Abstract
Study Objectives
Ambulatory tracking of sleep and sleep pathology is rapidly increasing with the introduction of wearable devices. The objective of this study was to evaluate a wearable device which used novel computational analysis of the electrocardiogram (ECG), collected over multiple nights, as a method to track the dynamics of sleep quality in health and disease.
Methods
This study used the ECG as a primary signal, a wearable device, the M1, and an analysis of cardiopulmonary coupling to estimate sleep quality. The M1 measures trunk movements, the ECG, body position, and snoring vibrations. Data from three groups of patients were analyzed: healthy participants and people with sleep apnea and insomnia, obtained from multiple nights of recording. Analysis focused on summary measures and night-to-night variability, specifically the intraclass coefficient.
Results:
Data were collected from 10 healthy participants, 18 people with positive pressure–treated sleep apnea, and 20 people with insomnia, 128, 65, and 121 nights, respectively. In any participant, all nights were consecutive. High-frequency coupling (HFC), the signal biomarker of stable breathing and stable sleep, showed high intraclass coefficients (ICCs) in healthy participants and people with sleep apnea (0.83, 0.89), but only 0.66 in people with insomnia. The only statistically significant difference between weekday and weekend in healthy subjects was HFC duration: 242.8 ± 53.8 vs. 275.8 ± 57.1 minutes (89 vs. 39 total nights), F(1,126) = 9.86, p = .002.
Conclusions
The M1 and similar wearable devices provide new opportunities to measure sleep in dynamic ways not possible before. These measurements can yield new biological insights and aid clinical management.
Keywords: cardiopulmonary coupling, ECG, sleep, ambulatory, insomnia, apnea
Statement of Significance
Wearable devices are starting to enable ambulatory tracking of sleep quality and sleep apnea. The M1-SleepImageTM system uses the electrocardiogram and a novel computational analysis (cardiopulmonary coupling) to estimate sleep stability, based on a concept of nonrapid eye movement (NREM) sleep bimodality (stable and unstable forms). High-frequency coupling is the signal biomarker of stable NREM sleep. Multiple night recordings were performed in healthy individuals, people with sleep apnea, and insomnia. Stable individual patterns of sleep stability resulted in low intra-individual variability, greater in health than in insomnia. Pharmacological effects were demonstrable, specifically an increase in high-frequency coupling induced by benzodiazepine. Sleep apnea treatment effects including complex sleep apnea could also be tracked.
Introduction
Ambulatory tracking of detailed sleep state physiology is as highly desirable in sleep medicine as glucometers, and loop recorders have been in their respective fields. The gold standard method of recording sleep, polysomnography (PSG), is not a feasible solution for large-scale use due to prohibitive cost burden and inconvenience, resulting in a move to home sleep apnea testing. Moreover, repeated PSG for disease tracking is impractical. However, technological advances are now rendering accessible ambulatory acquisition of numerous relevant physiological signals such as respiration, electrocardiogram (ECG), and even electroencephalogram (EEG) [1, 2]. Although recording technology has been available for decades, the miniaturization of devices has progressed substantially in recent years. This shift in capability is reflected in the medical monitoring arena as well as the profusion of consumer-facing wearable devices [3] that generally employ activity and/or autonomic sensors to estimate sleep quality and quantity [4].
The advantage of at-home feasibility comes at a potential risk: measuring limited channels of physiological information may yield insufficient biological information to make clinically relevant determinations of sleep state. Even with the rich physiology of conventional PSG, the “biological worth” of any given stage does not clearly map onto clinical decision making. Extensive experimental literature supports stage-specific roles (such as REM and memory, or N3 and the homeostatic process) [5], but the clinical utility of measuring stages on a single night does not translate readily into long-term management applications. A related challenge is that the dominant stage of sleep in any given night (by percentage of time), N2, is often ignored at the expense of attention paid to REM and N3—so much so that consumer devices such as the S+ (ResMed, Inc.) (and sometimes even clinical reports) falsely lump stages N1 and N2 together as “light” nonrapid eye movement (NREM).
We previously described an approach to characterize sleep based on the interaction of autonomic and respiratory oscillations (cardiopulmonary coupling, CPC), termed the ECG-derived sleep spectrogram [6]. Sleep spectrogram analysis reveals that NREM sleep has a distinct bimodal-type structure marked by distinct alternating and abruptly varying periods of strong high- and low-frequency CPC power (HFC and LFC, respectively). Much of HFC, which is associated with noncyclic alternating pattern (non-CAP) EEG, occurs during stage N2 and is associated with periods of stable breathing, a paucity of phasic EEG transients, and physiologic blood pressure dipping [6, 7]. The amount of HFC is reduced by processes that fragment sleep such as sleep apnea and fibromyalgia [8, 9]. The ECG-CPC technique has been shown to capture treatment effects in sleep apnea [10–13] and insomnia [14, 15]. In this way, the NREM sleep phenotype extends beyond conventional scoring and its reliability on absolute δ power. These disparities between high quality NREM sleep and conventionally scored N3 are especially apparent in individuals over the age of 40–50 years, in whom stage N3 makes up less than 20% of the sleep period [16]. Computational analytical approaches of limited numbers of continuously acquired signals offer one possible solution to characterization of sleep quality, especially enabling dynamic tracking over time.
The SleepImageTM system (www.sleepimage.com) is a Health Insurance Portability and Accountability Act (HIPAA)-compatible Cloud Computing system current hosted in the Amazon Cloud, using a small wearable device called the M1. The system enables collecting ECG, actigraphy, body position, and snoring information on multiple nights, followed by generation of CPC measures. We used this system to estimate signal characteristics and biological stability over multiple nights in healthy participants and people with sleep apnea and insomnia. Our primary hypotheses were as follows: (1) Sleep stability metrics would show strong interindividual stability over multiple nights. (2) Stable sleep as estimated by HFC would show reductions in people with apnea and insomnia relative to healthy controls.
Methods
Participants
We analyzed three groups for this work. First, we enrolled healthy adult subjects at the Beth Israel Deaconess Medical Center, free of medical, neurological, and psychiatric disease, as determined by medical history and clinical examination. Habitual snoring and a body mass index (BMI) over 30 kg/m2 were clinical exclusion criteria. Each underwent PSG to exclude sleep apnea, followed by 14 consecutive nights of at-home M1 recordings and sleep diaries. Second, we analyzed data from sleep clinic people with sleep apnea at the Beth Israel Deaconess Medical Center who underwent M1 recordings performed for clinical tracking purposes. These included assessment of medication effects.
