Abstract
Objectives
To describe the prevalence of degenerative changes on MRI and conventional radiographs of the spine in a young population with suspicion of axial spondyloarthritis (axSpA) and assess whether it is possible to discriminate between degenerative changes and lesions associated with axSpA.
Methods
Whole spine MRI and cervical and lumbar radiographs of patients ≥18 years with chronic back pain (≥3 months, ≤3 years, onset <50 years) were assessed for degeneration by two readers, and for SpA lesions by two other readers, all blinded for clinical information and results of the other readers. Degenerative scores were adjudicated in case of disagreement (by a third reader). Patients fulfilling and not fulfilling the Assessment of SpondyloArthritis international Society axSpA criteria were compared for prevalence of degenerative lesions. Scores for degenerative and SpA lesions were compared, and overlap was defined as the presence of both types of lesions in a single vertebral unit (VU).
Results
In 456/648 (70.4%) patients (46.8% men, mean age 33.6), degenerative lesions were found with similar percentages in patients with no axSpA and with axSpA (72.4% and 69.2%, p=0.45). Modic changes were found more often in patients with no axSpA (29/239, 12.1%) versus patients with axSpA (19/409, 4.6%, p=0.01). Other lesions were evenly distributed. Overlap was minimal in 19 patients (3.0%) and 32/14 674 (0.2%) VUs for SpA reader 1 and in 23 patients (3.6%) and 34/14 674 VUs (0.2%) for SpA reader 2.
Conclusion
The prevalence of degeneration is high in an early inflammatory back pain cohort. Discrimination between degeneration and axSpA lesions is very well possible with little overlap between degenerative and axSpA readings.
Keywords: ankylosing spondylitis, low back pain, magnetic resonance imaging, spondyloarthritis
Key messages.
What is already known about this subject?
Abnormalities associated with axial spondyloarthritis (axSpA) on radiographs and MRI of the spine may mimic abnormalities associated with degenerative lesions.
What does this study add?
More than 70% of the patients suspected of axSpA have degenerative lesions of the spine.
It is very well possible to discriminate between lesions associated with axSpA and degenerative disease in the spine in patients with chronic back pain with an age at onset below 50 years.
How might this impact on clinical practice?
Evaluators can be trained to discriminate between abnormalities on radiographs and MRI of the spine belonging to axSpA and degenerative disease.
Introduction
MRI and conventional radiographs are both used in the screening for and diagnosis of axial spondyloarthritis (axSpA). MRI is a relatively new imaging technique in the field of axSpA. The advantage is the early detection of bone marrow oedema on short tau inversion recovery (STIR) sequences, consistent with inflammation. MRI of the sacroiliac (SI) joints is introduced in the imaging arm of the Assessment of SpondyloArthritis international Society (ASAS) definition of axSpA in 2009.1 Although MRI of the spine is not used for classification and its additional value has to be determined, it may play a role in the evaluation of (potential) patients with axSpA.2–4
Lesions in the spine associated with axSpA have been well documented and various scoring systems have been developed.3 5–7 However, in addition to lesions associated with axSpA, concomitant degenerative changes in the spine are prevalent both in patients with axSpA and in the general population.8–10 Degenerative changes in the spine can become relevant when they mimic axSpA. For example, the presence of degenerative changes of the endplates might be misinterpreted for inflammatory lesions since both are associated with bone marrow oedema. On radiographs, syndesmophytes and spondylophytes are both a bony spur arising from the corner of vertebral bodies and are distinguished by the angle that they form with the vertebra, but this is sometimes challenging.11 Until now, it is unknown whether misinterpretation is an issue when assessing MRIs and radiographs of the spine.
Therefore, the aim of this study is to describe the prevalence and location of degenerative changes in the spine in a young population with suspicion of axSpA. In addition, this study aims to assess whether it is possible to discriminate reliably between degenerative changes and lesions associated with axSpA.
Methods
Imaging studies and clinical data collected at baseline of patients included in the DESIR cohort (DEvenir des Spondylarthropathies Indifférenciées Récentes) were used (database was locked on 30 June 2010).12 Inclusion criteria were age over 18 and under 50 years; inflammatory back pain in the buttock(s), lumbar spine or thoracic spine fulfilling either the Calin or the Berlin criteria for inflammatory back pain for longer than 3 months but no longer than 3 years13 14; and a SpA probability score of at least 5/10 on a numerical rating scale (0, not suggestive at all; 10, highly suggestive) as determined by the local investigator. For exclusion criteria and more details, see the original article written by Dougados et al12 introducing the cohort. Based on the ASAS axSpA criteria, patients were categorised as no-axSpA (not fulfilling ASAS axSpA criteria) or axSpA (fulfilling ASAS criteria) based on the central reading (see ‘Axial spondyloarthritis parameters’ section below).15
Imaging
Sagittal T1-weighted spin echo and STIR MR images of the whole spine and sagittal cervical and lumbar radiographs were used. All four readers were educated on degenerative and axSpA findings on radiographs as well as MRI (supervised by MR and AF, musculoskeletal radiologists with an extensive experience). Readers were blinded for clinical information, the reading of the SpA lesions and the other imaging modality.
Degenerative parameters
Two well-calibrated readers (FdB, MOT) assessed all MRIs and radiographs for degenerative lesions. Adjudication was performed (MR for MRI and AF for radiographs) on a per-lesion basis, except for the disc degeneration grade on MRI: in case of a difference of one grade, the lower grade was used, and in case of a difference of two grades, adjudication was performed. From hereon we refer to this reading as the ‘degenerative reading’.
