Abstract
Patient-specific stem cell technology from skin and other biopsy sources has transformed in vitro models of neurodegenerative disease, permitting interrogation of the effects of complex human genetics on neurotoxicity. However, the neuropathologic changes that underlie cognitive and behavioral phenotypes can only be determined at autopsy. To better correlate the biology of derived neurons with age-related and neurodegenerative changes, we generated leptomeningeal cell lines from well-characterized research subjects that have undergone comprehensive postmortem neuropathologic examinations. In a series of proof of principle experiments, we reprogrammed autopsy leptomeningeal cell lines to human-induced pluripotent stem cells (hiPSCs) and differentiated these into neurons. We show that leptomeningeal-derived hiPSC lines can be generated from fresh and frozen leptomeninges, are pluripotent, and retain the karyotype of the starting cell population. Additionally, neurons differentiated from these hiPSCs are functional and produce measurable Alzheimer disease-relevant analytes (Aβ and Tau). Finally, we used direct conversion protocols to transdifferentiate leptomeningeal cells to neurons. These resources allow the generation of in vitro models to test mechanistic hypotheses as well as diagnostic and therapeutic strategies in association with neuropathology, clinical and cognitive data, and biomarker studies, aiding in the study of late-onset Alzheimer disease and other age-related neurodegenerative diseases.
Keywords: Alzheimer disease (AD), Amyloid beta (Aβ), Human-induced pluripotent stem cells (hiPSCs), Leptomeninges, Neurodegenerative disease, Neurons
INTRODUCTION
Alzheimer disease (AD) is a devastating dementing neurologic disorder predicted to affect 1 in 85 people worldwide by 2050 (1). Human-induced pluripotent stem cells (hiPSCs) have the potential to be a powerful preclinical model for numerous complex disorders, including AD, with human genetic and biologic properties that cannot be realized in animal models. Published hiPSC models of AD have documented cellular phenotypes relevant to AD pathology (2) and have begun to interrogate the role of AD-associated risk variants in individual genetic backgrounds (3). A limitation of most studies published to date is reliance on clinical diagnosis in the absence of postmortem neuropathologic assessment; dementia is a multifactorial condition, neurodegenerative processes precede phenotypic penetrance, and not all of them are visible with in vivo imaging. Previous reports of hiPSC generation from dura mater and scalp demonstrate that postmortem tissue is a viable source for cell line generation (4, 5). Because of its proximity to brain and relative protection from environmental exposures, we hypothesized that leptomeninges (arachnoid and pia mater) is a robust source of cells for reprogramming to hiPSCs and direct conversion to neurons. We generated leptomeningeal cell lines from autopsies from extremely well-characterized research participants in 3 longitudinal brain aging/neurodegenerative disease studies. We demonstrate efficient production of leptomeninges-derived hiPSC lines capable of differentiating into neurons that are functionally active and produce measurable levels of amyloid beta (Aβ) peptides. We show that postmortem leptomeningeal cells can be directly converted to neurons using transdifferentiation strategies (6). This approach may be useful for future studies examining the epigenetics of these derived neurons as leptomeningeal cells have theoretically been exposed to the brain macroenvironment. Neurons generated from these leptomeningeal cell lines, both via hiPSCs or direct transdifferentiation, have the potential to accelerate both mechanistic insights into diverse neurodegenerative and other neurologic diseases and promote development of diagnostic tools and therapeutic strategies for these conditions.
MATERIALS AND METHODS
Case Selection
Leptomeninges were sampled from sequential autopsies performed by the University of Washington Neuropathology and Targeted Molecular Testing (UW NPTMT) Core, and include study subjects from the Kaiser-Permanente Washington/UW Adult Changes in Thought (ACT) study (7), the UW Alzheimer’s Disease Research Center (ADRC), and the Seattle Longitudinal Study (SLS) (8). Each participant has detailed clinical history, cognitive, pharmacologic, genomic, and environmental exposure data collected through their respective studies. All protocols and procedures followed guidelines outlined and approved by the UW Institutional Review Board.
Neuropathologic Examinations
NPTMT Core autopsies include rapid dissection of 1 hemisphere and collection of 60 or more fresh frozen samples and comprehensive neuropathologic evaluation according to the latest consensus guidelines (9).
Leptomeninges Tissue Sampling and Cell Line Generation
Leptomeninges tissue was dissected away from the superior frontoparietal cortex under sterile conditions in 1–3 cm2 sections immediately after removal of the skull at autopsy. The meninges were evaluated for evidence of abnormalities, including thrombus, hemorrhage, and inflammatory infiltrates. Samples were then placed in collection media (phosphate-buffered saline [PBS], 2% fetal bovine serum [FBS], 100 mM HEPES, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin; Gibco, Waltham, MA) and stored at 4°C, or frozen to −80°C at a controlled rate of −1°C/minute in tissue cryoprotectant (water + 342.3 g/mol sucrose + 10% dimethylsulfoxide (DMSO); Sigma Aldrich, St. Louis, MO) until time of processing.
