Abstract
STUDY QUESTION
Is there an association between intake of fruits and vegetables and risk of laparoscopically confirmed endometriosis?
SUMMARY ANSWER
Higher intake of fruits, particularly citrus fruits, is associated with a lower risk of endometriosis.
WHAT IS KNOWN ALREADY
Two case–control studies have examined the associations between fruit and vegetable intake and endometriosis risk with contrasting results. Diets rich in fruits and vegetables include higher levels of pro-vitamin A nutrients (alpha-carotene, beta-carotene, beta-cryptoxanthin) and women with endometriosis have been reported to have lower intake of vitamin A than women without endometriosis.
STUDY DESIGN SIZE, DURATION
A prospective cohort study using data collected from 70 835 premenopausal women from 1991 to 2013 as part of the Nurses’ Health Study II cohort.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Diet was assessed with a validated food frequency questionnaire (FFQ) every 4 years. Cases were restricted to laparoscopically confirmed endometriosis. Cox proportional hazards models were used to calculate rate ratios (RR) and 95% CI.
MAIN RESULTS AND THE ROLE OF CHANCE
During 840 012 person-years of follow-up, 2609 incident cases of laparoscopically confirmed endometriosis were reported (incidence rate = 311 per 100 000 person-years). We observed a non-linear inverse association between higher fruit consumption and risk of laparoscopically confirmed endometriosis (Psignificance of the curve = 0.005). This inverse association was particularly evident for citrus fruits. Women consuming ≥1 servings of citrus fruits/day had a 22% lower endometriosis risk (95% CI = 0.69–0.89; Ptrend = 0.004) compared to those consuming <1 serving/week. No association was observed between total vegetable intake and endometriosis risk. However, women consuming ≥1 servings/day cruciferous vegetables had a 13% higher risk of endometriosis (95% CI = 0.95–1.34; Ptrend = 0.03) compared to those consuming <1 serving/week. Of the nutrients examined, only beta-cryptoxanthin intake was significantly associated with lower endometriosis risk (RR fifth quintile = 0.88; 95% CI = 0.78–1.00; Ptrend = 0.02).
LIMITATIONS REASONS FOR CAUTION
Some error in the self-reporting of dietary intake is expected, however, use of a validated FFQ and examining diet prospectively across multiple time points, make it unlikely that this non-differential misclassification strongly influenced the results.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings suggest that a higher intake of fruits, particularly citrus fruits, is associated with a lower risk of endometriosis, and beta-cryptoxanthin in these foods may partially explain this association. In contrast to what we hypothesized, consumption of some vegetables increased endometriosis risk which may indicate a role of gastrointestinal symptoms in both the presentation and exacerbation of endometriosis-related pain; however, it is not clear what components of these foods might underlie the observed associations. Future studies examining dietary patterns that consider different combinations of food intake may help clarify these associations.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by research grants HD4854, HD52473 and HD57210 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and grant P30 DK046200 from the National Institute of Diabetes and Digestive and Kidney Diseases. The Nurses’ Health Study II is supported by the Public Health Service grant UM1 CA176726 from the National Cancer Institute, National Institutes of Health. HRH is supported by the National Cancer Institute, National Institutes of Health (K22 CA193860). No competing interests.
TRIAL REGISTRATION NUMBER
n/a.
Keywords: endometriosis, diet, fruits, vegetables, beta-cryptoxanthinIntroduction
Introduction
Endometriosis is a hormone-dependent disorder characterized by the presence of endometrial-like tissue in extra-uterine sites, often resulting in a chronic, inflammatory reaction. It affects ~10% of reproductive age women (Missmer and Cramer, 2003) and current evidence indicates that genetic, environmental, immunological and inflammatory factors all play roles in its pathogenesis (Treloar et al., 1999; Lebovic et al., 2001; Nothnick, 2001; Missmer et al., 2004; Eisenberg et al., 2012; Rahmioglu et al., 2014). Dietary factors may have a role in the etiology of endometriosis through, e.g. influences on steroid hormones, inflammation or food contaminants.
To our knowledge, only two studies have examined the associations between fruit and vegetable intake and endometriosis risk with contrasting results. An Italian hospital-based case–control study reported that those with laparoscopically confirmed endometriosis had a significantly lower intake of both fresh fruit and green vegetables (Parazzini et al., 2004), while a population-based case–control study in Washington state, USA found no association with vegetable consumption but greater fruit intake among women with endometriosis (Trabert et al., 2010). Diets rich in fruits and vegetables include higher levels of pro-vitamin A nutrients (alpha-carotene, beta-carotene, beta-cryptoxanthin) and women with endometriosis have been reported to have a lower intake of vitamin A than women without endometriosis (Mier-Cabrera et al., 2009). In-vitro studies also suggest that vitamin A may influence endometriosis (Casey et al., 1994; Sawatsri et al., 2000; Tee et al., 2006). For example, in human endometrial cells retinoic acid has been shown to suppress interleukin-6 (IL-6) mRNA expression levels (Sawatsri et al., 2000) and elevated levels of IL-6 have been observed in the peritoneal fluid of women with endometriosis (Harada et al., 1997; Punnonen et al., 1996)
In this study, we used data from the US prospective Nurses’ Health Study II to investigate whether intake of fruits, vegetables, nutrients concentrated in these foods (alpha-carotene, beta-carotene, beta-cryptoxanthin, lutein, zeaxanthin and lycopene), or retinol activity equivalents were associated with incident laparoscopically confirmed endometriosis over a 22-year follow-up period. We also examined whether the associations between these factors and endometriosis varied according to the fertility status of the participants and whether the associations were modified by cigarette smoking.
Materials and Methods
Study population
The Nurses’ Health Study II (NHS II) is an ongoing prospective cohort that was established in 1989 when 116 429 female registered nurses, aged 25–42 years, completed a baseline questionnaire that collected information on demographic and lifestyle factors, anthropometric variables and disease history. Follow-up questionnaires are sent biennially to participants with questions updating the information on incident disease risk factors. Further details on the study have been provided elsewhere (Solomon et al., 1997).
Follow-up for the current analyses began in 1991, when 97 527 NHS II participants returned the dietary assessment, and concluded in 2013. We excluded participants who had an implausible total energy intake (<800 or >4200 kcal/day) or left more than 70 food items blank on the 1991 food frequency questionnaire (FFQ) (n = 2356). Participants were also excluded if they reported a diagnosis of endometriosis (n = 5442), history of infertility (n = 10 975), or a cancer diagnosis (other than non-melanoma skin cancer) (n = 1221) prior to June 1991. The analytical cohort was limited to women who were premenopausal and had intact uteri as endometriosis rarely occurs incidentally among postmenopausal women or subsequent to a hysterectomy. After these exclusions, 70 835 premenopausal women with dietary information remained.
Ethical approval
This study was approved by the Institutional Review Boards of the Harvard School of Public Health and Brigham and Women’s Hospital, Boston, MA, USA. Implied consent was assumed upon completion and return of the questionnaires.
Dietary assessment
Diet was assessed in 1991, 1995, 1999, 2003, 2007 and 2011 using a FFQ listing over 130 food items. Participants were asked how often, on average, they had consumed each type of food or beverage during the previous year. Nine responses were possible, ranging from never or less than once per month to six or more times per day. Participants were also asked to report whether they used other nutrient supplements, and to provide the brand and dose. Intakes of the nutrients of interest, were calculated by multiplying the portion size of a single serving of each food by its reported frequency of intake, then multiplying the total amount consumed by the nutrient content of the food, and summing the nutrient contributions of all food items using the US Department of Agriculture food composition data (Nutrient Data Laboratory ARS, 1999), while also taking dietary supplements into account.
The reproducibility and validity of the FFQ has been reported elsewhere (Salvini et al., 1989; Willett et al., 1985; Yuan et al., 2017). The FFQ has been shown to provide valid estimates of fruit, vegetable, and nutrient intake with deattenuated correlation coefficients for fruits and vegetables between the FFQ and 1-week diet records ranging from 0.16 for yellow squash to 0.80 for apples. The coefficients for most fruits and vegetables were above 0.40 (Salvini et al., 1989). Retinol activity equivalents (RAE), alpha-carotene, beta-carotene, lutein/zeaxanthin, lycopene and beta-cryptoxanthin had deattenuated correlation coefficients ranging from 0.57 to 0.72 (Yuan et al., 2017). Carotenoid intake has also been validated using plasma carotenoid levels with reported correlations between dietary intake and blood levels of 0.27 for beta-carotene, 0.48 for alpha-carotene, 0.32 for beta-cryptoxanthin, 0.21 for lycopene and 0.27 lutein (Michaud et al., 1998). Intakes of all nutrients were adjusted for total energy intake using the residual method (Willett, 2013).
Ascertainment and definition of endometriosis
Starting in 1993, participants were asked on each biennial questionnaire if they had ‘ever had physician-diagnosed endometriosis’, and, if so, the date of diagnosis and whether it had been confirmed by laparoscopy. The validity of self-reported endometriosis in this cohort has been described previously (Missmer et al., 2004). Briefly, a diagnosis of endometriosis was confirmed by medical records in 96% of those who reported laparoscopic confirmation. However, a review of the medical records of those without laparoscopic confirmation indicated a clinical diagnosis of endometriosis in only 54%. In addition, a diagnosis of endometriosis at the time of hysterectomy was confirmed in 80% of the cases, but endometriosis was the primary indication for hysterectomy in only 6% of those for whom an indication was available. Therefore, in order to minimize the magnitude of misclassification and prevent confounding by indication for hysterectomy, we restricted our definition of incident diagnosis of endometriosis to women who reported laparoscopic confirmation of their diagnosis.