Chronic insomnia participants were enrolled at the Massachusetts General Hospital. Diagnosis was made following evaluation by a board-certified Sleep Medicine specialist. All participants presented with sleep initiation or maintenance symptoms with estimated self-reported total sleep time less than 6 hr at initial presentation or wake after sleep onset of greater than 30 minutes, with subjective impairment of daytime function and quality of life. None had any risk features for sleep apnea or restless legs. Participants were asked to monitor sleep with M1 and diary in the home for 4–8 nights. This group was heterogeneous in the etiology of insomnia, use of hypnotic medications, and comorbidities. Known untreated obstructive sleep apnea (OSA) was an exclusion. PSG was not consistently obtained. All participants were free of arrhythmias that could degrade the ECG signal—including use of pacemakers, continuous of frequent ventricular ectopy, and atrial fibrillation or flutter.
Polysomnography
Conventional-attended PSG was performed in the General Clinical Research Center at the Beth Israel Deaconess Medical Center, Boston, MA, USA. The standard montage included frontal, central, and occipital EEG, electrooculogram, chin electromyogram, respiratory flow (thermistor and nasal pressure), effort, tibialis anterior electromyogram, and finger pulse oximetry. Scoring used standard 2016 Update American Academy of Sleep Medicine rules (http://www.aasmnet.org/scoringmanual/default.aspx) for sleep stages, respiratory variables, and arousals.
The M1 wearable device (MyCardio, LLC; Broomfield, CO. 80021, USA)
This small wearable recorder (Figure 1) measures continuous ECG, sampled at 600 Hz, expressed in millivolts, 12-bit quantization, with one adhesive pad under the device and a thin wire across the chest to a second pad. Activity and body position is measured by internal accelerometers and gyroscopes, and snoring is detected by induced vibration. The data are uploaded to the SleepImageTM website, and automatic analysis generating CPC variables, the sleep spectrogram graphs (Figure 2) total sleep time (actigraphic), actigraphic wake (after sleep onset), and transient awakenings (by movement). The images have been touched up for information on the x- and y-axis for Figures 2–4. Figures 5 and [6] show the original x- and y-axis information. None of the graphic CPC information was manipulated.
Figure 1.
M1: A wrist-worn actigraph is shown for comparison/size.
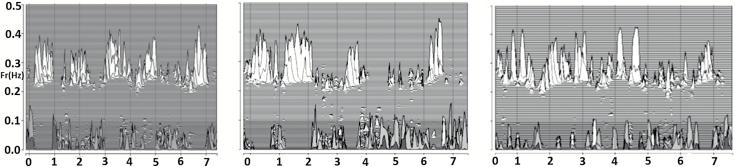
Figure 2.
Sleep spectrogram and night-to-night stability of HFC. Nights 1, 7, and 15 in a 22-year-old healthy male participant. Note overall similarity of individual nights with respect to the proportion of the recording spent in HFC (upper spectral clusters, ~0.3 Hz).
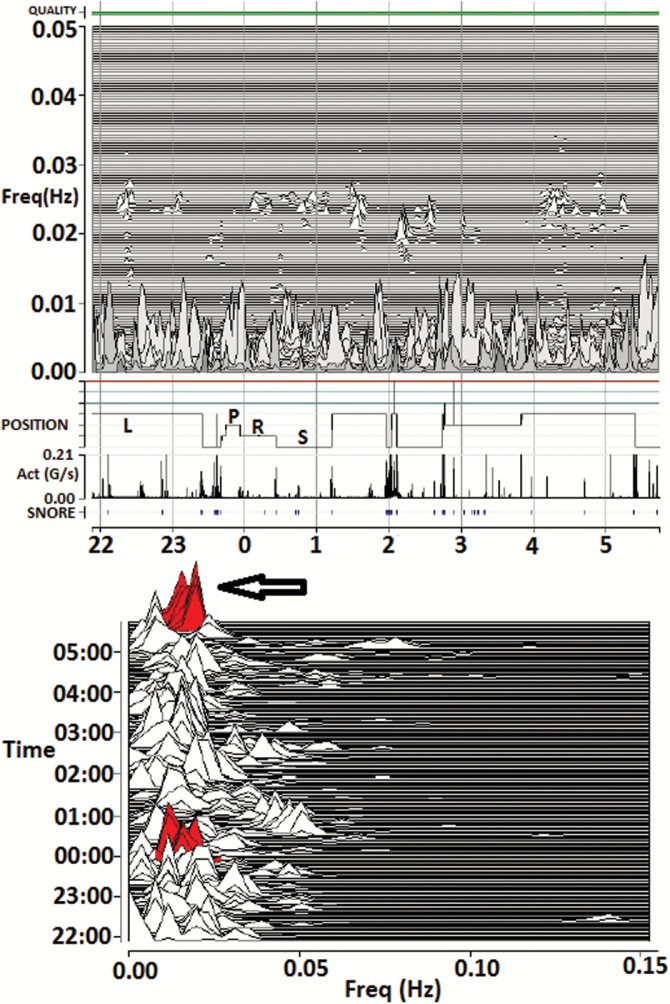
Figure 3.
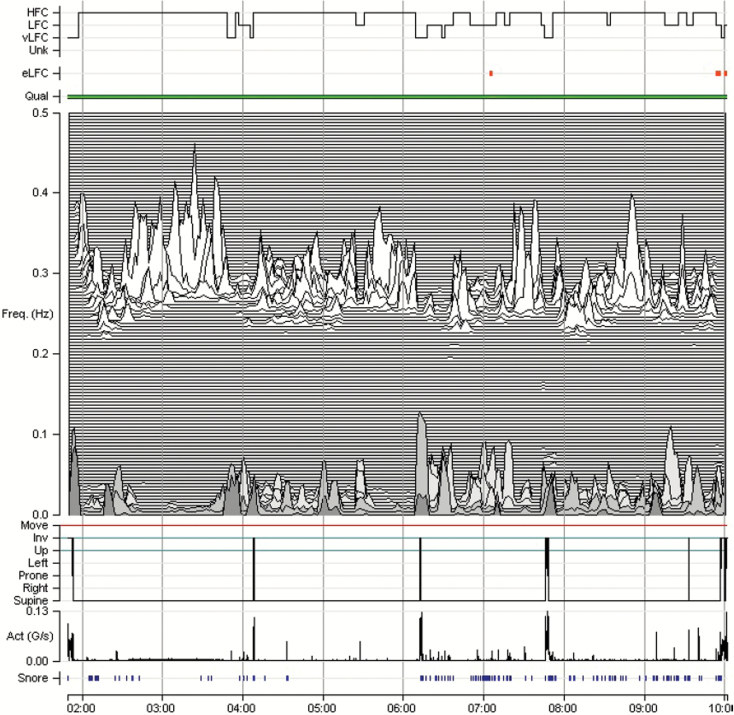
Tracking treatment effects in a patient with complex/treatment-emergent sleep apnea. M1 (home) recording 4 weeks after initiating CPAP, showing reduced HFC amount, as well as narrow-band coupling. Note minimal HFC and an expansion of the low-frequency coupling zone. The red coloring/arrow is the detection of e-LFCNB, signifying periods of repetitive central apneas, or sustained periodic breathing. As the narrow-band marker requires sustained self-similar oscillations lasting 15+ minutes, shorter bursts or single events will not be detected. On the other hand, mixed obstructive and central disease may show as “periodic” based on the oscillatory dispersion of coupling power. Thus, phenotype information that complements standard approaches can be obtained from the M1 recordings. Act = trunk actigraphy; g/second = Gravitational acceleration units/second; FREQ = frequency in Hz; Quality = ECG signal quality; SNORE = snoring vibration estimated by the accelerometer.
