Abstract
Purpose
Verinurad (RDEA3170) is a selective uric acid reabsorption inhibitor in clinical development for treatment of gout and asymptomatic hyperuricemia. This study evaluated verinurad pharmacokinetics, pharmacodynamics, and tolerability in healthy Japanese and non-Asian adult male subjects.
Methods
This was a Phase I, randomized, single-blind, placebo-controlled study. Panels of 8 Japanese subjects were randomized to receive oral verinurad (2.5–15 mg) or placebo administered as a single dose in a fasted and fed state and as once-daily doses for 7 days in a fed state. Eight non-Asian subjects received verinurad 10 mg as a single dose (fasted and fed) and multiple doses in the fed state. Serial plasma/serum and urine samples were assayed for verinurad and uric acid. Safety was assessed by adverse events and laboratory data.
Results
Of 48 randomized subjects, 46 (Japanese, 39; non-Asian, 7) completed the study. Following single or multiple doses in Japanese subjects, maximum plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC) increased in a near dose-proportional manner. Time to Cmax (Tmax) was ~1.25–2.0 hours with fasting. A moderate-fat meal delayed Tmax (range 3.0–5.0 hours) and had a variable effect on AUC (0%–97% increase) and Cmax (0%–26% increase) across the dose groups. Following multiple verinurad 10 mg doses, Cmax and AUC were 38% and 23% higher, respectively, in Japanese vs non-Asian subjects, largely due to body weight differences. Mean reduction of serum urate following multiple verinurad 10 mg doses was 46% and 44% after 24 hours in Japanese and non-Asian subjects, respectively. Verinurad was well tolerated at all doses.
Conclusion
Verinurad monotherapy lowered serum urate and was well tolerated in both healthy Japanese and non-Asian males, while small differences in plasma pharmacokinetics were observed. These data support further evaluation of once-daily verinurad as a treatment for gout and asymptomatic hyperuricemia.
Keywords: pharmacokinetics, pharmacodynamics, selective uric acid reabsorption inhibitor, serum urate, urinary uric acid
Introduction
Gout is an inflammatory arthritis induced by deposition of monosodium urate monohydrate crystals in joints, tissues, and organs.1,2 It is associated with hyperuricemia, which is defined as a serum urate (sUA) level of ≥6.8 mg/dL, the limit of urate solubility at physiologic body temperature and pH.3 The primary objective of chronic therapy for gout is to reduce sUA levels to <6.0 or <5.0 mg/dL in severe disease, which over time supports crystal dissolution and alleviates gout symptoms.4–6
In 85% of Japanese patients with hyperuricemia, elevated sUA results from both inefficient renal excretion and overproduction of uric acid.7,8 The xanthine oxidase inhibitors (XOIs), allopurinol and febuxostat, which lower sUA via inhibition of urate production, are recommended as first-line therapy for gout.9,10 However, the majority of patients with gout fail to achieve the target goal of sUA <6 mg/dL using an XOI alone.11,12 Treatment guidelines then recommend switching to another treatment or combining treatments with different mechanisms, such as an XOI with a uricosuric.5,6,13
Lesinurad, a selective uric acid reabsorption inhibitor, was recently approved in the USA and Europe in combination with an XOI for the treatment of hyperuricemia associated with gout in patients who fail to achieve target sUA levels on an XOI alone.14 Lesinurad inhibits the uric acid transporter URAT1, which is responsible for most reabsorption of urate from the renal tubule.15,16 By inhibiting URAT1, lesinurad increases the excretion of uric acid and lowers sUA.
Verinurad (RDEA3170) is a potent and selective URAT 1 inhibitor in clinical development for the treatment of gout and asymptomatic hyperuricemia. Although verinurad has the same mechanism of action as lesinurad, it is structurally distinct from lesinurad.17 Previously, the pharmacokinetics (PK), pharmacodynamics (PD), and tolerability of verinurad monotherapy following single and multiple ascending doses was assessed in healthy, non-Asian, adult male subjects using an immediate-release formulation.18 It is important that any differences in response associated with ethnic origin be identified early to guide future studies. Therefore, the current study evaluated the PK, PD, and tolerability of verinurad monotherapy in healthy Japanese subjects and compared the results with non-Asian adult male subjects using a modified-release formulation.
Methods
The study (RDEA3170-104; NCT01872832) was conducted at the PAREXEL Early Phase Clinical Research Unit (Glendale, CA, USA) in accordance with Good Clinical Practice and the Declaration of Helsinki and with institutional review board (Aspire Institutional Review Board, Santee, CA, USA) approval. Written informed consent was obtained from all subjects before starting the study.
Subjects
Healthy adult Japanese or non-Asian male subjects between 18 and 55 years of age, with body weight ≥50 kg, body mass index ≥18 and ≤30 kg/m2, and a screening sUA >4.5 mg/dL were eligible. Japanese subjects had to be born in Japan of Japanese parents and grandparents. All subjects were to be in good health with laboratory parameters within normal limits, no clinically significant diseases, no relevant abnormalities in vital signs, and no history of cardiac abnormalities. Subjects who had a history or suspicion of kidney stones were excluded.