Due to the complex relation between endometriosis and infertility within this restricted case definition, we examined risk factors by two ‘subtypes’ of endometriosis: women who never reported infertility (those with no past or concurrent infertility), and women with concurrent infertility. At baseline, the prevalence of infertility (defined as attempting to become pregnant for >1 year without success) was greater among women with laparoscopic confirmation (20%) than among those who were clinically diagnosed without laparoscopic confirmation (4%). This may result in the over-sampling of those with otherwise ‘asymptomatic’ endometriosis and also those who may have altered their diet due to infertility prior to enrollment. While pelvic pain information is not available in the NHS II, endometriosis case women with infertility will have a greater prevalence of being asymptomatic in terms of pelvic pain compared to those who never experienced infertility, because during this time period most underwent an exploratory laparoscopy to identify the cause of their infertility during which the endometriosis was discovered. Because endometriosis with infertility may have a higher prevalence of asymptomatic disease secondary to other primary causes of infertility, the etiology, and thus risk factors, for endometriosis with infertility could differ from those for endometriosis without concurrent infertility.
Statistical analysis
Participants contributed follow-up time from the return of the 1991 questionnaire until self-report of laparoscopically confirmed endometriosis diagnosis, diagnosis of any cancer (except non-melanoma skin cancer), death, loss to follow-up, hysterectomy, menopause or until return of the 2013 questionnaire, whichever occurred first. In addition, women were censored at time of self-report of infertility, because infertility in this population is strongly correlated with diagnosis of endometriosis via laparoscopy. Therefore, the person-time denominator for the incidence rate consists of women with neither diagnosed endometriosis nor infertility.
We used Cox proportional hazards regression models with age and questionnaire period as the time scale to estimate incidence rate ratios (RR), and 95% CI using the lowest category of each food or nutrient intake as the reference. We examined the possibly non-linear relation between intake of selected fruit and vegetable groups and endometriosis with restricted cubic splines (Durrleman and Simon, 1989). In addition, as the temporal relation between these foods/nutrients and risk of endometriosis is uncertain, dietary intake was examined multiple ways: baseline intake (1991 FFQ), varying lag-time intake and cumulative average intake. The cumulative average method captures long-term dietary intake and reduces measurement error due to within-person variation over time (Hu et al., 1999). The varying lag-time intake allows us to examine dietary intake closer to endometriosis onset as there is often a lengthy delay between the emergence of clinical symptoms and definitive diagnosis. We examined lag times of 2–4 (simple update), 4–6 and 6–8 years. For example, for a lag time of 2–4 years before diagnosis we used dietary intake from the 1991 questionnaire for an endometriosis diagnosis reported from June 1991 to June 1995, intake from 1995 for diagnosis from 1995 to 1999, and so forth. For a lag time of 4–6 years before diagnosis, we used dietary intake reported on the 1991 questionnaire for diagnosis from 1995 to 1999, intake from 1995 for diagnosis from 1999 to 2003, and so forth. For a lag time of 6–8 years we used dietary intake from 1991 for a diagnosis from 1997 to 2001, and intake from 1995 for follow-up from 2001 to 2005. The cumulative average method was used in all analyses except when comparing analytic approaches.
Total caloric intake was included in both age-adjusted and multivariable models (Willett, 2013). Multivariable models were further adjusted for the following potential confounders: age at menarche, parity, length of menstrual cycle and BMI as these factors have previously been associated with endometriosis risk. Covariates were updated throughout the analysis whenever new information was available from the biennial questionnaires. Missing data were handled via the missing indicator method, with categories created for missing data included in the regression model (Miettinen, 1985). Income and marital status were also evaluated as potential confounders but did not materially influence the effect estimates so were not included in the final models. Tests for linear trend for the exposures of interest were performed by assigning the median value of each category to all participants in that group. Tests for heterogeneity comparing the effect estimates among endometriosis cases groups were calculated with a Wald statistic for each food/nutrient group.
We assessed the association for each food/nutrient group by smoking status, as previous studies have suggested that smoking may modify the effect of antioxidant intake, and smoking has been observed to modify the effect of fat intake on endometriosis risk within this cohort (Missmer et al., 2010). Participants were classified as ever or never smokers based on biennial questionnaire data. While smoking status data abstracted from medical records is often quite poor, self-reported smoking status, including among NHS participants, has been demonstrated previously to be highly reliable and valid (Al-Delaimy et al., 2002; Patrick et al., 1994). Effect modification was assessed with a likelihood ratio test that compared the model with the cross-product term between the exposure variable and smoking status with the model with main effects only. All tests of statistical significance were two-sided and all statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
During 840 012 person-years of follow-up contributed by 70 835 women, 2609 incident cases of laparoscopically confirmed endometriosis were reported. Of these, 2114 never reported infertility and 313 cases reported an infertility evaluation during the same follow-up period as their laparoscopic confirmation of endometriosis. Women with the greatest intake of fruits were slightly younger, less likely to be Caucasian, less likely to be current smokers, nulliparous and obese than those with lower total fruit intake. Those who had the greatest intake of vegetables were more likely to be physically active and had an earlier age at menarche than those with lower total vegetable intake (Table I).
Table I.
Distribution of potential risk factors for endometriosis according to total fruit and total vegetable intake among women in the Nurses Health Study II at baseline (1991)1.
| Total Fruits | Total Vegetables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <1/day | 1/day | 2/day | 3/day | >3/day | ≤2/day | 3/day | 4/day | 5/day | ≥6/day | |
| No. of women | 15 075 | 5495 | 24 305 | 14 624 | 10 993 | 20 224 | 17 930 | 13 413 | 8343 | 10 582 |
| Age (years), mean (SD) | 36.1 (4.6) | 36.1 (4.7) | 36.2 (4.6) | 36.2 (4.6) | 35.8 (4.7) | 35.5 (4.8) | 36.0 (4.6) | 36.3 (4.5) | 36.5 (4.6) | 36.8 (4.5) |
| Caucasian (%) | 96 | 96 | 96 | 96 | 94 | 95 | 96 | 96 | 96 | 94 |
| BMI (kg/m2) (%) | ||||||||||
| <25 | 65 | 66 | 66 | 68 | 70 | 68 | 68 | 67 | 66 | 64 |
| 25–29.9 (overweight) | 20 | 21 | 21 | 20 | 19 | 19 | 20 | 21 | 22 | 22 |
| ≥30 (obese) | 15 | 14 | 13 | 12 | 11 | 14 | 12 | 12 | 12 | 14 |
| Physical activity (MET-h/week) | 23 (68) | 25 (64) | 27 (67) | 30 (63) | 37 (76) | 23 (66) | 26 (65) | 29 (67) | 33 (76) | 37 (71) |
| Cigarette smoking (%) | ||||||||||
| Never | 61 | 64 | 67 | 70 | 71 | 68 | 67 | 67 | 65 | 63 |
| Past | 21 | 22 | 23 | 22 | 22 | 19 | 22 | 23 | 24 | 25 |
| Current | 19 | 15 | 11 | 9 | 7 | 13 | 11 | 11 | 11 | 12 |
| Age at menarche (%) | ||||||||||
| <12 years | 9 | 9 | 9 | 8 | 9 | 8 | 8 | 9 | 10 | 11 |
| 12 years | 37 | 35 | 37 | 36 | 35 | 36 | 37 | 36 | 36 | 37 |
| 13 years | 33 | 34 | 33 | 35 | 33 | 34 | 34 | 34 | 33 | 32 |
| ≥14 years | 22 | 22 | 21 | 21 | 23 | 22 | 22 | 21 | 21 | 20 |
| Menstrual cycle length (%) | ||||||||||
| <26 days | 13 | 11 | 11 | 11 | 11 | 12 | 11 | 11 | 11 | 12 |
| 26–31 days | 67 | 67 | 67 | 67 | 67 | 67 | 68 | 67 | 67 | 66 |
| 32–50 days | 16 | 17 | 18 | 18 | 17 | 17 | 17 | 17 | 18 | 17 |
| 51+ days or irregular | 4 | 5 | 4 | 5 | 5 | 4 | 4 | 5 | 4 | 5 |
| Oral contraceptive use (%) | ||||||||||
| Ever | 85 | 85 | 84 | 82 | 81 | 84 | 84 | 83 | 83 | 81 |
| Nulliparous (%) | 29 | 27 | 26 | 26 | 24 | 29 | 25 | 24 | 25 | 28 |
| Total energy intake (kcal) | 1484 (485) | 1611 (489) | 1736 (483) | 1948 (495) | 2221 (527) | 1509 (478) | 1732 (487) | 1875 (505) | 1990 (514) | 2171 (552) |
1All data shown are standardized to the age distributions of the 1991 cohort. MET, metabolic equivalent.