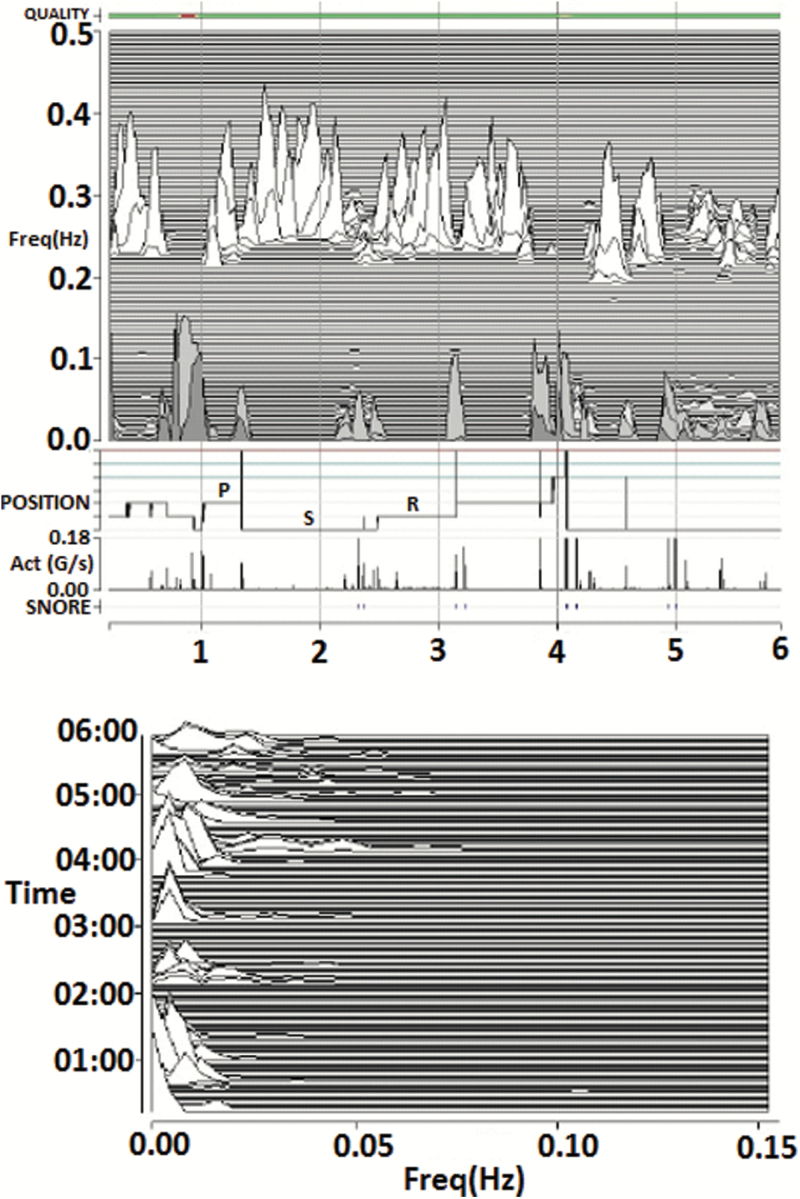
Figure 4.
Tracking treatment effects in a patient with complex/treatment-emergent sleep apnea. M1 (home) recording in the same participant as Figure 5 now treated with CPAP, low-dose acetazolamide, and a nonvented mask. Note the marked increase in HFC, consistent with stable breathing periods resulting from improved control of sleep apnea, and absence of e-LFCNB. Act = trunk actigraphy, g/second = Gravitational acceleration units/second; FREQ = frequency in Hz; Quality = ECG signal quality; SNORE = snoring vibration estimated by the accelerometer.
Figure 5.
Pharmacological effects. A 48-year-old male with complex sleep apnea and recurrent nocturnal arousals. Baseline: HFC = high-frequency coupling; LFC = low-frequency coupling; e-LFC = elevated-LFC; VLFC = very low frequency coupling; UNK = unknown (oscillations falling outside the other coupling ranges). Qual = ECG quality; Freq = frequency in Hz; Act = trunk actigraphy; g/second = gravitational acceleration/second.
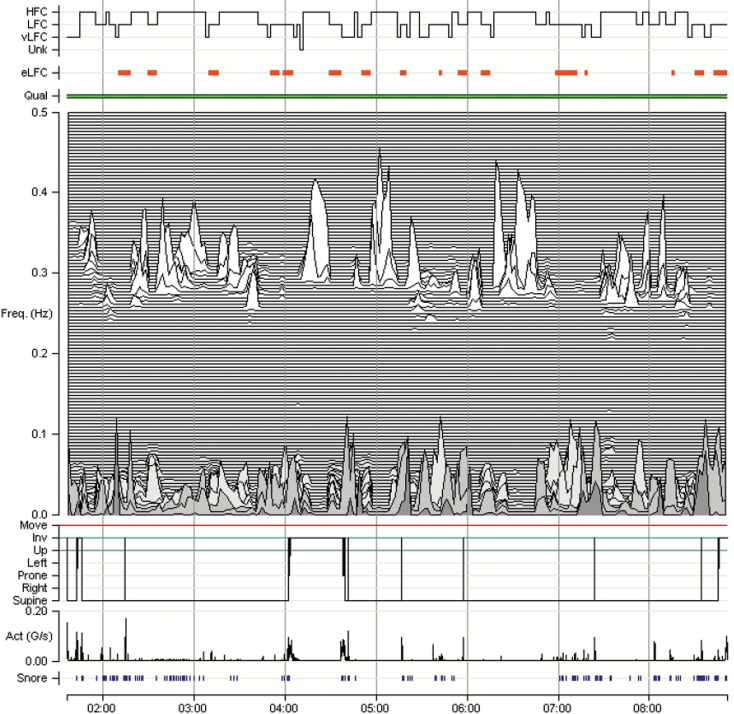
Figure 6.