Study design
This was a Phase I, randomized, single-blind, placebo-controlled study. Panels of 8 Japanese male subjects were randomized in a 3:1 ratio to receive verinurad (n=6) or placebo (n=2) administered as a single dose in a fasted (day 1) or fed (day 6) state and as multiple once-daily doses in a fed state for 7 days (days 6–12). Verinurad dosages were 2.5, 5, 10, and 15 mg, administered as modified-release formulation 2.5 mg tablets. A panel of 8 non-Asian male subjects received single and multiple doses of verinurad 10 mg or placebo as described for the Japanese subjects.
Subjects were confined to the research facility from 2 days before dose administration until 72 hours after the last dose. Prior to dosing on day 1 and day 6, subjects fasted overnight for at least 10 hours. Study medication was administered with ~240 mL of water. No breakfast was eaten on day 1, and no food was allowed for 4 hours after the administration of study medication. Water was allowed as desired except for 1 hour before and 1 hour after the administration of study medication. On days 6 to 12, study medication was administered in the fed state after a moderate-fat (~30%–40% of calories) and calorie (~642–800 calories) breakfast.
Serial PK plasma samples were collected on days 1 and 12 up to 72 hours postdose and on day 6 up to 24 hours postdose. Additional PK samples were collected predose on days 8–11. Serial PD serum samples were collected predose on days 1, 6, and 12 and up to 72 hours postdose on days 1 and 12 and up to 24 hours postdose on day 6. Additional PD samples were collected predose on days 8–11. Urine samples (total catch) for PK/PD were collected at 6- or 12-hour intervals prior to dosing and up to 72 hours postdose on days 1 and 12 and up to 24 hours postdose on day 6. All plasma and urine PK samples were stored at −70°C until analyzed for verinurad concentrations by Ardea Biosciences, Inc. (San Diego, CA, USA) as previously described.18 The lower limit of quantification for verinurad in plasma and urine was 0.100 and 10.0 ng/mL, respectively, and showed % Theoretical (% CV) during study analyses of 92.7%–95.7% (3.6%–6.0%) and 104%–106% (2.8%–3.4%), respectively. Serum PD samples were analyzed for sUA and creatinine and urine samples for uric acid and creatinine by Covance Central Laboratory Services (Indianapolis, IN, USA).
PK/PD assessments
PK and PD were assessed as described previously.18 PK parameters were derived using WinNonlin Professional, version 5.2 (Pharsight Corporation, Mountain View, CA, USA) and noncompartmental analysis. Plasma PK parameters included maximum observed plasma concentration (Cmax), time to Cmax (Tmax), area under the plasma concentration–time curve (AUC) from 0–24 hours (AUC0–24) or from zero to infinity (AUC0–∞), and terminal half-life (t1/2). Urinary PK parameters included fraction of the verinurad dose (% dose) excreted unchanged in urine from time 0–24 hours post-dose (fe0–24) and renal clearance of verinurad (CLR0–24). The latter was calculated as the amount of verinurad excreted unchanged in urine from time 0–24 hours postdose divided by the verinurad plasma AUC0–24.
PD parameters were calculated using SAS® software (SAS Institute Inc., Cary, NC, USA). The maximum percent change from baseline and the time of the maximum percent change in sUA were obtained directly from the concentration versus time data. Renal clearance of uric acid (CLUR0–24) was obtained by dividing the amount of uric acid in urine (AeUR) by the plasma urate AUC from time 0–24 hours postdose. Fractional excretion of uric acid (FEUA0–24) was calculated as CLUR0–24 divided by creatinine clearance × 100.
Safety assessment
Subjects were assessed daily for adverse events (AEs), which were documented, along with any remedial action, in the subject’s clinical report form. The investigator assessed the severity of an AE according to Rheumatology Common Toxicity Criteria version 2.0. Blood and urine samples were collected for clinical laboratory evaluations at screening, admission, and days 2, 5, 7, 13, and at follow-up. Supine blood pressure, pulse rate, respiratory rate, and oral body temperature were also measured at specific times during the study. A physical examination and standard digital 12-lead electrocardiograms were recorded at screening and follow-up.
Statistical analyses
The PK and PD populations consisted of all subjects who received verinurad and had evaluable PK data and PD data, respectively. The safety population consisted of all subjects who received verinurad.
To assess the effect of food on the PK of verinurad, the natural log-transformed PK parameters for day 6 (fed) were compared against those under fasted conditions (day 1) following a single dose. An estimate of the geometric mean ratios (GMR) of the PK parameters with the corresponding 90% CIs was generated.
Dose proportionality assessments were performed on Cmax and AUC0–24 following multiple doses of 2.5, 5, 10, and 15 mg using a power model:
where the slope b measures the proportionality between dose and AUC or Cmax. When slope b is close to unity (1.0) with 95% CI within (0.8, 1.25), the relationship between dose and the PK parameter is concluded to be dose proportional for the dose range studied.