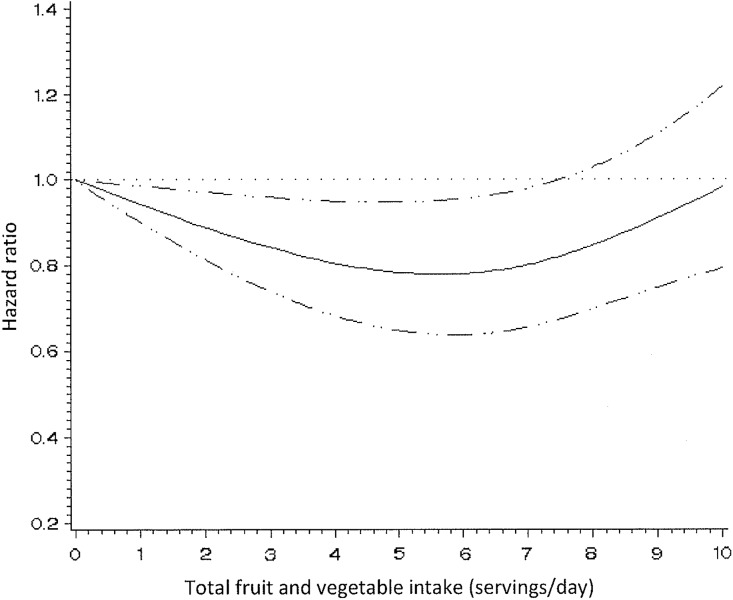
Higher consumption of total fruits and vegetables was associated with reduced endometriosis risk. Women consuming 3, 4, 5 and ≥6 servings/day of fruits and vegetables had 9% (95% CI = 0.77–1.07), 10% (95% CI = 0.76–1.06), 18% (95% CI = 0.69–0.97) and 12% (95% CI = 0.75–1.03) reduced risks for endometriosis compared to women consuming ≤2 servings/day, respectively (Ptrend = 0.32) (Table II). In a model using restricted cubic splines, the relation between total fruit and vegetable intake and endometriosis appeared non-linear (Pnon-linearity = 0.01) and was statistically significant (Psignificance of the curve = 0.02) (Fig. 1).
Table II.
Hazard ratios and 95% CIs for laparoscopically confirmed endometriosis according to intake of fruits and vegetables by infertility status among 70 835 women in the Nurses’ Health Study II, 1991–2013.
| Intake in servings per day or week | Case definition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All women (n = 2609) | Never infertile1 (n = 2114) | Concurrent infertility1 (n = 313) | ||||||||||
| Cases | Age-adjusted HR (95% CI) | Multivariable2 HR (95% CI) | Cases | Multivariable2 HR (95% CI) | Cases | Multivariable2 HR (95% CI) | P het 3 | |||||
| Total fruits and vegetables | ||||||||||||
| ≤2/day | 224 | 1.00 | Referent | 1.00 | Referent | 181 | 1.00 | Referent | 33 | 1.00 | Referent | 0.43 |
| 3/day | 359 | 0.89 | (0.75, 1.05) | 0.91 | (0.77, 1.07) | 298 | 0.92 | (0.76, 1.11) | 39 | 0.79 | (0.49, 1.27) | |
| 4/day | 455 | 0.88 | (0.75, 1.03) | 0.90 | (0.76, 1.06) | 358 | 0.85 | (0.71, 1.02) | 56 | 0.98 | (0.63, 1.53) | |
| 5/day | 411 | 0.81 | (0.68, 0.95) | 0.82 | (0.69, 0.97) | 337 | 0.81 | (0.67, 0.98) | 44 | 0.81 | (0.51, 1.29) | |
| ≥6/day | 1160 | 0.89 | (0.76, 1.03) | 0.88 | (0.75, 1.03) | 940 | 0.86 | (0.72, 1.02) | 141 | 1.06 | (0.70, 1.61) | |
| Ptrend4 | 0.54 | 0.32 | 0.21 | 0.28 | ||||||||
| Total vegetables | ||||||||||||
| ≤2/day | 711 | 1.00 | Referent | 1.00 | Referent | 568 | 1.00 | Referent | 100 | 1.00 | Referent | 0.75 |
| 3/day | 663 | 0.94 | (0.84, 1.04) | 0.95 | (0.85, 1.06) | 535 | 0.94 | (0.83, 1.05) | 76 | 0.94 | (0.69, 1.28) | |
| 4/day | 508 | 0.93 | (0.82, 1.04) | 0.93 | (0.82, 1.04) | 429 | 0.95 | (0.84, 1.09) | 52 | 0.89 | (0.63, 1.26) | |
| 5/day | 321 | 0.98 | (0.86, 1.13) | 0.97 | (0.84, 1.11) | 264 | 0.97 | (0.83, 1.13) | 32 | 0.93 | (0.61, 1.41) | |
| ≥6/day | 406 | 1.11 | (0.97, 1.27) | 1.04 | (0.91, 1.19) | 318 | 0.99 | (0.85, 1.15) | 53 | 1.22 | (0.84, 1.76) | |
| Ptrend4 | 0.11 | 0.57 | 0.97 | 0.37 | ||||||||
| Green vegetables5 | ||||||||||||
| ≤1/week | 329 | 1.00 | Referent | 1.00 | Referent | 258 | 1.00 | Referent | 53 | 1.00 | Referent | 0.05 |
| 2–6/week | 1486 | 0.99 | (0.87, 1.11) | 0.96 | (0.85, 1.09) | 1219 | 1.00 | (0.87, 1.15) | 162 | 0.80 | (0.58, 1.10) | |
| 1/day | 329 | 0.97 | (0.83, 1.13) | 0.93 | (0.79, 1.08) | 274 | 0.97 | (0.82, 1.16) | 29 | 0.61 | (0.38, 0.97) | |
| >1/day | 465 | 1.01 | (0.87, 1.17) | 0.93 | (0.80, 1.08) | 363 | 0.92 | (0.78, 1.08) | 69 | 1.01 | (0.69, 1.48) | |
| Ptrend4 | 0.81 | 0.28 | 0.18 | 0.78 | ||||||||
| Orange vegetables6 | ||||||||||||
| <1/week | 313 | 1.00 | Referent | 1.00 | Referent | 249 | 1.00 | Referent | 49 | 1.00 | Referent | 0.88 |
| 1/week | 359 | 0.94 | (0.81, 1.10) | 0.97 | (0.83, 1.13) | 285 | 0.94 | (0.79, 1.12) | 48 | 1.05 | (0.70, 1.57) | |
| 2–6/week | 1566 | 0.93 | (0.82, 1.06) | 0.97 | (0.86, 1.10) | 1276 | 0.95 | (0.83, 1.10) | 175 | 1.10 | (0.79, 1.54) | |
| ≥1/day | 371 | 0.96 | (0.82, 1.12) | 0.97 | (0.83, 1.14) | 304 | 0.96 | (0.80, 1.14) | 41 | 1.04 | (0.67, 1.61) | |
| Ptrend4 | 0.94 | 0.88 | 0.88 | 0.94 | ||||||||
| Cruciferous vegetables7 | ||||||||||||
| <1/week | 266 | 1.00 | Referent | 1.00 | Referent | 213 | 1.00 | Referent | 40 | 1.00 | Referent | 0.05 |
| 1/week | 316 | 0.90 | (0.77, 1.06) | 0.92 | (0.78, 1.09) | 261 | 0.93 | (0.78, 1.12) | 35 | 0.84 | (0.53, 1.33) | |
| 2–6/week | 1730 | 0.98 | (0.86, 1.12) | 1.01 | (0.88, 1.15) | 1390 | 0.97 | (0.84, 1.12) | 213 | 1.26 | (0.89, 1.78) | |
| ≥1/day | 297 | 1.16 | (0.98, 1.38) | 1.13 | (0.95, 1.34) | 250 | 1.12 | (0.93, 1.36) | 25 | 0.90 | (0.54, 1.51) | |
| Ptrend4 | 0.006 | 0.03 | 0.05 | 0.94 | ||||||||
| Tomatoes | ||||||||||||
| <1/week | 130 | 1.00 | Referent | 1.00 | Referent | 107 | 1.00 | Referent | 18 | 1.00 | Referent | 0.59 |
| 1/week | 282 | 0.90 | (0.73, 1.10) | 0.94 | (0.76, 1.16) | 225 | 0.90 | (0.71, 1.13) | 41 | 1.20 | (0.68, 2.09) | |
| 2–6/week | 1740 | 0.82 | (0.69, 0.99) | 0.88 | (0.74, 1.06) | 1421 | 0.84 | (0.68, 1.02) | 199 | 1.19 | (0.72, 1.94) | |
| ≥1/day | 457 | 0.91 | (0.74, 1.11) | 0.94 | (0.77, 1.15) | 361 | 0.85 | (0.68, 1.07) | 55 | 1.27 | (0.73, 2.21) | |
| Ptrend4 | 0.63 | 0.81 | 0.53 | 0.52 | ||||||||
| Legumes8 | ||||||||||||
| <1/week | 273 | 1.00 | Referent | 1.00 | Referent | 204 | 1.00 | Referent | 54 | 1.00 | Referent | 0.57 |
| 1/week | 328 | 0.95 | (0.81, 1.12) | 0.99 | (0.85, 1.17) | 270 | 1.06 | (0.88, 1.27) | 40 | 0.82 | (0.54, 1.23) | |
| 2–6/week | 1781 | 0.96 | (0.84, 1.10) | 1.04 | (0.91, 1.18) | 1455 | 1.06 | (0.91, 1.23) | 194 | 1.06 | (0.77, 1.45) | |
| ≥1/day | 227 | 1.03 | (0.86, 1.24) | 1.07 | (0.89, 1.29) | 185 | 1.11 | (0.90, 1.36) | 25 | 1.01 | (0.61, 1.67) | |
| Ptrend4 | 0.52 | 0.39 | 0.43 | 0.63 | ||||||||
| Total fruits | ||||||||||||
| <1/day | 516 | 1.00 | Referent | 1.00 | Referent | 432 | 1.00 | Referent | 59 | 1.00 | Referent | 0.53 |