Pharmacological effects. A 48-year-old male with complex sleep apnea and recurrent nocturnal arousals. One milligram clonazepam at bedtime. Note the marked increase in high frequency coupling (from 23.3% to 82%) with 1-mg dose. HFC = high-frequency coupling; LFC = low-frequency coupling; e-LFC = elevated-LFC; VLFC = very low-frequency coupling; UNK = unknown (oscillations falling outside the other coupling ranges). Qual = ECG quality; Freq = frequency in Hz; Act = trunk actigraphy; g/second = gravitational acceleration/second.
The device itself has the following dimensions: height 79.6 mm, width 48.7 mm, thickness 11.7 mm, weight 20 g, and a storage capacity of 500MB. The accelerometer within the device has the following specifications: 12-bit quantization, units are in gravitational acceleration “g” units. The Z channel is sampled at 300 Hz, the Y channel at 37.5 Hz, and the X channel at 37.5 Hz. Collectively, the X, Y, and Z channels are referred to as the “gravity channels,” and used to compute actigraphy, body position, and snore vibrations.
The M1 starts recording when the ECG is sensed, and stops when the ECG is no longer sensed. Thus, if participants place the device when they are ready to sleep (and not just get into bed) and take the device off on awakening, the analysis is constrained mostly to the sleep period. This minimizes unwanted large periods of wake recorded prior to sleep onset and complements the actigraphic analysis to more accurately capture pathology and physiology within the real total sleep time. This approach, however, may reduce accuracy of sleep onset latencies in those with prolonged latencies to sleep onset, so an alternate strategy is placing the device when getting into bed.
The SleepImage system allows review of raw data, to the resolution of individual ECG complexes, snoring bursts, and activity-driven sensor displacements. No important arrhythmia was noted in any participant.
Experimental protocol
Healthy participants
Participants were asked to wear the M1, placed at the time of “usual bedtime,” and taken off “on final awakening.” The target duration was 14 nights. The only constraints placed were to avoid more than two drinks before bedtime, use of prescription, or over the counter sedative-hypnotics, stimulants drugs, and tobacco or marijuana smoking. No toxic drug screens were performed.
People with sleep apnea
Participants were asked to use positive pressure therapy as usual and to maximize time nonsupine during sleep. No change in therapy or medications was done during the recording periods. The reason for the recording was to assess sleep quality in the context of persistent fatigue, despite highly compliant CPAP use.
People with insomnia
No study-specific constraints were placed on the participants, who maintained a sleep log in addition.
CPC analysis and sleep spectrograms
The CPC analysis of the ECG signal is performed as described in detail [6, 17]. Briefly, the method uses single-channel ECG to extract heart rate variability and ECG-derived respiration (EDR; amplitude variations in the QRS complex due to shifts in the cardiac electrical axis relative to the electrodes during respiration and changes in thoracic impedance as the lungs fill and empty). Time series of normal-to-normal sinus (N–N) intervals and the time series of the EDR associated with these N–N intervals are then extracted from the original R–R (QRS to QRS) interval time series. Outliers due to false or missed R-wave detections are removed using a sliding window average filter with a window of 41 data points and rejection of central points lying outside 20% of the window average. The resulting N–N interval series and its associated EDR are then resampled using cubic splines at 2 Hz. The cross-spectral power and coherence of these two signals are calculated over a 1,024 sample (8.5 minutes) window using the fast Fourier transform applied to the three overlapping 512 sample subwindows within the 1,024 coherence window. The 1,024 coherence window is then advanced by 256 samples (2.1 minutes) and the calculation repeated until the entire N–N interval/EDR series is analyzed.
For each 1,024 window, the product of the coherence and cross-spectral power is used to calculate the ratio of coherent cross power in the low-frequency (0.01–0.1 Hz) band to that in the high-frequency (0.1–0.4 Hz) band. The logarithm of the high-to-low frequency cardiopulmonary coupling ratio (log [HFC/LFC]) is then computed to yield a continuously varying measure of CPC. The output is thus a moving average of overlapping CPC windows. The graph of CPC at relevant frequencies (ordinate) vs. time (abscissa) provides a sleep spectrogram. Periods of very low-frequency coupling (VLFC) with detected movement are considered wake, and without activity, it is considered REM sleep [6]. When REM sleep has apneas, the coupling signatures are indistinguishable from e-LFC, but e-LFCNB does not occur during REM sleep.
When the power of LFC is considered, a subset, named elevated-LFC (e-LFC), detects apnea–hypopnea or sleep fragmentation [17]. Periods of e-LFC coincided with periods of scored apnea/hypopnea in the PhysioNet Sleep Apnea Database (http://www.physionet.org/physiobank/database/apnea-ecg/). Optimal detection thresholds required that the minimum low-frequency power be >0.05 normalized units and that the low-to-high frequency ratio be >30 to define periods of probable apnea/hypopnea. Since the apneas and hypopneas in this database were scored in 60-second epochs and CPC measurements made every 2.1 minutes, 60-second linear interpolation between consecutive 2.1-minute measurements was done. The 70 recordings in this database contained a total of 34,243 minutes of which 13,062 (38%) were scored as containing episodes of apnea/hypopnea. Sensitivities and specificities for minute-by-minute apnea detection were calculated for a range of LFC powers and low/high coupling ratios. Receiver–operator curves were then calculated, and the thresholds giving the maximum combined sensitivity and specificity for apnea/hypopnea detection were selected as optimal. Thus, e-LFC is defined here as a subset of low-frequency coupled cardiopulmonary oscillations, periods of which correlated significantly with periods of manually scored apneas and hypopneas in the PhysioNet Sleep Apnea Database. However, e-LFC is not restricted to apnea and is increased in conditions associated with sleep fragmentation such as depression [18] and fibromyalgia [8].