To establish primary cell lines, leptomeninges tissue was washed in sterile PBS and dissected into 2–4 mm2 sections free of blood vessels. Leptomeninges sections were digested in 1000 U/mL collagenase type IA solution (Gibco) in fibroblast growth media for 60 minutes at 37°C, with agitation at 20 and 40 minutes. Fibroblast growth media contained Dulbecco's modified Eagle’s medium (DMEM), 15% FBS, 1% nonessential amino acids (NEAA), 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, 2.5 μg/mL amphotericin B (Fungizone; Gibco). Excess cold fibroblast media was added, tissue vortexed, and passed through 100-μm cell strainer. Cell suspension was centrifuged at 1000 rpm for 5 minutes at 4°C, media was aspirated, cell pellet was resuspended in fibroblast media and plated into a 48-well plate coated in 0.1% gelatin (Sigma Aldrich). Cells were passaged using 0.25% trypsin-1 mM ethylenediaminetetraacetic acid (Thermo Fisher Scientific, Waltham, MA) for 5 minutes at 37°C. Penicillin, streptomycin, and Fungizone were removed from media after 2 weeks. Leptomeningeal cells were collected with trypsinization and stored in cryoprotectant (90% FBS + 10% DMSO) in liquid nitrogen freezer starting at passage 2. Before reprogramming, cells were tested for mycoplasma using MycoAlert Mycoplasma Kit (Lonza, Basel, Switzerland).
Leptomeninges Cell Line Characterization
Leptomeningeal cell lineage was characterized by immunostaining for Fibronectin 1:2000 (Abcam, Cambridge, MA), Vimentin 1:1000 (EMD Millipore, Temecula, CA), and PDGF Receptor alpha 1:200 (Cell Signaling, Danvers, MA) (see Supplementary Data Methods).
hiPSC Line Generation
Leptomeningeal cell lines were reprogrammed using an episomal protocol (10). Reprogrammed cells were plated onto irradiated mouse embryonic fibroblasts, in the presence of Y-27632 dihydrochloride (Abcam). Visible hiPSC colonies were selected and amplified on mouse embryonic fibroblasts using Dispase (Thermo Fisher) (see Supplementary Data Methods).
TRA181-Positive Magnetic Bead Sorting of hiPSC Lines
hiPSC colonies were purified using the cell surface marker TRA181. Briefly, cells were dissociated, washed with IMAG solution (PBS + 0.5% bovine serum albumin [Sigma Aldrich] + 2 mM ethylenediaminetetraacetic acid [Thermo Fisher Scientific]), and incubated with R-phycoerythrin (PE) anti-TRA181 (BD Biosciences, San Jose, CA). Subsequently, cells were incubated in anti-PE magnetic particles and pulled down with a rare-earth magnet. Purified hiPSCs were resuspended in mTESR (StemCell Technologies, Vancouver, Canada) + 1 μg/mL rho-associated protein kinase (ROCK) inhibitor and plated into Matrigel-coated wells. ROCK inhibitor was removed after 24 hours (see Supplementary Data Methods).
hiPSC Embryoid Body Formation
hiPSC colonies were dissociated with Dispase II (Gibco) and transferred to 10-cm nonadherent plate to form embryoid bodies (EBs). Plates were incubated at 37°C and agitated daily. On day 8, EBs were harvested for RT-qPCR analysis (see Supplementary Data Methods).
RT-qPCR
RNA was extracted with TRIzol reagent (Life Technologies, Carlsbad, CA) and digested with DNase I (Ambion, Waltham, MA). Five micrograms RNA was reverse-transcribed using the Superscript IV amplification system (Life Technologies). 18S ribosomal RNA (Rn18s) primers were used as internal control; fibronectin 1 (FN1) and vimentin (VIM) primers were used as fibroblast markers; progesterone receptor (PGR) and somatostatin receptor 2 (SSTR2) primers were used as meningothelial markers; platelet and endothelial cell adhesion molecule 1 (PECAM1) and smooth muscle actin alpha 2 (ACTA2) primers were used as vascular markers; nestin (NES), RNA-binding fox-1 homolog 3 (RBFOX3), allograft inflammatory factor 1 (AIF1), oligodendrocyte transcription factor 2 (OLIG2), and glial fibrillary acidic protein (GFAP) primers were used as brain parenchymal markers; POU class 5 homeobox 1 (POU5F1), nanog homeobox (NANOG), and SRY-box 2 (SOX2) primers were used as iPSC markers. For EB analysis, primers for alpha-fetoprotein (AFP, endoderm marker), decorin (DCN, mesoderm marker), nestin (NES, ectoderm marker), and collagen (Col1A1, ectoderm marker) were run in the paired hiPSC lines and EBs. As previously published, induction of germ layer markers above 2-fold was considered positive for germ layer induction (3). Germ layer primers used were published previously (3). All other primer sequences are found in the Supplementary Data Table S2. Real-time PCR was performed on an Applied Biosystems ViiA 7 Real-Time PCR System (Foster City, CA). cDNA was amplified for 45 cycles. Expression levels were calculated based on the 2−ΔΔCT method, using normalization to Rn18s expression for all calculations and the meningeal fibroblast line with the highest target gene expression (relative to Rn18s expression) as calibrator for each target gene. All PCR reactions were performed as duplicates and with the same amount of cDNA.