Disc degeneration was scored on a five-point scale, combining signal loss and loss of height of the intervertebral discs on STIR images.16 Afterwards, discs were categorised as normal (grades 1 and 2) or degenerated (grades 3, 4 and 5). The high-intensity zone (HIZ), indicating an annular tear or fissure, is seen as an area of high signal intensity located in the posterior annulus fibrosis on STIR images.17 We considered bulging of a disc as either protrusion or extrusion; the distinction is based on whether the maximal diameter in any direction is at the base of the displacement (protrusion) or not (extrusion).18 Canal stenosis was defined as reduction of the anterior–posterior diameter of the spinal canal with compression on the spinal cord (at cervical and thoracic level) or as contact between the nerve root and disc material with obliteration of perineural intraforaminal fat or compression of the nerve root (at lumbar level).19 20 We used the Modic classification to assess degenerative changes of the vertebral endplates. This is a three-point scale with grade I defined as bone marrow oedema, grade II is described as fatty changes and grade III as sclerotic changes.21 22 Schmorls nodes are defined as an indentation of the (cranial or caudal) endplate with herniation of intervertebral disc material into the vertebra, with or without oedema.23 24
On the radiographs, loss of disc height was defined as narrowing of the disc space in comparison with two adjacent (healthy) discs. By definition, the lumbar disc spaces should increase in height in the cranial to caudal direction, except disc L5–S1, which normally is smaller than disc L4–L5. Osteophytes, in the spine also called spondylophytes, are described by reactive bone hypertrophy, seen as bony spurs arising from the vertebral body close to the vertebral endplate in a horizontal configuration (maximum of 45-degree angle with the endplate). Sclerosis was defined by an increased bone density and calcification adjacent to the vertebral endplates. Schmorls nodes were defined as a radiolucent contour defect of the vertebral endplate with sclerotic margins.24 Facet joint osteoarthritis was defined as sclerotic joint surfaces and osteophyte formation.
Axial spondyloarthritis parameters
Initially, all images were read by the local radiologist and/or rheumatologist. In addition, two independent readers (MdH, J-BP) scored all images of all centres using the Berlin method and the Canada–Denmark for MRI and the modified Stoke Ankylosing Spondyloarthritis Syndesmophyte Score (mSASSS) for radiographs. Data of the two readers were kept separate as we did not perform an adjudication for each individual lesion. From hereon we refer to this central reading as ‘SpA reader 1’ and ‘SpA reader 2’.3 5 25–27 These readers were blinded for clinical information, the other modality and the scores of the other reader and the local reading. For the current study, we used bone marrow oedema (BME) lesions on MRI (from the Berlin score system), fatty corner lesion and corner and non-corner erosion on MRI (both from the Canada–Denmark scoring system) and syndesmophytes on radiographs (from the mSASSS) of SpA reader 1 and 2.
Statistical analysis
For the degenerative reading, consensus scores between the two readers or consensus by two out of three readers in case of adjudication have been used. For axSpA lesions, the scores of the two individual readers have been used. Summary statistics of continuous variables were reported in means and SD, for categorical data in percentages, median and frequencies to describe the prevalence of degenerative lesions. The difference between patients with no axSpA and with axSpA was analysed by comparing individual degenerative changes based on various cut-off points (at least one until at least three lesions) by the χ2 test. Cohen’s kappa was used for interobserver agreement (weighted kappa for Pfirrmann grading). A p value less than 0.05 was considered statistically significant. No adjustment for multiple testing was performed.
To analyse the overlap between lesions scored as degenerative or as SpA, four pairs of lesions were selected based on (potential) resemblance: Modic type I and SpA-associated BME (Berlin method); Modic type II and fatty lesions (Canada–Denmark scoring system); Schmorls nodes and non-corner erosions (Canada–Denmark); spondylophytes and syndesmophytes (mSASSS). Overlap was defined in case both the degenerative reading and a SpA reader marked a vertebral unit (VU) positive for the two corresponding lesions. Overlap was reported with absolute numbers and percentages on a patient and on a VU level.
Results
In total, 648 patients with complete radiographic and MRI at baseline were used. Mean age was 33.6 (SD 8.6) and 46.8% were men. ASAS classification criteria were fulfilled in 409/648 patients (63.1%) of which 173 (26.7%) patients were in the clinical arm and 236 (36.4%) patients were in the imaging arm. Moreover, 59.1% patients were HLA B27 positive and mean symptom duration was 18.2 months (SD 10.5).
Degeneration
Kappa scores were fair (0.41–0.60) for Schmorls nodes on radiographs and HIZ, and substantial (0.61–0.80) for disc degeneration, facet joint osteoarthritis, central stenosis, Schmorls nodes on MRI, extrusion, protrusion, loss of disc height, osteophytes and good (0.81–1.00) for Modic changes.28 Adjudication for at least one lesion per patient was performed in 286/648 patients (44.2%).
Degenerative changes were found in 456/648 patients (70.4%) (table 1). Degenerated discs and Schmorls nodes had the highest prevalence and were present in 278/648 patients (42.9%) and 238/648 patients (36.7%), respectively. More male patients had degenerative changes (226/303 (74.6%) males vs 230/345 (66.7%) females (p=0.03)), and this difference is almost completely explained by the higher prevalence of Schmorls nodes (136/303 (44.9%) males and 102/345 (29.6%) females). Prevalence of other degenerative changes on both MRI and radiograph were comparable in both genders. On radiographs, degenerative changes were found in 191/648 patients (29.5%). Male and female patients were equally represented; 92/303 men (30.4%) and 99/345 women (28.7%) (p=0.2). Prevalence of degeneration was lower on radiographs compared with MRI; loss of disc height was most prevalent on the radiographs and was found in 153/648 patients (23.6%) (table 1).
