Potato miR160 is crucial for both local and SAR responses to the late blight pathogen Phytophthora infestans and modulates antagonistic cross-talk between auxin-mediated growth and salicylic acid-mediated defense responses.
Keywords: Auxin–salicylic acid antagonism, microRNA, miR160, Phytophthora infestans, Solanum chacoense, Solanum tuberosum, systemic acquired resistance
Abstract
To combat pathogen infection, plants employ local defenses in infected sites and elicit systemic acquired resistance (SAR) in distant tissues. MicroRNAs have been shown to play a significant role in local defense, but their association with SAR is unknown. In addition, no such studies of the interaction between potato and Phytophthora infestans have been reported. We investigated the role of miR160 in local and SAR responses to P. infestans infection in potato. Expression analysis revealed induced levels of miR160 in both local and systemic leaves of infected wild-type plants. miR160 overexpression and knockdown plants exhibited increased susceptibility to infection, suggesting that miR160 levels equivalent to those of wild-type plants may be necessary for mounting local defense responses. Additionally, miR160 knockdown lines failed to elicit SAR, and grafting assays indicated that miR160 is required in both local and systemic leaves to trigger SAR. Consistently, SAR-associated signals and genes were dysregulated in miR160 knockdown lines. Furthermore, analysis of the expression of defense and auxin pathway genes and direct regulation of StGH3.6, a mediator of salicylic acid–auxin cross-talk, by the miR160 target StARF10 revealed the involvement of miR160 in antagonistic cross-talk between salicylic acid-mediated defense and auxin-mediated growth pathways. Overall, our study demonstrates that miR160 plays a crucial role in local defense and SAR responses during the interaction between potato and P. infestans.
Introduction
Upon infection with a pathogen, plants mount defense responses in both local infected and systemic non-infected sites. At the local site, plants recognize pathogen-associated molecular patterns (PAMPs) through their pattern recognition receptors and activate PAMP-triggered immunity (PTI) (Chisholm et al., 2006). Pathogens release effector proteins into the plant cell to suppress PTI. In this ‘arms race’, plants have in turn evolved resistance proteins that can distinguish effectors and activate effector-triggered immunity (ETI) (Chisholm et al., 2006; Dodds and Rathjen, 2010). The activation of local defense responses also induces systemic signals that result in broad-spectrum resistance at distant sites, termed systemic acquired resistance (SAR) (Kachroo and Robin, 2013). Salicylic acid (SA) is one of the well-studied and necessary components of the SAR process (Gaffney et al., 1993; Wildermuth et al., 2001). In Arabidopsis and tobacco, SA levels increase both locally and systemically upon SAR induction (Yalpani et al., 1991; Enyedi et al., 1992; Summermatter et al., 1995). Furthermore, recent studies have identified methyl salicylate (MeSA) (Park et al., 2007), azelaic acid (Jung et al., 2009), glycerol-3-phosphate (Chanda et al., 2011), dehydroabietinal (Chaturvedi et al., 2012), and pipecolic acid (Návarová et al., 2012) as potential mobile SAR signals. A lipid transfer protein, DIR1 (DEFECTIVE IN INDUCED RESISTANCE 1), was shown to be crucial for the induction of SAR by many of these mobile signals (Jung et al., 2009; Liu et al., 2011; Chanda et al., 2011; Chaturvedi et al., 2012).
In the past decade, microRNAs (miRNAs) have emerged as major contributors to the immune responses of plants, especially in PTI and ETI. miRNAs are small (~21–24 nt), endogenous, non-coding RNAs that act as negative regulators of gene expression (Jones-Rhoades et al., 2006). In Arabidopsis, miR393 was shown to be involved in PTI responses against bacterial pathogens by facilitating the suppression of the auxin signaling pathway (Navarro et al., 2006). Similarly, Arabidopsis miR160, miR398, and miR773 were shown to be associated with PTI (Li et al., 2010), and tomato miR482 and miR5300 (Shivaprasad et al., 2012; Ouyang et al., 2014) and tobacco miR6019 (Li et al., 2012) were demonstrated to play important roles in ETI. These reports have established the role of miRNAs in PTI and ETI responses of various plants. However, the role of miRNAs in the regulation of SAR is unknown.
SAR has been widely studied in the model plants Arabidopsis and tobacco; however, only a few studies have explored SAR in crop plants. Potato, the fourth most important food crop worldwide, is severely affected by late blight disease caused by the oomycete pathogen Phytophthora infestans, which leads to massive crop loss. Although much understanding has been gained regarding local defense responses and various resistance genes in potato (van Ooijen et al., 2007), our knowledge of SAR in potato is still rudimentary. Currently, we understand only that SA is important for SAR induction (Yu et al., 1997) and that MeSA acts as a mobile SAR signal in potato (Manosalva et al., 2010). Furthermore, the role of miRNAs in the interaction between potato and P. infestans has not been investigated. In light of recent findings that effector proteins of Phytophthora spp. can suppress host small RNA-mediated defense responses (Qiao et al., 2015; Ye and Ma, 2016), studying the role of potato miRNAs in mediating the local defense and SAR responses against P. infestans could be of great importance toward understanding late blight disease.
In this study, from an initial screening of 10 candidate miRNAs, we focused on the function of miR160. We show that the expression of miR160 is induced in both local and systemic leaves of wild-type (WT) potato upon P. infestans infection. Both overexpression (OE) and knockdown (KD) of miR160 result in enhanced susceptibility. Additionally, SAR and grafting assays on miR160 transgenic lines provide crucial insights regarding the role of miR160 in mounting an effective SAR response. Expression analysis of auxin and SAR genes and the interaction of StARF10 protein with the StGH3.6 promoter suggest the involvement of miR160 in auxin–SA antagonistic cross-talk. Overall, our findings indicate that miR160 plays an important role in modulating local defense and SAR responses in potato during P. infestans infection.
Materials and methods
Plant and pathogen growth conditions
All WT and transgenic potato (Solanum chacoense and Solanum tuberosum cv. Désirée) plants were grown and maintained as described previously (Banerjee et al., 2006b). The oomycete pathogen Phytophthora infestans strain A2 was maintained at 18 °C in pea agar media, corn media, and potato slices, and confirmed by amplifying part of the internal transcribed spacer 2 (ITS2) ribosomal DNA using primer sets PINF and ITS5 (Trout et al., 1997) (Supplementary Fig. S1A at JXB online). The bacterial pathogen Ralstonia solanacearum was maintained in nutrient agar medium and confirmed by performing PCR as described previously (Lee and Wang, 2000) (Supplementary Fig. S1B).
P. infestans infection and arachidonic acid treatment
For all infection experiments, P. infestans sporangia were used at a concentration of 2 × 105 ml–1 and treated plants were incubated at 18 °C and 90% humidity. Time-course expression analysis of miR160 and StARF10 in S. chacoense was performed by inoculating 10 µl of sporangia solution on the abaxial side of the eighth to 11th leaves, counted from the top of the plant. Control plants were inoculated with sterile water. Inoculated local leaves (leaves 8–11) and non-inoculated systemic leaves (leaves 5–7) were harvested at 12, 24, 48, and 96 hours post-inoculation (hpi). For local infection analysis, S. tuberosum WT and miR160 transgenic plants were sprayed with P. infestans sporangia and disease progression was monitored for 14 days. Samples were collected on 0, 2, 5, 7, 9, 11, and 14 days post-inoculation (dpi) for various analyses. For arachidonic acid (AA) treatment analysis, plants were treated with 30 µl of 0.05 mM AA. Local (AA-treated) and systemic (AA-untreated) leaves were collected at 0, 24, 48, 72, and 96 hours post-treatment (hpt).
Detection and qRT–PCR of miRNAs
A 1 µg quantity of total RNA was used for cDNA preparation using the respective stem-loop primers of miRNAs, followed by endpoint PCR or quantitative real-time (qRT)–PCR as described by Varkonyi-Gasic et al. (2007). GAPDH was used as normalization gene.
Northern blot analysis
Northern blot analysis for miR160 and U6 was carried out following a previously described protocol (Rio, 2014) with minor modifications, such as capillary transfer of RNA to nylon membrane and a hybridization temperature of 30 °C. Probe details are provided in Supplementary Table S2.
Prediction of miR160 targets and cleavage site mapping
To predict target genes of miR160 in potato, the target prediction software packages psRNATarget (http://plantgrn.noble.org/psRNATarget/; accessed 12/02/2018) (Dai and Zhao, 2011) and TAPIR (http://bioinformatics.psb.ugent.be/webtools/tapir/; accessed 12/02/2018) (Bonnet et al., 2010) were used. The S. tuberosum transcript library (http://solanaceae.plantbiology.msu.edu/pgsc_download.shtml; accessed 12/02/2018) was used as the target database. For in planta validation of the miR160 targets StARF10 and StARF16, a modified 5ʹ-RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) technique was performed as described previously (Bhogale et al., 2014).
Gene expression analysis
For expression analysis of mRNA transcripts, 1 µg of total RNA was used for oligo(dT) cDNA preparation with Superscript-III Reverse Transcriptase (Invitrogen), and qRT–PCR reactions were carried out using KAPA SYBR Green Mix (Kapa Biosystems). Data were analyzed using the 2−∆Ct method (Livak and Schmittgen, 2001) for all the experiments except the AA treatment experiment, in which the 2−∆∆Ct method was used. GAPDH was used as the normalization gene in all experiments.
miR160 overexpression and knockdown construct generation
For the generation of an miR160 OE construct, pBI121-35S::pre160 cDNA was prepared from potato leaf RNA using oligo(dT) primers. The miR160 precursor (St-pre160) was amplified and cloned into the binary vector pBI121 under the 35S CaMV constitutive promoter. For KD lines, the endogenous target mimicry (eTM) approach was employed using the construct pCAMBIA-35S::ath-eTM160 (Wu et al., 2013). Plant transformation was performed as described previously (Banerjee et al., 2006b).
