Abstract
Inadequate sleep and problematic drinking are prevalent among high school students and are significant public health issues. Inadequate sleep may contribute to alcohol use through impairments in emotion regulation or cognitive functioning, whereas alcohol use may lead to inadequate sleep through the biological effects of alcohol or social influences. However, the directionality of the associations between sleep and drinking variables remains unclear as most prior studies were cross-sectional. This study utilizes longitudinal data from the NEXT Generation Health Study to examine bidirectional associations between alcohol use and sleep adequacy in a nationally representative sample across 3 years of high school. Students reported usual bedtimes and waketimes for scheduled- and free-days, alcohol use, and heavy episodic drinking. Estimates of sleep duration, chronotype, and social jetlag were calculated. Cross-lagged autoregressive models revealed evidence of alcohol use predicting subsequent sleep duration and timing, and sleep timing predicting subsequent alcohol use. Specifically, previous-wave alcohol use predicted shorter free-day sleep duration and later chronotype at 11th and 12th grade, and more social jetlag at 12th grade; similar results were obtained for heavy episodic drinking. Eleventh grade social jetlag predicted subsequent year current alcohol use; eleventh grade chronotype and social jetlag predicted subsequent year heavy episodic drinking. Bidirectional findings suggest that alcohol use and sleep may reflect mutually reinforcing life style choices. Understanding these bidirectional associations could inform risk prevention interventions. Given the implications of poor sleep for adolescents, further research on possible social influences on the alcohol-sleep relations is merited. Clinical Trial Registration: NCT01031160.
Keywords: sleep duration, chronotype, social jetlag, adolescent drinking, heavy episodic drinking, cross-lagged modeling
Statement of Significance
Adolescent sleep is linked to health and well-being, but there is a sparsity of longitudinal studies on possible associations of alcohol consumption with sleep duration and timing during the high school years. This study extends previous findings on sleep duration by also examining chronotype and social jetlag. Our longitudinal findings point toward possible causal pathways between alcohol use and sleep health. Both inadequate sleep and early alcohol use may contribute to negative long-term outcomes related to mental and physical health. Thus, the relations between sleep and alcohol use over time are useful to understand. If as proposed here, alcohol use represents a social influence on sleep sufficiency, strategies to reduce alcohol use may also encourage more healthful sleep.
Introduction
The problem of pervasive and chronically inadequate sleep among adolescents is increasingly recognized as a public health issue [1–3]. The social, psychological, and biological changes that are the hallmarks of adolescence have profound effects on the timing and duration of adolescents’ sleep, which, in turn, have implications for health and well-being [4]. During adolescence, there is a shift in biologic preference to later sleep timing, which often conflicts with socially prescribed scheduling demands, such as early school start times [4]. Short sleep duration and evening chronotype (i.e., preference for later bedtime and morning wake time) may contribute to alcohol use through impairments in emotion regulation, executive function, response inhibition, and decision making [5, 6]. Notably, the prevalence of drinking and heavy episodic drinking increases during high school [7], the same period in which sleep duration declines, and in which evening chronotype and other sleep problems are on the rise [8, 9]. Thus, relations between inadequate sleep and alcohol use are of interest during this developmental period.
The National Heart, Lung, and Blood Institute recommends that adolescents get 9 to 10 hr of sleep per day [10]. However, research in the United States and internationally indicates that adolescents are not meeting these recommendations [3]. Research from US national surveys, each defining inadequate sleep differently, demonstrates the prevalence of the problem. Data from the 2007–2013 Youth Risk Behavior Surveillance Survey (YRBS) indicated that the majority of 9th (59.7%)–12th (76.6%) grade students reported usually getting less than 8 hr of sleep on school nights, with significantly more students reporting short sleep duration in the higher grades [11]. Data from the National Longitudinal Study of Adolescent Health (Add Health) also indicate increased prevalence of short sleep duration (less than 6 hr) through adolescence and continuing into early adulthood [12]. Moreover, data from the 1991–2012 Monitoring the Future surveys (MTF) indicate that the proportion of adolescents reporting inadequate sleep (<7 hr per night) has increased over time [13]. Thus, inadequate sleep among adolescents is pervasive and increasingly a problem.
In 2015, prevalence of current drinking (30-day report) was estimated to be 21.5% and 35.3% among 10th and 12th graders, respectively [7]. Similarly, heavy episodic drinking (five or more drinks on one occasion in the last 2 weeks) was 10.9% and 17.2% among 10th and 12th graders, respectively [7]. Cross-sectional studies of adolescents have found associations of shorter sleep duration with increased frequency of alcohol use [6, 14] and quantity of alcohol consumed on one occasion, for which higher ranges would be equivalent to binge drinking [15]. In prospective research, Wong et al. reported a negative association between initial sleep duration, when participants were on average 16 years of age, and binge drinking 1 year later, but not between sleep duration at the second assessment and binge drinking 5 years afterwards [6]. In a study of middle school students, shorter sleep duration was associated with early initiation of alcohol use (first full drink) and heavy episodic drinking within a 4-year period [16].
In addition to sleep duration, adequate sleep is defined by the timing of the sleep period. Considering most school start times range from 07:30 to 08:30 am, optimal weekday bedtime for high school students is estimated to be between 10:30 and 11:30 pm [2]. Using Add Health data, McGlinchey et al. examined sleep timing, operationalized as bedtime in summer months, in relation to several health behaviors, including alcohol abuse [17]. Cross-sectional and prospective analyses revealed associations between late bedtime (after 01:00 am) and a greater likelihood of alcohol abuse, defined as being drunk more than once in a week in the last year. Similar associations of late bedtime with emotional distress, criminal activity, cigarette use, and illicit drug use were reported. O’Brien and Mindell reported that a greater discrepancy between weekday and weekend bedtimes was associated with increased alcohol use [14]. However, no significant associations of alcohol use with weekday bedtime or sleep onset latency were reported in a recent meta-analysis [18]. The difference in finding may reflect the dissimilarities in definitions of bedtime, variability between weekday and weekend bedtimes, and differential contributions of bedtime and duration to overall sleep adequacy.
Delayed bedtimes during adolescence may be due in part to chronotype, which refers to the relation of an individual’s endogenous circadian clock to the 24-hr day [19]. People’s preferences fall on a continuum of later versus earlier evening bedtime and morning wake times [20]. Although chronotype is generally measured by circadian biomarkers, recent research has demonstrated the midpoint of sleep on free-days (adjusted for potential oversleeping) to be a useful estimate [21]. Sleep phase shifts to later during adolescence, reaching its latest point at around age 20, after which it gradually shifts to earlier [21]. The change in timing is sensitive to social and lifestyle factors, but chronotype has strong and consistent input from a central circadian pacemaker in the brain and clock genes that regulate sleep and circadian-linked functions at the cellular and organ system level [19]. Although the shift to later is normative, having a late chronotype relative to same age peers is associated with increased likelihood of alcohol use. German [22], Hungarian [23], and Japanese [24] youth with evening chronotype have been found to have a higher frequency of alcohol consumption.
Social jetlag refers to the discrepancy between sleep timing on scheduled days (e.g., school or work days) and unscheduled or free days (e.g., weekend days). A shift in sleep timing on unscheduled days occurs due to the corrective input of biological drives for optimal sleep period timing when there is a conflict with lifestyle or environmental demands. These conflicts result can result in suboptimal sleep period timing on scheduled days. Social jetlag has been posited as an explanation for the associations of late chronotype with tobacco and alcohol use [22], and academic performance [25]. Social jetlag tends to be most pronounced in evening chronotypes, whose preference for later bed and wake times conflicts with environmentally determined sleep schedules or lifestyle choices [22]. Prospective studies among adults have indicated that greater instability of sleep timing increased risk for alcohol and other drug independent of and in greater magnitude than the risk associated with short sleep duration [26]. Circadian systems are slow to adapt to the rapid changes in sleep or wake cycles common during adolescence, making social jetlag particularly interesting in this age group [27]. Tavernier et al. directly tested the hypothesis that social jetlag explained the relation between chronotype (participants’ perceived morningness or eveningness) and substance use (a composite of alcohol and marijuana use) in a longitudinal study of first year undergraduates. Perceived eveningness was associated with greater substance use at the subsequent time point, social jetlag was not. Moreover, both perceived eveningness and substance use were associated with subsequent social jetlag. Notably, greater substance use was not associated with subsequent perceived eveningness. The authors conclude that social jetlag is not the mechanism by which perceived eveningness affects subsequent substance use [28].
There are arguments for bidirectional prospective associations between inadequate sleep and alcohol use [8, 29]. Specifically, short sleep duration and mistiming of the sleep period both have deleterious effects on adolescent functioning that may lead to increased risk of alcohol use [29, 30]. Conversely, alcohol use may contribute to deficient sleep, which could contribute to decrements in regulation of affective and cognitive functioning [27]. Pasch examined cigarette, alcohol, and marijuana use in relation to sleep duration, and two measures of weekday–weekend sleep discrepancy, weekend sleep delay (defined as the difference between weekday and weekend bedtime), and weekend oversleeping, at two time points [29]. Short sleep duration predicted greater subsequent cigarette and marijuana use, but not alcohol use. Alcohol use was associated with subsequent decreases in weekend sleep duration and weekend oversleep, but not weekday sleep duration, total sleep duration, or weekend sleep period delay. Additional studies are warranted to explicate further the interplay between sleep adequacy and alcohol use.