Table 1.
Number of patients with a degenerative change split by gender
| Total (n=648) |
Female (n=345) |
Male (n=303) |
|
| MRI | |||
| Canal stenosis | 12 (1.9) | 6 (1.7) | 6 (2.0) |
| Extrusion | 106 (16.4) | 61 (17.7) | 45 (14.9) |
| High-intensity zone | 172 (26.5) | 96 (27.8) | 76 (25.1) |
| Modic type I | 48 (7.4) | 32 (9.3) | 16 (5.3) |
| Modic type II | 31 (4.7) | 17 (4.9) | 14 (4.6) |
| Protrusion | 108 (16.7) | 62 (17.9) | 46 (15.2) |
| Disc degeneration grade >2 | 278 (42.9) | 150 (43.5) | 128 (42.2) |
| Schmorls node without oedema | 238 (36.7) | 102 (29.6) | 136 (44.9) |
| Schmorls node with oedema | 18 (2.8) | 6 (1.7) | 12 (4.0) |
| Radiographs | |||
| Loss of disc height | 146 (22.5) | 73 (21.2) | 73 (24.1) |
| Osteophytes | 94 (14.5) | 49 (14.2) | 45 (14.9) |
| Sclerosis | 80 (12.3) | 45 (13.0) | 35 (11.6) |
| Facet joint osteoarthritis | 17 (2.6) | 7 (2.0) | 10 (3.3) |
| Schmorls node | 29 (4.5) | 14 (4.1) | 15 (4.9) |
Equal number of patients with axSpA and without axSpA had one or more positive degenerative parameters on MRI (173/239 (72.4%) patients with no axSpA and 283/409 (69.2%) patients with axSpA, p=0.45) and on radiograph (87/239 (36.4%) patients with no axSpA and 104/409 (26.9%) patients with axSpA, p=0.14). When patients with axSpA and without axSpA were compared for cut-off points of individual degenerative changes, more patients in the no-axSpA group had Modic changes compared with patients from the axSpA group; Modic type I change was found in 29/239 (14.9%) patients with no axSpA and in 19/409 (4.2%) patients with axSpA (p=0.001). For Modic type II changes, this was 20/239 (10.3%) patients with no axSpA and 11/409 (2.4%) patients with axSpA (p=0.002). Only few patients from both groups had more than one Modic I or Modic II change. No statistically significant differences were found between the two patient groups for any of the other cut-off points of any of the degenerative changes. Table 2 lists the prevalence of cut-off points and p values for the comparison of patients with no axSpA and with axSpA.
Table 2.
Degenerative changes according to various cut-offs of the lesions in the no-axSpA and the axSpA groups
| No-axSpA (n=239) |
axSpA (n=409) |
P values | ||
| MRI | ||||
| Canal stenosis | 1 | 5 (2.1) | 7 (1.7) | 0.31 |
| 2 | 4 (1.7) | 5 (1.2) | 0.56 | |
| 3 | 3 (1.3) | 4 (0.99) | 0.74 | |
| Extrusion | 1 | 48 (20.1) | 58 (14.3) | 0.07 |
| 2 | 17 (8.8) | 14 (3.1) | 0.05 | |
| 3 | 5 (2.6) | 4 (0.99) | 0.41 | |
| High-intensity zone | 1 | 66 (34.0) | 106 (23.3) | 0.70 |
| 2 | 12 (6.2) | 31 (6.8) | 0.91 | |
| 3 | 1 (0.5) | 6 (1.3) | 0.99 | |
| Modic type I | 1 | 29 (14.9) | 19 (4.2) | 0.01* |
| 2 | 1 (0.5) | 1 (0.2) | 1 | |
| 3 | – | 1 (0.2) | 1 | |
| Modic type II | 1 | 20 (10.3) | 11 (2.4) | 0.02* |
| 2 | 3 (1.5%) | 1 (0.2) | 0.29 | |
| 3 | 2 (1.0%) | – | 0.26 | |
| Protrusion | 1 | 37 (19.1) | 71 (15.6) | 0.42 |
| 2 | 11 (5.7) | 23 (5.1) | 0.73 | |
| 3 | 3 (1.5) | 8 (1.8) | 0.76 | |
| Pfirrmann grade >2 | 1 | 87 (44.8) | 191 (42.1) | 0.57 |
| 2 | 44 (22.7) | 94 (20.7) | 0.65 | |
| 3 | 17 (8.8) | 34 (7.5) | 0.70 | |
| Schmorls node without oedema | 1 | 67 (34.5) | 171 (37.7) | 0.50 |
| 2 | 48 (24.7) | 125 (27.5) | 0.52 | |
| 3 | 40 (20.6) | 91 (20.0) | 0.95 | |
| Schmorls node with oedema | 1 | 6 (3.1) | 12 (2.6) | 0.95 |
| 2 | 1 (0.5) | 5 (1.1) | 0.79 | |
| 3 | – | 1 (0.2) | 1 | |
| Radiographs | ||||
| Loss of disc height | 1 | 53 (27.3) | 93 (20.5) | 0.09 |
| 2 | 21 (10.8) | 31 (6.8) | 0.14 | |
| 3 | 7 (3.6) | 13 (2.9) | 0.83 | |
| Osteophytes | 1 | 35 (18.0) | 59 (13.0) | 0.14 |
| 2 | 15 (7.7) | 22 (4.8) | 0.23 | |
| 3 | 8 (4.1) | 15 (3.3) | 0.81 | |
| Sclerosis | 1 | 32 (16.5) | 48 (10.6) | 0.06 |
| 2 | 14 (7.2) | 16 (3.5) | 0.07 | |
| 3 | 6 (3.1) | 8 (1.8) | 0.46 | |
| Facet joint osteoarthritis | 1 | 6 (3.1) | 11 (2.4) | 0.86 |
| 2 | 1 (0.5) | 2 (0.4) | 1 | |
| 3 | – | – | – | |
| Schmorls node | 1 | 8 (4.1) | 21 (4.6) | 0.90 |
| 2 | 3 (1.5) | 8 (1.8) | 1 | |
| 3 | 1 (0.5) | 4 (0.9) | 0.99 |
*P<0.05.
