Abstract
OBJECTIVES
Bicuspid aortic valve (BAV) is the most common congenital valvular abnormality and frequently presents with accelerated calcific aortic valve disease, requiring aortic valve replacement (AVR) and thoracic aortic aneurysm and dissection. Supporting evidence for Association Guidelines of aortic dimensions for aortic resection is sparse. We sought to determine whether concurrent repair of dilated or aneurysmal aortic disease during AVR in patients with BAV substantially improves morbidity and mortality outcomes.
METHODS
Mortality and reoperation outcomes of 1301 adults with BAV and dilated aorta undergoing AVR-only surgery were compared to patients undergoing AVR with aortic resection (AVR-AR) using Cox proportional hazards modelling and patient matching.
RESULTS
Clinically important differences in patient characteristics, aortic valve function and aortic dimensions were identified between cohorts. Event rates were low, with rates of reoperation and death within 1 year of only 1.8% and 5.4%, respectively, and no aortic dissection observed during follow-up. There were no significant differences in reoperation or mortality outcomes between the AVR-only and AVR-AR cohorts. Age, aortic dimension or a combination thereof was not associated with better or worse outcomes after each AVR-AR compared with AVR.
CONCLUSIONS
We conclude AVR-only and AVR-AR surgery have low morbidity and mortality and have utility over a wide range of age and aortic sizes. Our results do not provide support for the 45-mm aortic dimension recommended in the current guidelines for aortic resection while performing AVR or any other specific dimension.
Keywords: Bicuspid aortic valve, Aortic valve replacement, Aortic aneurysm, Aorta, Survival
INTRODUCTION
Bicuspid aortic valve (BAV) is the most common congenital valvular abnormality, with an overall prevalence of 0.5–2% [1]. Patients with BAV often present with accelerated calcific aortic valve disease, requiring aortic valve replacement (AVR), more frequently and earlier than do patients with a tricuspid aortic valve [2, 3]. Of patients with echocardiographic diagnosis of BAV, 50% will eventually require AVR [4].
The incidence of ascending aortic dissection in patients with BAV is estimated to be 8 times higher than that in the general population [4]. Yet single-centre analyses focusing on long-term risk for dissection after isolated AVR in patients with BAV have yielded conflicting findings [5–8]. The indications for concomitant intervention on the thoracic aorta at the time of AVR are therefore controversial [9–11]. Joint Society Guideline recommendations for surgical replacement of the ascending aorta based on aortic size that currently state ‘Replacement of the ascending aorta is reasonable in patients with BAV undergoing AVR because of severe aortic stenosis or aortic regurgitation when the diameter of the ascending aorta is greater than 4.5 cm’ [12, 13]. However, the evidence supporting this recommendation is not definitive [6, 10, 11, 14, 15], and such an aggressive surgical treatment strategy of BAV-associated aortopathy has been questioned [5, 16–18].
We sought to test the central hypothesis that concurrent repair of dilated or aneurysmal aortic disease during AVR in patients with BAV improves morbidity and mortality outcomes and that there is an aortic dimension, above which aortic repair yields improved patient outcomes. We tested this hypothesis by comparing long-term outcomes of mortality and reoperation, for adult patients with BAV undergoing primary AVR, with or without concomitant aortic repair over ranges of aortic dimension and age, while accounting for other causes of mortality and reoperation in an observational 2-institution study. The overall goal of this study was to provide substantial increase in the level of evidence that the Society Guidelines are based upon.
MATERIALS AND METHODS
Patient selection
From the medical records of Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH) with institutional review board approval, 2148 adults with imaging-confirmed BAV undergoing their first aortic valve surgery between 1 January 2002 and 30 June 2014 were identified from institutional Society of Thoracic Surgeons (STS) and hospital databases.
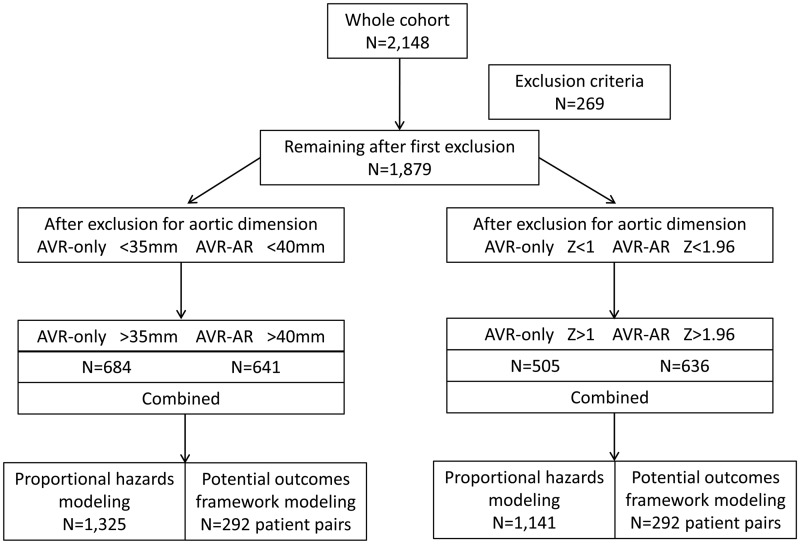
Exclusion criteria were age <18 or ≥90 years, connective tissue disease, previous AVR or repair, previous thoracic aortic surgery including coarctation repair, congenital heart disease other than BAV and patients who underwent transcatheter or transapical AVR. Patients undergoing AVR for endocarditis, aortic dissection or aortic resection for a calcified aorta were also excluded. These criteria yielded 1879 BAV patients available for analysis (CONSORT diagram, Fig. 1).