Results
Subject disposition and characteristics
A total of 40 subjects (32 Japanese, 8 non-Asian) were randomized, and 46 (31 Japanese, 7 non-Asian) completed the study. One Japanese subject was withdrawn from the study due to an AE of urticaria. One non-Asian subject was withdrawn following withdrawal of consent for personal reasons. Treatment groups within the Japanese cohort were generally well balanced. Demographic characteristics of the enrolled subjects are shown in Table 1. Mean body weight and body mass index were ~14% and 7% lower, respectively, in Japanese than non-Asian subjects.
Table 1.
Demographics
| Japanese subjects (n=32) | Non-Asian subjects (n=8) | |
|---|---|---|
| Age, years, mean (SD) | 35 (10.7) | 35 (8.5) |
| Age range, years | 21–53 | 21–45 |
| Body weight, kg, mean (SD) | 69.5 (8.1) | 80.6 (12.0) |
| Body mass index, kg/m2, mean (SD) | 23.5 (2.1) | 25.2 (3.1) |
| Race, n (%) | ||
| Black | 0 | 3 (37.5) |
| White | 0 | 5 (62.5) |
| Asian | 32 (100) | 0 |
Verinurad PK in Japanese subjects
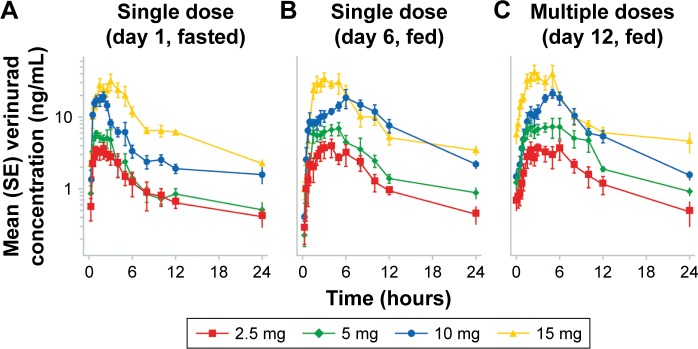
The verinurad plasma concentration−time profiles in Japanese subjects following single ascending doses in the fasted and fed states and multiple ascending doses in the fed state are shown in Figure 1. Following single oral doses of verinurad after fasting, verinurad was readily absorbed with a median Tmax ranging from 1.25 to 2.0 hours postdose, after which plasma concentrations of verinurad declined with average half-life values ranging between 8 and 12 hours (Table 2). A moderate-fat meal delayed Tmax (range, 3.0–5.0 hours post-dose) and had a variable effect on plasma verinurad AUC0–∞ (0%–97% increase) and plasma Cmax (0%–26% increase) across the dose groups. Following once-daily multiple oral doses of verinurad, the accumulation ratio ranged from 1.01 to 1.24 for Cmax and 0.886 to 1.34 for AUC0–24 (Table 2).
Figure 1.
Mean (SE) verinurad plasma concentration–time profiles in Japanese subjects following single ascending doses in fasted (A) and fed states (B) and following multiple ascending doses in the fed state (C).
Abbreviation: SE, standard error.
Table 2.
Summary of verinurad PK following a single dose in the fasted (day 1) or fed state (day 6) and multiple doses in the fed state (day 12) – Japanese subjects
| Dose | Day | Fed | Tmax, ha | Cmax, ng/mL | AUC0–24, ng·h/mL | AUC0–∞, ng·h/mL | t1/2, h | fe0–24, % | CLR0–24, mL/min |
|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 1 | Fasted | 1.25 (0.50–3.00) | 4.89 (3.49–6.84) | 23.2 (14.9–36.1) | 29.9 (18.2–49.0) | 10.4 (6.91–15.5) | 0.896 (0.620–1.29) | 16.1 (9.41–27.6) |
| 6 | Fed | 3.50 (2.50–8.00) | 5.66 (4.06–7.91) | 33.8 (23.4–49.0) | 39.2 (25.7–59.6) | 8.65 (6.55–11.7) | 1.27 (0.659–2.47) | 15.7 (9.54–25.8) | |
| GMRb | 116 (105–128) | 146 (101–211) | 131 (82.9–208) | ||||||
| 12 | Fed | 4.00 (2.00–6.00) | 5.74 (4.31–7.64) | 36.0 (24.0–53.9) | NA | 14.8 (7.84–27.8) | 1.89 (1.38–2.59) | 21.8 (13.4–35.5) | |
| Racc | 1.10 (0.714–1.44) | 1.06 (0.672–1.68) | |||||||
| 5 | 1 | Fasted | 1.25 (0.50–5.00) | 7.30 (5.05–10.6) | 32.6 (20.5–50.2) | 42.3 (24.9–72.1) | 12.2 (7.65–19.6) | 0.640 (0.279–1.47) | 16.4 (11.3–23.7) |
