 term infants remained for analysis, all of whom had information on asthma.
Figure 1.
Study population flow chart, Collaborative Perinatal Project, United States, 1959–1965.
Bilirubin measurements
Bilirubin was measured by the diazo method in 11 of the 12 centers and by direct spectrophotometry in the other center. The coefficient of variation of standard specimens among CPP laboratories was approximately 10%, and the intralaboratory coefficient of variation was approximately 2% (13). For all infants with birthweights of 2,250 g or more, the study protocol required measuring total serum bilirubin (TSB) levels at 36–60 hours postpartum (as close to 48 hours postpartum as possible). If a TSB level was higher than 10 mg/dL, a second test was obtained 24 hours later. If the second TSB level exceeded 10 mg/dL, a third test was required at 4–5 days of age. Additional bilirubin measurements were obtained at the discretion of physicians at each study site. We used the 48-hour TSB and maximum TSB data in this analysis.
Diagnosis of asthma
Detailed information on child health was collected, and various physical examinations were performed in each visit at 8 months and 1, 3, 4, and 7 years of age (14). All recorded health and disease information for each child through the age of 7 years was reviewed, summarized, and confirmed by a group of pediatricians and pediatric neurologists. Asthma diagnosis and related information in the current analysis was extracted mainly from the summarized data file. The diagnosis of asthma was recoded as “suspected,” “definite,” or “history only” according to the medical history at 1 year and 7 or 8 years of age. We used “definite” asthma as an event in this study. This classification scheme was consistent with a previous study of asthma that used the same data (15). A total of 169 subjects who were diagnosed with asthma at 1 year of age and without asthma by 7 years of age were recoded as “nonasthma.”
Confounders
Prenatal factors that may affect the relationship between neonatal bilirubin level and asthma were chosen as potential confounders according to previous literature (16). Maternal characteristics included maternal age at delivery (<20, 20–29, 30–39, or ≥40 years), marital status at pregnancy (no/yes); race (white, black, or other); educational level (<9, 10–12, or >12 years); number of previous deliveries (0, 1, or ≥2); smoking during pregnancy (0, 1–9, or ≥10 cigarettes per day), and maternal allergic diseases (no/yes). Child characteristics included sex, Apgar score at 5 minutes (0–6 or 7–10), mode of delivery (vaginal, assisted vaginal, or cesarean), birthweight (2,500–2,999, 3,000–3,499, 3,500–3,999, or ≥4,000 g), and breastfeeding (no/yes). Data on gestational age were based on the last menstrual period.
Statistical analysis
We first examined the differences in maternal and child baseline characteristics between children with and without asthma by using the χ2 test. We plotted separately the prevalence rates of asthma by the level of TSB at 48 hours postpartum and by maximum TSB level. Smoothed curves using the locally weighted scatterplot smoothing method were added. We conducted a trend analysis to evaluate the exposure-response relationship between bilirubin level and prevalence of childhood asthma with adjustment for confounders (17). The distribution of maximum TSB levels was left skewed with dual modality (Figure 2) and could not be transformed to a normal distribution. We therefore categorized the TSB data in tertiles with cutoff points of 3 mg/dL (cumulative percent: 32.2%) and 6 mg/dL (cumulative percent: 64.9%). Because TSB levels lower than 9 mg/dL at 48 hours postpartum would put a child in the low-risk zone with no need for phototherapy, and because phototherapy would not begin until TSB levels reached 15 mg/dL (18), the levels of bilirubin were further categorized into the following 5 groups: ≤3, 3.1–6, 6.1–9, 9.1–15, and >15 mg/dL. Children from the same family share similar genetic and home environments; thus, we used the generalized estimating equation model to correct for intracluster correlation with the GENMOD procedure in SAS, version 9.2, software (SAS Institute, Inc., Cary, North Carolina) with family number as the repeated subject. We started the model with the association of asthma and bilirubin level, and each potential confounder was then put into the model, 1 at a time. A variable was considered a confounder if it changed the β coefficient of the main effect by more than 10%. Eventually, maternal race, sex, gestational age, and study center were included in the analysis.
Figure 2.

Prevalence distribution of neonates by maximum total serum bilirubin levels, Collaborative Perinatal Project, United States, 1959–1965.