Figure 1:
CONSORT diagram. AVR: aortic valve replacement; AR: aortic resection.
Data collection
Patient demographics and hospital outcomes from electronic medical records were coded and defined according to the STS specifications [19]. Natural language queries combined with individual review of hospital discharge summaries, surgical records and transthoracic and transoesophageal echocardiographic reports were performed for the diagnosis of BAV.
Aortic dimensions were obtained from the most recent transthoracic and transoesophageal echocardiogram, computed tomography and magnetic resonance imaging obtained within 6 months prior to surgery. No accounting was made for possible systematic differences in dimensions between imaging techniques. Patients with inadequate imaging or reporting of the aortic root and ascending aortic diameters were remeasured by a single trained observer. Patients without further imaging were excluded.
Mortality data were obtained from institutional follow-up protocols, internal research data repositories and the US Social Security Death Index. The primary composite outcome of interest was composite all-cause mortality or reoperation upon the ascending aorta. The time to a long-term event was calculated from the date of first surgery to the first documented qualifying event or to 30 October 2016, if none occurred. Patients were followed up for a median of 6.6 (10th–90th percentiles; 3.3–11.5) years.
Analysis plan
To test the hypothesis that concurrent repair of dilated or aneurysmal aortic disease during AVR in patients with BAV substantially improves morbidity and mortality outcomes, we examined patients undergoing AVR-only surgery who had the largest aortic dimension ≥35 mm, compared to patients undergoing AVR with aortic resection (AVR-AR) who had the largest aortic dimension ≥40 mm at the levels of the sinuses of Valsalva or ascending aorta of each patient. Patients with largest aortic dimensions >59 mm were excluded, as they were not informative to the hypothesis, yielding 1301 patients for analyses. We additionally normalized aortic dimensions to Z-scores using a robust population-based algorithm [20] that provides separate estimations for both the sinuses of Valsalva and the ascending aorta supplement using identical methods (Supplementary Material).
Statistical analysis
Group differences in variables were compared by the Fisher’s exact test, Student’s t-test or Mann–Whitney’s U-test, as appropriate and were expressed as percentages, means (SD) or medians (10–90th percentiles). All statistical tests were 2-sided, and a P-value <0.05 was considered statistically significant. We performed both multivariable stepwise proportional hazards regression modelling of the composite outcome of mortality or reoperation using entry and exit P-values of 0.1. To control for potential selection bias, we also performed propensity matching using the potential outcomes framework (Supplementary Material).
RESULTS
Study cohort characteristics
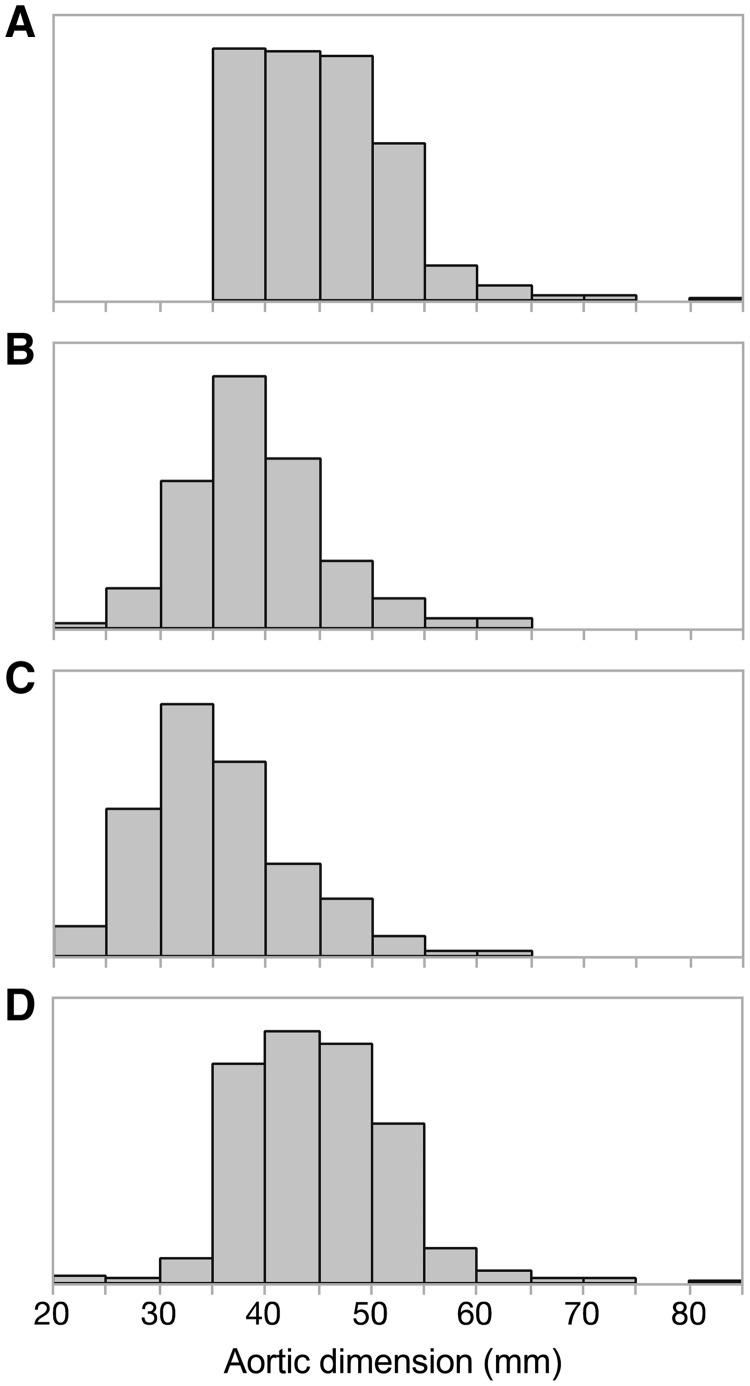
We observed differences in preoperative patient characteristics, aortic valve function and aortic dimensions between patient cohorts comprising 1301 patients with BAV, 683 undergoing AVR alone and 618 undergoing AVR with aortic root replacement (n = 47) or ascending aortic surgery (n = 571) (Table 1). Patients in the AVR-AR cohort were younger with larger overall aortic dimensions and had less severe aortic stenosis than patients in the AVR-only cohort. Five hundred twenty (81%) patients had the largest aortic dimension ≥45 mm; 249 (6%) patients had ≥50 mm. The AVR-only cohort also had a higher prevalence of moderate or severe mitral regurgitation and concurrent mitral valve surgery with 110 (16%) patients having the largest aortic dimension of ≥45 mm and 40 (6%) patients having ≥50 mm. Distributions of patient characteristics at the 2 institutions were similar (Supplementary Material, Tables S2 and S3). Distribution of aortic dimensions and operation is shown in Figs 2 and 3.
