Abstract
Aims
The aim of this study was to investigate the effect of contact-to-balloon time on mortality in ST-segment elevation myocardial infarction (STEMI) patients with and without haemodynamic instability.
Methods and results
Using data from the prospective, multicentre Feedback Intervention and Treatment Times in ST-Elevation Myocardial Infarction (FITT-STEMI) trial, we assessed the prognostic relevance of first medical contact-to-balloon time in n = 12 675 STEMI patients who used emergency medical service transportation and were treated with primary percutaneous coronary intervention (PCI). Patients were stratified by cardiogenic shock (CS) and out-of-hospital cardiac arrest (OHCA). For patients treated within 60 to 180 min from the first medical contact, we found a nearly linear relationship between contact-to-balloon times and mortality in all four STEMI groups. In CS patients with no OHCA, every 10-min treatment delay resulted in 3.31 additional deaths in 100 PCI-treated patients. This treatment delay-related increase in mortality was significantly higher as compared to the two groups of OHCA patients with shock (2.09) and without shock (1.34), as well as to haemodynamically stable patients (0.34, P < 0.0001).
Conclusions
In patients with CS, the time elapsing from the first medical contact to primary PCI is a strong predictor of an adverse outcome. This patient group benefitted most from immediate PCI treatment, hence special efforts to shorten contact-to-balloon time should be applied in particular to these high-risk STEMI patients.
Clinical Trial Registration
Keywords: ST-segment elevation myocardial infarction (STEMI), Percutaneous coronary intervention (PCI) , Cardiogenic shock , Out-of-hospital cardiac arrest , Contact-to-balloon time , Mortality
Introduction
Ischaemic time duration is a major determinant of infarct size in patients with ST-segment elevation myocardial infarction (STEMI), and prompt recognition and early management of acute STEMI is critical in reducing morbidity and mortality.1,2 Given the importance of primary percutaneous coronary intervention (PCI) for mortality in STEMI patients, the majority of clinical studies focus mainly on the door-to-balloon time.3–12 In contrast to door-to-balloon time as a potential quality marker for improved treatment protocols, much less is known about the prognostic significance of first medical contact-to-balloon time and of key components of pre-hospital delay on early STEMI outcomes.13,14 Systematic studies investigating the impact of pre-hospital ‘process of care’ factors on treatment delay and clinical outcome are currently limited and, moreover, often biased due to sampling errors which are related to different modes of presentation to the hospital such as emergency medical service (EMS) transportation, inter-facility transport to a receiving PCI centre, and ambulatory patient’s self-transport. In addition, the high incidence of out-of-hospital cardiac arrest (OHCA) requiring instant cardiopulmonary resuscitation as well as cardiogenic shock (CS) in STEMI patients may impact on the transport time between first medical contact and arrival at a PCI centre for coronary angioplasty. Currently, no data are available regarding the impact of contact-to-balloon time on mortality in patients with CS and/or OHCA. Using data from the large and multicentre Feedback Intervention and Treatment Times in ST-Elevation Myocardial Infarction (FITT-STEMI) trial, we addressed the prognostic significance of treatment delay from first medical contact to coronary reperfusion on short-term mortality in these high-risk patients.
Methods
Study design
The FITT-STEMI trial is an ongoing multicentre study to prospectively determine the additional benefit of systematic and formalized data assessment and interactive feedback on time to interventional treatment for patients with STEMI. The primary aim of FITT-STEMI is to implement standardized feedback-driven quality management for timely reperfusion therapy in existing regional cardiac care networks for the treatment of STEMI patients. Details on the study design, data collection, and outcome measures including first clinical results have been described.15,16 Participation in the FITT-STEMI consortium required that all participating hospitals endorsed the key strategies of the American College of Cardiology initiative for the management of patients with STEMI,17 which are the establishment of multidisciplinary treatment teams to develop guideline-based written protocols for triaging and managing patients presenting with symptoms suggestive of STEMI, activation of the catheterization laboratory through a single-call system by the emergency department physician, and prompt availability of a skilled cardiac intervention team within 30 min. Feedback quality control of the participating PCI centres was performed quarterly during the first month of each quarter beginning in the second quarter after joining the FITT-STEMI consortium. Outcome data for each local study site were discussed on a regular basis in interactive sessions with members of the participating interdisciplinary treatment teams, including local EMS, physicians, and nurses working in the emergency department and the emergency responding system, staff members in the catheterization laboratory and interventional cardiologists. The formalized feedback presentations were prepared centrally by the study initiator (K.H.S.) for use in regular site monitor visits and included slide presentations on site-specific descriptive statistics regarding clinical characteristics, times of transportation as well as procedural delays during the time from the first medical contact to direct handoff in the cardiac catheterization laboratory. In addition, the following pre-defined key quality indicators were routinely assessed for each participating study site: percentage of pre-hospital electrocardiogram (ECG) recordings within and longer than 10 min after the first medical contact (i.e. arrival-time of EMS), percentage of patients with telephone announcement in advance, proportion of patients with telemetry-ECG transmission, as well as average and median components of times to treatment, including those from the first medical contact to balloon inflation.
Participating hospitals
Inclusion criteria for participating hospitals were 24-h PCI capability for at least 1 year before study enrolment, at least two affiliated interventional cardiologists who could take calls, and a minimum of 250 PCI procedures per year with 50 annual procedures in STEMI patients. Study hospitals joined the FITT-STEMI consortium at different points in time. The consortium started off in 2006 consisting of one infarct treatment network with a central PCI hospital and two referring non-PCI-capable hospitals.15 The study was extended to a group of six further STEMI-treatment networks at the end of 2007 and the beginning of 2008.16 Since 2009 a number of new PCI hospitals have entered into the FITT-STEMI program every year. At the end of 2015, a total of 48 hospitals scattered throughout Germany participated in the consortium including five university hospitals. The PCI hospitals are obligated to prospectively enrol without exception all consecutive STEMI patients who presented within less than 24 h after onset of symptoms. To ensure that all ECG-diagnosed STEMI patients were included in the FITT-STEMI trial, written consent was obtained from the responsible head of the cardiology department and/or the interventional cardiologist in charge at each affiliated PCI hospital before patient enrolment was started.
Completeness of patient enrolment was checked by comparing data from two independent infarct surveys obtained from all participating PCI hospitals: (i) insurance reimbursement data regarding the ICD-10 (International Statistical Classification of Diseases and Related Health Problems) codes I21.0 to I21.3 (which included transmural infarction up to 28 days) and (ii) data from the German Hospital Quality Report on PCI procedures for the indication ‘ST-segment-elevation myocardial infarction (MI) within 24 h after ECG diagnosis’ (also including subacute infarctions),18 which was mandatory for all certified PCI-capable catheterization laboratories up to the year 2016. These data checks showed that the percentages of annually included FITT-STEMI study participants among ICD-coded patients with ‘transmural infarction’ (69.7 ± 6.8 percentage points) and patients registered by the German Hospital Quality Report (95.6 ± 11.5 percentage points) were stable. Although the inclusion criteria differed between the three data bases, the large overlap nevertheless underscored the integrity of the enrolment strategy.