The purpose of this paper is to examine bidirectional longitudinal associations between sleep and alcohol use in a nationally representative contemporary sample of high school-aged youth. This study provides a test of the relations among measures of sleep duration, chronotype, social jetlag, and alcohol use, using autoregressive cross-lagged models. We expand on previous work by focusing on a younger, larger sample than in previous studies and including heavy episodic drinking in addition to alcohol use. We also examine the role of social jetlag in the association between chronotype and alcohol use to test whether the finding of Tavernier et al. is evident in a younger sample [28]. We include in our analyses measures of parent monitoring, which has extensively been studied in relation to adolescent alcohol use [31, 32] and is considered relevant to adolescent sleep health [3]. Additionally, we include a measure of depressive symptoms which has a demonstrated association with increased alcohol use [33–36] and poorer sleep [37–40].
Methods
Data are from the first 3 years (waves) of the NEXT Generation Health Study (NEXT) spanning the last 3 years of high school. NEXT is an on-going 7-year cohort study of multiple health indicators and behaviors in a nationally representative sample of US adolescents followed prospectively. School districts were the primary sampling units, stratified by the nine US census divisions. Out of 137 schools with 10th grade selected, 81 (64%) agreed to participate. Classrooms within schools were randomly selected for inclusion. Baseline data were collected during the 2009–2010 school year; however, due to timing of approval for participation, baseline assessments for one school (n = 260) occurred during the second wave (2010–2011, 11th grade). Participants completed self-administered surveys annually. Over the first three waves, 2785 participants completed the survey, with 86% retention in Wave 3 (W3). Parents provided informed consent for their child’s participation; youth provided assent if less than18 years of age, consent was obtained once participants turned 18 years. The study protocol was approved by the institutional review board at the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Measures
Sleep Variables
Duration
Participants at W1, W2, and W3 were asked to report the time (hour, minute, and designate AM or PM) that they usually go to sleep and wake up on days that they went to school, work, or similar activity (scheduled-days) and, separately, on days that they did not have to get up at a certain time (free-days, e.g., weekends and vacations). Separate duration variables were calculated for scheduled- and free-days by calculating the length of the sleep period. Duration values of <5 and >15 hr were considered questionable. To address cases in which participants reported improbable AM and PM designations, the frequency of AM versus PM designations for each hour was examined. AM and PM designations were uniformly assigned to be consistent with the hours that the majority (92% or greater) of participants reported either “AM” or “PM.” Thus, wake time hours of 5–11 were designated as “AM” and wake time hours of 12, 1, and 2 were designated as “PM”; sleep time hours of 8–11 were designated as “PM” and sleep time hours of 12 and 1–4 were designated as “AM.” This resulted in changing an “AM” or “PM” designation for 147 (5.92%), 151 (6.29%), and 160 (6.70%) participants at W1, W2, and W3 respectively. Duration values that still met the criteria for implausible (<5 or >15 hr) were set to missing. The numbers (percent) set to missing due to duration of less than 5 hr on scheduled days were 68 (1.88%), 62 (2.05%), and 85 (2.67%), and on unscheduled days were 44 (1.87%), 45 (1.34%), and 33 (0.85%) for W1, W2, and W3, respectively. The number (percent) set to missing due to a duration of more than 15 hr on scheduled days was 82 (3.30%), 98 (3.19%), and 112 (5.44%), and on unscheduled days was 227 (9.53%), 232 (9.56%), and 228 (9.98%) for W1, W2, and W3, respectively.
Chronotype
Corrected mid-sleep time on free-days was used as an estimate of chronotype. Consistent with Roenneberg and colleagues, the midpoint of free-day sleep duration corrected for oversleeping due to sleep debt [Chronotype = free-day sleep midpoint − 0.5 × (scheduled-day sleep duration − weekly sleep duration)] [19].
Social JetLag
Social jetlag [22] was calculated as the absolute difference between free-day sleep mid-point and scheduled-day sleep mid-point.
Alcohol Use
Prevalence of alcohol use was assessed by asking the number of occasions participants drank alcohol in the last 30 days (seven response options were never, 1–2, 3–5, etc., to 40 times or more). Heavy episodic drinking was assessed as the number of times in the past 30 days participants had five (for boys) or four (for girls) drinks in a row on an occasion (response options were none, 1, 2, 3–5, 6–9, 10, or more). Because of skewed distributions, variables were dichotomized as never versus ever.
Covariates
Baseline demographic variables included the following: the participant reported gender, race/ethnicity (White, African American, Hispanic, and other), perceived family affluence using the Family Affluence Scale (FAS) (family car ownership, child having their own bedroom, etc.) [41], family structure (two biologic parents, biological and step-parent, single parent, and other), and parent-reported parent education (high school or less, some college or technical school, and bachelor’s degree or higher).
Parent monitoring [42] (five items) was assessed at W1 as participants’ perception of how much their mother and father (separately) know about who their friends are, how they spend their time and money, and where they go after school and at night (response options were knows a lot, knows a little, doesn’t know anything, and don’t have or see mother/father, the last two categories were combined).
Depressive symptoms at W1 were assessed using the Modified Depression Scale [43] which consists of six items assessing how often participants were sad, grouchy or irritable, hopeless, as well as changes in sleep and eating and reduced ability to concentrate at school (response options ranged from 1 = never to 5 = always). This measure has demonstrated utility in school-based measurement of depressive symptoms [43].
Statistical Analysis
Summary statistics for the sleep and alcohol variables were tabulated across waves. Bivariate associations of sleep and alcohol use with the demographic variables, parent monitoring, and depressive symptoms were examined. The cross-lagged autoregressive models were conducted in multiple steps. First, a stability model was fitted to estimate the concurrent associations between the sleep variable and the alcohol variable, the autoregressive associations for sleep and alcohol use, and the association of the demographic variables, W1 maternal monitoring, paternal monitoring, and depressive symptoms with the W1 sleep and alcohol variables. Based on modification indices, a path from the W1 to W3 sleep variable was added, which improved fit for the stability models. Second, the cross-lagged paths were added to the model. Third, models that included both chronotype and social jetlag were examined. All analyses accounted for the complex survey design, which included the primary sampling unit (school districts or groups of small school districts), census division, and a sampling weight to adjust the racial composition of the sample to represent that of the US population of 10th grade students. In assessing model fit, we relied on CFI (>0.95), TLI (>0.95), RMSEA (<0.05) [44], and WRMR (≤1.0) [45] as chi-square is sensitive to sample size and is less appropriate for large samples. Path analysis models were run in MPlus 7.4 [46] using robust weighted least-squares with mean and variance adjustments (WLSMV) estimator and θ parameterization [46]. MPlus utilizes all participants with complete data on all covariates (survey design, demographic, parent monitoring, and depressive symptoms); thus, the number of participants included in all models was 2336. Weighted least-squares estimation uses all present pairwise data for each coefficient. As recommended by Cribbie [47], a false discovery rate (FDR) [48] control (α set at p = .05) was used to determine statistically significant pathways.
Results
Descriptive Analyses
Slightly more than half of the sample were female and whites comprised the largest race/ethnic group at W1. Most participants lived with their biological parents. Most parents reported less than a bachelor degree. Participants reported a mean sleep duration for scheduled-days (weighted, and hereafter) of 7.61 hr at W1, 7.64 hr at W2, and 7.50 hr at W3 and a mean sleep duration for free-days of 9.36 hr for W1, 9.36 hr for W2, and 9.07 hr for W3. Measures of sleep timing were consistent across the waves, with participants reporting an average chronotype of about 4.5 hr or 04:30 am, and between 2.4 and 2.8 hr of social jetlag, or the equivalent of between 2 hr, 24 min and 2 hr, 48 min. Participant report of alcohol use increased from 35% to 38% from 10th to 12th grade. Prevalence of any heavy episodic drinking was 27%, 23%, and 27% at W1, W2, and W3, respectively. Means for sleep duration, sleep timing, and alcohol by demographic characteristics are presented in Tables 1, 2, and 3; significant associations are noted. Results of the bivariate regression between demographic characteristics and the sleep variables are found in Supplementary Tables S1 and S2. Bivariate associations of W1 assessments of maternal monitoring, paternal monitoring, and depressive symptoms with sleep duration, sleep timing, and alcohol use are found in Table 4. Correlations of the sleep variables were positive for maternal monitoring and negative for depressive symptoms; almost all were statistically significant with small coefficients. Paternal monitoring was significantly and positively associated with sleep timing (except W2 social jetlag) with small coefficients. The associations with alcohol use were statistically significant with those who never use alcohol having higher scores on maternal monitoring and paternal monitoring, and fewer depressive symptoms.