| 6 | Fed | 4.00 (1.50–5.00) | 9.21 (6.94–12.2) | 60.3 (45.7–79.6) | 73.2 (60.3–88.9) | 7.98 (5.19–12.3) | 1.09 (0.730–1.64) | 15.1 (11.8–19.3) | |
| GMRb | 126 (89.1–179) | 185 (121–283) | 173 (108–278) | ||||||
| 12 | Fed | 4.00 (2.00–10.0) | 11.5 (7.53–17.5) | 80.9 (61.9–106) | NA | 7.85 (5.49–11.2) | 1.65 (0.959–2.84) | 17.0 (11.8–24.5) | |
| Racc | 1.24 (1.01–1.53) | 1.34 (1.01–1.53) | |||||||
| 10 | 1 | Fasted | 1.75 (1.00–2.00) | 21.9 (18.2–26.4) | 93.4 (74.2–118) | 119 (89.8–157) | 8.79 (6.53–11.8) | 0.733 (0.558–0.964) | 13.1 (10.1–16.9) |
| 6 | Fed | 5.00 (1.00–10.0) | 21.9 (15.5–30.8) | 195 (149–256) | 215 (180–255) | 6.25 (3.73–10.5) | 1.63 (1.24–2.16) | 14.0 (11.5–25.2) | |
| GMRb | 99.7 (85.1–117) | 209 (172–253) | 197 (181–215) | ||||||
| 12 | Fed | 4.50 (2.00–6.00) | 24.9 (20.1–30.9) | 173 (134–222) | NA | 6.93 (5.34–9.00) | 1.77 (1.38–2.25) | 17.0 (9.43–25.4) | |
| Racc | 1.14 (0.774–1.64) | 0.886 (0.679–1.16) | |||||||
| 15 | 1 | Fasted | 2.00 (0.75–3.00) | 32.2 (19.5–53.0) | 212 (154–290) | 252 (174–365) | 9.57 (3.90–23.5) | 0.788 (0.424–1.45) | 9.30 (5.46–15.9) |
| 6 | Fed | 3.00 (1.5–5.00) | 38.2 (24.6–59.4) | 239 (137–416) | 272 (159–464) | 6.91 (5.85–8.16) | 1.14 (0.907–1.45) | 12.0 (6.06–23.7) | |
| GMRb | 112 (90.6–139) | 107 (89.1–128) | 100 (83.1–120) | ||||||
| 12 | Fed | 2.50 (2.50–5.00) | 41.5 (22.6–76.2) | 286 (177–460) | NA | 9.79 (4.98–19.3) | 1.83 (1.23–2.73) | 16.0 (8.22–31.3) | |
| Racc | 1.09 (0.823–1.44) | 1.20 (0.979–1.46) |
Notes:
Median (range);
(%) (day 6/day 1);
Rac, accumulation ratio (day 12/day 6). Data are geometric mean (95% CI).
Abbreviations: Cmax, maximum plasma concentration; Tmax, time to Cmax; AUC0–24, area under verinurad concentration–time curve from 0 to 24 hours; AUC0–∞, area under verinurad concentration–time curve from zero to infinity; t1/2, terminal half-life; fe0–24, fraction of dose excreted in urine from 0 to 24 hours postdose; CLR0–24, renal clearance from 0 to 24 hours postdose; GMR, geometric mean ratio; NA, not applicable; PK, pharmacokinetics.
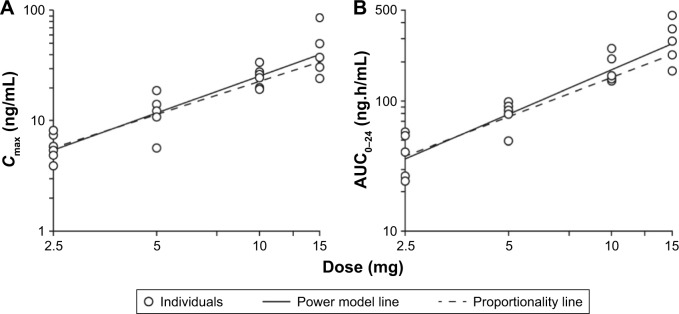
Figure 2 shows the dose proportionality for verinurad Cmax and AUC following multiple dosing in the fed state. Both Cmax (proportionality exponent b: 1.10, 95% CI: 0.882–1.31) and AUC0–24 (b: 1.15, 95% CI: 0.950–1.34) generally showed a near dose-proportional increase with no major deviation from dose proportionality. Similar results were observed following single doses of verinurad in the fasted or fed state.
Figure 2.
Dose proportionality of verinurad exposure Cmax (A) and AUC0–24 (B) in Japanese subjects following multiple dosing in the fed state.
Abbreviations: AUC0–24, area under verinurad concentration–time curve from 0 to 24 hours; Cmax, maximum plasma concentration.
Verinurad PK: Japanese versus non-Asian subjects
The verinurad plasma Cmax and AUC0–24 for Japanese and non-Asian subjects following a single 10 mg dose in fasted and fed states and multiple once-daily 10 mg doses in the fed state are shown in Table 3. When the data were normalized to body weight of 70 kg, exposure was similar for Japanese and non-Asian subjects following multiple dosing, with modest differences between the 2 groups following a single dose (Table 3).
Table 3.