P. infestans DNA quantification
For absolute quantification, a standard curve for the P.infestans-specific sequence O8 was generated using different concentrations of P. infestans genomic DNA (Supplementary Fig. S12). Genomic DNA was isolated from leaf samples collected from P. infestans-infected plants at 14 dpi. 50 pg of DNA was used for qRT–PCR analysis of the O8 sequence using the primers O8-3 and O8-4 (Judelson and Tooley, 2000).
SAR and grafting assays
Four-week-old plants were subjected to primary infection with P. infestans followed by secondary infection with R. solanacearum. For the primary infection, 50 µl of 2 × 105 ml−1P. infestans sporangia was swabbed on to the two lowest leaves and plants were incubated at 18 °C. Mock inoculation was carried out with sterile water. Four days after primary infection, three upper leaves were infiltrated with 106 colony-forming units ml−1 (OD600 ~0.1) of R. solanacearum and plants were incubated at 28 °C. After 5 days of secondary infection, a 1 cm2 piece of leaf tissue was excised and crushed in sterile water. The sample was serially diluted and plated on nutrient agar medium, and the bacterial count was recorded. For grafting analysis, two types of homografts (WT/WT and eTM160-26/eTM160-26) and heterografts (WT/eTM160-26 and eTM160-26/WT) were generated following the protocol described by Banerjee et al. (2006a). SAR assays were performed as described above. In brief, two leaves of grafted stock plants were inoculated with either P. infestans or sterile water (mock treatment). Four days after the primary inoculations, systemic scion leaves of all the grafts were infiltrated with R. solanacearum. After 5 days of secondary infection, systemic scion leaves were harvested and the bacterial count was recorded.
Quantification of salicylic acid
For quantification of SA levels, modification of a previous protocol was followed (Forcat et al., 2008). A sample of 50 mg of ground leaf tissue was extracted in 400 µl of 10% methanol containing 1% glacial acetic acid. The mixture was vigorously vortexed and incubated on ice for 30 min, followed by centrifugation at 13000 g for 10 min at 4 °C to obtain the supernatant. This process was repeated once and the supernatant volume was adjusted to 1 ml using a volumetric flask. Samples were resolved through a Thermo Scientific Hypersil Gold column of particle size 1.9 μm and dimensions 50 × 2.1 mm with a flow rate of 0.2 ml min–1 and a gradient solvent program of 10 min (0.0 min, 10% methanol/water; 0.5 min, 10% methanol/water; 3.0 min, 50% methanol/water; 10 min, 50% methanol/water). Formic acid (0.1% LC-MS grade) was also added to the water. MS and MS/MS experiments were performed in electrospray ionization (ESI)-negative ion mode using the tune method: sheath gas flow rate 45, auxiliary gas flow rate 10, sweep gas flow rate 2, spray voltage (|KV|) 3.60, spray current (μA) 3.70, capillary temperature 320 °C, s-lens RF level 50, heater temperature 350 °C. ESI-MS data were recorded in full scan mode within the mass range m/z 100–1000 (Supplementary Fig. S13).
Quantification of methyl salicylate
Quantification of MeSA was carried out by a previously described protocol (Schmelz et al., 2004) with minor modifications. A sample of 100 mg of leaf tissue was ground in liquid nitrogen and extracted in 800 µl of extraction buffer (1-propanol:water:hydrochloric acid at a 2:1:0.005 ratio) with 10 ng of 3ʹ-methylacetophenone (internal standard). Samples were re-homogenized by adding 1 ml of dichloromethane (DCM). The DCM layer was separated by centrifugation at 12000 g for 30 s and collected in a 2 ml glass vial, and the sample was further concentrated to ~100 µl using inert nitrogen gas. A 1 µl aliquot of the sample was injected manually into the inlet injector port (held at a temperature of 250 °C) of a single quadrapole GC-MS system (Agilent 7890A GC and Agilent 5975-Inert XL EL/CL MSD MS). Compounds were separated on a SUPELCOWAX® 10 Capillary GC column (30 m×0.20 µm) (Sigma-Aldrich) with the column temperature initially set to 60 °C and then increased to 220 °C. Helium was used as the carrier gas with a flow rate of 1 ml min–1. The areas of the internal standard and MeSA were calculated by extracting the peak for m/z=134 and m/z=152, respectively (Supplementary Figs S14 and S15). The quantity of MeSA per gram of ground tissue was calculated from the unit area obtained for the internal standard.
Yeast one-hybrid assay
The coding sequence of StARF10 (PGSC0003DMT400020874) and the promoter sequences of StGH3.6 (PGSC0003DMT400049613) (~2.4 kb upstream) and AtGH3.5 (AT4G27260) (~3.0 kb upstream) were cloned into the pGEM-T Easy vector (Promega). All the constructs for the yeast one-hybrid (Y1-H) assay were generated by Gateway cloning technology (Thermo Fisher Scientific) (Deplancke et al., 2004). For preparation of bait expression vectors, promoters were transferred to the destination vector pMW#2 (Addgene) through the donor vector pDONRP4-P1r. Furthermore, the yeast strain YM4271 was transformed with bait expression vectors and selected in SD -His (Synthetic dropout without histidine) medium. The prey expression vector was prepared by transferring the coding sequence of StARF10 to the destination vector pDEST-2µ-Gal4-AD via the donor vector pDONR221. The yeast strain Yα1867 was transformed with the prey expression vector and selected in SD -Trp (Synthetic dropout without tryptophan) medium. To study the interaction between the promoters and StARF10, the prey yeast (Yα1867-StARF10) and either of the bait yeasts (YM4271-prom-StGH3.6 or YM4271-prom-AtGH3.5) were mated by mixing in a 1:1 ratio and allowed to grow in YPDA medium. The mated yeast clones were then selected on SD -His, -Trp medium. The interaction was further confirmed by growing the mated yeast clones on SD -His, -Trp medium supplemented with increasing concentrations of 3-amino-1,2,4-triazole (3-AT).
Electrophoretic mobility shift assay
For StARF10-6×His protein preparation, the coding sequence of StARF10 was PCR amplified and cloned into the pET28a+ vector (Novagen). Protein expression was performed using Escherichia coli BL21(DE3) cells as host, followed by Ni-NTA affinity column-based protein purification. For bait DNA preparation, promoter fragments P1, P2, and P3 of prom:StGH3.6 were PCR amplified from potato genomic DNA, and promoter fragment P4 of prom:AtGH3.5 was PCR amplified from Arabidopsis genomic DNA. All the fragments were cloned in the pGEM-T Easy sub-cloning vector (Promega) and their sequences were verified. For the electrophoretic mobility shift assay (EMSA), probes were prepared by labeling promoter fragments with γ-32P-ATP using a KinaseMax End-Labeling kit (Ambion). The binding reactions were carried out as described previously (Chen et al., 2004).
Primer information
Details of all primers are provided in Supplementary Table S2.
Accession numbers
GAPDH: PGSC0003DMT400044944, StARF10: PGSC0003 DMT400020874, StARF16: PGSC0003DMT400062489, U6: X60506, StPR1: AY050221, StYUCCA1: PGSC0003DMT400067103, StLAX4: PGSC0003DMT400049377, StTIR1: PGSC0003DMT400029517, StIAA16: PGSC0003DMT400050101, StGH3.6: PGSC0003DMT 400049613, StNPR1: XM_006357647, StMES1: PGSC0003DMT 400019806, StBSMT1: XM_006354611.
Results
Potato miRNAs exhibit altered expression upon P. infestans infection
To decipher the role of miRNAs in the potato–P. infestans interaction, 10 miRNAs (miR156, miR159, miR160, miR166, miR169, miR171, miR172, miR396, miR414, and miR1533) were shortlisted based on previous reports of their predicted role in biotic interactions in various plants (Bazzini et al., 2007; Lu et al., 2007; Xin et al., 2010; Guo et al., 2011; Amin et al., 2011; Lang et al., 2011). WT S. chacoense, a susceptible variety widely employed in Indian potato-breeding projects, was used for infection studies with P. infestans. While all 10 miRNAs were detected in S. chacoense (Supplementary Fig. S2A), differential expression upon infection was observed for only five miRNAs (miR159, miR160, miR166, miR172, and miR396) (Supplementary Fig. S2B–G). Although all these miRNAs were promising candidates, the most interesting were miR159, miR160, and miR166, because they showed significant up-regulation as early as 24 hpi. We chose to characterize miR160 in detail as it is known to play an important role in auxin signaling (Mallory et al., 2005; Turner et al., 2013) and auxin has been shown to be associated with SAR responses (Truman et al., 2010).