Table 1:
Demographic, medical and surgical characteristics and aortic dimensions of 1301 patients without exclusion criteria and with aortic dimensions ≥35 mm and <59 mm (for AVR cohort) or ≥ 40 mm (for AVR with ascending aortic aneurysm repair cohort) and the largest aortic dimension ≤59 mm
| AVR-only (n = 683) | AVR and AAA (n = 618) | P-value | |
|---|---|---|---|
| Preoperative data | |||
| Age at AVR (years), n (%) | |||
| <50 | 37 (6) | 45 (7) | <0.0001 |
| 50–59 | 81 (12) | 103 (16) | |
| 60–69 | 151 (22) | 155 (25) | |
| 70–79 | 225 (33) | 227 (37) | |
| ≥80 | 188 (28) | 88 (14) | |
| Gender (female), n (%) | 148 (22) | 151 (24) | 0.24 |
| Race (Caucasian), n (%) | 640 (94) | 596 (96) | 0.031 |
| BMI >30 kg/m2, n (%) | 208 (31) | 188 (30) | 0.98 |
| Smoker past or current, n (%) | 236 (35) | 193 (31) | 0.20 |
| COPD, n (%) | 84 (12) | 54 (9) | 0.035 |
| Diabetes (NIDDM or IDDM), n (%) | 111 (16) | 41 (7) | <0.0001 |
| Dyslipidaemia, n (%) | 454 (67) | 326 (53) | <0.0001 |
| Hypertension, n (%) | 412 (61) | 343 (56) | 0.039 |
| Preoperative creatinine, mean (SD) | 1.12 (0.61) | 1.04 (0.45) | 0.004 |
| Preoperative dialysis, n (%) | 5 (1) | 4 (1) | 0.85 |
| Cancer, n (%) | 108 (16) | 82 (13) | 0.19 |
| Prior stroke, n (%) | 45 (7) | 31 (5) | 0.22 |
| Peripheral vascular disease, n (%) | 48 (7) | 99 (16) | <0.0001 |
| Medications, n (%) | |||
| Beta-blocker | 245 (36) | 275 (44) | 0.006 |
| ACEI/ARB | 499 (73) | 397 (64) | 0.001 |
| Lipid lowering | 612 (89) | 570 (92) | 0.04 |
| Prior cardiac status | |||
| NYHA class, n (%) | |||
| I or II | 170 (59) | 213 (73) | 0.002 |
| III | 99 (34) | 66 (24) | |
| IV | 21 (7) | 14 (5) | |
| Heart failure, n (%) | 135 (20) | 91 (15) | 0.016 |
| Prior CABG surgery, n (%) | 29 (6) | 3 (1) | <0.0001 |
| Prior non-aortic valve surgery, n (%) | 14 (3) | 8 (2) | 0.39 |
| Coronary and valve disease | |||
| Coronary artery disease, n (%) | 342 (50) | 221 (36) | <0.0001 |
| Aortic insufficiency, n (%) | |||
| None, trace or mild | 452 (66) | 362 (59) | 0.0003 |
| Moderate | 121 (18) | 167 (27) | |
| Severe | 109 (16) | 89 (14) | |
| Aortic valve area (cm2), mean (SD) | 0.80 (0.30) | 0.92 (0.39) | <0.0001 |
| Mitral incompetence, n (%) | |||
| None, trace or mild | 567 (83) | 546 (88) | 0.020 |
| Moderate | 86 (13) | 50 (8) | |
| Severe | 29 (4) | 22 (4) | |
| LV ejection fraction (%), n (%) | |||
| <30 | 45 (7) | 14 (2) | 0.0003 |
| 30–49 | 75 (11) | 58 (10) | |
| ≥50 | 552 (82) | 557 (88) | |
| Aortic measurements (mm) | |||
| Aortic root dimension | |||
| Mean (SD)/n | 37.3 (5.3)/287 | 39.1 (6.4)/349 | 0.0001 |
| Median (10–90% CI) | 37 (31–44) | 39 (31–47) | 0.0006 |
| Sinotubular junction dimension | |||
| Mean (SD)/n | 32.7 (5.7)/394 | 36.4 (6.6)/361 | <0.0001 |
| Median (10–90% CI) | 32 (26–40) | 36 (29–46) | <0.0001 |
| Ascending aorta dimension | |||
| Mean (SD)/n | 39.7 (4.7)/557 | 47.3 (4.7)/603 | <0.0001 |