RESULTS
Maternal and child characteristics of this study population are presented by asthma status in Table 1. There were no significant differences between asthma and nonasthma groups in most of the characteristics considered. Both asthma and nonasthma groups were predominantly vaginal deliveries to married, nonsmoking mothers. The asthma group had a higher proportion of allergic mothers, male infants, and black race (P < 0.05).
Table 1.
Maternal and Child Characteristics by Asthma Status in the Collaborative Perinatal Project, United States, 1959–1965
| Characteristic | Nonasthma (n = 27,293) |
Asthma (n = 1,514) |
P Valuea | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Maternal race | <0.01 | ||||
| White | 14,018 | 51.4 | 645 | 42.6 | |
| Black | 12,323 | 45.2 | 785 | 51.9 | |
| Other | 952 | 3.5 | 84 | 5.6 | |
| Maternal age, years | 0.33 | ||||
| <20 | 5,998 | 22.0 | 351 | 23.2 | |
| 20–29 | 15,767 | 57.8 | 838 | 55.4 | |
| 30–39 | 5,046 | 18.5 | 297 | 19.6 | |
| ≥40 | 482 | 1.8 | 28 | 1.9 | |
| Married at pregnancy | 21,485 | 78.7 | 1,151 | 76.0 | 0.04 |
| Maternal education | 0.34 | ||||
| Less than high school | 7,455 | 27.3 | 439 | 29.0 | |
| High school | 16,344 | 59.9 | 890 | 58.8 | |
| College and above | 3,494 | 12.8 | 185 | 12.2 | |
| Smoking during pregnancy | 0.27 | ||||
| Nonsmoker (0 cigarettes/day) | 14,824 | 54.3 | 810 | 53.5 | |
| Light smoker (1–9 cigarettes/day) | 7,878 | 28.9 | 456 | 30.1 | |
| Heavy smoker (≥10 cigarettes/day) | 4,425 | 16.2 | 244 | 16.1 | |
| Maternal allergic diseases | 3,039 | 11.1 | 203 | 13.4 | <0.01 |
| Parity | 0.09 | ||||
| 0 | 7,921 | 29.0 | 469 | 31.0 | |
| 1 | 6,118 | 22.4 | 355 | 23.5 | |
| ≥2 | 13,199 | 48.4 | 685 | 45.2 | |
| Mode of delivery | 0.58 | ||||
| Vaginal | 25,363 | 92.9 | 1,407 | 92.9 | |
| Assisted vaginal | 548 | 2.0 | 35 | 2.3 | |
| Cesarean | 1,361 | 5.0 | 72 | 4.8 | |
| Birthweight, g | 0.44 | ||||
| 2,500–2,999 | 6,945 | 25.5 | 389 | 25.7 | |
| 3,000–3,499 | 12,221 | 44.8 | 701 | 46.3 | |
| 3,500–3,999 | 6,469 | 23.7 | 332 | 21.9 | |
| ≥4,000 | 1,658 | 6.1 | 92 | 6.1 | |
| Gestational age, weeks | 0.13 | ||||
| 37 | 1,646 | 6.0 | 104 | 6.9 | |
| 38 | 3,040 | 11.1 | 194 | 12.8 | |
| 39 | 5,400 | 19.8 | 314 | 20.7 | |
| 40 | 6,860 | 25.1 | 338 | 22.3 | |
| 41 | 5,421 | 19.9 | 305 | 20.3 | |
| 42 | 2,922 | 10.7 | 151 | 10.0 | |
| 43 | 1,306 | 4.8 | 71 | 4.7 | |
| 44 | 698 | 2.6 | 37 | 2.4 | |
| Male sex | 13,785 | 50.5 | 975 | 64.4 | <0.01 |
| Apgar score of 0–6 at 5 minutes | 851 | 3.1 | 47 | 3.1 | 0.80 |
| Breastfeeding | 4,766 | 17.5 | 243 | 16.1 | 0.25 |
| Indirect Coombs’ test positiveb | 713 | 2.6 | 44 | 2.9 | 0.78 |
| Exchange transfusionc | 251 | 0.9 | 15 | 1.0 | 0.77 |
a P values were determined by χ2 test.
b Indirect Coombs’ test positive detects antibodies against red blood cells that are present unbound in the patient's serum.
c Medical treatment in which apheresis is used to remove patient's red blood cells or platelets and replace them with transfused blood products.