| 1/day | 218 | 0.98 | (0.84, 1.15) | 1.00 | (0.85, 1.17) | 175 | 0.95 | (0.80, 1.13) | 29 | 1.29 | (0.82, 2.03) | |
| 2/day | 941 | 0.91 | (0.82, 1.02) | 0.92 | (0.82, 1.02) | 753 | 0.87 | (0.77, 0.98) | 106 | 1.07 | (0.77, 1.48) | |
| 3/day | 541 | 0.86 | (0.76, 0.98) | 0.86 | (0.76, 0.98) | 437 | 0.83 | (0.72, 0.95) | 69 | 1.15 | (0.80, 1.66) | |
| ≥4/day | 393 | 0.93 | (0.81, 1.08) | 0.93 | (0.80, 1.07) | 317 | 0.89 | (0.76, 1.05) | 50 | 1.16 | (0.77, 1.77) | |
| Ptrend4 | 0.20 | 0.16 | 0.11 | 0.58 | ||||||||
| Citrus fruits9 | ||||||||||||
| <1/week | 408 | 1.00 | Referent | 1.00 | Referent | 339 | 1.00 | Referent | 56 | 1.00 | Referent | 0.76 |
| 1/week | 308 | 0.82 | (0.70, 0.95) | 0.84 | (0.72, 0.97) | 244 | 0.80 | (0.68, 0.94) | 42 | 0.99 | (0.66, 1.49) | |
| 2–6/week | 1277 | 0.83 | (0.74, 0.93) | 0.86 | (0.76, 0.96) | 1027 | 0.82 | (0.72, 0.93) | 138 | 0.93 | (0.67, 1.28) | |
| ≥1/day | 616 | 0.77 | (0.67, 0.87) | 0.78 | (0.69, 0.89) | 504 | 0.75 | (0.65, 0.87) | 77 | 0.90 | (0.63, 1.29) | |
| Ptrend4 | 0.003 | 0.004 | 0.006 | 0.56 | ||||||||
| Rosaceae fruits10 | ||||||||||||
| <1/week | 167 | 1.00 | Referent | 1.00 | Referent | 141 | 1.00 | Referent | 21 | 1.00 | Referent | 0.18 |
| 1/week | 193 | 0.90 | (0.73, 1.11) | 0.93 | (0.76, 1.15) | 156 | 0.89 | (0.70, 1.11) | 23 | 1.06 | (0.58, 1.92) | |
| 2–6/week | 1548 | 0.86 | (0.73, 1.01) | 0.91 | (0.77, 1.07) | 1238 | 0.84 | (0.71, 1.01) | 198 | 1.31 | (0.83, 2.07) | |
| ≥1/day | 701 | 0.82 | (0.68, 0.97) | 0.87 | (0.73, 1.03) | 579 | 0.83 | (0.69, 1.01) | 71 | 1.05 | (0.63, 1.74) | |
| Ptrend4 | 0.07 | 0.15 | 0.27 | 0.48 | ||||||||
| Fruit juice | ||||||||||||
| <1/week | 426 | 1.00 | Referent | 1.00 | Referent | 368 | 1.00 | Referent | 43 | 1.00 | Referent | 0.02 |
| 1/week | 294 | 1.04 | (0.90, 1.21) | 1.09 | (0.93, 1.26) | 239 | 1.02 | (0.86, 1.20) | 38 | 1.63 | (1.05, 2.53) | |
| 2–6/week | 1128 | 1.01 | (0.90, 1.14) | 1.06 | (0.94, 1.19) | 910 | 0.99 | (0.87, 1.12) | 112 | 1.32 | (0.92, 1.89) | |
| ≥1/day | 761 | 0.91 | (0.80, 1.03) | 0.96 | (0.84, 1.09) | 597 | 0.87 | (0.76, 1.00) | 120 | 1.64 | (1.13, 2.37) | |
| Ptrend4 | 0.02 | 0.09 | 0.01 | 0.03 | ||||||||
1Infertility is defined as attempting to become pregnant for >1 year without success. Cases with ‘no past or concurrent infertility’ are women who never reported infertility. Cases with ‘concurrent infertility’ are women who reported an infertility evaluation in the same follow-up cycle as laparoscopic-confirmation of endometriosis.
2Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51– days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), BMI (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2) and energy (continuous).
3Test for heterogeneity comparing the effect of fruit/vegetable consumption among women with no past or current infertility to those with concurrent infertility.
4Determined using category medians.
5Green vegetables include kale, spinach, and head, romaine or leaf lettuce.
6Orange vegetables include carrots, orange winter squash and yams/sweet potatoes.
7Cruciferous vegetables include broccoli, cauliflower, cabbage and Brussel sprouts.
8Legumes include peas, lima beans, lentils and tofu/soy.
9Citrus fruits include oranges, orange juice, grapefruit and grapefruit juice.
10Rosaceae fruits include prunes, prune juice, apples, apple juice, apple sauce, strawberries and peaches. HR, hazard ratios.
Figure 1.
Hazard ratios of endometriosis by total fruit and vegetable intake. Test for non-linearity = 0.007, Psignificance of the curve = 0.02.
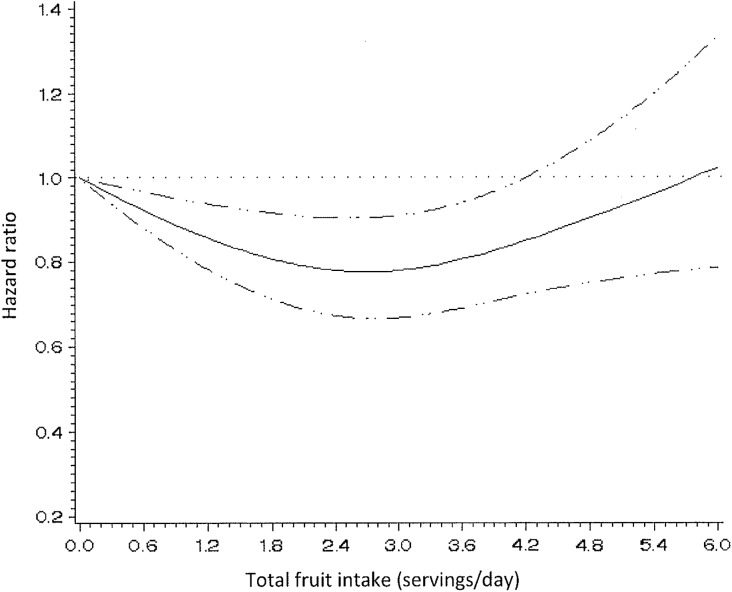
When fruits and vegetables were examined separately, total fruit consumption was associated with a lower endometriosis risk. Women consuming 3 servings of fruits/day had a 14% lower risk of endometriosis compared to women consuming <1 serving/day (95% CI = 0.76–0.98); however, the association was not significant for those consuming ≥4 servings/day (RR = 0.93; 95% CI = 0.80–1.07) and there was no significant linear trend (Ptrend = 0.16) (Table II). In a model using restricted cubic splines, the relation between fruit intake and endometriosis appeared non-linear (Pnon-linearity = 0.002) and was statistically significant (Psignificance of the curve = 0.005) (Fig. 2).
Figure 2.
Hazard ratios of endometriosis by total fruit intake. Test for non-linearity = 0.002, Psignificance of the curve = 0.005.
No association was observed between total vegetable intake and endometriosis risk: the RR for women consuming ≥6 servings/day of vegetables was 1.04 (95% CI = 0.91–1.19; Ptrend = 0.57) compared to women consuming ≤2 servings/day. No departure from linearity was observed for total vegetable intake (results not shown).
When the association between specific fruit groups and endometriosis risk was examined, citrus fruits (oranges, grapefruit, orange juice, grapefruit juice) were associated with a lower risk of endometriosis. Women consuming ≥1 servings of citrus fruits/day had a 22% lower endometriosis risk (95% CI = 0.69–0.89; Ptrend = 0.004) compared to those consuming <1 serving/week (Table II). When this relationship was evaluated by case subtype, this inverse association was only observed among women who had never reported infertility (RR = 0.75; 95% CI = 0.65–0.87; Ptrend = 0.006); however, the test for heterogeneity between the two case types was not significant (Pheterogeneity = 0.76). When individual citrus fruits were examined, oranges demonstrated the strongest association with endometriosis risk (Table III).
Table III.