| Median (10–90% CI) | 39 (35–46) | 47 (42–53) | <0.0001 |
| Largest aortic dimension | |||
| Mean (SD)/n | 40.2 (4.2)/682 | 47.9 (4.1)/618 | <0.0001 |
| Median (10–90% CI) | 39 (35–46) | 48 (43–54) | <0.0001 |
| Operation | |||
| Hospital, n (%) | |||
| BWH | 245 (36) | 275 (45) | 0.002 |
| MGH | 437 (64) | 343 (55) | |
| Year of operation, n (%) | |||
| 2002–03 | 61 (9) | 35 (6) | <0.0001 |
| 2004–05 | 74 (11) | 68 (11) | |
| 2006–07 | 131 (19) | 88 (14) | |
| 2008–09 | 110 (16) | 153 (25) | |
| 2010–11 | 128 (19) | 146 (24) | |
| 2012–14 | 178 (26) | 128 (21) | |
| Urgency, n (%) | |||
| Elective | 551 (81) | 531 (86) | 0.013 |
| Non-elective | 131 (19) | 87 (14) | |
| CABG performed, n (%) | 158 (23) | 100 (16) | 0.002 |
| DHCA used, n (%) | 1 (0) | 339 (55) | <0.0001 |
| Aortic valve implant type, n (%) | |||
| Mechanical | 129 (21) | 143 (26) | 0.039 |
| Bioprosthesis | 489 (79) | 407 (74) | |
| Mitral valve repair or replacement, n (%) | 47 (7) | 20 (3) | 0.003 |
| Postoperative outcomes | |||
| Duration of follow-up (years), median (10–90% CI) | 6.5 (3.2–12.1) | 6.8 (3.4–11.4) | 0.68 |
| Operative mortality, n (%) | 5 (1) | 2 (0) | 1.00 |
| Death or aortic reoperation, n (%) | |||
| 1 year | 13/668 (1.9) | 16/602 (2.7) | 0.34 |
| 5 years | 36/445 (8.1) | 28/431 (6.5) | 0.33 |
| 10 years | 42/145 (29) | 23/110 (21) | 0.19 |
| Second operation type, n (%) | |||
| Aortic repair or replacement | 0 (0) | 4 (0) | 1.00 |
| AVR and aortic repair or replacement | 1 (0) | 4 (0) | |
AAA: ascending aortic aneurysm; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blockers; AVR: aortic valve replacement; BMI: body mass index; BWH: Brigham and Women’s Hospital; CABG: coronary artery bypass grafting; CI: confidence interval; COPD: chronic obstructive pulmonary disease; DHCA: deep hypothermic circulatory arrest; IDDM: insulin-dependent diabetes mellitus; LV: left ventricular; MGH: Massachusetts General Hospital; NIDDM: non-insulin-dependent diabetes mellitus; NYHA: New York Heart Association; SD: standard deviation.
Figure 2:
Aortic dimensions observed in the 1301 patient cohort. The number of patients with an aortic dimension in millimetres within each 5 mm category, for the largest observed aortic dimension for an individual patient (A), the aortic sinuses (B), the sinotubular junction (C) and the tubular ascending aorta (D).
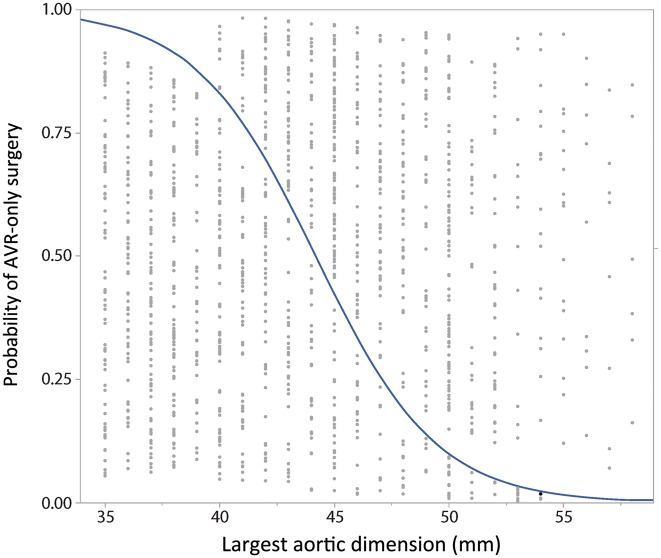
Figure 3:
Logistic plot of probability of undergoing AVR-only surgery for the largest observed aortic dimension. The logistic probability of undergoing an aortic valve replacement without concurrent aortic repair is displayed against the largest observed aortic dimension (mm). Individual patients are indicated by grey dots, and the probability is displayed as a blue line. Patients below the blue line underwent aortic valve replacement, whereas those above the line underwent AVR with aortic resection. AVR: aortic valve replacement.