Table 1.
Demographic Characteristics for the Full Sample and by Sleep Duration (Weighted Percent, Means, and SE)
| Full sample | Wave 1 | Wave 2 | Wave 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scheduled-day duration (hr) | Free-day duration (hr) | Scheduled-day duration (hr) | Free-day duration (hr) | Scheduled-day duration (hr) | Free-day duration (hr) | ||||||||
| Percent | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Overall | 7.61 | 0.04 | 9.36 | 0.06 | 7.64 | 0.05 | 9.36 | 0.04 | 7.50 | 0.05 | 9.07 | 0.06 | |
| Gendera | |||||||||||||
| Male | 44.56 | 7.60 | 0.06 | 9.10 | 0.07 | 7.69 | 0.05 | 9.26 | 0.06 | 7.54 | 0.06 | 8.90 | 0.08 |
| Female | 55.44 | 7.61 | 0.05 | 9.57 | 0.09 | 7.60 | 0.06 | 9.45 | 0.07 | 7.47 | 0.06 | 9.19 | 0.08 |
| Raceb | |||||||||||||
| Hispanic | 19.12 | 7.65 | 0.12 | 9.34 | 0.17 | 7.69 | 0.11 | 9.36 | 0.13 | 7.44 | 0.15 | 8.92 | 0.25 |
| African American | 14.38 | 7.37 | 0.07 | 9.17 | 0.15 | 7.36 | 0.09 | 9.15 | 0.14 | 7.15 | 0.05 | 8.92 | 0.17 |
| White | 61.95 | 7.65 | 0.04 | 9.47 | 0.05 | 7.71 | 0.05 | 9.44 | 0.07 | 7.63 | 0.05 | 9.18 | 0.07 |
| Other | 4.54 | 7.61 | 0.17 | 8.75 | 0.38 | 7.53 | 0.09 | 9.12 | 0.13 | 7.46 | 0.22 | 8.80 | 0.17 |
| Family structurec | |||||||||||||
| Both biological parents | 54.93 | 7.66 | 0.04 | 9.50 | 0.05 | 7.64 | 0.05 | 9.45 | 0.06 | 7.51 | 0.05 | 9.13 | 0.09 |
| Biological and step parent | 19.45 | 7.63 | 0.10 | 9.14 | 0.12 | 7.79 | 0.10 | 9.23 | 0.10 | 7.49 | 0.09 | 8.92 | 0.12 |
| Single parent | 18.02 | 7.54 | 0.09 | 9.32 | 0.16 | 7.58 | 0.08 | 9.22 | 0.14 | 7.63 | 0.11 | 9.17 | 0.14 |
| Other | 7.60 | 7.40 | 0.10 | 9.10 | 0.24 | 7.53 | 0.13 | 9.44 | 0.22 | 7.41 | 0.12 | 8.93 | 0.16 |
| Parent education | |||||||||||||
| High school or less | 32.35 | 7.62 | 0.07 | 9.30 | 0.15 | 7.73 | 0.08 | 9.43 | 0.10 | 7.52 | 0.07 | 9.08 | 0.15 |
| Some college | 40.45 | 7.59 | 0.05 | 9.28 | 0.11 | 7.63 | 0.06 | 9.29 | 0.09 | 7.46 | 0.07 | 9.07 | 0.07 |
| Bachelor degree or more | 27.20 | 7.61 | 0.05 | 9.52 | 0.10 | 7.62 | 0.08 | 9.38 | 0.06 | 7.56 | 0.07 | 9.03 | 0.10 |
Associations assessed by regression (complete results in Supplementary Table 1).
aSignificantly associated with gender (referent = female): free-day duration, male, W1 p < .001, W3 p = .01.
bSignificantly associated with race (referent = White): scheduled-day duration, African American, W1 p < .001, W2 p < .001, W3 p < .001.
cSignificantly associated with family structure (referent = both biological parents): scheduled-day duration; other, W1 p < .05.
Table 2.
Sleep Timing by Demographic Variables (Means and Standard Deviations)
| Wave 1 | Wave 2 | Wave 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chronotype (hr past midnight) | Social jetlag (hr) | Chronotype (hr past midnight) | Social jetlag (hr) | Chronotype (hr past midnight) | Social jetlag (hr) | |||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Overall | 4.55 | 0.08 | 2.77 | 0.06 | 4.50 | 0.06 | 2.59 | 0.05 | 4.46 | 0.06 | 2.36 | 0.05 |
| Gendera | ||||||||||||
| Male | 4.72 | 0.09 | 2.74 | 0.09 | 4.54 | 0.08 | 2.52 | 0.07 | 4.56 | 0.08 | 2.31 | 0.06 |
| Female | 4.40 | 0.10 | 2.79 | 0.07 | 4.47 | 0.08 | 2.65 | 0.06 | 4.38 | 0.06 | 2.40 | 0.06 |
| Raceb | ||||||||||||
| Hispanic | 4.62 | 0.14 | 2.77 | 0.12 | 4.57 | 0.13 | 2.58 | 0.11 | 4.53 | 0.18 | 2.35 | 0.11 |
| African American | 4.95 | 0.20 | 3.17 | 0.18 | 4.78 | 0.11 | 2.93 | 0.10 | 4.55 | 0.08 | 2.62 | 0.12 |
| White | 4.43 | 0.09 | 2.68 | 0.07 | 4.41 | 0.09 | 2.52 | 0.07 | 4.41 | 0.08 | 2.30 | 0.06 |
| Other | 4.30 | 0.19 | 2.47 | 0.15 | 4.43 | 0.24 | 2.36 | 0.15 | 4.51 | 0.36 | 2.18 | 0.23 |
| Family structurec | ||||||||||||
| Both biological parents | 4.34 | 0.07 | 2.57 | 0.06 | 4.37 | 0.10 | 2.13 | 0.07 | 4.33 | 0.05 | 2.29 | 0.04 |
| Biological and step parent | 4.68 | 0.16 | 2.95 | 0.11 | 4.55 | 0.10 | 2.36 | 0.13 | 4.54 | 0.14 | 2.37 | 0.11 |
| Single parent | 4.88 | 0.14 | 3.01 | 0.09 | 4.75 | 0.12 | 2.68 | 0.11 | 4.63 | 0.13 | 2.46 | 0.12 |
| Other | 4.83 | 0.21 | 3.03 | 0.19 | 4.87 | 0.17 | 2.77 | 0.12 | 4.86 | 0.14 | 2.57 | 0.12 |
| Parent educationd | ||||||||||||
| High school or less | 4.73 | 0.17 | 2.80 | 0.11 | 4.62 | 0.10 | 2.75 | 0.10 | 4.45 | 0.10 | 2.37 | 0.10 |
| Some college | 4.58 | 0.07 | 2.61 | 0.07 | 4.55 | 0.08 | 2.64 | 0.08 | 4.51 | 0.07 | 2.46 | 0.08 |
| Bachelor degree or more | 4.26 | 0.13 | 2.30 | 0.07 | 4.32 | 0.13 | 2.36 | 0.09 | 4.40 | 0.13 | 2.20 | 0.08 |
Associations assessed by linear regression (complete results in Supplementary Table 2); significant associations, p < .05.
aGender (ref = female): chronotype, male, W1, W3.
bRace (ref = White): chronotype, African American, W1, W2; social jetlag, African American, W2, W3.
cFamily structure (ref = both biological parents): chronotype, biological and step parent, W1; single parent, W1, W2, W3; other, W1, W2, W3; social jetlag, biological and step parent, W1; single parent, W1, W2; other, W1, W2, W3.
dParent education (ref = Bachelor’s degree or more): chronotype, high school or less, W1, some college, W1; social jetlag, high school or less, W1, W2, some college, W1, W2, W3.
Table 3.