There were 1,514 asthmatic children among the 28,807 subjects, with an overall prevalence of 5.26%. A total of 88.6% of neonates had maximum bilirubin levels lower than 9 mg/dL, and 97.6% of neonates had levels lower than 15 mg/dL (Table 2). Figure 3 shows that the prevalence of asthma increased significantly with both maximum TSB level and TSB level at 48 hours postpartum after adjustment for confounders (P < 0.01). Children with TSB levels higher than 15 mg/dL had the highest prevalence of asthma (8.01% for TSB at 48 hours postpartum and 7.99% for maximum TSB). The prevalence of asthma was associated with increased maximum TSB level, and the odds ratios in the groups of ≤3, 3.1–6, 6.1–9, 9.1–15, and >15 mg/dL were 1.00, 1.08, 1.22, 1.19, and 1.61, respectively, after adjustment for maternal race, sex, gestational age (by week), and study center (Supplementary Data and Supplementary Data).
Table 2.
Prevalence of Asthma by TSB Level at 48 Hours Postpartum and Maximum TSB Level and Odds Ratios in the Collaborative Perinatal Project, United States, 1959–1965
| TSB, mg/dL | No. (Total n = 28,807) | No. of Asthma Cases (Total n = 1,514) | Prevalence of Asthma, %a | Crude Analysis |
Adjusted Analysisb |
Trend Analysisc |
|||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Adjusted OR | 95% CI | P Value | Adjusted P Value | ||||
| 48-Hour level | 0.0045 | 0.0093 | |||||||
| ≤3 | 5,945 | 286 | 4.81 | 1.00 | Referent | 1.00 | Referent | ||
| 3.1–6 | 9,754 | 490 | 5.02 | 1.05 | 0.85, 1.38 | 1.07 | 0.92, 1.26 | ||
| 6.1–9 | 8,747 | 492 | 5.62 | 1.19 | 1.05, 1.36 | 1.23 | 1.05, 1.44 | ||
| 9.1–15 | 4,089 | 224 | 5.48 | 1.15 | 1.03, 1.28 | 1.17 | 1.02, 1.33 | ||
| >15 | 272 | 22 | 8.09 | 1.68 | 1.11, 2.25 | 1.59 | 0.89, 2.30 | ||
| Maximum level | 0.0022 | 0.0078 | |||||||
| ≤3 | 5,792 | 269 | 4.64 | 1.00 | Referent | 1.00 | Referent | ||
| 3.1–6 | 9,572 | 477 | 4.98 | 1.08 | 0.91, 1.25 | 1.08 | 0.93, 1.27 | ||
| 6.1–9 | 8,443 | 475 | 5.63 | 1.20 | 1.15, 1.82 | 1.22 | 1.04, 1.42 | ||
| 9.1–15 | 4,099 | 221 | 5.39 | 1.17 | 0.96, 1.42 | 1.19 | 0.91, 1.46 | ||
| >15 | 901 | 72 | 7.99 | 1.70 | 1.07, 2.45 | 1.61 | 1.04, 2.08 | ||
Abbreviations: CI: confidence interval; OR, odds ratio; TSB, total serum bilirubin.
a Overall prevalence of asthma = 5.26%.
b Generalized estimating equation model adjusted for maternal race, sex, gestational age, and study center with family number as repeated subject.
c Trend analysis adjusted for maternal race, sex, gestational age, and study center.
Figure 3.
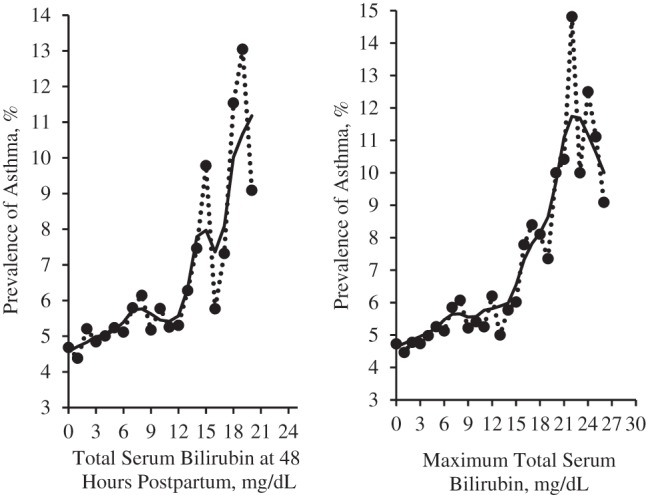
Prevalence of asthma by total serum bilirubin level, Collaborative Perinatal Project, United States, 1959–1965. Total serum bilirubin level at 48 hours postpartum (left panel) and maximum total serum bilirubin (right panel).