Patient outcomes
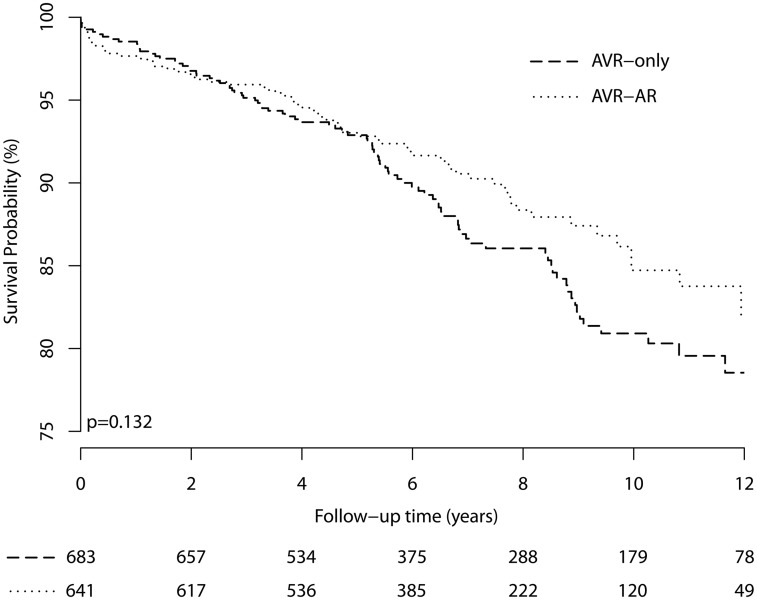
There were no significant differences in reoperation or mortality outcomes by surgeon, hospital or the operation performed (Supplementary Material, Tables S4 and S5). Event rates were low, with rates of reoperation or death within 1 year of only 0.3% and 2.3%, respectively (Table 1 and Fig. 4). Aortic dissection was not reported during follow-up after AVR-only or AVR-AR surgery. There were very few reoperations to permit proportional hazards modelling of reoperative risk alone; therefore, the death and reoperation outcomes were combined for all analyses.
Figure 4:
The Kaplan–Meier plot of the composite reoperation and mortality outcomes. Outcomes for an aortic dimension-based analysis of 1301 patients without exclusion criteria and with aortic dimensions ≥35 mm (for the AVR cohort) or 40 mm (for the AVR with ascending aortic aneurysm repair cohort) and the largest aortic dimension ≤59 mm, compared between patients undergoing either AVR or AVR with ascending aortic aneurysm repair. AVR: aortic valve replacement; AR: aortic resection.
Older age, smoking, cancer and coronary artery disease and its risk factors, dialysis and urgent procedures were associated with the composite mortality and reoperation outcome (Table 2). There was no significant association between aortic dimension, type of operation or an interaction term of these 2 predictors, with outcome occurrence (Table 2 and Fig. 4). Although the frequency of AVR-only and AVR-AR operations changed during the study period, there was no association of year of operation with outcomes. Although the frequency of events was greater for patients with larger aortic dimensions, this did not reach statistical significance in multivariate analysis. Analysis using Z-scores of aortic dimensions and reoperation yielded similar conclusions (Supplementary Material). Examination of 91 patients with a dilated (>40 mm) aortic root did not identify a beneficial effect of aortic root surgery.
Table 2:
Cox proportional hazard model of the reoperation and mortality outcomes of 1301 patients without exclusion criteria and with aortic dimensions ≥35 mm (for the AVR cohort) or 40 mm (for the AVR with ascending aortic aneurysm repair cohort) and the largest aortic dimension ≤59 mm
| Univariate analysis |
Multivariate aortic dimension-based analysis (n = 1301) |
|||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | Overall P-value | HR (95% CI) | P-value | Overall P-value | |
| Preoperative data | ||||||
| Age at AVR (years) | ||||||
| <50 | 1 | <0.0001 | 1 | <0.0001 | ||
| 50–59 | 2.45 (1.15–4.71) | 0.02 | 1.94 (0.90–4.18) | 0.09 | ||
| 60–69 | 4.20 (2.05–8.60) | <0.0001 | 2.62 (1.25–5.49) | 0.010 | ||
| 70–79 | 10.0 (4.95–20.4) | <0.0001 | 5.42 (2.55–11.5) | 0.0001 | ||
| ≥80 | 16.3 (7.33–36.4) | <0.0001 | 9.73 (4.18–22.6) | <0.0001 | ||
| Gender (female) | 1.04 (0.71–1.53) | 0.84 | ||||
| Race (Caucasian) | 0.99 (0.50–1.96) | 0.98 | ||||
| BMI >30 kg/m2 | 0.79 (0.52–1.15) | 0.22 | ||||
| Smoker past or current | 1.76 (1.27–2.43) | 0.0006 | ||||
| COPD | 3.02 (2.06–4.42) | <0.0001 | 2.40 (1.61–3.57) | 0.0002 | ||
| Diabetes (NIDDM or IDDM) | 1.86 (1.21–2.86) | 0.005 | ||||
| Hypertension | 0.41 (0.28–0.59) | <0.0001 | 0.72 (0.49–1.06) | 0.10 | ||
| Preoperative creatinine (mg/dl) | 1.39 (1.20–1.62) | <0.0001 | ||||
| Preoperative dialysis | 4.30 (1.37–13.5) | 0.013 | 6.31 (1.97–20.2) | 0.002 | ||
| Cancer | 2.80 (1.97–3.98) | <0.0001 | 1.71 (1.18–2.49) | 0.005 | ||
| Peripheral vascular disease | 1.67 (1.10–2.53) | 0.017 | 1.63 (1.06–2.52) | 0.027 | ||
| Medications | ||||||
| Beta-blocker | 0.56 (0.35–0.89) | 0.014 | ||||
| ACEI/ARB | 1.02 (0.57–1.85) | 0.94 | ||||
| Lipid lowering | 1.32 (0.93–1.87) | 0.12 | ||||
| Prior cardiac status | ||||||
| NYHA class (n = 600) | ||||||
| I or II | 1 | 0.002 | ||||
| III | 2.16 (1.29–3.76) | 0.003 | ||||
| IV | 3.13 (1.37–7.14) | 0.007 | ||||