Cell Line Karyotyping
Karyotyping analysis was performed on leptomeningeal and hiPSC lines by Diagnostic Cytogenetics, Inc. (Seattle, WA).
hiPSC Neuronal Differentiation
hiPSCs were differentiated to cortical neurons using dual SMAD inhibition in Basal Neural Maintenance Media (1:1 DMEM/F12 + glutamine media/neurobasal media, 0.5% N-2 supplement, 1% B-27 supplement, 0.5% GlutaMax, 0.5% insulin-transferrin-selenium-sodium pyruvate, 0.2% β-mercaptoethanol, 0.5% NEAA; Gibco) + 10 μM SB-431542 + 0.5 μM LDN-193189 (Biogems, Westlake Village, CA) for 12 days and then further differentiated for 3 weeks with neurotrophic factors in Neuron Differentiation media (DMEM-F12 + glutamine + 1% B-27 supplement + 0.5% N-2 supplement + 0.2 μg/mL brain-derived neurotrophic factor [PeproTech, Rocky Hill, NJ] + 0.2 μg/mL glial-cell-derived neurotrophic factor [PeproTech], 0.5 M dbcAMP [Sigma Aldrich]) and refreshed every 2 days for 3 weeks (see Supplementary Data Methods).
Immunocytochemistry
hiPSC-derived neurons were immunostained with microtubule-associated protein 2 (MAP2) primary antibody at 1:1000 (M2320, Sigma Aldrich) + DAPI (2.5 μg/mL final, Alfa Aesar, Reston, VA) (see Supplementary Data Methods).
Electrophysiology
Whole cell recordings were performed at 37°C with borosilicate glass pipettes (3.5–6.5 mOhm) filled with 120 mM l-aspartic acid, 20 mM KCl, 5 mM NaCl, 1 mM MgCl2, 3 mM Mg2+-ATP, 5 mM EGTA, and 10 mM HEPES (pH 7.2, 314 mOsm). External solution (Tyrode’s solution) was composed of 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM HEPES (pH 7.4, 319 mOsm). Recordings were made with a patch clamp EPC10 amplifier (HEKA, Lambrecht, Germany) and analyzed using Patchmaster (HEKA) software.
Direct Neuronal Conversion
Leptomeningeal cells were cultured in DMEM: F12 medium + 15% FBS, 1% sodium pyruvate, 1% NEAA, and 1% GlutaMax. Cells were transduced with lentiviral vectors for EtO and XTP-Ngn2:2A:Ascl1 (N2A) (6) and expanded in the presence of G418 (100 μg/mL) and puromycin (0.5 μg/mL). Neuronal conversion was induced by doxycycline treatment (see Supplementary Data Methods).
Amyloid Beta and Phospho (Thr 231)/Total Tau Measurements
Aβ peptides from hiPSC-derived neurons were measured as previously described (3). Briefly, neurons were purified, replated, and cultured for 5 days. Secreted Aβ peptides were measured from collected neuronal culture media using an ELISA assay (Meso Scale Discovery, Rockville, MD). From the same cultures, cells were lysed in MSD lysis buffer (Meso Scale Discovery) and phospho and total tau were measured using an ELISA assay (Meso Scale Discovery).
RESULTS
Leptomeningeal and Human-Induced Pluripotent Cell Lines: Generation and Characterization
We successfully generated leptomeningeal cell lines from 8 of 11 autopsies using both fresh and frozen tissue (Table). Clinical and neuropathologic details for cases with leptomeningeal lines are presented in the Supplementary Data Table S1 and demonstrate the diversity of cases available through the various studies including AD and nondemented controls in this initial series of cases. After initial plating, cells grew slowly but growth rate increased with cell density.
Table.