Data collection
Collected data included time of arrival on the scene and the durations of out-of-hospital treatment, transport to the PCI centre, and transfer to the catheterization laboratory as well as time of puncture and first balloon inflation. For each patient, data were collected on a case-report form which included clinical information on symptom onset, medical history, prior medication, comorbid diagnoses, blood pressure, heart rate, Killip classification, and the Thrombolysis in Myocardial Infarction (TIMI) risk score for STEMI, a commonly used and well-validated prognostication scheme categorizing the patient’s risk of death.19 In addition, results from coronary angiography and procedural critical time intervals were assessed. Cardiogenic shock (Killip class IV) was confirmed by trained cardiologists at hospital admission based on clinical criteria including hypotension (systolic blood pressure of <90 mmHg or the need for supportive measures to maintain a systolic blood pressure of ≥90 mmHg), signs of end-organ hypoperfusion, and a heart rate of ≥60 beats/min.20 Patients with bystander cardiopulmonary resuscitation were not excluded and were classified into the OHCA groups. Similarly, patients with brain damage were not excluded from the analysis, and pre-PCI fibrinolysis was not regarded as an exclusion criterion. Transfer of data from each local study site to the principal coordinating centre including data on in-hospital mortality was achieved using a web-based electronic data transfer system allowing for independent monitoring for data validation (‘source data verification’). The study was approved by the ethics committee of the Medical Faculty at the University of Göttingen and the local ethics committees of all participating PCI centres.
Study participants
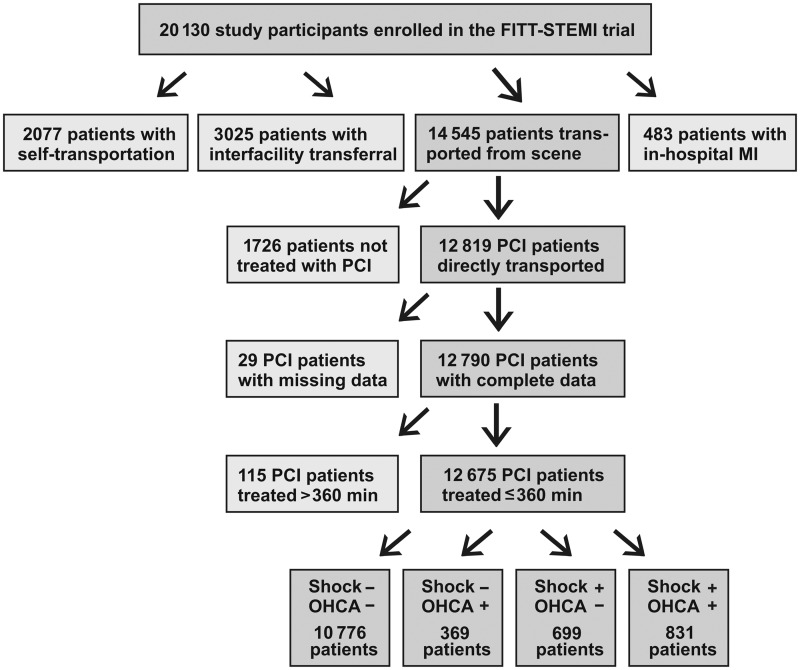
All patients presenting within 24 h of symptom onset of STEMI (n = 20 130) during 1 January 2006 to 30 November 2015, were included in the FITT-STEMI trial. Study participants who had interfacility transferral from a non-PCI-capable hospital to a receiving hospital with on-site PCI (n = 3025), arrived at the interventional hospital by self-transport (n = 2077), and suffered the infarction during in-hospital treatment at the PCI hospital (n = 483) were excluded from the analysis to prevent selection bias with respect to both treatment times and mortality in these heterogeneous patient groups. The final study population comprised all those STEMI patients with complete data who arrived at the primary PCI hospital via EMS transportation and had treatment times of less than 360 min from arrival at the scene to balloon inflation (n = 12 675). A flow diagram of the patient cohort is presented in Figure 1.
Figure 1.
Flow diagram of patient cohort including the four groups of percutaneous coronary intervention-treated ST-segment elevation myocardial infarction patients as stratified by out-of-hospital cardiac arrest (OHCA) and cardiogenic shock (Shock).
Statistical analysis
Raw time data were used to calculate the interval between first medical contact and balloon inflation for each study patient. For some of the analyses, the study cohort was classified along the guideline-recommended cut-off level for contact-to-balloon time equal or less than 90 min. Categorical outcomes were compared between the two groups using the χ2 tests. Continuous data were reported as means and standard deviations and were compared using the Student’s t-tests. In order to identify procedural and clinical parameters that impacted on treatment time, we computed a linear regression model using backward selection with contact-to-balloon time as the dependent variable and a set of potential confounders known to be associated with outcome. In addition, two logistic regression models were calculated to identify independent predictors of in-hospital mortality using either dichotomized or continuous data for contact-to-balloon time as the independent variable. These models were adjusted for the following potential confounders: age, sex, comorbid diabetes mellitus, hyperlipoproteinaemia, family history of MI, smoker status, chronic vessel occlusion in a non-infarct-related coronary artery (NIRA), localization of the culprit coronary artery, TIMI flow after PCI (score ≤2 vs. 3), as well as OHCA and CS, including their interaction term. In additional models, TIMI risk score was entered as an additional independent variable. The influence of hospital caseload on outcome was assessed using two independent approaches. Estimation of hospital case volume was based on the mean number of STEMI patients treated per quarter within each hospital over the entire study period. Alternatively, the total number of quarters in the study in which each PCI centre participated was used as an independent variable. Model diagnostics based on generalized additive models suggested the inclusion of a quadratic term for contact-to-balloon time when used as continuous (non-dichotomized) predictor. The results from these regression analyses are presented as odds ratios (OR) and 95% confidence intervals (95% CI). For the purpose of illustration, a linear trend was fitted to predicted in-hospital mortality risks depending on contact-to-balloon time, for contact-to-balloon times ranging from 60 to 180 min. Within this time interval, the predicted risks depended roughly linearly on the contact-to-balloon time. The reported P-values are all two-sided, and P-values <0.05 were considered statistically significant. No formal adjustment for multiple testing was carried out. All statistical analyses were performed on a personal computer using the statistical software SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Characterization of the study population
Of the 12 675 STEMI patients who received EMS transportation and were treated with PCI, 144 patients had pre-PCI fibrinolysis (1.1%). As temporary mechanical circulatory assist devices, intra-aortic balloon counter-pulsation (IABP) was used in 339 patients (2.7%), while 114 (0.9%) patients were treated with extracorporeal membrane oxygenation (ECMO) and/or ventricular assist devices (VAD) (Table 1). The majority (10 776; 85%) had no pre-hospital resuscitation and reached the PCI hospital in stable condition. Among the resuscitated STEMI patients (n = 1200, 9.5%), 369 patients had stable conditions and 831 patients presented with CS at the PCI hospital (Table 1). Another group of 699 STEMI patients (5.5%) had no OHCA but presented with CS. These four groups differed with respect to age, gender, and risk-factor distribution, as a higher proportion of patients with OHCA were male (78%) and, on average, younger (60.4 ± 12.3 and 61.3 ± 12.7 years) and had less hypertension, diabetes mellitus, hyperlipidaemia, and family history of MI (for all, P < 0.0001). ST-segment elevation myocardial infarction patients with CS and no OHCA were older (67.2 ± 13.2 years), had more diabetes mellitus and renal failure, higher TIMI risk scores, and a higher proportion of multi-vessel and/or left-main disease as compared to the three other groups (for all, P < 0.0001). In-hospital mortality was lowest in haemodynamically stable patients (2.7%) and highest in CS patients with (45%) and without (39%) OHCA. As compared to the two CS groups, mortality was lower in patients presenting with OHCA and no CS (16%). The demographic, clinical, and cardiological data on the study cohort as stratified by CS and OHCA are represented in Table 1. Supplementary material online, Figure S1 demonstrates boxplots of contact-to-balloon times stratified by the four groups.