axSpA, axial spondyloarthritis.
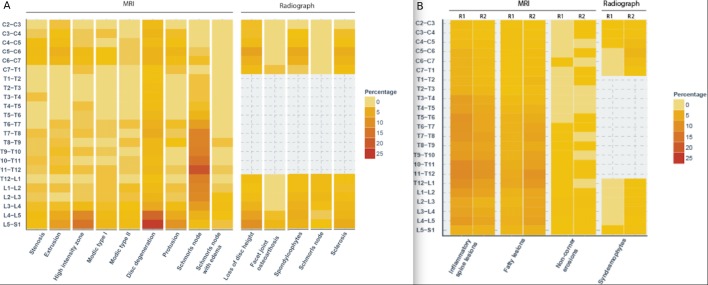
For both MRI and radiographic images, degenerative changes were found predominantly in the lumbar spine. Especially at L4–L5 and L5–S1, the prevalence of degeneration was high. Overall, 292/366 (79.8%) patients with degeneration in the lumbar spine had degeneration at this location. Degenerated discs (grade 2 or higher) were found in 211/366 patients (57.7%) at level L4–L5, L5–S1 and herniation in 149/366 patients (40.7%) in L4–L5 or L5–S1. Schmorls nodes were an exception as these were most prevalent in the lower thoracic spine. Figure 1 shows the distribution of the degenerative changes in the spine per VU.
Figure 1.
(A) Heatmap of all degenerative changes per vertebral unit. Graphical representation of the prevalence of all degenerative changes per vertebral unit. The colour key on the right side indicates the prevalence. (B) Heatmap of SpA lesions used for comparison with degenerative changes. Graphical representation of the prevalence and distribution of axSpA lesions (which were used for comparison with the degenerative lesions). Reader 1 (R1) and reader 2 (R2) are compared side by side. Colour key used same increments and colours as in figure 1A (for comparison). axSpA, axial spondyloarthritis.
Overlap
Both teams of readers assessed 638 patients with 14 674 VUs on MRI and 7656 VUs on radiographs. Figure 1b shows the distribution of the axSpA lesions in the spine per VU. AxSpA lesions on MRI are more evenly distributed in the spine with a slightly higher prevalence in the lower thoracic spine. Although reader 1 scored more BME lesions and reader 2 scored more fatty changes, the overall distribution was comparable.
In 19/638 (3.0%) and in 23/638 (3.6%) of the patients, at least one VU with overlap existed between the degenerative reading and axSpA reader 1 or 2, respectively. Table 3 shows the overlap for specific combinations of lesions. Examples of images are presented in figures 2–4. Of the patients with overlap between the degenerative reading and SpA reader 1, 16/19 (84.2%) patients had axSpA and for reader 2, this was 17/23 (73.9%) patients. Remarkably, there was no overlap on the radiographs between syndesmophytes and spondylophytes.
Table 3.
Number of patients with overlap between the degenerative reading and the SpA reading per compared lesion pair
| SpA reader 1 and degenerative reading (n=638) |
SpA reader 2 and degenerative reading (n=638) |
|
| MRI | ||
| Modic type I and BME lesions | 12 (1.9%) | 6 (0.9%) |
| Modic type II and fatty changes | 9 (1.4%) | 11 (1.7%) |
| Schmorls nodes and non-corner erosions | 5 (0.8%) | 11 (1.7%) |
| Radiographs | ||
| Spondylophytes and syndesmophytes | 0 | 0 |
BME, bone marrow oedema; SpA, spondyloarthritis.
Figure 2.