Autopsy Leptomeninges Cell Lines
| Case Number | Postmortem Interval (h) | Tissue Storage Method | Postautopsy Interval (h) | Meninges Line Banked | hiPSC Line Banked |
|---|---|---|---|---|---|
| 6661 | 5.5 | Media, 4°C | 12 | Yes | Yes |
| 6671 | 18 | Media, 4°C | 84 | Failed | |
| 6672 | 4 | Media, 4°C | 36 | Yes | |
| 6674 | 5.5 | Media, 4°C | 12 | Yes | |
| 6675 | 6.2 | Media, 4°C | 12 | Failed | |
| 6679 | 2.9 | Cryoprotectant, −80°C | N/A | Yes | Yes |
| 6682 | >11* | Media, 4°C | 12 | Yes | |
| 6684 | 8.2 | Media, 4°C | 24 | Failed | |
| 6686 | 4.9 | Media, 4°C | 48 | Yes | Yes |
| 6687 | 5 | Media, 4°C | 72 | Yes | |
| 6688 | 3.5 | Media, 4°C | 24 | Yes | Yes |
hiPSC, human-induced pluripotent stem cell.
Exact time of death unknown.
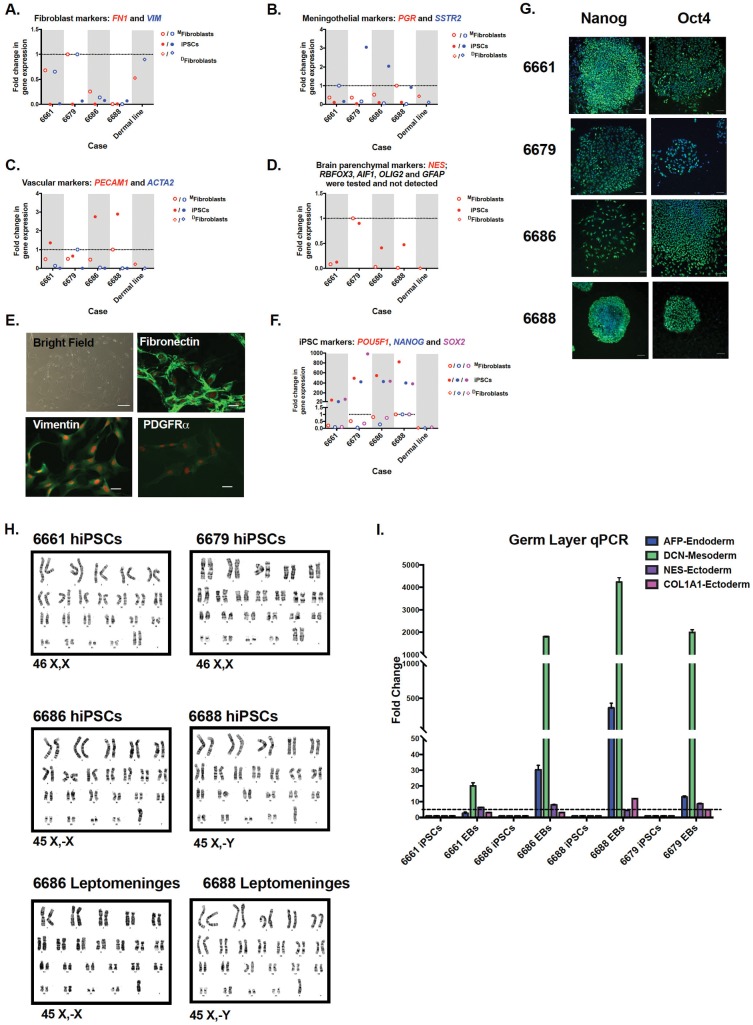
In order to generate pluripotent cells to derive neurons and other cell types for future mechanistic and therapeutic studies, leptomeningeal cells were reprogrammed by Yamanaka factors using an episomal strategy (10). We reprogrammed 4 of 8 meningeal cell lines which represented the first subjects from which the leptomeninges were harvested and include 3 patients with variable AD pathology and 1 control. As leptomeningeal tissue is a novel source for hiPSC generation, we characterized both the parental leptomeningeal cell lines and the subsequent reprogrammed hiPSCs at the level of gene expression and morphology. The leptomeninges is a combination of arachnoid and pia mater tissue of which the individual cell types are not well characterized. We reasoned that due to the composition of the leptomeninges and our method of harvesting the tissue, the cell lines that grew from the dissected tissue may contain fibroblast-like cells, vascular-like cells, or brain parenchyma cells. We therefore designed a novel qPCR panel to test for the presence of fibroblast markers, meningothelial markers, vascular markers, brain parenchymal markers, and pluripotent stem cell markers. We found that for the most part, the leptomeningeal cells showed similar expression patterns to dermal fibroblasts for the markers fibronectin (FN1) and vimentin (VIM) (Fig. 1A). The leptomeningeal lines had slightly elevated expression over dermal fibroblasts in terms of meningothelial markers progesterone receptor (PGR) and somatostatin receptor (SSTR2) (Fig. 1B) and 2 of the lines had a slight increase in vascular markers platelet endothelial cell adhesion marker (PECAM1 also known as CD31) and smooth muscle actin (ACTA2) (Fig. 1C), indicating that some lines may be more endothelial in nature. We next looked at gene expression that would indicate the presence of mature cells from the brain parenchyma. One leptomeningeal line showed some expression of Nestin (NES), a generic neural stem cell marker, suggesting the potential of culturing some neural progenitor type cells from this tissue, a phenomenon previously reported in mice (11) (Fig. 1D). However, Nestin expression was not increased in the other lines, indicating that any neural stem or progenitor cells would represent a very small percentage of the cellular population. We were unable to detect any expression of mature CNS cell types: NeuN (RBFOX3, neuronal marker), Iba1 (AIF1, microglial marker), Olig1 (OLIG1, oligodendrocyte marker), or Gfap (GFAP, astrocyte marker) (Fig. 1D), confirming that the meninges dissection is clean and separated from brain parenchymal cells. Upon morphologic examination, the leptomeningeal cells had a spindle-shaped appearance with elongated nucleus and bland cytoplasm akin to skin fibroblasts (Fig. 1E). Immunofluorescence studies revealed leptomeningeal cells were positive for fibroblast markers fibronectin, vimentin, and platelet-derived growth factor receptor alpha (Fig. 1E), consistent with the gene expression data that leptomeningeal cells share characteristics to dermal fibroblasts. Finally, we tested the expression of pluripotency markers in the hiPSC lines derived from the leptomeningeal cell lines. We found that all hiPSC lines derived from leptomeninges are highly enriched (>20-fold) for the known pluripotency genes Pou5F1 (Oct4), Nanog, and Sox2, indicating that regardless of the gene expression profile of the parental cell line, these cells all generate pluripotent stem cell lines (Fig. 1F). We next performed further characterization of the hiPSC lines generated from the leptomeningeal cells. All hiPSC cell lines were immunopositive for Oct4 and Nanog (Fig. 1G), maintained the karyotype evident from the starting cell population (Fig. 1H) and differentiated into 3 germ layers upon EB formation as evidenced by increased expression of endoderm gene AFP, mesoderm gene DCN, and ectoderm genes NES and Col1a1 (Fig. 1I). Interestingly, 2 of the 4 parental meningeal cell lines had a sex chromosome missing: lost X chromosome in case 6686, lost Y chromosome in case 6688 (Fig. 1H).
Figure 1.
Leptomeningeal cell and human-induced pluripotent stem cell (hiPSC) characterization. MFibroblasts refers to cell lines made from the meninges, DFibroblasts refers to cell line made from dermis. (A) Quantitative PCR (qPCR) analysis of fibroblast markers fibronectin (FN1) and Vimentin (VIM). (B) qPCR analysis of meningothelial markers progesterone receptor (PGR) and somatostatin receptor (SSTR2). (C) qPCR analysis of vascular markers platelet endothelial cell adhesion marker (PECAM1) and smooth muscle actin (ACTA2). (D) qPCR analysis of brain parenchymal markers nestin (NES), NeuN (RBFOX3), Iba-1 (AIF1), Olig2 (OLIG2), and Gfap (GFAP). (E) Representative images of primary leptomeningeal cells. Brightfield microscopy shows cytomorphology; scale bar = 10 μM. Cells are immunopositive for fibronectin, vimentin, and platelet-derived growth factor receptor alpha (PDGFRα); scale bar = 20 μM. (F) qPCR analysis of pluripotent stem cell markers Oct4 (POU5F1), Nanog (NANOG), and Sox2 (SOX2). (G) Representative immunofluorescence images of hiPSC lines reprogrammed from 4 autopsy leptomeningeal cell lines. All hiPSC lines exhibit the pluripotency markers OCT4 and Nanog. Scale bar = 500 μm. (H) hiPSC karyotype analysis from 6679 and 6661 cell lines shows normal female karyotypes. Karyotype analysis of 2 Alzheimer’s disease lines (6686, 6688) shows sex chromosome loss is present in hiPSC and starting leptomeninges cell lines. (I) All hiPSC lines differentiate into the 3 germ layers upon embryoid body generation as analyzed by ≥2-fold increase in endodermal (AFP), mesodermal (DCN), and ectodermal (NES, Col1a1) genes.