Table 1.
Demographic, clinical and angiographic characteristics of ST-segment elevation myocardial infarction patients with (+) and without (−) cardiogenic shock and out-of-hospital cardiac arrest (OHCA), respectively
| Variable | Total study population (n = 12 675) | Group 1 | Group 2 | Group 3 | Group 4 | P-value | |
|---|---|---|---|---|---|---|---|
| Shock– | Shock– | Shock+ | Shock+ | ||||
| OHCA– | OHCA+ | OHCA– | OHCA+ | ||||
| (n = 10 776) | (n = 369) | (n = 699) | (n = 831) | ||||
| Demographic data | |||||||
| Male gender | 9338 (74%) | 7909 (73%) | 287 (78%) | 490 (70%) | 652 (78%) | 0.0004 | |
| Age ± SD (years) | 63.6 ± 12.9 | 63.6 ± 12.9 | 60.4 ± 12.3 | 67.2 ± 13.2 | 61.3 ± 12.7 | <0.0001 | |
| Age > 80 years | 1311 (10%) | 1115 (10%) | 23 (6%) | 121 (17%) | 52 (6%) | <0.0001 | |
| Body mass index (kg/m2) | 27.5 ± 4.6 | 27.5 ± 4.6 | 26.9 ± 4.2 | 27.4 ± 4.4 | 27.3 ± 4.5 | 0.0451 | |
| Clinical data | |||||||
| Hypertension | 7475 (59%) | 6484 (60%) | 187 (51%) | 411 (59%) | 393 (47%) | <0.0001 | |
| Diabetes mellitus | 2179 (17%) | 1853 (17%) | 46 (12%) | 160 (23%) | 120 (14%) | <0.0001 | |
| Prior angina pectoris | 1656 (13%) | 1453 (13%) | 26 (7%) | 82 (12%) | 95 (11%) | 0.0009 | |
| Hyperlipidaemia | 3696 (29%) | 3300 (31%) | 73 (20%) | 158 (23%) | 165 (20%) | <0.0001 | |
| Family history | 2426 (19%) | 2214 (21%) | 48 (13%) | 85 (12%) | 79 (10%) | <0.0001 | |
| Current smoker | 5347 (42%) | 4657 (43%) | 161 (44%) | 222 (32%) | 307 (37%) | <0.0001 | |
| Previous MI | 1378 (11%) | 1168 (11%) | 32 (9%) | 92 (13%) | 86 (10%) | 0.1180 | |
| Previous stroke | 522 (4%) | 424 (4%) | 21 (6%) | 41 (6%) | 36 (4%) | 0.0331 | |
| Previous angioplasty | 1396 (11%) | 1196 (11%) | 32 (9%) | 87 (12%) | 81 (10%) | 0.1742 | |
| Previous CABG | 286 (2%) | 232 (2%) | 8 (2%) | 25 (4%) | 21 (3%) | 0.0964 | |
| Renal failure | 597 (5%) | 482 (4%) | 11 (3%) | 60 (9%) | 44 (5%) | <0.0001 | |
| TIMI risk score | <0.0001 | ||||||
| 0–2 | 4516 (36%) | 4347 (40%) | 106 (29%) | 21 (3%) | 42 (5%) | ||
| 3–4 | 3642 (29%) | 3279 (30%) | 102 (28%) | 92 (13%) | 169 (20%) | ||
| 5–8 | 3907 (31%) | 2902 (27%) | 140 (38%) | 395 (57%) | 470 (57%) | ||
| >8 | 610 (5%) | 248 (2%) | 21 (6%) | 191 (27%) | 150 (18%) | ||
| System-related data and supportive treatment | |||||||
| Off-hours (nights/weekends) | 7253 (57%) | 6095 (57%) | 247 (67%) | 402 (58%) | 509 (61%) | <0.0001 | |
| Pre-hospital ECG | 11 522 (91%) | 9912 (92%) | 321 (87%) | 612 (88%) | 677 (81%) | <0.0001 | |
| Telemetry ECG | 2923 (23%) | 2587 (24%) | 65 (18%) | 157 (22%) | 114 (14%) | <0.0001 | |
| Pre-announce-ment by telephone | 10 414 (82%) | 8910 (83%) | 309 (84%) | 562 (80%) | 633 (76%) | <0.0001 | |
| Angiographic results | |||||||
| No. coronary arteries narrowed | <0.0001 | ||||||
| 0 | 32 (0.3%) | 25 (0.2%) | 2 (0.5%) | 0 (0%) | 5 (0.6%) | ||
| 1 | 5059 (40%) | 4382 (41%) | 162 (44%) | 198 (28%) | 317 (38%) | ||
| 2 | 3917 (31%) | 3371 (31%) | 116 (31%) | 205 (29%) | 225 (27%) | ||
| 3 | 3569 (28%) | 2945 (27%) | 86 (23%) | 264 (38%) | 274 (33%) | ||
| LMCA | 93 (0.7%) | 48 (0.4%) | 3 (0.8%) | 32 (4.6%) | 10 (1.2%) | ||
| CTO in NIRA | 1399 (11%) | 1074 (10%) | 42 (11%) | 140 (20%) | 143 (17%) | ||
| STEMI recanalization vessel | <0.0001 | ||||||
| LAD | 5569 (44%) | 4661 (43%) | 196 (53%) | 290 (41%) | 422 (51%) | ||
| RCA | 5248 (41%) | 4624 (43%) | 105 (28%) | 270 (39%) | 249 (30%) | ||
| LCX | 1609 (13%) | 1329 (12%) | 63 (17%) | 87 (12%) | 130 (16%) | ||
| LMCA | 119 (1%) | 54 (1%) | 4 (1%) | 39 (6%) | 22 (3%) | ||
| Graft | 126 (1%) | 104 (1%) | 1 (0.3%) | 13 (2%) | 8 (1%) | ||
| ECG (STEMI site) | <0.0001 | ||||||
| Anterior | 5602 (44%) | 4642 (43%) | 204 (55%) | 323 (46%) | 433 (52%) | ||
| Inferior | 6306 (50%) | 5531 (51%) | 133 (36%) | 329 (47%) | 313 (38%) | ||
| Lateral | 641 (5%) | 531 (5%) | 27 (7%) | 30 (4%) | 53 (6%) | ||
| LBBB | 122 (1%) | 68 (1%) | 5 (1%) | 17 (2%) | 32 (4%) | ||
| TIMI angiographic flow grade before PCI | <0.0001 | ||||||
| Score 0–2 | 11 693 (92%) | 9889 (92%) | 343 (93%) | 673 (96%) | 788 (95%) | ||
| Score 3 | 961 (8%) | 869 (8%) | 26 (7%) | 23 (3%) | 43 (5%) | ||
| TIMI angiographic flow grade after PCI | <0.0001 | ||||||
| Score 0–2 | 867 (7%) | 604 (6%) | 20 (5%) | 127 (18%) | 116 (14%) | ||
| Score 3 | 11 793 (93%) | 10 160 (94%) | 349 (95%) | 569 (81%) | 715 (86%) | ||
| Treatment and outcome | |||||||
| Fibrinolysis | 144 (1.1%) | 22 (0.2%) | 13 (3.5%) | 17 (2.4%) | 92 (11.1%) | <0.0001 | |
| IABP | 339 (2.7%) | 43 (0.4%) | 18 (4.9%) | 129 (18.5%) | 149 (17.9%) | <0.0001 | |
| ECMO/VAD | 114 (0.9%) | 25 (0.2%) | 6 (1.6%) | 48 (6.9%) | 35 (4.2%) | <0.0001 | |
| In-hospital mortality | 994 (8%) | 289 (3%) | 60 (16%) | 273 (39%) | 372 (45%) | <0.0001 | |
Data are presented as means and standard deviations or percentages. P-values refer to the comparisons between the four groups.