| Heart failure | 2.81 (1.97–4.02) | <0.0001 | 1.90 (1.30–2.76) | 0.0008 | ||
| Prior CABG or non-aortic valve surgery | 2.73 (1.58–4.74) | 0.0003 | ||||
| Prior MI | 3.03 (2.02–4.54) | <0.0001 | ||||
| Prior CVA | 1.06 (0.52–2.16) | 0.87 | ||||
| Coronary and valve disease | ||||||
| Coronary artery disease | 2.44 (1.76–3.38) | <0.0001 | ||||
| Aortic insufficiency | ||||||
| None, trace or mild | 1 | 0.0017 | ||||
| Moderate | 0.81 (0.55–1.21) | 0.30 | ||||
| Severe | 0.38 (0.21–0.71) | 0.002 | ||||
| Aortic valve area (cm2) | 1.00 (0.99–1.01) | 0.99 | ||||
| Mitral incompetence | ||||||
| None, trace or mild | 1 | 0.0005 | ||||
| Moderate | 2.13 (1.40–3.24) | 0.0004 | ||||
| Severe | 2.38 (1.25–4.54) | 0.007 | ||||
| LV ejection fraction (%) | ||||||
| <30 | 3.54 (2.17–5.77) | <0.0001 | <0.0001 | |||
| 30–49 | 2.11 (1.37–3.24) | 0.0007 | ||||
| ≥50 | 1 | |||||
| Aortic measurements | ||||||
| Largest aortic dimension (mm) | ||||||
| 35–39 | 1 | 0.40 | 1 | 0.23 | ||
| 40–44 | 0.69 (0.44–1.08) | 0.10 | 0.82 (0.51–1.31) | 0.40 | ||
| 45–49 | 0.86 (0.57–1.32) | 0.49 | 1.25 (0.73–2.16) | 0.42 | ||
| ≥50 | 0.77 (0.49–1.21) | 0.25 | 1.46 (0.80–2.67) | 0.22 | ||
| Largest aortic Z-score | ||||||
| <1.96 | 1 | 0.18 | ||||
| 1.96–2.99 | 0.74 (0.48–1.15) | 0.19 | ||||
| ≥3.0 | 0.70 (0.48–1.01) | 0.054 | ||||
| Operation | ||||||
| Hospital | ||||||
| BWH | 1 | |||||
| MGH | 1.12 (0.80–1.58) | 0.50 | ||||
| Year of operation | ||||||
| 2002–03 | 1 | 0.10 | ||||
| 2004–05 | 1.71 (0.93–3.15) | 0.086 | ||||
| 2006–07 | 1.56 (0.85–2.84) | 0.15 | ||||
| 2008–09 | 1.04 (0.54–2.00) | 0.91 | ||||
| 2010–11 | 1.15 (0.58–2.29) | 0.69 | ||||
| 2012–14 | 0.71 (0.31–1.65) | 0.43 | ||||
| Urgency | ||||||
| Elective | 1 | |||||
| Urgent or more | 2.23 (1.57–3.15) | <0.0001 | ||||
| CABG performed | 3.08 (2.23–4.26) | <0.0001 | 1.92 (1.36–2.71) | 0.0002 | ||
| DHCA used | 1.10 (0.77–1.57) | 0.59 | ||||
| Operation | ||||||
| AVR only | 1 | 1 | ||||
| AVR and Asc Ao | 0.76 (0.55–1.05) | 0.099 | 0.84 (0.52–1.35) | 0.47 | ||
| Aortic valve implant type | ||||||
| Mechanical | 1 | |||||
| Bioprosthesis | 2.22 (1.38–3.57) | 0.001 | ||||
| Mitral valve repair or replacement | 1.86 (1.05–3.28) | 0.033 | ||||
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blockers; Asc Ao: ascending aorta; AVR: aortic valve replacement; BMI: body mass index; BWH: Brigham and Women‘s Hospital; CABG: coronary artery bypass grafting; CI: confidence interval; COPD: chronic obstructive pulmonary disease; CVA: cardiovascular accident; DHCA: deep hypothermic circulatory arrest; HR: hazard ratio; IDDM: insulin-dependent diabetes mellitus; LV: left ventricular; MI: myocardial infarction; MGH: Massachusetts General Hospital; NIDDM: non-insulin-dependent diabetes mellitus; NYHA: New York Heart Association.
DISCUSSION
This observational study compared mortality and aortic reoperation in adults with BAV who underwent AVR with a dilated aortic root or ascending aorta to determine the effect of concurrent aortic resection upon a composite outcome of mortality or aortic reoperation. Our aims were to determine age and aortic dimensions where concurrent repair of dilated or aneurysmal aortic disease during AVR in patients with BAV substantially improves morbidity and mortality outcomes.
Our principal finding was there was a very low incidence of mortality and reoperation, leading to a conclusion that both operations are reasonable choices. We were unable to identify an age or aortic dimension, or a combination thereof, associated with better or worse outcomes after each operation, including those with aortic root aneurysm. The use of Z-scores to account for patient morphometry did not improve the association of aortic size with outcomes. These findings lead us to the conclusions that both operations are safe, and aortic dimension within the dimensions examined in this study should not be a definitive criterion for aortic replacement at the time of AVR.