Neuron Generation: hiPSC Differentiation and Direct Conversion
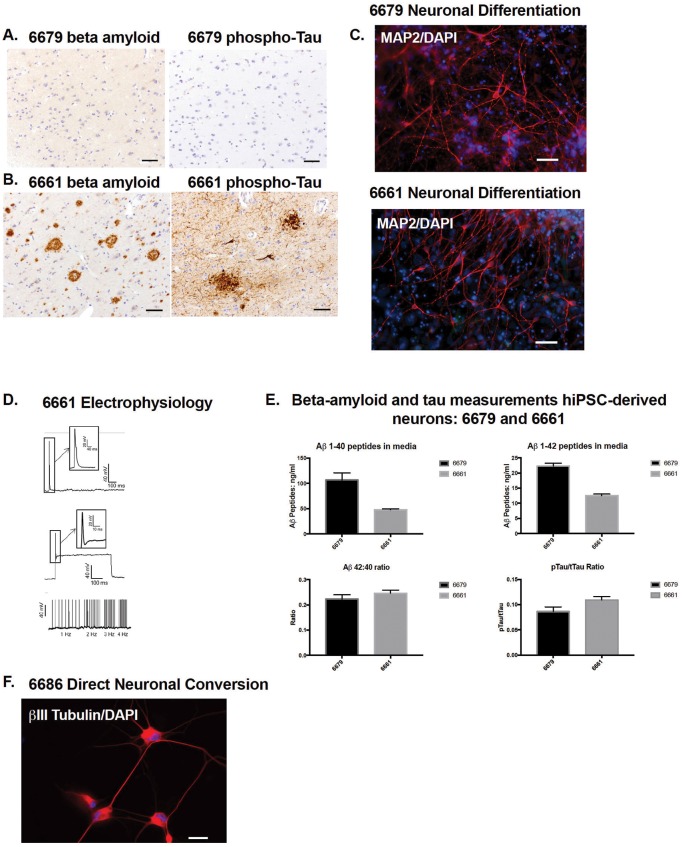
Next, we generated neurons from hiPSCs 6661 and 6679. We chose these subjects as being representative of AD and control neuropathology. Subject 6679 was nondemented at the time of last cognitive testing and had primary age-related tauopathy (Fig. 2A). Subject 6661 had dementia and high AD pathology (Fig. 2B). hiPSC-derived neurons from both subjects were positive for MAP2 (Fig. 2C). As a proof-of-principle, we tested whether neurons generated from line 6661 were electrophysiologically active and could detect action potentials after a depolarizing pulse (Fig. 2D). We subsequently differentiated neurons from the other hiPSC lines (6686 and 6688), demonstrating that all leptomeningeal-derived hiPSCs generate neurons in vitro (Supplementary Data Fig. S1A). From lines 6661 and 6679 we observed variable Aβ1-40 and Aβ1-42 levels secreted in culture media, although the calculation of Aβ 42:40 ratio normalizes these levels (Fig. 2E). We also measured detectable levels of phosphorylated (Thr231) and total Tau from these neurons and calculated a phospho/total Tau ratio (Fig. 2E). Finally, because reprogramming of somatic cells to hiPSCs erases epigenetic marks (12) we tested whether leptomeningeal cells are a viable starting cell source for direct neuron conversion. Following a published 3-week protocol (6), we transdifferentiated line 6686 directly to neurons (Fig. 2F).
Figure 2.
Leptomeningeal cells generate neurons by human-induced pluripotent stem cell (hiPSC)-directed differentiation and direct conversion protocols. (A, B) Immunohistochemistry analysis of prefrontal cortex. (A) Subject 6679 shows primary age-related tauopathy, with negative Aβ (6E10 antibody) and phosphorylated tau (p-tau) pathology (Tau2 antibody). (B) Subject 6661 shows high Alzheimer disease (AD) pathology, with dense core and diffuse Aβ plaques (6E10 antibody) and p-tau-positive neurofibrillary tangles and neuritic plaques (Tau2 antibody). Scale bar = 50 μm. (C) Immunocytochemistry using neuronal marker MAP2 of hiPSC-generated neurons from subjects 6679 and 6661. Scale bar = 50 μm. (D) Patch-clamp electrophysiology of neurons derived from 6661 hiPSC line shows action potentials after a depolarizing pulse (top). Repetitive firing was recorded in response to 1-, 2-, 3-, and 4-Hz stimulations (bottom) but not in response to a single depolarizing current (middle). (E) Aβ peptides 1-40 and 1-42 were measured from hiPSC-differentiated neuron culture media for both nondemented and AD subjects (6679 and 6661), and Aβ 42:40 ratio calculated. Phospho (Thr231) and total tau proteins were measured from the hiPSC-differentiated neuronal lysates and the pTau/tTau ratio calculated. (F) Neurons directly converted from leptomeningeal cells (subject 6686) are positive for the neuronal-specific marker βIII-tubulin. Scale bar = 10 μm.