CTO, chronic total occlusion; LAD, left anterior descending artery; LBBB, left bundle branch block; LCX, left circumflex artery; LMCA, left main coronary artery; RCA, right coronary artery; SD, standard deviation.
Haemodynamic instability results in treatment delay
Next, we performed a driver analysis by computing an estimate of the importance of various independent clinical and procedural variables in predicting the time interval from the first medical contact to reperfusion therapy. As expected, some procedural parameters were associated with significantly shorter contact-to-balloon time. Savings in contact-to-balloon time were on average 5.4 min for pre-hospital ECG, 17.5 min for pre-announcement of the patient’s transport by telephone, and 33.2 min for bypassing the emergency department and direct transport to the catheterization laboratory (for all P ≤ 0.0001; Table 2). Recording an ECG within the guideline-recommended first 10 min after EMS arrival resulted in an additional gain of 4.2 min in the time to reperfusion. In contrast, time to final reperfusion therapy was prolonged in the case of hypertension (3.2 min), diabetes mellitus (4.3 min), renal failure (6.8 min), and previous coronary artery bypass grafting (CABG) (11.4 min). Notably, OHCA (17.3 min) and CS upon hospital arrival (6.9 min) both significantly contributed to system delay.
Table 2.
Drivers of contact-to-balloon time as determined by regression analysis
| Variable | Estimate (delay in minutes) | Standard error | T-value | P-value |
|---|---|---|---|---|
| Patient-related parameters | ||||
| Age (per year) | 0.247 | 0.029 | 8.65 | <0.0001 |
| Arterial hypertension | 3.244 | 0.743 | 4.37 | <0.0001 |
| Diabetes mellitus | 4.343 | 0.943 | 4.61 | <0.0001 |
| CABG | 11.449 | 2.334 | 4.90 | <0.0001 |
| Renal failure | 6.844 | 1.710 | 4.00 | <0.0001 |
| OHCA | 17.268 | 1.459 | 11.84 | <0.0001 |
| Cardiogenic shock | 6.941 | 1.317 | 5.27 | <0.0001 |
| Performance-related parameters | ||||
| Off-hour care | 7.746 | 0.716 | 10.82 | <0.0001 |
| Pre-hospital ECG | −5.364 | 1.414 | 3.79 | 0.0001 |
| Pre-hospital ECG within 10 min | −4.156 | 0.759 | 5.47 | <0.0001 |
| Pre-announcement by telephone | −17.533 | 1.010 | 17.36 | <0.0001 |
| Direct transmission to catheterization laboratory | −33.250 | 0.781 | 42.55 | <0.0001 |
| TIMI angiographic flow grade after PCI (Score ≤ 2 vs. 3) | 8.565 | 1.403 | 6.11 | <0.0001 |
Data are demonstrated as estimates with their corresponding standard errors and T-values.
Contact-to-balloon time linked to outcome
Among the total study cohort, there were n = 994 in-hospital deaths (7.8%). In-hospital mortality was lower in patients with a contact-to-balloon time of ≤90 min as compared to their counterparts with a time to PCI treatment of >90 min (3.9% vs. 12.2%, P < 0.0001, Supplementary material online, Figure S2A). A particular high risk of death was observed in the group of patients with contact-to-balloon times ranging from 150 to 180 min, as one-fifth of all patients out of this group died after PCI (20.0%, Supplementary material online, Figure S2A). Patients with CS had a much higher mortality rate (645 deaths out of 1530; 42%) as compared to STEMI patients with no clinical signs of haemodynamic instability (289 deaths of 10776 patients, 2.7%, P < 0.0001, Take home figure and Supplementary material online, Figure S2B). In resuscitated patients with OHCA, there was also a high mortality rate (432 deaths out of 1200; 36%). We found pronounced beneficial effects on survival, when patients with either CS or OHCA were treated within 90 min after first medical contact (OR 0.49, 95% CI 0.36–0.66, P < 0.0001 and OR 0.56, 95% CI 0.38–0.82, P = 0.0031, respectively). By reducing the contact-to-balloon time to less than 90 min, one additional life could be saved out of five patients treated with CS. However, this number needed to treat was much higher in stable patients (one life additionally saved out of 53 patients).
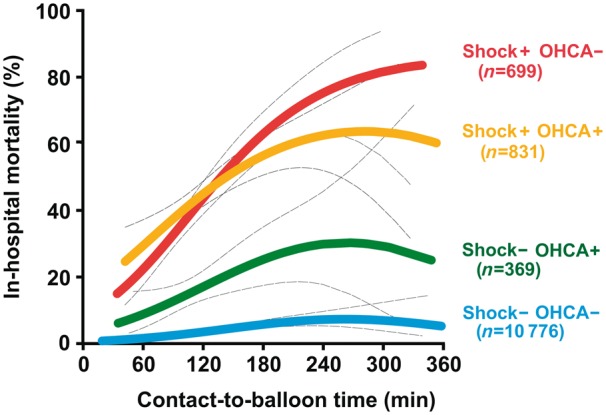
Take home figure.

In-hospital mortality of percutaneous coronary intervention-treated ST-segment elevation myocardial infarction patients by contact-to-balloon time. The figure displays the calculated probabilities of adverse outcome (coloured thick lines) from a logistic regression model with their corresponding 95% confidence intervals (dashes thin black lines) as stratified by the four groups of patients with and without cardiogenic shock (Shock) and out-of-hospital cardiac arrest (OHCA), respectively, including their interaction term.
