Abstract
Studies have yet to include minimally symptomatic Ebola virus (EBOV) infections and unrecognized Ebola virus disease (EVD) in Ebola-related transmission chains and epidemiologic risk estimates. We conducted a cross-sectional, sero-epidemiological survey from October 2015 to January 2016 among 221 individuals living in quarantined households from November 2014 to February 2015 during the Ebola outbreak in the village of Sukudu, Sierra Leone. Of 48 EBOV-infected persons, 25% (95% confidence interval [CI], 14%–40%) had minimally symptomatic EBOV infections and 4% (95% CI, 1%–14%) were unrecognized EVD cases. The pattern of minimally symptomatic EBOV infections in the transmission chain was nonrandom (P < .001, permutation test). Not having lived in the same house as an EVD case was significantly associated with minimally symptomatic infection. This is the first study to investigate a chain of EBOV transmission inclusive of minimally symptomatic EBOV infections and unrecognized EVD. Our findings provide new insights into Ebola transmission dynamics and quarantine practices.
Keywords: Ebola virus, Ebola virus infection, public health, epidemiology, transmission chain, Africa, Sierra Leone
We reconstructed a chain of Ebola virus (EBOV) transmission, inclusive of minimally symptomatic infection and unrecognized Ebola virus disease (EVD) cases. Minimally symptomatic EBOV infections did not transmit EBOV, were likely to live in EVD-unaffected households, and were non-randomly located in the transmission chain.
To date, there have been 35 confirmed outbreaks of Ebola with nearly 13000 reported deaths [1, 2]. Prior studies have shown that the clinical manifestations of Ebola virus (EBOV) infection range from asymptomatic infection and mild illness to severe disease and death [3–5]. By the end of the 2013–2016 Ebola outbreak in West Africa, there were 28646 probable, confirmed, and suspected cases of Ebola virus disease (EVD) [6]. However, the World Health Organization acknowledges that these are likely underestimates [7].
The number of individuals with minimally symptomatic EBOV infection and unrecognized EVD cases who did not present to an Ebola Treatment Unit in the West African outbreak is not known [8]. A systematic review and prior Ebola serosurveys suggest that minimally symptomatic EBOV infections and unrecognized EVD cases may account for approximately 15%–40% of EBOV infections [4, 9, 10]. Moreover, the use of serosurveys to ascertain the burden of unrecognized EVD cases has been quite limited. The burden of unrecognized survivors may be quite significant [11] because case identification during the outbreak relied on overburdened and under-resourced community- and facility-based surveillance systems [12–14].
Village-level serosurveys can identify Ebola-seropositive individuals who had both minimally symptomatic and symptomatic EBOV infections but who were not idenitified by public health surveillance systems. These surveys can inform the construction of transmission networks through a fine-grained depiction of potential person-to-person transmission and epidemiologic risk estimates and, together with risk factor analyses, provide important guidance for outbreak control. Few studies of the West African Ebola epidemic have depicted chains of transmission from aggregated outbreaks [15] or reported risk factors of EBOV transmission [16]. No study has reconstructed EBOV transmission chains inclusive of minimally symptomatic infection and unrecognized EVD.
In the village of Sukudu, a serosurvey demonstrated that 14 (7%) of 187 exposed persons without a previous history of EVD were found to be seropositive, and this subgroup accounted for 14 (29%) of 48 EBOV infections [17]. We sought to reconstruct an EBOV chain of transmission, which included minimally symptomatic EBOV infections along with reported and unrecognized EVD cases, to estimate epidemiologic risk for 1 Ebola-affected village from the 2014–2015 Ebola outbreak in Sierra Leone.
METHODS
Ethics Statement
The study protocol was approved by the Sierra Leone Ethics and Scientific Review Committee and the Stanford University and University of California, San Francisco Institutional Review Boards. Written consent was obtained for all participants, and permission to access the Viral Hemorrhagic Fever (VHF) database was given by the Kono District Ebola Response Center (DERC). The DERC was a government facility that acted as a coordinating body for Ebola-related activities.
Study Setting and Population
We conducted a cross-sectional, sero-epidemiological study in the village of Sukudu, which is located in Kono District, Sierra Leone. Sukudu had a population of approximately 900 persons during its Ebola outbreak. Ebola virus was introduced into Sukudu after 7 family members traveled from Sukudu to Koidu Town (the capital of the district) to care for a sick woman whose husband died from EVD. The sick woman developed EVD symptoms on November 20, 2014, and was diagnosed with EVD on November 28, 2014. These seven family members then traveled back to Sukudu. In Sukudu, 2 of the 7 family members developed EVD, and the first EVD diagnosis was made on December 15, 2014. This chain of transmission in the village ended 6 weeks later (Figure 1).