Alcohol Use by Demographic Characteristics (Weighted % Ever Used)
| Wave 1 | Wave 2 | Wave 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | Heavy episodic drinking | Alcohol | Heavy episodic drinking | Alcohol | Heavy episodic drinking | |||||||
| N | (% ever) | N | (% ever) | N | (% ever) | N | (% ever) | N | (% ever) | N | (% ever) | |
| Overall | 2497 | 35.33 | 2477 | 27.23 | 2155 | 33.82 | 2151 | 22.84 | 2144 | 38.30 | 2135 | 26.79 |
| Gender | 2492 | 2472 | 2401 | 2389 | 2375 | 2365 | ||||||
| Male | 34.23 | 28.84 | 35.78 | 24.09 | 39.24 | 30.56 | ||||||
| Female | 36.11 | 25.87 | 33.41 | 23.48 | 37.44 | 23.78 | ||||||
| Racea | 2488 | 2468 | 2395 | 2383 | 2369 | 2359 | ||||||
| Hispanic | 34.49 | 27.67 | 29.75 | 21.41 | 29.49 | 20.90 | ||||||
| African American | 31.92 | 18.48 | 28.20 | 12.58 | 29.11 | 16.11 | ||||||
| White | 36.26 | 29.47 | 37.45 | 28.03 | 43.69 | 32.42 | ||||||
| Other | 38.68 | 29.90 | 41.37 | 21.63 | 42.27 | 21.36 | ||||||
| Family structure | 2497 | 2477 | 2155 | 2151 | 2144 | 2135 | ||||||
| Both biological parents | 33.37 | 23.93 | 31.71 | 24.24 | 38.14 | 27.91 | ||||||
| Biological and step parent | 37.61 | 34.39 | 35.48 | 19.92 | 40.92 | 28.10 | ||||||
| Single parent | 35.83 | 27.84 | 36.71 | 22.49 | 39.06 | 25.59 | ||||||
| Other | 40.57 | 29.34 | 43.71 | 32.26 | 34.19 | 25.11 | ||||||
| Parent education | 2336 | 2321 | 2198 | 2189 | 2178 | 2178 | ||||||
| High school | 34.41 | 29.36 | 33.20 | 22.77 | 37.08 | 25.88 | ||||||
| Some college | 35.20 | 27.36 | 33.93 | 22.14 | 37.41 | 25.94 | ||||||
| Bachelor degree or more | 36.84 | 25.34 | 37.61 | 26.73 | 41.16 | 29.46 | ||||||
aSignificant differences by Race: Alcohol use, W3 Rao-Scott Chi Square=10.89, p = .012; Binge drinking, W2 Rao-Scott Chi Square = 13.34, p = .004, W3 Rao-Scott Chi Square = 8687, p = .001
Table 4.
Bivariate Associations of Correlates (Parent Monitoring and Depressive Symptoms) with Sleep Duration, Sleep Timing, and Alcohol Use
| Wave 1 maternal monitoring | Wave 1 paternal monitoring | Wave 1 depressive symptoms | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Correlation | P | N | Correlation | P | N | Correlation | P | |||||||
| Sleep duration | |||||||||||||||
| W1 scheduled-day | 2325 | 0.08 | .00 | 2320 | 0.07 | .00 | 2330 | −0.09 | .00 | ||||||
| W2 scheduled-day | 2005 | 0.05 | .02 | 2000 | 0.02 | .26 | 2008 | −0.04 | .05 | ||||||
| W3 scheduled-day | 1966 | 0.02 | .35 | 1961 | 0.01 | .56 | 1960 | −0.08 | .00 | ||||||
| W1 free-day | 2188 | 0.07 | .00 | 2185 | 0.01 | .65 | 2191 | −0.00 | .83 | ||||||
| W2 free-day | 1908 | 0.04 | .09 | 1906 | 0.05 | .02 | 1909 | −0.07 | .00 | ||||||
| W3 free-day | 1892 | 0.06 | .01 | 1889 | 0.04 | .07 | 1896 | −0.03 | .13 | ||||||
| Sleep timing | |||||||||||||||
| W1 chronotype | 2054 | −0.13 | .00 | 2052 | −0.10 | .00 | 2057 | 0.11 | .00 | ||||||
| W2 chronotype | 1798 | −0.13 | .00 | 1796 | −0.08 | .00 | 1799 | 0.16 | .00 | ||||||
| W3 chronotype | 1758 | −0.08 | .00 | 1755 | −0.08 | .00 | 1761 | 0.10 | .00 | ||||||
| W1 social jetlag | 2054 | −0.07 | .00 | 2052 | −0.07 | .00 | 2057 | 0.11 | .00 | ||||||
| W2 social jetlag | 1798 | −0.12 | .00 | 1796 | −0.04 | .12 | 1799 | 0.10 | .00 | ||||||
| W3 social jetlag | 1758 | −0.04 | .12 | 1755 | −0.10 | .00 | 1761 | 0.06 | .01 | ||||||
| N | Mean | t-test | P | N | Mean | t-test | P | N | Mean | t-test | P | ||||
| Ever | Never | Ever | Never | Ever | Never | ||||||||||
| Current alcohol use | |||||||||||||||
| W1 | 2486 | 2.23 | 2.43 | 7.71 | .00 | 2479 | 2.08 | 2.25 | 6.16 | .00 | 2491 | 2.55 | 2.24 | 8.24 | .00 |
| W2 | 2144 | 2.24 | 2.43 | 6.30 | .00 | 2139 | 2.12 | 2.23 | 3.38 | .00 | 2148 | 2.44 | 2.29 | 3.96 | .00 |
| W3 | 2129 | 2.29 | 2.39 | 3.62 | .00 | 2125 | 2.11 | 2.24 | 4.43 | .00 | 2134 | 2.41 | 2.31 | 2.63 | .01 |
| Heavy episodic drinking | |||||||||||||||
| W1 | 2465 | 2.19 | 2.42 | 7.98 | .00 | 2458 | 2.11 | 2.23 | 3.80 | .00 | 2469 | 2.57 | 2.27 | 6.95 | .00 |
| W2 | 2140 | 2.19 | 2.41 | 6.50 | .00 | 2135 | 2.11 | 2.21 | 2.76 | .01 | 2144 | 2.47 | 2.29 | 3.59 | .00 |
| W3 | 2121 | 2.24 | 2.40 | 5.20 | .00 | 2116 | 2.07 | 2.23 | 4.70 | .00 | 2126 | 2.43 | 2.32 | 2.54 | .01 |
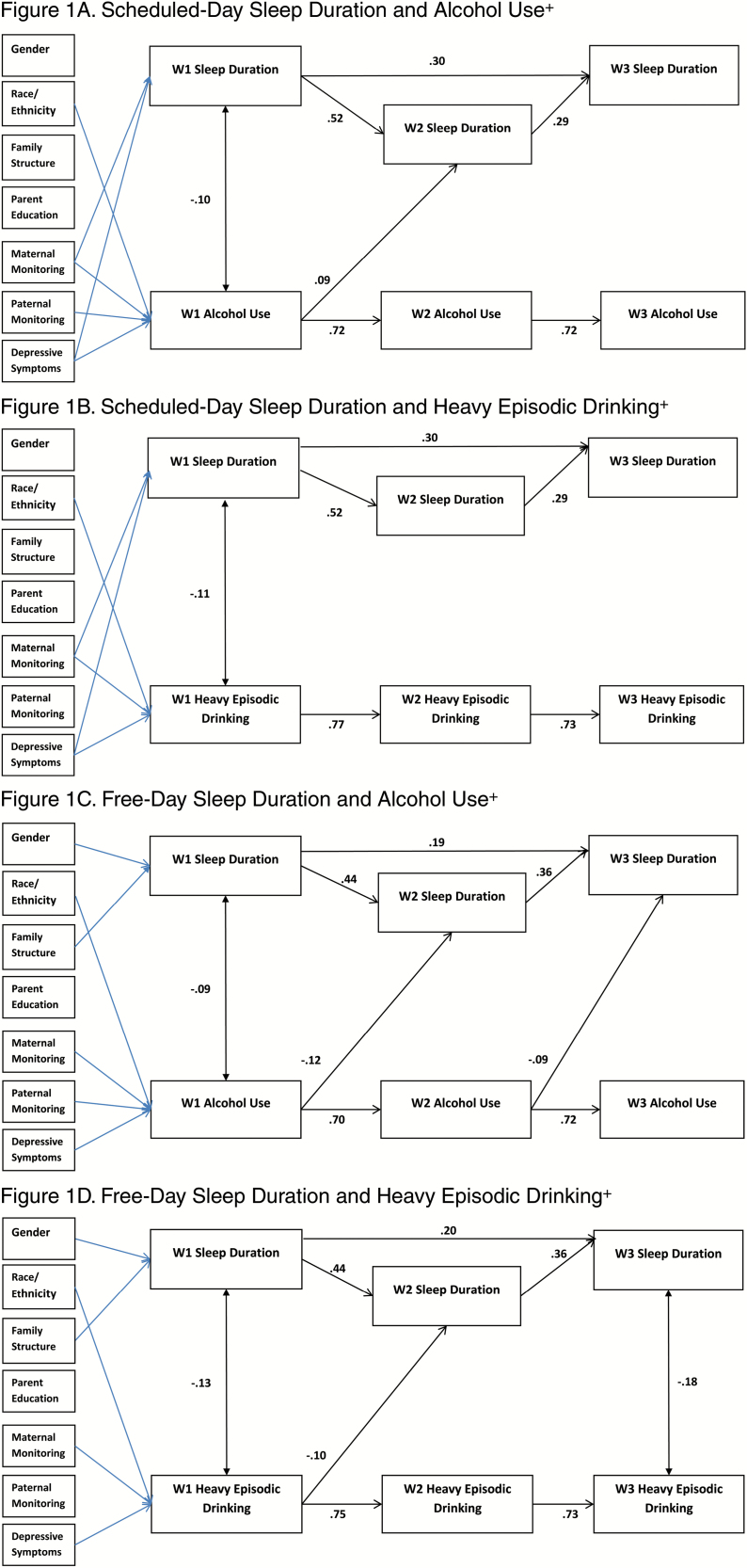
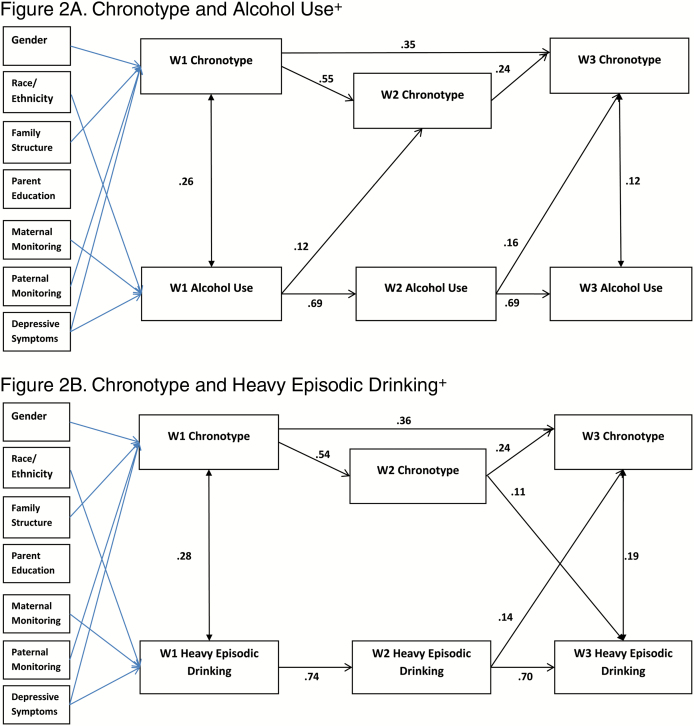
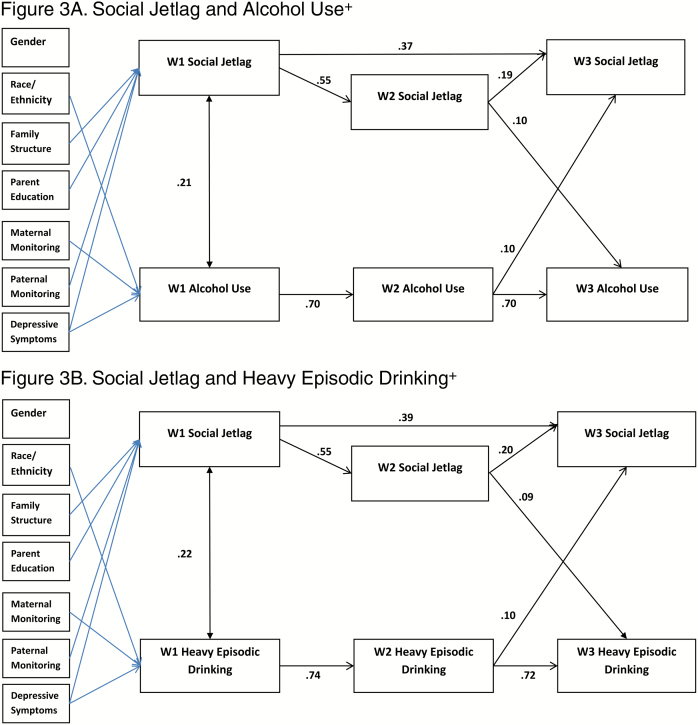
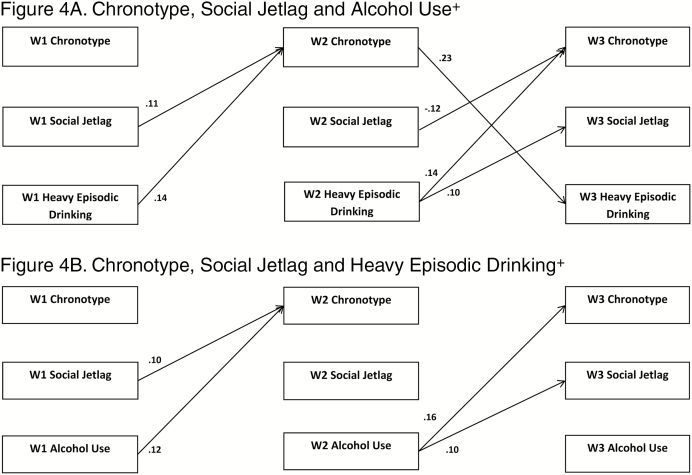
Cross-Lagged Models