DISCUSSION
Neonatal hyperbilirubinemia and asthma are frequently encountered in children. In the CPP, we found that, without the complication of phototherapy, the risks of asthma increased with both maximum TSB level and TSB level at 48 hours postpartum (P for trend < 0.01). Neonatal maximum TSB levels greater than 15 mg/dL were associated with a 61% increase in the risk of childhood asthma (odds ratio = 1.61, 95% CI: 1.04, 2.08) after adjustment for confounding variables.
To the best of our knowledge, there are only 3 articles published on this topic (8, 9, 19). Bu using data from the Swedish Hospital Discharge Register, Aspberg et al. (19) first reported the association between neonatal jaundice (defined as neonates who experienced phototherapy) and hospitalized childhood asthma (odds ratio = 1.27, 95% CI: 1.08, 1.50). In a subsequent analysis based on data in the Swedish Prescribed Drug Register (8), the authors confirmed that phototherapy/neonatal jaundice was associated with the use of antiasthma drugs before 12 years of age (odds ratio = 1.30, 95% CI: 1.16, 1.47). In a recent retrospective study, Ku et al. (9) showed a similar relationship between neonatal jaundice and asthma (odds ratio = 1.64, 95% CI: 1.36, 1.98).
All subjects in these studies were exposed to both neonatal jaundice and phototherapy. In addition, the diagnosis of neonatal jaundice was obtained from data in hospital discharge systems without specific values of bilirubin or timing of the measurements, which may have resulted in a certain degree of misclassification. Nonetheless, the magnitude of the relationship between neonatal jaundice and asthma in previous studies was surprisingly similar to our finding, suggesting that this association is less likely to be spurious. Furthermore, judging by the magnitude of the relationship between our results and previous studies, the interaction between neonatal icterus and phototherapy is less likely. Given that phototherapy was not used in our subjects, our study indicates that jaundice, not phototherapy, is associated with asthma. However, the underlying biological mechanisms remain unknown.
Physiological neonatal hyperbilirubinemia, especially in preterm infants, is caused mainly by increased bilirubin production (due to both relative polycythemia and shorter red blood cell lifespan), decreased clearance (due to decreased hepatic uptake and conjugation), and increased enterohepatic circulation (20). It has been found that neonates born at 37 weeks were 4 times more likely than those born at 40 weeks to have a bilirubin level greater than 13 mg/dL (21). This immaturity at birth is also associated with decreased childhood lung function (22). Moreover, poor enteral intake and delayed stooling in immature infants may alter intestinal flora, which may also be related to both hyperbilirubinemia and asthma (23). Thus, it is critical to control for gestational age in examining the association between bilirubin level and asthma.
Bilirubin levels vary even in neonates of the same gestational age. Genetic polymorphisms of certain enzymes, such as uridine diphosphate glucuronyltransferase and glutathione S-transferase (GST), have been suggested to be associated with hyperbilirubinemia (24, 25). For example, GSTs, including GSTM1, GSTT1, and GSTP1, participate in binding to nonsubstrate ligands such as bilirubin. Neonates with the GSTM1 null genotype are at high risk of developing hyperbilirubinemia (26). Genetic variations of GSTs were also associated with the ability to handle oxidative stress, which was considered an important pathological process in asthma. It has been found that histamine and immunoglobulin E responses to air pollution oxidants and allergens were increased in GSTM1 null genotypes (27, 28). More efforts are necessary to examine genetic predisposition in the association between hyperbilirubinemia and asthma in future studies.
Bilirubin itself may affect the development of the immune system, as well. One study showed that intracellular accumulation of unconjugated bilirubin inhibits the production of interleukin-2 (29), which may cause a T-helper type 1/T-helper type 2 cytokine imbalance and immunoglobulin E class switch. Changes in cytokine profile as a response to neonatal exposures may lead to asthma and allergic diseases later in childhood (30).