Multivariable1 adjusted HR and 95% CIs for laparoscopically confirmed endometriosis according to intake of selected fruits and vegetables among 70,835 women in the Nurses’ Health Study II, 1991–2013.
| <1/month | 1-3/month | 1-3/week | ≥4/week | P trend 2 | |
|---|---|---|---|---|---|
| Fruits | |||||
| Oranges | 1.00 (Referent) | 0.92 (0.84, 1.01) | 0.87 (0.76, 0.98) | 0.73 (0.58, 0.93) | 0.005 |
| Orange juice | 1.00 (Referent) | 0.96 (0.86, 1.07) | 0.99 (0.87, 1.11) | 0.89 (0.78, 1.01) | 0.10 |
| Grapefruit/grapefruit juice | 1.00 (Referent) | 0.89 (0.82, 0.98) | 0.95 (0.82, 1.10) | 0.89 (0.69, 1.16) | 0.20 |
| Apples/pears | 1.00 (Referent) | 0.99 (0.85, 1.14) | 0.88 (0.76, 1.03) | 0.96 (0.81, 1.14) | 0.32 |
| Apple juice/cider | 1.00 (Referent) | 0.96 (0.88, 1.04) | 0.93 (0.81, 1.06) | 0.76 (0.58, 0.98) | 0.02 |
| Strawberries | 1.00 (Referent) | 0.95 (0.85, 1.06) | 1.08 (0.94, 1.24) | 1.04 (0.79, 1.37) | 0.08 |
| Peaches/plums | 1.00 (Referent) | 0.93 (0.85, 1.03) | 1.03 (0.90, 1.16) | 0.92 (0.70, 1.20) | 0.63 |
| Avocado3 | 1.00 (Referent) | 0.95 (0.85, 1.05) | 0.63 (0.39, 1.00) | 0.03 | |
| Vegetables | |||||
| Broccoli | 1.00 (Referent) | 0.85 (0.73, 0.99) | 0.89 (0.76, 1.04) | 0.94 (0.75, 1.18) | 0.83 |
| Raw cabbage/coleslaw3 | 1.00 (Referent) | 1.09 (0.97, 1.23) | 1.26 (1.05, 1.51) | 0.02 | |
| Cauliflower3 | 1.00 (Referent) | 1.03 (0.94, 1.12) | 1.16 (1.02, 1.33) | 0.03 | |
| Brussel sprouts3 | 1.00 (Referent) | 1.11 (1.01, 1.22) | 1.30 (1.04, 1.63) | 0.02 | |
| Romaine/leaf lettuce | 1.00 (Referent) | 0.96 (0.85, 1.08) | 0.96 (0.85, 1.08) | 0.85 (0.73, 0.99) | 0.09 |
| Peas/lima beans3 | 1.00 (Referent) | 1.06 (0.96, 1.17) | 1.25 (1.10, 1.42) | 0.0002 | |
| Corn | 1.00 (Referent) | 0.99 (0.86, 1.15) | 1.15 (0.97, 1.35) | 1.51 (1.12, 2.02) | <0.0001 |
| Carrots | 1.00 (Referent) | 1.05 (0.87, 1.26) | 1.05 (0.87, 1.27) | 1.06 (0.88, 1.28) | 0.40 |
| Green/red pepper | 1.00 (Referent) | 0.90 (0.82, 1.00) | 0.94 (0.83, 1.06) | 0.88 (0.72, 1.06) | 0.49 |
1Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51+ days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), BMI (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2), and energy (continuous).
2Determined using category medians.
3When there were ≤30 cases endometriosis cases in the ≥4/week category the ≥4/week and 1–3/week categories were combined.
When types of vegetables were examined, women consuming ≥1 servings/day cruciferous vegetables (broccoli, cauliflower, cabbage and Brussel sprouts) had a 13% higher risk of endometriosis (95% CI = 0.95–1.34; Ptrend = 0.03) (Table II). This positive association was apparent only among women who had never reported infertility (RR = 1.12; 95% CI = 0.93–1.36; Ptrend = 0.05) while no higher risk was observed among women reporting concurrent infertility (RR = 0.90; 95% CI = 0.54–1.51; Ptrend = 0.94) (Pheterogeneity = 0.045). When individual cruciferous vegetables were examined Brussel sprouts, raw cabbage/coleslaw, and cauliflower were all related to higher endometriosis risk (Table III). Among other types of vegetables, intake of corn and peas/lima beans was also associated with a higher risk while romaine/leaf lettuce was associated with a lower risk (Table III).
Beta-cryptoxanthin intake had an inverse association with endometriosis risk (RRfifth quintile = 0.88; 95% CI = 0.78–1.00; Ptrend = 0.02) (Table IV). As expected, the association between beta-cryptoxanthin and endometriosis was no longer significant after adjustment for citrus fruits, the main source of beta-cryptoxanthin in this population. No statistically significant associations were observed for any of the other nutrients examined (RAE, beta-carotene, alpha-carotene, lycopene and lutein/zeaxanthin) and endometriosis risk (Table IV).
Table IV.
Hazard ratios1 and 95% CIs for laparoscopically confirmed endometriosis according to nutrient intake by infertility status among 70 835 women in the Nurses’ Health Study II, 1991–2013.
| Nutrient quintiles | Case definition | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All women (n = 2609) | Never infertile2 (n = 2114) | Concurrent infertility2 (n = 313) | ||||||||
| Cases | RR (95% CI) | Cases | RR (95% CI) | Cases | RR (95% CI) | P heterogeneity 3 | ||||
| Total retinol activity equivalents | ||||||||||
| 1 | 574 | 1.00 | Referent | 469 | 1.00 | Referent | 74 | 1.00 | Referent | 0.84 |
| 2 | 515 | 0.97 | (0.86, 1.09) | 427 | 0.97 | (0.85, 1.11) | 55 | 0.98 | (0.69, 1.39) | |
| 3 | 468 | 0.95 | (0.84, 1.07) | 387 | 0.97 | (0.84, 1.11) | 45 | 0.99 | (0.68, 1.44) | |
| 4 | 524 | 1.03 | (0.91, 1.17) | 423 | 1.04 | (0.91, 1.19) | 57 | 1.07 | (0.75, 1.52) | |
| 5 | 528 | 0.93 | (0.83, 1.05) | 408 | 0.91 | (0.79, 1.04) | 82 | 1.13 | (0.82, 1.55) | |
| Ptrend4 | 0.48 | 0.29 | 0.36 | |||||||
| Retinol activity equivalents from foods | ||||||||||
| 1 | 562 | 1.00 | Referent | 448 | 1.00 | Referent | 73 | 1.00 | Referent | 0.29 |
| 2 | 548 | 1.06 | (0.94, 1.19) | 463 | 1.11 | (0.97, 1.26) | 53 | 0.97 | (0.68, 1.39) | |
| 3 | 498 | 0.98 | (0.87, 1.11) | 417 | 1.02 | (0.89, 1.16) | 49 | 0.94 | (0.65, 1.36) | |
| 4 | 469 | 0.91 | (0.80, 1.03) | 377 | 0.90 | (0.78, 1.03) | 59 | 1.15 | (0.81, 1.63) | |
| 5 | 532 | 0.95 | (0.84, 1.07) | 409 | 0.91 | (0.80, 1.04) | 79 | 1.13 | (0.82, 1.57) | |
| Ptrend4 | 0.11 | 0.02 | 0.29 | |||||||
| Total beta carotene | ||||||||||
| 1 | 563 | 1.00 | Referent | 461 | 1.00 | Referent | 66 | 1.00 | Referent | 0.46 |
| 2 | 490 | 0.94 | (0.84, 1.07) | 394 | 0.92 | (0.80, 1.05) | 64 | 1.25 | (0.88, 1.77) | |
| 3 | 517 | 1.03 | (0.91, 1.16) | 420 | 1.01 | (0.88, 1.15) | 64 | 1.36 | (0.96, 1.93) | |
| 4 | 536 | 1.08 | (0.95, 1.21) | 425 | 1.04 | (0.91, 1.18) | 64 | 1.33 | (0.94, 1.89) | |
| 5 | 503 | 0.98 | (0.86, 1.10) | 414 | 0.98 | (0.86, 1.13) | 55 | 1.03 | (0.71, 1.48) | |
| Ptrend4 | 0.81 | 0.71 | 0.97 | |||||||
| Beta carotene from food | ||||||||||
| 1 | 603 | 1.00 | Referent | 488 | 1.00 | Referent | 75 | 1.00 | Referent | 0.90 |
| 2 | 469 | 0.84 | (0.75, 0.95) | 386 | 0.84 | (0.74, 0.97) | 55 | 0.93 | (0.65, 1.32) | |
| 3 | 500 | 0.93 | (0.82, 1.05) | 403 | 0.91 | (0.79, 1.04) | 60 | 1.13 | (0.80, 1.59) | |
| 4 | 522 | 0.98 | (0.87, 1.10) | 428 | 0.98 | (0.85, 1.11) | 58 | 1.10 | (0.78, 1.56) | |
| 5 | 515 | 0.93 | (0.83, 1.05) | 409 | 0.91 | (0.79, 1.03) | 65 | 1.09 | (0.77, 1.53) | |
| Ptrend4 | 0.92 | 0.62 | 0.47 | |||||||
| Total alpha carotene | ||||||||||
| 1 | 585 | 1.00 | Referent | 462 | 1.00 | Referent | 87 | 1.00 | Referent | 0.99 |