Figures 1–4 depict the results for the path models that include all significant cross-lagged pathways. Standardized linear regression coefficients are presented for paths terminating with sleep duration, chronotype, and social jetlag, and interpreted as standard deviation change in a sleep variable per standard deviation increase in a predictor. Standardized probit regression coefficients are presented for paths terminating with alcohol use and heavy episodic drinking, and interpreted as the change in Z-score of the probability of alcohol use per standard deviation increase in a predictor. The stability models (include no cross-lagged pathways) showed good fit to the data with concurrent positive associations between the sleep variable and the alcohol use variable. Additionally, positive autoregressive associations were found for the sleep and alcohol use variables (stability model fit, path coefficients, and p-values provided in Supplementary Tables S3–S7). All cross-lagged models demonstrated good fit to the data (Table 5; coefficients and p-values for the cross-lagged models provided in Supplementary Tables S4–S7).
Figure 1.
Significant paths (FDR adjusted) with standardized coefficients. Blue lines indicate significant pathways for covariates. Survey design variables were included in models. +Any use in past 30 days. FDR adjustments: Figure 1A = p ≤ .021, Figure 1B = p ≤ .009, Figure 1C = p ≤ .026, Figure 1D = p ≤ .015.
Figure 2.
Significant paths (FDR adjusted) with standardized coefficients. Blue lines indicate significant pathways for covariates. Survey design variables were included in models. +Any use in past 30 days. FDR adjustments: Figure 2A =p<.009, Figure 2B= p<.024.
Figure 3.
Significant paths (FDR adjusted) with standardized coefficients. Blue lines indicate significant pathways for covariates. Survey design variables were included in models. +Any use in past 30 days. FDR adjustments: Figure 3A= p<.028, Figure 3B= p<.023.
Figure 4.
Significant paths (FDR adjusted) with standardized coefficients. Demographic variables, maternal monitoring, paternal monitoring, and depressive symptoms were regressed on W1 variables; survey design variables were included in models. +Any use in past 30 days. FDR adjustments: Figure 4A= p<.020, Figure 4B= p<.029
Table 5.
Model Fit Statistics for Cross-lagged Models
| Model | CFI | TLI | RMSEA | RMSEA 90% CI | WRMR | |
|---|---|---|---|---|---|---|
| 1a. Scheduled-day sleep duration and alcohol use | 0.980 | 0.963 | 0.012 | 0.000 | 0.021 | 0.749 |
| 1b. Scheduled-day sleep duration and heavy episodic drinking | 0.990 | 0.981 | 0.010 | 0.000 | 0.019 | 0.693 |
| 1c. Free-day sleep duration and alcohol use | 0.983 | 0.968 | 0.011 | 0.000 | 0.020 | 0.727 |
| 1d. Free-day sleep duration and heavy episodic drinking | 0.988 | 0.979 | 0.010 | 0.000 | 0.020 | 0.710 |
| 2a. Chronotype and alcohol use | 0.986 | 0.975 | 0.014 | 0.000 | 0.022 | 0.720 |
| 2b. Chronotype and heavy episodic drinking | 0.991 | 0.984 | 0.012 | 0.000 | 0.021 | 0.694 |
| 3a. Social jetlag and alcohol use | 0.981 | 0.965 | 0.014 | 0.002 | 0.022 | 0.760 |
| 3b. Social jetlag and heavy episodic drinking | 0.989 | 0.979 | 0.012 | 0.000 | 0.021 | 0.720 |
| 4a. Chronotype, social jetlag, and alcohol use | 0.994 | 0.988 | 0.011 | 0.000 | 0.018 | 0.639 |
| 4b. Chronotype, social jetlag, and heavy episodic drinking | 0.996 | 0.991 | 0.010 | 0.000 | 0.017 | 0.619 |
Criteria: Comparative fit index (CFI) > 0.96, Tucker–Lewis Index (TLI) > 0.96, root mean square error (RMSEA) < 0.05, weighted root mean square residual (WRMR) ≤ 1.0.
Sleep Duration
In the model examining scheduled-day sleep duration, the concurrent inverse association between sleep duration and alcohol use was significant at W1, and the autoregressive associations for sleep duration and for alcohol use were positive and significant (Figures 1A–D). Significant positive cross-lagged associations between W1 alcohol use and W2 sleep duration were found. No cross-lagged associations were statistically significant for heavy episodic drinking.
In the model examining free-day sleep duration and alcohol use, the W1 concurrent association was significant and negative, and the autoregressive associations were positive. Both cross-lagged paths from alcohol use to subsequent year free-day sleep duration were negative and significant. In the model examining free-day sleep duration and heavy episodic drinking, W1 and W3 concurrent associations were negative and significant, and the autoregressive associations were positive. The cross-lagged path from W1 heavy episodic drinking and W2 sleep duration was negative and significant.