Strengths and limitations
Our study has several strengths. As the largest prospective birth cohort study in the United States, the CPP was carefully conducted with a high long-term follow-up rate (mean = 88%) (11). In this study, serum bilirubin levels were assessed in newborns with birthweights of 2,250 g or more according to a uniform protocol that is unlikely to be duplicated in contemporary studies. The distribution of bilirubin levels was consistent with that reported in the literature (18). Moreover, the exposure of hyperbilirubinemia in this study was not confounded by phototherapy, making the CPP particularly suitable to study the association between untreated neonatal jaundice and childhood asthma.
Our study also has limitations. First, asthma was defined in the 1960s as “a disease of the respiratory passages characterized by dyspnea of an obstructive type which is predominantly expiratory, reversible at least partially, and of varying severity and duration” (31). The immunoglobulin E test, the skin test for allergens, and spirometry had been recently introduced to clinical use at that time. In the absence of laboratory test results, asthma may be underdiagnosed when children have persistent nocturnal coughs but no wheezing or breathlessness and coughing after exercise (32, 33). However, even today it is difficult to monitor lung function in children younger than 7 years (34). In practice, the diagnosis of asthma in young children is often made largely on the basis of symptom patterns and a careful clinical assessment of family history and physical findings, which is not very different from the method used 50 years ago (35).
On the other hand, misclassification of asthma may not be a serious issue in our study because we included only “definite asthma” confirmed by pediatricians and neurologists and excluded suspected asthma. Moreover, although asthma diagnosis has changed with time, the misclassification of asthma may not necessarily differ in high- and low-bilirubin groups. This nondifferential misclassification in terms of bilirubin level may bias the results toward the null (i.e., underestimating the true effect size). In our data, the prevalence of asthma was associated with maternal allergic diseases. The trend of asthma was similar to that reported in the literature when stratified by other characteristics, such as marital status, number of previous deliveries, and breastfeeding (16). All of the above patterns indicate that the diagnosis of asthma in the CPP may still be acceptable even according to contemporary criteria.
However, cesarean delivery and maternal smoking had no relationship with asthma in this study, which was inconsistent with results of previous studies. This may be attributable to the fact that preterm births and low-birthweight infants were excluded in our study. Because these outcomes are significantly related to smoking during pregnancy, the exclusion of disproportionately more smokers may undermine the association between smoking and asthma. Furthermore, in the 1960s, the cesarean delivery rate was very low (<5%), and babies born via cesarean delivery were likely to have serious health problems. Child deaths and losses to follow-up may also have led to a lower prevalence of asthma among cesarean deliveries and smokers than reported in other studies (16).
In this study, data on gestational age were based on the last menstrual period, which is subject to error. Some term births might actually be preterm (36), causing a false association between hyperbilirubinemia and asthma. However, we found that the magnitude of the association was consistent with that in previous studies, in which gestational age data were more accurate (8). Thus, error in gestational age is an unlikely explanation for our positive finding.
Given that both asthma and neonatal jaundice are common conditions, more studies are warranted to confirm our finding. Comprehension of the underlying biological mechanisms of this association may shed light on the etiology and pathogenesis of asthma. However, it remains possible that both jaundice and asthma are associated with an unmeasured confounder, rendering this association noncausal.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliation: MOE-Shanghai Key Laboratory of Children's Environmental Health, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (Lisu Huang, Xiaoping Lei, Jun Zhang); Department of Pediatrics, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China (Lisu Huang, Yixiao Bao, Yan Chen, Yongjun Zhang); and Epidemiology Branch, National Institute of Environmental Health Sciences, Durham, North Carolina (Zongli Xu).
Lisu Huang and Yixiao Bao contributed equally to the work and should both be considered as first author.
This work was supported by the Natural Science Foundation of China (grant 81273091/H2605).
We thank Dr. Allen J Wilcox and the staff of the Epidemiology Branch at the National Institute of Environmental Health Sciences for their valuable advice.
Conflict of interest: none declared.
