Abstract
Background and Aims
Medical management of fistulising Crohn’s disease [CD] is constrained by the limited number of available therapies. We evaluated the efficacy of vedolizumab, a gut-selective α4β7 integrin antagonist approved for treating moderately to severely active CD, in a subpopulation of patients with fistulising CD who participated in the GEMINI 2 trial [NCT00783692].
Methods
Exploratory analyses of data from the GEMINI 2 trial were conducted in 461 responders to 6-week vedolizumab induction therapy who received maintenance placebo [VDZ/PBO, N = 153] or vedolizumab [VDZ/VDZ, N = 308]. Fistula closure rates were assessed at Weeks 14 and 52, and the time to fistula closure was analysed by the Cox proportional hazards model with adjustments for significant covariates.
Results
At entry into the maintenance period, 153 [33%] patients had a history of fistulising disease and 57 [12%] patients had ≥1 active draining fistula. By Week 14, 28% of VDZ/VDZ-treated patients compared with 11% of VDZ/PBO-treated patients (95% confidence interval [CI], –11.4 to 43.9) achieved fistula closure. Corresponding rates at Week 52 were 31% and 11% (absolute risk reduction [ARR]: 19.7%; 95% CI, –8.9 to 46.2). Similarly, VDZ/VDZ-treated patients had faster time to fistula closure and were more likely to have fistula closure at Week 52 [33% vs 11%; HR: 2.54; 95% CI, 0.54–11.96]. Prior failure of antibiotic therapy was a negative predictor of fistula closure [HR: 0.217; 95% CI, 0.059–0.795; p = 0.021], whereas trough vedolizumab concentrations did not affect closure rates.
Conclusions
Our findings are consistent with the beneficial effect of vedolizumab treatment for fistulising CD.
Keywords: Vedolizumab, Crohn’s disease, fistula
1. Introduction
The development of fistulae is an important complication of Crohn’s disease [CD] that affects 40–50% of patients.1–3 The pathophysiology of fistula formation is complex, likely due to the interplay between multiple factors, including cryptoglandular disease, uncontrolled transmural inflammation, luminal stenosis, and bacterial sepsis.4,5 Patients with fistulising disease often experience symptoms of anal pain, discharge, and incontinence, which result in high morbidity and impaired health-related quality of life.1
Treatment of fistulae is challenging and usually requires a combination of medical and surgical interventions.5 While medical treatment with antibiotics and immunosuppressants has demonstrated some benefit, tumour necrosis factor [TNF] antagonists are most effective for treating complex or refractory perianal fistulae.1 In clinical trials of infliximab and adalimumab, both agents demonstrated superiority to placebo in achieving fistula closure (46% [infliximab] vs 13% [placebo]; 33% [adalimumab] vs 13% [placebo]).6–8 However, the percentage of patients who do not achieve complete fistula closure is high, and even among the patients with an initial response, many have continuing disease activity.9,10 Furthermore, loss of response, defined by reopening of a fistula’s external orifice, is common in patients who initially achieve complete closure with a TNF antagonist when the TNF antagonist is discontinued as per the study protocol.6 Thus, fistulising CD presents an unmet medical need for which additional drug therapies are required.
The efficacy and safety of vedolizumab, a gut-selective α4β7 integrin antagonist, were demonstrated in the Phase 3 placebo-controlled GEMINI 2 trial [ClinicalTrials.gov ID: NCT00783692] conducted in patients with moderately to severely active CD.11 In the induction phase of this trial, 410 [37%] patients had a history of fistulising disease, and 165 [15%] patients had active draining fistulae at baseline [Week 0].11 Notably, 58% of participants in the trial had previously experienced failure of treatment with at least one TNF antagonist.11 Herein, we present exploratory analyses of the GEMINI 2 maintenance intent-to-treat [ITT] population to evaluate the potential efficacy of vedolizumab for the treatment of fistulising CD.