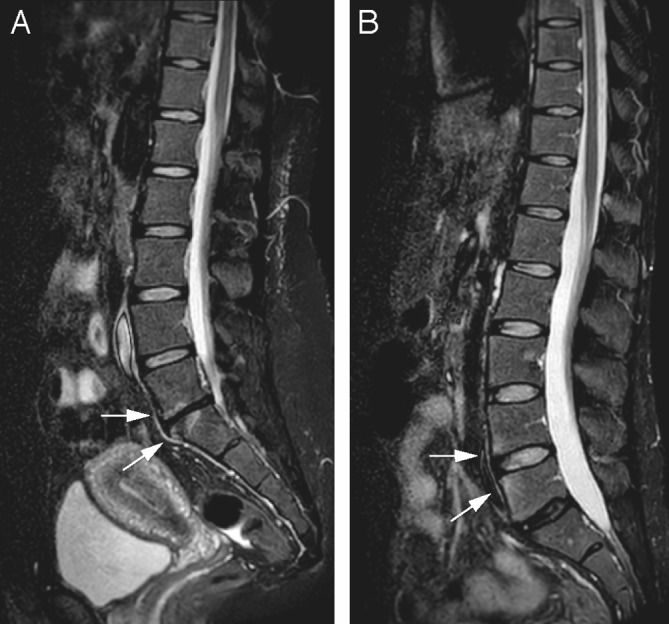
Sagittal short tau inversion recovery images of the lower spine of two different patients. (A) At level L5–S1, disc space narrowing with a low signal intensity of the disc is seen, with concomitant high signal intensity of the endplates of L4 and L5 (arrows). This is consistent with disc degeneration with Modic endplate changes. (B) At L5–S1, disc degeneration is present without endplate changes; the disc space narrowing is less pronounced than in (A). At level L3–4, however, the anterior corners of the endplates show focal high signal intensity despite a normal disc (arrows). This is based on osteitis secondary to axial spondyloarthritis.
Figure 3.
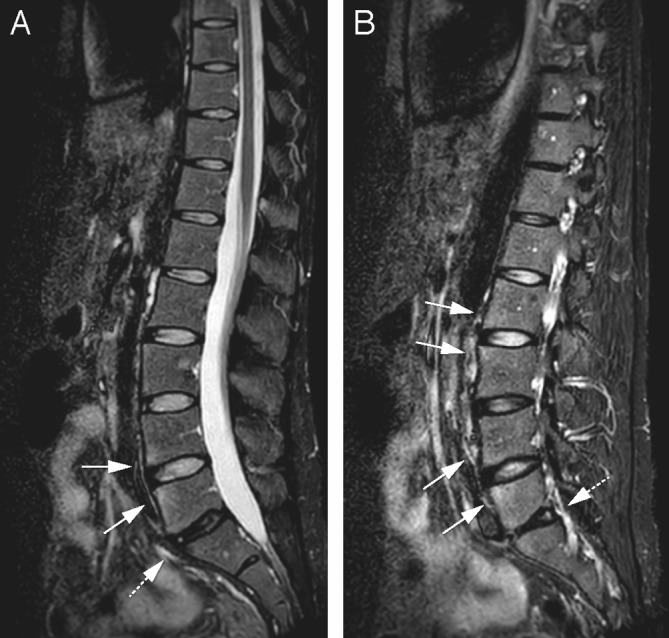
Same patient as 1b. On another sagittal short tau inversion recovery slice, the high signal intensity at the corners of the endplates, signs of axial spondyloarthritis, are more clearly seen at L2–3 as well (arrows). The degenerated disc L5–S1 (dotted arrow) has a high-intensity zone at the posterior part (B).
Figure 4.
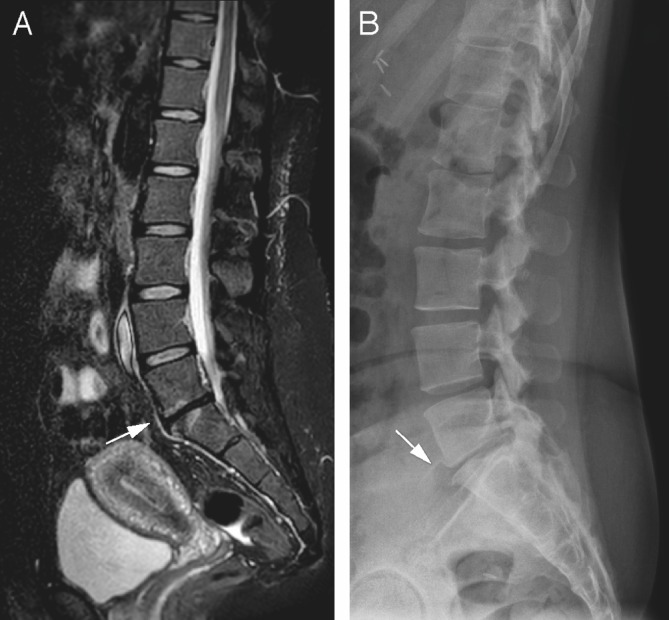
(A, B) Sagittal short tau inversion recovery image with a degenerated disc L5–S1 (arrow), with classic spondylophytes on the conventional radiograph (arrow).
In table 4, we present cross-tables per VU and per mimicking lesion between the degenerative reading and each SpA reader. It is apparent that both for the combination Modic type I and BME and for the combination Modic type II and fatty lesions, the axSpA lesion is more prevalent than the degenerative counterpart (eg, SpA reader 1 scored 898 VUs with BME and Modic type I was scored in 50 VUs). For Schmorls nodes and non-corner erosions, the opposite is true (eg, in 865 VUs a Schmorls node was scored by the degenerative reading and SpA reader 1 scored in 30 VUs a non-corner erosion). Nonetheless, the number of VUs with overlap was low in all cases (14 VUs in the first example and 9 in the latter). On radiographs, no VU with overlap between the degenerative reading and SpA readers was present.
Table 4.