| 2 | 501 | 0.96 | (0.85, 1.08) | 411 | 0.98 | (0.85, 1.12) | 58 | 0.94 | (0.67, 1.31) | |
| 3 | 487 | 0.97 | (0.86, 1.09) | 400 | 0.98 | (0.85, 1.12) | 57 | 1.00 | (0.71, 1.40) | |
| 4 | 531 | 1.07 | (0.95, 1.20) | 432 | 1.07 | (0.94, 1.23) | 52 | 0.97 | (0.69, 1.38) | |
| 5 | 505 | 0.97 | (0.86, 1.09) | 409 | 0.97 | (0.85, 1.11) | 59 | 0.94 | (0.67, 1.31) | |
| Ptrend4 | 0.61 | 0.69 | 0.79 | |||||||
| Total beta cryptoxanthin | ||||||||||
| 1 | 594 | 1.00 | Referent | 485 | 1.00 | Referent | 73 | 1.00 | Referent | 0.56 |
| 2 | 518 | 0.98 | (0.87, 1.10) | 410 | 0.93 | (0.82, 1.06) | 69 | 1.30 | (0.93, 1.81) | |
| 3 | 537 | 1.04 | (0.92, 1.16) | 444 | 1.03 | (0.90, 1.17) | 55 | 1.10 | (0.77, 1.56) | |
| 4 | 487 | 0.93 | (0.83, 1.05) | 390 | 0.90 | (0.78, 1.03) | 57 | 1.13 | (0.80, 1.61) | |
| 5 | 473 | 0.88 | (0.78, 1.00) | 385 | 0.87 | (0.76, 0.99) | 59 | 1.03 | (0.72, 1.45) | |
| Ptrend4 | 0.02 | 0.03 | 0.78 | |||||||
| Total lycopene | ||||||||||
| 1 | 586 | 1.00 | Referent | 473 | 1.00 | Referent | 82 | 1.00 | Referent | 0.91 |
| 2 | 495 | 0.94 | (0.83, 1.06) | 392 | 0.90 | (0.79, 1.03) | 65 | 1.06 | (0.76, 1.48) | |
| 3 | 501 | 0.99 | (0.87, 1.11) | 418 | 0.99 | (0.87, 1.13) | 52 | 0.95 | (0.67, 1.36) | |
| 4 | 475 | 0.96 | (0.85, 1.08) | 397 | 0.96 | (0.84, 1.10) | 46 | 0.93 | (0.64, 1.34) | |
| 5 | 552 | 1.04 | (0.93, 1.17) | 434 | 0.99 | (0.87, 1.13) | 68 | 1.07 | (0.77, 1.48) | |
| Ptrend4 | 0.29 | 0.77 | 0.83 | |||||||
| Total lutein and zeaxanthin | ||||||||||
| 1 | 549 | 1.00 | Referent | 442 | 1.00 | Referent | 65 | 1.00 | Referent | 0.62 |
| 2 | 532 | 1.06 | (0.94, 1.19) | 438 | 1.07 | (0.93, 1.22) | 56 | 1.14 | (0.79, 1.64) | |
| 3 | 452 | 0.90 | (0.79, 1.02) | 355 | 0.87 | (0.76, 1.00) | 59 | 1.14 | (0.80, 1.63) | |
| 4 | 524 | 1.04 | (0.92, 1.17) | 427 | 1.04 | (0.91, 1.19) | 67 | 1.31 | (0.93, 1.86) | |
| 5 | 552 | 1.04 | (0.92, 1.17) | 452 | 1.06 | (0.93, 1.21) | 66 | 1.06 | (0.75, 1.51) | |
| Ptrend4 | 0.53 | 0.38 | 0.74 | |||||||
1Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51+ days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), BMI (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2), and energy (continuous).
2Infertility is defined as attempting to become pregnant for >1 year without success. Cases with ‘no past or concurrent infertility’ are women who never reported infertility. Cases with ‘concurrent infertility’ are women who reported an infertility evaluation in the same follow-up cycle as laparoscopic-confirmation of endometriosis.
3Test for heterogeneity comparing the effect of nutrient consumption among women with no past or current infertility to those with concurrent infertility.
4Determined using category medians.
The associations between fruit and vegetable intake and endometriosis risk were consistent across different time intervals between dietary intake and endometriosis diagnosis (Table V). The strongest inverse associations were observed for fruit intake 4–6 years before endometriosis diagnosis; those consuming ≥4 servings/day had RR = 0.82 (95% CI = 0.68–0.99; Ptrend = 0.03) compared to those consuming <1 serving/day. This differential association was not observed for the citrus fruits. No associations were observed with total vegetable intake for any time interval. In addition, adjustment for intake of trans fat and omega-3 fatty acids, which have previously been associated with endometriosis in this cohort, did not influence the results.
Table V.
Hazard ratios and 95% CIs for laparoscopically confirmed endometriosis according to fruit intake at different time points among women in the Nurses’ Health Study II, 1991–2013.
| Baseline | Cumulative average | Simple update | 4–6 year lag | 6–8 year lag | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Multivariable1 HR (95% CI) | Cases | Multivariable1 HR (95% CI) | Cases | Multivariable1 HR (95% CI) | Cases | Multivariable1 HR (95% CI) | Cases | Multivariable1 HR (95% CI) | |
| Total fruit intake | ||||||||||
| <1/day | 604 | 1.00 (Referent) | 516 | 1.00 (Referent) | 615 | 1.00 (Referent) | 321 | 1.00 (Referent) | 231 | 1.00 (Referent) |
| 1/day | 200 | 0.93 (0.79, 1.09) | 218 | 1.00 (0.85, 1.17) | 206 | 0.95 (0.81, 1.11) | 101 | 0.89 (0.71, 1.11) | 74 | 0.91 (0.70, 1.18) |
| 2/day | 881 | 0.91 (0.82, 1.01) | 941 | 0.92 (0.82, 1.02) | 812 | 0.85 (0.76, 0.94) | 471 | 0.91 (0.79, 1.06) | 358 | 0.96 (0.81, 1.14) |
| 3/day | 502 | 0.85 (0.75, 0.97) | 541 | 0.86 (0.76, 0.98) | 460 | 0.80 (0.70, 0.91) | 260 | 0.83 (0.70, 0.99) | 214 | 0.95 (0.78, 1.15) |
| ≥4/day | 422 | 0.94 (0.82, 1.07) | 393 | 0.93 (0.80, 1.07) | 390 | 0.90 (0.78, 1.03) | 200 | 0.82 (0.68, 0.99) | 153 | 0.87 (0.70, 1.09) |
| Ptrend2 | 0.27 | 0.16 | 0.07 | 0.03 | 0.28 | |||||
| Total vegetable intake | ||||||||||
| ≤2/day | 780 | 1.00 (Referent) | 711 | 1.00 (Referent) | 730 | 1.00 (Referent) | 351 | 1.00 (Referent) | 272 | 1.00 (Referent) |
| 3/day | 667 | 1.01 (0.90, 1.12) | 663 | 0.95 (0.85, 1.06) | 555 | 0.86 (0.77, 0.96) | 367 | 1.02 (0.88, 1.18) | 284 | 1.04 (0.88, 1.24) |
| 4/day | 466 | 0.96 (0.85, 1.08) | 508 | 0.93 (0.82, 1.04) | 475 | 0.96 (0.85, 1.08) | 268 | 0.92 (0.78, 1.08) | 175 | 0.81 (0.66, 0.98) |
| 5/day | 291 | 0.96 (0.83, 1.10) | 321 | 0.97 (0.84, 1.11) | 311 | 1.00 (0.87, 1.15) | 160 | 0.91 (0.75, 1.11) | 137 | 1.04 (0.84, 1.29) |
| ≥6/day | 405 | 1.04 (0.91, 1.19) | 406 | 1.04 (0.91, 1.19) | 412 | 1.01 (0.88, 1.15) | 207 | 1.04 (0.87, 1.26) | 162 | 1.08 (0.88, 1.34) |
| Ptrend2 | 0.78 | 0.57 | 0.42 | 0.98 | 0.63 | |||||
1Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51– days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), BMI (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2), and energy (continuous).
2Determined using category medians.
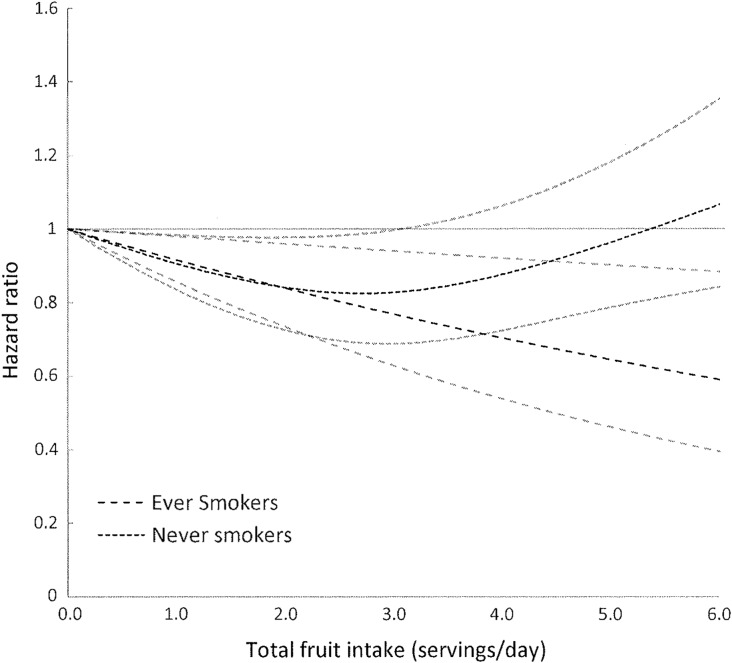
Finally, we assessed whether the associations between fruit and vegetable intake or related nutrients were modified by cigarette smoking. The protective effect of fruit consumption, total and specific groups, was stronger among ever smokers compared to never smokers (Table VI). This was particularly apparent for total fruits (Fig. 3) and rosaceae fruits, while for citrus fruits a protective effect was observed for both ever and never smokers. No effect modification by smoking was observed for vegetable intake, total or specific groups, or by nutrient intake (RAE, beta-carotene, alpha-carotene, beta-cryptoxanthin lycopene and lutein/zeaxanthin) (results not shown).