2. Methods
2.1. Patients
GEMINI 2 was a Phase 3, randomized, double-blind, placebo-controlled study consisting of separate induction and maintenance phases.11 Eligible patients aged 18–80 years had CD for ≥3 months and a CD Activity Index [CDAI] score of 220–450.11 After 6 weeks of induction treatment with vedolizumab [intravenous doses of 300 mg at Weeks 0 and 2], 461 responders were randomized to receive either placebo [termed VDZ/PBO] or vedolizumab every 8 weeks [Q8W] or every 4 weeks [Q4W] [termed VDZ/VDZ], and were entered into a 46-week maintenance phase.11 To evaluate outcomes through 52 weeks between randomized groups, the Week 6 responder [maintenance ITT VDZ/PBO and VDZ/VDZ] population was utilized. Given that the vedolizumab Q4W and Q8W dosing regimens are generally considered clinically equivalent,12 the data from the two dose groups were combined in these analyses to increase statistical power and precision of estimation.11 Fistula closure, a pre-specified exploratory endpoint, was defined as a patient with clinically no draining fistulae at the given visit. Fistulae were assessed at each visit [2–4 week intervals] until Week 52; for each patient, the number and location of fistulae were assessed (by gentle compression of the track by the examiner’s finger) and recorded.8
2.2. Statistical methods
Baseline characteristics of patients with and without draining fistula were assessed using descriptive statistical techniques. Limited by the small sample size, this post hoc exploratory subgroup analysis of GEMINI 2 was not powered to generate precise estimates of efficacy with meaningful p values. Therefore, we focused on the magnitude of the point estimate for treatment efficacy rather than the statistical significance of the result.
The proportion of patients with ≥1 draining fistula at baseline who achieved fistula closure at Weeks 14 and 52 was described, and the absolute difference with 95% confidence intervals [CIs] was calculated. The time to fistula closure was analysed in a Cox proportional hazards model with adjustment for pre-specified covariates, including treatment group, baseline CDAI score, concomitant use of corticosteroids, previous exposure to TNF antagonists and/or concomitant use of immunosuppressants, enrolment in Cohort 1 or 2 at induction, and geographical region. The results of this analysis were expressed as hazard ratios [HRs] and 95% CIs. A subgroup analysis was performed in a subpopulation of patients with only perianal fistula at baseline.
An additional Cox proportional hazards model was performed with the inclusion of the following additional covariates: disease duration, baseline C-reactive protein [CRP], number of fistulae at baseline, prior antibiotic failure, and Week 6 vedolizumab concentration to assess the significance of each covariate; HRs and 95% CIs were estimated after adding each covariate we explored as sensitivity to our pre-defined model. New draining fistulae (defined as draining fistulae that occurred at any point during the study among patients without a draining fistula at baseline) occurred at an insufficient rate for statistical analysis, and their occurrence is therefore only described according to treatment group. Patients who failed to respond to treatment during the maintenance phase and received an open-label drug in either treatment group were considered to have experienced treatment failure.
3. Results
3.1. Patients
Patients received either VDZ/VDZ [N = 308] or VDZ/PBO [N = 153]. One hundred and fifty three [33%] patients had a history of fistulising disease and 57 [12%] patients had ≥1 active draining fistula at entry into the maintenance period. Of the 57 patients with a draining fistula, all had a history of fistulising disease before entering the GEMINI 2 study; 44% of VDZ/PBO-treated patients and 49% of VDZ/VDZ-treated patients had prior failure of TNF antagonist therapy, and 39% of VDZ/PBO-treated patients and 54% of VDZ/VDZ-treated patients had prior surgery for CD [Table 1]. Three patients had fistula surgery [one during induction and two during maintenance], and in all of these patients, treatment was considered a failure in the analysis. The only patient who underwent bowel resection was in the VDZ/VDZ group, and this treatment was considered a failure. Prior antibiotic failure/discontinuation, defined as relating to those patients who had previously taken but were not taking antibiotics at baseline, was recorded on the case report form. At baseline, the percentages of patients in the VDZ/VDZ and VDZ/PBO groups receiving concomitant antibiotics were 54% and 33%, respectively. Most patients [n = 43/57] had only 1 draining fistula at baseline, 10 patients had 2, and 4 patients had ≥3 [Table 1]. The majority of patients [n = 45/57] had perianal fistulae.
Table 1.