Cross-tables between the degenerative reading and the SpA readers for the overlap per vertebral unit per mimicking lesion pair
| Degenerative reading | SpA reader 1 | SpA reader 2 | ||||||
| MRI | ||||||||
| Bone marrow oedema lesions (Berlin criteria) | ||||||||
| 1 | 0 | Total | 1 | 0 | Total | |||
| Modic type I | 1 | 14 | 36 | 50 | 1 | 8 | 42 | 50 |
| 0 | 884 | 13 740 | 14 624 | 0 | 619 | 14 005 | 14 624 | |
| Total | 898 | 13 776 | 14 674 | Total | 627 | 14 047 | 14 674 | |
| Fatty lesions (Canada–Denmark) | ||||||||
| 1 | 0 | Total | 1 | 0 | Total | |||
| Modic type II | 1 | 9 | 26 | 35 | 1 | 11 | 24 | 35 |
| 0 | 3 | 14 646 | 14 639 | 0 | 3 | 14 646 | 14 639 | |
| Total | 12 | 14 662 | 14 674 | Total | 14 | 14 660 | 14 674 | |
| Non-corner erosions (Canada–Denmark) | ||||||||
| 1 | 0 | Total | 1 | 0 | Total | |||
| Schmorls nodes | 1 | 9 | 856 | 865 | 1 | 15 | 850 | 865 |
| 0 | 21 | 13 788 | 13 809 | 0 | 23 | 13 786 | 13 809 | |
| Total | 30 | 14 644 | 14 674 | Total | 38 | 14 636 | 14 674 | |
| Radiographs | ||||||||
| Syndesmophytes (mSASSS) | ||||||||
| 1 | 0 | Total | 1 | 0 | Total | |||
| Osteophytes | 1 | 0 | 51 | 51 | 1 | 0 | 51 | 51 |
| 0 | 5 | 7600 | 7605 | 0 | 72 | 7533 | 7605 | |
| Total | 5 | 7651 | 7656 | Total | 72 | 7584 | 7656 | |
mSASSS, modified Stoke Ankylosing Spondyloarthritis Syndesmophyte Score (assessed on radiograph); SpA, spondyloarthritis; 1, present; 0, absent.
Discussion
We assessed the prevalence of degenerative changes on MRI and radiographs of the spine of young patients with short-term inflammatory back pain included in the DESIR cohort. The prevalence of degeneration in the spine is high and concentrated predominantly in the lower lumbar spine, while there is no difference between patients fulfilling or not fulfilling the axSpA criteria. There was only minimal overlap between the degenerative reading and the individual central readings for axSpA lesions, which indicates that readers are able to distinguish lesions as belonging to degenerative versus SpA lesions.
The prevalence of degeneration in the current study is comparable with results of the SPACE cohort (inclusion based on back pain symptom duration of more than 3 months but no longer than 2 years and onset back pain after 16 years but before 45).29 Of 274 patients, 245 (90%) patients had one or more parameters for positive parameters of degeneration, compared with 70% in the current study. In the SPACE study, the highest prevalence was also found in the lower lumbar spine, and no difference was found between patients fulfilling the ASAS criteria and patients not fulfilling the ASAS criteria.8 Degeneration was found as often in patients without axSpA as it was in patients with axSpA and cut-off points could not discriminate between patient groups. In the current study, Modic type I and II changes were exceptions; although Modic lesions were scarce in both groups, more patients without axSpA compared with patients with axSpA had one lesion. However, Modic changes are found in both groups, and thus the presence of a Modic lesion does not discriminate between patients with no axSpA and patients with axSpA. Also, it is possible to discriminate between Modic changes and BME and fatty changes associated with axSpA, meaning that Modic changes in patients with no axSpA are not likely to be misinterpreted as BME or fatty changes as we showed in the current study.
The overlap was low: 32 VUs in 19 patients for SpA reader 1 and 34 VUs in 23 patients for SpA reader 2. When we look at individual mimicking lesion pairs, we see that the overlap did not depend on the prevalence of specific lesions. For example, high signal intensity on STIR consistent with BME due to osteitis in axSpA was frequently found and not misinterpreted by the degenerative readers. In contrast, Schmorls nodes had a high prevalence and were not misinterpreted by the axSpA readers. In both cases, the degenerative and SpA readers were able to discriminate between them and only scored in case they were certain of the aetiology. This was true for the comparison between the degenerative reading and each of the two SpA readers.
These results can be explained by several reasons. First, the readers (both for degeneration and axSpA lesions) in the current study were trained and experienced. This was achieved by practising and reviewing cases with experienced musculoskeletal radiologists with an interest in axSpA. Knowledge of degenerative changes and axSpA lesions is pivotal for both the degeneration readers and axSpA readers to make the correct judgement in equivocal cases. Second, the clear definitions of degenerative changes and SpA lesions given by the literature aided in making the distinction. For example, Modic type I lesions are defined as high signal on fluid sensitive images over the full width of the vertebral endplate, while axSpA-associated BME typically is located in the anterior or posterior corner.7 21 In real life, there are of course cases where the distribution of oedema in the endplate is ambiguous. However, readers should adhere to the definitions as much as possible. Third, the distribution pattern of degeneration and axSpA lesions within the spine differed: degeneration is predominantly located in the lower lumbar spine and axSpA lesions were present in the whole spine, as can be seen in figure 1A and B. As an exception to this, Schmorls nodes are found in the lower thoracic spine most often. When in doubt, the location can give additional information about the (probable) origin of a lesion.
So, with proper training and adequate knowledge, readers are able to discriminate between SpA and degeneration. This is especially valuable in patients with early axSpA or patients with a suspicion of axSpA because in these patients lesions can be scarce and indistinct.