Expression of miR160 and its target StARF10 are altered in both local and systemic leaves upon infection
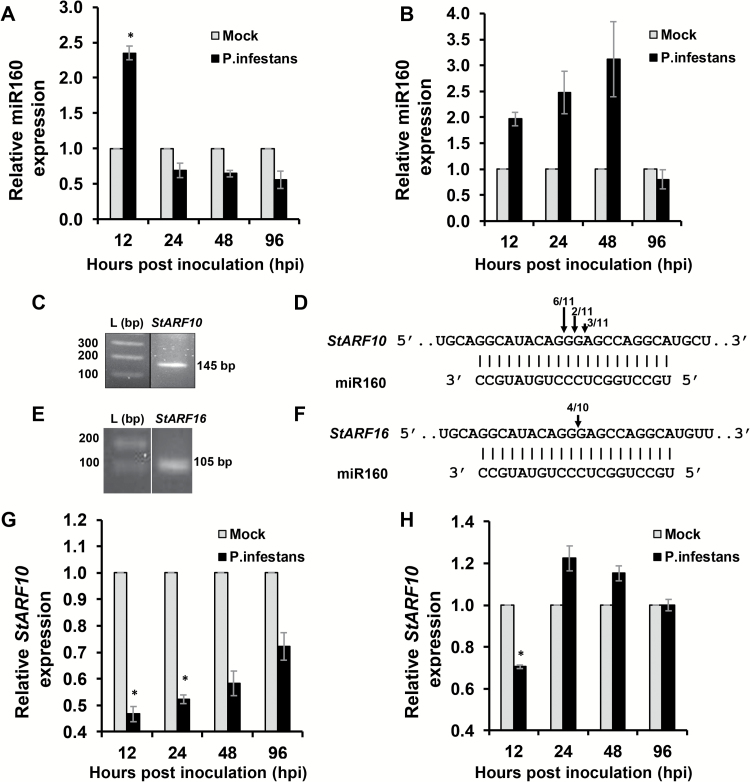
To analyze the involvement of miR160 in local defense and SAR responses, expression analysis was performed in local and systemic leaves of potato after P. infestans infection. In local leaves, miR160 levels were significantly enhanced at 12 hpi, followed by a decrease at later time points (Fig. 1A). However, in systemic leaves, miR160 levels constantly increased from 12 to 48 hpi, followed by a decrease at 96 hpi (Fig. 1B). We observed a corresponding increase in the accumulation of miR160 (~5–6-fold) in the phloem-enriched exudates of potato at 12 hpi (Supplementary Fig. S3).
Fig. 1.
Expression of miR160 and StARF10 in local and systemic leaves of wild-type potato (Solanum chacoense) upon Phytophthora infestans infection. (A, B) qRT–PCR analysis of miR160 levels in local leaves (A) and systemic leaves (B) at different time points post-infection. (C–F) RLM-RACE-based isolation of miR160 cleavage product of StARF10 (C) and StARF16 (E); partial mRNA sequence of StARF10 (D) and StARF16 (F) aligned with miR160. Numbers denote the fraction of cloned cleavage products that terminated at different positions (denoted by arrows). (G, H) qRT–PCR analysis of StARF10 in local leaves (G) and systemic leaves (H) at different time points post-infection. For miR160 and StARF10 expression analysis, data represent the mean±SE of two biological replicates with three technical replicates each, plotted by normalizing the P. infestans-treated values with mock-treated values for each time point. Asterisks indicate significant differences (P<0.05) between P. infestans-treated and mock-treated samples for the respective time point as analyzed by Student’s t-test.
Using target prediction software, we predicted six targets of miR160 in potato: five were auxin response factors (StARF10, StARF10-2, StARF16, StARF16-2, and StARF17) and one was a MAP kinase (StMAPK9) (Supplementary Table S1). Of these, only StARF10 (Fig. 1C, D) and StARF16 (Fig. 1E, F) were validated as true targets of miR160 through cleavage site mapping assay. StARF10 appeared to be the stronger target, as it had a higher cleavage frequency (11/11) than StARF16 (4/10) (Fig. 1D, F). To investigate the effect of P. infestans infection on targets of miR160, expression of StARF10 was analyzed in local and systemic leaves of S. chacoense. qRT–PCR analysis indicated an overall reduction in expression of StARF10 in local leaves compared with mock-treated plants (Fig. 1G). However, in P. infestans-treated plants, a steady increase in StARF10 expression was also observed over time (from 12 to 96 hpi) (Fig. 1G). In systemic leaves, StARF10 expression was reduced significantly at 12 hpi, followed by an increase at 24 and 48 hpi and a decrease at 96 hpi (Fig. 1H). Comparison of miR160 and StARF10 levels suggested a strong antagonistic relationship, specifically at 12 hpi, in both local (Fig. 1A, G) and systemic (Fig. 1B, H) leaves (Supplementary Fig. S4B). Overall, our findings suggested a possible role for miR160 and StARF10 in local defense and SAR responses of potato against P. infestans.
Both overexpression and knockdown of miR160 result in enhanced susceptibility
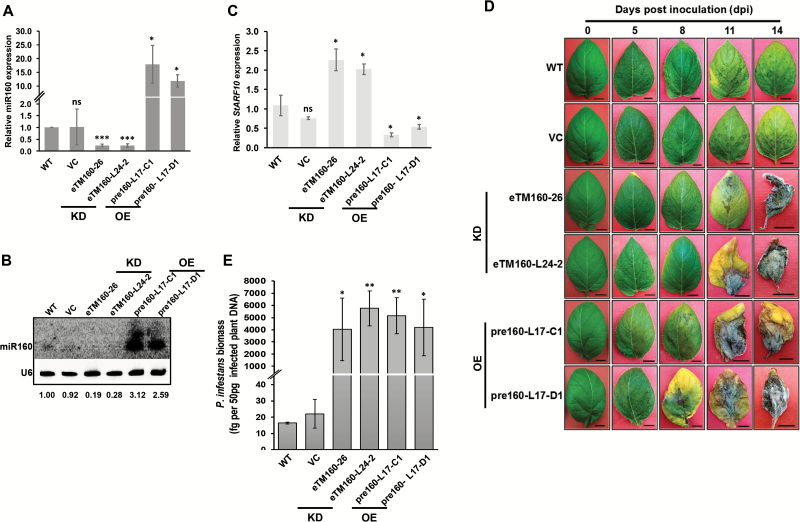
To understand the detailed function of miR160 in the potato–P. infestans interaction, OE and KD lines of miR160 were raised in potato (Supplementary Fig. S5). As S. chacoense is not amenable to transformation, S. tuberosum cv. Désirée—a susceptible variety—was used for the generation of transgenic lines. Based on the expression patterns of St-pre160 (the precursor of miR160), miR160, and StARF10 (Supplementary Fig. S5A, C), the OE lines pre160-L17-C1 and pre160-L17-D1 were selected for further analysis (Fig. 2A–C). Similarly, the KD lines eTM160-L24-2 and eTM160-26 were selected on the basis of the expression patterns of eTM160 (target mimic), miR160, and StARF10 (Supplementary Fig. S5B, D; Fig. 2A–C). No drastic morphological changes were observed in any of the miR160 OE and KD lines (Supplementary Fig. S6), indicating that their growth was comparable to that of WT plants. To assess the function of miR160 in the local defense response of potato, miR160 OE and KD lines were challenged with P. infestans and disease progression was monitored for a period of 14 days. Both miR160 OE and KD lines started developing early symptoms of infection at around 8 dpi, compared with 11 dpi in WT and vector control (VC) plants (Fig. 2D). By 14 dpi, most of the OE and KD lines exhibited severe disease symptoms compared with WT and VC plants (Fig. 2D; Supplementary Fig. S7). The P. infestans load was also significantly higher in these lines at 14 dpi (Fig. 2E). These findings suggest that both OE and KD of miR160 in potato results in enhanced susceptibility to P. infestans.
Fig. 2.
miR160 knockdown (KD) and overexpression (OE) lines are highly susceptible to P. infestans. (A, B) Relative levels of miR160 in two different KD (eTM160-26 and eTM160-L24-2) and OE (pre160-L17-C1 and pre160-L17-D1) lines, quantified by qRT–PCR (A) and northern blot analysis (B). (C) qRT–PCR analysis of StARF10 in miR160 KD and OE lines. (D) Late blight progression in wild-type (WT), vector control (VC; transformed with empty pBI121 vector), and miR160 KD and OE plants monitored for a period of 14 days. Scale bars=1 cm. (E) qRT–PCR analysis of P. infestans genomic DNA from infected plants at 14 days post-inoculation.