Table VI.
Hazard ratios and 95% CIs for laparoscopically confirmed endometriosis according to intake of fruits stratified by smoking status, Nurses’ Health Study II, 1991–2013.
| Intake in servings per day or week | Never smoker | Ever smoker | |||||
|---|---|---|---|---|---|---|---|
| Cases | Multivariable1 HR (95% CI) | Cases | Multivariable1 HR (95% CI) | P interaction 2 | |||
| Total fruits | |||||||
| <1/day | 313 | 1.00 | Referent | 203 | 1.00 | Referent | 0.05 |
| 1/day | 134 | 0.95 | (0.78, 1.15) | 84 | 1.08 | (0.85, 1.36) | |
| 2/day | 642 | 0.93 | (0.82, 1.04) | 296 | 0.90 | (0.78, 1.04) | |
| 3/day | 393 | 0.90 | (0.78, 1.03) | 147 | 0.79 | (0.66, 0.96) | |
| ≥4/day | 297 | 0.98 | (0.84, 1.14) | 95 | 0.80 | (0.63, 1.00) | |
| P trend 3 | 0.99 | 0.008 | |||||
| Citrus fruits4 | |||||||
| <1/week | 256 | 1.00 | Referent | 152 | 1.00 | Referent | 0.11 |
| 1/week | 198 | 0.83 | (0.70, 0.99) | 110 | 0.86 | (0.69, 1.06) | |
| 2–6/week | 871 | 0.87 | (0.77, 0.98) | 404 | 0.84 | (0.73, 0.96) | |
| ≥1/day | 454 | 0.81 | (0.71, 0.93) | 159 | 0.71 | (0.59, 0.85) | |
| Ptrend3 | 0.05 | 0.006 | |||||
| Rosaceae fruits5 | |||||||
| <1/week | 92 | 1.00 | Referent | 75 | 1.00 | Referent | 0.12 |
| 1/week | 128 | 1.02 | (0.81, 1.28) | 65 | 0.81 | (0.60, 1.08) | |
| 2–6/week | 1045 | 0.91 | (0.77, 1.07) | 499 | 0.91 | (0.76, 1.09) | |
| ≥1/day | 514 | 0.91 | (0.76, 1.09) | 186 | 0.78 | (0.63, 0.96) | |
| Ptrend3 | 0.70 | 0.04 | |||||
| Fruit juice | |||||||
| <1/week | 257 | 1.00 | Referent | 169 | 1.00 | Referent | 0.07 |
| 1/week | 187 | 1.07 | (0.90, 1.27) | 106 | 1.11 | (0.90, 1.38) | |
| 2–6/week | 767 | 1.08 | (0.95, 1.22) | 359 | 1.02 | (0.89, 1.18) | |
| ≥1/day | 568 | 1.00 | (0.87, 1.14) | 191 | 0.86 | (0.73, 1.03) | |
| Ptrend3 | 0.52 | 0.02 | |||||
1Multivariable model was stratified by age in months at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for age at menarche (<10, 10, 11, 12, 13, 14, 15, 16, >16 years), length of menstrual cycle (<26, 26–31, 32–50, 51– days), parity (nulliparous, 1, 2, 3, 4+ pregnancies lasting >6 months), BMI (<19, 19–20.4, 20.5–21.9, 22–24.9, 25–29.9, 30+ kg/m2), and energy (continuous).
2Test for interaction comparing the effect of fruit consumption by smoking status.
3Determined using category medians.
4Citrus fruits include oranges, orange juice, grapefruit and grapefruit juice.
5Rosaceae fruits include prunes, prune juice, apples, apple juice, apple sauce, strawberries and peaches.
Figure 3.
Hazard ratios of endometriosis by total fruit intake stratified by smoking status. Test for non-linearity among never smokers = 0.005, Psignificance of the curve = 0.02. Test for non-linearity among ever smokers > 0.05, Plinear relation = 0.009.
Discussion
In this cohort, we observed a non-linear inverse association between higher fruit consumption and risk of laparoscopically confirmed endometriosis. This inverse association was particularly evident for citrus fruits. In contrast, intake of cruciferous vegetables, corn and peas/lima beans was associated with an increased risk of endometriosis. No significant associations were observed with any of the nutrients examined after adjustment for intake of food sources of these nutrients. In addition, the observed association with fruits was strongest among ever smokers.
Few human studies have examined the association between diet and endometriosis, yielding conflicting results for the examined dietary factors. Two case–control studies have examined the associations between fruit and vegetable intake and endometriosis risk. An Italian hospital-based case–control study compared 504 cases of laparoscopically confirmed endometriosis and 504 controls admitted with non-gynecological conditions and observed a statistically significant lower consumption of green vegetables among cases (odds ratio [OR] = 0.3; 95% CI = 0.2–0.5) and of fresh fruit (OR = 0.6; 95% CI = 0.4–0.8) when comparing the third to first tertiles of intake. This study did not utilize a validated complete dietary assessment and was unable to adjust for total caloric intake or account for other dietary components (Parazzini et al., 2004). In contrast, a population-based case–control study among 284 cases and 660 controls who were members of a health care organization in WA state, USA, reported a greater odds of endometriosis with greater fruit intake (OR = 1.5; 95% CI = 1.2–2.3 for >2 servings/day versus ≤1/day) and no association with vegetable intake (OR = 1.0; 95% CI = 0.6–1.7 for >3 servings/day versus ≤1/day) (Trabert et al., 2010). A major limitation of both of these studies was the retrospective collection of dietary data that were limited to examining diet in the year preceding endometriosis diagnosis, while our results represent prospectively collected diet over 2 decades. Trabert et al. (2010) speculated that pesticide exposure through fruit intake might explain the increased endometriosis risk observed in their study. Our data do not support this hypothesis as we observed an inverse association with fruits consumed during a time period that overlapped with the WA study. In addition, the vegetables that we observed to be positively associated with endometriosis have a low pesticide residue burden (Chiu et al., 2015). In the WA case population the women reporting infertility (23% of endometriosis cases) may have increased their fruit intake during the previous year in an attempt to improve fertility and could have been diagnosed with endometriosis during an evaluation for prolonged time to conception resulting in the observed association.
In-vitro and in-vivo studies have suggested a number of nutrients that are present in fruits and vegetables that could influence endometriosis risk. Citrus fruits were the most strongly associated with reduced risk of endometriosis in our study and are high in vitamins A and C. Mier-Cabrera et al. (2009) compared antioxidant intake in 83 infertile women with endometriosis to 80 parous women undergoing tubal ligation and observed lower intake of vitamins A, C, and E among women with endometriosis. The growth and adhesion of endometrial cells in the peritoneal cavity may be influenced by free radicals and reactive oxygen species (ROS) and vitamin C may counteract the effect of free radicals and ROS (Jackson et al., 2005). We have previously observed in this NHS II population that vitamin C from foods, but not vitamin C from foods and supplements combined or supplements alone, was associated with endometriosis risk (Darling et al., 2013). This suggests that the vitamin C in citrus foods may not explain the reduced risk observed or that there may be a threshold of intake after which vitamin C does not influence endometriosis risk. Vitamin A intake could also influence risk as Sawatsri et al. (2000) have shown that retinoids may play a role in altering the aberrant production of cytokines in endometriosis, as retinoic acid has been found to suppress IL-6 molecular transcription and translational processes in a time and dose-dependent manner. In addition, vascular endothelial growth factor (VEGF) is thought to contribute to the angiogenesis of endometriosis lesions, and treatment of HL-60 cells differentiated into neutrophil granulocytes with all-trans retinoic acid (atRA) has been shown to suppress VEGF mRNA and protein (Tee et al., 2006). Consumption of citrus fruits that are high in beta-cryptoxanthin has been demonstrated to increase serum retinol (de Pee et al., 1998) suggesting a potential mechanism for our observed associations.
In contrast to the inverse association with citrus fruits, we observed that cruciferous vegetables, particularly cauliflower, cabbage, Brussel sprouts, were associated with increased endometriosis risk. This result was not what we had hypothesized considering that these vegetables contain various phytochemicals and nutrients that have been demonstrated to have health benefits, as well as being a good source of dietary fiber. However, cruciferous vegetables may not be as easily absorbed or digested, and some are high in fermentable oligo-, di- and monosaccharides and polyols (FODMAPs), which have been reported to exacerbate irritable bowel syndrome symptoms (Eswaran et al., 2016). Gastrointestinal symptoms are almost as common as gynecological symptoms in women with endometriosis, and presenting with these symptoms is often the first step toward obtaining a surgical confirmation of endometriosis (Maroun et al., 2009). Thus, the observed association could be due to increased abdominal pain in women consuming cruciferous vegetables that subsequently results in an endometriosis diagnosis. Given the difficulty in accurately quantifying general gastroenterologic distress symptoms and distinguishing them from chronic pelvic pain, we do not have the data necessary to validly stratify by presence or absence of gastrointestinal symptoms. However, the slightly stronger association between cruciferous vegetable intake and the never infertile case group, the case group most likely to have pain as the indication for their surgical diagnosis, provides some support for this hypothesis. In addition, previous studies have observed an increased risk of hypertension among women with higher intake of cruciferous vegetables (Borgi et al., 2016; Wang et al., 2012). The mechanism(s) behind this increased risk are unclear but cooking methods or use of pesticides are possible explanations (Borgi et al., 2016).