Comparison of plasma Cmax and AUC0–24 for Japanese vs non-Asian subjects following single or multiple verinurad 10 mg doses
| Treatment | Parameter | Japanese | Non-Asian | GMR, % (95% CI) | Bodyweight normalized to 70 kg
|
||
|---|---|---|---|---|---|---|---|
| Japanese | Non-Asian | GMR, % (95% CI) | |||||
| Single dose, fasted | Cmax, ng/mL | 21.9 | 11.0 | 199 (167–237) | 20.6 | 13.1 | 157 (132–187) |
| AUC0–24, ng·h/mL | 93.4 | 69.9 | 134 (111–161) | 87.9 | 83.3 | 106 (84.5–132) | |
| Single dose, fed | Cmax, ng/mL | 21.9 | 15.4 | 142 (107–189) | 20.6 | 18.3 | 112 (88.5–143) |
| AUC0–24, ng·h/mL | 195 | 111 | 176 (137–227) | 183 | 132 | 139 (114–170) | |
| Multiple dose, fed | Cmax, ng/mL | 24.9 | 18.0 | 138 (112–171) | 23.4 | 21.5 | 109 (94.4–126) |
| AUC0–24, ng·h/mL | 173 | 141 | 123 (93.0–162) | 163 | 168 | 96.9 (77.4–121) | |
Notes: Data are geometric least square means. GMR, Japanese/non-Asian.
Abbreviations: Cmax, maximum plasma concentration; AUC0–24, area under verinurad concentration–time curve from 0 to 24 hours; GMR, geometric mean ratio; PK, pharmacokinetics.
Renal clearance in Japanese subjects was lower than in non-Asian subjects following a single dose in fasted (GMR [90% CI]: 70.0% [53.7–91.4]) and fed (80.9% [65.0–101]) states, but was similar following multiple dosing (100% [79.7–126]). The fraction of drug excreted in urine was similar for Japanese and non-Asian subjects following a single dose in fasted (GMR [90% CI]: 93.7% [70.9–124]) and fed (143% [107–191]) states and following multiple dosing (123% [98.7–153]).
Verinurad PD
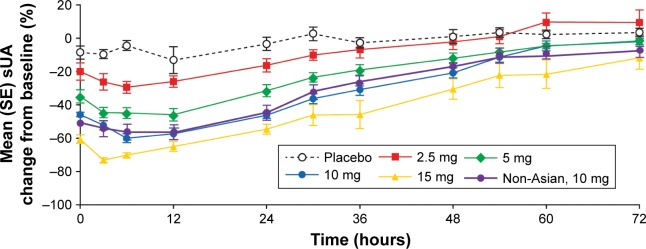
Figure 3 shows the mean (standard error) percentage change from baseline in sUA following multiple once-daily ascending doses of verinurad in Japanese subjects and in non-Asian subjects (10 mg dose) in the fed state. Maximum reduction in sUA was achieved by 12 hours postdose in both Japanese and non-Asian subjects. In Japanese subjects, the least square mean maximum percent change from baseline at 12 hours was −17%, −31%, −50%, −62%, and −74% for placebo, 2.5, 5, 10, and 15 mg verinurad, respectively, while the percent change from baseline at 24 hours was −3%, −16%, −31%, −46%, and −55%, respectively. The 74% maximum reduction in sUA with verinurad 15 mg resulted from a reduction in sUA concentration to <2 mg/dL. In non-Asian subjects following multiple once-daily dosing of verinurad 10 mg, the maximum percent change from baseline was −58% and at 24 hours postdose was −44%. The sUA-lowering effect for a 10 mg dose was generally comparable between Japanese and non-Asian subjects.
Figure 3.
Mean (SE) percent change from baseline in sUA concentration–time profiles following verinurad multiple ascending doses in Japanese subjects and in non-Asian subjects (10 mg dose) in the fed state.
Abbreviations: SE, standard error; sUA, serum urate.
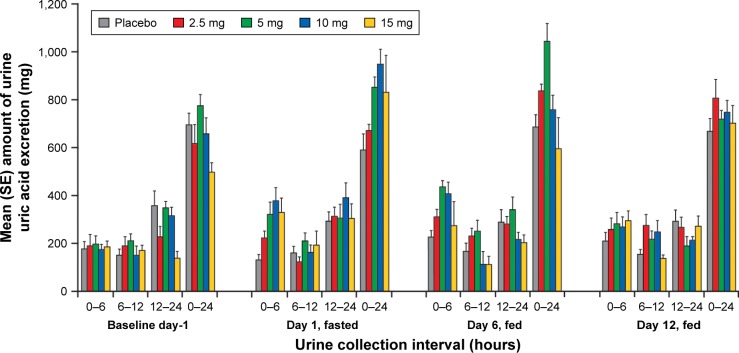
The percent change from baseline in AeUR in Japanese subjects was generally dose dependent after the first dose (day 1, fasted and day 6, fed) for doses ≤10 mg, with the largest percent change occurring in the first 6 hours post-dose (Figure 4). The percent changes for the 0–6 hour interval on day 1 were 18%, 59%, 114%, and 77% for verinurad 2.5, 5, 10, and 15 mg, respectively; on day 6 the changes were 62%, 114%, 131%, and 49% for the same doses, respectively. There was a trend toward reduced excretion at steady-state (day 12) compared with first dose (day 6) when the percent change over the 0–6 hours was −17%, −35%, −33%, and 6%, respectively. The percent change from baseline in AeUR over 0–6 hours with verinurad 10 mg was similar to that of Japanese subjects for non-Asian subjects (day 1, 70%; day 6, 141%; and day 12, -41%). The trend was generally the same for the 0–24 hour interval for both Japanese and non-Asian subjects (data not shown).