Chronotype
Concurrent positive associations between alcohol use and chronotype were positive and significant for W1 and W3, and the autoregressive associations were positive (Figures 2A and B). Both paths from alcohol use to subsequent chronotype (W1 to W2 and W2 to W3) were positive and significant. Regarding heavy episodic drinking, concurrent positive associations with chronotype were found at W1 and W3 and the autoregressive associations were positive. Significant positive cross-lagged association was found for W2 chronotype and W3 heavy episodic drinking, and for W2 heavy episodic drinking to W3 chronotype.
Social Jetlag
The concurrent associations between social jetlag and alcohol use were positive and significant at W1, and the autoregressive associations were positive (Figures 3A and B). Regarding the cross-lagged associations, significant positive associations were found between W2 social jetlag and W3 alcohol use, and W2 alcohol use and W3 social jetlag. In the model for heavy episodic drinking, a concurrent positive association between heavy episodic drinking and social jetlag at W1, the autoregressive associations were positive, and statistically significant positive cross-lagged associations were found for W2 social jetlag and W3 heavy episodic drinking, and for W2 heavy episodic drinking and W3 social jetlag.
Chronotype and Social JetLag
The final models included chronotype and social jetlag simultaneously (Figures 4A and B). For clarity, only the significant cross-lagged paths are depicted in the figures; the pattern of significant concurrent and autoregressive associations found in the previous models was maintained. In both models, chronotype and social jetlag were highly concurrently correlated (alcohol use model, 0.74–0.84; heavy episodic drinking, 0.72–0.82) at each wave. In the model for current alcohol use, the cross-lagged path W1 social jetlag to W2 chronotype was positive and significant. The paths from alcohol use to subsequent wave chronotype (W1 to W2 and W2 to W3) were positive and significant as was the cross-lagged path from W2 alcohol use to W3 social jetlag. In the model for heavy episodic drinking, the cross-lagged path from W2 chronotype to W3 heavy episodic drinking was positive and significant. Social jetlag was statistically significantly associated with subsequent chronotype such that the path coefficient from W1 to W2 was positive, and the path coefficient from W2 to W3 was negative. Both cross-lagged paths from heavy episodic drinking (W1 to W2 and W2 to W3) were positive and significantly associated.
Discussion
The average scheduled-day sleep duration reported by our sample of high school was shorter than the recommended 9 to 10 hr, and sleep durations were longer for free-days, consistent with others’ findings among similarly aged adolescents [2]. However, a smaller percentage of our sample reported usual weekday sleep of less than 8 hr (59%, 56%, and 64%, weighted, for grades 10, 11, and 12, respectively) than reported insufficient sleep (69%) in the YRBS sample [11]. This difference may be attributable in part to the YRBS data retaining those who reported less than 5 hr (about 16% of the YRBS sample), whereas we did not. A late chronotype (mean of about 4.5 or 04:30 am) was found across all time points. Social jetlag exceeded 2 hr, 20 min at each time point, indicating a considerable discrepancy between weekday and weekend sleep (e.g., 6 to 7 hr on school or work nights and 9 to 10 hr on weekend nights). Notably, both chronotype and social jetlag remained consistent across the three assessments. It had been expected that chronotype would shift later across the study period [19]. It is plausible that the study period was too brief to detect a shift in the means of our sleep variables. The bivariate associations between the sleep variables and the demographic characteristics were consistent with previous research. Males reported shorter sleep durations [3] and more social jetlag [49] than females; African American participants had the shortest sleep duration, latest chronotype, and greatest social jetlag [3] compared with other race and ethnic groups; non-two parent family structure and lower parent education were associated with later chronotype and higher social jetlag [3, 50]. The associations between parent monitoring and the sleep variables were consistent with previous research, indicating that parents may promote longer and better timed sleep [3]. Our findings on depressive symptoms were also consistent with previous research, indicating increased report of symptoms among those with poorer sleep.
Reported prevalence of 30-day alcohol use and heavy episodic drinking in our sample was consistent with other nationally representative adolescent samples [7]. Notably, our sample did not have as marked an increase in reported alcohol use as that reported in the 2015 YRBS [51]. The greater consistency in alcohol use in our sample may reflect that we assessed the same participants annually, whereas YRBS used successive cross-sectional survey.
Concurrent Associations in the Cross-Lagged Models
Concurrent associations were assessed in the paths between alcohol use and sleep variables at the same wave (i.e., W1 alcohol and W1 sleep, W2 alcohol with W2 sleep, and W3 alcohol and W3 sleep). In the stability models, there were significant negative correlations between sleep duration and alcohol use, and significant positive correlations found between sleep timing and alcohol use. In contrast, in the models that included the cross-lagged paths, significant concurrent correlations between alcohol use and sleep duration and social jetlag were found only at W1. Thus, when the prospective associations were included, the cross-sectional results were not maintained. Cross-sectional research regarding poor sleep as a risk factor for adolescent alcohol use attributes in relation to poorer emotion regulation, executive function, response inhibition, and decision making [3]. Possibly, multiple factors could contribute to inadequate sleep which might explain our lack of findings. For example, inadequate sleep may also result from increased engagement in academic (e.g., late night studying and crowded weekday time schedule) or other conventional behaviors (elite sports, etc.) that may interact with or be associated with alcohol use [52, 53].
Cross-Lagged Associations
The cross-lagged models enabled us to examine the association between alcohol use and sleep adequacy 1 year later and, simultaneously, the association between sleep adequacy and alcohol use 1 year later. Across the models, alcohol use compared with no use predicted the sleep variables 1 year later. Specifically, using alcohol predicted shorter free-day sleep duration in the subsequent wave, cross-lagged paths at W1 to W2 and W2 to W3 for alcohol use, and W1 to W2 for heavy episodic drinking. Alcohol use predicted later chronotype in the subsequent year at W1 (cross-lagged path from W1 to W2) and both W2 alcohol use and W2 heavy episodic predicted W3 chronotype. Alcohol use and heavy episodic drinking predicted increased social jetlag in the subsequent year, cross-lagged paths from W2 to W3. In the model examining chronotype and social jetlag simultaneously, these paths remained significant. The consistency in the associations with the sleep measures of both alcohol use and heavy episodic drinking increases confidence in the findings. This pattern of results is consistent with finding of Pasch et al. with regards to weekend sleep duration and extends them to chronotype and social jetlag [29]. Pasch et al. suggest that adolescents may use alcohol to cope with stress, which in turn has a negative impact on sleep [29]. It is possible that our findings also reflect some shared characteristics, such as stress or persistent depressed mood. Research has examined how alcohol negatively affects sleep, some of which has focused on biological effects of alcohol, such as lower levels of melatonin following alcohol consumption [54] or alterations in circadian rhythm of body temperature [55]. However, these effects are measured within short time frames, whereas our assessments were approximately 1 year apart. Alcohol abuse and addiction have been found to have disruptive effects on sleep that last after treatment [56]. Research has demonstrated early alcohol use to be predictive of poorer health and behavioral outcomes [57, 58], such as academic problems [59, 60], substance use [59, 61], delinquency [59], motor vehicle crash [62], depression [61], interpersonal violence [60], risky sexual behavior [60], and suicide [60]. Our findings add to our understanding of the pervasive and long-term impact of underaged drinking on adolescent health.
Support for bidirectional associations between sleep and alcohol use was also found, but only in the models examining sleep timing and between W2 and W3. Bidirectional positive associations were found in the model examining chronotype and heavy episodic drinking between W2 and W3, consistent with other research that has found evening chronotype may be linked to a propensity for alcohol use [22–24]. Additionally, bidirectional positive associations were found between W2 and W3 in the models examining social jetlag and both current alcohol use and heavy episodic drinking. Our results indicate that these might be mutually reinforcing unhealthy lifestyle choices. Different characteristics of sleep–wake regulation may be predictive of alcohol use at different time points, suggesting that there are changes across adolescent development.
Using analytic methods similar to those employed by Tavernier, we sought to examine whether social jetlag was a mechanism through which late chronotype was associated with increased alcohol use by conducting a cross-lagged model including social jetlag, chronotype, and alcohol use. In this model, chronotype, but not social jetlag, was predictive of later alcohol use (W2 to W3), consistent with findings reported by Tavernier [28]. In contrast to Tavernier, social jetlag predicted subsequent chronotype, and in different directions at different time points. This inconsistency in direction of association is difficult to explain. The differences with Tavernier’s findings may be attributed to how chronotype was measured in each study. Tavernier assessed perceived morningness or eveningness using survey questions, whereas our study calculated chronotype and social jetlag using reported sleep and wake times.