Stronger inverse associations were observed between fruit and endometriosis risk among women who were ever smokers. Higher oxidative stress and production of free radicals among smokers may explain the stronger protective association observed in ever smokers. These results are consistent with other studies that have observed stronger inverse associations in smokers between fruit intake and conditions including chronic obstructive pulmonary disease (Kaluza et al., 2017), cholecystectomy (Tsai et al., 2006) and cardiovascular disease (Hung et al., 2004). Further, among women who were ever smokers the risk of endometriosis decreased linearly with increasing fruit consumption while among women who had never smoked the inverse association was non-linear. This may indicate that the ideal level of fruit consumption in relation to endometriosis risk may be mediated by individual-level factors such as oxidative stress and free radical exposure.
Limitations of our study should be considered. Some error in the self-reporting of dietary intake is expected. However, the FFQ has been previously validated for both foods and nutrients (Michaud et al., 1998; Salvini et al., 1989; Willett, 2013; Willett et al., 1985; Yuan et al., 2017). In addition, we had dietary information collected at multiple time points, allowing quantification of cumulative average intake, which reduces measurement error due to within-person variation over time (Hu et al., 1999) and the prospective nature of our study makes it likely that any misclassification would be non-differential. Strengths include its large, prospective design with high follow-up rates over 22 years making it the largest study to date to examine the associations between fruits, vegetables, and related nutrients and laparoscopically confirmed endometriosis. In addition, we were able to apply rigorous modeling to adjust for total caloric intake and quantify the association with fruits and vegetables independent of other dietary components. In addition, we were able to examine varying diet windows in relation to likely endometriosis onset, to examine the associations by case subtype, and to explore potential effect modification by cigarette smoking.
In conclusion, our findings within this cohort of US nurses who had not been diagnosed with endometriosis nor experienced infertility prior to study start, suggest that higher intake of fruits, particularly citrus fruits, are associated with a lower risk of endometriosis and beta-cryptoxanthin in these foods may partially explain this association. In addition, consumption of specific vegetables increased endometriosis risk, which may indicate a role of gastrointestinal symptoms in both the presentation and exacerbation of endometriosis-related pain; however, it is not clear what components of these foods might underlie the observed associations. Future studies examining dietary patterns that consider different combinations of food intake may help clarify these associations.
Authors’ roles
S.A.M., H.R.H., A.C.E. and J.E.C. designed the research, H.R.H. performed statistical analyses, S.A.M., H.R.H., A.C.E. and J.E.C. interpreted the data, H.R.H. and A.C.E. wrote the article, S.A.M. and J.E.C. edited article drafts. All authors read and approved the final article.
Funding
Research Grants (HD4854, HD52473 and HD57210) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and Grant (P30 DK046200) from the National Institutes of Diabetes and Digestive and Kidney Diseases. The Nurses’ Health Study II is supported by the Public Health Service Grant (UM1 CA176726) from the National Cancer Institute, NIH, U.S. Department of Health and Human Services. HRH is supported by the National Cancer Institute, National Institutes of Health (K22 CA193860).
Conflict of interest
None.
References
- Al-Delaimy WK, Mahoney GN, Speizer FE, Willett WC. Toenail nicotine levels as a biomarker of tobacco smoke exposure. Cancer Epidemiol Biomarkers Prev 2002;11:1400–1404. [PubMed] [Google Scholar]
- Borgi L, Muraki I, Satija A, Willett WC, Rimm EB, Forman JP. Fruit and vegetable consumption and the incidence of hypertension in three prospective cohort studies. Hypertension 2016;67:288–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M, MacDonald P, Andersson S. 17 bets-Hydroxysteroid dehydrogenase type 2: chromosomal assignment and progestin regulation of gene expression in human endometrium. J Clin Invest 1994;94:2135–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, Hauser R, Chavarro JE. Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic. Hum Reprod 2015;30:1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling A, Chavarro J, Malspeis S, Harris H, Missmer S. A prospective cohort study of Vitamins B, C, E, and multivitamin intake and endometriosis. J Endometr 2013;5:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pee S, West CE, Permaesih D, Martuti S, Muhilal, Hautvast JG. Orange fruit is more effective than are dark-green, leafy vegetables in increasing serum concentrations of retinol and beta-carotene in schoolchildren in Indonesia. Am J Clin Nutr 1998;68:1058–1067. [DOI] [PubMed] [Google Scholar]
- Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–561. [DOI] [PubMed] [Google Scholar]
- Eisenberg VH, Zolti M, Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun Rev 2012;11:806–814. [DOI] [PubMed] [Google Scholar]
- Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE Guidelines in US adults with IBS-D. Am J Gastroenterol 2016;111:1824–1832. [DOI] [PubMed] [Google Scholar]
- Harada T, Yoshioka H, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, Terakawa N. Increased interleukin-6 in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol 1997;176:593–597. [DOI] [PubMed] [Google Scholar]
- Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol 1999;149:531–540. [DOI] [PubMed] [Google Scholar]
- Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst 2004;96:1577–1584. [DOI] [PubMed] [Google Scholar]
- Jackson LW, Schisterman EF, Dey-Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Hum Reprod 2005;20:2014–2020. [DOI] [PubMed] [Google Scholar]
- Kaluza J, Larsson SC, Orsini N, Linden A, Wolk A. Fruit and vegetable consumption and risk of COPD: a prospective cohort study of men. Thorax 2017;72:500–509. [DOI] [PubMed] [Google Scholar]
- Lebovic DI, Mueller MD, Taylor RN. Immunobiology of endometriosis. Fertil Steril 2001;75:1–10. [DOI] [PubMed] [Google Scholar]
- Maroun P, Cooper M, Reid G, Keirse M. Relevance of gastrointestinal symptoms in endometriosis. Aust N Z J Obstet Gynaecol 2009;49:411–414. [DOI] [PubMed] [Google Scholar]
- Michaud DS, Giovannucci EL, Ascherio A, Rimm EB, Forman MR, Sampson L, Willett WC. Associations of plasma carotenoid concentrations and dietary intake of specific carotenoids in samples of two prospective cohort studies using a new carotenoid database. Cancer Epidemiol, Biomarkers Prev 1998;7:283–290. [PubMed] [Google Scholar]
- Mier-Cabrera J, Aburto-Soto T, Burrola-Mendez S, Jimenez-Zamudio L, Tolentino M, Casanueva E, Hernandez-Guerrero C. Women with endometriosis improved their peripheral antioxidant markers after the application of a high antioxidant diet. Reprod Bio Endocrinol 2009;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen O. Theoretical Epidemiology: Principles of Occurrence Research in Medicine. New York: John Wiley & Sons, 1985. [Google Scholar]
- Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, Barbieri RL, Willett WC, Hankinson SE. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod 2010;25:1528–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am 2003;30:1–19. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 2004;160:784–796. [DOI] [PubMed] [Google Scholar]
- Nothnick WB. Treating endometriosis as an autoimmune disease. Fertil Steril 2001;76:223–231. [DOI] [PubMed] [Google Scholar]
- Nutrient Data Laboratory ARS Department of agriculture In: USDA Nutrient Database for Standard Reference, Release, 13 Washington, DC: US Department of Agriculture, 1999. [Google Scholar]
- Parazzini F, Chiaffarino F, Surace M, Chatenoud L, Cipriani S, Chiantera V, Benzi G, Fedele L. Selected food intake and risk of endometriosis. Hum Reprod 2004;19:1755–1759. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994;84:1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen J, Teisala K, Ranta H, Bennett B, Punnonen R. Increased levels of interleukin-6 and interleukin-10 in the peritoneal fluid of patients with endometriosis. Am J Obstet Gynecol 1996;174:1522–1526. [DOI] [PubMed] [Google Scholar]
- Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT. Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update 2014;20:702–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858–867. [DOI] [PubMed] [Google Scholar]
- Sawatsri S, Desai N, Rock J, Sidell N. Retinoic acid suppresses interleukin-6 production in human endometrial cells. Fertil Steril 2000;73:1012–1019. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Stampfer MJ, Speizer FE, Spiegelman D, Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. J Am Med Assoc 1997;278:1078–1083. [PubMed] [Google Scholar]
- Tee M, Vigne J, Taylor R. All-trans retinoic acid inhibits vascular endothelial growth factor expression in a cell model of neutrophil activation. Endocrinology 2006;147:1264–1270. [DOI] [PubMed] [Google Scholar]
- Trabert B, Peters U, De Roos AJ, Scholes D, Holt VL. Diet and risk of endometriosis in a population-based case-control study. Br J Nutr 2010;105:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar S, O’Connor D, O’Connor V, Martin N. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril 1999;71:701–710. [DOI] [PubMed] [Google Scholar]
- Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Fruit and vegetable consumption and risk of cholecystectomy in women. Am J Med 2006;119:760–767. [DOI] [PubMed] [Google Scholar]
- Wang L, Manson JE, Gaziano JM, Buring JE, Sesso HD. Fruit and vegetable intake and the risk of hypertension in middle-aged and older women. Am J Hypertens 2012;25:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology, 3rd edn Oxford: Oxford University Press, 2013. [Google Scholar]
- Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570–584. [DOI] [PMC free article] [PubMed] [Google Scholar]