Effects of 10-min treatment delay
For contact-to-balloon times ranging from 60 to 180 min, we found a nearly linear relationship between treatment time and mortality in all four patients groups (Take home figure). Every 10-min treatment delay resulted in 3.31 additional deaths in 100 PCI-treated CS patients with no OHCA. This treatment delay-related increase in mortality was significantly higher as compared to the two groups of OHCA patients with (2.09) and without CS (1.34), as well as to haemodynamically stable patients (0.34, P < 0.0001), respectively (Figure 2).
Figure 2.
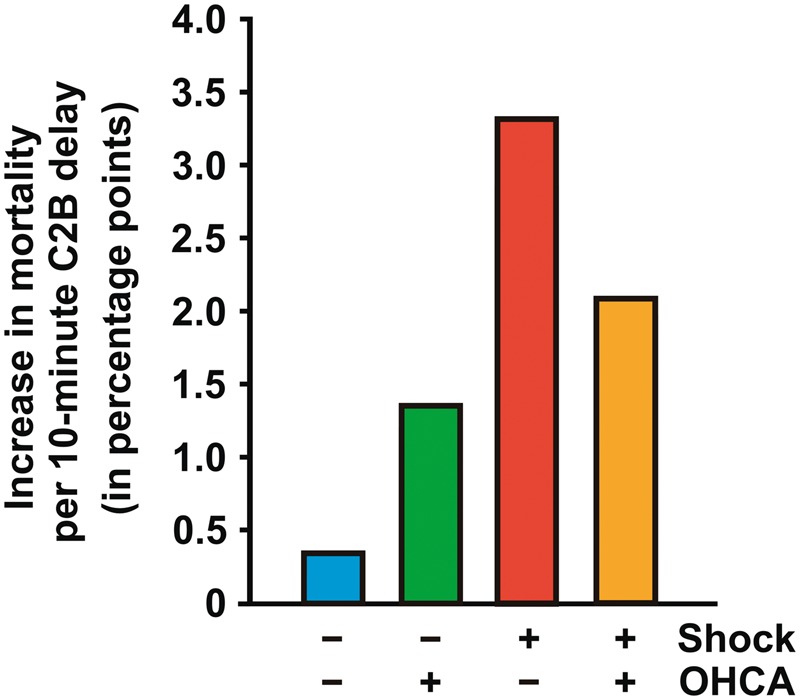
Increase in mortality risk (in percentage points) in percutaneous coronary intervention-treated patients with a contact-to-balloon time (C2B) between 60 and 180 min resulting from a 10-min delay as shown for the four groups of study participants with and without out-of-hospital cardiac arrest (OHCA) and cardiogenic shock, respectively. The standard error for each bar is less than 0.02.
Impact of treatment delay on mortality in adjusted models
Data from a stepwise multiple logistic regression model using in-hospital mortality as dependent variable and guideline-recommended contact-to-balloon time as independent variable adjusted for a variety of clinical and procedural confounders confirmed the result from the univariate analysis (Table 3). In this model, contact-to-balloon time of ≤90 min was a significant and independent predictor for better survival with an OR estimate of 0.57 (95% CI 0.47–0.70, P < 0.0001), when adjusted for OHCA and CS. Using a model with continuous instead of dichotomized data for contact-to-balloon time as a sensitivity analysis, this result remained stable, thus confirming the significant and independent predictive role of treatment delay on mortality (P < 0.0001; Supplementary material online, Table S1).
Table 3.
Results from a logistic regression model with in-hospital mortality as dependent variable and the guideline-recommended cut-off level of contact-to-balloon time (≤90 min) as independent variable adjusted to the indicated confounders, including the interaction term of out-of-hospital cardiac arrest and cardiogenic shock
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Contact-to-balloon time | 0.574 | 0.469–0.702 | <0.0001 |
| (≤90 min vs. >90 min) | |||
| Age (year) | 1.061 | 1.051–1.071 | <0.0001 |
| Female gender | 1.256 | 1.023–1.542 | 0.0292 |
| Diabetes mellitus | 1.530 | 1.225–1.910 | 0.0002 |
| Hyperlipoproteinaemia | 0.758 | 0.608–0.944 | 0.0132 |
| Family history | 0.574 | 0.405–0.812 | 0.0017 |
| Smoker | 0.785 | 0.623–0.988 | 0.0393 |
| Chronic total occlusion in NIRA | |||
| RCA | 1.877 | 1.314–2.621 | 0.0003 |
| LCX | 1.905 | 1.338–2.803 | 0.0007 |
| LAD | 1.677 | 1.152–2.376 | 0.0050 |
| Recanalization | |||
| Graft vs. LAD | 1.131 | 0.558–2.292 | 0.7323 |
| LMCA vs. LAD | 3.269 | 1.875–5.699 | <0.0001 |
| RCA vs. LAD | 0.767 | 0.620–0.948 | 0.0144 |
| LCX vs. LAD | 0.944 | 0.712–1.251 | 0.6879 |
| TIMI angiographic flow grade after PCI (score ≤ 2 vs. 3) | 3.632 | 2.822–4.675 | <0.0001 |
| OHCA | 9.233 | 6.331–13.466 | <0.0001 |
| Cardiogenic shock | 17.796 | 13.893–22.794 | <0.0001 |
| OHCA*cardiogenic shock | |||
| With OHCA and shock | 3.920 | 2.665–5.766 | <0.0001 |
| Without OHCA and shock | 2.034 | 1.537–2.691 | |
Data are presented as odds ratios and their 95% confidence intervals.
LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; NIRA, non-infarct-related artery; RCA, right coronary artery.
In addition, the TIMI risk score was included as an independent variable in a separate logistic regression model. Although higher TIMI risk scores were significantly associated with poorer outcome (P < 0.0010), the results demonstrated that contact-to-balloon time of >90 min remained a significant predictor of in-hospital mortality (P < 0.0001). However, neither hospital caseload (P = 0.2979) nor the duration of study participation per PCI centre (P = 0.6741) had any significant impact on mortality (Supplementary material online, Table S2).
Discussion
In this article, we present the outcome data from the prospective, multicentre FITT-STEMI trial which was aimed at assessing the impact of timeliness of invasive reperfusion therapy on in-hospital mortality in patients with STEMI. Our results demonstrate that, in STEMI patients, reduced contact-to-balloon time is associated with better survival, regardless of the adverse haemodynamic consequences of acute ischaemia-induced systolic dysfunction and cardiac arrest. We found that longer intervals from the first medical contact to revascularization enhance the risk of death, even when adjusted for OHCA and CS, which both significantly delayed the time to interventional treatment and were both linked to higher mortality. Most importantly, we observed that, in CS- and in OHCA patients, shorter times to reperfusion considerably improved the outcome, with surprisingly low mortality in OHCA patients in our study.