Some spectrograms from the PhysioNet Sleep Apnea Database demonstrated periods of near-constant frequency spectral peaks in the e-LFC region that was reminiscent of the sinusoidal oscillations of heart rate variability seen in Cheyne–Stokes respiration in people with heart failure, which has a relatively constant cycle length [17]. Since the period of central apnea can be as slow as 120 seconds or longer, we used the frequency band between 0.006 and 0.1 Hz to define narrow spectral band e-LFC (putative central sleep apnea, periodic breathing, or complex sleep apnea). We require (1) a minimum power in this band of 0.3 normalized units and (2) that the coupling frequency of each pair of consecutive measurements remains within 0.0059 Hz of each other over five consecutive sampling windows (totaling 17 continuous minutes) [17]. Periods of e-LFC not meeting these criteria are defined as broad spectral band e-LFC (e-LFCNB, putative pure OSA). The amounts of broad and narrow spectral band coupling in e-LFC bands are then expressed as the percentage of windows detected in relation to the total sleep period. Thus, the narrow spectral band e-LFC identified periods with oscillations that have a single dominant coupling frequency, suggesting central sleep apnea or periodic breathing [17]. The broad spectral band e-LFC (e-LFCBB) identified periods with oscillations that have variable coupling frequencies, suggesting an alternative mechanism, which we posited was dominance of anatomic upper airway obstructive processes. As it takes 17 minutes of continuous narrow-band CPC to reach the detection threshold, we estimate that this would be approximately equal to an averaged central apnea index of 5 per hour of sleep, assuming 6 hours of sleep and a periodic breathing cycle length of approximately 35 seconds.
Statistical analysis
Summary statistics were mean and standard deviation. Intraclass coefficients (ICCs) were computed for CPC variables, both across all nights and 2-week averages. CPC metrics were evaluated with three-way analysis of variance (ANOVA) with factors participant, night, and health state or disease. Sleep apnea and insomnia participant summary and intraclass coefficient data were estimated. All analysis was done using STATA 12. Significance thresholds were a p < .05. Pairwise comparisons of group results used multiple corrections (Tukey).
Results
Healthy participants and sleep apnea
Table 1 is a summary of the healthy controls (10 subjects) and sleep apnea (18 patients; 15/18 had periodic breathing noted during the titration polysomnograms). The polysomnograms of the healthy participants were unremarkable. All people with apnea were compliant (6.2 ± 0.9 hours) with positive pressure therapy, using during the entire self-reported sleep time (no missed nights), corroborated by data card review. The expected differences were present, including BMI, gender (male dominance in patients), and polysomnographic indices. Half of people with sleep apnea were hypertensive.
Table 1.
Participant characteristics
| Condition | Healthy controls mean ± SD | Sleep apnea (diagnostic study) mean ± SD | Insomnia mean ± SD |
|---|---|---|---|
| Sample size | 10 | 18 | 20 |
| Recording nights | 10–14 | 3–5 | 3–9 |
| Age | 26.5 ± 5.3 | 49.7 ± 10.4 | 45.15 ± 14.11 |
| Gender | 50% male | 70% male | 65% women |
| Race | 8 W, 1 A | 15 W, 3 AA, 2 A | 17W, 2 AA, 1A |
| Body mass index | 24.3 ± 2.3 | 32.5 ± 11.2 | |
| Hypertension | 0 | 50% | 0 |
| Total sleep time (TST) | 387 ± 55.6 | 332 ± 68.3 | |
| Wake after sleep onset | 54.2 ± 48.5 | 102.6 ± 35.7 | |
| Awakenings (number) | 20.4 ± 4.6 | 53.4 ± 19.3 | |
| Sleep efficiency (% TST) | 84.4 ± 10.7 | 78 ± 8.3 | |
| N1 | 9.4 ± 4.4 | 15.1 ± 9 | |
| N2 | 45.8 ± 9.7 | 61.8 ± 11.1 | |
| N3 | 23.3 ± 9.6 | 7.4 ± 12.3 | |
| REM | 21.4 ± 5.9 | 15.7 ± 7.5 | |
| AHI (4%) | 0 | 22 ± 7 | |
| RDI | 5.4 ± 9.1 | 46 ± 12 | |
| Minimum saturation | 95.3 ± 1.2 | 76.3 ± 8.2 | |
| Arousal index | 9.5 ± 11.3 | 52 ± 18 |
REM = rapid eye movement sleep; NREM = non-REM sleep; AHI = apnea–hypopnea index; RDI = respiratory disturbance index; N1, N2, N3 = standard NREM sleep stages; W = white; AA = African American; A = Asian.
Participants with insomnia
There were 20 participants with insomnia analyzed. The mean age was 45.15 ± 14.11 years. Thirteen were women, and race distribution was 17 white, two African American, and one Asian; none were Hispanic. Four used a sedative nightly or nearly nightly. Fourteen drank at least one cup of coffee per day, eight were social drinkers, and one was an active smoker. Self-reported total sleep time during the period of recording was 341 ± 125.4 minutes of sleep, significantly different from total sleep time estimated by M1 actigraphy (432.1 ± 82.8, p ≤ .001), and the Stanford Sleepiness Scale was 3.7 ± 1.4.
M1 data
Table 2 summarizes the M1 data. An average of 12.8 nights were recorded for the 10 healthy control subjects (range: 5 to 17, one participant did a few “extra nights” on own accord). Eighteen people with sleep apnea performed recordings for 3–4 nights. Twenty people with insomnia performed 121 recordings of 3–9 nights’ duration. Weekends were not always captured and thus did not enable weekday vs. weekend analysis. Overall, ANOVA was significant for the participant factor but not the night factor in all groups for all measures (all statistical significances for subject factor, p: <.001). The only statistically significant difference between weekday and weekend in healthy participants was HFC duration: 242.8 ± 53.8 vs. 275.8 ± 57.1 minutes (89 vs. 39 total nights), F(1,126) = 9.86, p = .002. There was an approximately 90-minute difference in self-reported vs. objective M1-estimated total sleep time in people with insomnia. Self-reported sleep time was not collected in people with sleep apnea, and there were missing data in healthy participants, not allowing direct comparisons.
Table 2.