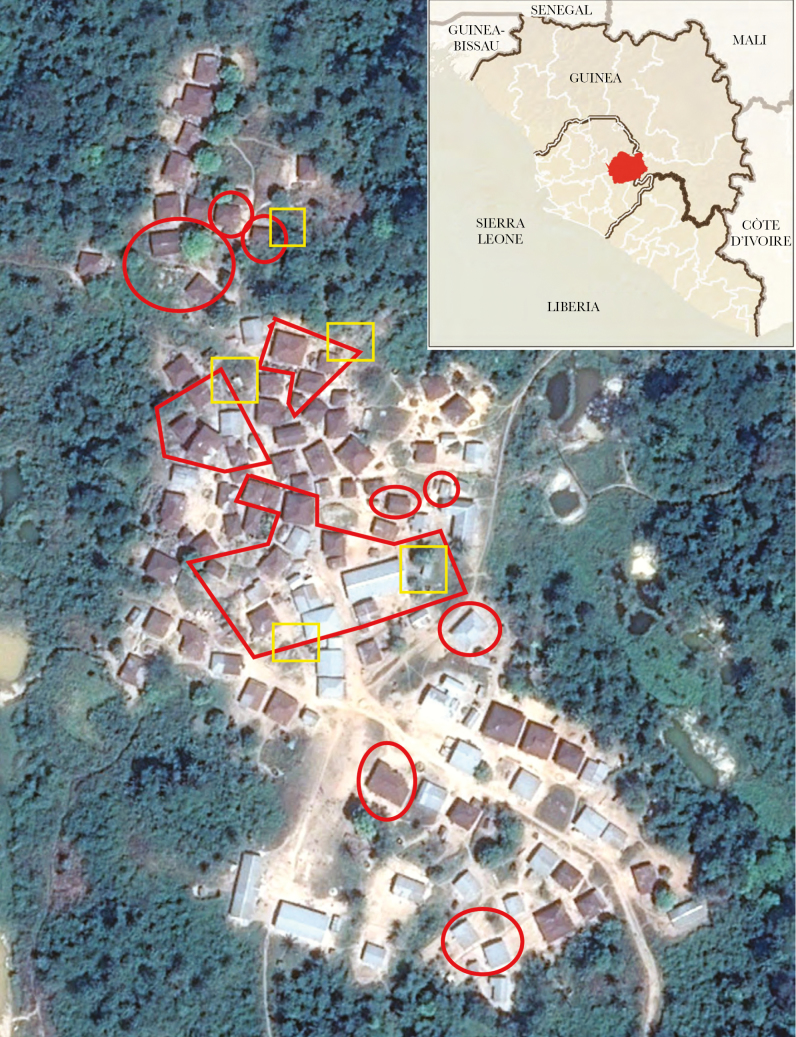
Figure 1.
Satellite image of village. Quarantined areas are bounded in red, public latrines in yellow. Source: Google Earth Pro. (Inset: Sierra Leone, Liberia, and Guinea; Kono District in red. Source: Centers for Disease Control and Prevention.)
We obtained a list of quarantined households and EVD cases from the Kono DERC. Quarantined households included persons whose exposure to EBOV was inferred based on living in a house or sharing a latrine with an EVD case. In Sukudu, there were several shared latrines near the houses of EVD cases. Given the poor condition of these sanitary facilities and the close proximity of the households, the DERC decided to quarantine people who shared a latrine with an EVD case. The DERC did not use any other quarantine practices in Sukudu. Persons living in the quarantined households of Sukudu were enrolled. The study was conducted from October 2015 to January 2016. The immunoassay was validated and then applied in a serosurvey that classified seronegative and seropositive persons based on antiglycoprotein immunoglobulin G serology with a cutoff of 4.7 U/mL. Details of the immunoassay validation and serosurvey can be found elsewhere [17].
Epidemiologic Data Collection
Participants were interviewed to obtain sociodemographic information. In instances where confirmed or probable EVD cases had died, we interviewed household members to obtain further information. We collected data on reasons for quarantine and identified those who were quarantined for having lived in the same house or having shared a latrine with an EVD case. Additional variables were collected from DERC records, including date of symptom onset, date of EVD diagnosis, date of hospitalization, date of death, family relationship, and context of transmission (community, health facility, or burial). The infectious period was defined as the difference between the date of symptom onset and whichever event occurred first: date of hospitalization, date of death, or date of burial.
Outcomes were EBOV-infected persons, EVD cases (reported and unrecognized), and minimally symptomatic EBOV-infected persons (defined below). Uninfected persons were defined as persons quarantined during the outbreak who had a seronegative blood test during the serosurvey. Ebola virus–infected persons were defined as quarantined persons with a seropositive test as well as reported and unrecognized EVD cases. Reported EVD cases were defined as EVD survivors, persons who died with laboratory-confirmed EVD, and persons who died with probable EVD and were epidemiologically linked to a laboratory-confirmed EVD case.
Signs and symptoms consistent with EVD included fever or unexplained bleeding or any 3 of the following: headache, myalgia, rash, vomiting, diarrhea, hiccups, breathing problems, or difficulty swallowing [18]. Unrecognized EVD cases were defined as seropositive persons who self-reported signs or symptoms consistent with EVD but were not identified during the Ebola outbreak. Ebola virus disease cases were defined as reported and unrecognized EVD cases. Minimally symptomatic EBOV-infected persons were defined as seropositive persons who did not self-report signs or symptoms consistent with EVD during the Ebola outbreak in Sukudu [17].