Increased susceptibility of miR160 transgenic lines may be related to auxin–salicylic acid antagonism
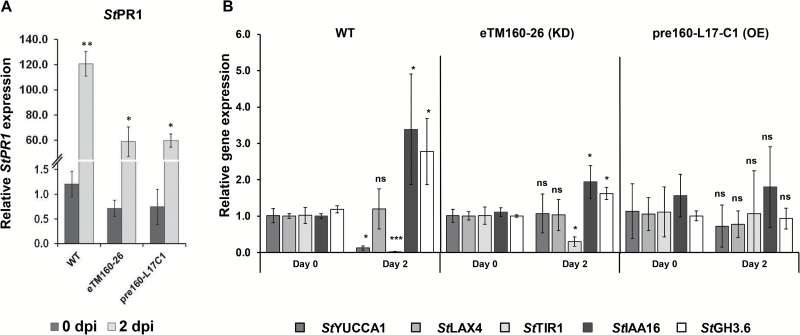
Auxin plays a critical role in plant defense (Wang et al., 2007; Truman et al., 2010) and antagonistic cross-talk between auxin and SA is crucial for mounting an effective defense response in plants (Huot et al., 2014). In other words, to mount an SA-dependent defense response during an infection, it is important for plants to attenuate the auxin signaling pathway. miR160 is a well-known player in the auxin pathway (Mallory et al., 2005; Hendelman et al., 2012; Turner et al., 2013; Huang et al., 2016). Hence, we investigated the enhanced susceptibility phenotype of miR160 OE and KD lines by analyzing the expression of various auxin pathway genes as well as the SA pathway gene StPR1. Upon P. infestans infection, the expression of StPR1 was induced in WT plants as well as in miR160 OE (pre160-L17-C1) and KD (eTM160-26) lines, however, the magnitude of induction was greater in WT plants (Fig. 3A). In infected WT plants, the expression of the positive auxin pathway regulators StYUCCA1 (auxin biosynthesis gene) and StTIR1 (TRANSPORT INHIBITOR RESPONSE 1, an auxin receptor) was significantly reduced relative to 0 dpi WT plants (Fig. 3B), whereas the levels of the negative regulators StIAA16 (INDOLE ACETIC ACID INDUCED PROTEIN 16, and auxin signaling repressor) and StGH3.6 (GRETCHEN HAGEN 3.6, an auxin/salicylic acid adenylating enzyme) were significantly elevated relative to 0 dpi WT plants (Fig. 3B). Expression of StLAX4 (LIKE AUXIN RESISTANT 4, an auxin influx carrier) remained unchanged in WT plants (Fig. 3B). The KD line eTM160-26 showed lower levels of StTIR1 and higher expression of StIAA16 and StGH3.6 upon infection relative to 0 dpi eTM160-26 plants (Fig. 3B); levels of StYUCCA1 and StLAX4 were not affected in this line. Although WT and eTM60-26 plants showed similar expression patterns for StTIR1, StIAA16, and StGH3.6, WT plants showed a greater magnitude of change in the expression of these genes. For StTIR1, we observed a ~59-fold decrease in WT plants and a ~3-fold decrease in KD lines. StIAA16 showed a ~3.4-fold and 1.75-fold increase in WT and KD lines, respectively; for StGH3.6, WT and KD lines showed a ~2.3-fold and 1.6-fold increase in expression, respectively. No significant changes in expression were observed for any of these genes in the OE line (pre160-L17-C1) upon infection with P. infestans (Fig. 3B). These results revealed that OE or KD of miR160 leads to an imbalance in auxin–SA antagonistic cross-talk, which might result in the enhanced susceptibility phenotype.
Fig. 3.
miR160 transgenic lines have altered expression of StPR1 and auxin pathway genes. (A) StPR1 expression after P. infestans infection. (B) Effect of P. infestans infection on expression of the auxin pathway genes StYUCC1, StLAX4, StTIR1, StIAA16, and StGH3.6 in WT and miR160 OE and KD plants. All data represent the mean±SD of three biological replicates with three technical replicates each. Analysis was carried out for each plant type separately by comparing expression of each gene at 2 days post-inoculation (dpi) with 0 dpi. *P<0.05, **P<0.01, ***P<0.005; ns, not significant (Student’s t-test).
miR160 knockdown, but not overexpression, leads to compromised SAR
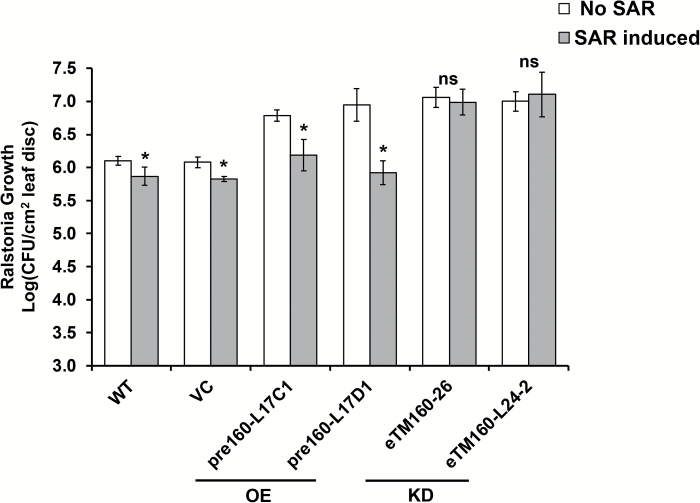
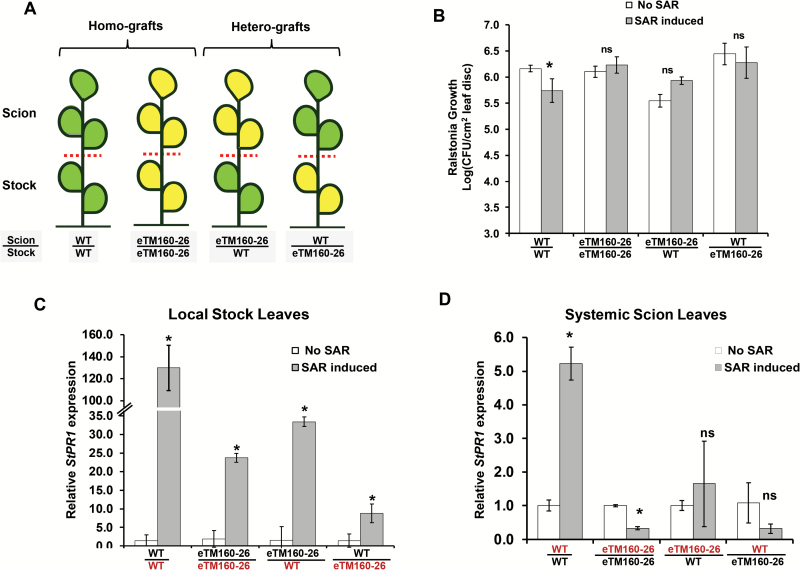
To understand the function of miR160 in the SAR of potato, the miR160 OE and KD lines were assessed for the effectiveness of their SAR response. Interestingly, the miR160 KD lines failed to mount an effective SAR, whereas the OE lines exhibited a significant SAR similar to that observed in WT and VC plants (Fig. 4). To understand the compromised SAR response of miR160 KD lines, we investigated whether the SAR defect was associated with the local or systemic leaves of KD lines. SAR assays were performed on homografts (WT/WT and eTM160-26/eTM160-26) and heterografts (eTM160-26/WT and WT/eTM160-26) generated with WT plants and the KD line eTM160-26 (Fig. 5A). The stocks of the grafted plants were treated with either sterile water or P. infestans, and the scions were treated with R. solanacearum. Consistent with our previous results (Fig. 4), WT/WT homografts showed significant SAR development and KD homografts (eTM160-26/eTM160-26) did not exhibit an SAR (Fig. 5B). This suggested that the grafting process had not affected the SAR response. Interestingly, neither of the heterograft types demonstrated a significant SAR, suggesting that KD of miR160 renders both local and systemic leaves defective in SAR. Consistent with this, the expression of the SAR marker gene StPR1 was induced in local stock leaves of all grafted plants, with the highest magnitude of induction in WT/WT homografts (Fig. 5C). In contrast, in systemic scion leaves, StPR1 expression was induced only in WT/WT grafts (Fig. 5D), reflecting the compromised SAR in the other grafts. Overall, these results indicated that miR160 is required in both local and systemic leaves for effective SAR development.
Fig. 4.
SAR response of miR160 KD and OE lines. miR160 KD lines showed a compromised SAR response. OE lines were able to induce a significant SAR response similar to that of wild-type (WT) and vector control (VC) plants, as evidenced by the reduced growth of Ralstonia solanacearum. Data represent the mean±SD of at least three biological replicates with three technical replicates each. ‘No SAR’ represents primary treatment with sterile water and ‘SAR induced’ represents primary treatment with P. infestans. Asterisks indicate values that were significantly different from the No SAR plants for each plant type (P<0.05; Student’s t-test). ns, not significant.
Fig. 5.
miR160 is required in both local and systemic leaves to induce an SAR response. (A) Schematics of grafts developed using WT plants and the miR160 KD line eTM160-26: homografts (WT/WT and eTM160-26/eTM160-26) and heterografts (WT/eTM160-26 and eTM160-26/WT). (B) SAR responses of the homografts and heterografts. All SAR data represent the mean±SD of at least three biological replicates with three technical replicates each. (C, D) qRT–PCR analysis of StPR1 expression in the inoculated local stock leaves (C) and non-inoculated systemic scion leaves (D) of all the grafted plants after 4 days of primary treatment. Data represent the mean±SD of two biological replicates with three technical replicates each. ‘No SAR’ represents primary treatment with sterile water and ‘SAR induced’ represents primary treatment with P. infestans. Asterisks indicate values that were significantly different (P<0.05; Student’s t-test) from the No SAR plants for each plant type or graft type. ns, not significant.