This study has several strengths. The study population in the current study is large, 648 patients, with both patients fulfilling and not fulfilling the ASAS axSpA criteria, making a comparison of the two groups possible. The DESIR cohort is a multicentre study and standardised imaging protocols ensure uniform radiographs and MRI scans. Available adjudicated degenerative scores provide an excellent source of comparison with the central SpA readings.
We have analysed the results of two well-trained central readers, whereas the images in the DESIR cohort were also assessed by a local radiologist and/or rheumatologist for the presence of axSpA lesions. Although comparing the local readers with the degenerative reading would make an interesting analysis, this was not possible since the local readers assessed the images only on the presence of inflammatory lesions and on the presence of structural lesions for the whole spine and SI joints. A limitation of the study is that the current results cannot be extrapolated to non-trained readers.
In addition, the way we defined overlap (a positive score by both readings anywhere in a VU) does not necessarily mean that the degenerative reading and SpA reading scored the exact same lesion, for example, a Schmorls node in the superior endplate and an erosion in the inferior endplate of the same VU is regarded in the current analysis as overlap; thus, the actual overlap by double interpretation of a lesion is potentially even lower.
In conclusion, we confirm that the prevalence of degeneration is high in an early inflammatory back pain cohort without difference between patients fulfilling and not fulfilling ASAS axSpA criteria. Most importantly, we show that it is very well possible to discriminate between degeneration and axSpA lesions by trained readers.
Acknowledgments
The DESIR cohort is conducted under the control of Assistance Publique Hopitaux de Paris via the Clinical Research Unit Paris Centre and under the umbrella of the French Society of Rheumatology and Institut national de la sante et de la recherche medicale (Inserm). Database management is performed within the Department of Epidemiology and Biostatistics (Professor Jean-Pierre Daures, D.I.M., Nımes, France). We also wish to thank the different regional participating centres: Professor Maxime Dougados (Paris-Cochin B), Professor Andre Kahan (Paris-Cochin A), Professor Philippe Dieudé (Paris-Bichat), Professor Bruno Fautrel (Paris-La Pitie-Salpetriere), Professor Francis Berenbaum (Paris-Saint-Antoine), Professor Pascal Claudepierre (Creteil), Professor Maxime Breban (Boulogne-Billancourt), Dr Bernadette Saint-Marcoux (Aulnay-sous-Bois), Professor Philippe Goupille (Tours), Professor Jean Francis Maillefert (Dijon), Dr Emmanuelle Dernis (Le Mans), Professor Daniel Wendling (Besancon), Professor Bernard Combe (Montpellier), Professor Liana Euller-Ziegler (Nice), Professor Pascal Richette (Paris Lariboisiere), Professor Pierre Lafforgue (Marseille), Dr Patrick Boumier (Amiens), Professor Martin Soubrier (Clermont-Ferrand), Dr Nadia Mehsen (Bordeaux), Professor Damien Loeuille (Nancy), Professor Rene-Marc Flipo (Lille), Professor Alain Saraux (Brest), Professor Xavier Mariette (LeKremlin-Bicetre), Professor Alain Cantagrel (Toulouse) and Professor Olivier Vittecoq (Rouen). We wish to thank the research nurses, the staff members of the Clinical Research Unit of Paris Centre, the staff members of the Biological Resource Center of Bichat Hospital, the staff members of the Department of Statistics of Nımes and all the investigators, and in particular Jerome Allain, Emmanuelle Dernis, Salah Ferkal, Clement Prati, Marie-Agnes Timsit and Eric Toussirot, for active patient recruitment and monitoring.
Footnotes
Contributors: FdB, MOT, AF, MdH, J-BP and LG collected data for the study. MD, DvdH, AF, JLB and MR drafted the study design. FdB and DvdH drafted the manuscript. FdB performed the statistical analysis. All authors interpreted the data. All authors approved the final manuscript for submission.
Funding: The DESIR study is conducted as a Programme Hospitalier de Recherche Clinique with Assistance Publique Hopitaux de Paris as the sponsor. The DESIR study is also under the umbrella of the French Society of Rheumatology, which financially supports the cohort. An unrestricted grant from Pfizer has been allocated for the first 10 years.
Competing interests: None declared.