Baseline characteristics of patients with and without draining fistulae at maintenance baseline [Week 6]*.
| Patients with draining fistulae at baseline [n = 57] |
Patients without draining fistulae at baseline [n = 404] |
|||
|---|---|---|---|---|
| VDZ/PBO [n = 18] | VDZ/VDZ [n = 39] | VDZ/PBO [n = 135] | VDZ/VDZ [n = 269] | |
| Age [year], mean ± SDa | 35.8 ± 11.0 | 32.6 ± 9.5 | 37.4 ± 12.1 | 35.3 ± 12.5 |
| Male sex, n [%] | 8 [44] | 20 [51] | 64 [47] | 130 [48] |
| Current smoker, n [%] | 5 [28] | 10 [26] | 43 [32] | 77 [29] |
| Disease duration [year], mean ± SDa | 10.8 ± 9.9 | 9.1 ± 7.8 | 9.5 ± 8.7 | 7.9 ± 6.9 |
| CDAI score, mean ± SDa | 318.4 ± 74.0 | 340.6 ± 73.4 | 326.1 ± 64.3 | 318.4 ± 66.2b |
| CRP [mg/L], median | 15.1 | 12.4 | 9.6 | 8.9 |
| Disease site, n [%] | ||||
| Ileum only | 2 [11] | 3 [8] | 17 [13] | 60 [22] |
| Colon only | 7 [39] | 11 [28] | 36 [27] | 63 [23] |
| Ileum and colon | 9 [50] | 25 [64] | 82 [61] | 146 [54] |
| Prior surgery for CD, n [%] | 7 [39] | 21 [54] | 50 [37] | 97 [36] |
| Prior TNF antagonist failure, n [%] | 8 [44] | 19 [49] | 70 [52] | 140 [52] |
| Concomitant medication, n [%] | ||||
| CS | 5 [28] | 17 [44] | 51 [38] | 100 [37] |
| IMM | 2 [11] | 8 [21] | 21 [16] | 50 [19] |
| CS and IMM | 5 [28] | 6 [15] | 21 [16] | 39 [14] |
| Neither CS nor IMM | 6 [33] | 8 [21] | 42 [31] | 80 [30] |
| History of fistulising disease, n [%] | 18 [100] | 39 [100] | 39 [29] | 57 [21] |
| Concomitant antibiotic use, n [%] | ||||
| Yes | 6 [33] | 21 [54] | N/A | N/A |
| No | 12 [67] | 18 [46] | ||
| Number of draining fistulae per patient, n [%] | ||||
| 1 | 13 [72] | 30 [77] | N/A | N/A |
| 2 | 4 [22] | 6 [15] | ||
| ≥3 | 1 [6] | 3 [8] | ||
| Patients with perianal fistulae, n [%] | 13 [72] | 32 [82] | N/A | N/A |
Abbreviations: CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CRP, C-reactive protein; CS, corticosteroids; IS, immunosuppressants; ITT, intent-to-treat; N/A, not applicable; PBO, placebo; SD, standard deviation; TNF, tumour necrosis factor; VDZ, vedolizumab.
Patients in the VDZ/PBO maintenance group received VDZ in the 6-week induction phase and then PBO in the 46-week maintenance phase. Patients in the VDZ/VDZ maintenance group received VDZ during induction and VDZ every 8 or 4 weeks during maintenance.
aMean values were similar to the median values [not shown] based on the distribution of the data. bn = 267.