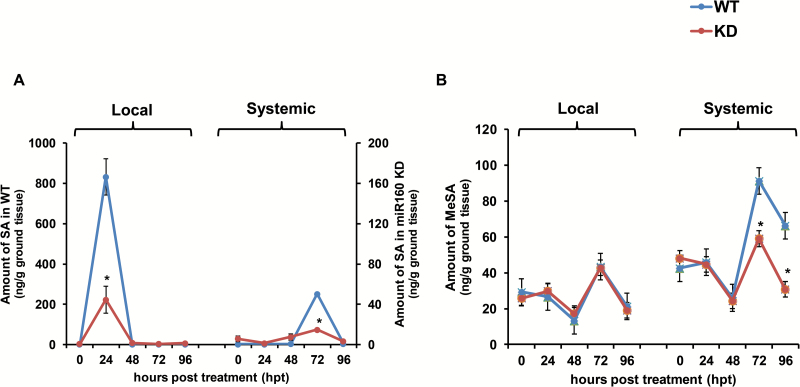
Salicylic acid and methyl salicylate levels are reduced in miR160 KD lines
To investigate the SAR-defective phenotype of the miR160 KD lines, the SAR-associated signals SA and MeSA were measured in local and systemic leaves of miR160 KD line eTM160-26 and WT plants after AA treatment. AA, a PAMP of P. infestans, has been used in previous studies of the SAR in potato because of its potential to trigger an effective SAR response (Coquoz et al., 1995; Yu et al., 1997). In our analysis, the amount of SA peaked in local leaves at 24 hpt and in systemic leaves at 72 hpt for both WT and eTM160-26 plants (Fig. 6A). However, SA levels were lower in the KD line compared with the WT plants at these time points (Fig. 6A). This observation suggested reduced SA signaling in both local and systemic leaves of miR160 KD plants. Levels of MeSA, the only known mobile SAR signal in potato (Manosalva et al., 2010), did not differ in local leaves of WT and miR160 KD plants (Fig. 6B). However, MeSA levels were lower in systemic leaves of the KD line at 72 and 96 hpt compared with the levels in WT plants (Fig. 6B). This analysis indicated that KD of miR160 affects SA levels in both local and systemic leaves, and MeSA levels in systemic leaves, of potato.
Fig. 6.
Analysis of salicylic acid (SA) and methyl salicylate (MeSA) levels in WT and eTM160-26 KD lines after arachidonic acid (AA) treatment. (A) HR-MS-based analysis of SA accumulation and (B) GC-MS-based analysis of MeSA accumulation in local and systemic leaves of WT and eTM160-26 KD lines at different time points after treatment with 0.05 mM AA. Samples were analyzed at 0, 24, 48, 72, and 96 hours post-treatment in local (AA-treated) and systemic (AA-untreated) leaves. Data represent the mean±SD of three biological replicates with three technical replicates each. Asterisks indicate statistically significant differences (P<0.05; Student’s t-test). ns, not significant.
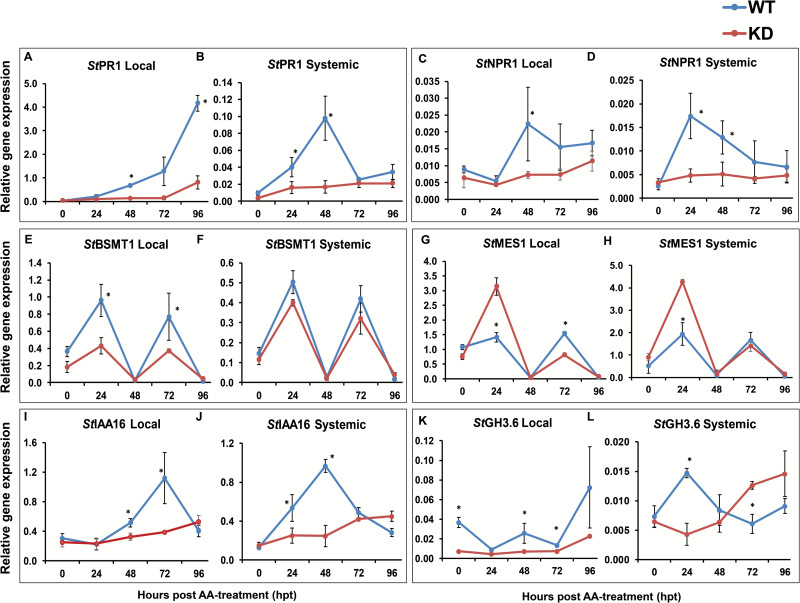
SAR pathway genes are affected in miR160 KD lines
We also performed expression analysis of some important SAR and auxin pathway genes in local and systemic leaves of WT and miR160 KD (eTM160-26) plants. After AA treatment, the expression of StPR1 increased in both local and systemic leaves of WT plants (Fig. 7A, B). An equivalent increase in StPR1 expression was not observed in miR160 KD plants (Fig. 7A, B). Similarly, the expression of StNPR1 (NONEXPRESSOR OF PR1), the master regulator of SAR, was also induced in local and systemic leaves of WT plants, whereas the miR160 KD line failed to exhibit a similar increase in StNPR1 expression (Fig. 7C, D). Furthermore, StBSMT1 (BENZOIC ACID/SA CARBOXYL METHYL TRANSFERASE 1), the gene involved in the conversion of SA to MeSA in local leaves, exhibited an oscillating expression pattern, with peaks at 24 and 72 hpt in both local and systemic leaves of WT and KD plants (Fig. 7E, F). The magnitude of StBSMT1 expression was reduced in local leaves of miR160 KD plants relative to WT; however, no significant changes were observed in systemic leaves (Fig. 7E, F). StMES1 (METHYL ESTERASE 1), the gene involved in the conversion of MeSA to SA in systemic leaves, also exhibited an oscillating expression pattern similar to that of StBSMT1, but StMES1 expression was higher in both local and systemic leaves of miR160 KD lines at 24 hpt (Fig. 7G, H). Additionally, the expression of the auxin signaling repressor StIAA16 increased in both local and systemic leaves of WT plants after AA treatment; a similar increase of StIAA16 expression was not observed in miR160 KD plants (Fig. 7I, J). StGH3.6, a potential auxin and SA conjugator in potato, had lower expression in local leaves of miR160 KD plants, relative to WT, at all time points (Fig. 7K). However, in systemic leaves of the KD line, its levels were low at 24 hpt and high at 72 and 96 hpt (Fig. 7L). Unlike all the other genes tested, the expression pattern of StGH3.6 in systemic leaves of the KD plants was opposite to that of WT plants, suggesting a possible dysregulation of this gene specifically in systemic leaves of miR160 KD plants. These results indicate that miR160 KD plants failed to induce the negative regulators of auxin signaling, which could potentially lead to unsuccessful attenuation of the auxin pathway. Taken together, these analyses reveal dysregulation of several genes involved in the SAR pathway associated with miR160 KD, and provide further evidence that auxin–SA antagonistic cross-talk appears to play role in the SAR responses of potato.
Fig. 7.
Multiple SAR-associated genes are affected in miR160 KD lines. (A, B) StPR1 expression in local (A) and systemic (B) leaves. (C, D) StNPR1 expression in local (C) and systemic (D) leaves. (E, F) StBSMT1 expression in local (E) and systemic (F) leaves. (G, H) StMES1 expression in local (G) and systemic (H) leaves. (I, J) StIAA16 expression in local (I) and systemic (J) leaves. (K, L) StGH3.6 expression in local (K) and systemic (L) leaves. All data represent the mean±SD of three biological replicates with three technical replicates each. *P<0.05 (Student’s t-test). ns, not significant.
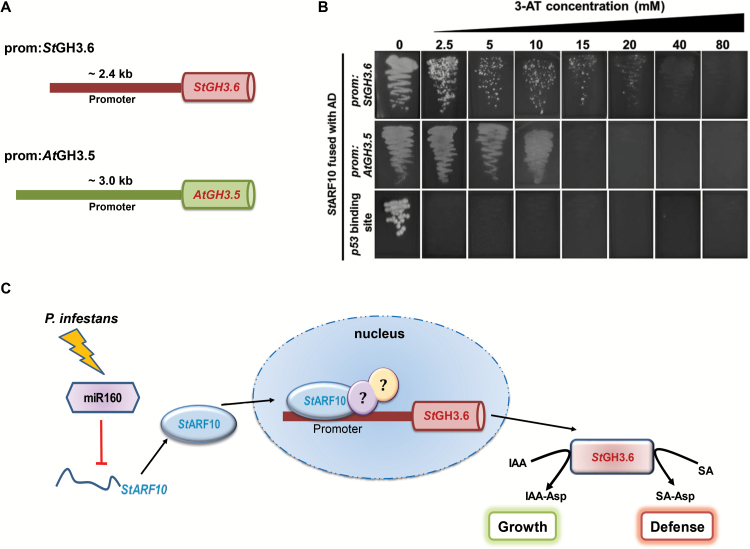
StGH3.6, a mediator of auxin–SA cross-talk, is regulated by StARF10
As mentioned above, auxin–SA antagonistic cross-talk is important for mounting an effective defense response in plants. A recent study demonstrated the role of Arabidopsis Gretchen Hagen 3.5 (AtGH3.5) as a mediator of this cross-talk, since it can conjugate both indole-3-acetic acid (IAA), an auxin, and SA (Westfall et al., 2016). AtGH3.5 has also been shown to be involved in local defense and SAR responses (Zhang et al., 2007). In our study, we observed differential expression of StGH3.6, a potato homolog of AtGH3.5 (sequence similarity of 77.6%; Supplementary Fig. S8), in miR160 OE and KD lines (Supplementary Fig. S9), as well as under infective conditions (Figs 3B and 7K, L). Previous studies have demonstrated the control of ARF transcription factors on the GH3 family of genes (Ulmasov et al., 1997; Yang et al., 2006). This prompted us to hypothesize that miR160 could regulate StGH3.6 expression through StARF10 in potato. To test this hypothesis, Y1-H analysis and EMSA were performed using StARF10 protein and the promoters of potato StGH3.6 (prom-StGH3.6) and Arabidopsis AtGH3.5 (prom-AtGH3.5) (Fig. 8A; Supplementary Fig. S11A). For Y1-H assays, the interaction of StARF10 protein was explored with the ~2.4 kb promoter of StGH3.6 and the ~3.0 kb promoter of AtGH3.5 (Fig. 8A). Mated yeast colonies containing StARF10 with the promoter of either StGH3.6 or AtGH3.5 grew robustly on the selection media (SD -His -Trp) with increasing concentrations of 3-AT (Fig. 8B). This suggested the binding of StARF10 to both the promoter sequences. As the protein sequences of StARF10 and AtARF10 are highly similar (70.3%; Supplementary Fig. S10), it is possible that Arabidopsis AtARF10 also binds to these promoter sequences. EMSA analysis further confirmed the interaction of StARF10 protein with the promoter fragments of prom:StGH3.6 and prom:AtGH3.5 (Supplementary Fig. S11). Together, our results suggest that a miR160-StARF10 module could possibly affect StGH3.6 expression, thereby modulating the cross-talk between auxin-mediated growth and the SA-mediated defense response during the potato–P. infestans interaction (Fig. 8C).