Our analysis of >12 500 STEMI patients demonstrates a steep incremental increase in mortality resulting from longer times to PCI treatment. One death among 12 STEMI patients can be prevented in the total study cohort when the time from the first medical contact to reperfusion is reduced to the guideline-recommended treatment time to less than 90 min.21 As an important finding of our analysis, STEMI patients with CS have substantially benefitted from early reperfusion, regardless of whether or not OHCA had occurred. However, the benefit of rapid revascularization was more pronounced in the group of STEMI patients with CS who had not experienced prior OHCA. For contact-to-balloon times ranging from 60 to 180 min, every 10-min treatment delay led to 3.3 additional deaths in 100 PCI-treated patients in this group. This treatment delay-related increase in mortality rate within the early hours of infarction was 10-fold higher in shock patients as compared to haemodynamically stable patients. Thus, our data clearly show the need for immediate PCI treatment in STEMI patients with haemodynamic instability for whom data on timely reperfusion therapy are currently not available.
The SHOCK investigators have published important studies assessing the effects of revascularization within 36 h of onset of MI in patients with CS due to left ventricular failure complicating MI.20,22 They found that there was no benefit from early revascularization, as the overall mortality at 30 days was not significantly reduced using either PCI or CABG (152 patients) as compared to initial medical stabilization (150 patients).20 However, there was statistically significant mortality improvement at 6-month20 and 1-year follow-up.22 The authors showed that restoration of coronary blood flow was a major predictor of survival in patients with predominant left ventricular dysfunction.23,24 Trends in the use of early mechanical revascularization for patients with acute MI complicated by CS obtained for the National Registry of Myocardial Infarction (NRMI) support this observation.25 However, so far no data were available regarding the impact of contact-to-balloon-time on mortality in patients with CS complicating MI.
Another important finding of our study is that, in comparison to OHCA patients presenting with CS, the mortality of OHCA patients without CS was surprisingly low (45% vs. 16%). The observed low mortality rate indicated that our study protocol allowed us to indeed clearly distinguish between these two OHCA groups. We assume that the ischaemia-induced loss of contractile myocardium is a pre-requisite for the development of CS and appears to be the main predictor of cardiac outcome, whereas potentially reversible arrhythmic episodes can be successfully treated with instant defibrillation, which may contribute to the comparably low mortality in the subgroup of OHCA patients without shock. This assumption is supported by our group comparison (shown in Table 1), indicating significant differences in angiographic findings, including chronic total occlusion of a NIRA and TIMI angiographic flow rate before and after PCI. The predictive role of these angiographic parameters was confirmed in our study cohort (Table 3).
Furthermore, we confirm that pre-hospital diagnosis of STEMI and early notification of on-call cardiac catheterization teams by a physician trained in emergency medicine, who in Germany is a regular member in the EMS transportation team, minimizes prognosis-relevant delays in the time to PCI treatment, which results in a time saving of more than 15 min. Moreover, we observed significant time saving benefits of nearly 10 min by recording an ECG within the recommended first 10 min after arrival of the EMS transportation team at the scene as compared to patients without a pre-clinical ECG, which contributed to the improved outcome. This result is in good agreement with data from the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry-Get With The Guidelines (GWTG) study showing that pre-hospital ECG use is associated with a 10-min reduction in contact-to-balloon time.26 In order to improve prognosis, pre-hospital ECG transmission and STEMI identification linked to prompt activation of the cardiac catheterization laboratory have been reported in some studies as a feasible strategy to shorten the time from arrival at the hospital to reperfusion.27–29 The benefit of early ECG recording on short-term survival most probably accounts for faster decision processes during patient management. This should be most important for STEMI patients with CS and/or OHCA.
Limitations
Several issues merit consideration in the interpretation of the present study. First and foremost, the data are observational and registry based and, therefore, susceptible to unmeasured confounding and selection bias such as resuscitation-specific variables (e.g. quality of chest compressions), the use of additional treatments (e.g. therapeutic hypothermia or pre-PCI fibrinolysis), or non-system reasons for delay. Second, owing to the cross-sectional nature of the FITT-STEMI trial, only in-hospital mortality was completely collected in this database, while future investigations are needed to examine the association with longitudinal outcomes at longer follow-up periods. Third, as shown by our multivariate driver analysis, prolonged treatment times resulted from serious clinical conditions. Thus, late arrival of very sick STEMI patients could be the consequence of their serious clinical conditions rather than the cause of increased hospital mortality, which might be of relevance even within the groups of CS and OHCA. Nevertheless, our logistic regression models indicated that contact-to-balloon time was a strong and significant prognostic factor independent of OHCA, CS, and other patient-related factors. The validity of this important finding was strengthened by including TIMI risk score as an additional independent variable. Fourth, no conclusion about causality between the duration of contact-to-balloon time and outcome can be reached from our observational study which influences the generalizability of our results. Fifth, the findings from our study conducted in Germany may not be directly applicable to STEMI management care systems in other countries, in which exclusively paramedics are part of the EMS transportation teams and not physicians experienced in emergency medicine. The attendance of both physicians and paramedics might have accounted for the beneficial effects of shortened treatment times on survival seen in our OHCA patients. Finally, it is unknown how the exclusion of formalized data analysis and interactive feedback intervention would affect the results from similar investigations, given that this approach was an integral key element of the FITT-STEMI study design.
In summary, our data demonstrate that, in STEMI patients, the time from first medical contact to primary PCI is strongly and inversely linked to short-term survival. In addition, we show that CS patients have maximum benefit from rapid reperfusion treatment with short contact-to-balloon times. In this high-risk group, the treatment delay-related increase in mortality was 10-fold higher as compared to haemodynamically stable patients. Thus, our data suggest that efforts to shorten the time to PCI therapy should be applied to all STEMI patients, and that particularly patients with haemodynamic instability may benefit most from future improvements in STEMI treatment protocols.
Supplementary material
Supplementary material is available at European Heart Journal online.
List of contributors
(In order of number of patients included up to November 30, 2015).