Reconstructing the Chain of Ebola Virus Transmission
Based on interviews we conducted and/or case investigation forms from the VHF database, we assessed how likely each potential epidemiological link was between an EVD case and contact who was EBOV-infected. A link from EVD case A to person B was classified as type 1 if person B reported contact with case A only and then person B developed EVD symptoms during his/her incubation period. An incubation period was defined as the 21-day period after last infectious contact. A link from EVD case A to person B was classified as type 2 if person B reported contact with multiple EVD cases, including EVD case A, and then person B developed EVD symptoms during his/her incubation period. If person B denied contact with any known EVD case, a type 3 link connected person B with EVD case A and/or other EVD cases based on geospatial, temporal, and social network inferences. Type 1 links were considered more likely to be true epidemiological links than type 2, and type 2 more likely than type 3.
We classified transmission into successive generations based on the temporal relationship of EVD cases established in the epidemiological link assessment described above. The first generation was defined as the number of primary EVD cases in the chain, the second generation was defined as the number of EVD cases who acquired EBOV infection from a member of the first generation, and so forth.
Using the data about the types of epidemiological links and generations of transmission, we reconstructed a complete chain of EBOV transmission in a geospatial array. The distance between any 2 persons (nodes) in the transmission chain (graph) is the minimum number of steps (arcs/arrows) needed to move from one individual to another [19]. We used the transmission network to assess randomness of transmission events resulting in minimally symptomatic EBOV infections as follows: We computed the graph theoretic distance [19] between any 2 minimally symptomatic EBOV infections (ie, the number of arcs of the shortest path connecting 2 minimally symptomatic EBOV-infected persons). We also reconstructed a complete chain of transmission of reported EVD cases in a temporal array based on onset of symptoms. We excluded persons with minimally symptomatic EBOV infections and unrecognized EVD cases from the temporal array because persons with minimally symptomatic EBOV infections were assumed to be noninfectious, and unrecognized EVD cases were not asked to recall their date of symptom onset.
Data Analyses
For the epidemiologic analysis, we included the following covariates: age, sex, occupation, educational level, and reason for being quarantined. We excluded persons aged <15 years from the educational-level variable. We calculated the proportion of minimally symptomatic EBOV infections and unrecognized EVD cases from the total number of EBOV infections and the proportion of deaths from EVD cases, and we reported binomial exact confidence intervals (Cis). We analyzed the relationship among the covariates, risk of EBOV infection, and risk of minimally symptomatic EBOV infection. We fit bivariate logistic regression models, and covariates with a P value ≤ .20 were included in cluster-adjusted, multivariate logistic regression models. The mean and standard deviations (SDs) of the infectious period for EVD cases were described, and we used the t test to calculate differences in means. Additional cluster-based analyses were conducted to identify risk factors for reported EVD among uninfected, quarantined persons (Supplementary Data 1). Analyses were performed in STATA/IC 13.1 (STATA Corporation, College Station, TX).
For the transmission chain analysis, we estimated the number of EVD cases in each successive generation of transmission. To estimate the effective reproduction number, R(t), for each generation of transmission, we divided the total number of new EVD cases in each generation by the number of EVD cases in the previous generation. For the first generation, we calculated binomial exact confidence intervals using the number infected out of the number exposed. Poisson exact confidence intervals were calculated for subsequent generations (in the absence of a known denominator). We also used a permutation test to assess whether the mean nearest neighbor distance between minimally symptomatic EBOV infections in the transmission chain was smaller than would be expected if the pattern of minimally symptomatic infections was random (Supplementary Data 2). Finally, we repeated the analysis for each method of assessment of linkages (Supplementary Data 2). These analyses were performed in R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Quarantined Population
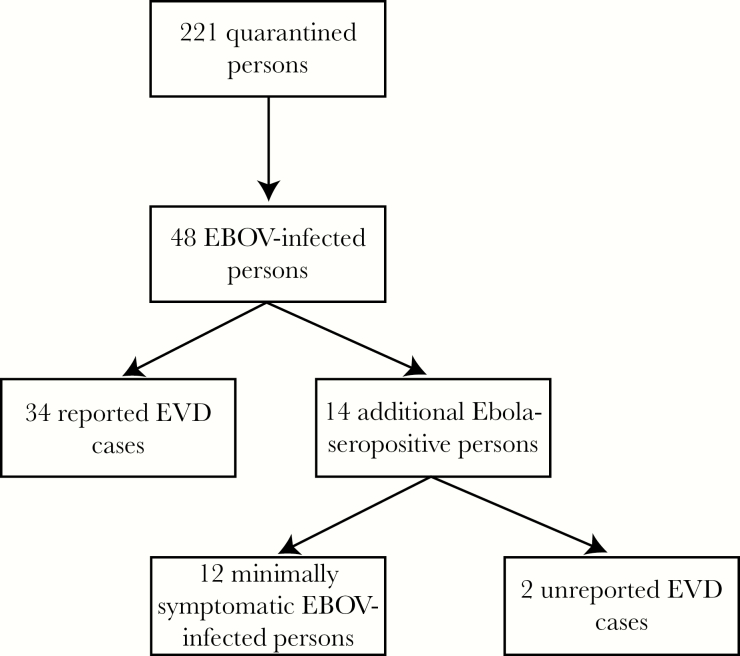
Of 221 quarantined persons, 48 (22%) were categorized as EBOV-infected persons. Among EBOV-infected persons, 34 (71%) were reported EVD cases, 12 (25%; 95% CI, 14%–40%) were minimally symptomatic EBOV infections, and 2 (4%; 95% CI, 1%–14%) were unrecognized EVD cases (Figure 2). In total, there were 36 (75%) EVD cases. Twenty-five houses were quarantined.