Our findings support the contention that adolescent alcohol use may contribute to deficient sleep. However, a great deal more needs to be learned about possible explanations for the prospective associations between alcohol and inadequate sleep. One possibility is that it is not only the effect of drinking, but the social context of drinking that drives the association. To develop a better understanding of this, contextual, developmental, and social factors would need to be considered. Although social support for healthful sleep is important [27, 63], there are few studies of social influences on sleep behavior. Parental monitoring of bedtimes, overall sleep schedule, and decreased nighttime media use have been associated with increased total sleep time [18]. In the current study, increased parent monitoring was associated with earlier chronotype and shorter social jetlag. In a study of peer influence, both substance use and sleep behavior were found to spread through friendship networks, with those most central to the network most likely to engage in marijuana use and have the most inadequate sleep [64]. Moreover, friend sleep behaviors were predictive of similar participant future sleep behavior and marijuana use, providing evidence that sleep behavior is subject to social influences in ways similar to other health behaviors. Potentially, homophily in chronotype among friends is reinforced by shared social schedules. Our findings that alcohol use predicts subsequent wave chronotype may reflect a shared life style or selection effect. Social jetlag describes the discrepancy between sleep periods on scheduled versus unscheduled days. Often the constraints on scheduled days are thought of in terms of structural demands, such as school or work start times [25, 65], but in adolescence, increased academic and social engagement as well as lifestyle choices are likely to contribute to increased social jetlag [27]. Given that alcohol use is most often a social behavior [66, 67] and is well documented to be influenced by peer behavior [68–71], it is possible that alcohol use in this study reflects a social influence on sleep behavior. Further research on possible social influences (e.g., changes in parental monitoring and peer norms and activities) on the alcohol-sleep relations is merited.
Limitations
The national sample, longitudinal design, and measurement of multiple dimensions of sleep are assets of the NEXT study. However, there are limitations as well. These analyses relied on self-reported sleep times and alcohol use assessed once each year. More frequent assessments of both sleep and alcohol use could be more informative. Participants were asked to report their usual sleep and wake times, which cannot capture night-to-night variability in sleep duration or sleep that occurs at other times of the day. It is plausible that acute stressors (e.g., a relationship break-up or school stress) could lead to brief periods of poor sleep and alcohol use that would not be captured in the current measures of sleep. There are also potential confounders that were not included that were not included in the study, for example, impulse control [27] and decision making [72] that merit further study. The sleep variables reported here are perceptions of usual sleep patterns rather than reports of actual sleep times. Moreover, participants may conflate bedtime and actual sleep time, thus overestimating sleep duration by an unmeasured latency to sleep, which has been estimated as approximately 17 min [2]. We relied on self-reports of drinking over the last 30 days, which may be subject to recall bias. The reports of heavy episodic drinking as operationalized did not capture occasions of extremely heavy episodic drinking, which has a higher likelihood of serious consequences [73]. Additionally, in the probit model for alcohol use, linear effect of sleeping variables are assumed, but potentially the relationship could be nonlinear. For example, it is possible that both too long and too short sleep durations are associated with increased alcohol use.
Conclusion
This study adds to our understanding of the interplay among sleep habits, chronotype, and alcohol use among adolescents. These longitudinal findings clarify the directionality of the sleep-alcohol use relations during a critical developmental stage [29]. Addressing sleep issues among adolescents is likely to have far reaching benefits to their health and well-being. If as proposed here, in addition to its psycho-physiological effects, alcohol use represents a social influence on sleep sufficiency, strategies to reduce alcohol use may also encourage healthier sleep. For example, parental monitoring has a demonstrated protective effect on underage drinking [71, 74] and improved sleep adequacy [18]. Strategies encouraging increased monitoring could be expanded to include parental enforcement of healthy sleep schedules and decreased social media. It is plausible that such limitations would have similar effects by disrupting the self-perpetuating pattern of alcohol use and less optimal sleep found in this study.
Supplementary Material
Supplementary material is available at SLEEP online.
Funding
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Contract No. HHSN275201200001I), the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, and Maternal and Child Health Bureau of the Health Resources and Services Administration, with supplemental support from the National Institute on Drug Abuse.
Disclosure Statement
None declared.
References
- 1. Colten H, Altevogt BM.. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington DC: National Academies Press; 2006. http://www.nap.edu/catalog/11617.html Accessed May 5, 2017. [PubMed] [Google Scholar]
- 2. Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011; 12(2): 110–118. [DOI] [PubMed] [Google Scholar]
- 3. Owens J. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014; 134(3): e921–e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004; 1021: 276–291. [DOI] [PubMed] [Google Scholar]
- 5. Pieters S, Burk WJ, Van der Vorst H, Dahl RE, Wiers RW, Engels RC. Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. J Youth Adolesc. 2015; 44(2): 379–388. [DOI] [PubMed] [Google Scholar]
- 6. Wong MM, Robertson GC, Dyson RB. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcohol Clin Exp Res. 2015; 39(2): 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miech RA, Johnston LD, O’Malley PM, Bachman JG, Shulenberg JE. Monitoring the future national survey results on drug use, 1975–2015: Volume 1, Secondary school students. Ann Arbor: Institute for Social Research; 2016. [Google Scholar]
- 8. Conroy DA, Arnedt JT. Sleep and substance use disorders: an update. Curr Psychiatry Rep. 2014; 16(10): 487. [DOI] [PubMed] [Google Scholar]
- 9. Hasler BP, Soehner AM, Clark DB. Circadian rhythms and risk for substance use disorders in adolescence. Curr Opin Psychiatry. 2014; 27(6): 460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Heart Lung and Blood Institute. How Much Sleep Is Enough?2012. https://www.nhlbi.nih.gov/health/health-topics/topics/sdd/howmuch Accessed May 5, 2017.
- 11. Wheaton AG, Olsen EO, Miller GF, Croft JB. Sleep duration and injury-related risk behaviors among high school students–United States, 2007-2013. MMWR Morb Mortal Wkly Rep. 2016; 65(13): 337–341. [DOI] [PubMed] [Google Scholar]
- 12. Maslowsky J, Ozer EJ. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014; 54(6): 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keyes KM, Maslowsky J, Hamilton A, Schulenberg J. The great sleep recession: changes in sleep duration among US adolescents, 1991-2012. Pediatrics. 2015; 135(3): 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005; 3(3): 113–133. [DOI] [PubMed] [Google Scholar]
- 15. Morioka H, Itani O, Kaneita Y et al. Associations between sleep disturbance and alcohol drinking: a large-scale epidemiological study of adolescents in Japan. Alcohol. 2013; 47(8): 619–628. [DOI] [PubMed] [Google Scholar]
- 16. Miller MB, Janssen T, Jackson KM. The prospective association between sleep and initiation of substance use in young adolescents. J Adolesc Health. 2017; 60(2): 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGlinchey EL, Harvey AG. Risk behaviors and negative health outcomes for adolescents with late bedtimes. J Youth Adolesc. 2015; 44(2): 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartel KA, Gradisar M, Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015; 21: 72–85. [DOI] [PubMed] [Google Scholar]
- 19. Roenneberg T, Kuehnle T, Pramstaller PP et al. A marker for the end of adolescence. Curr Biol. 2004; 14(24): R1038–R1039. [DOI] [PubMed] [Google Scholar]
- 20. Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry. 2014; 26(2): 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003; 18(1): 80–90. [DOI] [PubMed] [Google Scholar]
- 22. Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006; 23(1-2): 497–509. [DOI] [PubMed] [Google Scholar]
- 23. Urbán R, Magyaródi T, Rigó A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiol Int. 2011; 28(3): 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ishihara K, Miyasita A, Inugami M, Fukuda K, Yamazaki K, Miyata Y. Differences in the time or frequency of meals, alcohol and caffeine ingestion, and smoking found between ‘morning’ and ‘evening’ types. Psychol Rep. 1985; 57(2): 391–396. [DOI] [PubMed] [Google Scholar]
- 25. Haraszti RÁ, Ella K, Gyöngyösi N, Roenneberg T, Káldi K. Social jetlag negatively correlates with academic performance in undergraduates. Chronobiol Int. 2014; 31(5): 603–612. [DOI] [PubMed] [Google Scholar]