Abbreviations
- CI
confidence interval
- CPP
Collaborative Perinatal Project
- GST
glutathione S-transferase
- TSB
total serum bilirubin
REFERENCES
- 1.Vollmer WM, Osborne ML, Buist AS. 20-year trends in the prevalence of asthma and chronic airflow obstruction in an HMO. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1079–1084. doi: 10.1164/ajrccm.157.4.9704140. [DOI] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, Von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Murk W, Risnes KR, Bracken MB. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics. 2011;127(6):1125–1138. doi: 10.1542/peds.2010-2092. [DOI] [PubMed] [Google Scholar]
- 4.Burke H, Leonardi-Bee J, Hashim A, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012;129(4):735–744. doi: 10.1542/peds.2011-2196. [DOI] [PubMed] [Google Scholar]
- 5.Bernsen RM, van der Wouden JC, Nagelkerke NJ, et al. Early life circumstances and atopic disorders in childhood. Clin Exp Allergy. 2006;36(7):858–865. doi: 10.1111/j.1365-2222.2006.02518.x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SM. Jaundice in the full-term newborn. Pediatr Nurs. 2006;32(3):202–208. [PubMed] [Google Scholar]
- 7.Punnoose AR, Schwartz LA, Golub RM. JAMA patient page. Neonatal hyperbilirubinemia. JAMA. 2012;307(19):2115. doi: 10.1001/jama.2012.4070. [DOI] [PubMed] [Google Scholar]
- 8.Aspberg S, Dahlquist G, Kahan T, et al. Confirmed association between neonatal phototherapy or neonatal icterus and risk of childhood asthma. Pediatr Allergy Immunol. 2010;21(4 Pt 2):e733–e739. doi: 10.1111/j.1399-3038.2010.01038.x. [DOI] [PubMed] [Google Scholar]
- 9.Ku MS, Sun HL, Sheu JN, et al. Neonatal jaundice is a risk factor for childhood asthma: a retrospective cohort study. Pediatr Allergy Immunol. 2012;23(7):623–628. doi: 10.1111/j.1399-3038.2012.01345.x. [DOI] [PubMed] [Google Scholar]
- 10.Dobbs RH, Cremer RJ. Phototherapy. Arch Dis Child. 1975;50(11):833–836. doi: 10.1136/adc.50.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy JB. The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol. 2003;13(5):303–311. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- 12.Von Mutius E. Paediatric origins of adult lung disease. Thorax. 2001;56(2):153–157. doi: 10.1136/thorax.56.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman TB, Klebanoff MA. Neonatal hyperbilirubinemia and long-term outcome: another look at the Collaborative Perinatal Project. Pediatrics. 1993;92(5):651–657. [PubMed] [Google Scholar]
- 14.Klebanoff MA. The Collaborative Perinatal Project: a 50-year retrospective. Paediatr Perinat Epidemiol. 2009;23(1):2–8. doi: 10.1111/j.1365-3016.2008.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen TC, Leviton A. Asthma and eczema in children born to women with migraine. Arch Neurol. 1990;47(11):1227–1230. doi: 10.1001/archneur.1990.00530110087022. [DOI] [PubMed] [Google Scholar]
- 16.Metasälä J, Kilkkinen A, Kaila M, et al. Perinatal factors and the risk of asthma in childhood—a population-based register study in Finland. Am J Epidemiol. 2008;168(2):170–178. doi: 10.1093/aje/kwn105. [DOI] [PubMed] [Google Scholar]
- 17.Hothorn LA, Vaeth M, Hothorn T. Trend tests for the evaluation of exposure-response relationships in epidemiological exposure studies. Epidemiol Perspect Innov. 2009;6:1. doi: 10.1186/1742-5573-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alkalay AL, Simmons CF. Hyperbilirubinemia guidelines in newborn infants. Pediatrics. 2005;115(3):824–825. doi: 10.1542/peds.2004-2442. [DOI] [PubMed] [Google Scholar]
- 19.Aspberg S, Dahlquist G, Kahan T, et al. Is neonatal phototherapy associated with an increased risk for hospitalized childhood bronchial asthma? Pediatr Allergy Immunol. 2007;18(4):313–319. doi: 10.1111/j.1399-3038.2006.00518.x. [DOI] [PubMed] [Google Scholar]