Figure 2.
A flow diagram of Ebola virus transmission among the 221 quarantined persons. Abbreviations: EBOV, Ebola virus; EVD, Ebola virus disease.
Characteristics of the quarantined population by EBOV infection status are shown in Table 1. Of quarantined persons, 26 (12%) were aged >45 years, 130 (59%) were male, 18 (8%) had an occupation of housework, and 105 (69%) had an educational level of primary school or less. Among quarantined persons, 125 (57%) were quarantined for having lived in the same house with an EVD case, and 96 (43%) were quarantined for having shared a latrine with an EVD case.
Table 1.
Characteristics of Quarantined Persons, Ebola virus–Infected Persons, Minimally Symptomatic Ebola Virus–Infected Persons, and Ebola Virus Disease Cases
| Quarantined persons (n = 221) |
EBOV-infected persons (n = 48) |
Minimally symptomatic EBOV-infected persons (n = 12) |
EVD cases (n = 36) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | No. | % | No. | % | No. | % | No. | % | |
| Sex | Male | 130 | 58.8 | 29 | 60.4 | 6 | 50.0 | 23 | 63.9 |
| Female | 91 | 41.2 | 19 | 39.6 | 6 | 50.0 | 13 | 36.1 | |
| Age, y | <15 | 70 | 31.7 | 5 | 5.5 | 2 | 16.7 | 3 | 8.3 |
| 15–45 | 125 | 56.6 | 33 | 68.8 | 9 | 75.0 | 24 | 66.7 | |
| >45 | 26 | 11.7 | 10 | 20.8 | 1 | 8.3 | 9 | 25.0 | |
| Occupation | Student | 81 | 36.7 | 10 | 20.1 | 2 | 16.7 | 8 | 22.2 |
| Works outdoors | 122 | 55.2 | 32 | 66.7 | 8 | 66.7 | 24 | 66.7 | |
| Housework | 18 | 8.1 | 6 | 12.5 | 2 | 16.7 | 4 | 11.1 | |
| Highest level of school completeda | Primary or less | 105 | 69.1 | 26 | 60.5 | 5 | 50.0 | 21 | 63.6 |
| Middle school or above | 47 | 30.9 | 17 | 39.5 | 5 | 50.0 | 12 | 36.4 | |
| Having lived in the same house as an EVD case | No | 96 | 43.4 | 8 | 16.7 | 6 | 50.0 | 2 | 5.6 |
| Yes | 125 | 56.6 | 40 | 83.3 | 6 | 50.0 | 34 | 94.4 | |
| Exposure relationship to EVD case | Family | 29 | 80.6 | ||||||
| Infectious periodb | Mean days | 4 | SD ± 2.9 | ||||||
| Transmission context | Community | 22 | 61.1 | ||||||
| Burial | 13 | 36.1 | |||||||
| Healthcare facility | 1 | 2.8 | |||||||
| Admitted to a hospital | Yes | 28 | 77.8 | ||||||
| EVD death | Yes | 28 | 77.8 | ||||||
Abbreviations: EBOV, Ebola virus; EVD, Ebola virus disease; SD, standard deviation.
aEducation level excluded persons aged <15 years in the analysis. There were 152 quarantined persons, 43 EBOV-infected persons, 10 minimally symptomatic EBOV-infected persons, and 33 reported EVD cases.
bData were available for the 34 reported EVD cases only.
Chain of Ebola Virus Transmission
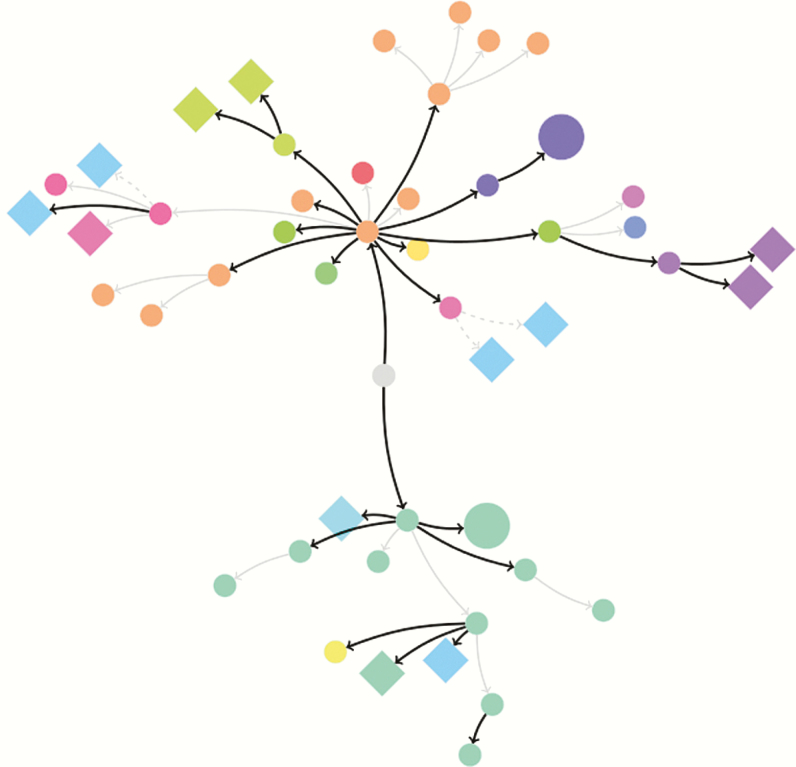
Ebola virus was transmitted through the village during a 6-week period over 4 generations of transmission. The overall number of EBOV infections (cluster size) was 48. Twenty-seven persons had a type 1 link (EVD case to contact), 18 persons had a type 2 link (EVD cases to contact), and 3 persons had a type 3 link (inference of EVD case to contact) (Figure 3).