- 26. Hasler BP, Soehner AM, Clark DB. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 2015; 49(4): 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dahl RE, Lewin DS. Pathways to adolescent health sleep regulation and behavior. J Adolesc Health. 2002; 31(6 Suppl): 175–184. [DOI] [PubMed] [Google Scholar]
- 28. Tavernier R, Munroe M, Willoughby T. Perceived morningness-eveningness predicts academic adjustment and substance use across university, but social jetlag is not to blame. Chronobiol Int. 2015; 32(9): 1233–1245. [DOI] [PubMed] [Google Scholar]
- 29. Pasch KE, Latimer LA, Cance JD, Moe SG, Lytle LA. Longitudinal bi-directional relationships between sleep and youth substance use. J Youth Adolesc. 2012; 41(9): 1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Owens JA, Dearth-Wesley T, Lewin D, Gioia G, Whitaker RC. Self-regulation and sleep duration, sleepiness, and chronotype in adolescents. Pediatrics. 2016; 138(6): e20161406. [DOI] [PubMed] [Google Scholar]
- 31. Clark DB, Kirisci L, Mezzich A, Chung T. Parental supervision and alcohol use in adolescence: developmentally specific interactions. J Dev Behav Pediatr. 2008; 29(4): 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dickson DJ, Laursen B, Stattin H, Kerr M. Parental supervision and alcohol abuse among adolescent girls. Pediatrics. 2015; 136(4): 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alati R, Lawlor DA, Najman JM, Williams GM, Bor W, O’Callaghan M. Is there really a ‘J-shaped’ curve in the association between alcohol consumption and symptoms of depression and anxiety? Findings from the Mater-University Study of Pregnancy and its outcomes. Addiction. 2005; 100(5): 643–651. [DOI] [PubMed] [Google Scholar]
- 34. Caldwell TM, Rodgers B, Jorm AF et al. Patterns of association between alcohol consumption and symptoms of depression and anxiety in young adults. Addiction. 2002; 97(5): 583–594. [DOI] [PubMed] [Google Scholar]
- 35. Rodgers B, Korten AE, Jorm AF, Jacomb PA, Christensen H, Henderson AS. Non-linear relationships in associations of depression and anxiety with alcohol use. Psychol Med. 2000; 30(2): 421–432. [DOI] [PubMed] [Google Scholar]
- 36. Skogen JC, Harvey SB, Henderson M, Stordal E, Mykletun A. Anxiety and depression among abstainers and low-level alcohol consumers. The Nord-Trøndelag Health Study. Addiction. 2009; 104(9): 1519–1529. [DOI] [PubMed] [Google Scholar]
- 37. Harvey A, Lundervold A, Hysing M, Sivertsen B. Sleep problems and depression in adolescence: results from a large population-based study of Norwegian adolescents aged 16–18 years. Eur Child Adolesc Psychiatry. 2014; 23(8): 681–689. [DOI] [PubMed] [Google Scholar]
- 38. Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BW. Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety. 2016; 33(1): 75–83. [DOI] [PubMed] [Google Scholar]
- 39. Haraden DA, Mullin BC, Hankin BL. The relationship between depression and chronotype: A longitudinal assessment during childhood and adolescence. Depress Anxiety. 2017; 34(10): 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Short MA, Gradisar M, Lack LC, Wright HR. The impact of sleep on adolescent depressed mood, alertness and academic performance. J Adolesc. 2013; 36(6): 1025–1033. [DOI] [PubMed] [Google Scholar]
- 41. Currie CE, Elton RA, Todd J, Platt S. Indicators of socioeconomic status for adolescents: the WHO health behaviour in school-aged children Survey. Health Educ Res. 1997; 12(3): 385–397. [DOI] [PubMed] [Google Scholar]
- 42. Rispens JH, Meeus WJ.. Opvoeden in Nederland [Parenting in the Netherlands]. Assen: Van Gorcum; 1996. [Google Scholar]
- 43. Dunn EC, Johnson RM, Green JG. The Modified Depression Scale (MDS): a brief, no-cost assessment tool to estimate the level of depressive symptoms in students and schools. School Ment Health. 2012; 4(1): 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal. 1999; 6(1): 1–55. [Google Scholar]
- 45. Yu C. Evaluating Cutoff Criteria of Model Fit Indices for Latent Variable Models With Binary and Continuous Outcome. Los Angeles, CA: University of California; 2002. https://www.statmodel.com/download/Yudissertation.pdf, Accessed May 5, 2017 [Google Scholar]
- 46. Muthen LK, Muthen BO.. MPlus User’s Guide. 7th ed. Los Angeles, CA: Muthen & Muthen; 1998. –2015. [Google Scholar]
- 47. Cribbie RA. Evaluating the importance of individual parameters in structural equation modeling: the need for type I error control. Personal Individ Differ. 2000; 29(3): 567–577. [Google Scholar]
- 48. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statistical Soc, Series B. 1995; 57(1): 289–300. [Google Scholar]
- 49. Chandrakar P. Social jetlag in school students: evidence to suggest that sleep deprivation during work days is common. Biolog Rhythm Res. 2017; 48(1): 99–112. [Google Scholar]
- 50. Fitzgerald CT, Messias E, Buysse DJ. Teen sleep and suicidality: results from the youth risk behavior surveys of 2007 and 2009. J Clin Sleep Med. 2011; 7(4): 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kann L, McManus T, Harris WA et al. Youth risk behavior surveillance – United States, 2015. MMWR Surveill Summ. 2016; 65(6): 1–174. [DOI] [PubMed] [Google Scholar]
- 52. Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. The J Pediatrics. 2005; 147(6): 830–834. [DOI] [PubMed] [Google Scholar]
- 53. Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998; 69(4): 875–887. [PubMed] [Google Scholar]
- 54. Rupp TL, Acebo C, Carskadon MA. Evening alcohol suppresses salivary melatonin in young adults. Chronobiol Int. 2007; 24(3): 463–470. [DOI] [PubMed] [Google Scholar]
- 55. Danel T, Touitou Y. Chronobiology of alcohol: from chronokinetics to alcohol-related alterations of the circadian system. Chronobiol Int. 2004; 21(6): 923–935. [DOI] [PubMed] [Google Scholar]
- 56. Hasler BP, Martin CS, Wood DS, Rosario B, Clark DB. A longitudinal study of insomnia and other sleep complaints in adolescents with and without alcohol use disorders. Alcohol Clin Exp Res. 2014; 38(8): 2225–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. U.S.Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Rockville, MD: U.S. Department of Health and Human Services, Office of the Surgeon General; 2007. [PubMed] [Google Scholar]
- 58. Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med. 2006; 160(7): 739–746. [DOI] [PubMed] [Google Scholar]
- 59. Ellickson PL, Tucker JS, Klein DJ. Ten-year prospective study of public health problems associated with early drinking. Pediatrics. 2003; 111(5 Pt 1): 949–955. [DOI] [PubMed] [Google Scholar]
- 60. Miller JW, Naimi TS, Brewer RD, Jones SE. Binge drinking and associated health risk behaviors among high school students. Pediatrics. 2007; 119(1): 76–85. [DOI] [PubMed] [Google Scholar]
- 61. Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: predictors and substance abuse outcomes. J Consult Clin Psychol. 2002; 70(1): 67–78. [PubMed] [Google Scholar]
- 62. Hingson RW, Heeren T, Edwards EM. Age at drinking onset, alcohol dependence, and their relation to drug use and dependence, driving under the influence of drugs, and motor-vehicle crash involvement because of drugs. J Stud Alcohol Drugs. 2008; 69(2): 192–201. [DOI] [PubMed] [Google Scholar]
- 63. Maume DJ. Social ties and adolescent sleep disruption. J Health Soc Behav. 2013; 54(4): 498–515. [DOI] [PubMed] [Google Scholar]
- 64. Mednick SC, Christakis NA, Fowler JH. The spread of sleep loss influences drug use in adolescent social networks. PLoS One. 2010; 5(3): e9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Díaz-Morales JF, Escribano C, Jankowski KS. Chronotype and time-of-day effects on mood during school day. Chronobiol Int. 2015; 32(1): 37–42. [DOI] [PubMed] [Google Scholar]
- 66. Beck KH, Summons TG, Thombs DL. A factor analytic study of social context of drinking in a high school population. Psychology of Addictive Behaviors. 1991; 5(2): 66–77. [Google Scholar]
- 67. Stewart C, Power TG. Identifying patterns of adolescent drinking: a tri-ethnic study. J Stud Alcohol. 2002; 63(2): 156–168. [DOI] [PubMed] [Google Scholar]
- 68. Ajilore O, Amialchuk A, Egan K. Alcohol consumption by youth: peers, parents, or prices?Econ Hum Biol. 2016; 23: 76–83. [DOI] [PubMed] [Google Scholar]
- 69. Jacobs W, Goodson P, Barry AE, McLeroy KR, McKyer EL, Valente TW. Adolescent social networks and alcohol use: variability by gender and type. Subst Use Misuse. 2017; 52(4): 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pedersen ER, Osilla KC, Miles JN et al. The role of perceived injunctive alcohol norms in adolescent drinking behavior. Addict Behav. 2017; 67: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang C, Hipp JR, Butts CT, Jose R, Lakon CM. Coevolution of adolescent friendship networks and smoking and drinking behaviors with consideration of parental influence. Psychol Addict Behav. 2016; 30(3): 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Harrison PA, Luxenberg MG. Comparisons of alcohol and other drug problems among Minnesota adolescents in 1989 and 1992. Arch Pediatr Adolesc Med. 1995; 149(2): 137–144. [DOI] [PubMed] [Google Scholar]
- 73. Hingson RW, White A. Trends in extreme binge drinking among US high school seniors. JAMA Pediatr. 2013; 167(11): 996–998. [DOI] [PubMed] [Google Scholar]
- 74. McCann M, Perra O, McLaughlin A, McCartan C, Higgins K. Assessing elements of a family approach to reduce adolescent drinking frequency: parent-adolescent relationship, knowledge management and keeping secrets. Addiction. 2016; 111(5): 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.