*Patients with draining fistulae at baseline: n = 141, [14.7%] VDZ; n = 23 [15.5%] PBO
3.2. Fistula closure
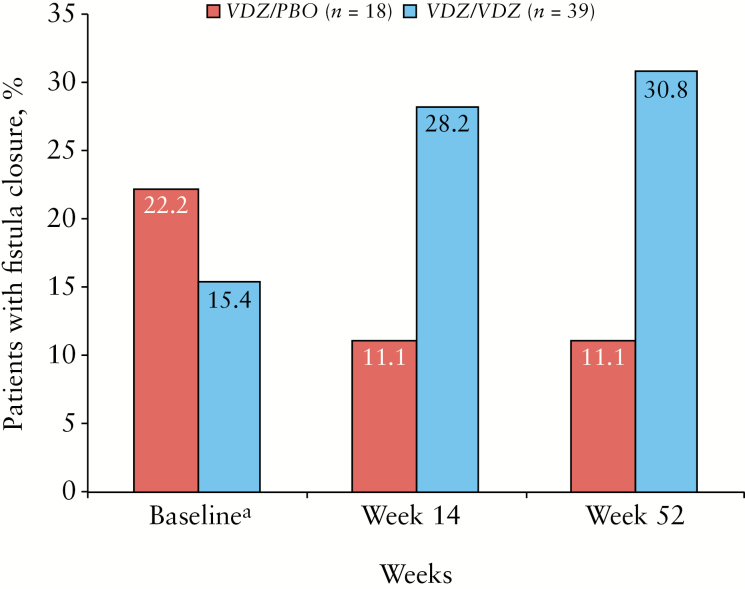
By Week 14, 28% [n = 11] of patients with draining fistulae treated with VDZ/VDZ had fistula closure compared with 11% [n = 2] treated with VDZ/PBO (absolute risk reduction [ARR]: 17.1%; 95% CI, –11.4 to 43.9) [Figure 1]. Similar results were observed in the subpopulation of patients with only perianal fistulae [VDZ/VDZ, 34%; VDZ/PBO, 15%] and across vedolizumab dose groups [Q8W: 29.4%, n = 17; Q4W: 27.3%, n = 22]. Corresponding values at Week 52 were 31% [n = 12] for VDZ/VDZ and 11% [n = 2] [ARR: 19.7%; 95% CI, –8.9 to 46.2] [Figure 1].
Figure 1.
Fistula closure in patients with ≥1 draining fistula at baseline, Week 14 and Week 52 by treatment group. All patients in this analysis [n = 57] received induction treatment with vedolizumab [Weeks 0–6, not shown]. Black bars represent the VDZ/PBO maintenance group [n = 18] and grey bars represent the VDZ/VDZ maintenance group [n = 39]. aMaintenance baseline, Week 6. Abbreviations: PBO, placebo; VDZ, vedolizumab.
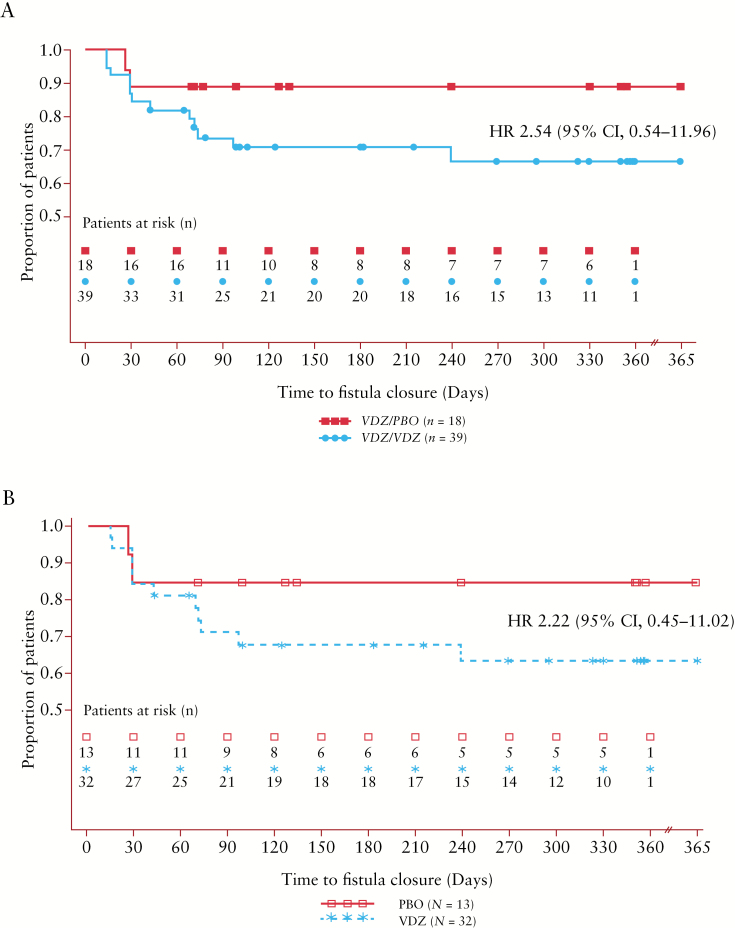
The time to fistula closure is shown in Figure 2. Patients who continued treatment with vedolizumab were more likely to have fistula closure by Week 52 than those who received placebo [HR: 2.54; 95% CI, 0.54–11.96] [Figure 2A]. Results were similar in the subpopulation of patients with only a perianal fistula at baseline [HR: 2.22; 95% CI, 0.45–11.02] [Figure 2B]. The additional Cox model assessing significance of covariates indicated that patients who, at the time of baseline, had previously experienced failure of antibiotic therapy for CD were significantly less likely to achieve fistula closure by Week 52 [HR: 0.2172; 95% CI, 0.059–0.795; p = 0.021]. No other covariates were significantly associated with fistula closure, including concomitant antibiotic use during the maintenance phase [HR: 0.7200; 95% CI, 0.212–2.441; p = 0.5979], baseline faecal calprotectin [HR: 0.9998; 95% CI, 0.999–1.000; p = 0.4725] and CRP change from baseline at Week 6 [HR: 0.9895; 95% CI, 0.928–1.055; p = 0.7452]. The inclusion of the above covariates as they relate to sensitivity to our pre-defined model did not alter the findings from the main model.