Figure 4.
Mean (SE) amount of urine uric acid excretion in Japanese subjects following single (day 1, fasted; day 6, fed) and multiple doses (day 12, fed) of verinurad.
Abbreviation: SE, standard error.
Verinurad increased 24 hour FEUA and CLUR in a generally dose-dependent manner for both single and multiple doses ≤10 mg (Table 4). The increases in both FEUA and CLUR with verinurad 10 mg were similar between Japanese and non-Asian subjects.
Table 4.
FEUA0–24 h and CLUR0–24 in Japanese and non-Asian subjects following single and multiple doses
| Treatment | FEUA0–24, %
|
CLUR0–24, mL/min
|
||||
|---|---|---|---|---|---|---|
| Single dose, fasted | Single dose, fed | Multiple dose, fed | Single dose, fasted | Single dose, fed | Multiple dose, fed | |
| Japanese subjects | ||||||
| Baseline | 6.81 (5.29–8.32) | 6.46 (5.55–7.36) | 6.46 (5.55–7.36) | 8.67 (6.74–10.6) | 7.68 (5.71–9.65) | 7.68 (5.71–9.65) |
| Placebo | 6.09 (4.59–7.59) | 6.75 (5.49–8.00) | 6.24 (5.33–7.15) | 6.70 (4.86–8.53) | 8.23 (6.76–9.70) | 8.92 (6.91–10.9) |
| Verinurad 2.5 mg | 7.72 (6.01–9.43) | 7.75 (6.80–8.70) | 7.57 (6.74–8.41) | 7.82 (6.90–8.74) | 11.4 (9.74–13.0) | 12.2 (10.3–14.2) |
| Verinurad 5 mg | 9.05 (7.20–10.9) | 11.2 (7.42–14.9) | 14.5 (9.93–19.1) | 10.9 (8.88–12.9) | 16.0 (11.7–20.3) | 13.7 (9.35–18.1) |
| Verinurad 10 mg | 10.8 (7.87–13.6) | 20.5 (11.3–29.6) | 13.7 (10.8–16.6) | 14.3 (10.7–17.9) | 16.0 (9.88–22.1) | 19.7 (16.7–22.7) |
| Verinurad 15 mg | 14.5 (7.08–22.0) | 9.61 (2.96–16.3) | 16.0 (10.7–21.2) | 15.0 (6.40–23.6) | 12.1 (4.04–20.2) | 23.9 (14.9–32.9) |
| Non-Asian subjects | ||||||
| Verinurad 10 mg | 10.4 (4.84–16.0) | 11.7 (6.44–17.0) | 13.2 (8.45–17.9) | 15.3 (7.35–23.3) | 18.6 (8.95–28.2) | 20.6 (13.7–27.4) |
Note: Data are mean (95% CI).
Abbreviations: FEUA0–24, fractional excretion of uric acid from time 0 to 24 hours; CLUR0–24, renal clearance of uric acid from time 0 to 24 hours.
Safety
Verinurad was well tolerated by healthy male Japanese and non-Asian subjects at single and multiple once-daily doses ranging from 2.5 to 15 mg. The overall incidence of treatment-emergent AEs (TEAEs) was low. Five TEAEs were reported by Japanese subjects across all verinurad dose levels, with 0–2 TEAEs reported for each dose level, including the pooled placebo group (Table 5). There was no difference in the incidence of TEAEs between Japanese (n=2) and non-Asian subjects (n=2) at the 10 mg dose level. No individual TEAE was reported more than once in any group. None occurred in more than 1 subject, except for rash that occurred in 1 subject who received placebo and 1 who received verinurad 10 mg. TEAEs were mostly mild in severity, except for 2 TEAEs of moderate severity. One Japanese subject was withdrawn from the study due to a moderately severe TEAE of urticaria, which commenced 2 days after the subject received a single dose of verinurad 15 mg and resolved after 11 days; the AE was considered to be possibly related to verinurad by the investigator. A non-Asian subject reported a moderately severe muscle strain on day 13 following daily dosing with verinurad 10 mg, which was considered unlikely to be related to verinurad by the investigator; the subject completed the study. No deaths, other serious AEs, or other significant AEs of interest were reported during the study.
Table 5.