- 20.Piazza AJ, Stoll BJ. Jaundice and hyperbilirubinemia in the newborn. In: Kliegman RM, Behrman RE, Jenson HB, et al., editors. Nelson Textbook of Pediatrics. 18th ed. Philadelphia, PA: WB Saunders; 2007. pp. 756–766. [Google Scholar]
- 21.Sarici SU, Serdar MA, Korkmaz A, et al. Incidence, course, and prediction of hyperbilirubinemia in near-term and term newborns. Pediatrics. 2004;113(4):775–780. doi: 10.1542/peds.113.4.775. [DOI] [PubMed] [Google Scholar]
- 22.Lu FL, Hsieh CJ, Caffrey JL, et al. Body mass index may modify asthma prevalence among low-birth-weight children. Am J Epidemiol. 2012;176(1):32–42. doi: 10.1093/aje/kwr484. [DOI] [PubMed] [Google Scholar]
- 23.Kalliomaki M, Isolauri E. Role of intestinal flora in the development of allergy. Curr Opin Allergy Clin Immunol. 2003;3(1):15–20. doi: 10.1097/00130832-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Prachukthum S, Nunnarumit P, Pienvichit P, et al. Genetic polymorphisms in Thai neonates with hyperbilirubinemia. Acta Paediatr. 2009;98(7):1106–1110. doi: 10.1111/j.1651-2227.2009.01275.x. [DOI] [PubMed] [Google Scholar]
- 25.Abdel Ghany EA, Hussain NF, Botros SK. Glutathione S-transferase gene polymorphisms in neonatal hyperbilirubinemia. J Investig Med. 2012;60(1):18–22. doi: 10.2310/JIM.0b013e318235479a. [DOI] [PubMed] [Google Scholar]
- 26.Karam RA, Pasha HF, El-Shal AS, et al. Impact of glutathione-S-transferase gene polymorphisms on enzyme activity, lung function and bronchial asthma susceptibility in Egyptian children. Gene. 2012;497(2):314–319. doi: 10.1016/j.gene.2012.01.059. [DOI] [PubMed] [Google Scholar]
- 27.Tamer L, Calikoglu M, Ates NA, et al. Glutathione-S-transferase gene polymorphisms (GSTT1, GSTM1, GSTP1) as increased risk factors for asthma. Respirology. 2004;9(4):493–498. doi: 10.1111/j.1440-1843.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 28.Minelli C, Granell R, Newson R, et al. Glutathione-S-transferase genes and asthma phenotypes: a Human Genome Epidemiology (HuGE) systematic review and meta-analysis including unpublished data. Int J Epidemiol. 2010;39(2):539–562. doi: 10.1093/ije/dyp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung EK, Miller RL, Wilson MT, et al. Antenatal risk factors, cytokines and the development of atopic disease in early childhood. Arch Dis Child Fetal Neonatal Ed. 2007;92(1):F68–F73. doi: 10.1136/adc.2006.106492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Li P, Lu J, et al. Bilirubin possesses powerful immunomodulatory activity and suppresses experimental autoimmune encephalomyelitis. J Immunol. 2008;181(3):1887–1897. doi: 10.4049/jimmunol.181.3.1887. [DOI] [PubMed] [Google Scholar]
- 31.Meneely GR, Renzetti AD, Jr, Steele JD, et al. Chronic bronchitis, asthma, and pulmonary emphysema: a statement by the Committee on Diagnostic Standards for Nontuberculous Respiratory Diseases. Am Rev Respir Dis. 1962;85:762–768. [Google Scholar]
- 32.Williams H, McNicol KN. Prevalence, natural history, and relationship of wheezy bronchitis and asthma in children. An epidemiological study. Br Med J. 1969;4(5679):321–325. doi: 10.1136/bmj.4.5679.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speight AN. Is childhood asthma being underdiagnosed and undertreated? Br Med J. 1978;2(6133):331–332. doi: 10.1136/bmj.2.6133.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemanske RF, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125(2 suppl 2):S95–S102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen SE, Hurd SS, Lemanske RF, et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1–17. doi: 10.1002/ppul.21321. [DOI] [PubMed] [Google Scholar]
- 36.Dietz PM, England LJ, Callaghan WM, et al. A comparison of LMP-based and ultrasound-based estimates of gestational age using linked California livebirth and prenatal screening records. Paediatr Perinat Epidemiol. 2007;21(suppl 2):62–71. doi: 10.1111/j.1365-3016.2007.00862.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.