Figure 2.
[A] Time to fistula closure in all patients with fistula at baseline and [B] the subgroup of patients with perianal fistula only by treatment group. All patients in this analysis had ≥1 draining fistula at baseline [n = 57] and received previous induction treatment with vedolizumab [Weeks 0–6]. Kaplan–Meier 12-month estimate of fistula closure with VDZ/VDZ treatment is shown. Abbreviations: CI, confidence interval; HR, hazard ratio; PBO, placebo; VDZ, vedolizumab.
In a separate Cox model conducted on patients with evaluable pharmacokinetics data including Week 6, a total of 450 [of 461] patients in the maintenance ITT population had evaluable pharmacokinetic data, including for Week 6 vedolizumab serum concentrations. With vedolizumab concentration as an additional covariate, prior antibiotic failure for CD remained the only significant covariate [HR: 0.2027; 95% CI, 0.053–0.773; p = 0.0194]. Vedolizumab serum concentrations [mean, SD] at Week 6 did not differ between those in patients who achieved fistula closure at Week 52 [26.8 ± 23.4 µg/mL; n = 12] and those who did not [35.0 ± 29.8 µg/mL; n = 26]. Based on this analysis, no significant correlation was observed between early drug levels and clinical response with vedolizumab at Week 52 in patients with fistula closure.
3.3. Development of new draining fistulae
Over the course of the study, a total of 8 [3%] VDZ/VDZ-treated and 5 [4%] VDZ/PBO-treated patients developed new draining fistulae; of these patients, 5 [63%] VDZ/VDZ-treated and 4 [80%] VDZ/PBO-treated patients had a history of fistulising disease. Because of the small number of patients in each group, estimates of developing draining fistulae could not be determined with precision.
4. Discussion
In this exploratory analysis, a greater percentage of CD patients with draining fistulae at baseline who continued vedolizumab treatment after induction achieved fistula closure by Week 52 than those who were re-randomized to placebo. Although these results are not statistically significant and must be interpreted in the context of the relatively small sample size, the magnitude of the point estimate for treatment efficacy is clinically meaningful and encourages further evaluation of vedolizumab for this indication.
We found that prior failure of antibiotics was a negative predictor of fistula closure at Week 52. Interestingly, higher vedolizumab trough concentrations at Week 6 were not predictive of fistula closure in VDZ/VDZ-treated patients. In a recent study of infliximab in luminal CD patients, trough concentrations in the range of 3–5 µg/mL were only a modest predictor of disease activity; however, those falling below a threshold of <3 µg/mL were potentially indicative of greater risk for disease activity and inflammation.13 While the small sample size of our study may have limited our ability to detect a relationship between drug levels and clinical response, it is possible that higher concentrations are required for fistula closure, and/or that those below a certain threshold may correlate with poorer clinical outcomes.