Ethics approval: Each study center.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mau W, Zeidler H, Mau R, et al. Clinical features and prognosis of patients with possible ankylosing spondylitis. Results of a 10-year followup. J Rheumatol 1988;15:1109–14. [PubMed] [Google Scholar]
- 2.de Hooge M, van den Berg R, Navarro-Compán V, et al. Patients with chronic back pain of short duration from the SPACE cohort: which MRI structural lesions in the sacroiliac joints and inflammatory and structural lesions in the spine are most specific for axial spondyloarthritis? Ann Rheum Dis 2016;75:1308–14. 10.1136/annrheumdis-2015-207823 [DOI] [PubMed] [Google Scholar]
- 3.Lambert RGW, Pedersen SJ, Maksymowych WP, et al. Active inflammatory lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis—definitions, assessment system, and reference image set. J Rheumatol Suppl 2009;84:3–17. 10.3899/jrheum.090616 [DOI] [Google Scholar]
- 4.Hermann KG, Baraliakos X, van der Heijde DM, et al. Descriptions of spinal MRI lesions and definition of a positive MRI of the spine in axial spondyloarthritis: a consensual approach by the ASAS/OMERACT MRI study group. Ann Rheum Dis 2012;71:1278–88. 10.1136/ard.2011.150680 [DOI] [PubMed] [Google Scholar]
- 5.Ostergaard M, Maksymowych WP, Pedersen SJ, et al. Structural lesions detected by magnetic resonance imaging in the spine of patients with spondyloarthritis—definitions, assessment system, and reference image set. J Rheumatol Suppl 2009;84:18–34. 10.3899/jrheum.090617 [DOI] [Google Scholar]
- 6.Sieper J, Rudwaleit M, Baraliakos X, et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis 2009;68(Suppl 2):ii1–44. 10.1136/ard.2008.104018 [DOI] [PubMed] [Google Scholar]
- 7.Braun J, Baraliakos X, Golder W, et al. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: evaluation of a new scoring system. Arthritis Rheum 2003;48:1126–36. 10.1002/art.10883 [DOI] [PubMed] [Google Scholar]
- 8.de Bruin F, ter Horst S, Bloem HL, de BF, ter HS, et al. Prevalence of degenerative changes of the spine on magnetic resonance images and radiographs in patients aged 16–45 years with chronic back pain of short duration in the Spondyloarthritis Caught Early (SPACE) cohort. Rheumatology 2016;55:56–65. 10.1093/rheumatology/kev283 [DOI] [PubMed] [Google Scholar]
- 9.Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990:1178–84. [PubMed] [Google Scholar]
- 10.Weishaupt D, Zanetti M, Hodler J, et al. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology 1998;209:661–6. 10.1148/radiology.209.3.9844656 [DOI] [PubMed] [Google Scholar]
- 11.Baraliakos X, Listing J, Rudwaleit M, et al. Progression of radiographic damage in patients with ankylosing spondylitis: defining the central role of syndesmophytes. Ann Rheum Dis 2007;66:910–5. 10.1136/ard.2006.066415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougados M, d’Agostino MA, Benessiano J, et al. The DESIR cohort: a 10-year follow-up of early inflammatory back pain in France: study design and baseline characteristics of the 708 recruited patients. Joint Bone Spine 2011;78:598–603. 10.1016/j.jbspin.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 13.Rudwaleit M, Metter A, Listing J, et al. Inflammatory back pain in ankylosing spondylitis: a reassessment of the clinical history for application as classification and diagnostic criteria. Arthritis Rheum 2006;54:569–78. 10.1002/art.21619 [DOI] [PubMed] [Google Scholar]
- 14.Calin A, Porta J, Fries JF, et al. Clinical history as a screening test for ankylosing spondylitis. JAMA 1977;237:2613–4. 10.1001/jama.1977.03270510035017 [DOI] [PubMed] [Google Scholar]
- 15.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. 10.1136/ard.2009.108233 [DOI] [PubMed] [Google Scholar]
- 16.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 2001;26:1873–8. 10.1097/00007632-200109010-00011 [DOI] [PubMed] [Google Scholar]
- 17.Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol 1992;65:361–9. 10.1259/0007-1285-65-773-361 [DOI] [PubMed] [Google Scholar]
- 18.Fardon DF, Milette PC. Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine 2001;26:E93–113. [DOI] [PubMed] [Google Scholar]
- 19.Okada E, Matsumoto M, Ichihara D, et al. Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine 2009;34:706–12. 10.1097/BRS.0b013e31819c2003 [DOI] [PubMed] [Google Scholar]
- 20.Maus TP. Imaging of spinal stenosis: neurogenic intermittent claudication and cervical spondylotic myelopathy. Radiol Clin North Am 2012;50:651–79. 10.1016/j.rcl.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 21.Modic MT, Steinberg PM, Ross JS, et al. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 1988;166:193–9. 10.1148/radiology.166.1.3336678 [DOI] [PubMed] [Google Scholar]
- 22.Modic MT, Masaryk TJ, Ross JS, et al. Imaging of degenerative disk disease. Radiology 1988;168:177–86. 10.1148/radiology.168.1.3289089 [DOI] [PubMed] [Google Scholar]
- 23.Moore RJ. The vertebral endplate: disc degeneration, disc regeneration. Eur Spine J 2006;15(Suppl 3):S333–7. 10.1007/s00586-006-0170-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pfirrmann CW, Resnick D. Schmorl nodes of the thoracic and lumbar spine: radiographic-pathologic study of prevalence, characterization, and correlation with degenerative changes of 1,650 spinal levels in 100 cadavers. Radiology 2001;219:368–74. 10.1148/radiology.219.2.r01ma21368 [DOI] [PubMed] [Google Scholar]
- 25.Creemers MC, Franssen MJ, van’t Hof MA, et al. Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 2005;64:127–9. 10.1136/ard.2004.020503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudwaleit M, Schwarzlose S, Listing J, et al. Is there a place for magnetic resonance imaging (MRI) in predicting a major clinical response (BASDAI 50%) to TNF alpha blockers in ankylosing spondylitis? [abstract]. Arthritis Rheum 2003;50:8211. [Google Scholar]
- 27.Lukas C, Braun J, van der Heijde D, et al. Scoring inflammatory activity of the spine by magnetic resonance imaging in ankylosing spondylitis: a multireader experiment. J Rheumatol 2007;34:862–70. [PubMed] [Google Scholar]
- 28.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- 29.van den Berg R, de Hooge M, van Gaalen F, et al. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the Spondyloarthritis Caught Early (SPACE) cohort: Table 3. Rheumatology 2015;54:1336 10.1093/rheumatology/kev208 [DOI] [PubMed] [Google Scholar]