DISCUSSION
The accessibility of viable, age-appropriate human neural tissue for the mechanistic study of AD and other neurodegenerative disorders is challenging. Murine and other animal models of human neurodegenerative disease have limited application due to factors such as widely different genomic backgrounds and short lifespan. Immortalized human cell lines are also used to model neurodegenerative disease but lack specific genetic variations that may underlie neuropathologic risk or resilience. hiPSCs generated from dermal fibroblasts of AD patients and controls have generated promising data, but correlation of neuropathology with neuroimaging, cognitive, and psychometric data cannot be performed without a definitive diagnosis.
For these reasons, we sought to develop a cell line resource that includes leptomeningeal cell lines and matched hiPSC lines, capable of generating neurons for experimentation, from pathologically defined cases with detailed clinical and behavioral histories. We tested the hypothesis that leptomeninges-derived cells could be expanded and banked for reprogramming to hiPSCs or directly converted into neurons as a resource to study AD and related neurodegenerative diseases. In agreement with previous reports (5), we generated cell lines from autopsy leptomeningeal tissue that could be readily expanded and reprogrammed to hiPSCs. These cells are morphologically similar to fibroblasts and express similar cellular marker, such as fibronectin and vimentin. However, across 4 leptomeningeal-derived cell lines, we also detected vascular and meningothelial markers as well. These levels of gene expression are different from skin-derived fibroblasts, but do not appear to influence the generation of hiPSCs from leptomeningeal tissue. From our initial set of 11 cases, approximately 70% were successfully used to generate leptomeningeal cell lines, but our efficiency has improved to near 100% since completing this study (data not shown). Of the 8 meningeal cell lines, 4 (of 4) were successfully reprogrammed to hiPSCs using standard techniques. After routine cytogenetic karyotyping, 2 of the 4 hiPSC lines were found to be missing a sex chromosome (X chromosome in case 6686, Y chromosome in case 6688). To determine whether this was intrinsic to the cells or an artifact of the reprogramming process, we tested the parental meningeal cell lines and found that they were missing the same sex chromosome. Sex chromosome aneuploidy is a common occurrence in aged cells and is also associated with increased AD risk (13, 14). Interestingly, both lines with sex chromosome loss were from pathologically confirmed AD cases. We next performed a neuronal differentiation on the leptomeningeal-derived hiPSC lines. We observed that all 4 hiPSC lines from leptomeninges differentiated to Map2-positive neurons and exhibited classical neuronal morphology.
hiPSCs and their derived progeny are increasingly utilized as preclinical screening tools (15), demonstrating the value of patient-derived cells to test novel compounds or existing drugs. Therefore, it is imperative that these derived cells have detectable levels of analytes that would be used as phenotypic read-outs. To this end, we measured Aβ production by hiPSC-derived neurons. Aβ production variability is commonly observed in hiPSC lines with nondeterministic, sporadic AD genetic backgrounds (3). More lines must be characterized to determine whether secreted Aβ levels correlate with neuropathologic findings. However, detectable levels of Aβ peptides are useful to investigate modulators of APP processing and Aβ clearance. We also show that we can detect phosphorylated tau protein, demonstrating another pathogenic phenotype that can be manipulated in in vitro cell culture conditions. Additional studies are underway to investigate expression levels of other pathologic peptides, α-synuclein species, and neurotoxicity from these cells.
Although our sample size is too small to report definitive conclusions related to disease mechanisms, we have demonstrated successful primary cultures, hiPSCs, and neurons from brains of AD patients and controls. The generation of functional neurons from cases with well-characterized pathologic phenotypes and extensive clinical history will aid the development of accurate in vitro models of AD and other neurodegenerative diseases. Future studies are needed to generate other central nervous system cell types and potential organoid cultures.