The potential efficacy of vedolizumab in fistulising CD is further supported by findings from a recent integrated safety study for vedolizumab, in which exposure-adjusted incidence rates of fistulae reported as adverse events were lower in CD patients treated with vedolizumab [n = 1723] than those who received placebo [n = 355].14 Although higher rates of abscess formation, often a precursor to fistula development, have been reported with placebo [5.3 per 100 patient-years; 95% CI, 1.4–9.3] than with vedolizumab [4.8 per 100 patient-years; 95% CI, 3.9–5.6], further investigation will evaluate anal, rectal and perirectal abscess in fistulising CD.14
In this exploratory analysis of the GEMINI 2 trial, baseline CRP levels were included in the second Cox proportional hazards model, but were not predictive of fistula closure at Week 52. To assess whether fistula healing was more due to reduction of mucosal inflammation and healing, we added ‘CRP change from baseline at Week 6’ to the Cox model, but did not observe a significant effect between this parameter and fistula healing at Week 52. Baseline faecal calprotectin levels were not collected in all patients at baseline and were not included in the initial Cox model. However, we were able to re-run the analysis to include faecal calprotectin levels at baseline, and we found that neither baseline faecal calprotectin nor CRP levels were significantly associated with fistula closure.
Our analyses had some limitations; notably, the small sample size resulting in relatively imprecise estimates of efficacy, the lack of randomization based on the presence of a draining fistula at baseline, and the absence of magnetic resonance testing evaluations to evaluate efficacy.15 Results reported in this manuscript regarding fistula closure in Week-6 responders are not generalizable to the non-responder population. In fact, the percentages [95% CI] for VDZ non-responder patients achieving fistula closure were 12% [5.8, 20.6] and 18% [9.5, 25.8] at Weeks 6 and 52, respectively. Considering these limitations, the findings are consistent with a benefit of vedolizumab and should be considered hypothesis generating. An ongoing uncontrolled clinical trial is evaluating perianal fistula healing at Week 30 in patients with fistulising CD who were treated with vedolizumab, and this study will provide a more detailed characterization of fistulae [NCT02630966].16
Complete and sustained closure of fistulae remains an unmet medical need for patients with fistulising CD. Clinical trial data for TNF antagonists have shown response rates to induction as high as 69%, but complete and sustained fistulae closure is observed in only one-third of responders.6–8,10 While cross-trial comparisons cannot be made, our data suggest that vedolizumab may be efficacious in patients with fistulising CD, and warrants further evaluation in dedicated prospective clinical studies.
Funding
This work was supported by Takeda Pharmaceuticals International, Inc.
Conflict of Interest
BGF has received grant support from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Genentech, J&J, Janssen, Millennium, Pfizer, Receptos, Roche, Tillotts and UCB; and has served as a consultant or advisory board member for AbbVie, ActoGeniX, Akros, Albireo, Amgen, AstraZeneca, Avaxia Biologics, Avir Pharma, Baxter Healthcare Corp, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, enGene, Ferring Pharmaceuticals, Galapagos, Genentech/Roche, GiCare Pharma, Gilead, Given Imaging, GSK, Inception IBD Inc., Ironwood Pharmaceuticals, J&J, Janssen, Japan Tobacco, Kyowa Hakko Kirin Co. Ltd, Lexicon, Lilly, Lycera Biotech, Merck, Mesoblast Ltd, Millennium, Nektar, Nestlé, Novartis, Novo Nordisk, Pfizer, Prometheus Therapeutics & Diagnostics, Protagonist, Receptos, Salix, Shire, Sigmoid Pharma, Synergy Pharmaceuticals Inc., Takeda, Teva Pharmaceutical Industries Ltd, TiGenix, Tillotts, UCB, Vertex Pharmaceuticals, VHsquared Ltd, Warner Chilcott, Wyeth, Zealand Pharma and Zyngenia. DS has received grant support from AbbVie and UCB, and served as a consultant for AbbVie, Janssen, Takeda, TiGenix and UCB. SD has served as a consultant for AbbVie, AstraZeneca, Boehringer Ingelheim, Ferring Pharmaceuticals, Hospira, J&J, Janssen, MSD, Pfizer, Sandoz, Takeda and Vifor Pharma; and served as a speaker for, Janssen, MSD, Pfizer and Takeda. DTR has served as a consultant for AbbVie, AbGenomics, Allergan, Inc., Amgen, Celgene Corporation, Forward Pharma, Genentech/Roche, Janssen, Merck, Miraca Life Sciences, Napo Pharmaceuticals, Pfizer, Salix Pharmaceuticals, Samsung Bioepis, Sandoz Pharmaceuticals, Shire, and Takeda; has received grant support from AbbVie, Genentech/Roche, Janssen, Prometheus Laboratories, Shire, Takeda, and UCB Pharma; has served on the Amgen Board of Trustees; and is Co-Founder/CFO of Cornerstones Health, Inc., and Co-Founder of GoDuRn, LLC. TWL and KL are employees of Takeda Pharmaceuticals USA, Inc, Deerfield, IL, USA. JX is an employee of Takeda Pharmaceuticals International Co, Cambridge, MA, USA.