The decision to operate on the dilated bicuspid ascending aorta is complex with competing risk factors. The relative risk of aortic dissection may be higher in patients with BAV, but the absolute risk of aortic dissection is low. Even though the relative risk of aortic dissection increases with increased aortic size in both BAV and TAV patients, the majority of aortic dissections occur at low aortic dimensions. Further, some individuals with BAV have progressive aortic dilation after AVR and may have increased risk of aortic dissection or progress above a Guideline dimension for aortic resection, thus indicating a reoperation [21, 22],
Absolute and relative risk of aortic dissection
The principal rationale for performing aortic replacement concurrently with surgical AVR in patients with BAV is to prevent subsequent aortic dissection and to reduce the need for later reoperative surgery for aortic aneurysm. The relative abundance of aortic dilation, aneurysm and aortic dissection in patients with BAV is recognized [23] but not uniformly reported [8, 15, 24], and it is not clear whether the absolute risk of aortic rupture or dissection in patients with BAV mandates a different surgical approach compared with TAV. Although prior studies reported high rates of dissection and aortic dilation [10, 11], the absolute rate of aortic dissection in patients with BAV is low, especially over the last decade [4, 6–8, 25] and may not exceed the risk in patients with TAV at a comparable aortic dimension [26]. Further, the majority of aortic dissections occur at an aortic dimension <55 mm [27], and aortic size measured at dissection is considerably larger than that measured prior to dissection [28]. Thus, prior studies may have overestimated aortic size and ascribed increased risk to much larger aortic dimensions than that present before dissection [28]. These conflicting findings have generated considerable debate and recent revision of the Guidelines [12]; however, these Guidelines do not yet consider patient characteristics, such as age, untreated hypertension, renal or cardiac disease, family history or genetic findings.
There are known systematic differences in the methods of aortic dimension measurement between imaging modalities and the use of anatomical landmarks for estimating aortic dimension. These differences were probably small [29] and unlikely to affect surgical decision-making.
Limitations
Retrospective cohort study design has inherent limitations but is the only feasible method to assess long-term BAV outcomes. Patients were not randomly assigned to undergo concurrent aortic resection, so there is considerable potential for bias by clinical presentation or surgical practices that are possibly unaccounted for in this study. We used all-cause rather than cardiac-specific mortality in all analyses, which can be disadvantageous, as it includes mortality that is not due the primary disease. However, it does allow for a more complete accounting of mortality and accounts for competing risks of death and potential biases observed in cause-of-death reporting. We are unable to identify reoperations or aortic dissection that occurred at other institutions, not causing mortality. This may result in systematic under-reporting of aortic dissection that may bias study findings towards favouring one or other operation. Additionally, both AVR and aortic aneurysm repair carry a significant risk of stroke and other morbidity that were not examined in this study. The additional risk of aortic aneurysm repair or reoperation may have significant impact of patient morbidity that is not yet accounted for. Thus, there may be unconsidered bias against reoperation in those with aortic dilation after AVR. We were unable to identify an age or aortic dimension, or combination thereof, associated with better or worse outcomes after each operation, including those with aortic root aneurysm.
Because of the institutional referral patterns, there were patients who were followed up locally rather than our own institutions. This may cause under-reporting of reoperation, notwithstanding that most of these patients would be referred to our hospital because of the health system referral patterns.
CONCLUSIONS
Our results do not establish an aortic dimensional threshold to guide decision-making for an individual patient within the range of 40–60 mm nor provide support for the 45 mm aortic dimension recommended in the current Guidelines for aortic resection while performing AVR [12]. Although there are strong patient and provider forces favouring aortic resection at relatively low aortic dimensions to avoid a need for reoperation or aortic dissection, this study reports a very low rate of reoperation for aortic dilation.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This work was supported by the National Institutes of Health [R01HL114823 to S.C.B.].
Conflict of interest: none declared.