Fig. 8.
StARF10 binds to the promoters of StGH3.6 and AtGH3.5. (A) Diagrammatic representation of the promoters used for the yeast one-hybrid (Y1-H) assay: regions ~2.4 kb upstream of StGH3.6 (prom:StGH3.6) and ~3.0 kb upstream of AtGH3.5 (prom:AtGH3.5) were used. (B) Yeast strains containing StARF10 prey protein and prom:StGH3.6 bait grew in media containing up to 20 mM 3-amino-1,2,4-triazole (3-AT); strains containing StARF10 prey and prom:AtGH3.5 bait grew in media containing up to 10 mM 3-AT. The growth of yeast strains indicates the binding of StARF10 to both StGH3.6 and AtGH3.5 promoters. The p53 binding site was used as a negative control; inhibition of yeast growth at all the concentrations of 3-AT suggests no interaction between the p53 binding site and StARF10 protein. AD, activation domain. (C) A proposed model showing the control of StGH3.6 by a miR160-StARF10 module. Upon infection by P. infestans, changes in the expression of miR160 affect the levels of StARF10 mRNA. This in turn affects the levels of StARF10 protein that could enter the nucleus. In the nucleus, StARF10 protein binds to the promoter of StGH3.6 and affects its expression, possibly in combination with unknown partners (represented by circles containing question marks). Altered StGH3.6 protein levels might potentially affect the auxin–SA antagonistic cross-talk and the balance of the defense and growth pathways. This may be one of the pathways by which potato plants regulate local and SAR defense responses during P. infestans infection. IAA, indole-3-acetic acid; IAA-Asp, conjugated IAA–aspartic acid; SA, salicylic acid; SA-Asp, conjugated SA–aspartic acid.
Discussion
Several reports have demonstrated differential expression of miRNAs during a variety of plant–pathogen interactions (Bazzini et al., 2007; Lu et al., 2007; Xin et al., 2010; Guo et al., 2011; Amin et al., 2011; Lang et al., 2011; Zhao et al., 2012; Li et al., 2014). However, no study has yet explored the role of miRNAs in the potato–P. infestans interaction. Understanding this process could enhance our knowledge toward combating late blight disease. Additionally, while miRNAs are well demonstrated to be involved in local defenses, their role in SAR has not been explored. In this study, from a shortlist of 10 potential miRNAs, we selected miR160 and investigated its role in the defense responses of potato against P. infestans. By using a combination of approaches such as expression profiling, transgenic studies, infection analysis, and grafting, we show that miR160 is crucial for eliciting local defense and SAR responses during the potato–P. infestans interaction.
The miR160 expression pattern is important for eliciting local defense and SAR responses in potato
miR160, a conserved miRNA among various plant species, is known to play a vital role in plant growth and development (Mallory et al., 2005; Hendelman et al., 2012; Turner et al., 2013; Huang et al., 2016); however, its role in defense was discovered only recently (Li et al., 2010, 2014). Previous reports have demonstrated the induction of miR160 in local infected leaves of various plants upon bacterial or fungal infection (Xin et al., 2010; Lang et al., 2011; Pérez-Quintero et al., 2012). Similarly, we observed the induction of miR160 expression in local leaves of potato upon P. infestans infection (Fig. 1A). In addition, systemic leaves also exhibited induced expression of miR160 (Fig. 1B), indicating a potential role for miR160 in both local and SAR responses of potato. The enhanced susceptibility to P. infestans observed in both miR160 OE and KD lines is an indication of the breakdown of local defense responses (Fig. 2D, E; Supplementary Fig. S7). Our findings in the miR160 OE lines contrasted with previous observations. In Arabidopsis, miR160 OE plants were shown to have no difference in basal resistance to bacterial infection compared with WT plants (Li et al., 2010). In rice, however, miR160 OE plants exhibited increased basal resistance against Magnaporthe oryzae relative to WT plants (Li et al., 2014). Such contradictory responses of miR160 OE plants could be due to the different types of plant–pathogen interaction studied. Furthermore, the enhanced susceptibility observed in both miR160 OE and KD lines in our study came as a surprise. Similar observations were made with a plasmodesmata localizing protein, PDLP5, in Arabidopsis. Lim et al. (2016) have shown that both 35S-PDLP5 (OE) and pdlp-5 (KD) plants show a similar phenotype of a compromised SAR response. These results indicated that it is not improbable for a similar phenotype to develop in an OE and a KD line for a given gene.
The SAR phenotype was, however, different in the miR160 OE and KD lines we studied. The miR160 KD lines failed to elicit a SAR, whereas the OE lines successfully mounted a SAR (Fig. 4). Similar to the miR160 KD lines, many SAR-deficient mutants of Arabidopsis, such as npr1 (Cao et al., 1997), pad4 (Jirage et al., 1999), and sid2 (Wildermuth et al., 2001), are affected in basal defense as well. Furthermore, the Arabidopsis mutant eds5 is affected in basal defense but capable of mounting a SAR response, similar to our miR160 OE lines (Rogers and Ausubel, 1997). We propose that the compromised SAR response of the miR160 KD lines could be because of either or both of the following possibilities: (i) local leaves of KD lines have failed to generate and/or transport the SAR signal to systemic leaves, (ii) systemic leaves have failed to perceive and/or process the SAR signal transported from local leaves. Several previous studies have used grafting as a powerful tool to delineate questions associated with SAR responses in plants (Vernooij et al., 1994; Park et al., 2007). Our grafting study with WT and KD plants suggested that the miR160 KD lines were defective in both local and systemic responses (Fig. 5).
On the basis of these findings, we contend that a dynamic expression pattern of miR160 in local and systemic leaves is important for mounting local and SAR responses in potato. An initial increase of miR160 expression at 12 hpi followed by a decrease at later time points in the local leaves of WT plants (Fig. 1A) appears to be critical for the establishment of local defense responses. This notion is supported by the fact that both miR160 OE and KD plants (which constitutively overexpress or knockdown miR160 and thus cannot achieve similar dynamic expression patterns) show a breakdown of local defense (Fig. 2). In the systemic leaves of WT plants, the increased miR160 expression up to 48 hpi seems to be vital for the development of SAR (Fig. 1B). We conclude this because a compromised SAR response is observed only in miR160 KD lines and not in OE lines (Fig. 4).
miR160 may be involved in the SA pathway
To elucidate the defects observed in the local and systemic leaves of miR160 KD lines, SAR-associated signals (SA and MeSA); (Fig. 6), as well as the expression of selected SAR pathway genes (Fig. 7), were analyzed in miR160 KD lines after treatment with AA. We observed reduced levels of SA, StNPR1, and StPR1 in both local and systemic leaves of miR160 KD lines compared with WT plants (Figs 6A and 7A–D). This finding suggested that miR160 could positively regulate SA accumulation in both local and systemic leaves by some, as yet unknown, mechanism. In addition, reduced SA accumulation in the miR160 KD lines might have resulted in low levels of StNPR1 and StPR1. In potato, MeSA was shown to act as a mobile SAR signal and StMES1 was found to be involved in the conversion of MeSA to SA in systemic leaves (Manosalva et al., 2010). However, the role of StBSMT1, a homolog of the Arabidopsis SA to MeSA converting enzyme (Chen et al., 2003), is unknown in potato. We observed reduced StBSMT1 (Fig. 7E, F) and increased StMES1 (Fig. 7G, H) expression in miR160 KD lines relative to WT plants; however, it is not clear how miR160 affects the expression of these genes. Although local leaves of the KD lines had low SA (Fig. 6A) and reduced StBSMT1 expression (Fig. 7E), their accumulation of MeSA was comparable to that of WT plants (Fig. 6B). This might have resulted from any of the following possibilities: (i) the low SA levels in KD plants were perhaps sufficient to produce an optimum amount of MeSA; (ii) the conversion of SA to MeSA might have been done by other homologs of StBSMT1; or (iii) MeSA is not effectively transported from the local leaves of KD plants. The lower levels of MeSA observed in systemic leaves at later time points relative to WT (Fig. 6B) suggest that MeSA transport was possibly affected in the miR160 KD plants. This could be the cause of the reduced SA levels in the systemic leaves of KD plants (Fig. 6A). Taken together, our results suggest that miR160 may be involved in the SA pathway and is crucial for SAR in potato.