Author Contributions
All authors contributed to the concept, design, analysis and interpretation of data. All authors provided critical review and approved the final manuscript for submission.
Acknowledgments
Medical writing assistance was provided by Gina DeStefano, PhD, and Bomina Yu, PhD, CMPP of inVentiv Medical Communications and funded by Takeda Pharmaceuticals U.S.A., Inc. Additional statistical analyses and support with interpreting the data were provided by Alexandra James, MSc and Maria Rosario of Takeda Pharmaceuticals International during manuscript preparation. A subset of these findings was presented at the European Crohn′s and Colitis Organisation 2015 [P508] and at the Digestive Disease Week 2015 [Sa1261].
Glossary
Abbreviations:
- CDAI
CD Activity Index
- CRP
C-reactive protein
- CS
corticosteroids
- IS
immunosuppressants
- ITT
intent-to-treat
- N/A
not applicable
- TNF
tumour necrosis factor
- VDZ/PBO
responders to vedolizumab induction therapy who received placebo during maintenance
- VDZ/VDZ
responders to vedolizumab induction therapy who received vedolizumab every 8 or 4 weeks during maintenance.
References
- 1. Vavricka SR, Rogler G. Fistula treatment: the unresolved challenge. Dig Dis 2010;28:556–64. [DOI] [PubMed] [Google Scholar]
- 2. Cosnes J, Cattan S, Blain A et al. . Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 3. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017;389:1741–55. [DOI] [PubMed] [Google Scholar]
- 4. Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn’s disease. World J Gastrointest Pathophysiol 2014;5:205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gecse KB, Bemelman W, Kamm MA et al. ; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut 2014;63:1381–92. [DOI] [PubMed] [Google Scholar]
- 6. Sands BE, Anderson FH, Bernstein CN et al. . Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85. [DOI] [PubMed] [Google Scholar]
- 7. Colombel JF, Sandborn WJ, Rutgeerts P et al. . Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 8. Present DH, Rutgeerts P, Targan S et al. . Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999;340: 1398–405. [DOI] [PubMed] [Google Scholar]
- 9. Yassin NA, Askari A, Warusavitarne J et al. . Systematic review: the combined surgical and medical treatment of fistulising perianal Crohn’s disease. Aliment Pharmacol Ther 2014;40:741–9. [DOI] [PubMed] [Google Scholar]
- 10. Geltzeiler CB, Wieghard N, Tsikitis VL. Recent developments in the surgical management of perianal fistula for Crohn’s disease. Ann Gastroenterol 2014;27:320–30. [PMC free article] [PubMed] [Google Scholar]
- 11. Sandborn WJ, Feagan BG, Rutgeerts P et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 12. Takeda. Entyvio (vedolizumab) [package insert]. Deerfield, IL: Takeda Pharmaceuticals America, Inc; 2014. http://general.takedapharm.com/content/file.aspx?filetypecode=ENTYVIOPI&cacheRandomizer=3b1c1685-2767-4d48-acbe-10e550693ce0. Accessed March 8, 2017. [Google Scholar]
- 13. Levesque BG, Greenberg GR, Zou G et al. . A prospective cohort study to determine the relationship between serum infliximab concentration and efficacy in patients with luminal Crohn’s disease. Aliment Pharmacol Ther 2014;39:1126–35. [DOI] [PubMed] [Google Scholar]
- 14. Colombel JF, Sands BE, Rutgeerts P et al. . The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 2017;66:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panés J, García-Olmo D, Van Assche G et al. ; ADMIRE CD Study Group Collaborators Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016;388:1281–90. [DOI] [PubMed] [Google Scholar]
- 16. Takeda; National Institutes of Health. Vedolizumab IV 300 mg in the treatment of fistulizing Crohn’s disease (ENTERPRISE) ClinicalTrials.gov identifier: NCT02630966. 2017; https://clinicaltrials.gov/ct2/show/NCT02630966. Accessed March 8, 2017.