M1 Data
| Measure | 1: 10 healthy participants (128 nights) mean ± SD | 2: 18 sleep apnea participants (65 nights) mean ± SD | 3: 20 insomnia participants (121 nights) mean ± SD | Pairwise comparison, Tukey (p: <.05) |
|---|---|---|---|---|
| Total sleep time (TST, actigraphic) | 395.7 ± 62.3 | 376 ± 51.7 | 432.8 ± 90 | NS |
| Sleep efficiency | 88.6 ± 12.1 | 86.7 ± 10.2 | 78.8 ± 4.2 | NS |
| Wake after sleep onset (actigraphic) | 50.3 ± 20 | 62.9 ± 47.8 | 75.1 ± 34 | 3 > 1 |
| Arousals (actigraphic, total, TST) | 45.6 ± 22.9 | 28.2 ± 22.1 | 68.1 ± 36.4 | 3 > 2 |
| HFCP | 55.2 ± 11 | 48.9 ± 32.3 | 44 ± 13.8 | NS |
| HFCD | 218 ± 56.7 | 180.3 ± 121.4 | 225.7 ± 80.9 | 3 > 2 |
| LFCP | 28.7 ± 11.3 | 38.6 ± 22.1 | 35.6 ± 12.8 | NS |
| LFCD | 113.6 ± 51.3 | 145.1 ± 83.1 | 185.2 ± 82.9 | 3 > 1, 2 2 > 1 |
| VLFCP | 17.3 ± 10 | 13.5 ± 11.7 | 20.1 ± 6.4 | NS |
| VLFCD | 68.5 ± 27.9 | 50.8 ± 44 | 102 ± 41.9 | 3 > 2 |
| e-LFCBBP | 13.8 ± 7.7 | 15.8 ± 14.9 | 19.9 ± 10.5 | NS |
| e-LFCBBD | 64.2 ± 38.5 | 59.4 ± 56 | 104.5 ± 65.1 | 3 > 2 |
| e-LFCNBP | 0.005 ± 0.01 | 12.7 ± 6.3 | 0.8 ± 1.7 | 3 > 1, 2 |
| e-LFCNBD | 1.9 ± 4.7 | 47.8 ± 23.7 | 4.5 ± 8.3 | 2 > 1, 3 |
| Supine sleep % actigraphic TST | 51.1 ± 22.3 | 38.6 ± 12.5 | * | p: <.01** |
| Subjective total sleep time | * | * | 341.3 ± 125.4 | |
| Stanford Sleepiness Scale | * | * | 3.7 ± 1.4 |
HFC = high-frequency coupling; D = duration; LFC = low-frequency coupling; P = percentage; VLFC = very low-frequency coupling; BB, NB = broad and narrow band; e = elevated; TST = total sleep time (minutes); SD = standard deviation; NS = not significant.
*Data not available or incomplete.
**t-test.
Intraclass coefficients
Intra-individual stability of the signal was highest for HFC and actigraphic arousals. The ICC was higher when averaged by week (2 weeks for healthy participants) than when estimating variance across all days of recordings. Body position also showed moderate ICCs, especially the supine position. All participants with sleep apnea were encouraged to sleep nonsupine, but showed substantial supine sleep [19]. Total sleep time varied substantially across days of the week within individuals, but when 2 weeks were considered, the ICC was 0.54. Sleep apnea participants were similar in ICC. The highest ICCs in healthy participants and people with sleep apnea were noted for actigraphic arousals in healthy participants (0.94 and 0.88, respectively). Narrow band coupling, a marker of sustained periods of periodic breathing or central apneas, showed a high ICC (0.86) in people with sleep apnea. People with insomnia had a somewhat different pattern. Overall, the ICC’s were lower, consistent with greater intra-individual variability relative to healthy participants and people with sleep apnea. The ICC values for “nights” were very low (<0.05), not significant in any group. We were unable to detect any sex differences in CPC metrics and ICC’s, but were not powered to conclusively address this dimension.
Pharmacological effects
One participant each was evaluated pre- or post-pramipexole for periodic limb movement disorder, suvorexant for persistent nonrefreshing sleep, and clonazepam for persistent awakenings, despite optimal positive airway pressure therapy in a patient with complex sleep apnea. Only the use of clonazepam in the patient with complex apnea showed a clinically meaningful difference (Figures 3 and 4).
An analysis of nights with (47, complete or partial missing data in five participants) and without (56) sedative use in people with insomnia showed no statistically significant difference, but the HFC percentage (46.7 ± 14.9 vs. 41.8 ± 13.2, p: .07) and duration in minutes (230.6 ± 80.6 vs. 204.8 ± 71.2, p: .08) averages were the closest to significance.
Tracking sleep apnea
Sleep physiology improvements in people with sleep apnea after initiation of treatment may be tracked and quantified with the M1 (Figures 5 and 6). The mean HFC increased from 23.7 ± 18.3% total sleep period on the diagnostic polysomnogram to 48.9 ± 32.3% total actigraphic-estimated sleep period during therapy with positive airway pressure, t-test, p: <.02. There was a reduction in LFC (53.7 ± 21.6 to 38.6 ± 22.1, p: .02) but no significant change in e-LFCNB (15.8 ± 14.9 vs. 12.7 ± 6.3, p: .13).
Comparison of health, apnea, and insomnia groups
CPC and M1 metrics across groups are shown in Table 3. Important differences included increased actigraphic wake after sleep onset in people with insomnia, reduced high frequency duration in people with apnea, increased LFC duration in people with apnea and insomnia, and increased broad-band LFC duration in people with insomnia.
Table 3.
Intra-individual vs. interindividual variance (intraclass coefficients)
| Measure | Healthy participants by day | Healthy participants by week | Sleep apnea participants by day | Insomnia participants by day |
|---|---|---|---|---|
| Total sleep time (TST, actigraphic) | 0.0 | 0.54 | 0.66 | 0.33 |
| Wake after sleep onset (actigraphic) | 0.24 | 0.58 | 0.54 | 0.54 |
| Arousals (actigraphic, total, TST) | 0.62 | 0.94 | 0.88 | 0.39 |
| HFCP | 0.44 | 0.83 | 0.89 | 0.66 |
| HFCD | 0.35 | 0.72 | 0.87 | 0.54 |
| LFCP | 0.31 | 0.58 | 0.76 | 0.60 |
| LFCD | 0.44 | 0.64 | 0.69 | 0.56 |
| VLFCP | 0.20 | 0.64 | 0.60 | 0.52 |
| VLFCD | 0.47 | 0.89 | 0.74 | 0.50 |
| e-LFCBBP | 0.48 | 0.63 | 0.73 | 0.50 |
| e-LFCBBD | 0.45 | 0.64 | 0.69 | 0.51 |
| e-LFCNBP | 0.01 | 0.41 | 0.86 | 0.20 |
| e-LFCNBD | 0.08 | 0.44 | 0.88 | 0.11 |
| Supine | 0.58 | 0.72 | 0.73 | * |
| Left | 0.33 | 0.66 | 0.58 | * |
| Right | 0.37 | 0.68 | 0.46 | * |
HFC = high-frequency coupling; D = duration; LFC = low-frequency coupling; P = percentage; VLFC = very low-frequency coupling; BB, NB = broad and narrow bands; e = elevated; TST = total sleep time (minutes)
*Data not available or incomplete.