Figure 3.
A geospatial representation of the chain of Ebola virus (EBOV) transmission. The cluster size of 48 EBOV-infected persons in Sukudu orginated from the Ebola virus disease (EVD) index case (center: gray circle) in Koidu Town, Kono District, Sierra Leone. Small circles represent the reported EVD cases, large circles represent unrecognized EVD cases, and diamonds represent minimally symptomatic EBOV-infected persons. Arrows demonstrate the directionality of the EBOV transmission between persons and epidemiological type of link. Black arrows represent type 1 links, solid gray arrows represent type 2 links, and dashed grey arrows represent type 3 links. Color describes the households in which persons acquired EBOV.
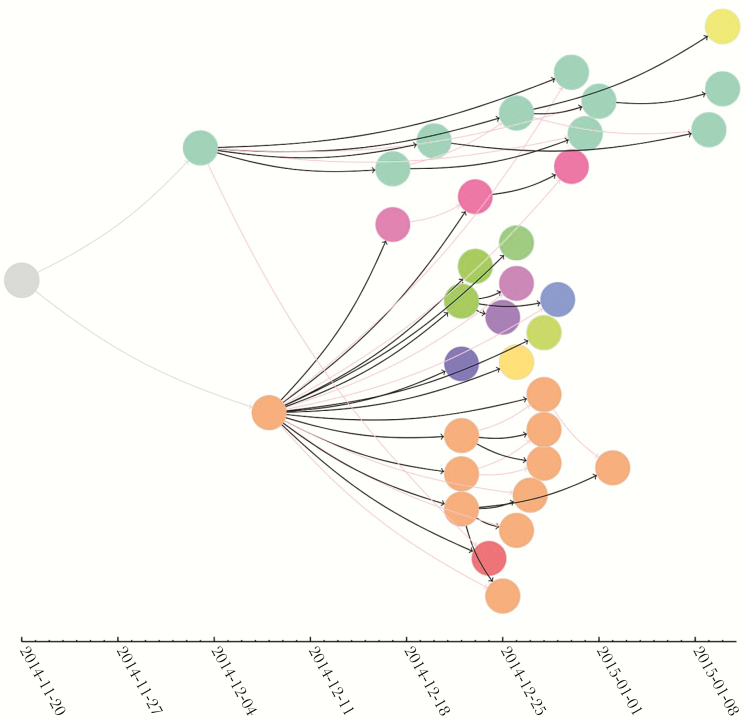
Given that minimally symptomatic EBOV-infected persons were assumed to be noninfectious, we included the 36 EVD cases in the effective reproductive numbers. In the first generation of transmission, 1 person transmitted EBOV to 2 persons (R(1), 2; 95% CI, .25–4.97). In the second generation of transmission, 2 persons transmitted EBOV to 18 persons (R(2), 9.0; 95% CI, 5.33–14.22). In the third generation of transmission, 18 persons transmitted EBOV to 15 persons (R(3), 0.83; 95% CI, .47–1.37). In the fourth generation of transmission, 15 persons transmitted EBOV to 1 person (R(4), 0.07; 95% CI, .002–.37) (Figure 4). The results were not substantially changed when we accounted for uncertainty in the links of successive generations of transmission (Supplementary Data 1).
Figure 4.
A temporal representation of the chain of transmission and epidemic curve for Ebola virus disease (EVD) cases. The EVD index case (left: gray circle) in Koidu Town initiated a chain of 34 reported EVD cases in Sukudu. Each circle represents the date of onset of EVD symptoms reported for each reported EVD case. Arrows demonstrate the directionality of the Ebola virus (EBOV) transmission between persons. Solid black arrows represent the primary contact of the EVD case, and red arrows represent secondary contacts. Color describes the households of EVD cases. We excluded the minimally symptomatic EBOV-infected persons and unrecognized EVD cases because they were assumed to be noninfectious or were not asked to recall their date of symptom onset.
Finally, we found evidence that the nearest neighbor distance between minimally symptomatic EBOV infections in the transmission chain was substantially smaller than would be expected at random (expected mean nearest neighbor distance = 3.60; observed mean nearest neighbor distance = 2.14; P < .001).