Auxin–SA cross-talk and the miR160-StARF10- StGH3.6 module
For plants, the maintenance of both defense and development can be energy intensive. The antagonistic cross-talk of auxin and SA is one of the mechanisms adopted by plants to mediate trade-offs between growth and defense to mount an effective defense response (Huot et al., 2014). In other words, during an infection, plants seem to redistribute their energy by attenuating auxin signaling and directing resources to trigger defense responses (Kazan and Manners, 2009). Our results suggest that miR160 is involved in this cross-talk through its target StARF10. In our local infection experiments, we observed that infected WT potato plants were able to repress positive (StYUCCA1 and StTIR1) and induce negative (StIAA16 and StGH3.6) regulators of the auxin pathway (Fig. 3B), leading to enhanced expression of StPR1 (Fig. 3A). However, similar attenuation of the auxin pathway (Fig. 3B) and induction of StPR1 expression (i.e. the SA pathway; Fig. 3A) was not observed in the miR160 KD and OE plants. This observation suggested that OE and KD of miR160 affected auxin-SA cross-talk and basal defense responses in potato.
In Arabidopsis, one of the mediators of this cross-talk is Gretchen Hagen 3.5 (AtGH3.5), an enzyme that conjugates both auxin and SA (Westfall et al., 2016) and is implicated in both local defense and SAR responses (Zhang et al., 2007). The molecular mechanism involved in the transcriptional control of AtGH3.5 is not known. Here, we show that the expression of StGH3.6, a potato homolog of AtGH3.5, is affected in miR160 OE and KD lines (Figs 3B and 7K, L). Several studies have reported that ARFs can regulate some GH3 family genes (Hagen and Guilfoyle, 2002; Staswick et al., 2005), hence we wanted to understand whether StARF10 (the target of miR160) could affect StGH3.6 expression. Through Y1-H and EMSA analysis, we confirmed that the potato protein StARF10 can directly bind to the promoter of both StGH3.6 and AtGH3.5 (Fig. 8; Supplementary Fig. S11). Further experiments could determine: (i) whether such binding has a positive or negative effect on StGH3.6 transcription, and (ii) whether StARF10 forms dimers with itself or any other ARF partner, similar to Arabidopsis (Korasick et al., 2014), to bring about this effect. Our findings provide insights regarding one of the possible mechanisms by which miR160 could be modulating auxin–SA cross-talk in potato (Fig. 8C).
Taken together, our findings demonstrate that miR160 plays a crucial role in local defense and SAR responses during the potato–P. infestans interaction. Our study provides new insights regarding the SAR process in potato and various genes involved in its regulation. Furthermore, we showed that miR160 is involved in antagonistic cross-talk between SA-mediated pathogen defense processes and auxin-mediated growth. The binding of the StGH3.6 promoter by StARF10 protein could be one of the mechanisms by which miR160 might modulate SA–auxin cross-talk. We speculate that the miR160-StARF10 module functions differentially in local and systemic leaves to regulate StGH3.6 expression. Our investigation has raised several novel questions, regarding whether miR160 has a role in the SAR response of other plant–pathogen interactions; whether, given the increased accumulation of miR160 in phloem-enriched exudates, it could be acting as a phloem-mobile SAR signal; and what is the role of other differentially expressed miRNAs in local and SAR responses of potato. Future investigations are required to shed light on these important questions.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Detection and confirmation of Phytophthora infestans and Ralstonia solanacearum.
Fig. S2. Detection and post-infection expression analysis of miRNAs in potato.
Fig. S3. Analysis of miR160 in phloem-enriched exudates of S. chacoense.
Fig. S4. Examining the inverse correlation between the expression of miR160 and StARF10.
Fig. S5. Confirmation of miR160 overexpression and knockdown lines in potato.
Fig. S6. Morphological phenotype of potato miR160 transgenic lines.
Fig. S7. miR160 overexpression and knockdown lines are highly susceptible to P. infestans infection.
Fig. S8. Sequence similarity between AtGH3.5 and StGH3.6 protein sequences.
Fig. S9. Expression of StGH3.6 in miR160 transgenic lines.
Fig. S10. Sequence similarity between StARF10 and AtARF10 protein sequences.
Fig. S11. Electrophoretic mobility shift assay to understand the interaction of StARF10 protein with promoter fragments of StGH3.6 and AtGH3.5.
Fig. S12. Standard graph for absolute quantification of P. infestans biomass.
Fig. S13. HR-MS analysis of salicylic acid.
Fig. S14. GC-MS analysis of internal standard, 3ʹ-methylacetophenone.
Fig. S15. GC-MS analysis of methyl salicylate.
Table S1. Target prediction analysis of miR160 from potato.
Table S2. Details of the primers used in this study.
Acknowledgements
We thank IISER Pune for core funding and the Department of Biotechnology (DBT), Govt. of India (grant no. BT/14449/BRB/10/847/2010) for financial support. BN, HSK and NM acknowledge the graduate fellowship obtained from IISER Pune. Thanks go to K.S. Supreeth for help with eTM160-26 knockdown line generation, Dipesh Jadav for technical help with GC-MS analysis, and Nitish Lahigude and Dr M.M. Jana for help in plant maintenance. We thank Prof. Xiu-Jie Wang for ath-eTM160 constructs, Dr Suvendra Ray for Ralstonia solanacearum, Dr Rita Ulloa for Solanum tuberosum cv. Désirée, and Prof. Albertha Walhout for yeast strains and constructs. Interesting discussions with Prof. Neelima Sinha are gratefully acknowledged. We thank Prof. Pradeep Kachroo for critical reading and editing of our manuscript. All authors declare that they have no conflict of interest.
References
- Amin I, Patil BL, Briddon RW, Mansoor S, Fauquet CM. 2011. A common set of developmental miRNAs are upregulated in Nicotiana benthamiana by diverse begomoviruses. Virology Journal 8, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AK, Chatterjee M, Yu Y, Suh SG, Miller WA, Hannapel DJ. 2006a. Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. The Plant Cell 18, 3443–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AK, Prat S, Hannapel DJ. 2006b. Efficient production of transgenic potato (S. tuberosum L. ssp. andigena) plants via Agrobacterium tumefaciens-mediated transformation. Plant Science 170, 732–738. [Google Scholar]
- Bazzini AA, Hopp HE, Beachy RN, Asurmendi S. 2007. Infection and coaccumulation of tobacco mosaic virus proteins alter microRNA levels, correlating with symptom and plant development. Proceedings of the National Academy of Sciences, USA 104, 12157–12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogale S, Mahajan AS, Natarajan B, Rajabhoj M, Thulasiram HV, Banerjee AK. 2014. MicroRNA156: a potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp. andigena. Plant Physiology 164, 1011–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet E, He Y, Billiau K, Van de Peer Y. 2010. TAPIR, a web server for the prediction of plant microRNA targets, including target mimics. Bioinformatics 26, 1566–1568. [DOI] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. 1997. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88, 57–63. [DOI] [PubMed] [Google Scholar]
- Chanda B, Xia Y, Mandal MK et al. 2011. Glycerol-3-phosphate is a critical mobile inducer of systemic immunity in plants. Nature Genetics 43, 421–427. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Venables B, Petros RA, Nalam V, Li M, Wang X, Takemoto LJ, Shah J. 2012. An abietane diterpenoid is a potent activator of systemic acquired resistance. The Plant Journal 71, 161–172. [DOI] [PubMed] [Google Scholar]
- Chen H, Banerjee AK, Hannapel DJ. 2004. The tandem complex of BEL and KNOX partners is required for transcriptional repression of ga20ox1. The Plant Journal 38, 276–284. [DOI] [PubMed] [Google Scholar]
- Chen F, D’Auria JC, Tholl D, Ross JR, Gershenzon J, Noel JP, Pichersky E. 2003. An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. The Plant Journal 36, 577–588. [DOI] [PubMed] [Google Scholar]
- Chisholm ST, Coaker G, Day B, Staskawicz BJ. 2006. Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124, 803–814. [DOI] [PubMed] [Google Scholar]
- Coquoz JL, Buchala AJ, Metraux J. 1995. Arachidonic acid induces local but not systemic synthesis of salicylic acid and confers systemic reistance in potato plants to Phytophthora infestans and Alternaria solani. Phytopathology 85, 1219–1224. [Google Scholar]
- Dai X, Zhao PX. 2011. psRNATarget: a plant small RNA target analysis server. Nucleic Acids Research 39, W155–W159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplancke B, Dupuy D, Vidal M, Walhout AJ. 2004. A gateway-compatible yeast one-hybrid system. Genome Research 14, 2093–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant–pathogen interactions. Nature Reviews Genetics 11, 539–548. [DOI] [PubMed] [Google Scholar]
- Enyedi AJ, Yalpani N, Silverman P, Raskin I. 1992. Localization, conjugation, and function of salicylic acid in tobacco during the hypersensitive reaction to tobacco mosaic virus. Proceedings of the National Academy of Sciences, USA 89, 2480–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcat S, Bennett MH, Mansfield JW, Grant MR. 2008. A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA and SA in plants following biotic and abiotic stress. Plant Methods 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. 1993. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754–756. [DOI] [PubMed] [Google Scholar]
- Guo N, Ye WW, Wu XL, Shen DY, Wang YC, Xing H, Dou DL. 2011. Microarray profiling reveals microRNAs involving soybean resistance to Phytophthora sojae. Genome 54, 954–958. [DOI] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle T. 2002. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Molecular Biology 49, 373–385. [PubMed] [Google Scholar]
- Hendelman A, Buxdorf K, Stav R, Kravchik M, Arazi T. 2012. Inhibition of lamina outgrowth following Solanum lycopersicum AUXIN RESPONSE FACTOR 10 (SlARF10) derepression. Plant Molecular Biology 78, 561–576. [DOI] [PubMed] [Google Scholar]