Supplementary Material
REFERENCES
- 1. Della Corte A, Body SC, Booher AM, Schaefers HJ, Milewski RK, Michelena HI. et al. Surgical treatment of bicuspid aortic valve disease: knowledge gaps and research perspectives. J Thorac Cardiovasc Surg 2014;147:1749–57, 1757.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM. et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008;117:2776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts WC, Ko JM.. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005;111:920–5. [DOI] [PubMed] [Google Scholar]
- 4. Michelena HI, Khanna AD, Mahoney D, Margaryan E, Topilsky Y, Suri RM. et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011;306:1104–12. [DOI] [PubMed] [Google Scholar]
- 5. Girdauskas E, Disha K, Borger MA, Kuntze T.. Long-term prognosis of ascending aortic aneurysm after aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Thorac Cardiovasc Surg 2014;147:276–82. [DOI] [PubMed] [Google Scholar]
- 6. Svensson LG, Kim KH, Blackstone EH, Rajeswaran J, Gillinov AM, Mihaljevic T. et al. Bicuspid aortic valve surgery with proactive ascending aorta repair. J Thorac Cardiovasc Surg 2011;142:622–9, 629.e1–3. [DOI] [PubMed] [Google Scholar]
- 7. McKellar SH, Michelena HI, Li Z, Schaff HV, Sundt TM 3rd. Long-term risk of aortic events following aortic valve replacement in patients with bicuspid aortic valves. Am J Cardiol 2010;106:1626–33. [DOI] [PubMed] [Google Scholar]
- 8. Kim JB, Spotnitz M, Lindsay ME, MacGillivray TE, Isselbacher EM, Sundt TM 3rd. Risk of aortic dissection in the moderately dilated ascending aorta. J Am Coll Cardiol 2016;68:1209–19. [DOI] [PubMed] [Google Scholar]
- 9. Svensson LG, Kim KH, Lytle BW, Cosgrove DM.. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg 2003;126:892.. [DOI] [PubMed] [Google Scholar]
- 10. Borger MA, Preston M, Ivanov J, Fedak PW, Davierwala P, Armstrong S. et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg 2004;128:677–83. [DOI] [PubMed] [Google Scholar]
- 11. Russo CF, Mazzetti S, Garatti A, Ribera E, Milazzo A, Bruschi G. et al. Aortic complications after bicuspid aortic valve replacement: long-term results. Ann Thorac Surg 2002;74:S1773–6. [DOI] [PubMed] [Google Scholar]
- 12. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease Representative Members, Hiratzka LF, Creager MA, Isselbacher EM, Svensson LG,. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease Representative Members et al. Surgery for aortic dilatation in patients with bicuspid aortic valves: a statement of clarification from the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016;133:680–6.26637530 [Google Scholar]
- 13. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA. et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440–92. [DOI] [PubMed] [Google Scholar]
- 14. Park CB, Greason KL, Suri RM, Michelena HI, Schaff HV, Sundt TM 3rd. Fate of nonreplaced sinuses of Valsalva in bicuspid aortic valve disease. J Thorac Cardiovasc Surg 2011;142:278–84. [DOI] [PubMed] [Google Scholar]
- 15. Yasuda H, Nakatani S, Stugaard M, Tsujita-Kuroda Y, Bando K, Kobayashi J. et al. Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: comparison with tricuspid aortic valve. Circulation 2003;108:II291–4. [DOI] [PubMed] [Google Scholar]
- 16. Disha K, Rouman M, Secknus MA, Kuntze T, Girdauskas E.. Are normal-sized ascending aortas at risk of late aortic events after aortic valve replacement for bicuspid aortic valve disease? Interact CardioVasc Thorac Surg 2016;22:465–71. [DOI] [PubMed] [Google Scholar]
- 17. Patel HJ. Comparison of long-term risk of thoracic aortic aneurysm and dissection in patients with bicuspid aortic valve and marfan syndrome after aortic valve replacement. J Am Coll Cardiol 2015;65:2370–1. [DOI] [PubMed] [Google Scholar]
- 18. Sundt TM., 3rd Replacement of the ascending aorta in bicuspid aortic valve disease: where do we draw the line? J Thorac Cardiovasc Surg 2010;140:S41–4. [DOI] [PubMed] [Google Scholar]
- 19. Jacobs JP, Shahian DM, Prager RL, Edwards FH, McDonald D, Han JM. et al. Introduction to the STS National Database Series: outcomes analysis, quality improvement, and patient safety. Ann Thorac Surg 2015;100:1992–2000. [DOI] [PubMed] [Google Scholar]
- 20. Campens L, Demulier L, De Groote K, Vandekerckhove K, De Wolf D, Roman MJ. et al. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am J Cardiol 2014;114:914–20. [DOI] [PubMed] [Google Scholar]
- 21. Matsuyama K, Usui A, Akita T, Yoshikawa M, Murayama M, Yano T. et al. Natural history of a dilated ascending aorta after aortic valve replacement. Circ J 2005;69:392–6. [DOI] [PubMed] [Google Scholar]
- 22. Thanassoulis G, Yip JW, Filion K, Jamorski M, Webb G, Siu SC. et al. Retrospective study to identify predictors of the presence and rapid progression of aortic dilatation in patients with bicuspid aortic valves. Nat Clin Pract Cardiovasc Med 2008;5:821–8. [DOI] [PubMed] [Google Scholar]
- 23. Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG.. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation 2000;102:III35–9. [DOI] [PubMed] [Google Scholar]
- 24. La Canna G, Ficarra E, Tsagalau E, Nardi M, Morandini A, Chieffo A. et al. Progression rate of ascending aortic dilation in patients with normally functioning bicuspid and tricuspid aortic valves. Am J Cardiol 2006;98:249–53. [DOI] [PubMed] [Google Scholar]
- 25. Girdauskas E, Disha K, Raisin HH, Secknus MA, Borger MA, Kuntze T.. Risk of late aortic events after an isolated aortic valve replacement for bicuspid aortic valve stenosis with concomitant ascending aortic dilation. Eur J Cardiothorac Surg 2012;42:832–7. [DOI] [PubMed] [Google Scholar]
- 26. Eleid MF, Forde I, Edwards WD, Maleszewski JJ, Suri RM, Schaff HV. et al. Type A aortic dissection in patients with bicuspid aortic valves: clinical and pathological comparison with tricuspid aortic valves. Heart 2013;99:1668–74. [DOI] [PubMed] [Google Scholar]
- 27. Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'gara PT, Evangelista A. et al. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007;116:1120–7. [DOI] [PubMed] [Google Scholar]
- 28. Rylski B, Blanke P, Beyersdorf F, Desai ND, Milewski RK, Siepe M. et al. How does the ascending aorta geometry change when it dissects? J Am Coll Cardiol 2014;63:1311–9. [DOI] [PubMed] [Google Scholar]
- 29. Park JY, Foley TA, Bonnichsen CR, Maurer MJ, Goergen KM, Nkomo VT. et al. Transthoracic echocardiography versus computed tomography for ascending aortic measurements in patients with bicuspid aortic valve. J Am Soc Echocardiogr 2017;30:625–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.