Summary of treatment-emergent AEs in Japanese and non-Asian subjects
| Japanese subjects
|
Non-Asian subjects
|
|||
|---|---|---|---|---|
| Pooled placebo (n=8) | Pooled verinurad (n=24) | Pooled placebo (n=2) | Pooled verinurad (n=6) | |
| Any AE, n (%) [events] | 2 (25.0) [7] | 5 (20.8) [5] | 0 | 2 (33.3) [2] |
| Mild severity | 2 (25.0) [7] | 4 (16.7) [4] | 0 | 1 (16.7) [1] |
| Moderate severity | 0 | 1 (4.2) [1] | 0 | 1 (16.7) [1] |
| Severe severity/life-threatening | 0 | 0 | 0 | 0 |
| AE possibly related to study drug, n (%) [events] | 1 (12.5) [1] | 3 (12.5) [3] | 0 | 1 (16.7) [1] |
| Mild severity | 1 (12.5) [1] | 2 (8.3) [2] | 0 | 1 (16.7) [1] |
| Moderate severity | 0 | 1 (4.2) [1] | 0 | 0 |
| Severe severity/life-threatening | 0 | 0 | 0 | 0 |
| Subjects withdrawn due to AEs, n (%) [events] | 0 | 1 (4.2) [1] | 0 | 0 |
| SAEs, n (%) [events] | 0 | 0 | 0 | 0 |
Abbreviations: AEs, adverse events; SAEs, serious adverse events.
One Japanese subject had a serum creatinine elevation 1.5× baseline 3 days after receiving a single 2.5 mg dose of verinurad that resolved by day 14.
No safety concerns were identified for any of the serum chemistry, hematology, coagulation, or urinalysis parameters assessed. No clinically significant findings were found for vital signs, 12-lead electrocardiograms, and physical examinations performed during the study.
Discussion
PK and PD characteristics of verinurad were carefully studied in both Japanese and non-Asian healthy subjects. In Japanese subjects, verinurad exposure was found to increase in a near dose-proportional manner following single doses in fed and fasted states as well as following multiple once-daily doses in the fed state. A moderate-fat meal delayed the absorption of verinurad by several hours and had a variable effect on verinurad exposure ranging from no effect to an approximate doubling of AUC. This result differs from that reported previously where a moderate-fat meal reduced verinurad exposure.18 In that study, an immediate-release formulation of verinurad was used, rather than the modified-release formulation used here. Following once-daily multiple doses, there was modest accumulation of verinurad. Both Cmax and AUC were higher in Japanese versus non-Asian subjects, but when corrected for body weight, the difference was minimal following once-daily 10 mg doses for 10 days.
Consistent with its mechanism of action as a selective reabsorption inhibitor of URAT1, verinurad increased Aeur in a generally dose-dependent manner. The increase in Aeur led to reductions in sUA that were associated with verinurad dose. The increase in Aeur and reduction in sUA were generally similar in healthy Japanese and non-Asian males, while small differences in plasma PK were observed.
The increased urinary excretion of uric acid by verinurad may have the potential to induce uric acid microcrystallization in the renal tubules, which could increase the risk for renal-related AEs. In Phase 1 clinical trials with verinurad combined with an XOI (which reduces uric acid production), urinary excretion of uric acid was reduced,19,20 which may reduce risk of microcrystallization. Reduced urinary uric acid excretion with the addition of an XOI to a URAT1 inhibitor was also seen with lesinurad, where low rates of kidney stones were observed with lesinurad + XOI treatment in Phase 3 clinical trials.21–23 Verinurad appeared to be well tolerated by all subjects when given single doses in the fasted or fed state or multiple daily doses in the fed state. Verinurad had no apparent effect on the safety and laboratory parameters measured.
Possible limitations of this study were the exclusive inclusion of healthy male subjects, rather than male and female patients with hyperuricemia associated with gout. However, it is standard to conduct these studies in healthy subjects, most often males with no risk of pregnancy, to provide information for future Phase II/III studies to be conducted in the target population. Although there were very specific criteria for defining Japanese subjects, environment could have affected the results because the study was not conducted in Japan. In addition, although diets for this study were the same for Japanese and non-Asian subjects, the diet may differ from a traditional Japanese diet.
In summary, single and multiple doses of verinurad were well tolerated and yielded dose-proportional increases in verinurad exposure. Sustained reductions in sUA were achieved in in both Japanese and non-Asian healthy volunteers due to increased excretion of uric acid. Verinurad had similar PD properties in healthy Japanese and non-Asian males, despite small differences in plasma PK. These data support further evaluation of once-daily verinurad for the management of hyperuricemia in patients with gout in Japan and elsewhere.
Acknowledgments
Funding was provided by Ardea Biosciences/AstraZeneca. Editorial support was provided by Tom Claus, PhD, of PAREXEL and funded by AstraZeneca. Ardea Biosciences, Inc. is a member of the AstraZeneca group. This paper was presented at the 2016 ACR/ARHP Annual Meeting, November 11–16, 2016, Washington, DC as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Arthritis Rheumatol. 2016;68(suppl 10): http://acrabstracts.org/abstract/pharmacokinetics-pharmacodynamics-and-tolerability-of-verinurad-a-selective-uric-acid-reabsorption-inhibitor-in-healthy-japanese-male-subjects/.
Footnotes
Disclosure
Jesse Hall, Zancong Shen, Caroline Lee, Sha Liu, Shakti Valdez, and Jeffrey N Miner are former employees of Ardea Biosciences, Inc. Mike Gillen is an employee of AstraZeneca Pharmaceuticals. The authors report no other conflicts of interest in this work.