One drawback to cell models generated from hiPSCs is that the required reprogramming erases important epigenetic features that undoubtedly contribute significantly to disease pathophysiology and progression. Recent work has demonstrated that neurons directly converted from fibroblasts, by-passing a pluripotent intermediate, may retain age-related epigenetic and transcriptomic signatures (6, 15). Therefore, transdifferentiated neurons are likely to be more representative of the aged epigenome than reprogrammed hiPSCs (6, 15). As this may be a useful alternative strategy for producing human neurons to studying AD in vitro, we applied a direct conversion protocol (6) to leptomeningeal cells in culture and observed that the cells had developed neuronal morphology and expressed neuronal specific protein β-III tubulin, suggesting that these cells can also be directly converted to neurons as well as reprogrammed to hiPSCs. Therefore, for the first time, we show that leptomeningeal cells can be both reprogrammed and differentiated as well as directly converted to neurons, an important step toward interrogation of the effects of cellular age and other epigenetic regulatory mechanisms. Epigenetic regulation may be relevant to AD pathology, particularly the accumulation of age-related DNA changes. Additionally, side-by-side comparisons of hiPSC-derived neurons and direct-conversion neurons may aid in determining the contribution of pure genetics versus environment toward disease mechanisms. Although a detailed comparative study is warranted, leptomeningeal cells share the brain macroenvironment and may harbor neurogenic potential (11). This cell source may be a preferable alternative to dermal fibroblasts, which are exposed to a host of epigenetically modifying environmental insults not shared by subdural tissue. Thus, the ability to generate human neurons from autopsy leptomeninges tissue and directly compare cellular phenotypes back to the same brain is an important step toward a precision medicine approach for AD modeling and drug discovery.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Sam Josephson, Victoria Smith, Lisa Keene and Samantha Rice for technical support, and Allison Beller for administrative support.
REFERENCES
- 1. Brookmeyer R, Johnson E, Ziegler-Graham K et al. , Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 2007;3:186–91http://dx.doi.org/10.1016/j.jalz.2007.04.381 [DOI] [PubMed] [Google Scholar]
- 2. Mungenast AE, Siegert S, Tsai LH.. Modeling Alzheimer's disease with human induced pluripotent stem (iPS) cells. Mol Cell Neurosci 2016;73:13–31http://dx.doi.org/10.1016/j.mcn.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young JE, Boulanger-Weill J, Williams DA et al. , Elucidating molecular phenotypes caused by the SORL1 Alzheimer's disease genetic risk factor using human induced pluripotent stem cells. Cell Stem Cell 2015;16:373–85http://dx.doi.org/10.1016/j.stem.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bliss LA, Sams MR, Deep-Soboslay A et al. , Use of postmortem human dura mater and scalp for deriving human fibroblast cultures. PLoS One 2012;7:e45282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sproul AA, Vensand LB, Dusenberry CR et al. , Generation of iPSC lines from archived non-cryoprotected biobanked dura mater. Acta Neuropathol Commun 2014;2:4.http://dx.doi.org/10.1186/2051-5960-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mertens J, Paquola AC, Ku M et al. , Directly reprogrammed human neurons retain aging-associated transcriptomic signatures and reveal age-related nucleocytoplasmic defects. Cell Stem Cell 2015;17:705–18http://dx.doi.org/10.1016/j.stem.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cholerton B, Larson EB, Baker LD et al. , Neuropathologic correlates of cognition in a population-based sample. J Alzheimers Dis 2013;36:699–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaie KW, Willis SL.. The Seattle longitudinal study of adult cognitive development. ISSBD Bull 2010;57:24–9 [PMC free article] [PubMed] [Google Scholar]
- 9. Montine TJ, Phelps CH, Beach TG et al. , National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol 2012;123:1–11http://dx.doi.org/10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Okita K, Matsumura Y, Sato Y et al. , A more efficient method to generate integration-free human iPS cells. Nat Methods 2011;8:409–12http://dx.doi.org/10.1038/nmeth.1591 [DOI] [PubMed] [Google Scholar]
- 11. Bifari F, Decimo I, Pino A et al. , Neurogenic radial glia-like cells in meninges migrate and differentiate into functionally integrated neurons in the neonatal cortex. Cell Stem Cell 2017;20:360–73http://dx.doi.org/10.1016/j.stem.2016.10.020 [DOI] [PubMed] [Google Scholar]
- 12. Huh CJ, Zhang B, Victor MB et al. , Maintenance of age in human neurons generated by microRNA-based neuronal conversion of fibroblasts. Elife 2016;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dumanski JP, Lambert JC, Rasi C et al. , Mosaic loss of chromosome Y in blood is associated with Alzheimer disease. Am J Hum Genet 2016;98:1208–19http://dx.doi.org/10.1016/j.ajhg.2016.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yurov YB, Vorsanova SG, Liehr T et al. , X chromosome aneuploidy in the Alzheimer's disease brain. Mol Cytogenet 2014;7:20.http://dx.doi.org/10.1186/1755-8166-7-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brownjohn PW, Smith J, Portelius E et al. , Phenotypic screening identifies modulators of amyloid precursor protein processing in human stem cell models of Alzheimer's disease. Stem Cell Rep 2017;8:870–82http://dx.doi.org/10.1016/j.stemcr.2017.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.