Characteristics of Ebola Virus–Infected Persons, Minimally Symptomatic Ebola Virus–Infected Persons, and Ebola Virus Disease Cases
Of 48 EBOV-infected persons, 10 (21%) were aged >45 years, 29 (60%) were male, 6 (13%) had an occupation of housework, 26 (61%) had an educational level of primary school or less, 40 (83%) were quarantined for having lived in the same house as an EVD case, and 8 (17%) were quarantined for having shared a latrine with an EVD case.
Among the 12 minimally symptomatic EBOV-infected persons, 1 (8%) was aged >45 years, 6 (50%) were male, 2 (17%) had an occupation of housework, 5 (50%) had an educational level of primary school or less; 6 (50%) were quarantined for having lived in the same house as an EVD case; and 6 (50%) were quarantined for having shared a latrine with an EVD case.
Among the 36 EVD cases, 9 (25%) were aged >45 years, 23 (64%) were male, 4 (11%) had an occupation of housework, 21 (64%) had an educational level of primary school or less, 34 (94%) were quarantined for having lived in the same house as an EVD case, and 29 (81%) were family members. The transmission context was as follows: 22 (61%) were in the community, 13 (36%) also attended a traditional burial, and 1 (2.8%) was a healthcare worker. The mean infectious period was 5 days (SD ± 2.9) (Table 1). The mean infectious period was 2.9 days (SD ± 1.5) for persons who were hospitalized and 8.8 days (SD ± 3.2) for persons who were not hospitalized (P < .01, t test). The mean infectious period for people who survived was 2.3 days (SD ± 1.6), whereas the infectious period for people who died was 4.4 days (SD ± 3.0; P = .12, t test). Twenty-eight (78%) were admitted to a hospital or Ebola treatment unit, and 28 (78%; 95% CI, 61%–90%) died from EVD.
Factors Associated With Risk for Ebola Virus Infection and for Minimally Symptomatic Ebola Virus Infection
In unadjusted analyses, EBOV infection was associated with being an adult, being a person who does housework, having completed middle school or above, and having lived in the same house as an EVD case (Table 2). After adjustment, factors associated with EBOV infection included being age 15–45 years (adjusted odds ratio [AOR], 3.74; 95% CI, 1.05–13.27; referent group, <15 years), having an occupation of housework (AOR, 4.36; 95% CI, 1.86–10.17; referent group, student), and having lived in the same house as an EVD case (AOR, 7.61; 95% CI, 1.67–34.65).
Table 2.
Factors Associated With Risk for Ebola Virus Infection Among Persons Who Lived Within Quarantine and Risk for Minimally Symptomatic Infection Among Ebola Virus–Infected Persons
| Risk of EBOV infection from quarantine (uninfected, n = 173; infected, n = 48) |
Risk of minimally symptomatic infection from EBOV infection (minimally symptomatic, n = 12; EVD, n = 36) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Unadjusted OR | Adjusted OR | Unadjusted OR | Adjusted OR | |||||
| OR | 95% CI | AOR | 95% CI | OR | 95% CI | AOR | 95% CI | ||
| Sex | Male | Ref | Ref | Ref | Ref | ||||
| Female | 0.91 | .48–1.76 | 1.77 | 0.47–6.62 | |||||
| Age | <15 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 15–45 | 4.66 | 1.72–12.58 | 3.74 | 1.05–13.27 | 0.56 | .08–3.94 | .49 | .07–3.54 | |
| >45 | 8.24 | 2.49–27.27 | 5.96 | .89–39.84 | 0.17 | .01–2.56 | .15 | .003–7.38 | |
| Occupation | Student | Ref | Ref | Ref | Ref | Ref | Ref | ||
| Works outdoors | 2.52 | 1.16–5.48 | 1.35 | .52–3.54 | 1.33 | .23–7.62 | |||
| Housework | 3.55 | 1.09–11.58 | 4.36 | 1.86–10.17 | 2.00 | .20–19.91 | |||
| Highest level of school completed | Primary or less | Ref | Ref | Ref | Ref | Ref | Ref | ||
| Middle school or above | 1.85 | .91–3.80 | 2.00 | .99–4.04 | 1.85 | .48–7.23 | |||
| Having lived in the same house as an EVD case |
No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Yes | 5.18 | 2.29–11.70 | 7.61 | 1.67–34.65 | 0.06 | .01–0.36 | .06 | .004–.75 | |
Abbreviations: AOR, adjusted odds ratio; EBOV, Ebola virus; EVD, Ebola virus disease; OR, odds ratio; Ref, referent.
Bold indicates P ≤ .05.
aEducation level excluded persons aged <15 years in the analysis. There were 152 quarantined persons, 43 EBOV-infected persons, 10 minimally symptomatic EBOV-infected persons and 33 reported EVD cases;
Among EBOV-infected persons, being a child and having not been quarantined in an EVD-infected house were associated with minimally symptomatic EBOV infections in unadjusted analyses. After adjustment, having not lived in the same house as an EVD case remained associated with minimally symptomatic EBOV infections and was a strongly protective factor for EVD (AOR, 0.06; 95% CI, .004–.75). Notably, the AOR of 0.06 corresponds to an OR of 16.67 when considering EVD as the reference group.