Universitätsklinikum Göttingen (Claudius Jacobshagen; Kristina Schröder; Swetlana Hartmann; Lars S. Maier), Universitätsklinikum Würzburg (Björn Lengenfelder, Verena Reinhart, Sebastian K. G. Meier), St. Bernward-Krankenhaus Hildesheim (Karl H. Scholz; Dorothee Ahlersmann), Helios Klinikum Krefeld (Rainer Ott; Heinrich G. Klues; Alexander Bufe), Klinikum Oldenburg (Albrecht Elsässer; Susanne Grafmüller; Annette Schütz), Klinikum Wolfsburg (Claus Fleischmann; Rolf Engberding; Rüdiger Becker), Klinikum Darmstadt (Gerald S. Werner; Hiller Moehlis), Asklepios Klinik Langen (Hans G. Olbrich, Kerstin Eck), Städtisches Klinikum München Neuperlach (Harald Mudra; Martin Hug; Anamaria Stote), Klinikum Ingolstadt (Harald Franck; Monika-Krista Zackl; Karlheinz Seidl), Klinik am Eichert Göppingen (Stephen Schröder; Marion Steindl; Josef Steindl; Sophia Atseles), Klinikum Worms (Jens Jung; Birgit Nicklas), Klinikum Augsburg (Christian Thilo, Georg Waidhauser; Wolfgang v. Scheidt), Universitätsklinikum Jena (Attila Yilmaz; Hans R. Figulla; Daniel Kretzschmar; Corinna Schneider; Christian Schulze); Klinikum Lüneburg (Christian Weiß; Claus H. Müller), Krankenhaus Buchholz (Werner Raut; Klaus Hertting), Krankenhaus Landshut-Achdorf (Bernhard Zrenner; Josef Haimerl; Ute Zrenner), KRH Klinikum Hannover-Siloah (Andreas Franke; Jan Fürste), Klinikum Lippe-Detmold (Dirk Härtel; Melanie Kriete; Ulrich Tebbe; Stephan Gielen), Klinikum Leer (Christian Vahlhaus; Ralf-G. Pretzsch), Klinikum Ludwigsburg (Ralph Berroth; Joachim Geiger; Friederike Wunsch; Christian Wolpert), Robert-Bosch-Krankenhaus Stuttgart (Stephan Hill; Andrea Bullinger; Udo Sechtem), Klinikum Deggendorf (Edmond Skenderaj; Ulrich Valta-Seufzer; Martin Giesler), Kreiskrankenhaus Eschwege (Marco Lubitz; Peter Schott), Regio Klinikum Pinneberg (Konrad Gorski; Thomas Hofmann), Klinikverbund Kempten-Oberallgäu (Carsten Bauer; Wulf Ito), Klinikum Viersen (Nicolas v. Beckerath), Medizinische Hochschule Hannover (Jörn Tongers; Benedikta Ritter; Karin Hohenleitner), Sana Kliniken Lübeck (Hans-Martin Grusnick; Joachim Weil), Klinikum St. Elisabeth Straubing (Sebastian K.G. Maier; Elke Grassl), Klinikum REGIOMED-Kliniken Coburg (Caroline Kleinecke; Andrea Linss; Kerstin Truthan; Hans-Joachim Goller; Johannes Brachmann), Asklepios Harzklinik Goslar (Gaby Lehnert; Stefan Lange; Tobias Steffen; Arnd B. Buchwald; Christoph Engelhardt), Kliniken Ostallgäu-Kaufbeuren, Füssen (Simon Delladio; Martin Hinterseer; Myriam Parvanov), Hermann-Josef-Krankenhaus Erkelenz (Klaus-Dieter Winter; Christina Ziesen), Kliniken Maria Hilf Mönchengladbach (Jürgen vom Dahl; Dierk Rulands), SLK Kliniken Heilbronn (Marcus Hennersdorf; Jens-Martin Maier; Eva Schropp), Krankenhaus Rothenburg ob der Tauber (Christian Wacker), Kreiskrankenhaus Dormagen (Benjamin Orth; Georg Haltern), Marienkrankenhaus Soest (Roland Bürger; Markus Flesch), SLK Kliniken Am Plattenwald Bad Friedrichshall (Thomas Dengler), Universitätsklinikum Regensburg (Christina Strack; Dierk Endemann; Lars S. Maier), Klinikum Landkreis Erding (Lorenz Bott-Flügel), Klinikum Neumarkt (Veronika Lingg), Krankenhaus Bethanien Moers (Alexander D. Donath; Stefan Möhlenkamp), Vinzenzkrankenhaus Hannover (Beate Bugdoll; Petra Wucherpfennig; Jan Bernd Schüttert; Christian Zellerhoff), Krankenhaus Henriettenstift Hannover (Thomas Weiss; Thorsten Grundmann), Evangelisches Krankenhaus Bethesda Mönchengladbach (Thomas Lickfeld), DRK-Krankenhaus Clementinenhaus Hannover (Heinz-Peter Remmlinger).
Funding
Deutsche Herzstiftung and Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausärzte to K.H.S.
Conflict of interest: Tim Friede reports personal fees for consultancies (including data monitoring committees) from Novartis, Bayer, Biogen, AstraZeneca, Janssen, Grünenthal, Pharmalog, SGS, Boehringer Ingelheim, Daiichi-Sankyo, Mediconomics, and Roche, all outside the submitted work. Furthermore, he has received research funding by the European Commission for statistical analyses on the EUTrigTreat (NCT01209494) and EU-CERT-ICD (NCT02064192) clinical studies. All relationships declared are modest. All other authors declare no conflict of interest.
Supplementary Material
References
- 1. De Luca G, Suryapranata H, Ottervanger JP, Antman EM.. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation 2004;109:1223–1225. [DOI] [PubMed] [Google Scholar]
- 2. Brodie BR, Webb J, Cox DA, Qureshi M, Kalynych A, Turco M, Schultheiss HP, Dulas D, Rutherford B, Antoniucci D, Stuckey T, Krucoff M, Gibbons R, Lansky A, Na Y, Mehran R, Stone GW; EMERALD Investigators. Impact of time to treatment on myocardial reperfusion and infarct size with primary percutaneous coronary intervention for acute myocardial infarction (from the EMERALD Trial). Am J Cardiol 2007;99:1680–1686. [DOI] [PubMed] [Google Scholar]
- 3. McNamara RL, Wang Y, Herrin J, Curtis JP, Bradley EH, Magid DJ, Peterson ED, Blaney M, Frederick PD, Krumholz HM; NRMI Investigators. Effect of door-to-balloon time on mortality in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol 2006;47:2180–2186. [DOI] [PubMed] [Google Scholar]
- 4. Brodie BR, Hansen C, Stuckey TD, Richter S, Versteeg DS, Gupta N, Downey WE, Pulsipher M.. Door-to-balloon time with primary percutaneous coronary intervention for acute myocardial infarction impacts late cardiac mortality in high-risk patients and patients presenting early after the onset of symptoms. J Am Coll Cardiol 2006;47:289–295. [DOI] [PubMed] [Google Scholar]
- 5. Rathore SS, Curtis JP, Chen J, Wang Y, Nallamothu BK, Epstein AJ, Krumholz HM; National Cardiovascular Data Registry. Association of door-to-balloon time and mortality in patients admitted to hospital with ST elevation myocardial infarction: national cohort study. BMJ 2009;338:1807.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Terkelsen CJ, Sørensen JT, Maeng M, Jensen LO, Tilsted HH, Trautner S, Vach W, Johnsen SP, Thuesen L, Lassen JF.. System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA 2010;304:763–771. [DOI] [PubMed] [Google Scholar]
- 7. Shiomi H, Nakagawa Y, Morimoto T, Furukawa Y, Nakano A, Shirai S, Taniguchi R, Yamaji K, Nagao K, Suyama T, Mitsuoka H, Araki M, Takashima H, Mizoguchi T, Eisawa H, Sugiyama S, Kimura T; CREDO-Kyoto AMI Investigators. Association of onset to balloon and door to balloon time with long term clinical outcome in patients with ST elevation acute myocardial infarction having primary percutaneous coronary intervention: observational study. BMJ 2012;344:e3257.. [DOI] [PubMed] [Google Scholar]