Discussion
The key points from our research study are as follows: (1) ambulatory tracking of sleep quality using the M1-SleepImage system is feasible and provides clinically meaningful information. (2) ECG-CPC measurements of sleep stability show substantial stability of patterns within individuals over time in health and disease. (3) As HFC on the ECG-CPC tracks slow wave power, the results could provide new insights into night-to-night sleep homeostatic mechanisms. (4) In people with sleep apnea, therapeutic efficacy may be tracked, including detection of persistence or emergence of high loop gain features (treatment-emergent central sleep apnea/complex sleep apnea). (5) Dissociation between self-reported and objective sleep quantity in people with insomnia complaints may identify individuals with aberrant somnoperception, potentially providing insights into insomnia and nonrestorative sleep.
The results show the feasibility of using the M1-SleepImage system for tracking sleep quality and stability in health and disease. Several nights’ recording provides information on night-to-night variability within and across individuals. In health, variability may reflect past sleep debt (e.g., restriction) and current modifiers (e.g., stress, affective state, pain, body position, and sleep disorders). For example, the higher HFC on the weekends could reflect rebound sleep with mild chronic restriction during weeknights in the healthy group. The interindividual stability of HFC percentage across 2 weeks of recordings is consistent with similar degrees of expression of sleep homeostatic drive on a night-to-night basis, i.e., a sleep bioprint for the individual. A remarkable feature of sleep–wake regulation is a high degree of stable trait-like interindividual variability in sensitivity or resistance to sleep deprivation effects [20–25]. Proposed mechanisms have included genomic factors [26–29], electrocortical activity [30], and brain network physiology [31]. Our results show that stable NREM sleep has sustained night-to-night stability within individuals and substantial interindividual differences.
The healthy participants in this study were minimally constrained, unlike strict instructions often provided in most clinical studies. This may have enabled detection of interesting patterns, such as substantial variance of total sleep time across a week but having at least moderate week-to-week constancy, possibly constrained by the needs for adaptive sleep homeostasis. Despite no significant ICC for total sleep time across the week, there was moderate ICC for HFC percentage, consistent with a minimum and individually determined amount of stable sleep regardless of total sleep time. The weekday or weekend differences in HFC duration probably detected catch up weekend stable sleep.
The results in participants with insomnia showed patterns consistent with the known night-to-night variability of sleep times and sleep quality in this population [32–34]. As variability is a treatment target, successful behavioral or pharmacological treatments can be expected to reduce variability and increase measured ICC’s by methods such as the M1. Relative to healthy controls, HFC percentage for M1 total sleep time was reduced in participants with insomnia, but the total HFC duration was similar. Total M1 estimated sleep time was longer in people with insomnia, one possible explanation is increased time in bed to compensate for poor quality. M1-estimated total sleep time is not the same as actigraphic total sleep time, but the sum of time spent in HFC, LFC, and VLFC associated with absence of actigraphic movement (presumptive REM sleep, though quiet wake will mimic REM and cannot be easily distinguished). Thus, if there is movement detected by actigraphy but the CPC estimate is that of any of the LFC metrics, that period is considered sleep. Movements during HFC are almost never seen (personal observation of over 1,000 nights of recorded data).
Regarding misperception seen in the insomnia group, there is increasing interest in this phenomenon beyond mechanistic interest. Objective measurement of sleep duration appears to be crucial for insomnia phenotyping, as recent epidemiology data suggest that the medical and psychiatric morbidity previously associated with self-reported insomnia or short sleep duration is isolated to those insomniacs with objective short sleep duration on PSG testing [35] The degree of misperception appears state-like, as it can be induced in healthy adults [36] and can be improved in people with OSA during positive pressure titration [37]. Introducing objective device-based monitoring for people with insomnia holds promise not only for diagnostic phenotyping, but could be used to provide therapeutic feedback, as previously reported by Harvey et al. using actigraphy [38].
Improved ambulatory tracking of the effects of sleep apnea therapies can have clinical utility. As the ECG-CPC method can also detect sustained central apnea and periodic breathing, phenotyping residual sleep apnea is possible, enabling targeted therapies. For example, we previously showed that CPC metrics predicted PAP failure [17]. Ambulatory tracking of sleep quality, such as the method described here, could provide objective evidence of benefits and feedback for the patient regarding effectiveness of pharmacological sleep therapy. Increased night-to-night variability and dissociation between subjective perception of sleep quantity or quality and objectively measured sleep occur in subsets of patients presenting with insomnia. Tracking with the M1-SleepImage system can enable one view of these processes.
Limitations or caveats of our study include the unconstrained nature of recordings, but these could also more closely reflect real-life heterogeneity of factors influencing sleep from night to night. Well-matched (e.g., age and BMI) and head-to-head comparisons of different clinical groups are not possible from our data. Our people with insomnia did not have PSG, and mild sleep apnea could have been present. We did not perform simultaneous M1 recording with PSG, but we believe that the primary signal (ECG) is so robust that the analysis results of repeated PSG or M1-acquired ECG analysis would be identical. We have not directly evaluated in detail the potential uses of the M1 technology, so the use of this and other devices in the realm of dynamic tracking of sleep remain promising but speculative.
In conclusion, the M1-SleepImage system shows clinically useful characteristics which can be applied to sleep medicine practice. Tracking the dynamics of sleep over prolonged periods of time, by whatever method, is likely to provide unique insights into sleep regulation in health and disease.
Funding
This study was funded by National Heart, Lung and Blood Institute grant 1RC1HL099749-01 to Robert J. Thomas.
Notes
Disclosure Statement. RJT reports the following: (1) Patent, license, and royalties from MyCardio, LLC, for an ECG-based method to phenotype sleep quality and sleep apnea; (2) Grant support, license, and intellectual property (patent submitted) from DeVilbiss Healthcare; (3) GLG consulting for general sleep medicine; (4) Intellectual Property (patent) for a device using CO2 for central/complex sleep apnea. MTB reports the following: (1) Funding from Massachusetts General Hospital, the Center for Integration of Medicine and Innovative Technology, the Milton Family Foundation, and currently receives funding from the Department of Neurology, the MGH-MIT Grand Challenge, and the American Sleep Medicine Foundation. (2) A patent pending on a home sleep monitoring device. (3) Consulting and research agreements with MC10, Insomnisolv, McKesson, a medical monitor for Pfizer, and has provided expert testimony in sleep medicine.