DISCUSSION
This is the first study to investigate a chain of EBOV transmission that includes minimally symptomatic infections and unrecognized EVD cases. We found these minimally symptomatic EBOV infections did not transmit EBOV to others, were associated with not having lived in the same house as an EVD case, and were nonrandomly located in the transmission chain. More fine-grained investigations of EBOV transmission chains are needed to substantiate and confirm findings resulting from the inclusion of minimally symptomatic infections and unrecognized EVD cases.
In our study, there was no evidence of transmission from individuals with minimally symptomatic EBOV infection. These findings are consistent with prior evidence unable to prove transmission from asymptomatic or minimally symptomatic individuals, although sexual transmission from healthy EVD survivors with viral persistance suggests the possibility [7, 20]. Even if such events do not increase the effective reproductive number, they still represent instances where containment efforts failed to prevent human-to-human EBOV transmission. In addition, the clinical consequences of minimally symptomatic EBOV infection remain unknown, including whether such individuals develop post-Ebola clinical sequelea such as uveitis [21]. In future Ebola outbreaks, real-time study of transmission chains that include minimally symptomatic EBOV-infected persons may improve our scientific understanding of potential EBOV transmission from minimally symptomatic EBOV-infected persons to uninfected close contacts.
In Sukudu, not having lived in the same house as an EVD case was associated with minimally symptomatic EBOV infections and strongly protective against EVD. Close contacts who include friends and family of an EVD case may live outside of the EVD-infected house. Other studies have not reported risk for EBOV transmission based on quarantine practices, nor have these studies used the definition of a close contact to guide quarantine practices [16]. Including close contacts of nonquarantined houses in future studies will be essential to assess the efficacy of quarantine practices and to identify additional minimally symptomatic EBOV infections and unrecognized EVD cases.
Sukudu is a small, rural village that suffered from 1 major introduction of EBOV and 4 successive generations of transmission. We observed substantial transmission from EVD cases at the onset of the outbreak. After EVD incidence peaked, there was rapid, approximate 10-fold decline of EVD cases for each successive generation of infection (consistent, for example, with recent simple models of disease outbreaks [22]). We found the pattern of minimally symptomatic EBOV infections was not consistent with random occurrence in the transmission chain. It remains unclear whether such nonrandomness is indicative of clustering of susceptibility in households due to genetic factors, infectious dose, or exposure to dead or attenuated virus.
The effective reproductive number of Sukudu village changed over a short time, and there were less than a quarter of EBOV infections in the study population, which is in the range of most other serosurveys [4, 9, 10]. Since the West Africa outbreak ended, multiple antiglycoprotein immunoassays have been validated and evaluated with relatively similar sensitivity, ranging from 95.9% to 96.7% [17, 23, 24]. A recent article by Glynn and colleagues reported that 2.6% of asymptomatic contacts of EVD survivors tested positive for Ebola virus antibodies using a newly validated oral fluid antiglycoprotein immunoglobulin G capture assay [23]; however, their serosurvey may have captured a nonrepresentative sample of potentially exposed individuals because their investigation focused on contacts in survivor households and excluded both contacts outside the home as well as households with only fatal cases. In addition fatal cases may be more infectious than survivors [25].
Other studies attributed the control of the 2013–2016 Ebola outbreaks to local behavioral changes and improved international response activities [25–27]. Ebola response activities were being strengthened during this outbreak, so our village-level findings support the concept that behavioral changes and clinical care measures aided in extinguishing this outbreak, as opposed to micronetwork saturation of exposure to EVD cases.
Our study has several limitations. This study focuses on 1 village only, potentially limiting the generalizability of our findings. Although the sample size is small, the fine-grained results at the village level allow for assessment of epidemiological links, which might be missed when looking at aggregated data. In addition, our study was conducted a year after the outbreak in Sukudu, and self-reported EVD symptoms may be subject to recall error; however our in-depth interviews with entire households sought to verify symptomatology via collective memory. Our serosurvey results were also unable to prove seropositive persons had a definitive EBOV infection during the Ebola epidemic. Therefore, our data analysis in epidemiology risk models should be interpreted with caution. However, the small size of the village and outbreak increased confidence that recall bias regarding social contacts would be minimized in our analysis of potential transmission links. Finally, our findings cannot be extrapolated to locations with extended transmission histories or multiple reintroductions of infection or different quarantine practices given that the effective reproductive number may be widely dependent on these factors.
Despite these limitations, this study depicts a chain of EBOV transmission inclusive of minimally symptomatic infections and unrecognized EVD cases. We found these minimally symptomatic EBOV infections were nonrandomly located in the transmission chain, did not transmit EBOV to others, and were associated with not having lived in the same house as an EVD case. The low number of EBOV transmission events in the quarantined population suggests that behavioral changes and the international response were critical to containment. More fine-grained depictions of the chain of EBOV transmission, inclusive of close contacts, have the potential to inform minimally symptomatic EBOV transmission dynamics and assist in containment in future Ebola outbreaks.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to the staff of Wellbody Alliance and Partners In Health for their dedication to health equity in Sierra Leone. We graciously acknowledge Barbara Knust, Michael Sneller, Art Reingold, Alan Hubbard, and Paul Farmer for their input.