References
- 1.Perez-Ruiz F, Dalbeth N, Bardin T. A review of uric acid, crystal deposition disease, and gout. Adv Ther. 2015;32(1):31–41. doi: 10.1007/s12325-014-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rees F, Hui M, Doherty M. Optimizing current treatment of gout. Nat Rev Rheumatol. 2014;10(5):271–283. doi: 10.1038/nrrheum.2014.32. [DOI] [PubMed] [Google Scholar]
- 3.Loeb JN. The influence of temperature on the solubility of monosodium urate. Arthritis Rheum. 1972;15(2):189–192. doi: 10.1002/art.1780150209. [DOI] [PubMed] [Google Scholar]
- 4.Doherty M, Jansen TL, Nuki G, et al. Gout: why is this curable disease so seldom cured? Ann Rheum Dis. 2012;71(11):1765–1770. doi: 10.1136/annrheumdis-2012-201687. [DOI] [PubMed] [Google Scholar]
- 5.Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken) 2012;64(10):1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2016;76(1):29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 7.Japanese Society of Gout and Nucleic Acid Metabolism Diagnosis of hyperuricemia and gout . Guideline for the Management of Hyperuricemia and Gout. 2nd ed. Osaka: Medical Review; 2010. pp. 60–72. [Google Scholar]
- 8.Nakamura T. Guideline for the Managemnt of Hyperuricemia and Gout. Osaka: Medical Review; 2003. [Google Scholar]
- 9.Neogi T. Clinical practice. Gout. N Engl J Med. 2011;364(5):443–452. doi: 10.1056/NEJMcp1001124. [DOI] [PubMed] [Google Scholar]
- 10.Terkeltaub R, Edwards NL. Gout: Diagnosis and Management of Gouty Arthritis and Hyperuricemia. 3rd ed. West Islip, NY: Professional Communications, Inc.; 2013. [Google Scholar]
- 11.Becker MA, Fitz-Patrick D, Choi HK, et al. An open-label, 6-month study of allopurinol safety in gout: the LASSO study. Semin Arthritis Rheum. 2015;45(2):174–183. doi: 10.1016/j.semarthrit.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Singh JA, Akhras KS, Shiozawa A. Comparative effectiveness of urate lowering with febuxostat versus allopurinol in gout: analyses from large U.S. managed care cohort. Arthritis Res Ther. 2015;17:120. doi: 10.1186/s13075-015-0624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanaka H, Japanese Society of Gout and Nucleic Acid Metabolism Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1018–1029. doi: 10.1080/15257770.2011.596496. [DOI] [PubMed] [Google Scholar]
- 14.Zurampic (lesinurad) AstraZeneca. [Accessed January 6, 2017]. Available from: http://www.azpicentral.com/zurampic/zurampic.pdf#page=1.
- 15.Reginato AM, Mount DB, Yang I, Choi HK. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2012;8(10):610–621. doi: 10.1038/nrrheum.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120(6):1791–1799. doi: 10.1172/JCI42344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardet JL, Miner JN. Urate crystal deposition disease and gout – new therapies for an old problem. Annu Rep Med Chem. 2014;49:151–164. [Google Scholar]
- 18.Shen Z, Gillen M, Miner JN, Bucci G, Wilson DM, Hall JW. Pharmacokinetics, pharmacodynamics, and tolerability of verinurad, a selective uric acid reabsorption inhibitor, in healthy adult male subjects. Drug Des Devel Ther. 2017;11:2077–2086. doi: 10.2147/DDDT.S140658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall J, Gillen M, Yang X, Shen Z. Pharmacokinetics, pharmacodynamics, and tolerability of concomitant administration of verinurad and febuxostat in healthy male volunteers. Clin Pharmacol Drug Dev. 2018 Apr 24; doi: 10.1002/cpdd.463. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kankam M, Hall J, Gillen M, et al. Pharmacokinetics, pharmacodynamics, and tolerability of concomitant multiple dose administration of verinurad (RDEA3170) and allopurinol in adult male subjects with gout. J Clin Pharmacol. 2018 May 7; doi: 10.1002/jcph.1119. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saag KG, Fitz-Patrick D, Kopicko J, et al. Lesinurad combined with allopurinol: a randomized, double-blind, placebo-controlled study in gout patients with an inadequate response to standard-of-care allopurinol (a US-based study) Arthritis Rheumatol. 2017;69(1):203–212. doi: 10.1002/art.39840. [DOI] [PubMed] [Google Scholar]
- 22.Bardin T, Keenan RT, Khanna PP, et al. Lesinurad in combination with allopurinol: a randomised, double-blind, placebo-controlled study in patients with gout with inadequate response to standard of care (the multinational CLEAR 2 study) Ann Rheum Dis. 2017;76(5):811–820. doi: 10.1136/annrheumdis-2016-209213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalbeth N, Jones G, Terkeltaub R, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous gout: a phase III clinical trial. Arthritis Rheumatol. 2017;69(9):1903–1913. doi: 10.1002/art.40159. [DOI] [PMC free article] [PubMed] [Google Scholar]