- 8. Swaminathan RV, Wang TY, Kaltenbach LA, Kim LK, Minutello RM, Bergman G, Wong SC, Feldman DN.. Nonsystem reasons for delay in door-to-balloon time and associated in-hospital mortality: a report from the National Cardiovascular Data Registry. J Am Coll Cardiol 2013;61:1688–1695. [DOI] [PubMed] [Google Scholar]
- 9. Anderson LL, French WJ, Peng SA, Vora AN, Henry TD, Roe MT, Kontos MC, Granger CB, Bates ER, Hellkamp A, Wang TY.. Direct transfer from the referring hospitals to the catheterization laboratory to minimize reperfusion delays for primary percutaneous coronary intervention: Insights from the National Cardiovascular Data Registry. Circ Cardiovasc Interv 2015;8:e002477.. [DOI] [PubMed] [Google Scholar]
- 10. Pinto DS, Kirtane AJ, Nallamothu BK, Murphy SA, Cohen DJ, Laham RJ, Cutlip DE, Bates ER, Frederick PD, Miller DP, Carrozza JP Jr, Antman EM, Cannon CP, Gibson CM.. Hospital delays in reperfusion for ST-elevation myocardial infarction: implications when selecting a reperfusion strategy. Circulation 2006;114:2019–2025. [DOI] [PubMed] [Google Scholar]
- 11. Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, McNamara RL, Parkosewich J, Loeb JM, Krumholz HM.. Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med 2006;355:2308–2320. [DOI] [PubMed] [Google Scholar]
- 12. Brener SJ, Brodie BR, Guerchicoff A, Witzenbichler B, Guagliumi G, Xu K, Mehran R, Stone GW.. Impact of pre-procedural cardiopulmonary instability in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol 2014;114:962–967. [DOI] [PubMed] [Google Scholar]
- 13. Lønborg J, Schoos MM, Kelbæk H, Holmvang L, Steinmetz J, Vejlstrup N, Jørgensen E, Helqvist S, Saunamäki K, Bøtker HE, Kim WY, Terkelsen CJ, Clemmensen P, Engstrøm T.. Impact of system delay on infarct size, myocardial salvage index, and left ventricular function in patients with ST-segment elevation myocardial infarction. Am Heart J 2012;164:538–546. [DOI] [PubMed] [Google Scholar]
- 14. Bata I, Armstrong PW, Westerhout CM, Travers A, Sookram S, Caine E, Christenson J, Welsh RC; WEST Study Group. Time from first medical contact to reperfusion in ST elevation myocardial infarction: a Which Early ST Elevation Myocardial Infarction Therapy (WEST) substudy. Can J Cardiol 2009;25:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scholz KH, Hilgers R, Ahlersmann D, Duwald H, Nitsche R, von Knobelsdorff G, Volger B, Möller K, Keating FK.. Contact-to-balloon time and door-to-balloon time after initiation of a formalized data feedback in patients with acute ST-elevation myocardial infarction. Am J Cardiol 2008;101:46–52. [DOI] [PubMed] [Google Scholar]
- 16. Scholz KH, Maier SK, Jung J, Fleischmann C, Werner GS, Olbrich HG, Ahlersmann D, Keating FK, Jacobshagen C, Moehlis H, Hilgers R, Maier LS.. Reduction in treatment times through formalized data feedback: results from a prospective multicenter study of ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2012;5:848–857. [DOI] [PubMed] [Google Scholar]
- 17. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX;. CF/AHA Task Force. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127:529–555. [DOI] [PubMed] [Google Scholar]
- 18. Kaufmann-Kolle P, German Hospital Quality Report 2013. AQUA Institute for Applied Quality Improvement and Research in Health Care: Göttingen; 2015. p1–252.
- 19. Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E.. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation 2000;102:2031–2037. [DOI] [PubMed] [Google Scholar]
- 20. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH; SHOCK Investigators. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999;341:625–634. [DOI] [PubMed] [Google Scholar]
- 21. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 22. Hochman JS, Sleeper LA, White HD, Dzavik V, Wong SC, Menon V, Webb JG, Steingart R, Picard MH, Menegus MA, Boland J, Sanborn T, Buller CE, Modur S, Forman R, Desvigne-Nickens P, Jacobs AK, Slater JN, LeJemtel TH; SHOCK Investigators. One-year survival following early revascularization for cardiogenic shock. JAMA 2001;285:190–192. [DOI] [PubMed] [Google Scholar]
- 23. Webb JG, Lowe AM, Sanborn TA, White HD, Sleeper LA, Carere RG, Buller CE, Wong SC, Boland J, Dzavik V, Porway M, Pate G, Bergman G, Hochman JS; SHOCK Investigators. Percutaneous coronary intervention for cardiogenic shock in the SHOCK trial. J Am Coll Cardiol 2003;42:1380–1386. [DOI] [PubMed] [Google Scholar]
- 24. Webb JG, Sanborn TA, Sleeper LA, Carere RG, Buller CE, Slater JN, Baran KW, Koller PT, Talley JD, Porway M, Hochman JS; SHOCK Investigators. Percutaneous coronary intervention for cardiogenic shock in the SHOCK Trial Registry. Am Heart J 2001;141:964–970. [DOI] [PubMed] [Google Scholar]
- 25. Babaev A, Frederick PD, Pasta DJ, Every N, Sichrovsky T, Hochman JS.. NRMI Investigators. Trends in management and outcomes of patients with acute myocardial infarction complicated by cardiogenic shock. JAMA 2005;294:448–454. [DOI] [PubMed] [Google Scholar]
- 26. Shavelle DM, Chen AY, Matthews RV, Roe MT, de Lemos JA, Jollis J, Thomas JL, French WJ; ACTION-GWTG Investigators. Predictors of reperfusion delay in patients with ST elevation myocardial infarction self-transported to the hospital (from the American Heart Association's Mission: Lifeline Program). Am J Cardiol 2014;113:798–802. [DOI] [PubMed] [Google Scholar]
- 27. Brown JP, Mahmud E, Dunford JV, Ben-Yehuda O.. Effect of prehospital 12-lead electrocardiogram on activation of the cardiac catheterization laboratory and door-to-balloon time in ST-segment elevation acute myocardial infarction. Am J Cardiol 2008;101:158–161. [DOI] [PubMed] [Google Scholar]
- 28. Sejersten M, Sillesen M, Hansen PR, Nielsen SL, Nielsen H, Trautner S, Hampton D, Wagner GS, Clemmensen P.. Effect on treatment delay of prehospital teletransmission of 12-lead electrocardiogram to a cardiologist for immediate triage and direct referral of patients with ST-segment elevation acute myocardial infarction to primary percutaneous coronary intervention. Am J Cardiol 2008;101:941–946. [DOI] [PubMed] [Google Scholar]
- 29. Savage ML, Poon KK, Johnston EM, Raffel OC, Incani A, Bryant J, Rashford S, Pincus M, Walters DL.. Pre-hospital ambulance notification and initiation of treatment of ST elevation myocardial infarction is associated with significant reduction in door-to-balloon time for primary PCI. Heart Lung Circ 2014;23:435–443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.