Financial support. This study was supported by Univeristy of California, San Francisco; Global Health Sciences; Stanford University Center for Innovation in Global Health; and Partners In Health–Sierra Leone. T. C. P. acknowledges salary support through the US National Science Foundation (1515734, J. Pulliam, PI). E. T. R. acknowledges salary support of a KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst, The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health award KL2 TR001100).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: American Society of Tropical Medicine and Hyigene 65th Annual Meeting, November 2016, Atlanta, GA: Session 38–Ebola: Drivers of Transmission, Vaccines, Clinical Sequelae and Asymptomatic Infection.
References
- 1. World Health Organization. Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Org 1978; 56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Outbreaks chronology: Ebola hemorrhagic fever 2014. http://www.cdc.gov/vhf/ebola/resources/outbreak-table.html.
- 3. Leroy EM, Baize S, Volchkov VE et al. . Human asymptomatic Ebola infection and strong inflammatory response. Lancet 2000; 355:2210–5. [DOI] [PubMed] [Google Scholar]
- 4. Dean NE, Halloran ME, Yang Y, Longini IM. Transmissibility and pathogenicity of Ebola virus: a systematic review and meta-analysis of household secondary attack rate and asymptomatic infection. Clin Infect Dis 2016; 62:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schieffelin JS, Shaffer JG, Goba A et al. ; KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014; 371:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Ebola situation reports March 27, 2016. http://apps.who.int/ebola/ebola-situation-reports.
- 7. WHO Ebola Response Team. After Ebola in West Africa—unpredictable risks, preventable epidemics. N Engl J Med 2016; 375:587–96. [DOI] [PubMed] [Google Scholar]
- 8. Richardson ET, Barrie MB, Nutt CT et al. . The Ebola suspect’s dilemma. Lancet Glob Health 2017; 5:e254–6. [DOI] [PubMed] [Google Scholar]
- 9. Becquart P, Wauquier N, Mahlakõiv T et al. . High prevalence of both humoral and cellular immunity to Zaire Ebolavirus among rural populations in Gabon. PLoS One 2010; 5:e9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heffernan RT, Pambo B, Hatchett RJ, Leman PA, Swanepoel R, Ryder RW. Low seroprevalence of IgG antibodies to Ebola virus in an epidemic zone: Ogooué-Ivindo region, Northeastern Gabon, 1997. J Infect Dis 2005; 191:964–8. [DOI] [PubMed] [Google Scholar]
- 11. Meltzer MI, Atkins CY, Santibanez S et al. . Estimating the future number of cases in the Ebola epidemic—Liberia and Sierra Leone, 2014–2015. MMWR Suppl 2014; 63:1–14. [PubMed] [Google Scholar]
- 12. Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet 2011; 377:849–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson ET, Barrie MB, Kelly JD, Dibba Y, Koedoyoma S, Farmer PE. Biosocial approaches to the 2013–2016 Ebola pandemic. Health Hum Rights 2016; 18:115–28. [PMC free article] [PubMed] [Google Scholar]
- 14. Cancedda C, Davis SM, Dierberg KL et al. . Strengthening health systems while responding to a health crisis: lessons learned by a nongovernmental organization during the Ebola virus disease epidemic in Sierra Leone. J Infect Dis 2016; 214:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faye O, Boëlle PY, Heleze E et al. . Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: an observational study. Lancet Infect Dis 2015; 15:320–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brainard J, Hooper L, Pond K, Edmunds K, Hunter PR. Risk factors for transmission of Ebola or Marburg virus disease: a systematic review and meta-analysis. Int J Epidemiol 2016; 45:102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richardson ET, Kelly JD, Barrie MB et al. . Minimally symptomatic infection in an Ebola “hotspot”: a cross-sectional serosurvey. PLoS Negl Trop Dis 2016; 10:e0005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization. Case definition recommendations for Ebola or Marburg virus diseases 9 August 2014. http://apps.who.int/iris/bitstream/10665/146397/1/WHO_EVD_CaseDef_14.1_eng.pdf?ua=1&ua=1.
- 19. Bollobas B. Modern graph theory. Corrected ed. New York: Springer Verlag, 2013. [Google Scholar]
- 20. Diallo B, Sissoko D, Loman NJ et al. . Resurgence of Ebola virus disease in guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis 2016; 63:1353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Varkey JB, Shantha JG, Crozier I et al. . Persistence of Ebola virus in ocular fluid during convalescence. N Engl J Med 2015; 372:2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fisman DN, Hauck TS, Tuite AR, Greer AL. An IDEA for short term outbreak projection: nearcasting using the basic reproduction number. PLoS One 2013; 8:e83622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glynn JR, Bower H, Johnson S et al. . Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: a cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect Dis 2017; 17:645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kennedy SB, Bolay F, Kieh M et al. ; PREVAIL I Study Group Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med 2017; 377:1438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richards P, Amara J, Ferme MC et al. . Social pathways for Ebola virus disease in rural Sierra Leone, and some implications for containment. PLoS Negl Trop Dis 2015; 9:e0003567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Richards P. Ebola: how a people’s science helped end an epidemic. London: Zed Books Limited, 2016. [Google Scholar]
- 27. Atkins KE, Pandey A, Wenzel NS et al. . Retrospective analysis of the 2014–2015 Ebola epidemic in Liberia. Am J Trop Med Hyg 2016; 94:833–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.