- Huang J, Li Z, Zhao D. 2016. Deregulation of the OsmiR160 target gene OsARF18 causes growth and developmental defects with an alteration of auxin signaling in rice. Scientific Reports 6, 29938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot B, Yao J, Montgomery BL, He SY. 2014. Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Molecular Plant 7, 1267–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage D, Tootle TL, Reuber TL, Frost LN, Feys BJ, Parker JE, Ausubel FM, Glazebrook J. 1999. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proceedings of the National Academy of Sciences, USA 96, 13583–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B. 2006. MicroRNAs and their regulatory roles in plants. Annual Review of Plant Biology 57, 19–53. [DOI] [PubMed] [Google Scholar]
- Judelson HS, Tooley PW. 2000. Enhanced polymerase chain reaction methods for detecting and quantifying phytophthora infestans in plants. Phytopathology 90, 1112–1119. [DOI] [PubMed] [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. 2009. Priming in systemic plant immunity. Science 324, 89–91. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Robin GP. 2013. Systemic signaling during plant defense. Current Opinion in Plant Biology 16, 527–533. [DOI] [PubMed] [Google Scholar]
- Kazan K, Manners JM. 2009. Linking development to defense: auxin in plant–pathogen interactions. Trends in Plant Science 14, 373–382. [DOI] [PubMed] [Google Scholar]
- Korasick DA, Westfall CS, Goo S, Nanao MH, Dumas R, Hagen G, Guilfoyle TJ, Jez JM, Strader LC. 2014. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proceedings of the National Academy of Sciences, USA 111, 5427–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang QL, Zhou XC, Zhang XL, Drabek R, Zuo ZX, Ren YL, Li TB, Chen JS, Gao XL. 2011. Microarray-based identification of tomato microRNAs and time course analysis of their response to Cucumber mosaic virus infection. Journal of Zhejiang University Science B 12, 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Wang C. 2000. The design of specific primers for the detection of Ralstonia solanacearum in soil samples by polymerase chain reaction. Botanical Bulletin of Academia Sinica 41, 121–128. [Google Scholar]
- Li Y, Lu YG, Shi Y et al. 2014. Multiple rice microRNAs are involved in immunity against the blast fungus Magnaporthe oryzae. Plant Physiology 164, 1077–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Pignatta D, Bendix C, Brunkard JO, Cohn MM, Tung J, Sun H, Kumar P, Baker B. 2012. MicroRNA regulation of plant innate immune receptors. Proceedings of the National Academy of Sciences, USA 109, 1790–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Q, Zhang J, Wu L, Qi Y, Zhou JM. 2010. Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiology 152, 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GH, Shine MB, de Lorenzo L, Yu K, Cui W, Navarre D, Hunt AG, Lee JY, Kachroo A, Kachroo P. 2016. Plasmodesmata localizing proteins regulate transport and signaling during systemic acquired immunity in plants. Cell Host & Microbe 19, 541–549. [DOI] [PubMed] [Google Scholar]
- Liu PP, von Dahl CC, Park SW, Klessig DF. 2011. Interconnection between methyl salicylate and lipid-based long-distance signaling during the development of systemic acquired resistance in Arabidopsis and tobacco. Plant Physiology 155, 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu S, Sun YH, Amerson H, Chiang VL. 2007. MicroRNAs in loblolly pine (Pinus taeda L.) and their association with fusiform rust gall development. The Plant Journal 51, 1077–1098. [DOI] [PubMed] [Google Scholar]
- Mallory AC, Bartel DP, Bartel B. 2005. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. The Plant Cell 17, 1360–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manosalva PM, Park SW, Forouhar F, Tong L, Fry WE, Klessig DF. 2010. Methyl esterase 1 (StMES1) is required for systemic acquired resistance in potato. Molecular Plant-Microbe Interactions 23, 1151–1163. [DOI] [PubMed] [Google Scholar]
- Návarová H, Bernsdorff F, Döring AC, Zeier J. 2012. Pipecolic acid, an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. The Plant Cell 24, 5123–5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro L, Dunoyer P, Jay F, Arnold B, Dharmasiri N, Estelle M, Voinnet O, Jones JD. 2006. A plant miRNA contributes to antibacterial resistance by repressing auxin signaling. Science 312, 436–439. [DOI] [PubMed] [Google Scholar]
- Ouyang S, Park G, Atamian HS, Han CS, Stajich JE, Kaloshian I, Borkovich KA. 2014. MicroRNAs suppress NB domain genes in tomato that confer resistance to Fusarium oxysporum. PLoS Pathogens 10, e1004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. 2007. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113–116. [DOI] [PubMed] [Google Scholar]
- Pérez-Quintero ÁL, Quintero A, Urrego O, Vanegas P, López C. 2012. Bioinformatic identification of cassava miRNAs differentially expressed in response to infection by Xanthomonas axonopodis pv. manihotis. BMC Plant Biology 12, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Shi J, Zhai Y, Hou Y, Ma W. 2015. Phytophthora effector targets a novel component of small RNA pathway in plants to promote infection. Proceedings of the National Academy of Sciences, USA 112, 5850–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio DC. 2014. Northern blots for small RNAs and microRNAs. Cold Spring Harbor Protocols 2014, 793–797. [DOI] [PubMed] [Google Scholar]
- Rogers EE, Ausubel FM. 1997. Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. The Plant Cell 9, 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz EA, Engelberth J, Tumlinson JH, Block A, Alborn HT. 2004. The use of vapor phase extraction in metabolic profiling of phytohormones and other metabolites. The Plant Journal 39, 790–808. [DOI] [PubMed] [Google Scholar]
- Shivaprasad PV, Chen HM, Patel K, Bond DM, Santos BA, Baulcombe DC. 2012. A microRNA superfamily regulates nucleotide binding site-leucine-rich repeats and other mRNAs. The Plant Cell 24, 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. 2005. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. The Plant Cell 17, 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summermatter K, Sticher L, Metraux JP. 1995. Systemic responses in Arabidopsis thaliana infected and challenged with Pseudomonas syringae pv syringae. Plant Physiology 108, 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trout CL, Ristaino JB, Madritch M, Wangsomboondee T. 1997. Rapid detection of Phytophthora infestans in late blight-infected potato and tomato using PCR. Plant Disease 81, 1042–1048. [DOI] [PubMed] [Google Scholar]
- Truman WM, Bennett MH, Turnbull CG, Grant MR. 2010. Arabidopsis auxin mutants are compromised in systemic acquired resistance and exhibit aberrant accumulation of various indolic compounds. Plant Physiology 152, 1562–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M, Nizampatnam NR, Baron M, Coppin S, Damodaran S, Adhikari S, Arunachalam SP, Yu O, Subramanian S. 2013. Ectopic expression of miR160 results in auxin hypersensitivity, cytokinin hyposensitivity, and inhibition of symbiotic nodule development in soybean. Plant Physiology 162, 2042–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1997. ARF1, a transcription factor that binds to auxin response elements. Science 276, 1865–1868. [DOI] [PubMed] [Google Scholar]
- van Ooijen G, van den Burg HA, Cornelissen BJC, Takken FLW. 2007. Structure and function of resistance proteins in solanaceous plants. Annual Review of Phytopathology 45, 43–72. [DOI] [PubMed] [Google Scholar]
- Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP. 2007. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij B, Friedrich L, Morse A, Reist R, Kolditz-Jawhar R, Ward E, Uknes S, Kessmann H, Ryals J. 1994. Salicylic acid is not the translocated signal responsible for inducing systemic acquired resistance but is required in signal transduction. The Plant Cell 6, 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Pajerowska-Mukhtar K, Culler AH, Dong X. 2007. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Current Biology 17, 1784–1790. [DOI] [PubMed] [Google Scholar]
- Westfall CS, Sherp AM, Zubieta C, Alvarez S, Schraft E, Marcellin R, Ramirez L, Jez JM. 2016. Arabidopsis thaliana GH3.5 acyl acid amido synthetase mediates metabolic crosstalk in auxin and salicylic acid homeostasis. Proceedings of the National Academy of Sciences, USA 113, 13917–13922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414, 562–565. [DOI] [PubMed] [Google Scholar]
- Wu HJ, Wang ZM, Wang M, Wang XJ. 2013. Widespread long noncoding RNAs as endogenous target mimics for microRNAs in plants. Plant Physiology 161, 1875–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Wang Y, Yao Y, Xie C, Peng H, Ni Z, Sun Q. 2010. Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat (Triticum aestivum L.). BMC Plant Biology 10, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N, Silverman P, Wilson TM, Kleier DA, Raskin I. 1991. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. The Plant Cell 3, 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Han SJ, Yoon EK, Lee WS. 2006. ‘Evidence of an auxin signal pathway, microRNA167-ARF8-GH3, and its response to exogenous auxin in cultured rice cells’. Nucleic Acids Research 34, 1892–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Ma W. 2016. Filamentous pathogen effectors interfering with small RNA silencing in plant hosts. Current Opinion in Microbiology 32, 1–6. [DOI] [PubMed] [Google Scholar]
- Yu D, Liu Y, Fan B, Klessig DF, Chen Z. 1997. Is the high basal level of salicylic acid important for disease resistance in potato?Plant Physiology 115, 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Li Q, Li Z, Staswick PE, Wang M, Zhu Y, He Z. 2007. Dual regulation role of GH3.5 in salicylic acid and auxin signaling during Arabidopsis-Pseudomonas syringae interaction. Plant Physiology 145, 450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JP, Jiang XL, Zhang BY, Su XH. 2012. Involvement of microRNA-mediated gene expression regulation in the pathological development of stem canker disease in Populus trichocarpa. PLoS One 7, e44968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.