References
- 1. Barrett PM, et al. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(1):95.e11–95.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gargiulo G, et al. Wearable dry sensors with bluetooth connection for use in remote patient monitoring systems. Stud Health Technol Inform. 2010;161:57–65. [PubMed] [Google Scholar]
- 3. Russo K, et al. Consumer sleep monitors: is there a baby in the bathwater?Nat Sci Sleep. 2015;7(11):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ko PR, et al. Consumer sleep technologies: a review of the landscape. J Clin Sleep Med. 2015;11(12):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Llewellyn S,et al. Not only … but also: REM sleep creates and NREM Stage 2 instantiates landmark junctions in cortical memory networks. Neurobiol Learn Mem. 2015;122(7):69–87. [DOI] [PubMed] [Google Scholar]
- 6. Thomas RJ, et al.. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28(9):1151–1161. [DOI] [PubMed] [Google Scholar]
- 7. Bianchi MT, et al.. Technical advances in the characterization of the complexity of sleep and sleep disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas RJ, et al.. Impaired sleep quality in fibromyalgia: detection and quantification with ECG-based cardiopulmonary coupling spectrograms. Sleep Med. 2010;11(5): 497–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas RJ, et al.. Prevalent hypertension and stroke in the Sleep Heart Health Study: association with an ECG-derived spectrographic marker of cardiopulmonary coupling. Sleep. 2009;32(7):897–904. [PMC free article] [PubMed] [Google Scholar]
- 10. Choi JH, et al.. Sleep quality change after upper airway surgery in obstructive sleep apnea: electrocardiogram-based cardiopulmonary coupling analysis. Laryngoscope. 2015;125(7):1737–1742. [DOI] [PubMed] [Google Scholar]
- 11. Guo D, et al.. ECG-derived cardiopulmonary analysis of pediatric sleep-disordered breathing. Sleep Med. 2011;12(4):384–389. [DOI] [PubMed] [Google Scholar]
- 12. Lee WH, et al.. Cardiopulmonary coupling analysis: changes before and after treatment with a mandibular advancement device. Sleep Breath. 2014;18(4):891–896. [DOI] [PubMed] [Google Scholar]
- 13. Lee WH, et al.. A comparison of different success definitions in non-continuous positive airway pressure treatment for obstructive sleep apnea using cardiopulmonary coupling. J Clin Sleep Med. 2016;12(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schramm PJ, et al.. Quantitative measurement of sleep quality using cardiopulmonary coupling analysis: a retrospective comparison of individuals with and without primary insomnia. Sleep Breath. 2013;17(2):713–721. [DOI] [PubMed] [Google Scholar]
- 15. Schramm PJ, et al.. Sleep quality changes in chronically depressed patients treated with mindfulness-based cognitive therapy or the cognitive behavioral analysis system of psychotherapy: a pilot study. Sleep Med. 2016;17(1): 57–63. [DOI] [PubMed] [Google Scholar]
- 16. Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(Suppl 1):S7–S11. [DOI] [PubMed] [Google Scholar]
- 17. Thomas RJ, et al.. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30(12):1756–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang AC, et al.. Sleep state instabilities in major depressive disorder: detection and quantification with electrocardiogram-based cardiopulmonary coupling analysis. Psychophysiology. 2011;48(2):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Russo K, et al.. How reliable is self-reported body position during sleep?J Clin Sleep Med. 2016;12(1):127–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bakotic M, et al.. State-trait arousal and daytime sleepiness after sleep restriction. Int J Psychophysiol. 2013;88(2):164–170. [DOI] [PubMed] [Google Scholar]
- 21. Chua EC, et al.. Individual differences in physiologic measures are stable across repeated exposures to total sleep deprivation. Physiol Rep. 2014;2(9):e12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goel N, et al.. Phenotyping of neurobehavioral vulnerability to circadian phase during sleep loss. Methods Enzymol. 2015;552:285–308. [DOI] [PubMed] [Google Scholar]
- 23. Van Dongen HP. Shift work and inter-individual differences in sleep and sleepiness. Chronobiol Int. 2006;23(6): 1139–1147. [DOI] [PubMed] [Google Scholar]
- 24. Van Dongen HP, et al.. Individual differences in adult human sleep and wakefulness: Leitmotif for a research agenda. Sleep. 2005;28(4):479–496. [DOI] [PubMed] [Google Scholar]
- 25. Spaeth AM, et al.. Phenotypic vulnerability of energy balance responses to sleep loss in healthy adults. Sci Rep. 2015;5(10):14920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Satterfield BC, et al.. TNFα G308A polymorphism is associated with resilience to sleep deprivation-induced psychomotor vigilance performance impairment in healthy young adults. Brain Behav Immun. 2015;47(7):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maire M, et al.. Sleep ability mediates individual differences in the vulnerability to sleep loss: evidence from a PER3 polymorphism. Cortex. 2014;52(3):47–59. [DOI] [PubMed] [Google Scholar]
- 28. Reichert CF, et al.. Insights into behavioral vulnerability to differential sleep pressure and circadian phase from a functional ADA polymorphism. J Biol Rhythms. 2014;29(2): 119–130. [DOI] [PubMed] [Google Scholar]
- 29. Pellegrino R, et al.. A novel BHLHE41 variant is associated with short sleep and resistance to sleep deprivation in humans. Sleep. 2014;37(8):1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tarokh L, et al.. The spectrum of the non-rapid eye movement sleep electroencephalogram following total sleep deprivation is trait-like. J Sleep Res. 2015;24(4):360–363. [DOI] [PubMed] [Google Scholar]
- 31. Yeo BT, et al.. Functional connectivity during rested wakefulness predicts vulnerability to sleep deprivation. Neuroimage. 2015;111(5):147–158. [DOI] [PubMed] [Google Scholar]
- 32. Baron KG, et al.. Sleep variability among older adults with insomnia: associations with sleep quality and cardiometabolic disease risk. Behav Sleep Med. 2017;15(2):144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buysse DJ, et al.. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11(1):56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suh S, et al.. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012;13(5):469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vgontzas AN, et al.. Insomnia with short sleep duration: nosological, diagnostic, and treatment implications. Sleep Med Clin. 2013;8(3):309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bianchi MT, et al.. Sleep misperception in healthy adults: implications for insomnia diagnosis. J Clin Sleep Med. 2012;8(5):547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castillo J, et al.. Sleep-wake misperception in sleep apnea patients undergoing diagnostic versus titration polysomnography. J Psychosom Res. 2014;76(5):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harvey AG, et al.. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138(1):77–101. [DOI] [PMC free article] [PubMed] [Google Scholar]